1. Introduction
Nosocomial bacterial infections are associated with high mortality, especially with a growing number of drug-resistant pathogens seen in routine clinical practice. Described as part of the group of ESKAPE pathogens,
Staphylococcus aureus and
Pseudomonas aeruginosa are the cause of many hospital-acquired infections with potential life-threatening progress [
1,
2]. Early diagnosis and accurate localisation of these infections could improve patient outcome and would allow to monitor antibacterial treatment more quickly and precisely than current diagnostic techniques. Established methods include identification of bacteria through culture tests, which suffer from a long delay between sampling and obtaining of results [
3], or molecular imaging techniques like [
18F]FDG, among others, which rely on secondary inflammatory response of the host organism [
4,
5]. Novel radiopharmaceuticals for fast and specific detection of human pathogens could therefore provide a considerable benefit to clinical practice [
6].
Desferrioxamine B (deferoxamine, DFO-B), a naturally occurring substance produced by
Streptomyces pilosus, belongs to a group of biomolecules called siderophores, which show high binding affinity for iron, among other metals [
7]. These low-molecular weight (< 1 kDa) structures play a pivotal role in the iron metabolism of most fungal and bacterial species, including several human pathogenic microorganisms [
8]. Uptake of these siderophores involves highly specific transporter proteins, which are not found in human cells [
9]. Trivalent gallium as an isosteric diamagnetic substitute for ferric iron also forms stable complexes with siderophores [
10], facilitating targeted uptake into microbial cells. Labelling siderophores with positron-emitting Ga-68 could enable precise localisation of pathogens by means of Positron Emission Tomography (PET), a concept already proven in preclinical imaging studies of
S. aureus and
P. aeruginosa mouse infection models with [
68Ga]Ga-desferrioxamine B [
11]. Data from preclinical imaging of
Aspergillus fumigatus in a rat aspergillosis model suggests that this concept might also be transferable to fungal infections [
12].
Automation of synthesis and radiolabelling procedures for bringing novel radiopharmaceuticals into clinical settings has become increasingly important in recent years. A driving force of this development is the rising use of PET radionuclides like Ga-68, which is readily available on site from
68Ge/
68Ge radionuclide generators or cyclotron production [
13]. The high decay energy of such radioisotopes imposes a substantial radiation burden on the personnel performing radiopharmaceutical production, which can be reduced significantly through the use of automated synthesis modules [
14], while also increasing repeatability and providing complete documentation of the production process [
15].
This study aimed to establish automated production of [
68Ga]Ga-desferrioxamine B on two different synthesis modules for clinical trial application according to current scientific standards [
16,
17]. Specifications for routine production of [
68Ga]Ga-desferrioxamine B were set up based on existing monographs of the European Pharmacopeia (Ph. Eur.) for other Ga-68 labelled radiopharmaceuticals [
18,
19].
2. Results
2.1. Generator Elution and Reaction Conditions
With the described buffer system, a pH of 4.5 was reached in the reaction solution. Elution of the 68Ge/68Ga radionuclide generator was followed by pre-purification of the eluate on a strong cation-exchange cartridge. This process resulted in activity yields of 405.9 ± 26.3 MBq for method 1 and 431.6 ± 150.3 MBq for method 2 (both at EOS) with final radioactivity concentrations of 47.8 ± 3.1 MBq/mL (method 1) and 25.4 ± 8.8 MBq/mL (method 2). Molar activities were calculated as 3.5 ± 0.3 GBq/µmol for method 1 and 4.0 ± 1.4 GBq/µmol for method 2. Radiochemical yields based on the theoretical radioactivity of germanium-68 at the time of elution were 64.53 ± 6.09 % (method 1) and 49.61 ± 11.27 % (method 2). All values for both methods were determined with four individual batches each.
To keep the ethanol concentration in the final product below the pharmacopeial threshold of 10 % (v/v), the products were diluted with physiological saline to a final volume of 8.5 mL (method 1) or 17.0 mL (method 2). Gas chromatography analysis of the product formulations confirmed an ethanol concentration of 6.3 ± 0.2 % (v/v) for method 1 and 5.4 ± 0.4 % (v/v) for method 2.
2.2. ITLC & RP-HPLC Analysis and Stability Testing
Instant thin-layer chromatography analysis of the product formulations with mobile phases A and B showed a radionuclide incorporation of >97 % which remained stable during stability testing for up to 2 hours post preparation (n=4). ITLC with mobile phase A was only performed as part of process validation. ITLC with mobile phase B was defined as a release parameter in analogy to European Pharmacopoeia monographs [
17,
18]. Sample chromatograms for ITLC are shown in the supplementary material (
Figure S1).
