Submitted:
26 July 2024
Posted:
29 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Dental Resin Landscape—What Is on The Horizon?
3. Focus on Mechanical Properties
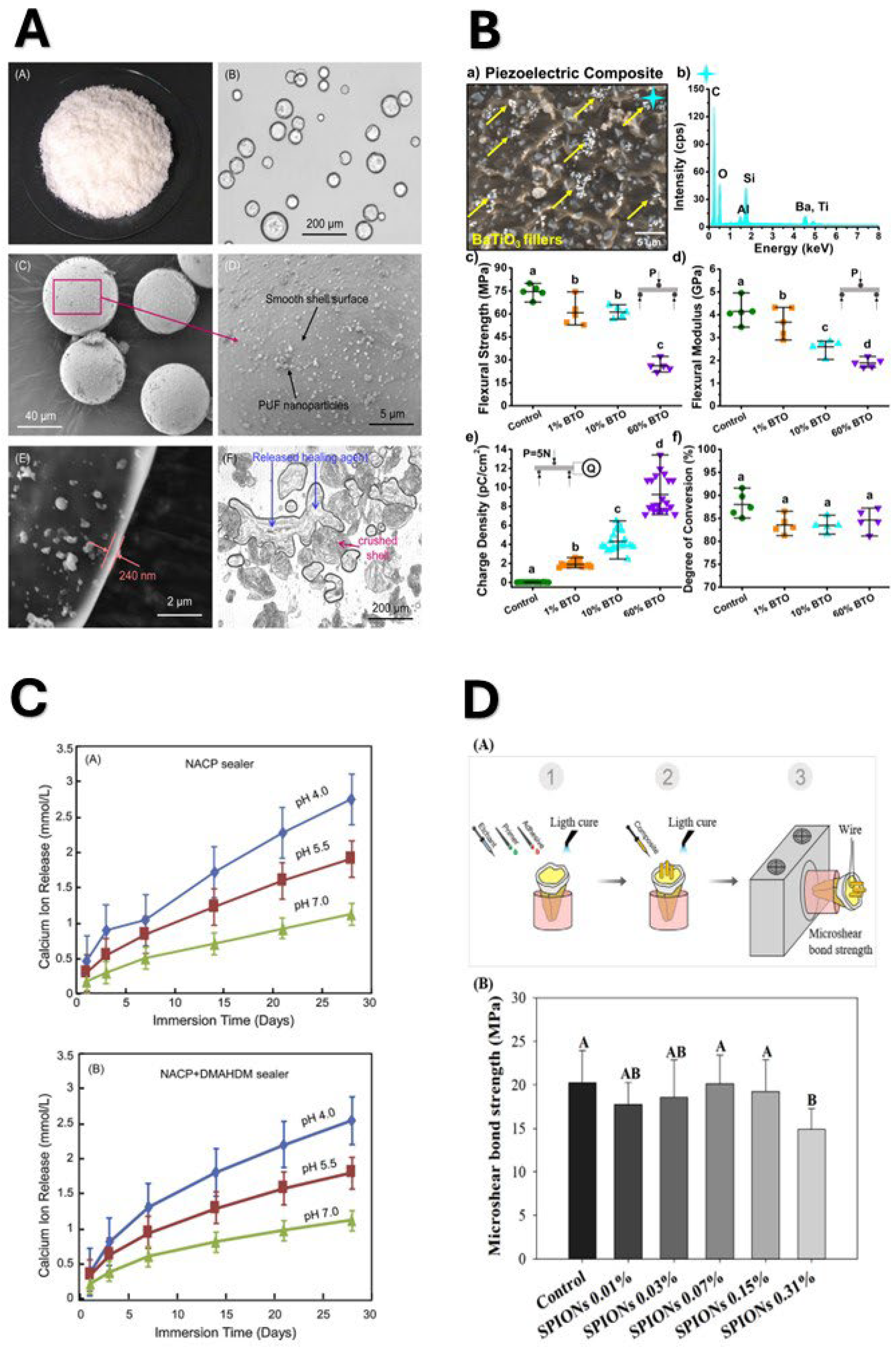
4. Shift Towards Health-Promoting Restorations
5. Dental Materials in the Context of Biomedical Smart Materials
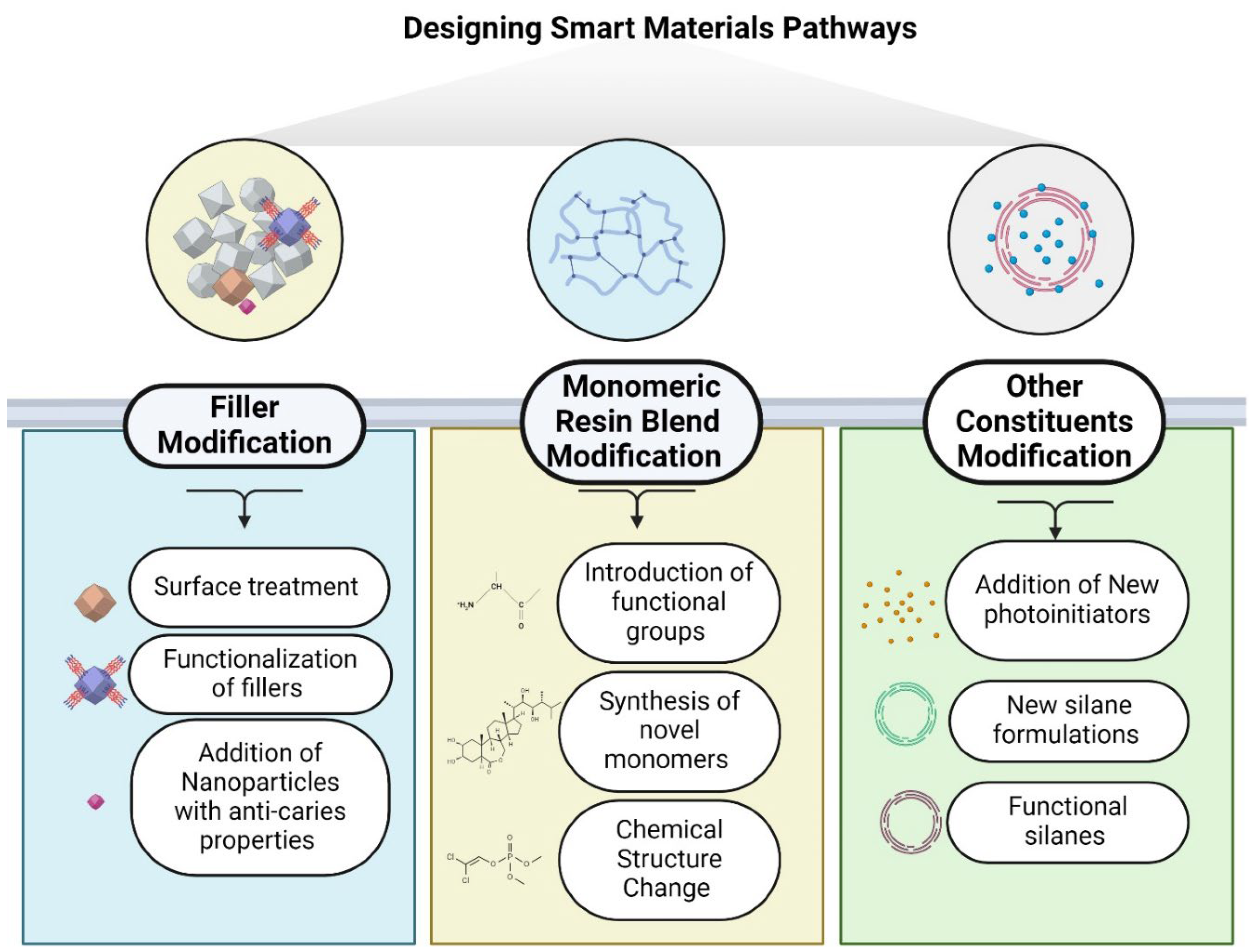
6. Designing Smart Materials Pathways
7. Filler Modification Pathway:
8. Monomeric Organic Resin Modification Pathway:
9. Modification of Other Constituents:
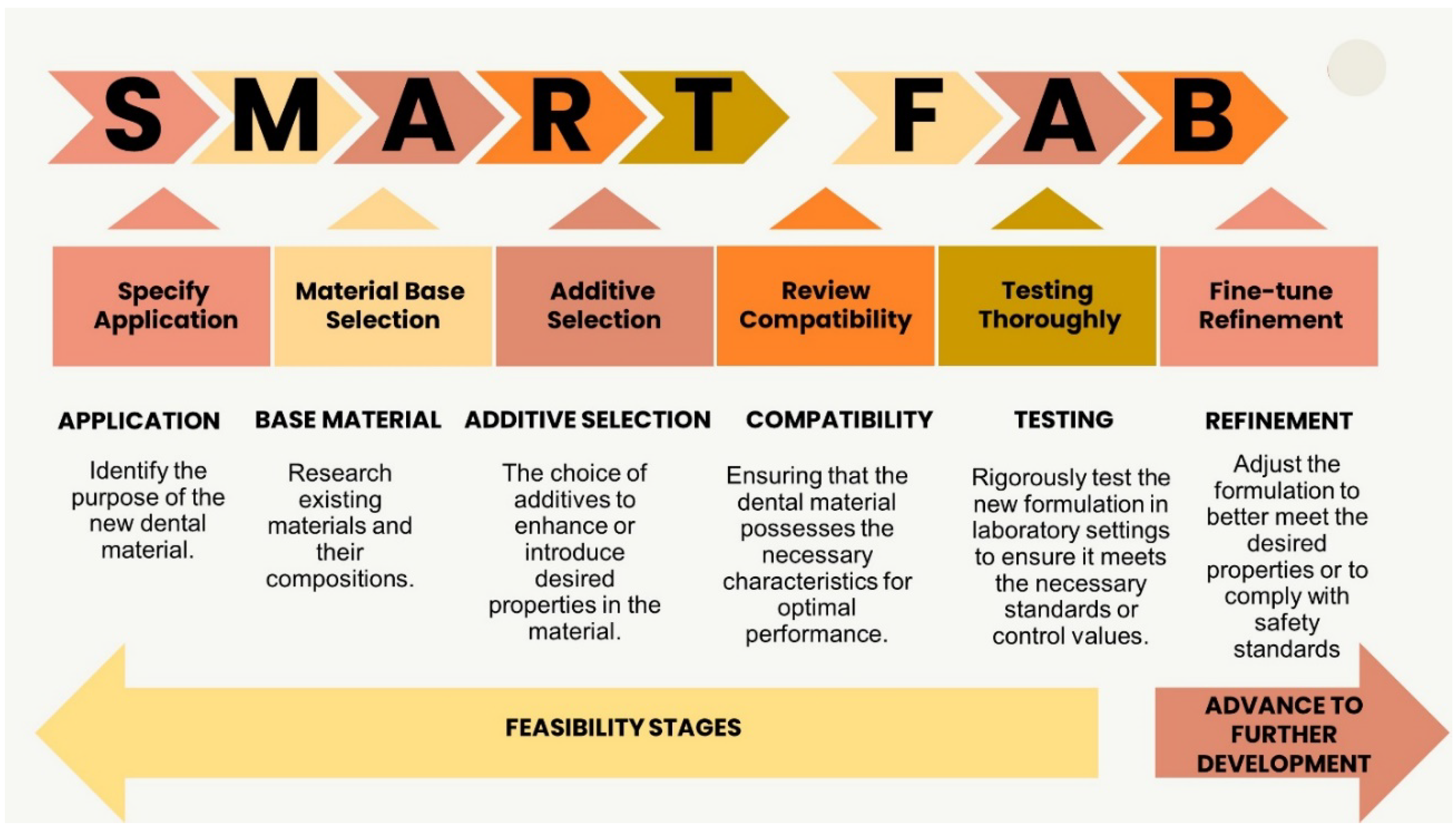
11. Concluding Remarks and Future Perspectives
References
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Cucchiarini, M.; Ramakrishna, S. A Review of Dental Composites: Challenges, Chemistry Aspects, Filler Influences, and Future Insights. Composites Part B: Engineering 2021, 216, 108852. [Google Scholar] [CrossRef]
- Shah, Y.; Shiraguppi, V.; Deosarkar, B.; Shelke, U. Long-Term Survival and Reasons for Failure in Direct Anterior Composite Restorations: A Systematic Review. J Conserv Dent 2021, 24, 415. [Google Scholar] [CrossRef]
- Pizzolotto, L.; Moraes, R.R. Resin Composites in Posterior Teeth: Clinical Performance and Direct Restorative Techniques. Dentistry Journal 2022, 10, 222. [Google Scholar] [CrossRef]
- Cho, K.; Rajan, G.; Farrar, P.; Prentice, L.; Prusty, B.G. Dental Resin Composites: A Review on Materials to Product Realizations. Composites Part B: Engineering 2022, 230, 109495. [Google Scholar] [CrossRef]
- Guo, X.; Yu, Y.; Gao, S.; Zhang, Z.; Zhao, H. Biodegradation of Dental Resin-Based Composite—A Potential Factor Affecting the Bonding Effect: A Narrative Review. Biomedicines 2022, 10, 2313. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Hilton, T.J. Polymerization Stress--Is It Clinically Meaningful? Dent Mater 2016, 32, 1–10. [Google Scholar] [CrossRef]
- Mokeem, L.S.; Garcia, I.M.; Melo, M.A. Degradation and Failure Phenomena at the Dentin Bonding Interface. Biomedicines 2023, 11, 1256. [Google Scholar] [CrossRef]
- Guo, X.; Yu, Y.; Gao, S.; Zhang, Z.; Zhao, H. Biodegradation of Dental Resin-Based Composite—A Potential Factor Affecting the Bonding Effect: A Narrative Review. Biomedicines 2022, 10, 2313. [Google Scholar] [CrossRef]
- Peskersoy, C.; Recen, D.; Kemaloğlu, H. The Effect of Composite Placement Technique on the Internal Adaptation, Gap Formation and Microshear Bond Strength. eor 2021, 0, 0–0. [Google Scholar] [CrossRef]
- Ástvaldsdóttir, Á.; Dagerhamn, J.; van Dijken, J.W.V.; Naimi-Akbar, A.; Sandborgh-Englund, G.; Tranæus, S.; Nilsson, M. Longevity of Posterior Resin Composite Restorations in Adults – A Systematic Review. Journal of Dentistry 2015, 43, 934–954. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.S.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.; Carvalho, R.M.; Tay, F.R.; et al. Strategies to Prevent Hydrolytic Degradation of the Hybrid Layer-A Review. Dent Mater 2013, 29, 999–1011. [Google Scholar] [CrossRef]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of Degradation of the Hybrid Layer in Adhesive Dentistry and Therapeutic Agents to Improve Bond Durability--A Literature Review. Dent Mater 2016, 32, e41–53. [Google Scholar] [CrossRef]
- Saikaew, P.; Sattabanasuk, V.; Harnirattisai, C.; Chowdhury, A.F.M.A.; Carvalho, R.; Sano, H. Role of the Smear Layer in Adhesive Dentistry and the Clinical Applications to Improve Bonding Performance. Jpn Dent Sci Rev 2022, 58, 59–66. [Google Scholar] [CrossRef]
- Mai, S.; Zhang, Q.; Liao, M.; Ma, X.; Zhong, Y. Recent Advances in Direct Adhesive Restoration Resin-Based Dental Materials With Remineralizing Agents. Front. Dent. Med 2022, 3, 868651. [Google Scholar] [CrossRef]
- Montoya, C.; Roldan, L.; Yu, M.; Valliani, S.; Ta, C.; Yang, M.; Orrego, S. Smart Dental Materials for Antimicrobial Applications. Bioactive Materials 2023, 24, 1–19. [Google Scholar] [CrossRef]
