Submitted:
25 July 2024
Posted:
29 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
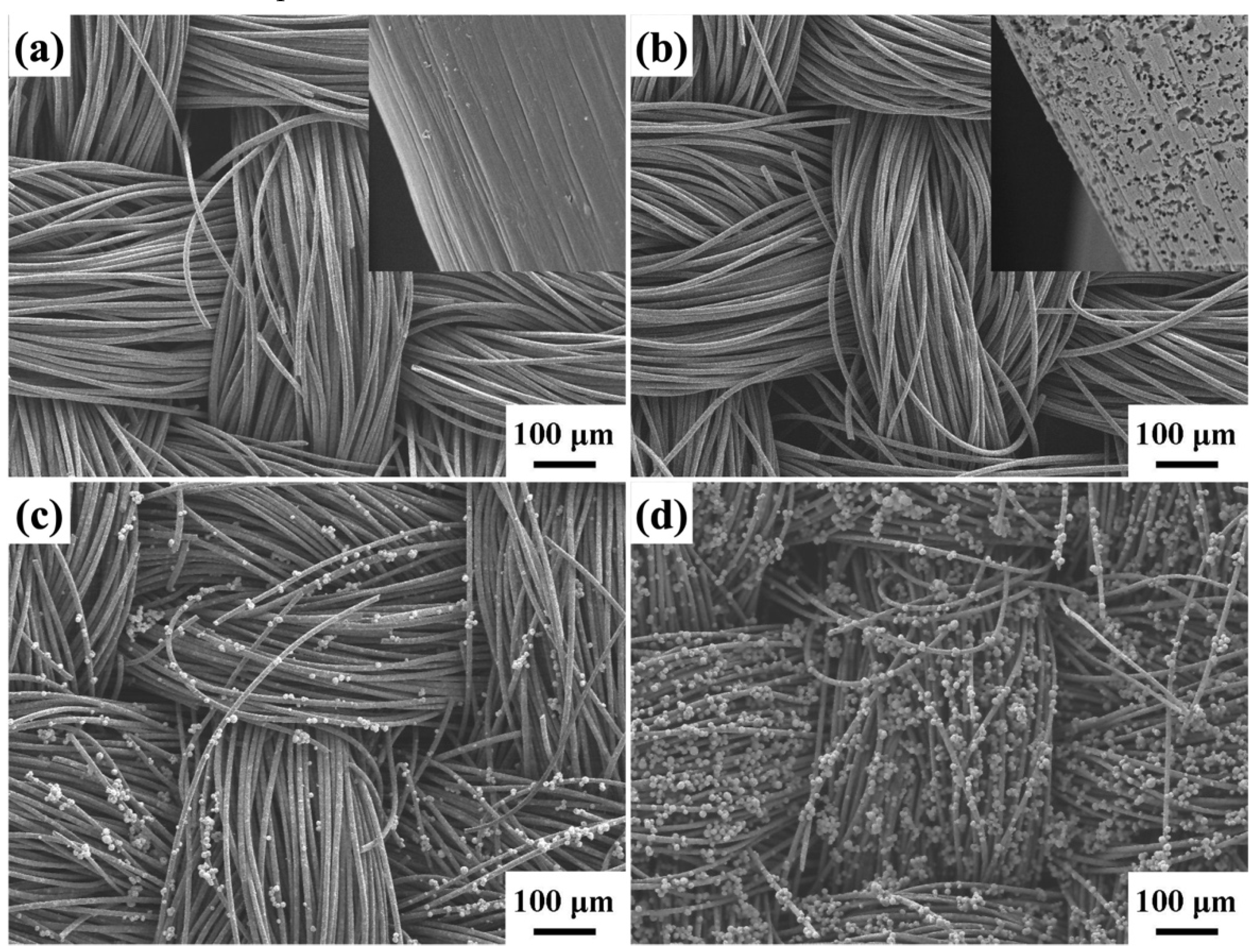
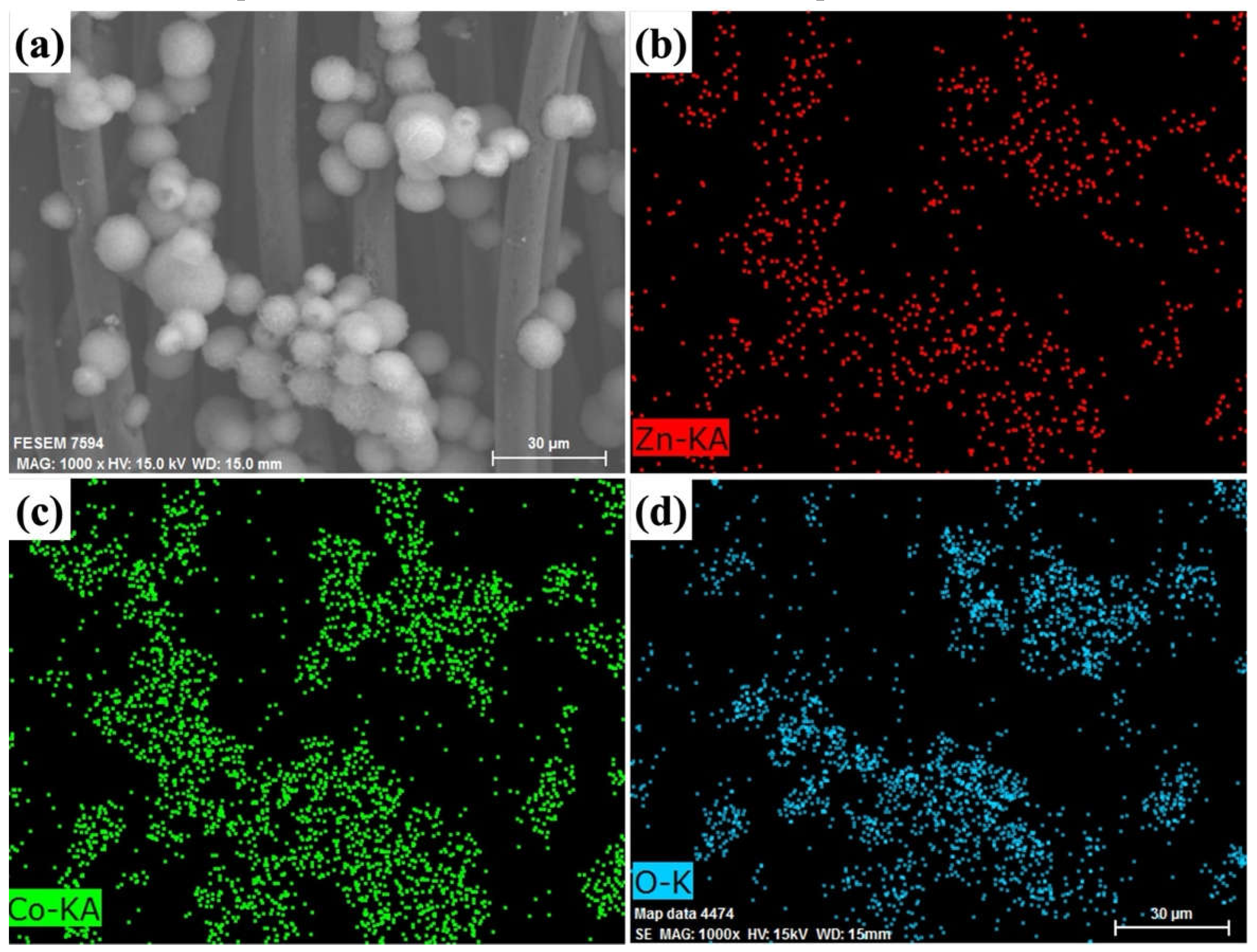
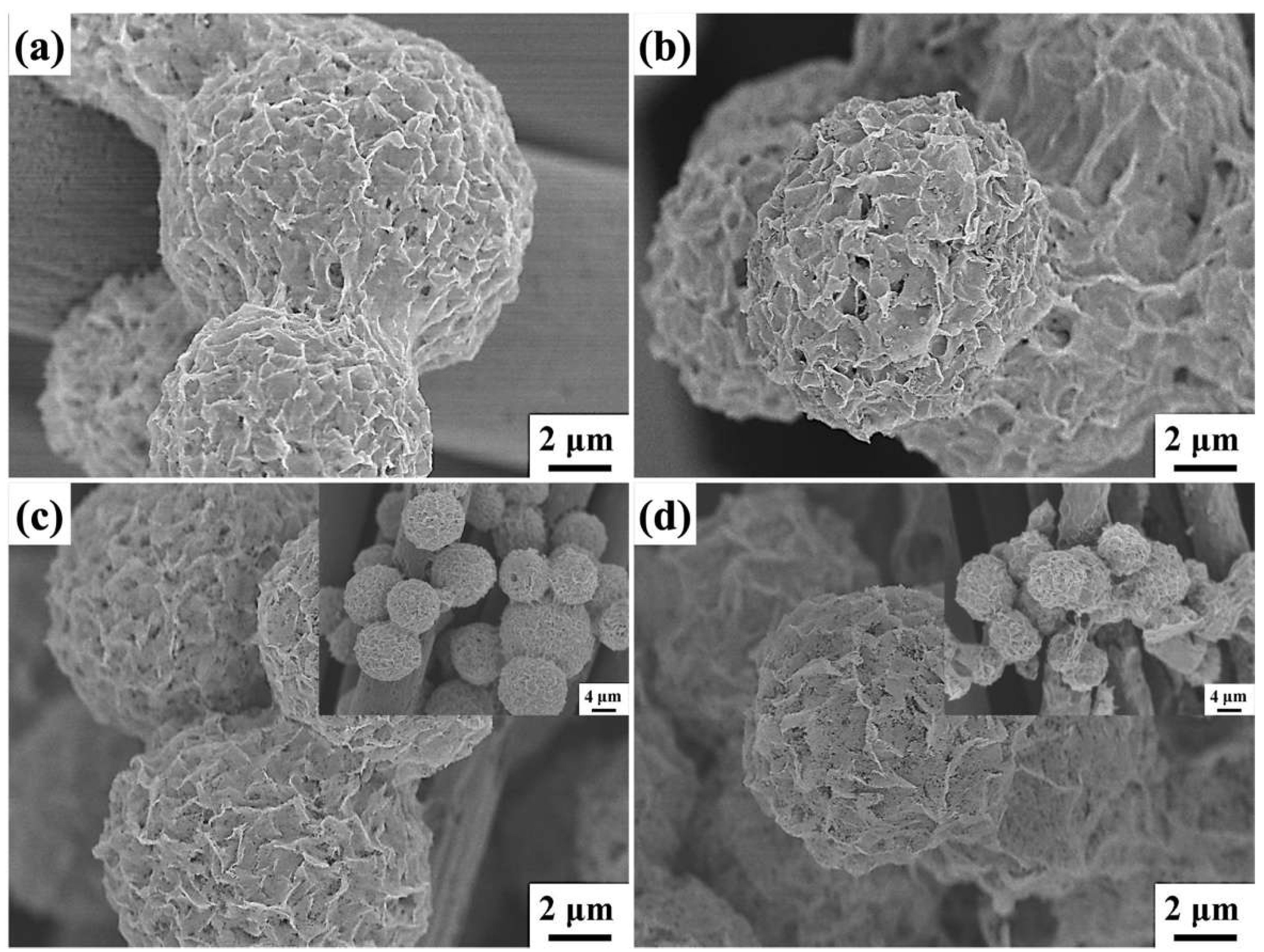
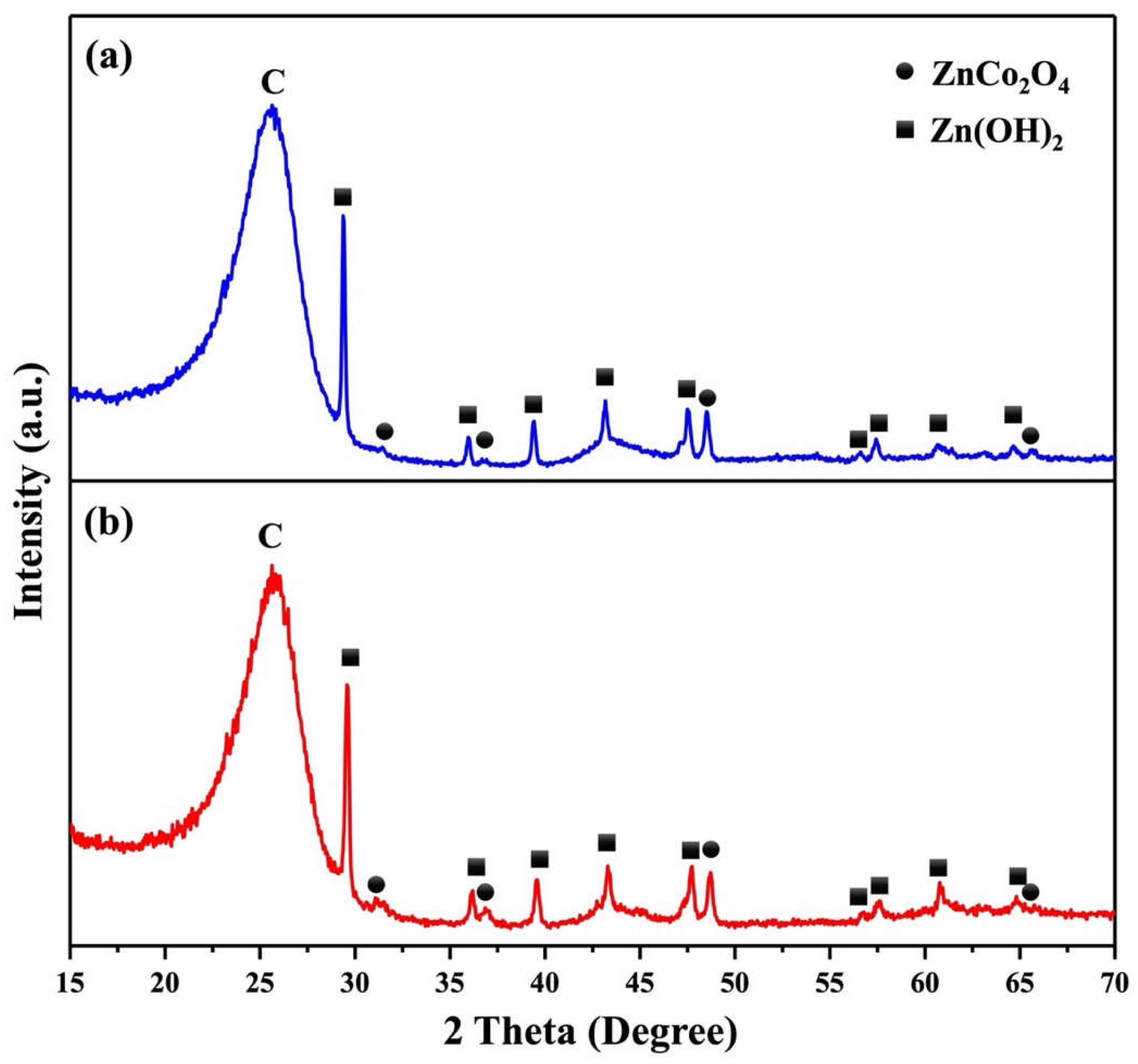
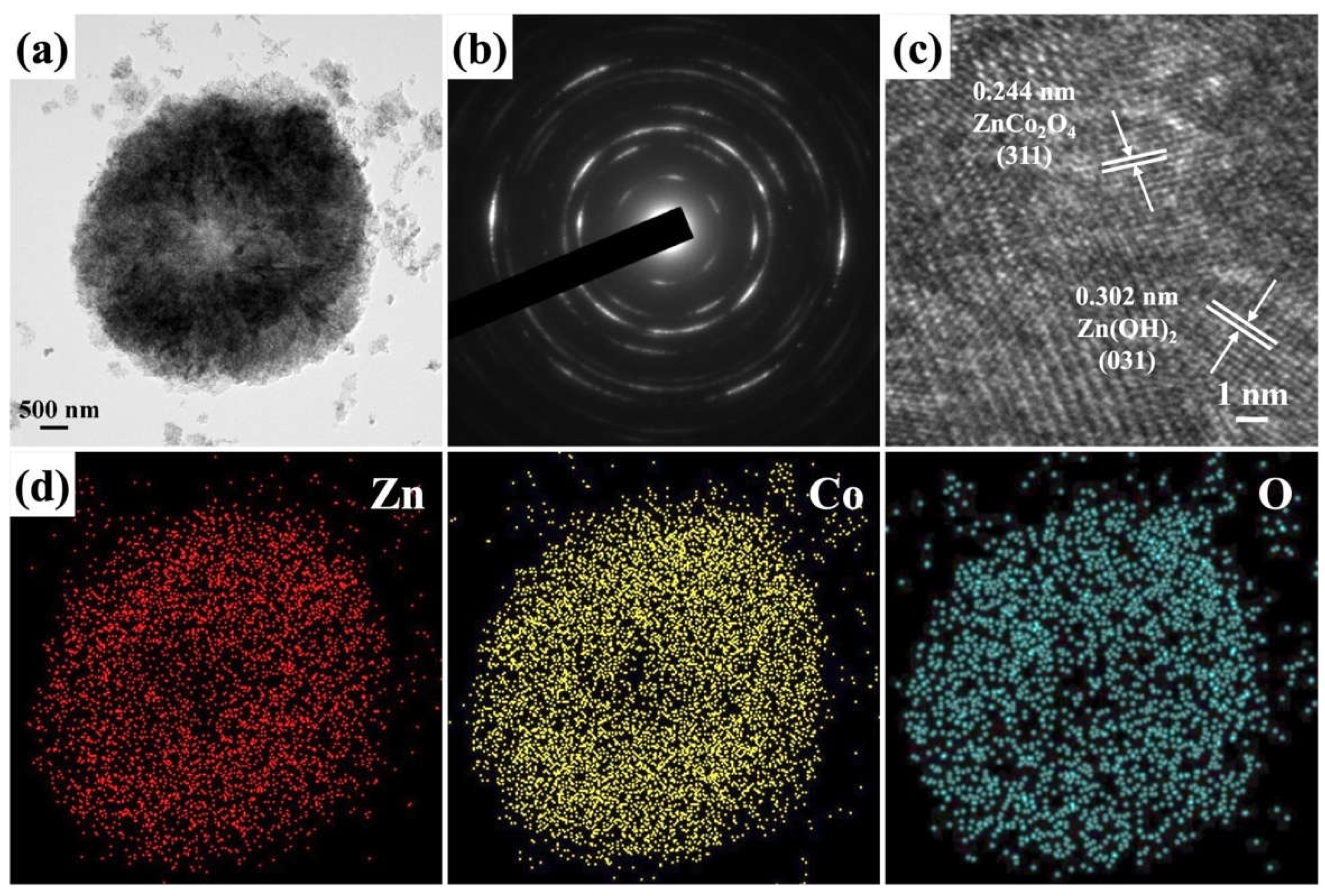
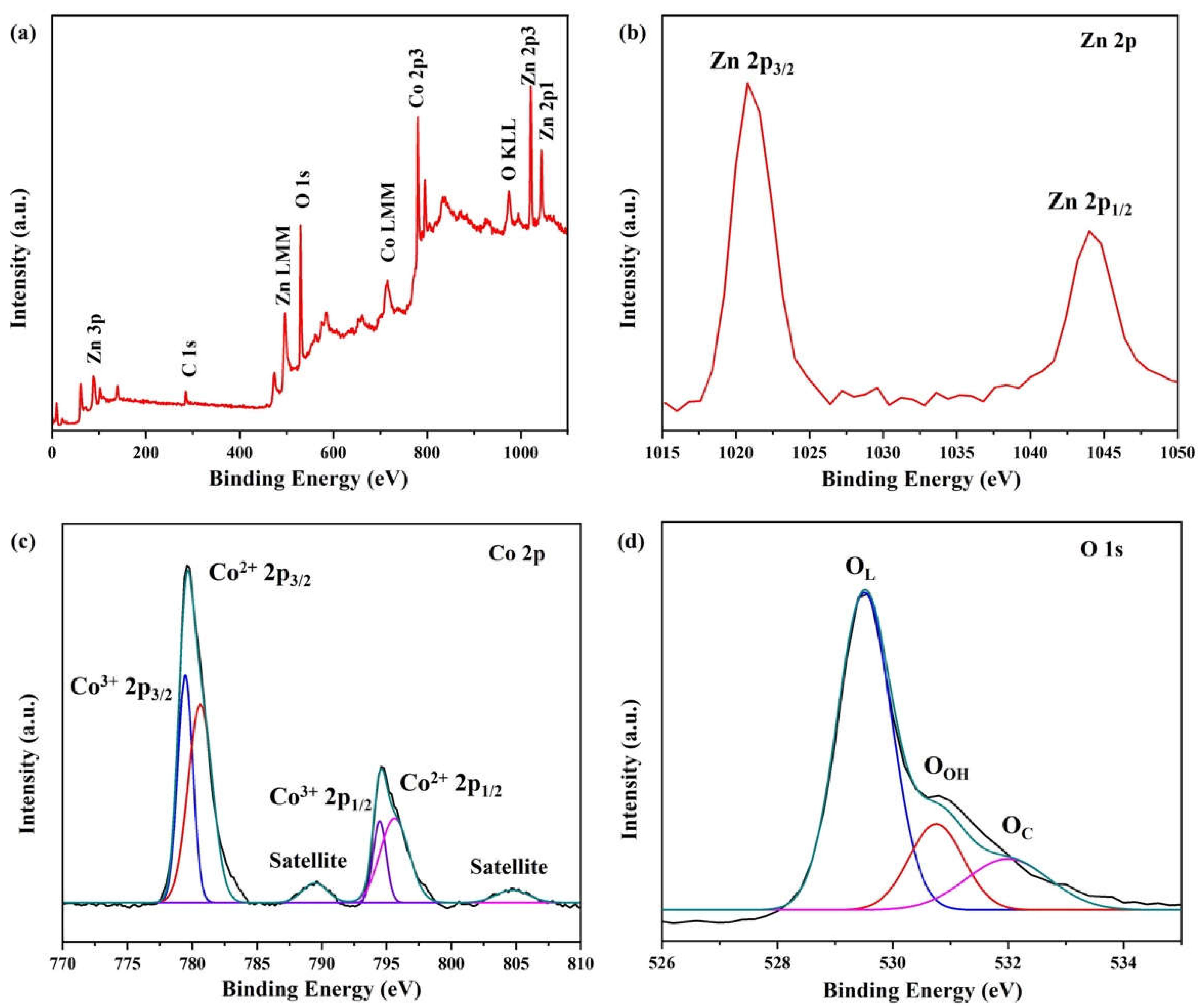
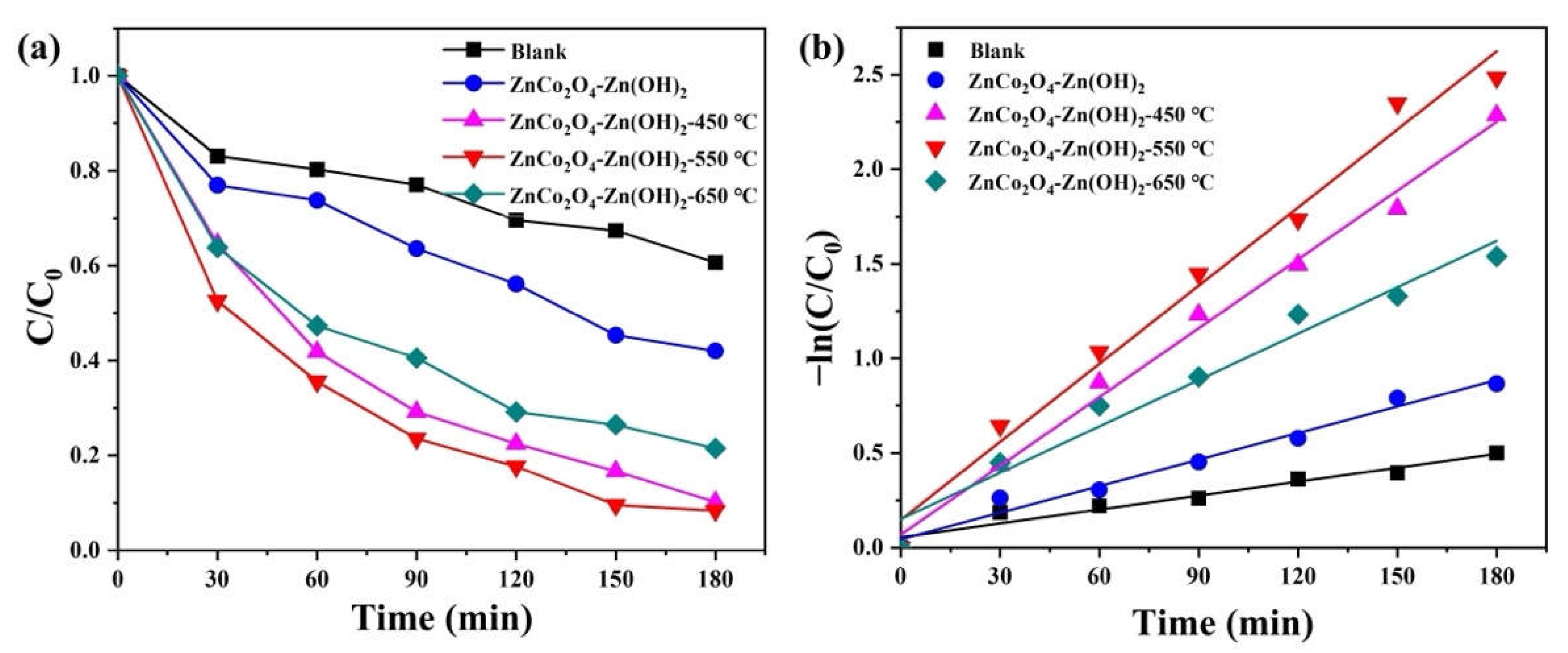
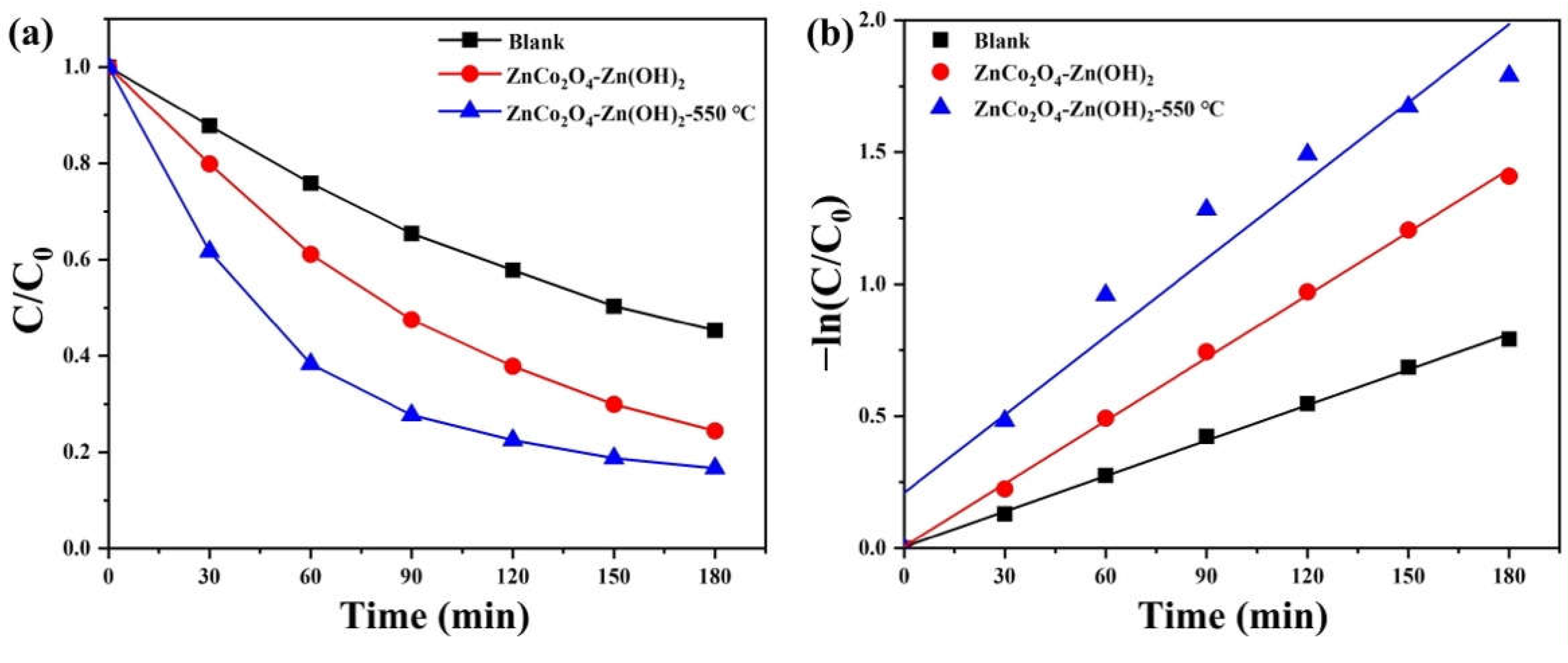
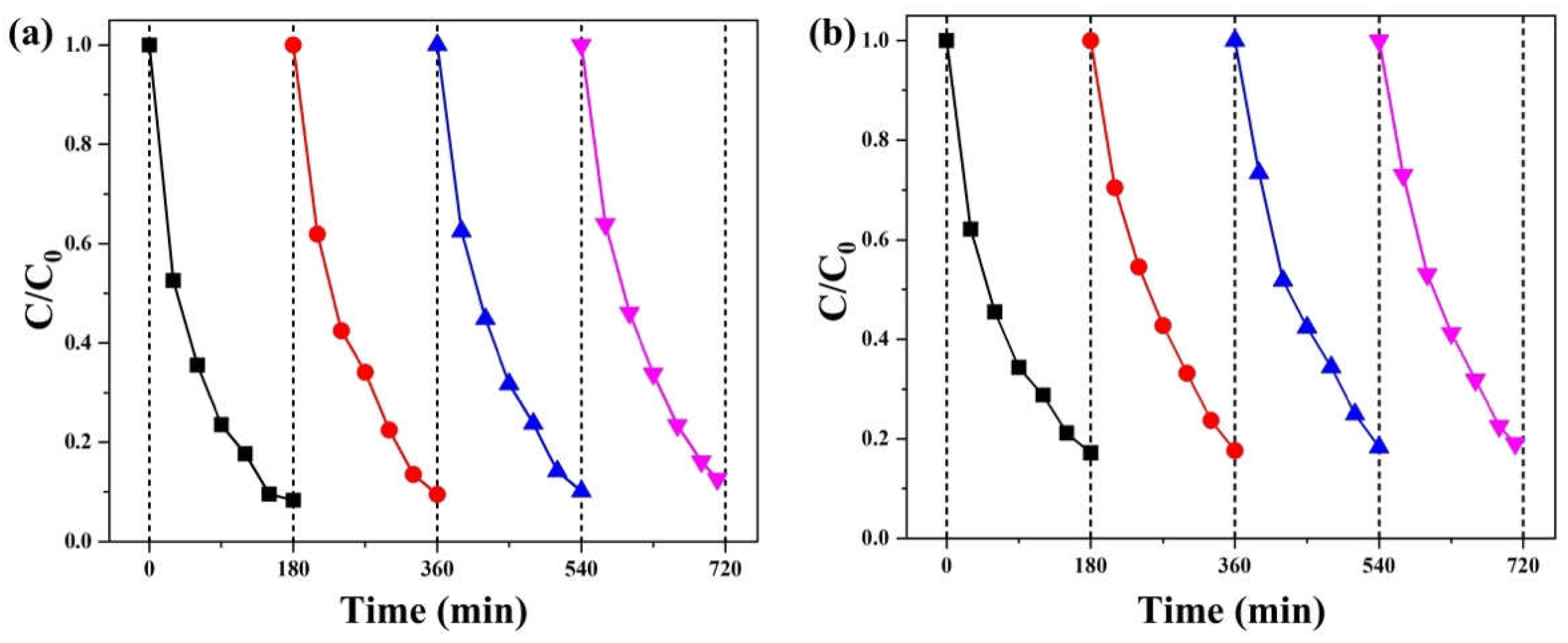
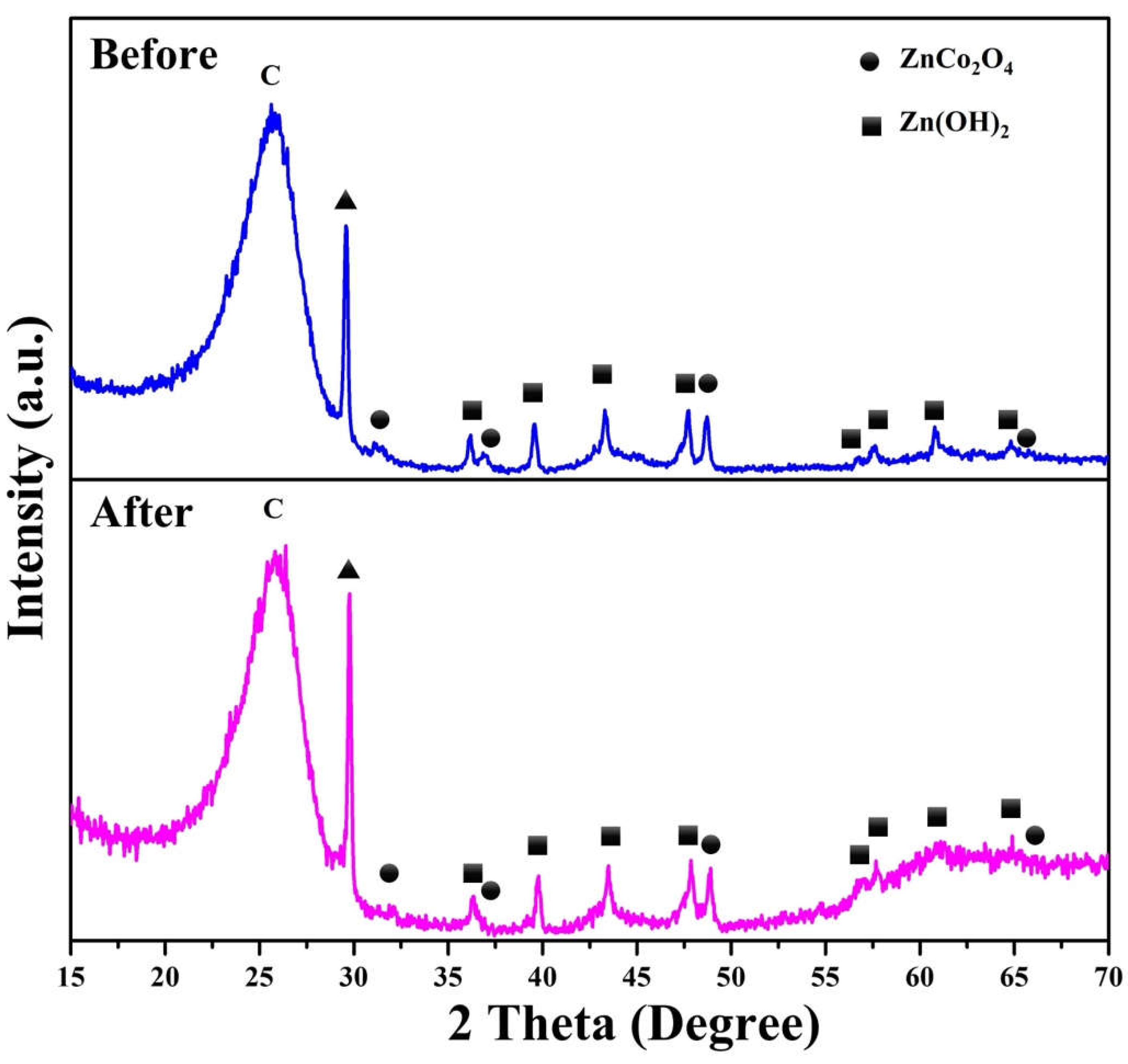
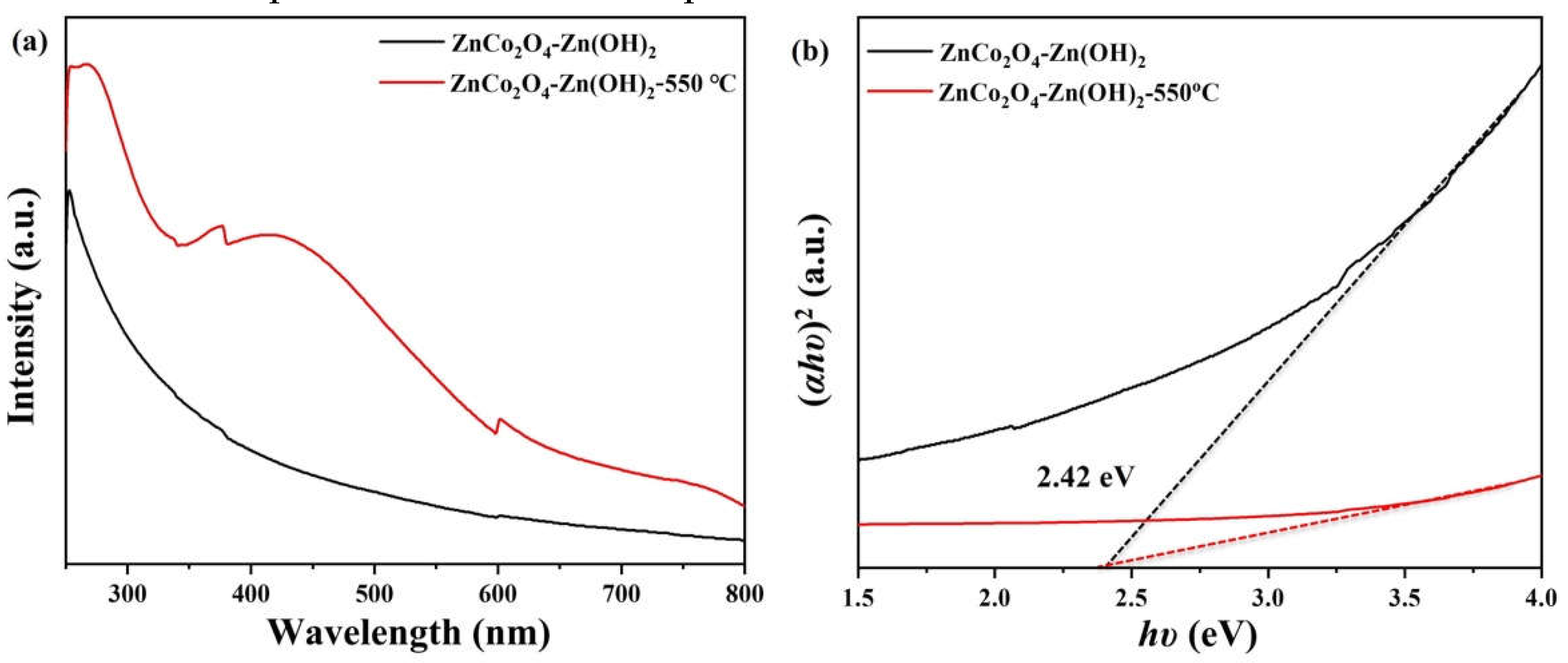
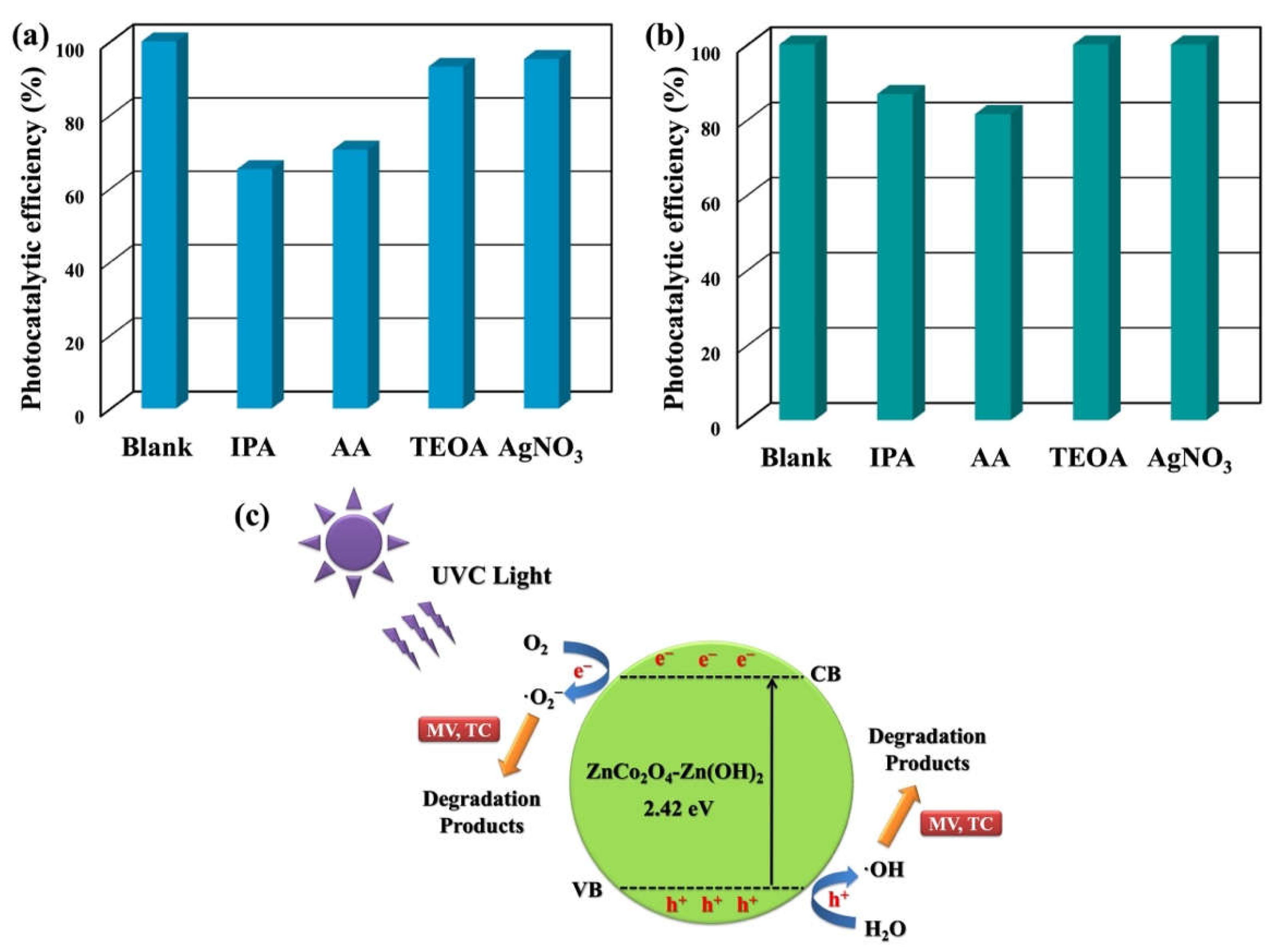
2. Results and Discussion
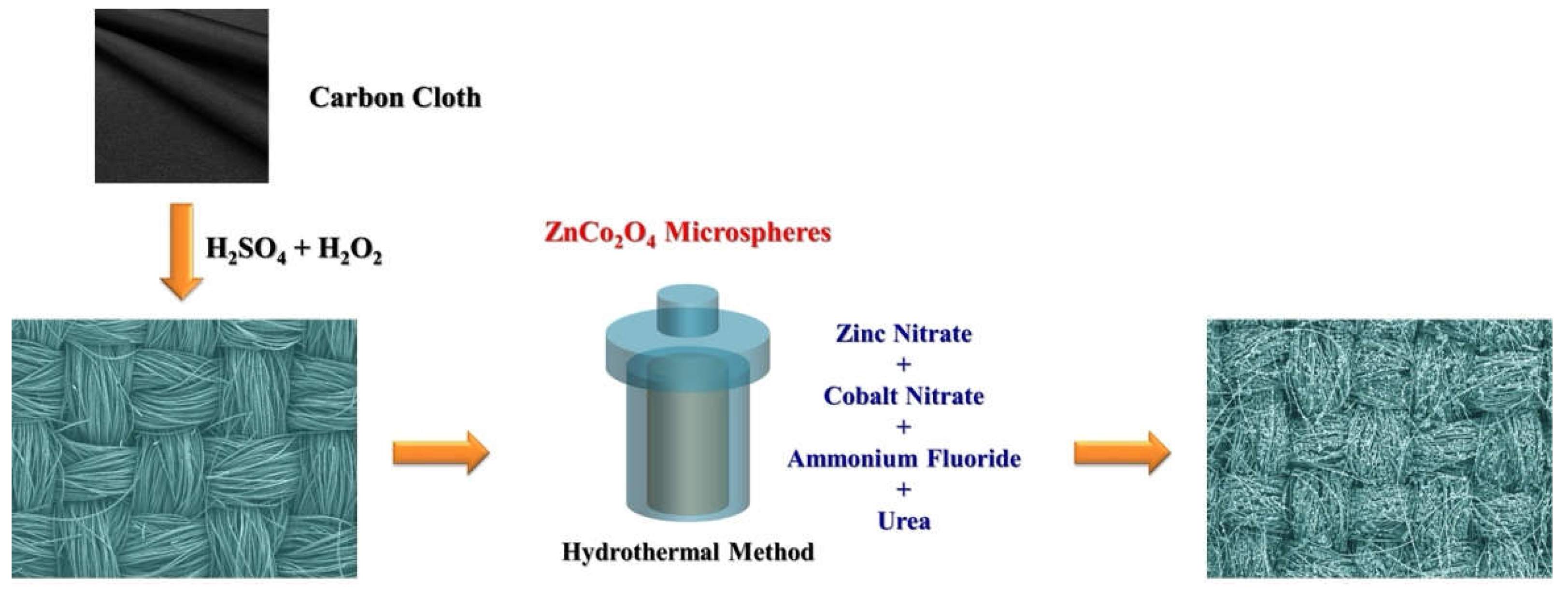
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Fabrication of ZnCo2O4-Zn(OH)2 Microspheres
3.3. Characterization
3.4. Photocatalytic Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandrasekaran, S.; Bowen, C.; Zhang, P.; Li, Z.; Yuan, Q.; Ren, X.; Deng, L. Spinel photocatalysts for environmental remediation, hydrogen generation, CO2 reduction and photoelectrochemical water splitting. J. Mater. Chem. A 2018, 6, 11078–11104. [Google Scholar] [CrossRef]
- Sriram, B.; Baby, J.N.; Wang, S.-F.; George, M.; Joseph, X.B.; Tsai, J.-T. Surface Engineering of Three-Dimensional-like Hybrid AB2O4 (AB = Zn, Co, and Mn) Wrapped on Sulfur-Doped Reduced Graphene Oxide: Investigation of the Role of an Electrocatalyst for Clioquinol Detection. ACS Applied Electronic Materials 2021, 3, 362–372. [Google Scholar] [CrossRef]
