1. Introduction
Over the past decade, the human immunodeficiency virus (HIV) /AIDS epidemic has led to the development of several products to prevent its spread [
1]. Novel prevention methods that combine advances in drug development and delivery can contribute significantly to slowing down the incidence of HIV infections. Most human HIV-1 infections are sexually transmitted, and young women in developing countries have little power to claim condom use for protection [
2]. HIV-1 is transmitted through infected semen and vaginal or cervical secretions containing infected lymphocytes [
3]. Microbicides can be applied to the vagina and rectum to reduce the transmission of sexually transmitted infections (STIs), including HIV-1. Vaginal microbicides are a potential means of protecting women against HIV-1 infection during sexual transmission. Early-generation microbicide candidates based on detergents and polyanions have failed to achieve protective effects in efficacy tests, and some products even promote viral transmission [
4]. Although tenofovir (TFV)-based gels have been shown to decrease HIV-1 infection acquisition [
2], the FACTS 001 clinical trial showed a failure of protection [
5].
The ampholytic polysaccharide sacran was extracted from cyanobacterium Aphanothece sacrum, a species indigenous to Japan that is massively cultured in rivers with high ionic concentrations and is rich in a high water content (97.5%) jelly-like extracellular matrix [
6,
7,
8,
9,
10]. Sacran is a heteropolysaccharide containing a variety of sugar residues, including the following: glucose, galactose, mannose, xylose, rhamnose, fucose, galacturonic acid, glucuronic acid, traces of alanine, galactosamine, and muramic acid. In addition, 11% of the monosaccharides contained sulfate groups, and 22% contained carboxyl groups. Sacran also has an extremely high molecular weight (approximately 20 MDa) and is surprisingly long (over 8 μm) [
6]. Sacran has potential as an anti-HIV-1 agent because a previous study demonstrated that the advantages of its anti-HIV-1 activity include its high molecular weight and high degree of sulfation [
11]. Sacran is a type of massively cultivated cyanobacteria and is considered a safe biomaterial because it has traditionally been used as a functional food to improve allergic tendencies and gastroenteritis in the Kyushu region of Japan. In addition, sacran can hold a large amount of water compared with hyaluronic acid or xanthangum and form a nanofilm on the platform [
10].
We investigated the effect of sacran as an anti-HIV-1 agent using a transwell mimic of HIV-1 transmission in vaginal tissue. Our findings suggest a potential application of sacran in preventing HIV-1 transmission.
2. Materials and Methods
2.1. Preparation of Sacran Gel
Sacran gel was kindly provided by Green Science Materials (Kumamoto, Japan), and analytical reagent-grade solvents were used for all experiments [
7,
8,
9]. The sacran gel was diluted with deionized double-distilled water.
2.2. Cell lines and Culture
TZM-bl, Jurkat
HXBc2 (4) and Jurkat
522F/Y cells were procured from the National Institutes of Health AIDS Reagent Program (Bethesda, MD, USA). Molt-4 T cell line was obtained from RIKEN cell bank (Tsukuba, Japan). MT-4 T cell line was kindly provided by Dr. Kazuhiko Ide (Kumamoto University, Kumamoto, Japan). The human T cell line PM1-CCR5, stably expressing human CCR5 [
12], was kindly gifted by Dr. Y. Maeda (Kumamoto University, Kumamoto, Japan). MT-4, MOLT-4, and PM1-CCR5 cells were maintained in RPMI 1640 medium (Fujifilm Wako Pure Chemical, Osaka, Japan) supplemented with penicillin 100 U/mL, streptomycin 100 μg/mL, and 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA) and cultured in a 5% CO
2 humidified incubator at 37 °C. Stably expressing HIV-1 env cell lines, Jurkat
HXBc2 (4), and Jurkat
522F/Y were used for cell-cell fusion assay and cultured in RPMI 1640 supplemented with 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, 1 µg/mL doxycycline, 200 µg/mL G418, and 200 µg/mL hygromycin. Jurkat
HXBc2 (4) expresses functional gp120 and gp41 glycoproteins (
Env), whereas Jurkat
522F/Y has an F/Y substitution at position 522 of gp41, which prevents fusion [
13]. HIV-1 env expression was induced by a Tet-Off system, whereby cells were washed with PBS and cultured in doxycycline-free medium for 3 d prior to fusion experiments[
14,
15]. TZM-bl cells were maintained in DMEM (Fujifilm Wako Pure Chemical) supplemented with penicillin 100 U/mL, streptomycin 100 μg/mL, and 10% fetal bovine serum.
2.3. Virus Preparation
HIV-1LAI/ⅢB was procured from the NIH AIDS Reagent Program and propagated in the MT-4 T cell line. After a few days, the supernatant was collected, examined for p24 levels using a p24 antigen ELISA kit (ZeptoMetrix Corp., Buffalo, NY, USA), and stored at -80 ˚C until use.