- Cao, W.; Wang, X.; Li, Q.; Ye, Z.; Xing, X. Mechanical Property and Antibacterial Activity of Silver-Loaded Polycation Functionalized Nanodiamonds for Use in Resin-Based Dental Material Formulations. Materials Letters 2018, 220, 104–107. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, Q.; Dai, Z.; Zhu, M.; Xiao, L.; Zhao, Z.; Bai, Y.; Zhang, K. Smart Dental Materials Intelligently Responding to Oral PH to Combat Caries: A Literature Review. Polymers 2023, 15, 2611. [Google Scholar] [CrossRef]
- Syed, M.R.; Bano, N.Z.; Ghafoor, S.; Khalid, H.; Zahid, S.; Siddiqui, U.; Hakeem, A.S.; Asif, A.; Kaleem, M.; Khan, A.S. Synthesis and Characterization of Bioactive Glass Fiber-Based Dental Restorative Composite. Ceramics International 2020, 46, 21623–21631. [Google Scholar] [CrossRef]
- Kolb, C.; Gumpert, K.; Wolter, H.; Sextl, G. Highly Translucent Dental Resin Composites through Refractive Index Adaption Using Zirconium Dioxide Nanoparticles and Organic Functionalization. Dental Materials 2020, 36, 1332–1342. [Google Scholar] [CrossRef]
- Cavalcante, L.M.; Ferraz, L.G.; Antunes, K.B.; Garcia, I.M.; Schneider, L.F.J.; Collares, F.M. Silane Content Influences Physicochemical Properties in Nanostructured Model Composites. Dental Materials 2021, 37, e85–e93. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Karabela, M.M. Effect of the Amount of 3-Methacyloxypropyltrimethoxysilane Coupling Agent on Physical Properties of Dental Resin Nanocomposites. Dental Materials 2009, 25, 1315–1324. [Google Scholar] [CrossRef]
- Cadenaro, M.; Josic, U.; Maravić, T.; Mazzitelli, C.; Marchesi, G.; Mancuso, E.; Breschi, L.; Mazzoni, A. Progress in Dental Adhesive Materials. J Dent Res 2023, 102, 254–262. [Google Scholar] [CrossRef]
- Ozimek, J.; Łukaszewska, I.; Pielichowski, K. POSS and SSQ Materials in Dental Applications: Recent Advances and Future Outlooks. IJMS 2023, 24, 4493. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Mokeem, L.; Sun, J. Bioactive Restorative Dental Materials—The New Frontier. Dental Clinics of North America 2022, 66, 551–566. [Google Scholar] [CrossRef]
- Montoya, C.; Roldan, L.; Yu, M.; Valliani, S.; Ta, C.; Yang, M.; Orrego, S. Smart Dental Materials for Antimicrobial Applications. Bioactive Materials 2023, 24, 1–19. [Google Scholar] [CrossRef]
- Melo, M. a. S.; Garcia, I.M.; Mokeem, L.; Weir, M.D.; Xu, H.H.K.; Montoya, C.; Orrego, S. Developing Bioactive Dental Resins for Restorative Dentistry. J Dent Res 2023, 102, 1180–1190. [Google Scholar] [CrossRef]
- Azmy, E.; Al-Kholy, M.R.Z.; Fattouh, M.; Kenawi, L.M.M.; Helal, M.A. Impact of Nanoparticles Additions on the Strength of Dental Composite Resin. International Journal of Biomaterials 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, F.; Xie, H.; Gu, N. Nanoparticle-Reinforced Resin-Based Dental Composites. J Dent 2008, 36, 450–455. [Google Scholar] [CrossRef]
- Ferracane, J.L. A Historical Perspective on Dental Composite Restorative Materials. Journal of Functional Biomaterials 2024, 15, 173. [Google Scholar] [CrossRef]
- Bapat, R.A.; Yang, H.J.; Chaubal, T.V.; Dharmadhikari, S.; Abdulla, A.M.; Arora, S.; Rawal, S.; Kesharwani, P. Review on Synthesis, Properties and Multifarious Therapeutic Applications of Nanostructured Zirconia in Dentistry. RSC Adv. 2022, 12, 12773–12793. [Google Scholar] [CrossRef]
- Mokeem, L.S.; Garcia, I.M.; Shahkarami, Y.; Blum, L.; Balhaddad, A.A.; Collares, F.M.; Williams, M.A.; Weir, M.D.; Melo, M.A.S. Core-Shell Nanostructures for Improving Dental Restorative Materials: A Scoping Review of Composition, Methods, and Outcome. Smart Materials in Medicine 2023, 4, 102–110. [Google Scholar] [CrossRef]
- Moraes, G.; Zambom, C.; Siqueira, W.L. Nanoparticles in Dentistry: A Comprehensive Review. Pharmaceuticals 2021, 14, 752. [Google Scholar] [CrossRef]
- Antonucci, J.M.; Regnault, W.F.; Skrtic, D. Polymerization Shrinkage and Stress Development in Amorphous Calcium Phosphate/Urethane Dimethacrylate Polymeric Composites. Journal of Composite Materials 2010, 44, 355–367. [Google Scholar] [CrossRef]
- Naebe, M.; Abolhasani, M.M.; Khayyam, H.; Amini, A.; Fox, B. Crack Damage in Polymers and Composites: A Review. Polymer Reviews 2016, 56, 31–69. [Google Scholar] [CrossRef]