- Amirzhanova, A.; Akmanşen, N.; Karakaya, I.; Dag, Ö. Mesoporous MnCo2O4, NiCo2O4, and ZnCo2O4 Thin-Film Electrodes as Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Solutions. ACS Applied Energy Materials 2021, 4, 2769–2785. [Google Scholar] [CrossRef]
- Zhang, G.; Li, C.; Cheng, F.; Chen, J. ZnFe2O4 tubes: Synthesis and application to gas sensors with high sensitivity and low-energy consumption. Sens. Actuators. B Chem. 2007, 120, 403–410. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B. Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment: Review. Sustainable Materials and Technologies 2020, 23, e00140. [Google Scholar] [CrossRef]
- Kanazawa, T.; Kato, K.; Yamaguchi, R.; Uchiyama, T.; Lu, D.; Nozawa, S.; Yamakata, A.; Uchimoto, Y.; Maeda, K. Cobalt Aluminate Spinel as a Cocatalyst for Photocatalytic Oxidation of Water: Significant Hole-Trapping Effect. ACS Catal. 2020, 10, 4960–4966. [Google Scholar] [CrossRef]
- Gambini, M.; Mazzoni, S.; Vellini, M. The Role of Cogeneration in the Electrification Pathways towards Decarbonization. Energies 2023, 16. [Google Scholar] [CrossRef]
- Maity, D.; Karmakar, K.; Pal, D.; Saha, S.; Khan, G.G.; Mandal, K. One-Dimensional p-ZnCo2O4/n-ZnO Nanoheterojunction Photoanode Enabling Photoelectrochemical Water Splitting. ACS Applied Energy Materials 2021, 4, 11599–11608. [Google Scholar] [CrossRef]
- Qu, F.; Thomas, T.; Zhang, B.; Zhou, X.; Zhang, S.; Ruan, S.; Yang, M. Self-sacrificing templated formation of Co3O4/ZnCo2O4 composite hollow nanostructures for highly sensitive detecting acetone vapor. Sens. Actuators. B Chem. 2018, 273, 1202–1210. [Google Scholar] [CrossRef]