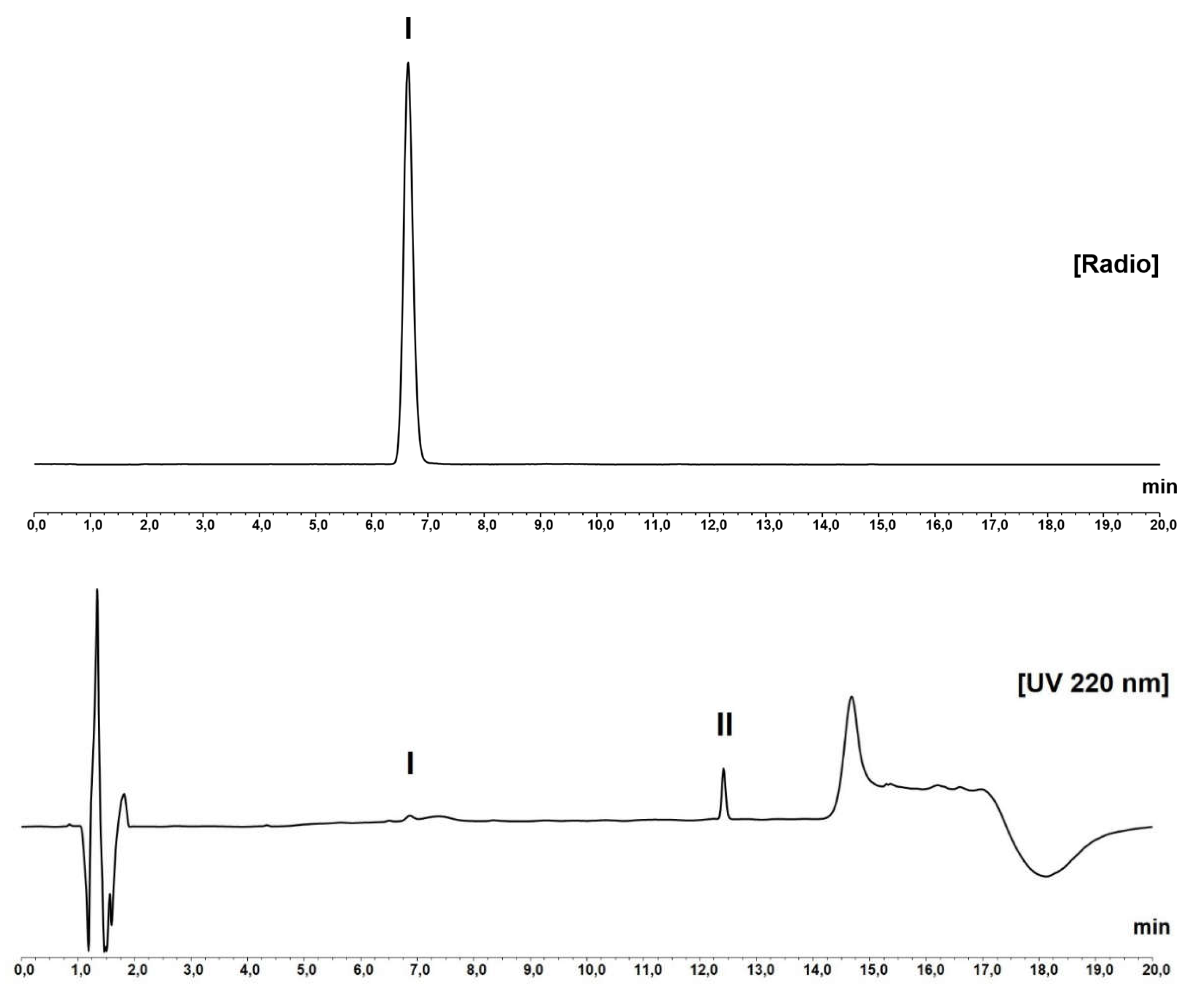
RP-HPLC analysis showed a main peak in the radiochromatogram at approximately 6.6 min (see
Figure 1) corresponding to the retention time of the reference chromatogram obtained for [
natGa]Ga-desferrioxamine B (see
Figure S2). The method provided an excellent separation from desferrioxamine B itself (R
t = 11.9 min, see
Figure S3) with a resolution of >20, and also separation of the two peaks in the deferoxamine for system suitability chemical reference standard (CRS) (
Figure S4) with a resolution of >3 for the impurity meeting the specifications in the Ph. Eur. monograph for deferoxamine mesilate [
20].
HPLC analysis performed immediately after end of synthesis showed a mean radiochemical purity of 98.2 ± 0.5 % for method 1 and 98.6 ± 1.3 % for method 2 (n=4). Stability testing performed at 2 and 4 hours confirmed high stability of the final product with RCP of 98.0 ± 0.6 % (2 h) and 97.6 ± 0.5 % (4 h), respectively (n=4 for both). Peptide concentration in the final formulation was assessed by integration of all visible peaks in the UV chromatogram and comparison to a calibration curve with desferrioxamine B finding a value of 103.7 ± 4.8 µg/V for method 1 and 96.9 ± 5.2 µg/V for method 2 (n = 4 for both; see
Table 1).