- Khvostenko, D.; Salehi, S.; Naleway, S.E.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Cyclic Mechanical Loading Promotes Bacterial Penetration along Composite Restoration Marginal Gaps. Dent Mater 2015, 31, 702–710. [Google Scholar] [CrossRef]
- Lewis, S.H.; Fugolin, A.P.P.; Bartolome, A.; Pfeifer, C.S. Relaxation Mechanisms in Low-Stress Polymer Networks with Alternative Chemistries. JADA Foundational Science 2024, 3. [Google Scholar] [CrossRef]
- Lima, A.F.; Salvador, M.V.O.; Dressano, D.; Saraceni, C.H.C.; Gonçalves, L.S.; Hadis, M.; Palin, W.M. Increased Rates of Photopolymerisation by Ternary Type II Photoinitiator Systems in Dental Resins. Journal of the Mechanical Behavior of Biomedical Materials 2019, 98, 71–78. [Google Scholar] [CrossRef]
- Chen, H.; Wei, S.; Wang, R.; Zhu, M. Improving the Physical-Mechanical Property of Dental Composites by Grafting Methacrylate-Polyhedral Oligomeric Silsesquioxane onto a Filler Surface. ACS Biomater Sci Eng 2021, 7, 1428–1437. [Google Scholar] [CrossRef]
- Azhar, S.; Rana, N.F.; Kashif, A.S.; Tanweer, T.; Shafique, I.; Menaa, F. DEAE-Dextran Coated AgNPs: A Highly Blendable Nanofiller Enhances Compressive Strength of Dental Resin Composites. Polymers (Basel) 2022, 14, 3143. [Google Scholar] [CrossRef]
- German, M.J. Developments in Resin-Based Composites. Br Dent J 2022, 232, 638–643. [Google Scholar] [CrossRef]
- Chen, L.; Suh, B.I.; Yang, J. Antibacterial Dental Restorative Materials: A Review. American Journal of Dentistry 2018, 31. [Google Scholar]
- Chen, F.; Dong, J.; Sun, W.; Di Iorio, D.; Wegner, S.V.; Zeng, W. Editorial: Construction of Smart Materials for Biomedical Application. Front Bioeng Biotechnol 2023, 11, 1278243. [Google Scholar] [CrossRef]
- Yin, Y.; Rogers, J.A. Introduction: Smart Materials. Chem. Rev. 2022, 122, 4885–4886. [Google Scholar] [CrossRef]
- Kim, Y.; Zhao, X. Magnetic Soft Materials and Robots. Chem. Rev. 2022, 122, 5317–5364. [Google Scholar] [CrossRef]
- Yildirim, M.; Candan, Z. Smart Materials: The next Generation in Science and Engineering. Materials Today: Proceedings. [CrossRef]
- Zeng, N.; He, L.; Jiang, L.; Shan, S.; Su, H. Synthesis of Magnetic/PH Dual Responsive Dextran Hydrogels as Stimuli-Sensitive Drug Carriers. Carbohydrate Research 2022, 520, 108632. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/Stimuli-Responsive Hydrogels: Cutting-Edge Platforms for Tissue Engineering and Other Biomedical Applications. Materials Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Fan, R.; Lee, S.; Jung, H.; Melo, M.A.; Masri, R. Piezoelectric Energy Harvester Utilizing Mandibular Deformation to Power Implantable Biosystems: A Feasibility Study. J Mech Sci Technol 2019, 33, 4039–4045. [Google Scholar] [CrossRef]
- Dong, L.; Jin, C.; Closson, A.B.; Trase, I.; Richards, H.C.; Chen, Z.; Zhang, J.X.J. Cardiac Energy Harvesting and Sensing Based on Piezoelectric and Triboelectric Designs. Nano Energy 2020, 76, 105076. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart Wound Dressings for Wound Healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Yang, J.; He, Y.; Li, Z.; Yang, X.; Gao, Y.; Chen, M.; Zheng, Y.; Mao, S.; Shi, X. Intelligent Wound Dressing for Simultaneous in Situ Detection and Elimination of Pathogenic Bacteria. Acta Biomaterialia 2024, 174, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, C.; Bosio, J.A.; Melo, M.A.S. Smart Flexible 3D Sensor for Monitoring Orthodontics Forces: Prototype Design and Proof of Principle Experiment. Bioengineering (Basel) 2022, 9, 570. [Google Scholar] [CrossRef]
- Wu, J.; Weir, M.D.; Melo, M.A.S.; Xu, H.H.K. Development of Novel Self-Healing and Antibacterial Dental Composite Containing Calcium Phosphate Nanoparticles. J Dent 2015, 43, 317–326. [Google Scholar] [CrossRef]
- Wu, J.; Weir, M.D.; Zhang, Q.; Zhou, C.; Melo, M.A.S.; Xu, H.H.K. Novel Self-Healing Dental Resin with Microcapsules of Polymerizable Triethylene Glycol Dimethacrylate and N,N-Dihydroxyethyl-p-Toluidine. Dental Materials 2016, 32, 294–304. [Google Scholar] [CrossRef]