2.4. Flow Cytometric Detection of HIV-1-Infected Cells
Viral infections were performed under three experimental settings. First, PM1-CCR5 T cell line (5×10
5 cells/ml), sacran gel (final concentration 0–0.2%), and HIV-1LAI (X4 tropic) (p24 concentration: 25 ng/mL) were cultured in a triple-layered medium for 48 h at 37 °C (
Figure S1A). Second, sacran gel (final concentration 0–0.2%) and HIV-1LAI (p24 concentration: 25 ng/mL) were co-treated with PM1-CCR5 cells and cultured for 48 h at 37 °C (
Figure S1B). Third, sacran gel (final concentration 0–0.2%) and HIV-1LAI (p24 concentration 25 ng/mL) were mixed in a 1.5-mL tube. Then, the mixed solution was treated to PM1-CCR5 cells and cultured for 48 h at 37 °C (
Figure S1C). Intracellular p24 positive cells were identified as HIV-1-infected using flow cytometric analysis. Briefly, cells were fixed with 1% paraformaldehyde/PBS for 20 min in the dark and permeabilized with 0.1% saponin in PBS. Following incubation on ice for 10 min, the cells were stained with anti-HIV-1 Gag p24-FITC mAb (Beckman Coulter, Fullerton, CA, USA) on ice for 30 min [
14,
16]. Stained cells were analyzed using an LSR II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The corrected data were analyzed using the FlowJo software (Tree Star, San Carlos, CA, USA).
2.5. Cell-to-Cell Fusion Inhibition Assay
Env-expressing cell lines (JurkatHXBc2 (4), Jurkat522F/Y) and MOLT-4 T cell lines were labeled using the PKH 67 Green Fluorescent Cell Linker Kit (Sigma-Aldrich) and PKH 26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich), respectively. The two cell populations were then cocultured at a ratio of 1:1 for 24 h and treated with sacran gel at a final concentration of 0–0.2%. The cells were analyzed using an LSR II flow cytometer, and double-positive cells for PKH67 and PKH26 were defined as fusion cells. Data were analyzed using FlowJo software.
2.6. Transwell Experiments to Test the Barrier Function of Sacran
We applied 50 µL of sacran gel to the membranes of 24-well Transwell plates (HTS Transwell®- 24 Well Plate, polycarbonate membrane, pore size = 8 µm Cat. no. 3422; Corning Inc., Corning, NY, USA). Each gel layer was challenged with a solution of 50 µL of HIV-1 virions (p24 concentration: 2 µg/mL), added to the upper chamber. The bottom compartment of each Transwell contained 600 µL of cell culture medium. The cell culture medium comprised DMEM supplemented with penicillin 100 U/mL, streptomycin 100 μg/mL, and 10% fetal bovine serum. Transwell plates were incubated in a 5% CO2 incubator at 37 ◦C for 24 h. Following incubation, the Transwell plate was removed from the incubator, and the solutions were collected from the upper (sacran gel-containing) and lower chambers. The p24 levels were measured in samples from both the upper and lower chambers using a p24 antigen ELISA kit.
2.7. Transwell Experiments for TZM-bl Assay
Three different solutions (37.5 µL each) were applied to the membranes of 96-well Transwell plates (HTS Transwell®- 96 Well Plate, polycarbonate membrane, pore size = 5 µm Cat. no. 3387; Corning Inc.): pure sacran gel, sacran gel mixed with an HIV-1 entry inhibitor (either AMD3100 or T-20), and sacran gel mixed with the HIV-1 reverse transcriptase inhibitor EFV. Each gel layer was challenged with a solution of 37.5 µL of HIV-1 virions (p24 concentration;533 ng/mL) added to the upper chamber. The bottom compartment of each Transwell contained 235 µL of the cell culture medium. The cell culture medium consisted of DMEM supplemented with penicillin 100 U/mL, streptomycin 100 μg/mL, and 10% fetal bovine serum. Transwell plates were incubated in a 5% CO2 incubator at 37 ◦C for 24 h. Following incubation, solutions from the bottom chambers were collected and used for the TZM-bl assay.
2.8. TZM-bl Assay
The TZM-bl assay was used to evaluate the synergistic effects of sacran and HIV-1 entry inhibitors [
17,
18]. TZM-bl cells were used as viral target cells, seeded in 96-well tissue culture plates at a density of 1×10
4 cells/well, and cultured for 24 h. The HIV-1 solution collected from the bottom chambers was used to infect the cells. Viral infectivity was assessed by measuring the HIV-1 Tat-mediated induction of β-galactosidase activity in the target cells using a mammalian β-galactosidase assay kit (Thermo Fisher Scientific Inc, Waltham, MA) at 48 h post-infection [
18]. The absorbance of the wells was measured at 405/595 nm using an iMark microplate absorbance reader (Bio-Rad, Hercules, CA, USA).
2.9. Quantification of Synergistic Effects of Sacran with HIV-1 Entry Inhibitors
The effects of the combination of sacran with HIV-1 entry inhibitors or HIV-1 reverse transcriptase inhibitors on HIV-1 infection were determined using CompuSyn software (ComboSyn, Inc., Paramus, NJ, USA) based on the quantitative analysis of the concentration-effect relationships of multiple drugs or enzyme inhibitors by Chou and Talalay [
19,
20]. The software calculates a combination index (CI) value to confirm synergistic effects and compares it to the effect of a single agent to further help determine the nature of the combination. CI values <1 indicate synergistic effects, CI values equal to 1 indicate a mean additive effect of the drugs, and CI values > 1 indicate antagonistic effects.
2.10. Statistical Analysis
Parametric statistical analyses were performed using the Student’s t-test. P-values set at > 0.05 were considered statistically significant.