2.3. Process Validation
Results from radiolabelling tests as well as specifications of Ph. Eur. monographs available for other
68Ga-labelled radiopharmaceuticals [
18,
19] were used to set up specifications for routine production. All four master batches produced for process validation of both production routes, respectively, complied with the pre-defined specifications (see
Table 1).
3. Discussion
[
68Ga]Ga-desferrioxamine B showed highly promising results in preclinical studies [
11], with a high sensitivity to detect infections with several clinically relevant bacteria including
Staphylococcus or
Pseudomonas ssp. Contrary to currently available PET radiotracers for imaging of infectious processes, in particular [
18F]FDG, which only visualise the effects of these pathogens, i.e. inflammatory response, [
68Ga]Ga-desferrioxamine B can potentially provide imaging data of the actual causative microorganism in vivo and would potentially allow to monitor the origin of infections in patients as well as treatment response. The short half-life of Ga-68 is suitable for this application, as the radiation burden for patients can be kept relatively low compared to other radionuclides, which would be suitable for radiolabelling of desferrioxamine B, e.g. Ga-67 or Zr-89 [
20,
21], but in particular, based on its availability from a radionuclide generator allows immediate and on-demand preparation of the PET radiopharmaceutical. Even though also other siderophores have been proposed, such as [
68Ga]Ga-pyoverdine which showed high specific uptake of this radiotracer in
Pseudomonas aeruginosa [
22], [
68Ga]Ga-desferrioxamine B was selected for a first in human clinical trial due to the availability of desferrioxamine B as a licensed pharmaceutical for human use.
As previously shown [
11], radiolabelling of desferrioxamine B with Ga-68 can be performed under mild conditions and yields a product of high radiochemical purity. For a clinical translation, automation of the process was required to allow a preparation of the radiopharmaceuticals according to current radiopharmaceutical standards and to reduce radiation burden to operators. The alternative of providing a kit formulation, as established for e.g. [
68Ga]Ga-DOTA-TOC or [
68Ga]Ga-PSMA-11, was discarded due to the additional efforts for a GMP compliant kit production [
23]. The synthesis was established on two different synthesis modules to enable inclusion of different centres into the planned clinical trial. Setup of the automated production process based on single use cassette systems was established in alignment with well-known procedures for
68Ga-radiolabelling [
24] and based on the preclinical data on [
68Ga]Ga-desferrioxamine B preparation [
11]. An acetate buffer system with pH 4.5, higher than for
68Ga-labelling of DOTA-based radiopharmaceuticals [
25], with ascorbic acid as radiation scavenger was used. 100 µg desferrioxamine B mesylate precursor were used to enable a stable labelling process, while guaranteeing sufficiently low amounts to ensure the tracer principle. Since desferrioxamine B is given in much higher amounts of up to 50 mg/kg body weight for treatment of iron overload, this limit was considered safe for patient use [
26]. Reaction takes place at ambient temperature or mild heating to 40 °C and the described synthesis approaches resulted in high yields and high radiochemical purity in the final product. One major difference between the two employed synthesis modules was, that for Method 2 a higher final volume was required as the applied synthesis setup requires larger ethanol amounts to elute the purified product from the C18 cartridge. Therefore, twice as much saline was required to not exceed the pharmacopeial limit of 10 % (v/v) ethanol in the final formulation [
18].
Overall, these conditions resulted in products of equivalent quality with high radiochemical purity on both synthesis modules and meeting quality specifications analogous to well established 68Ga-radiopharmaceuticals. For HPLC analysis reference standards from Ph. Eur. were available and the applied method resulted in excellent resolution between desferrioxamine B and its respective gallium-complex, which was also used to establish a method for system suitability testing and also sufficient separation of the impurity in the system suitability standard CRS from Ph. Eur.
HPLC analysis also allowed to determine the content of desferrioxamine B in the final product formulations by integration of the corresponding peaks in the UV chromatograms and comparison to a calibration curve. To include variations from precursor dilutions the specifications for the validation procedures were set to 150 µg desferrioxamine B per V.
Product stability was confirmed over 2 hours post-production through determination of radiochemical purity via RP-HPLC and ITLC. 4-hour measurements were also performed but were inconclusive due to short half-life of Ga-68, resulting in low detectability and low signal-to-noise ratio. Consequently, shelf life of the final products was limited to 2 hours.