- Fadel, V.S.; Furtado, P.R.P.; Meier, M.M. Benzoyl Peroxide Encapsulation in Poly(Urea-Formaldehyde) Microcapsules for Use in Dental Materials. Polymer Engineering & Science n/a. [CrossRef]
- Montoya, C.; Kurylec, J.; Baraniya, D.; Tripathi, A.; Puri, S.; Orrego, S. Antifungal Effect of Piezoelectric Charges on PMMA Dentures. ACS Biomater. Sci. Eng. 2021, 7, 4838–4846. [Google Scholar] [CrossRef]
- Roldan, L.; Montoya, C.; Solanki, V.; Cai, K.Q.; Yang, M.; Correa, S.; Orrego, S. A Novel Injectable Piezoelectric Hydrogel for Periodontal Disease Treatment. ACS Appl. Mater. Interfaces 2023, 15, 43441–43454. [Google Scholar] [CrossRef]
- Montoya, C.; Jain, A.; Londoño, J.J.; Correa, S.; Lelkes, P.I.; Melo, M.A.; Orrego, S. Multifunctional Dental Composite with Piezoelectric Nanofillers for Combined Antibacterial and Mineralization Effects. ACS Appl. Mater. Interfaces 2021, 13, 43868–43879. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; AlQarni, F.D.; Al-Dulaijan, Y.A.; Weir, M.D.; Oates, T.W.; Xu, H.H.K.; Melo, M.A.S. Tuning Nano-Amorphous Calcium Phosphate Content in Novel Rechargeable Antibacterial Dental Sealant. Materials (Basel) 2018, 11, 1544. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Balhaddad, A.A.; Garcia, I.M.; Collares, F.M.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. PH-Responsive Calcium and Phosphate-Ion Releasing Antibacterial Sealants on Carious Enamel Lesions in Vitro. J Dent 2020, 97, 103323. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Toward Dental Caries: Exploring Nanoparticle-Based Platforms and Calcium Phosphate Compounds for Dental Restorative Materials. Bioact Mater 2019, 4, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.S.; Weir, M.D.; Passos, V.F.; Powers, M.; Xu, H.H.K. Ph-Activated Nano-Amorphous Calcium Phosphate-Based Cement to Reduce Dental Enamel Demineralization. Artif Cells Nanomed Biotechnol 2017, 45, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Balhaddad, A.A.; Lan, Y.; Simionato, A.; Ibrahim, M.S.; Weir, M.D.; Masri, R.; Xu, H.H.K.; Collares, F.M.; Melo, M.A.S. Magnetic Motion of Superparamagnetic Iron Oxide Nanoparticles- Loaded Dental Adhesives: Physicochemical/Biological Properties, and Dentin Bonding Performance Studied through the Tooth Pulpal Pressure Model. Acta Biomater 2021, 134, 337–347. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.-Z.; Watari, F. Current Investigations into Magnetic Nanoparticles for Biomedical Applications. J Biomed Mater Res A 2016, 104, 1285–1296. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Xia, Y.; Ji, Y.; Ruan, J.; Weir, M.D.; Lin, X.; Nie, Z.; Gu, N.; Masri, R.; et al. Novel Magnetic Nanoparticle-Containing Adhesive with Greater Dentin Bond Strength and Antibacterial and Remineralizing Capabilities. Dent Mater 2018, 34, 1310–1322. [Google Scholar] [CrossRef]
- Mokeem, L.S.; Martini Garcia, I.; Balhaddad, A.A.; Lan, Y.; Seifu, D.; Weir, M.D.; Melo, M.A. Multifunctional Dental Adhesives Formulated with Silane-Coated Magnetic Fe3O4@m-SiO2 Core-Shell Particles to Counteract Adhesive Interfacial Breakdown. ACS Appl Mater Interfaces 2024, 16, 2120–2139. [Google Scholar] [CrossRef]
- Zhou, K.; Li, J.; Li, W.; Zhang, Y.; Wang, K.; Xiong, X.; Li, S.; Chen, X.; Cheng, H.-W.; Qiu, J.; et al. Preparation and Magnetic Manipulation of Fe3O4/Acrylic Resin Core-Shell Microspheres. Langmuir 2023, 39, 11459–11467. [Google Scholar] [CrossRef] [PubMed]
- Baras, B.H.; Wang, S.; Melo, M.A.S.; Tay, F.; Fouad, A.F.; Arola, D.D.; Weir, M.D.; Xu, H.H.K. Novel Bioactive Root Canal Sealer with Antibiofilm and Remineralization Properties. Journal of Dentistry 2019, 83, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Balhaddad, A.A.; Lan, Y.; Simionato, A.; Ibrahim, M.S.; Weir, M.D.; Masri, R.; Xu, H.H.K.; Collares, F.M.; Melo, M.A.S. Magnetic Motion of Superparamagnetic Iron Oxide Nanoparticles- Loaded Dental Adhesives: Physicochemical/Biological Properties, and Dentin Bonding Performance Studied through the Tooth Pulpal Pressure Model. Acta Biomater 2021, 134, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Nathanael, A.J.; Oh, T.H. Biopolymer Coatings for Biomedical Applications. Polymers 2020, 12, 3061. [Google Scholar] [CrossRef]