3. Results
3.1. Sacran Inhibited HIV-1 Infection
We performed HIV-1 infection with sacran gel treatment on the PM1-CCR5 cells [
12] in three experimental settings. A human CD4 and human CXCR4/CCR5-positive T cell line, PM1-CCR5 cells, were exposed to X4 tropic virus HIV-1LAI/IIIB with or without sacran gel. First, the target cells, sacran gel, and HIV-1 were cultured in a triple-layered medium for 48 h at 37 °C (
Figure 1A, upper panel: a). Intracellular expression of p24 decreased after sacran gel treatment in a concentration-dependent manner (
Figure 1B,C). Next, the target cells were treated with sacran gel and HIV-1 and cultured for 48 h at 37 °C (
Figure 1A, middle: b). Intracellular expression of p24 was decreased by sacran gel treatment in a concentration-dependent manner (
Figure 1B,C). Third, sacran gel and HIV-1 were mixed in a 1.5-mL tube. The target cells were then treated with sacran and HIV-1 mix solution for 48 h at 37 °C (
Figure 1A, lower panel: c). Intracellular p24 expression was decreased by sacran gel treatment in a concentration-dependent manner (
Figure 1B,D). These results indicated that sacran gel inhibited HIV-1 infection.
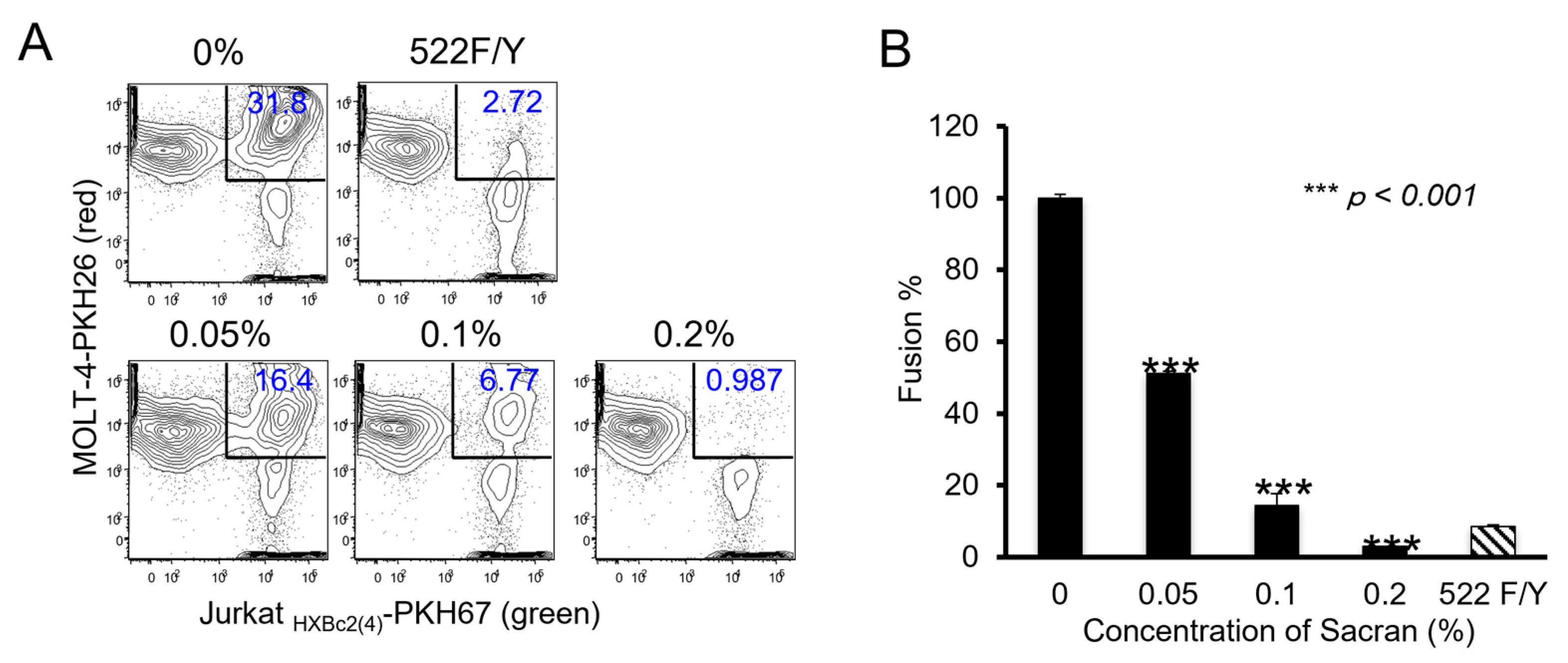
3.2. Sacran Inhibited HIV-1 Envelope-Dependent Cell Fusion
Membrane fusion between the virus-infected and target cells is an essential step in HIV-1 infection. Therefore, we used an in vitro fusion model system to evaluate the effect of sacran gel on HIV-1 envelope-dependent cell fusion [
13,
15]. As shown in
Figure 2A, cells double-positive for PKH67 (green fluorescence) and PKH26 (red fluorescence) were defined as fused cells, which decreased in a concentration-dependent manner following sacran gel treatment (
Figure 2A). Sacran gel significantly inhibited cell fusion (P <0.001) (
Figure 2B). These results demonstrate that sacran gel inhibits HIV-1 envelope-dependent cell-cell fusion. This suggests that sacran gel might protect against cell-virus interactions.
