1. Introduction
Oral medications remain the most preferred and accustomed method of drug delivery owing to several advantages they offer, including convenience for self-administration and high patient compliance amongst others [
1]. However, some patient groups, such as the geriatric, pediatric, individuals with Parkinson’s disease or Alzheimer’s disease, psychiatric conditions, and patients with dysphagia, often confront challenges in swallowing or chewing oral solid dosage forms [
1,
2,
3].
Thus, to overcome these issues, extensive efforts were taken to create novel oral drug delivery systems with the aim of providing drugs that could dissolve or disperse in the oral cavity and form solution or suspension without the requirement of water. These fast-dissolving oral drug delivery systems were first invented in the late 1970s and became prominent as oral mucosal dosage formulations. Currently, they are available in various forms, including adhesive tablets, gels, ointments, patches, and mouth-dissolving films for buccal delivery [
4].
These buccal drug delivery systems over time became a suitable alternative drug form for tablets or capsules. In addition, the oral cavity serves as an ideal route of administration due to its highly vascularized thin membranous structure. Apparently, the lower enzymatic activity in oral mucosa provides greater permeability and rapid absorption for several drugs, especially for those drugs with low aqueous solubility. It also bypasses first-pass metabolism, potentiates higher systemic bioavailability of active pharmaceutical ingredients (API), and ensures a rapid onset of action [
5].
Oral disintegrating tablets (ODTs) are a type of oral solid dosage form intended to disintegrate swiftly within seconds of placement on the tongue without necessitating water or chewing [
5,
6]. Orodispersible films (ODFs) are a drug delivery system that encompasses thin, flexible sheets manufactured with or without plasticizers that typically dissolve or disintegrate promptly, usually within seconds, when placed in the mouth. They are designed to be positioned in the buccal cavity either on the tongue or cheek and can be used to deliver a variety of medications, including prescription and non-prescription or over-the-counter drugs [
5]. ODFs have been reported to resolve many issues associated with conventional oral dosage forms that include but are not limited to: (i) accelerate the time of drug release; (ii) prolong the duration of drug action; (iii) reduce the number of dose administrations; and (iv) maximize the efficacy of API [
7]. Besides, this novel drug delivery system (film technology) offers an array of benefits during the pharmacokinetics and pharmacodynamic aspects of the drugs including: (i) improved absorption and metabolism; (ii) site-specific action; (iii) lowering of side effects; and (iv) most significantly enhancing the drug’s bioavailability. ODFs also confer expeditious dissolution, pertinent drug loading capacities, and improved stability and durability of drug formulations. Moreover, they are non-toxic, bio-resorbable, and biodegradable. Further, as ODTs evolved, the ODFs achieved patient compliance by circumventing discomfort due to their user-friendly advantages. Thus, the ODFs provide immediate release and disintegration, rapid onset of action, disease-specific, target-specific drug action that causes instant and effective reduction of symptoms, relieves discomfort, and restores normal physiological function. These advantages have compelled patients to adhere to their therapy and complete the duration of treatment with resultant improved quality of life (
Figure 1) [
8,
9,
10,
11].
Although there are several names employed to refer oral film dosage form like thin strip, oral thin film, oral film, orally dissolving film, quick dissolve film, melt-away film, and wafer, it is the officially named as ODF by European Medicines Agency, or, as soluble films by the United States Food and Drug Administration (U.S. FDA) [
14]. As per European Pharmacopeia (Ph. Eur.), ODFs are delineated as sheets, either single or multilayered, composed of appropriate materials and are intended for rapid dispersion in the mouth. In fact, they instantaneously disintegrate/disperse in saliva to form a solution or suspension, thus facilitating rapid absorption and distribution of the drug into the blood circulation [
5]. This review discusses the advantages of ODF in improving compliance and satisfaction in patients with erectile dysfunction (ED) and describes the bioequivalence and stability studies of sildenafil citrate ODF.
2. Search Strategy
An online PubMed literature search was conducted to identify English language publications from inception to March 2024 using combinations of the terms erectile dysfunction, ED, phosphodiesterase type 5 inhibitor, phosphodiesterase 5 inhibitor, PDE5 inhibitor, sildenafil, vardenafil, tadalafil, avanafil, drug formulations, drug delivery, buccal mucosa, orally dispersible, orally disintegrating, orodispersible, ODT, ODF, oral dispersible formulations, novel drug delivery, innovative technologies, manufacturing methods, stability studies, stress test, bioequivalence, regulatory requirements. Other relevant articles were identified by manually reviewing the reference lists of selected articles.