- Tigmeanu, C.V.; Ardelean, L.C.; Rusu, L.-C.; Negrutiu, M.-L. Additive Manufactured Polymers in Dentistry, Current State-of-the-Art and Future Perspectives-A Review. Polymers 2022, 14, 3658. [Google Scholar] [CrossRef]
- Garcia, I.M.; Mokeem, L.S.; Shahkarami, Y.; Blum, L.; Sheraphim, V.; Leonardo, R.; Balhaddad, A.A.; Melo, M.A.S. Tube-Shaped Nanostructures for Enhancing Resin-Based Dental Materials: A Landscape of Evidence and Research Advancement. Smart Materials in Medicine 2023, 4, 504–513. [Google Scholar] [CrossRef]
- Adeniji, O.O.; Ojemaye, M.O.; Okoh, A.I. Antibacterial Activity of Metallic Nanoparticles against Multidrug-Resistant Pathogens Isolated from Environmental Samples: Nanoparticles/Antibiotic Combination Therapy and Cytotoxicity Study. ACS Appl. Bio Mater. 2022, 5, 4814–4826. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.S.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.A.; Xu, H.H.K. Novel Dental Adhesives Containing Nanoparticles of Silver and Amorphous Calcium Phosphate. Dent Mater 2013, 29, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Collares, F.M.; Garcia, I.M.; Klein, M.; Parolo, C.F.; Sánchez, F.A.L.; Takimi, A.; Bergmann, C.P.; Samuel, S.M.W.; Melo, M.A.; Leitune, V.C. Exploring Needle-Like Zinc Oxide Nanostructures for Improving Dental Resin Sealers: Design and Evaluation of Antibacterial, Physical and Chemical Properties. Polymers (Basel) 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Toward Dental Caries: Exploring Nanoparticle-Based Platforms and Calcium Phosphate Compounds for Dental Restorative Materials. Bioact Mater 2019, 4, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Mitwalli, H.; AlSahafi, R.; Alhussein, A.; Oates, T.W.; Melo, M.A.S.; Xu, H.H.K.; Weir, M.D. Novel Rechargeable Calcium Fluoride Dental Nanocomposites. Dent Mater 2022, 38, 397–408. [Google Scholar] [CrossRef] [PubMed]
- AlSahafi, R.; Mitwalli, H.; Alhussein, A.; Balhaddad, A.A.; Alquria, T.A.; Melo, M.A.S.; Lynch, C.D.; Oates, T.W.; Zhang, K.; Xu, H.H.K.; et al. Novel Rechargeable Nano-Calcium Phosphate and Nano-Calcium Fluoride Resin Cements. J Dent 2022, 126, 104312. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Balhaddad, A.A.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Collares, F.M.; Melo, M.A.S. Antibacterial Response of Oral Microcosm Biofilm to Nano-Zinc Oxide in Adhesive Resin. Dent Mater 2021, 37, e182–e193. [Google Scholar] [CrossRef] [PubMed]
- Bhadila, G.; Baras, B.H.; Weir, M.D.; Wang, H.; Melo, M.A.S.; Hack, G.D.; Bai, Y.; Xu, H.H.K. Novel Antibacterial Calcium Phosphate Nanocomposite with Long-Term Ion Recharge and Re-Release to Inhibit Caries. Dent Mater J 2020, 39, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yu, B.; Ye, L.; Lin, D.; Zhang, W.; Zhong, H.-J.; He, J. Recent Advances in Quaternary Ammonium Monomers for Dental Applications. Materials (Basel) 2024, 17, 345. [Google Scholar] [CrossRef]
- Thongthai, P.; Kitagawa, H.; Kitagawa, R.; Hirose, N.; Noree, S.; Iwasaki, Y.; Imazato, S. Development of Novel Surface Coating Composed of MDPB and MPC with Dual Functionality of Antibacterial Activity and Protein Repellency. J Biomed Mater Res B Appl Biomater 2020, 108, 3241–3249. [Google Scholar] [CrossRef]
- Fugolin, A.P.; Dobson, A.; Mbiya, W.; Navarro, O.; Ferracane, J.L.; Pfeifer, C.S. Use of (Meth)Acrylamides as Alternative Monomers in Dental Adhesive Systems. Dental Materials 2019, 35, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Fugolin, A.P.; Lewis, S.; Logan, M.G.; Ferracane, J.L.; Pfeifer, C.S. Methacrylamide–Methacrylate Hybrid Monomers for Dental Applications. Dental Materials 2020, 36, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Saiprasert, P.; Tansakul, C.; Pikulngam, A.; Promphet, P.; Naorungroj, S.; Ratanasathien, S.; Aksornmuang, J.; Talungchit, S. Novel Hydrolytic Resistant Antibacterial Monomers for Dental Resin Adhesive. Journal of Dentistry 2023, 135, 104597. [Google