- Morán-Lázaro, J.P.; López-Urías, F.; Muñoz-Sandoval, E.; Blanco-Alonso, O.; Sanchez-Tizapa, M.; Carreon-Alvarez, A.; Guillén-Bonilla, H.; Olvera-Amador, M.D.; Guillén-Bonilla, A.; Rodríguez-Betancourtt, V.M. Synthesis, Characterization, and Sensor Applications of Spinel ZnCo2O4 Nanoparticles. Sensors 2016, 16. [Google Scholar] [CrossRef]
- Kim, T.W.; Woo, M.A.; Regis, M.; Choi, K.-S. Electrochemical Synthesis of Spinel Type ZnCo2O4 Electrodes for Use as Oxygen Evolution Reaction Catalysts. J. Phys. Chem. Lett. 2014, 5, 2370–2374. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ke, J.; Zhang, Y.; Huang, Y. Microwave-assisted rapid synthesis of mesoporous nanostructured ZnCo2O4 anode materials for high-performance lithium-ion batteries. J. Mater. Chem. A 2015, 3, 24303–24308. [Google Scholar] [CrossRef]
- Shih, G.-H.; Liu, W.-R. A facile microwave-assisted approach to the synthesis of flower-like ZnCo2O4 anode materials for Li-ion batteries. RSC Adv. 2017, 7, 42476–42483. [Google Scholar] [CrossRef]
- Liu, M.M.; Ma, S.Y.; Cai, Y.H.; Ma, N.N.; Wang, L.; Sheng, H. ZnO/ZnCo2O4 composite prepared by one-step hydrothermal method for high-performance ethylene glycol sensor. Ceram. Int. 2022, 48, 22305–22312. [Google Scholar] [CrossRef]
- Rajesh, J.A.; Min, B.-K.; Kim, J.-H.; Kang, S.-H.; Kim, H.; Ahn, K.-S. Facile hydrothermal synthesis and electrochemical supercapacitor performance of hierarchical coral-like ZnCo2O4 nanowires. J. Electroanal. Chem. 2017, 785, 48–57. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Wang, Y.; Hao, J.; Wang, S. Preparation of ZnCo2O4 porous nano-flower-like materials by one-step cryogenic hydrothermal method and study on their capacitive and photocatalytic properties. J. Mater. Sci. - Mater. Electron. 2023, 34, 531. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Heidari-Asil, S.A.; Salavati-Niasari, M. Recyclable magnetic ZnCo2O4-based ceramic nanostructure materials fabricated by simple sonochemical route for effective sunlight-driven photocatalytic degradation of organic pollution. Ceram. Int. 2021, 47, 8959–8972. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, S.; Mao, Y.; Liu, X. Preparation of ZnCo2O4/BiVO4 Z-Scheme heterostructures to enhance photocatalytic performance in organic pollutant and antibiotic removal. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130165. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Narsimulu, D.; Chinthakuntla, A.; Shilpa Chakra, C.; de Barros, A.L.F. Bimetallic MOF derived ZnCo2O4 nanocages as a novel class of high performance photocatalyst for the removal of organic pollutants. Inorg. Chem. Commun. 2022, 144, 109946. [Google Scholar] [CrossRef]
- Pan, J.H.; Dou, H.; Xiong, Z.; Xu, C.; Ma, J.; Zhao, X.S. Porous photocatalysts for advanced water purifications. J. Mater. Chem. 2010, 20, 4512–4528. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Wang, S.; Hao, J.; Liu, B. Preparation of ZnCo2O4 Nanosheets Coated on evenly arranged and fully separated Nanowires with high capacitive and photocatalytic properties by a One-Step Low-Temperature Water bath method. ChemistrySelect 2022, 7, e202200472. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Karthikeyan, V.; Ali, D.; Kumar, G.; Jenifer, S.G.; Yadav, V.K.; Choudhary, N.; Narayanan, V. Realization of rGO/ZnCo2O4 nanocomposites enhanced for the antimicrobial, electrochemical and photocatalytic activities. Diamond and Related Materials 2021, 120, 108677. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Wang, X.; Chen, G.; Chen, D.; Zhou, C.; Shen, G. Hierarchical Three-Dimensional ZnCo2O4 Nanowire Arrays/Carbon Cloth Anodes for a Novel Class of High-Performance Flexible Lithium-Ion Batteries. Nano Lett. 2012, 12, 3005–3011. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, W.; Yi, M.; Chen, Q.; Xu, C.; Cai, D.; Zhan, H. Metal-organic framework derived porous ternary ZnCo2O4 nanoplate arrays grown on carbon cloth as binder-free electrodes for lithium-ion batteries. Chem. Eng. J. 2018, 354, 454–462. [Google Scholar] [CrossRef]
- Chen, P.; Xu, K.; Fang, Z.; Tong, Y.; Wu, J.; Lu, X.; Peng, X.; Ding, H.; Wu, C.; Xie, Y. Metallic Co4N Porous Nanowire Arrays Activated by Surface Oxidation as Electrocatalysts for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2015, 54, 14710–14714. [Google Scholar] [CrossRef]
- Manjunatha, R.; Yuan, J.; Hongwei, L.; Deng, S.-Q.; Ezeigwe, E.R.; Zuo, Y.; Dong, L.; Li, A.; Yan, W.; Zhang, F.; et al. Facile carbon cloth activation strategy to boost oxygen reduction reaction performance for flexible zinc-air battery application. Carbon Energy 2022, 4, 762–775. [Google Scholar] [CrossRef]