2.1. Orodispersible Dosage Forms
Orodispersible dosage forms have a growing presence in the pharmaceutical market because their administration can improve the bioavailability of some drugs and their prescription can ameliorate patient adherence and/or compliance [
15]. This novel approach has transformed the conventional drug delivery methodology for oral drugs. These modified dosage forms, oral films have replaced oral tablets as they have achieved altered drug release characteristics and faster disintegration. Also, conventional tablets may get easily broken down necessitating considerable packaging during handling, storage, and transit, while oral films are flexible, handy, and can be kept for long-term use [
16].
An ideal fast dissolving delivery system is expected to possess the following properties: (i) optimal stability and transportability, (ii) uncomplicated handling and administration, (iii) no distinctive packaging material or processing requirements, (iv) should not require water for application, and (v) exhibit acceptable palatability. In addition, the film should be thin and elegant in appearance, available in various size and shapes, unobstructive, adhere to the oral cavity easily, process fast disintegration without requiring water and release the drug rapidly. In addition, the suitable drug candidate must exhibit these ideal characteristics. The drug should have a pleasant taste, lesser and moderate molecular weight, excellent stability and solubility in water and saliva, remain partially unionized at the neutral pH of oral cavity and easily permeate through the oral mucosal tissue [
4].
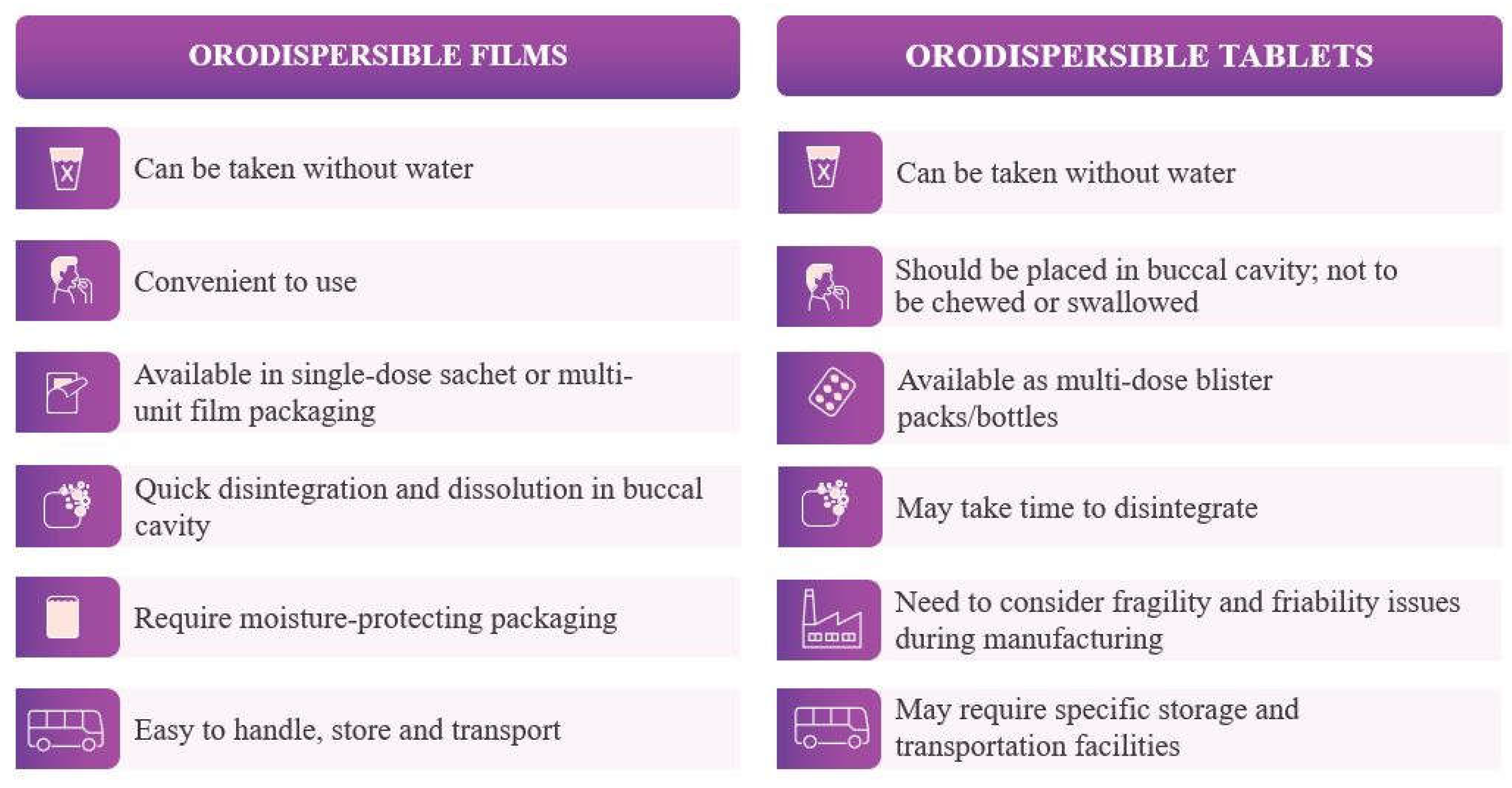
Table 1 and
Table 2 list the advantages and disadvantages of the ODF, respectively.
The existence of many polymers and production technologies has made it easy to develop a diverse range of ODFs [
11]. ODFs are manufactured in the form of a large sheet and cut according to the desired size and shape. The components of oral films can differ depending on the particular formulation and intended purpose [
18]. They are developed for a wide range of drugs providing local action or systemic action. For local action, ODFs are used for toothaches, local anesthetic, cold sores, and oral ulcers. For systemic action, they are used for treating cough, sore throat, migraine, gastric disorders, central nervous system disorder, pain, and nausea, or for delivering vitamins or nutraceutics [
6].