Scholar] [CrossRef] [PubMed]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin Based Restorative Dental Materials: Characteristics and Future Perspectives. Japanese Dental Science Review 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.; Sokolowski, J.; Bociong, K. The Photoinitiators Used in Resin Based Dental Composite—A Review and Future Perspectives. Polymers 2021, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Thadathil Varghese, J.; Cho, K.; Raju; Farrar, P. ; Prentice, L.; Prusty, B.G. Influence of Silane Coupling Agent on the Mechanical Performance of Flowable Fibre-Reinforced Dental Composites. Dental Materials 2022, 38, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Thadathil Varghese, J.; Cho, K.; Raju; Farrar, P. ; Prentice, L.; Prusty, B.G. Effect of Silane Coupling Agent and Concentration on Fracture Toughness and Water Sorption Behaviour of Fibre-Reinforced Dental Composites. Dental Materials 2023, 39, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Souza, V.S. de; Balhaddad, A.A.; Mokeem, L.; Melo, M.A.S. de; Scholten, J.D.; Collares, F.M. Ionic Liquid-Based Silane for SiO2 Nanoparticles: A Versatile Coupling Agent for Dental Resins. ACS Appl Mater Interfaces 2024. [Google Scholar] [CrossRef] [PubMed]
- Cuppini, M.; Garcia, I.M.; de Souza, V.S.; Zatta, K.C.; Visioli, F.; Leitune, V.C.B.; Guterres, S.S.; Scholten, J.D.; Collares, F.M. Ionic Liquid-Loaded Microcapsules Doped into Dental Resin Infiltrants. Bioact Mater 2021, 6, 2667–2675. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic Liquids Synthesis and Applications: An Overview. Journal of Molecular Liquids 2020, 297, 112038. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Rodell, C.B.; Burdick, J.A.; Anseth, K.S. Progress in Material Design for Biomedical Applications. Proc Natl Acad Sci U S A 2015, 112, 14444–14451. [Google Scholar] [CrossRef]
- Zhang, R.; Jones, M.M.; Moussa, H.; Keskar, M.; Huo, N.; Zhang, Z.; Visser, M.B.; Sabatini, C.; Swihart, M.T.; Cheng, C. Polymer–Antibiotic Conjugates as Antibacterial Additives in Dental Resins. Biomater. Sci. 2018, 7, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Al-Dulaijan, Y.A.; Balhaddad, A.A. Prospects on Tuning Bioactive and Antimicrobial Denture Base Resin Materials: A Narrative Review. Polymers (Basel) 2022, 15, 54. [Google Scholar] [CrossRef]
- Souza, A.F.; Souza, M.T.; Damasceno, J.E.; Ferreira, P.V.C.; Alves de Cerqueira, G.; Baggio Aguiar, F.H.; Marchi, G.M. Effects of the Incorporation of Bioactive Particles on Physical Properties, Bioactivity and Penetration of Resin Enamel Infiltrant. Clin Cosmet Investig Dent 2023, 15, 31–43. [Google Scholar] [CrossRef] [PubMed]
- ISO 4049:2019 Dentistry — Polymer-Based Restorative Materials 2019.
- ISO 3990:2023 Dentistry — Evaluation of Antibacterial Activity of Dental Restorative Materials, Luting Materials, Fissure Sealants and Orthodontic Bonding or Luting Materials 2023.
- Ibrahim, M.S.; Garcia, I.M.; Kensara, A.; Balhaddad, A.A.; Collares, F.M.; Williams, M.A.; Ibrahim, A.S.; Lin, N.J.; Weir, M.D.; Xu, H.H.K.; et al. How We Are Assessing the Developing Antibacterial Resin-Based Dental Materials? A Scoping Review. Journal of Dentistry 2020, 99, 103369. [Google Scholar] [CrossRef]
- Cuevas-Suárez, C.E.; Ramos, T.S.; Rodrigues, S.B.; Collares, F.M.; Zanchi, C.H.; Lund, R.G.; da Silva, A.F.; Piva, E. Impact of Shelf-Life Simulation on Bonding Performance of Universal Adhesive Systems. Dental Materials 2019, 35, e204–e219. [Google Scholar] [CrossRef]
- Karunakaran, H.; Krithikadatta, J.; Doble, M. Local and Systemic Adverse Effects of Nanoparticles Incorporated in Dental Materials- a Critical Review. The Saudi Dental Journal 2024, 36, 158–167. [Google Scholar] [CrossRef]
- Health, C.; for D. and R. Dental Composite Resin Devices - Premarket Notification [510(k)] Submissions - Guidance for Industry and FDA Staff Available online:. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/dental-composite-resin-devices-premarket-notification-510k-submissions-guidance-industry-and-fda (accessed on 3 July 2024).