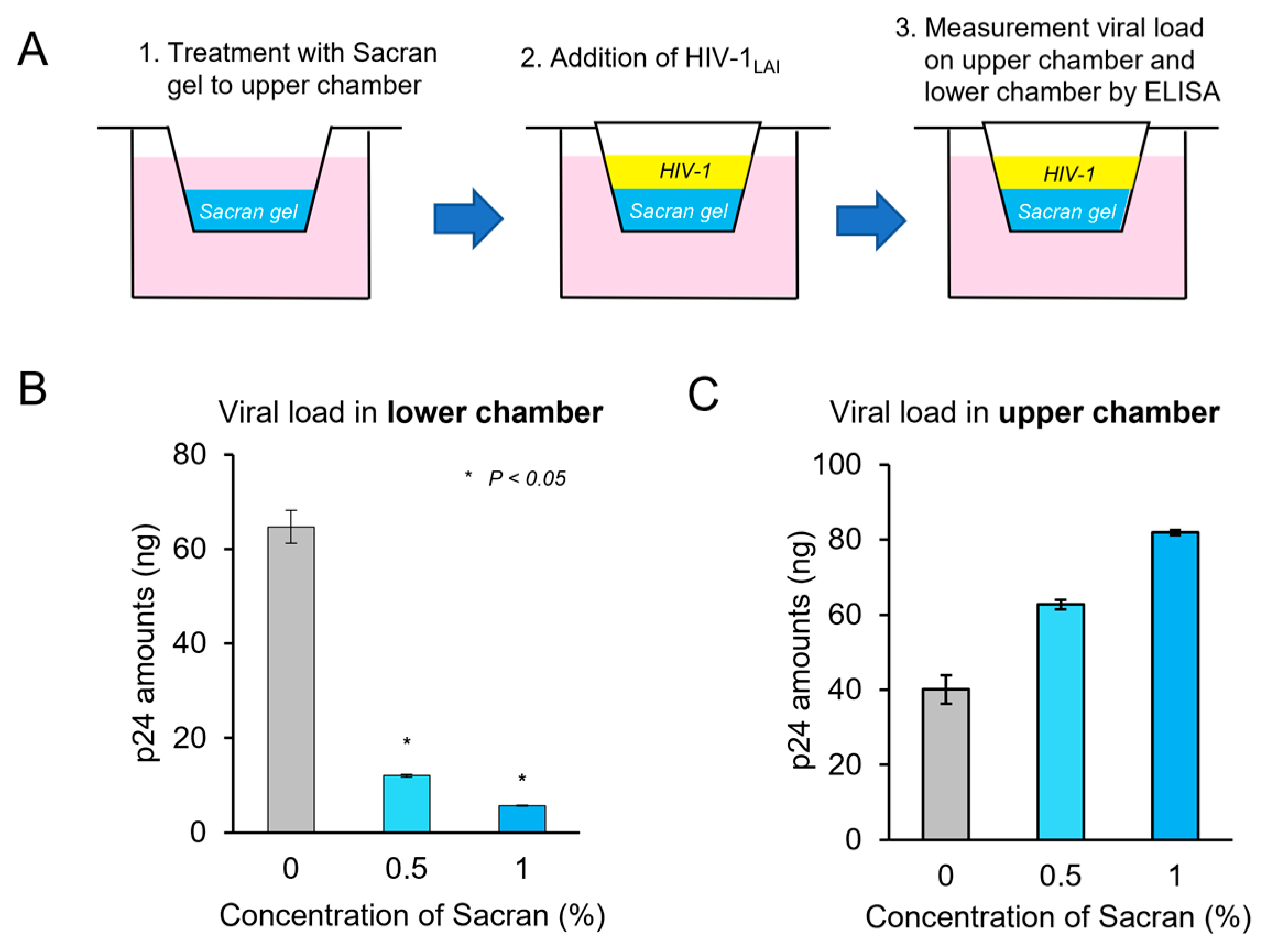
3.3. Sacran Inhibited Viral Diffusion and Captured HIV-1
Transwell assays were used to assess the impact of viral diffusion and the ability of sacran to capture the virus [
17]. Sacran gel was applied to the membranes of 24-well Transwell plates. Each gel layer was challenged with HIV-1 virions and added to the up-per chamber. Transwell plates were incubated in a 5% CO2 incubator at 37 ◦C for 24 h. Following incubation, solutions from the upper (sacran gel containing) and lower cham-bers were collected, and the p24 amounts of the samples were measured using a p24 an-tigen ELISA kit (
Figure 3A). As shown in
Figure 3B, following sacran treatment , p24 levels in the lower chamber decreased in a concentration-dependent manner. (
Figure 3B). The p24 levels in the upper chamber increased following sacran treatment in a concentration-dependent manner (
Figure 3C). These results demonstrate that sacran gel inhibits HIV-1 diffusion and has a viral capture ability.