This study was the basis for initiation of an academic clinical trial which is currently ongoing at the Medical University of Innsbruck under the EudraCT-No. 2020-002868-31 titled ”A Phase I/IIa study to evaluate safety, biodistribution, dosimetry and preliminary diagnostic performance of [68Ga]Ga-Deferoxamine for PET imaging in patients with bacterial infections”. The data presented herein were the basis of approval of [68Ga]Ga-desferrioxamine B as an Investigational Medicinal Product within this trial by the competent Austrian authority.
4. Materials and Methods
4.1. Materials and Synthesis Procedure
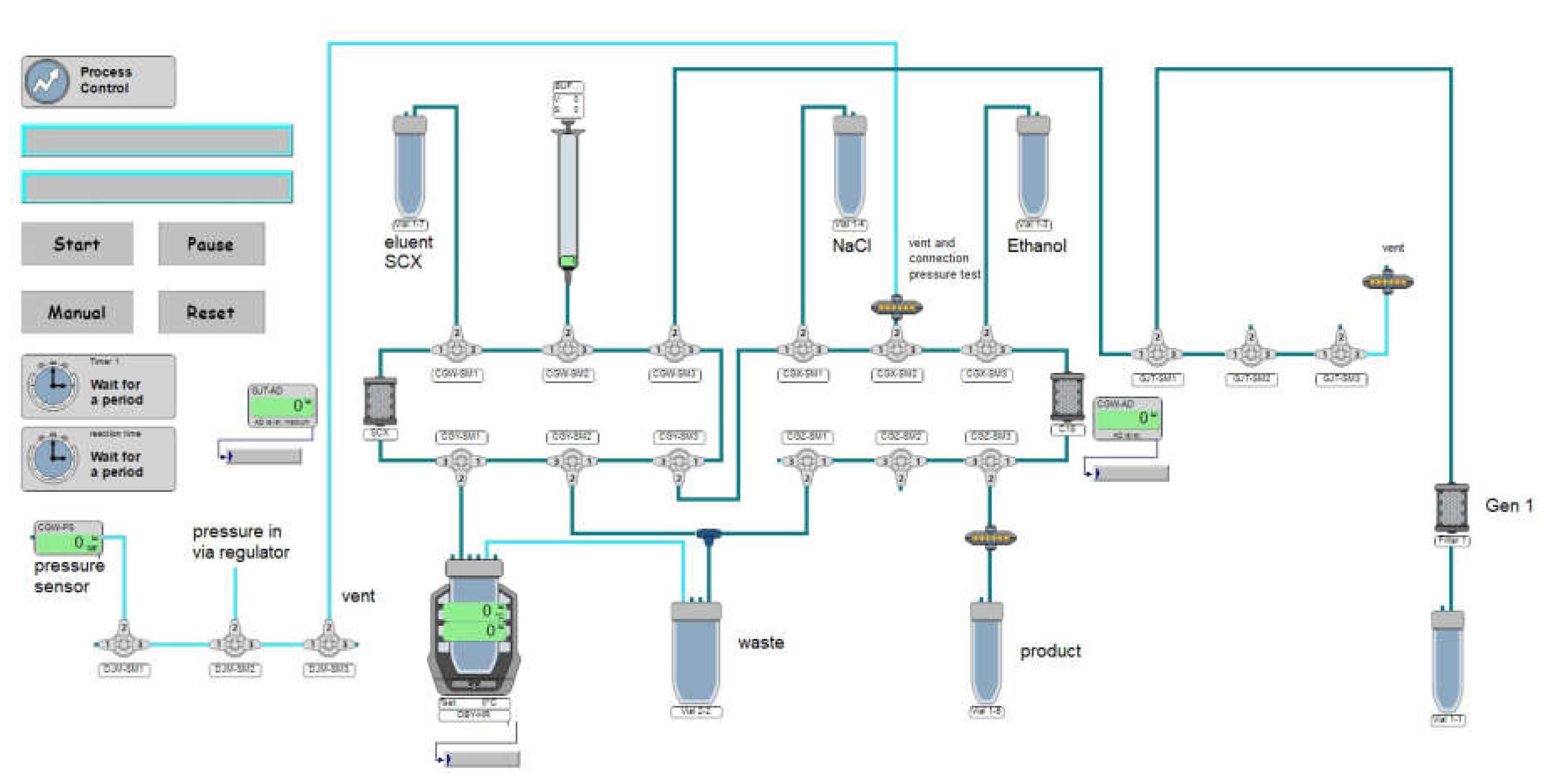
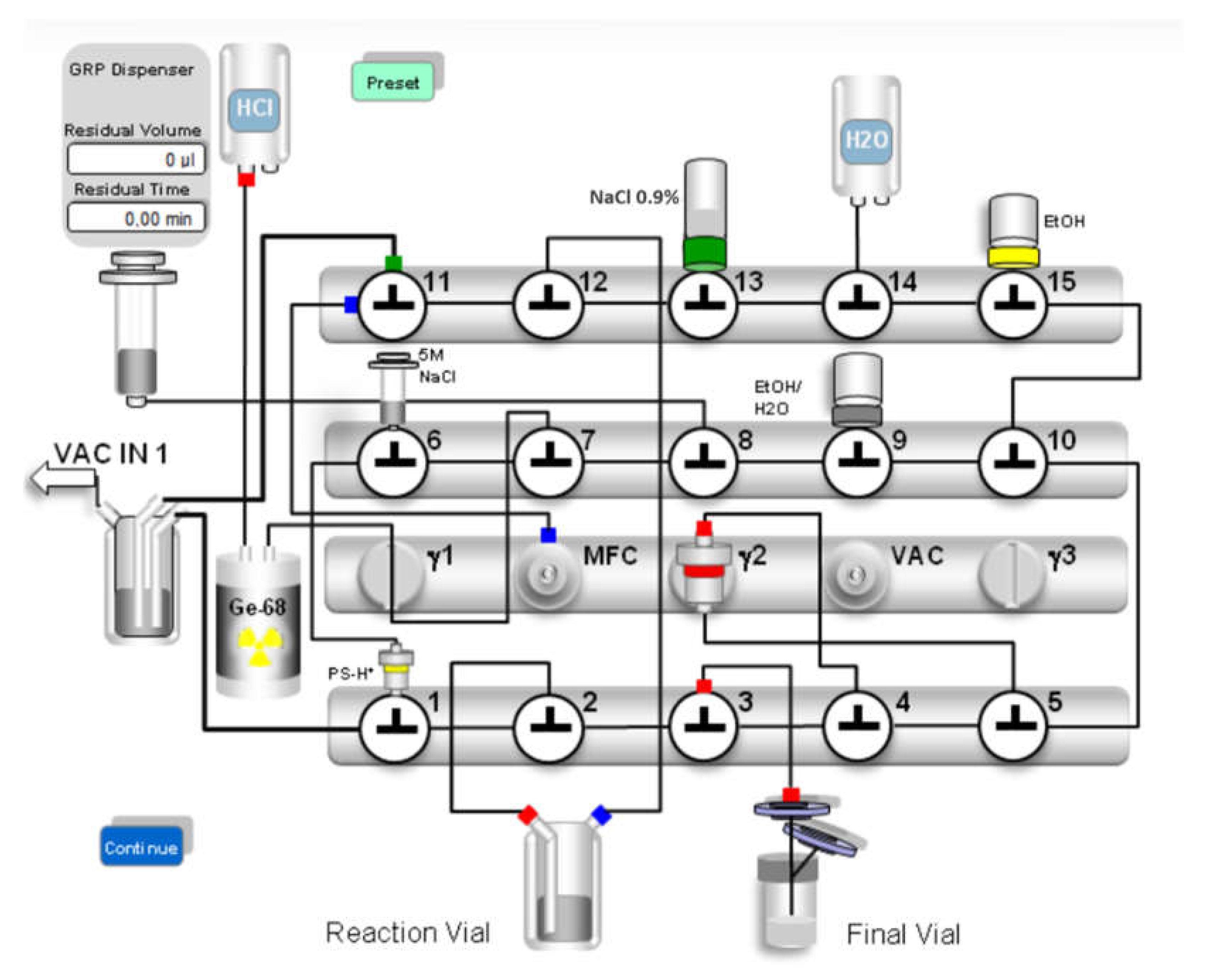
Automated synthesis was conducted on two different automated modules using similar synthesis approaches. Method 1: Modular-Lab PharmTracer® module (Eckert & Ziegler Radiopharma GmbH, Berlin, Germany) in conjunction with commercially available C4-GA-PEP cassettes (Eckert & Ziegler Radiopharma GmbH, Berlin, Germany); method 2: GRP 3V® module (Scintomics Molecular Applied Theranostics Technologies GmbH, Fürstenfeldbruck, Germany) in conjunction with commercially available SC-01 cassettes (ABX advanced biochemical compounds GmbH, Radeberg, Germany).
The synthesis process for both methods consisted of elution of the
68Ge/
68Ga radionuclide generator, radiolabelling at 40 °C, product purification via C18 cartridge, dilution with saline, sterile filtration and filter integrity testing. Schematic representations of the production process for both automated synthesis modules are shown in
Figure 2a and
Figure 2b, respectively.
[68Ga]GaCl3 used for radiolabelling was obtained from a licensed GalliaPharm® 68Ge/68Ga radionuclide generator (1850 MBq theoretical activity) by elution with ultrapure 0.1 M hydrochloric acid (both Eckert & Ziegler Radiopharma GmbH, Berlin, Germany).