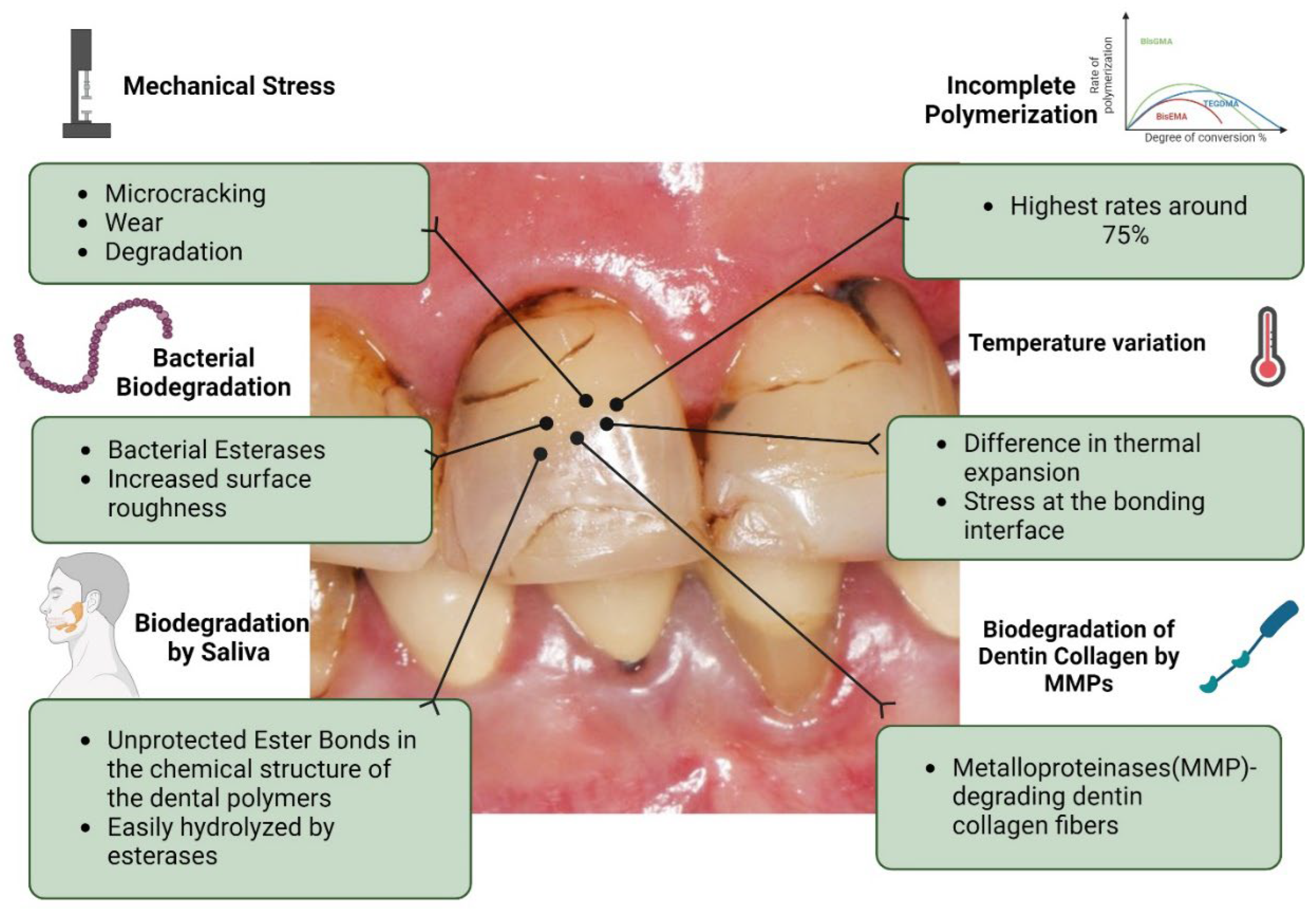
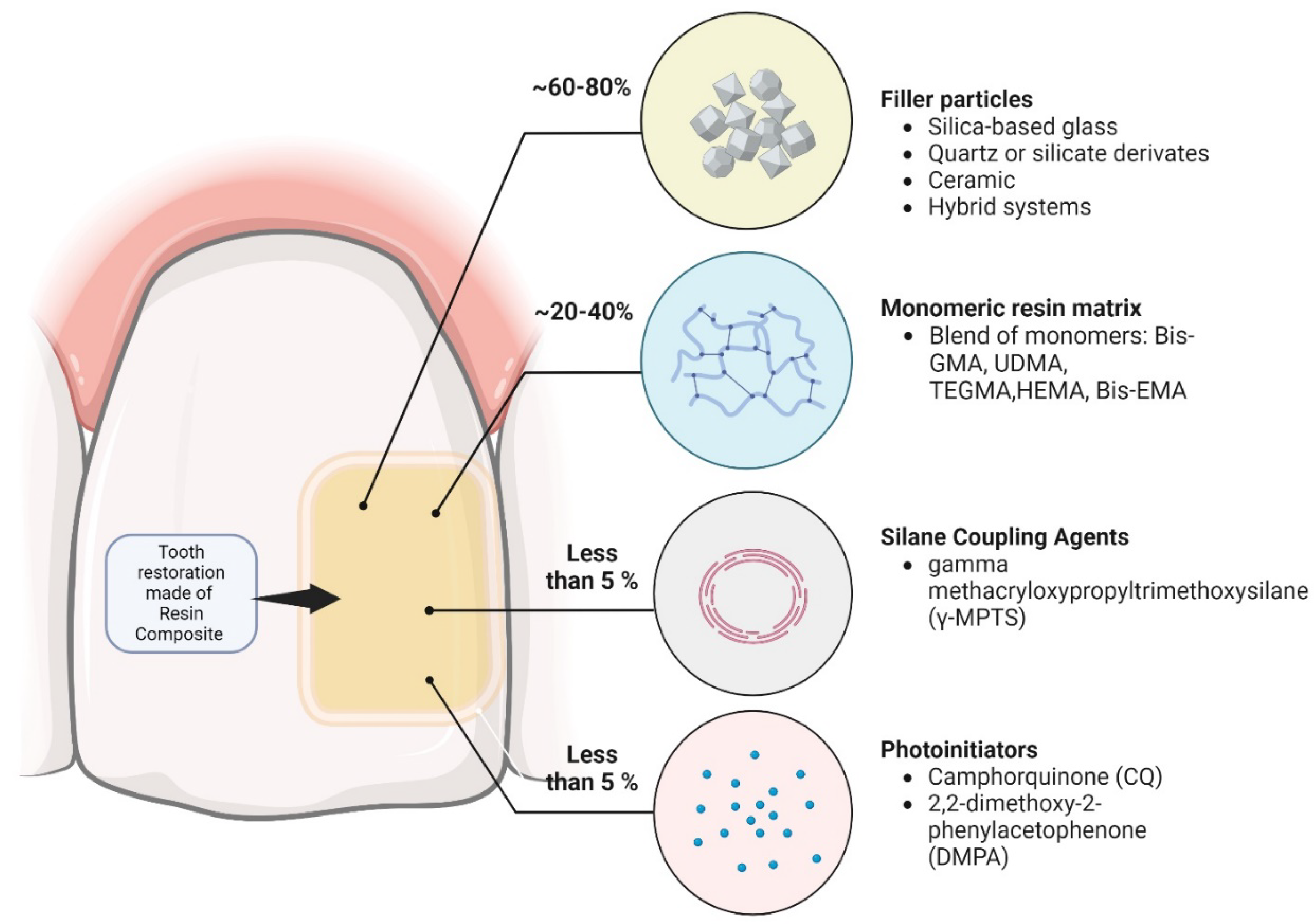
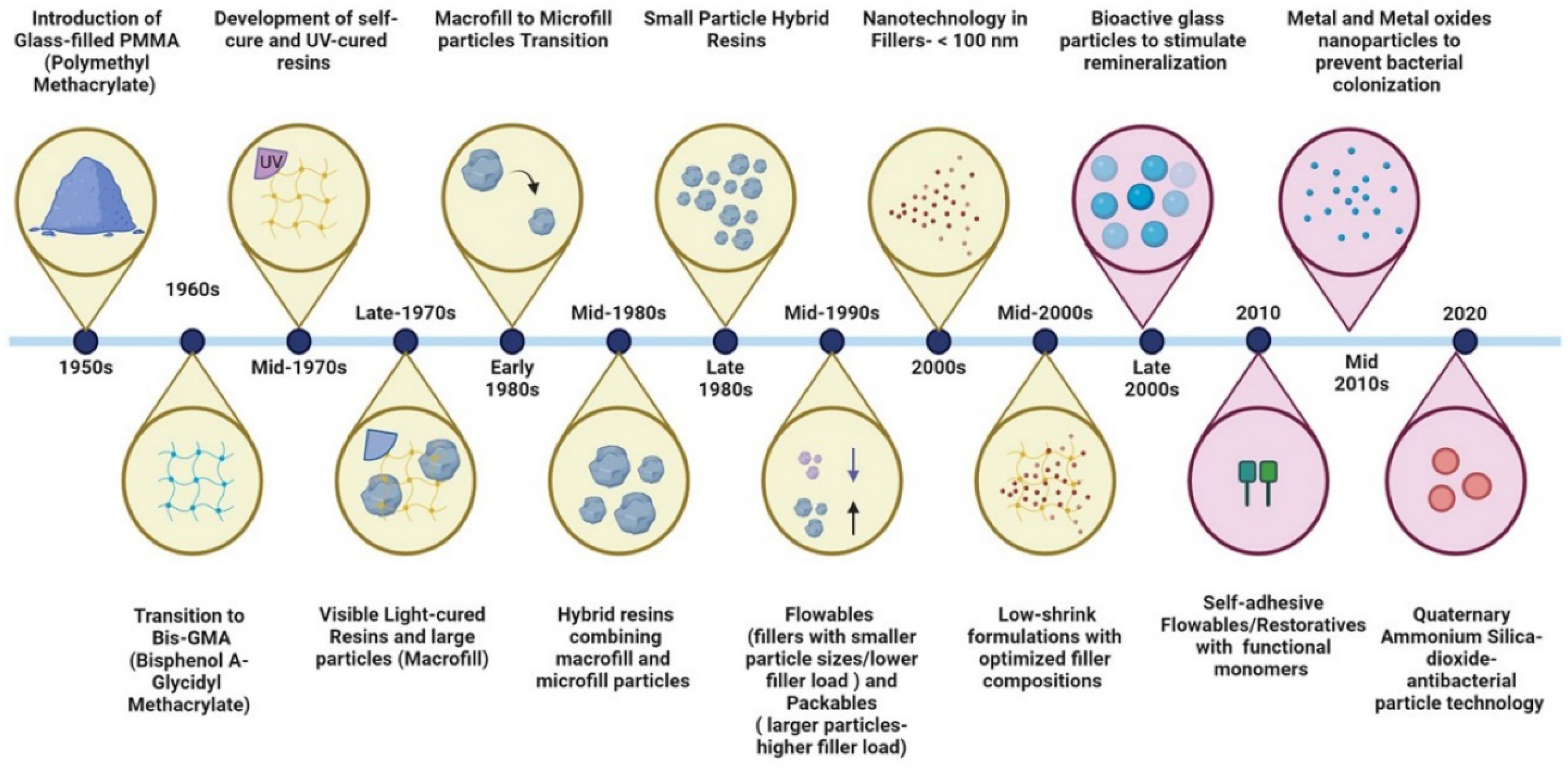
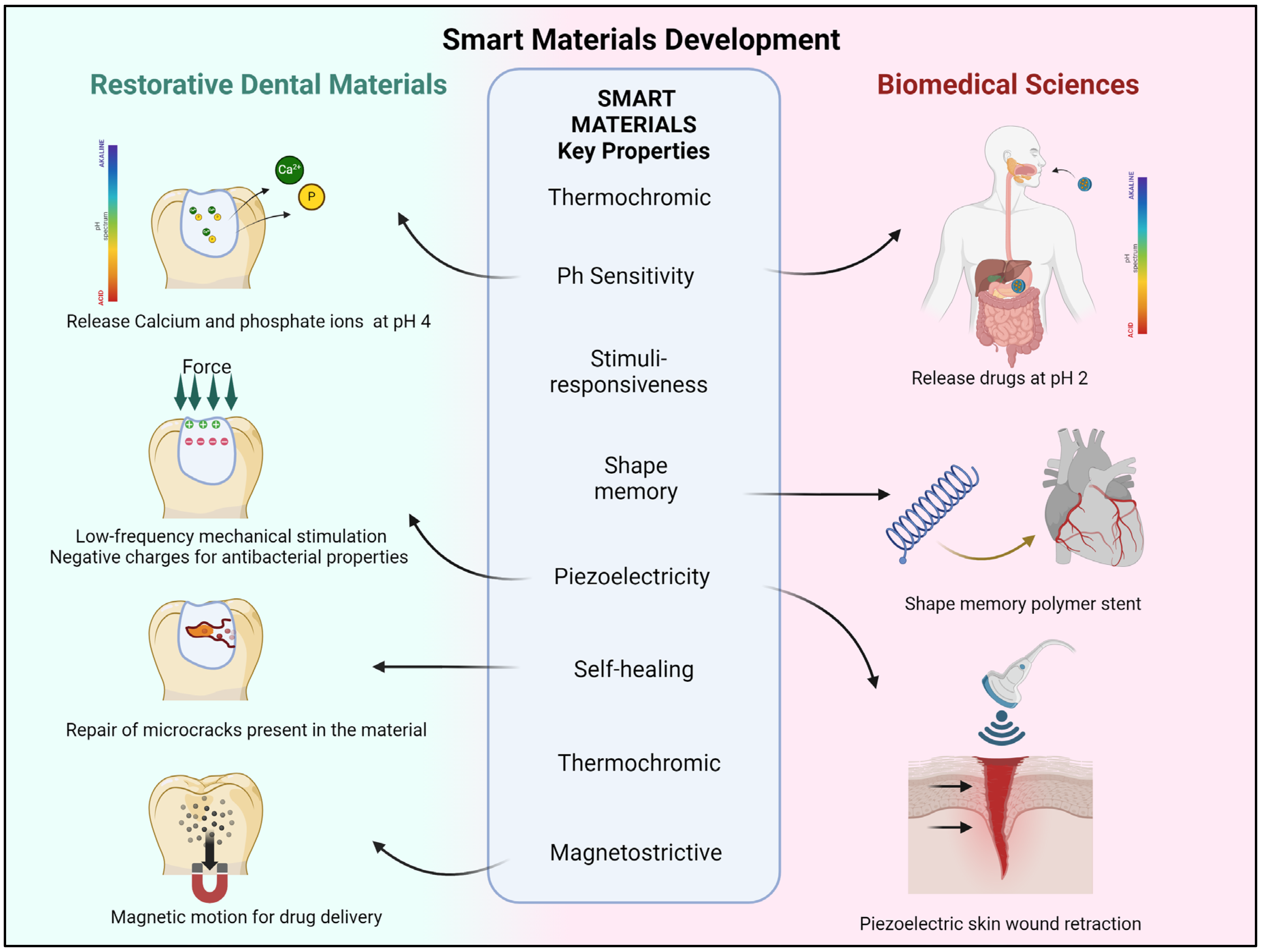
| NANOPARTICLE TYPE | INTENDED NEW PROPERTY | BASE RESTORATIVE MATERIAL |
|---|---|---|
| SILVER NANOPARTICLES | Antibacterial |
|
| ZINC OXIDE NANOPARTICLES | Antibacterial |
|
| TITANIUM COMPOUND:TITANIA NANOPARTICLES (TIO2) | Antibacterial |
|
| COPPER NANOPARTICLES:COPPER IODIDE, COPPER OXIDE | Antibacterial |
|
| NANODIAMONDS | Antibacterial |
|
| POLYMERIC/ORGANIC FILLERS | ||
| QUATERNARY AMMONIUM POLYETHYLENEIMINE (QAPEI) NANOPARTICLES | Antibacterial |
|
| CHITOSAN NANOPARTICLES | Antibacterial /Antifungal |
|
| CHLORHEXIDINE RELEASING FILLERS | Antibacterial |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