- Chen, R.; Wang, H.-Y.; Miao, J.; Yang, H.; Liu, B. A flexible high-performance oxygen evolution electrode with three-dimensional NiCo2O4 core-shell nanowires. Nano Energy 2015, 11, 333–340. [Google Scholar] [CrossRef]
- Ma, T.Y.; Ran, J.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Phosphorus-Doped Graphitic Carbon Nitrides Grown In Situ on Carbon-Fiber Paper: Flexible and Reversible Oxygen Electrodes. Angew. Chem. Int. Ed. 2015, 54, 4646–4650. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A.; Gallo, M.J.H.; Otero-Irurueta, G.; Mikhalev, S.; Staiti, P.; Lufrano, F. Energy storage of supercapacitor electrodes on carbon cloth enhanced by graphene oxide aerogel reducing conditions. Journal of Energy Storage 2020, 32, 101839. [Google Scholar] [CrossRef]
- Li, L.; Gao, J.; Cecen, V.; Fan, J.; Shi, P.; Xu, Q.; Min, Y. Hierarchical WS2@NiCo2O4 Core–shell Heterostructure Arrays Supported on Carbon Cloth as High-Performance Electrodes for Symmetric Flexible Supercapacitors. ACS Omega 2020, 5, 4657–4667. [Google Scholar] [CrossRef]
- Wang, H.-F.; Tang, C.; Wang, B.; Li, B.-Q.; Cui, X.; Zhang, Q. Defect-rich carbon fiber electrocatalysts with porous graphene skin for flexible solid-state zinc–air batteries. Energy Storage Materials 2018, 15, 124–130. [Google Scholar] [CrossRef]
- Kordek, K.; Jiang, L.; Fan, K.; Zhu, Z.; Xu, L.; Al-Mamun, M.; Dou, Y.; Chen, S.; Liu, P.; Yin, H.; et al. Two-Step Activated Carbon Cloth with Oxygen-Rich Functional Groups as a High-Performance Additive-Free Air Electrode for Flexible Zinc–Air Batteries. Advanced Energy Materials 2019, 9, 1802936. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Wang, Y.; Dou, S.; Yan, D.; Liu, D.; Xia, Z.; Wang, S. In Situ Exfoliated, Edge-Rich, Oxygen-Functionalized Graphene from Carbon Fibers for Oxygen Electrocatalysis. Adv. Mater. 2017, 29, 1606207. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, Q.; Nie, S.; Peng, X.; Hu, N. 3D ZnCo2O4 nanowires@MnO2 nanosheets core-shell structures grown on carbon cloth for excellent supercapacitor electrodes. Ceram. Int. 2016, 42, 19343–19348. [Google Scholar] [CrossRef]
- Mariappan, C.R.; Kumar, R.; Vijaya Prakash, G. Functional properties of ZnCo2O4 nano-particles obtained by thermal decomposition of a solution of binary metal nitrates. RSC Adv. 2015, 5, 26843–26849. [Google Scholar] [CrossRef]
- Jeong, G.H.; Jang, H.S.; Yoon, J.C.; Lee, Z.; Yang, J.; Jang, A.R.; Ryu, G.H. Morphologically Controlled Synthesis of Reduced-Dimensional ZnO/Zn(OH)2 Nanosheets. ACS Omega 2022, 7, 35834–35839. [Google Scholar] [CrossRef] [PubMed]
- Jheng, B.-R.; Chiu, P.-T.; Yang, S.-H.; Tong, Y.-L. Using ZnCo2O4 nanoparticles as the hole transport layer to improve long term stability of perovskite solar cells. Sci. Rep. 2022, 12, 2921. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Chen, P.-C. P-type spinel ZnCo2O4 thin films prepared using sol-gel process. Appl. Surf. Sci. 2020, 505, 144460. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiology and Molecular Biology Reviews 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Science of The Total Environment 2021, 753, 141975. [Google Scholar] [CrossRef]