However, there are common constituents typically found in oral film formulations, such as APIs, polymers, plasticizers, sweetening agents, flavoring agents, coloring agents, saliva stimulating agents, stabilizers, surfactants, and solvents. In the context of pharmaceutical ODF dosage form development, critical quality attributes (CQAs) comprise the physical, chemical, biological, or microbiological properties and characteristics that must fall within specified limits to ensure the desired quality of the product. These attributes can be influenced by essential material qualities, including the quality of the API and the physicochemical properties of excipients, as well as critical process parameters such as the order in which the raw materials are added, the sequence of introducing solutions during formulation, and the maintenance of appropriate temperature of water (
Table 3) [
19]
2.2. Manufacturing Methods
There are numerous parameters including tensile strength, pH, and thickness etc. along with the choice of polymer blend suitable for drug release that needs to be considered during preparation of orally disintegrating formulations. The crucial step in formulation of orodispersible film depends on judicial selection of polymers and polymer blends and appropriate method of preparation [
21]. The primary methods used in formulation of ODFs include the solvent casting technique, semisolid casting, solid dispersion extrusion electrospinning, hot-melt extrusion (HME), rolling and the emerging technique of 3D printing (3DP) for personalized dosing [
22]. An elaborated description of the manufacturing technologies leveraged in the production of orodispersible dosage formulations is outside the scope of this article.
In short, the diverse manufacturing technologies used in the production of ODTs is encompassed under the broad classifications of lyophilized systems and compressed tablet-based systems [
4,
11,
12,
22,
23]. The performance of ODTs relies on manufacturing processes that increase the porous structure of the tablet matrix, amalgamating a disintegrating agent, and the usage of highly water-soluble excipients to allow quick ingress of water, facilitating rapid oral disintegration [
24].
A striking balance between fast disintegration and fragility must be demonstrated to minimize challenges of packaging, storage, handling, and administration. In brief, lyophilization or freeze drying, a technology commonly used in the manufacture of ODTs, entails molding tablet-shaped units of a drug in suspension or solution along with other structural excipients, followed by freezing and lyophilization in the pack or mold. The very high porosity of the resulting ODTs allows rapid water or saliva penetration and rapid disintegration. On the other hand, compressed tablet-based systems use standard tableting technology by direct compression of the API and excipients deliberated to achieve the required disintegration performance and packaging requirements [
4,
8,
23].