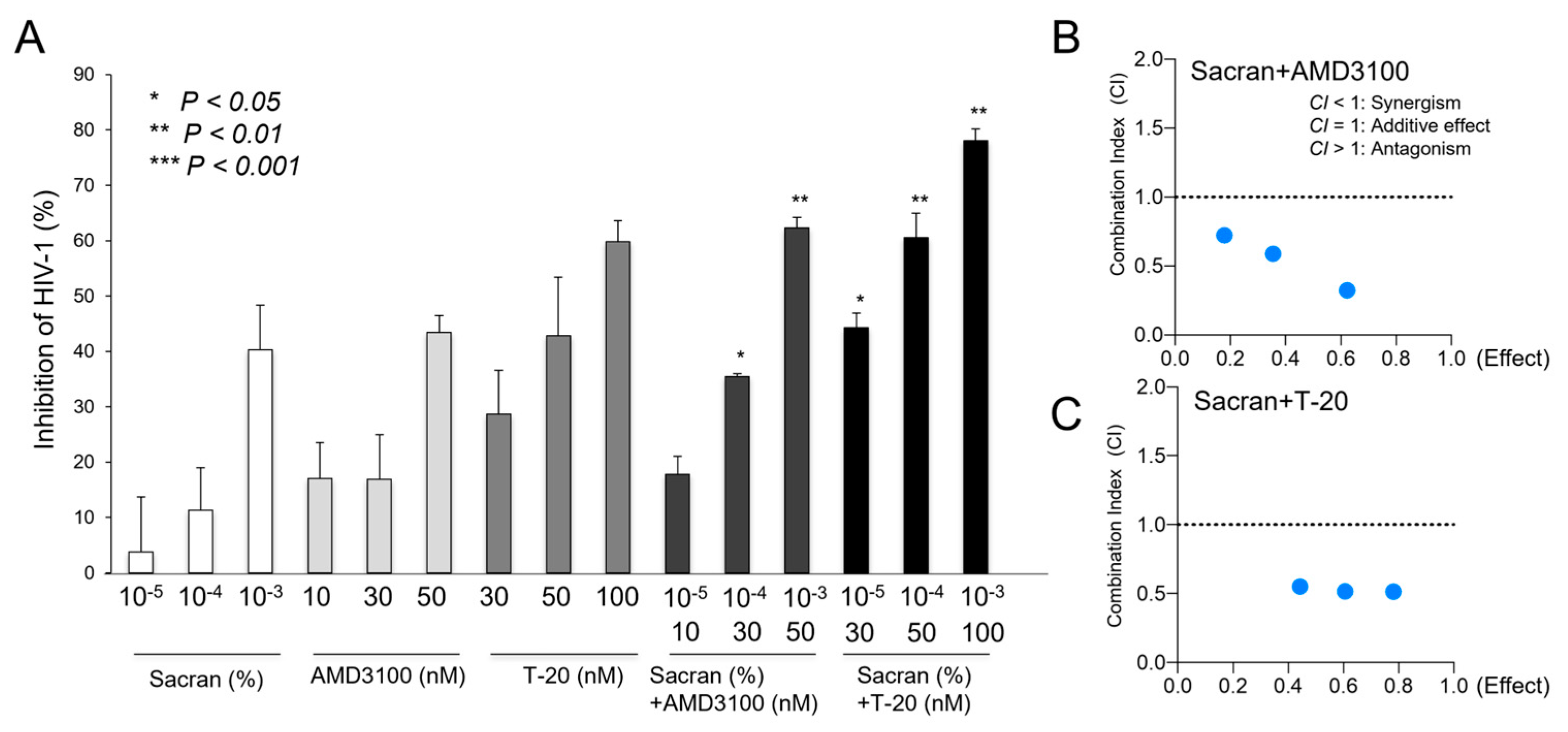
3.4. Synergistic HIV-1 Inhibition of Sacran and Anti-HIV-1 Drugs
Sacran gel or HIV-1 entry inhibitors, AMD3100 or T-20-containing sacran gel, were applied to the membranes of 96-well Transwell plates. Each gel layer was challenged with HIV-1 virions and added to the upper chamber. Transwell plates were incubated in a 5% CO2 incubator at 37 ◦C for 24 h. Following incubation, solutions from the bottom chambers were collected and used for the TZM-bl assay. As shown in
Figure 4A,B, the HIV-1 inhibitory activities of sacran with HIV-1 entry inhibitors were significantly enhanced in a concentration-dependent manner (
Figure 4A). The combination index was calculated using CompuSyn software [
18] according to the method previously established by Chou and Talalay [
19,
20] to evaluate the synergistic effect of sacran and HIV-1 entry inhibitors. The CI values for sacran and HIV-1 entry inhibitors were < 1 (
Table 1). This indicated that sacran exhibited synergism with AMD3100 and T-20 (
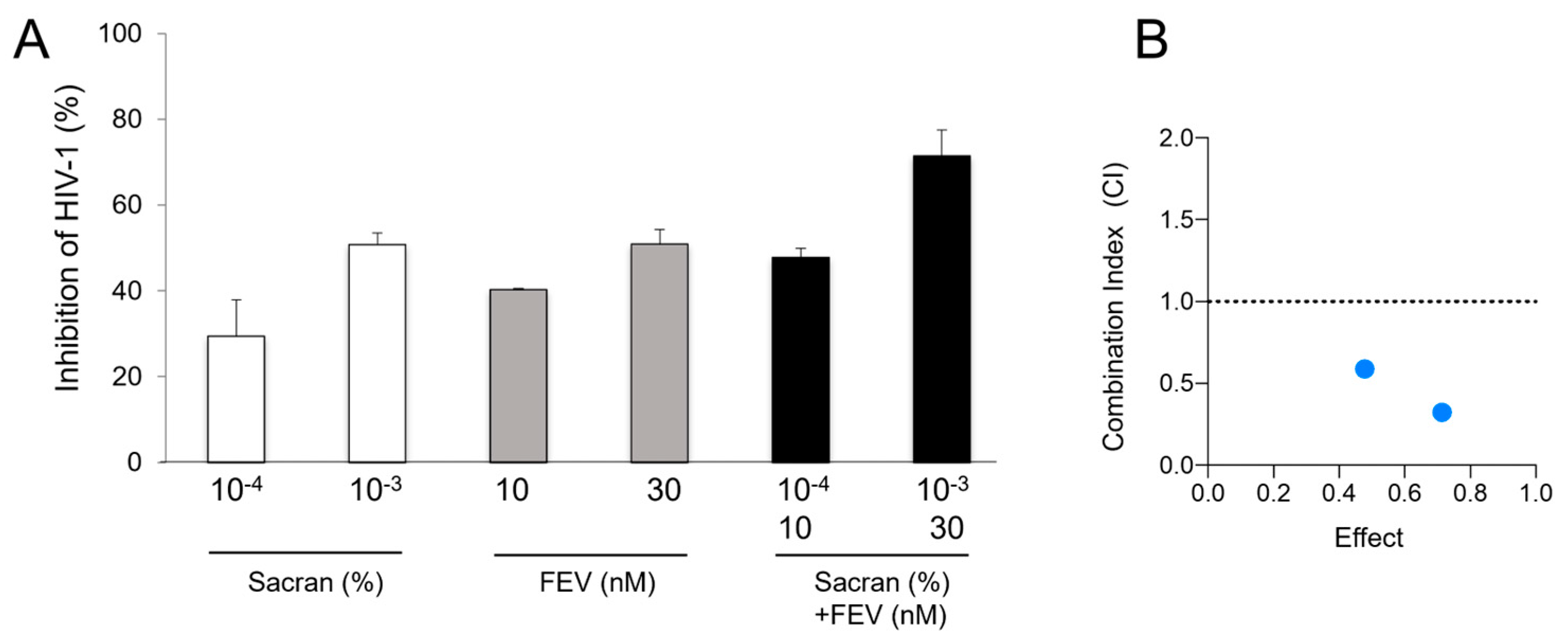
Figure 4B,C). Moreover, we evaluated the synergistic effect of sacran with HIV-1 reverse transcriptase inhibitor, Efavirenz (EFV), using the TZM-bl assay. Sacran gel or EFV-containing sacran gel was applied to the membranes of the 96-well Transwell plates. HIV-1-inhibiting activities of sacran with EFV were significantly enhanced in a concentration-dependent manner (
Figure 5A). The CI values for both the sacran and EFV were < 1 (
Table 1). This indicates that sacran acts synergistically with EFV (
Figure 5B). These results suggest synergism between sacran and anti-HIV-1 drugs.
4. Discussion
Sexual transmission is a major reason for the pandemic of HIV-1 infection. Among young women in developing countries, especially African countries, people living with HIV (PLWH) are still spreading it due to criminal heterosexual and homosexual transmission [
2]. The prevention of sexual transmission is beneficial for HIV-1 eradication. The development of tools to prevent viral infections has previously shown that TFV-based gels, such as PrEP, can decrease HIV-1 infection acquisition. The CAPRISA 004 trial was the first large trial to demonstrate the efficacy of 1% TFV gel, which reduced 39% of heterosexual HIV-1 infections [
2]. However, the FACTS 001 clinical trial, in which 1% TFV gel was prescribed within 12 h before and after sexual intercourse, showed no preventive effects in women [
5]. This study was evaluated based on the number of empty gel applicators returned and self-reported sexual activity; the lack of efficacy may be attributed to low adherence. HIV-1 entry inhibitors T-20 and Maraviroc, which are used in clinical HIV-1-infected individuals, suppress HIV-1 fusion with its target cells and prevent HIV-1 infection by specifically inhibiting the virus-receptor interactions or subsequent steps in the entry process [