Desferrioxamine B was obtained as desferrioxamine B mesylate from a licensed pharmaceutical (Desferal® vials 500 mg, Novartis Pharmaceuticals, Vienna, Austria). One vial was dissolved in 5 mL Aqua bidest for injection, 100 µL of this solution were diluted to 10 mL Aqua bidest for injection. 100 µL of this dilution (corresponding to 100 µg desferrioxamine B mesylate) were further diluted with 1.5 mL acetic acid/sodium acetate buffer 1.28 M with pH 4.5 containing 3 mg ascorbic acid. This mixture was used for radiolabelling. The eluate of the 68Ge/68Ga generator was absorbed on a strong cation exchange cartridge and [68Ga]GaCl3 was desorbed with 4.9 M NaCl/0.14 M HCl solution (method 1) or 5 M sodium chloride solution (method 2) as eluent solution to the buffer containing desferrioxamine B, the reaction solution was heated to 40 °C for 5 minutes.
Solid phase extraction (SPE) purification of the crude product was performed using a C18 Sep-Pak® Plus Light cartridge (Waters Corporation, Milford, Massachusetts, USA). Transfer of the labelling product to the SPE cartridge, rinsing of the reactor vial and all washing steps were carried out with physiological saline (method 1) or water for injection (method 2). The purified product was eluted from the cartridge with 50 % (v/v) ethanol (1 mL for method 1; 2 mL for method 2) and transferred to the product vial via a 0.22 µm filter (Millex-GV filter, 33 mm, Merck KGaA, Darmstadt, Germany). Subsequently, the product solution was diluted with physiological saline to a final volume of 8.5 mL (method 1) or 17 mL (method 2). Filter integrity testing of the sterile filter was performed immediately after the synthesis process.
[natGa]Ga-desferrioxamine B as an inactive reference compound was prepared by reacting 100 µg of desferrioxamine B mesylate with 12 µL of Gallium bromide solution (5 mg GaBr3/mL in 0.1 N HCl) and 10 µL of sodium acetate solution (155 mg sodium acetate trihydrate in 1 mL water) at 40 °C for 5 min. This solution was purified over a Sep-Pak C18 Plus Light cartridge (Waters Corporation, Milford, Massachusetts, USA), washed with water and eluted with ethanol 50 %. This stock solution was stored at -20 °C. Purity of [natGa]Ga-desferrioxamine B was assessed by RP-HPLC analysis. All chemicals used for synthesis and purification of [natGa]Ga-desferrioxamine B were reagent grade (Merck, Darmstadt, Germany) apart from desferrioxamine B mesylate which was available from Desferal®. Reference standards of desferrioxamine B mesylate and deferoxamine for system suitability were obtained as Chemical Reference Standard (CRS, EDQM; Strasbourg).
4.2. Quality Control
Quality control was carried out immediately at the end of synthesis. Stability of the final product was assessed up to 4 h post production.
Instant thin-layer chromatography (ITLC) was performed on ITLC-SG strips (Agilent Technologies, Vienna, Austria). The following mobile phases were used: (A) sodium citrate 0.1 M (pH = 5.0), (B) 1:1 v/v mixture of ammonium acetate 1 M and methanol. TLC analysis was conducted on a Scan-RAM radio-TLC scanner using the Laura radiochromatography analysis software (both LabLogic Systems Ltd, Sheffield, UK).
Reversed-phase high-performance liquid chromatography (RP-HPLC) analysis was performed using the following system (all UltiMate components: Thermo Fisher Scientific, Vienna, Austria): UltiMate 3000 RS UHPLC pump, UltiMate 3000 auto sampler, UltiMate 3000 column compartment with oven temperature set to 25 °C. Signal detection was carried out with an UltiMate 3000 Variable Wavelength Detector with UV detection at λ = 220 nm and a radiodetector (Gabi Star, Raytest; Straubenhardt, Germany) connected in series.
An ACE 3 C18 150 x 3.0 mm column (ACE-111-1503; Avantor, Radnor, Pennsylvania, USA) was used with 0.1 % TFA in water (A) and 0.1 % TFA in acetonitrile (B) as mobile phases with a flow rate of 0.6 mL/min and the following gradient: 8 % - 18 % B (0 - 12 min); 50 % B (12.1 - 15.0 min); 8 % B (15.1-20 min). A calibration curve for desferrioxamine B was created by injecting various amounts of deferoxamine mesylate CRS in a matrix similar to the final product formulation (6% ethanol in physiological saline).
An Orion 3-Star Benchtop pH meter (Thermo Fisher Scientific, Vienna, Austria) was used for pH measurement of buffer solutions and product solutions after radioactive decay. The pH of the final product was checked using pH indicator paper (1.09526.0003, Merck KGaA, Darmstadt, Germany).