The ODFs manufacturing has evolved from technologies that were used to produce transdermal patches over years. Despite the sophisticated manufacturing methods, the resultant dosage forms are stable, thin, and flexible with high mechanical and tensile strength. They also can be fabricated in varied sizes and shapes offering optimal transport and storage options [
8]. Sildenafil ODF is one of the novel formulations that have been recently made available that offers numerous merits over conventional FCT formulations for patients with ED [
25].
A study conducted in Japan, surveyed 25 patients with ED regarding the ease of consumption and portability with the use of sildenafil ODF formulation. The survey items included: (i) portability, (ii) storage, (iii) ease of consumption, (iv) comfortableness, (v) level of self-consciousness with their partner, (vi) thoughts on taking the film formulation, (vii) comparison with the tablets, and (viii) efficacy and adverse reaction. Of all the participants, 61.5% of the patients switched from tablets to film found that storage and portability were improved and were comfortable during consumption. The study reported that most of their patients felt more at ease (69.2% who switched from tablets to film and 75.0% who were prescribed film only) and more comfortable with the film (61.5% who switched from tablets to film and 66.7% who were prescribed film only). Mild hot flashes and headache were the only two adverse reactions with no severe adverse reaction reported by the study participants. The survey demonstrated improved ease of intake and portability with orodispersible film compared to tablets [
49].
2.3. Bioequivalence and Stability Testing
Several studies have demonstrated bioequivalence between sildenafil ODF formulation and conventional FCT. This display of bioequivalent pharmacokinetics between the two formulations supports and recommends the use of new ODF formulation as a suitable alternative to the customary oral solid dosage form. [
50,
51,
52].
Two randomized cross-over studies were conducted to examine the bioequivalence of 50 mg sildenafil citrate ODF with the marketed 50 mg sildenafil citrate FCT, with and without water. The study recruited 42 and 80 healthy male volunteers in the first and second study, respectively. Bioequivalence was demonstrated for sildenafil citrate ODF administered with water when compared with the sildenafil citrate FCT as the ratios of adjusted geometric means (90% confidence interval (CI)) were maximum plasma concentration: 1.02 (94.91–108.78) and area under the plasma concentration-time curve: 1.09 (104.49–113.21) for sildenafil citrate ODF administered with water vs sildenafil citrate FCT administered with water. The bioequivalence was demonstrated as these ratios were within the acceptance range of 80% to 125%. Further, the pharmacokinetic parameters in the successive study also established the bioequivalence for sildenafil citrate ODF (without water) with that of sildenafil citrate FCT administered with water. The ratios of adjusted geometric means (90% CI) were maximum plasma concentration: 1.02 (95.47–109.36) and area under the plasma concentration-time curve: 1.06 (103.42–108.40) for sildenafil citrate ODF administered without water vs sildenafil citrate FCT administered with water. The adverse events occurred at similar rates for both the formulations in all the studies and were of mild intensity. The study results demonstrated the bioequivalence and supported the interchangeability of new sildenafil citrate ODF formulation with the marketed FCT formulation [
25].
2.4. Stress Test
ODFs also depict a number of advantages including lack of friability, no risk of suffocation during administration, and ease of carrying the strips without the need for secondary container [
8]. Amongst other advantages, the sildenafil ODF offers incredible benefit to patients with erectile dysfunction in respecting their privacy. While the shame and the stigma culturally linked to the ED management, is considered a major unmet need of the patients treated with PDE5-Is, the ODF allows them to carry the drug in their pockets, in the most discreet way and then be placed on tongue before engaging in sexual activity [8, 53, 54]. Furthermore, the absence of the need to swallow the drug with water further increase the ease of use and the potential reduction of the stigma of being treated with a traditional pill. In fact, when the film is placed on the tongue or in the oral cavity, it gets immediately hydrated by saliva without any need of water followed by rapid disintegration and drug release for oro-mucosal and/or systemic absorption [
25].