21,
22,
23]. Several entry inhibitors have been shown to prevent SHIV infection in macaques through vaginal and/or rectal transmission [
24]. Notably, the gp120-binding inhibitor BMS-378806, gp41-targeted fusion inhibitor peptide C52L, and small-molecule CCR5 inhibitor CMPD167 were significantly protected by each compound alone and in combination in vivo. Additionally, the combination of the small-molecule CXCR4 inhibitors AMD3465 and CMPD167 inhibits R5X4 HIV-1[
25]. Therefore, the combination of entry inhibitors may improve the efficacy of microbicides. In this study, we demonstrated a combination assay of sacran with the small-molecule CXCR4 inhibitor AMD3100, gp41-targeted fusion inhibitory peptide T-20, and reverse transcriptase inhibitor efavirenz. Sacran gel alone was effective against HIV-1 infection, but its combination with anti-HIV-1 drugs strongly inhibited HIV-1 infection due to synergistic effects (
Figure 4 and 5). These results suggest the potent effects of sacran in combination with anti-HIV-1 drugs.
Sacran is a novel sulfated polysaccharide isolated from the cyanobacterium Aphanothece sacrum and has the potential for biomass applications [
7]. In this study, we investigated the anti-HIV-1 activity of sacran gel and found that it suppressed HIV-1 infectivity (
Figure 1). Cell-to-cell transmission plays a critical role in the spread of HIV-1 [
26]. The encounter of the HIV-1 envelope with the target cells causes transmission and influences its efficacy. As demonstrated here, sacran gel treatment inhibited HIV-1 infection derived from cell-to-cell fusion, which mimicked macrophage/dendritic cell and T lymphocyte transmission (
Figure 2).
Compounds extracted from marine and freshwater micro/macroalgae have shown antiviral potency against a variety of retroviruses (HIV-1/SIV), herpes viruses (HSV-1, HSV-2, and HCMV), rhabdoviruses (VSV), and paramyxoviruses (RSV) [
11]. Most studies have evaluated the anti-HIV effects of natural and synthetic sulfated polysaccharides because antiviral activity is affected by the degree of sulfation and high molecular weight. In addition, some compounds isolated from algae selectively inhibit the replication of enveloped viruses, such as HSV-2, HCMV, RSV, influenza A and B viruses, and SIV [
27]. Their modes of action against these viruses include an inhibitory effect on viral adsorption. We performed a Transwell assay to confirm the antiviral effect of the functional material. Following sacran gel treatment, the p24 levels in the lower chamber decreased, whereas those in the upper chamber increased in a concentration-dependent manner (
Figure 3). These results suggest that sacran has an antiviral diffusion ability and an inhibitory effect on viral adsorption.