The determination of ethanol content was performed on a GC-2010 Plus gas chromatograph with an AOC-20i autosampler (both Shimadzu Austria, Korneuburg, Austria), equipped with a Phenomenex Zebron ZB-624 column (7KM-G005-31). A flow rate of 3 mL/min and a FID temperature of 260 °C were applied.
4.3. Process Validation
For validation of the described synthesis approaches, four master batches of [68Ga]Ga-desferrioxamine B for each synthesis module were produced and analysed. The definition of product specifications was based on existing Ph.Eur. monographs for [68Ga]Ga-PSMA-11 and [68Ga]Ga-edotreotide.
5. Conclusions
The described methods for automated production of [68Ga]Ga-desferrioxamine B using the Modular-Lab PharmTracer synthesis module as well as the Scintomics GRP 3V module show high reproducibility and stable activity yields. All specifications set for quality control were met, including high radiochemical purity as demonstrated by RP-HPLC and ITLC analysis enabling reliable clinical production of this novel radiopharmaceutical for patient use, but also gives valuable reference for introduction of other 68Ga-radiopharmaceuticals in clinical research.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Sample chromatograms from ITLC of [68Ga]Ga-desferrioxamine B with silica gel as stationary phase; Figure S2: Sample UV chromatogram at 220 nm from RP-HPLC of [natGa]Ga-desferrioxamine B; Figure S3: Sample UV chromatogram at 220 nm from RP-HPLC of desferrioxamine B (Desferal®); Figure S4: Sample UV chromatogram at 220 nm from RP-HPLC of desferrioxamine B (CRS standard for system suitability).
Author Contributions
Conceptualization, Bernhard Nilica and Clemens Decristoforo; Formal analysis, Martin Kraihammer and Clemens Decristoforo; Funding acquisition, Bernhard Nilica and Clemens Decristoforo; Investigation, Martin Kraihammer, Miloš Petřík and Clemens Decristoforo; Methodology, Martin Kraihammer, Miloš Petřík, Hubertus Haas and Clemens Decristoforo; Project administration, Irene Virgolini and Clemens Decristoforo; Resources, Clemens Decristoforo; Supervision, Michael Gabriel and Clemens Decristoforo; Writing – original draft, Martin Kraihammer; Writing – review & editing, Miloš Petřík, Christine Rangger, Michael Gabriel, Hubertus Haas, Bernhard Nilica, Irene Virgolini and Clemens Decristoforo.
Funding
This research was funded in whole or in part by the Austrian Science Fund (FWF) [grant DOI: 10.55776/KLI909]. For open access purposes, the author has applied a CC BY public copyright license to any author accepted manuscript version arising from this submission.
Data Availability Statement
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Haque, M.; Sartelli, M.; McKimm, J.; Abu Bakar, M. Health Care-Associated Infections - an Overview. Infect Drug Resist 2018, 11, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Arias, C.A. ESKAPE Pathogens: Antimicrobial Resistance, Epidemiology, Clinical Impact and Therapeutics. Nat Rev Microbiol 2024. [Google Scholar] [CrossRef] [PubMed]
- Gerace, E.; Mancuso, G.; Midiri, A.; Poidomani, S.; Zummo, S.; Biondo, C. Recent Advances in the Use of Molecular Methods for the Diagnosis of Bacterial Infections. Pathogens 2022, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Lawal, I.; Zeevaart, J.; Ebenhan, T.; Ankrah, A.; Vorster, M.; Kruger, H.G.; Govender, T.; Sathekge, M. Metabolic Imaging of Infection. J Nucl Med 2017, 58, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Sollini, M.; Lauri, C.; Boni, R.; Lazzeri, E.; Erba, P.A.; Signore, A. Current Status of Molecular Imaging in Infections. Curr Pharm Des 2018, 24, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Alberto, S.; Ordonez, A.A.; Arjun, C.; Aulakh, G.K.; Beziere, N.; Dadachova, E.; Ebenhan, T.; Granados, U.; Korde, A.; Jalilian, A.; et al. The Development and Validation of Radiopharmaceuticals Targeting Bacterial Infection. J Nucl Med 2023, 64, 1676–1682. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X. Chemistry and Biology of Siderophores. Nat Prod Rep 2010, 27, 637–657. [Google Scholar] [CrossRef]
- Skaar, E.P. The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts. PLoS Pathog 2010, 6, e1000949. [Google Scholar] [CrossRef] [PubMed]