To further demonstrate the possibility to safely store the drug in the pocket, maximizing the need for a discreet treatment, a stress test has been conducted on sildenafil ODF by exposing it to elevated temperatures (60 degrees Celsius) for three weeks, mimicking extreme storage conditions and evaluate its physical and chemical stability. Ensuring the stability of pharmaceutical products is crucial to maintain their efficacy, safety, and quality throughout their shelf life, particularly critical in the case of sildenafil ODF. This test was conducted in Kyukyu Pharmaceutical Co., Ltd, Toyama Plant, Japan. A thin pale red colored ODF containing 50 mg sildenafil was formulated to dissolve in the oral cavity. Three batches of samples were subjected to a stability study at 60°C stress for three weeks. They were assessed for the amount of API assay (95.0% -105.0% of labelled amount), degradation, disintegration, dissolution rate (over 80% for 45 mm) and water content. All test items met the specifications and showed negligible differences between the samples. The amount of degradation products demonstrated an increasing trend over time in all conditions, but all met the specification. The purity testing for degradation product (0.2% or less) showed significant results (p value <0.05) at initial and 60°C in all the three samples.
The process was evaluated across a range of manufacturing conditions, and all intermediates and sildenafil ODF showed reliable performance that yields a consistent product meeting all specifications at release and after three weeks of storage under stressed conditions. These results, shown in
Table 4 indicate the robust performance of the sildenafil ODF across a range of manufacturing conditions. In addition, the water content stayed in a tight range regardless of the film casting parameters, and this remains true of the packaged product even after 3 weeks at 60
oC.
3. Discussion
ED is defined as the persistent inability to attain and maintain an erection sufficient to permit satisfactory sexual performance [25-28]. ED still prevails as an unprecedented diagnosis and undertreated condition which contributes to increased physical, and psychosocial burden. It negatively impacts the wellbeing, relationships, and health-related quality of life (HR-QoL) of patients and their partners [
29,
30] and thus, any treatment interventions that substantially revive sexual function have the prospect to reinforce patient health and overall HR-QoL.
ED may present with clinically relevant forms but also subclinical conditions deserving medical attention. Worldwide, various forms of ED are present in up to 150 million men and it has been predicted to upsurge to 322 million cases by 2025. ED has affected 52% of men aged between 40 and 70 years and 70% of men older than age 70 years [31-34]. The primary risk factors include diabetes mellitus, hypertension, hyperlipidaemia and modifiable risk factors like obesity, physical inactivity, alcoholism and cigarette smoking [
35,
36,
37]. In addition, there is increasing evidence that ED can be considered as the perfect gender-dependent (early) biomarker of non-communicable diseases (NCDs) including peripheral vascular disease, cardiovascular disease, with an increased risk of cardiovascular mortality as largely demonstrated by epidemiological studies [
38]. Indeed, ED can be considered a symptom for underlying NCD that may be explained as the classical canary in the coalmine [
39].
The management of ED includes the identification and control of risk factors and appropriate pharmacological and non-pharmacological therapy. The treatment strategies undoubtedly can reduce the initial symptoms and halt the progression of the disease. Some of the treatment approaches include psychosexological strategies, intraurethral or intracavernosal alprostadil self-injections, vacuum- assisted erection devices, low-intensity extracorporeal shock wave treatment, and penile implants. However, after the efforts to modify unhealthy lifestyle and control risk factors and comorbidities, pharmacological management with oral phosphodiesterase 5 inhibitors (PDE5-Is) remains the first-line treatment choice for ED. because of its proven efficacy and better safety profile [
8].
The PDE5-Is inhibit the PDE5 enzyme present in the smooth muscle cells in the blood vessels [
40]. Due to this inhibition PDE5-Is prevent the enzyme-initiated degradation of cyclic guanosine monophosphate (cGMP). Prevention of degradation of cGMP by PDE-5 leads to the accumulation of cGMP in the vascular smooth muscle, thereby leading to dilatation of the blood vessels through phosphorylation of different downstream effector molecules ensuing relaxation of smooth muscles [
41]. Inhibiting PDE5 produces a dilatation of the penile arteries that leads to a more prolonged and stable erection. In addition, PDE5-Is improve endothelial function and reduce apoptosis of vascular smooth muscle cells in the corpus cavernosum [
40,
42]. The PDE5 inhibitors approved by the US Food and Drug Administration and European Union (EU) include sildenafil, tadalafil, vardenafil, and avanafil [
29,
43,
44,
45].