Sacran has already been applied in cosmetic products, such as skin toners and face creams, because of its water retention capacity, moisturizing effect, and film-forming ability. Furthermore, it has been reported to have anti-inflammatory effects, particularly against dry sacran skin [
28,
28]. Notably, sacran controlled the itchiness and blood IgE concentrations. These findings indicate the safety of sacran.
Our findings suggest an anti-HIV-1 effect of sacran and a synergistic effect of sacran and anti-HIV-1 agents in in vitro models. However, the efficacy of sacran gel against HIV-1 infection in vivo requires further investigation to develop an effective microbicide.
5. Conclusions
We confirmed that sacran inhibited HIV-1 infection by reducing viral diffusion and capturing viral particles. The combination of sacran and anti-HIV-1 agents significantly reduces HIV-1 infection via synergistic effects. Our study suggests the potential of the use of sacran gel to protect against HIV-1 transmission.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Experimental design. Blocking of HIV-1 infection by sacran was set as three deferent conditions.
Author Contributions
Conceptualization, Ko.M. and S.O.; methodology, Ko.M.; validation, Ko.M., R.K. and S.O.; formal analysis, Ko.M.; investigation, Ko.M. and R.K.; resources, S.O.; data curation, Ke.M.; writing—original draft preparation, Ko.M.; writing—review and editing, S.O.; visualization, Ko.M. and R.K.; supervision, Ke.M. and S.O.; project administration, S.O.; funding acquisition, S.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Science and Technology Agency, grant number AS262Z02656P.
Institutional Review Board Statement
“Not applicable”.
Informed Consent Statement
“Not applicable.”.
Acknowledgments
We thank Ms. S. Fujikawa and Ms. I. Suzu for their technical assistance, and Ms. Y. Kanagawa for secretarial assistance. Dr. Shinichiro Kaneko (Greenscience Materials Co.) provided Sacran.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- UNAIDS, WHO. In Guidelines for Using Hiv Testing Technologies in Surveillance: Selection, Evaluation and Implementation: 2009 Update, edited by WHO UNAIDS. Geneva, 2009.
- Abdool Karim, Q., S. S. Abdool Karim, J. A. Frohlich, A. C. Grobler, C. Baxter, L. E. Mansoor, A. B. Kharsany, S. Sibeko, K. P. Mlisana, Z. Omar, T. N. Gengiah, S. Maarschalk, N. Arulappan, M. Mlotshwa, L. Morris, D. Taylor, and Caprisa Trial Group. "Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of Hiv Infection in Women." Science 329, no. 5996 (2010): 1168-74.
- McConville, C.; Boyd, P.; Major, I. Efficacy of Tenofovir 1% Vaginal Gel in Reducing the Risk of HIV-1 and HSV-2 Infection. Clin. Med. Insights: Women's Heal. 2014, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Veazey, R.S.; Ketas, T.A.; Klasse, P.J.; Davison, D.K.; Singletary, M.; Green, L.C.; Greenberg, M.L.; Moore, J.P. Tropism-independent protection of macaques against vaginal transmission of three SHIVs by the HIV-1 fusion inhibitor T-1249. Proc. Natl. Acad. Sci. 2008, 105, 10531–10536. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, J.M.; Ramjee, G.; Richardson, B.A.; Gomez, K.; Mgodi, N.; Nair, G.; Palanee, T.; Nakabiito, C.; van der Straten, A.; Noguchi, L.; et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. New Engl. J. Med. 2015, 372, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Okajima, M.K.; Bamba, T.; Kaneso, Y.; Hirata, K.; Fukusaki, E.; Kajiyama, S.; Kaneko, T. Supergiant Ampholytic Sugar Chains with Imbalanced Charge Ratio Form Saline Ultra-absorbent Hydrogels. Macromolecules 2008, 41, 4061–4064. [Google Scholar] [CrossRef]
- Okajima, M.K.; Miyazato, S.; Kaneko, T. Cyanobacterial Megamolecule Sacran Efficiently Forms LC Gels with Very Heavy Metal Ions. Langmuir 2009, 25, 8526–8531. [Google Scholar] [CrossRef]
- Okajima, M.K.; Nakamura, M.; Mitsumata, T.; Kaneko, T. Cyanobacterial Polysaccharide Gels with Efficient Rare-Earth-Metal Sorption. Biomacromolecules 2010, 11, 1773–1778. [Google Scholar] [CrossRef]
- Okajima, M.K.; Higashi, T.; Asakawa, R.; Mitsumata, T.; Kaneko, D.; Kaneko, T.; Ogawa, T.; Kurata, H.; Isoda, S. Gelation Behavior by the Lanthanoid Adsorption of the Cyanobacterial Extracellular Polysaccharide. Biomacromolecules 2010, 11, 3172–3177. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Tanida, Y.; Hata, K.; Hayashi, T.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Kaneko, S.; Arima, H. Potential Use of a Megamolecular Polysaccharide Sacran as a Hydrogel-Based Sustained Release System. Chem. Pharm. Bull. 2014, 62, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, D.J.; Krylov, V.S. Anti-HIV Activity of Extracts and Compounds from Algae and Cyanobacteria. Ecotoxicol. Environ. Saf. 2000, 45, 208–227. [Google Scholar] [CrossRef]