- Navalón, S.; Dhakshinamoorthy, A.; Álvaro, M.; Ferrer, B.; García, H. Metal–Organic Frameworks as Photocatalysts for Solar-Driven Overall Water Splitting. Chem. Rev. 2023, 123, 445–490. [Google Scholar] [CrossRef] [PubMed]
- Suram, S.K.; Newhouse, P.F.; Gregoire, J.M. High Throughput Light Absorber Discovery, Part 1: An Algorithm for Automated Tauc Analysis. ACS Combinatorial Science 2016, 18, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, J.; Lv, J.; Dai, K.; Liang, C. Plasmonic Ag2MoO4/AgBr/Ag composite: Excellent photocatalytic performance and possible photocatalytic mechanism. Appl. Surf. Sci. 2017, 396, 791–798. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Li, Y.; Jiang, L.; Zhang, G. Novel BiSbO4/BiOBr nanoarchitecture with enhanced visible-light driven photocatalytic performance: Oxygen-induced pathway of activation and mechanism unveiling. Appl. Surf. Sci. 2019, 498, 143850. [Google Scholar] [CrossRef]
- Xu, D.; Shi, W.; Song, C.; Chen, M.; Yang, S.; Fan, W.; Chen, B. In-situ synthesis and enhanced photocatalytic activity of visible-light-driven plasmonic Ag/AgCl/NaTaO3 nanocubes photocatalysts. Appl. Catal., B 2016, 191, 228–234. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Tasi, C.-L.; Ko, F.-H. Construction of ZnIn2S4/ZnO heterostructures with enhanced photocatalytic decomposition and hydrogen evolution under blue LED irradiation. Int. J. Hydrogen Energy 2021, 46, 10281–10292. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