- Wandersman, C.; Delepelaire, P. Bacterial Iron Sources: From Siderophores to Hemophores. Annu Rev Microbiol 2004, 58, 611–647. [Google Scholar] [CrossRef]
- Fadeev, E.A.; Luo, M.; Groves, J.T. Synthesis, Structure, and Molecular Dynamics of Gallium Complexes of Schizokinen and the Amphiphilic Siderophore Acinetoferrin. J Am Chem Soc 2004, 126, 12065–12075. [Google Scholar] [CrossRef]
- Petrik, M.; Umlaufova, E.; Raclavsky, V.; Palyzova, A.; Havlicek, V.; Pfister, J.; Mair, C.; Novy, Z.; Popper, M.; Hajduch, M.; et al. 68Ga-Labelled Desferrioxamine-B for Bacterial Infection Imaging. Eur J Nucl Med Mol Imaging 2021, 48, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Misslinger, M.; Petrik, M.; Pfister, J.; Hubmann, I.; Bendova, K.; Decristoforo, C.; Haas, H. Desferrioxamine B-Mediated Pre-Clinical In Vivo Imaging of Infection by the Mold Fungus Aspergillus Fumigatus. J Fungi (Basel) 2021, 7, 734. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F.; Spreckelmeyer, S. Good Practices for 68Ga Radiopharmaceutical Production. EJNMMI Radiopharm Chem 2022, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Kollaard, R.; Zorz, A.; Dabin, J.; Covens, P.; Cooke, J.; Crabbé, M.; Cunha, L.; Dowling, A.; Ginjaume, M.; McNamara, L. Review of Extremity Dosimetry in Nuclear Medicine. J Radiol Prot 2021, 41. [Google Scholar] [CrossRef] [PubMed]
- Boschi, S.; Malizia, C.; Lodi, F. Overview and Perspectives on Automation Strategies in (68)Ga Radiopharmaceutical Preparations. Recent Results Cancer Res 2013, 194, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Korde, A.; Mikolajczak, R.; Kolenc, P.; Bouziotis, P.; Westin, H.; Lauritzen, M.; Koole, M.; Herth, M.M.; Bardiès, M.; Martins, A.F.; et al. Practical Considerations for Navigating the Regulatory Landscape of Non-Clinical Studies for Clinical Translation of Radiopharmaceuticals. EJNMMI Radiopharm Chem 2022, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Gillings, N.; Hjelstuen, O.; Ballinger, J.; Behe, M.; Decristoforo, C.; Elsinga, P.; Ferrari, V.; Peitl, P.K.; Koziorowski, J.; Laverman, P.; et al. Guideline on Current Good Radiopharmacy Practice (CGRPP) for the Small-Scale Preparation of Radiopharmaceuticals. EJNMMI Radiopharm Chem 2021, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia. GALLIUM (68Ga) EDOTREOTIDE INJECTION. 01/2022:2482 (11th Edition) 2022.
- European Pharmacopoeia. GALLIUM (68Ga) PSMA-11 INJECTION. 04/2021:3044 (11th Edition) 2021.
- European Pharmacopoeia. DEFEROXAMINE MESILATE. 01/2018:0896 (11th Edition) 2018.
- Petrik, M.; Zhai, C.; Haas, H.; Decristoforo, C. Siderophores for Molecular Imaging Applications. Clin Transl Imaging 2017, 5, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Ioppolo, J.A.; Caldwell, D.; Beiraghi, O.; Llano, L.; Blacker, M.; Valliant, J.F.; Berti, P.J. 67Ga-Labeled Deferoxamine Derivatives for Imaging Bacterial Infection: Preparation and Screening of Functionalized Siderophore Complexes. Nucl Med Biol 2017, 52, 32–41. [Google Scholar] [CrossRef]
- Petrik, M.; Umlaufova, E.; Raclavsky, V.; Palyzova, A.; Havlicek, V.; Haas, H.; Novy, Z.; Dolezal, D.; Hajduch, M.; Decristoforo, C. Imaging of Pseudomonas Aeruginosa Infection with Ga-68 Labelled Pyoverdine for Positron Emission Tomography. Sci Rep 2018, 8, 15698. [Google Scholar] [CrossRef]
- Lepareur, N. Cold Kit Labeling: The Future of 68Ga Radiopharmaceuticals? Front Med (Lausanne) 2022, 9, 812050. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.; Knetsch, P.A.; Knopp, R.; Imperato, G.; Ocak, M.; von Guggenberg, E.; Haubner, R.; Silbernagl, R.; Decristoforo, C. Radiolabelling of Peptides for PET, SPECT and Therapeutic Applications Using a Fully Automated Disposable Cassette System. Nucl Med Commun 2011, 32, 887–895. [Google Scholar] [CrossRef] [PubMed]
- de Blois, E.; Sze Chan, H.; Naidoo, C.; Prince, D.; Krenning, E.P.; Breeman, W.A.P. Characteristics of SnO2-Based 68Ge/68Ga Generator and Aspects of Radiolabelling DOTA-Peptides. Appl Radiat Isot 2011, 69, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Desferal - Summary of Product Characteristics 2018.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).