- Yusa, K.; Maeda, Y.; Fujioka, A.; Monde, K.; Harada, S. Isolation of TAK-779-resistant HIV-1 from an R5 HIV-1 GP120 V3 Loop Library. J. Biol. Chem. 2005, 280, 30083–30090. [Google Scholar] [CrossRef]
- Cao, J.; Park, I.W.; Cooper, A.; Sodroski, J. Molecular determinants of acute single-cell lysis by human immunodeficiency virus type 1. J. Virol. 1996, 70, 1340–1354. [Google Scholar] [CrossRef]
- Matsuda, K.; Hattori, S.; Kariya, R.; Komizu, Y.; Kudo, E.; Goto, H.; Taura, M.; Ueoka, R.; Kimura, S.; Okada, S. Inhibition of HIV-1 entry by the tricyclic coumarin GUT-70 through the modification of membrane fluidity. Biochem. Biophys. Res. Commun. 2015, 457, 288–294. [Google Scholar] [CrossRef]
- Matsuda, K.; Hattori, S.; Komizu, Y.; Kariya, R.; Ueoka, R.; Okada, S. Cepharanthine inhibited HIV-1 cell–cell transmission and cell-free infection via modification of cell membrane fluidity. Bioorganic Med. Chem. Lett. 2014, 24, 2115–2117. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S.; Ide, K.; Nakata, H.; Harada, H.; Suzu, S.; Ashida, N.; Kohgo, S.; Hayakawa, H.; Mitsuya, H.; Okada, S. Potent Activity of a Nucleoside Reverse Transcriptase Inhibitor, 4′-Ethynyl-2-Fluoro-2′-Deoxyadenosine, against Human Immunodeficiency Virus Type 1 Infection in a Model Using Human Peripheral Blood Mononuclear Cell-Transplanted NOD/SCID Janus Kinase 3 Knockout Mice. Antimicrob. Agents Chemother. 2009, 53, 3887–3893. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.E.; Geonnotti, A.R.; DeSoto, M.G.; Montefiori, D.C.; Katz, D.F. Semi-solid gels function as physical barriers to human immunodeficiency virus transport in vitro. Antivir. Res. 2010, 88, 143–151. [Google Scholar] [CrossRef]
- Chutiwitoonchai, N.; Hiyoshi, M.; Mwimanzi, P.; Ueno, T.; Adachi, A.; Ode, H.; Sato, H.; Fackler, O.T.; Okada, S.; Suzu, S. The Identification of a Small Molecule Compound That Reduces HIV-1 Nef-Mediated Viral Infectivity Enhancement. PLOS ONE 2011, 6, e27696. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Dorr, P.; Westby, M.; Dobbs, S.; Griffin, P.; Irvine, B.; Macartney, M.; Mori, J.; Rickett, G.; Smith-Burchnell, C.; Napier, C.; et al. Maraviroc (UK-427,857), a Potent, Orally Bioavailable, and Selective Small-Molecule Inhibitor of Chemokine Receptor CCR5 with Broad-Spectrum Anti-Human Immunodeficiency Virus Type 1 Activity. Antimicrob. Agents Chemother. 2005, 49, 4721–4732. [Google Scholar] [CrossRef]
- Cervia, J. S., and M. A. Smith. "Enfuvirtide (T-20): A Novel Human Immunodeficiency Virus Type 1 Fusion Inhibitor." Clin Infect Dis 37, no. 8 (2003): 1102-6. [CrossRef]
- Matthews, T.; Salgo, M.; Greenberg, M.; Chung, J.; DeMasi, R.; Bolognesi, D. Enfuvirtide: the first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat. Rev. Drug Discov. 2004, 3, 215–225. [Google Scholar] [CrossRef]
- Veazey, R.S.; Klasse, P.J.; Schader, S.M.; Hu, Q.; Ketas, T.J.; Lu, M.; Marx, P.A.; Dufour, J.; Colonno, R.J.; Shattock, R.J.; et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus–cell fusion. Nature 2005, 438, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Ketas, T.J.; Schader, S.M.; Zurita, J.; Teo, E.; Polonis, V.; Lu, M.; Klasse, P.J.; Moore, J.P. Entry inhibitor-based microbicides are active in vitro against HIV-1 isolates from multiple genetic subtypes. Virology 2007, 364, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Sattentau, Q.J. Cell-to-Cell Spread of Retroviruses. Viruses 2010, 2, 1306–1321. [Google Scholar] [CrossRef] [PubMed]
- Damonte, E.; Neyts, J.; Pujol, C.; Snoeck, R.; Andrei, G.; Ikeda, S.; Witvrouw, M.; Reymen, D.; Haines, H.; Matulewicz, M.; et al. Antiviral activity of a sulphated polysaccharide from the red seaweed nothogenia fastigiata. Biochem. Pharmacol. 1994, 47, 2187–2192. [Google Scholar] [CrossRef]
- Ngatu, N. R., M. K. Okajima, M. Yokogawa, R. Hirota, M. Eitoku, B. A. Muzembo, N. Dumavibhat, M. Takaishi, S. Sano, T. Kaneko, T. Tanaka, H. Nakamura, and N. Suganuma. "Anti-Inflammatory Effects of Sacran, a Novel Polysaccharide from Aphanothece Sacrum, on 2,4,6-Trinitrochlorobenzene-Induced Allergic Dermatitis in Vivo." Ann Allergy Asthma Immunol 108, no. 2 (2012): 117-22.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).