Sildenafil citrate, the first-in-class selective PDE5-I, is a potent and selective inhibitor of cGMP-specific PDE5. Since its launch for the treatment of ED in 1998, there has been a strong evidence base established for the efficacy and safety of sildenafil in the treatment of ED. It is the most efficacious, from a vascular perspective, amongst other PDE5-Is [
55].
The proven efficacy of sildenafil in ED is irrespective of confounding factors such as age, baseline severity, or etiology of ED [
46]. Although, sildenafil is available at the doses of 25, 50, and 100 mg, 50 mg is the recommended dose for most of the patients with directions to be taken approximately 1 hour before sexual activity. The dose can be titrated to a maximum of 100 mg or decreased to 25 mg considering individual patient’s needs, tolerability and efficacy. Headache and flushing are the most commonly reported side effects [
47]. Sildenafil is available in both solid film-coated tablet (FCT) and orodispersible tablet formulations. The sildenafil ODT and ODFs are made available through implementation of innovative manufacturing technologies [
8,
56]. It is also available as chewable tablets and an orally soluble orodispersible film formulation [
29]. Owing to its unique pharmacological characteristics, quick disintegration within seconds, the novel sildenafil orodispersible film (ODF) will be widely prescribed and preferred among ED patients.
Although the efficacy of PDE5-Is has been thoroughly demonstrated, the documented evidence reveals that a prominent percentage of patients discontinue this pharmacological therapy prematurely. There is a gradual dissatisfaction noticed with the prescribed therapy among patients despite their successful intercourse. Studies depict that approximately 50% of men abandon their treatment with PDE5-Is in conventional formulations within a year [
48]. There is an immense need to understand individual patient’s needs and expectations regarding treatment for ED and also to comprehend their personal experiences and preferences. This will be instrumental in deciphering successful outcomes and satisfaction regarding the pharmacological therapy [
8]. ODFs were developed to overcome the above drawbacks of conventional oral solid dosing forms and to significantly improve patients’ compliance and acceptability to the pharmacological therapy.
ODFs are innovative and sophisticated drug delivery systems that are formulated to disperse and disintegrate rapidly when placed in the mouth, without the need for water. They unveil a prudent and convenient mode of administration, without risk of choking or difficulty in swallowing. These attributes of ODFs improves compliance specifically in patients with dysphagia, and children, elderly population with comorbidities (e.g., renal impairment or congestive heart failure) when compared with conventional tablets or capsules. This non-invasive drug delivery system offers an altered clinical profile as it surpasses the enterohepatic circulation. Since the drug gets absorbed majorly from buccal mucosal tissues, it also reduces the risk of formation of toxic metabolites due to lowered hepatic metabolism.
Additionally, their convenience, together with superior dosing accuracy and rapid onset of action, have transpired in strong patient preference for orodispersible formulations across a wide range of patient groups. Since each film or strip consists of precise quantities of the APIs and is devoid of physiological variability in GIT, there is minimal inter-subject variability in clinical response unveiled by ODFs [
57]. Given all these advantages, growing body of research illustrates that majority of patients and prescribers prefer orodispersible dosage forms over conventional oral solid dosage forms [
58,
59].
4. Conclusions
Sildenafil ODF, an innovative dosage form can be used for the treatment of ED as it better addresses the unmet needs and expectations of men with ED. Sildenafil ODF showed unwavering performance that yields a consistent product meeting all specifications at release and after three weeks of storage under stressed conditions (temperature up to 60°C). It offers unique and sustainable advantages to ED patients, including a pleasant mint taste, convenience, privacy and storage options, making it the potential drug of choice and the most preferred formulation.
Author Contributions
All authors substantially contributed to the manuscript. All authors contributed to the conceptualization or design of the work; the acquisition, analysis, or interpretation of data; drafting, editing, reviewing, and revising it critically for important intellectual content; for final approval of the version to be published; and agreeing to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
The stress test study was funded by Viatris Inc.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
Writing and editorial support was provided by Mamatha K. Ph D and Shantha Kumar V Ph D.
Conflicts of Interest
EAJ is Professor of endocrinology and sexual medicine, University of Rome Tor Vergata, Rome, Italy. He is paid speaker for several pharmaceutical companies like Bayer, Ibsa, Menarini, Otsuyka, Recordati, Pfizer, Viatris. SV is employee of Mylan Pharmaceuticals Pvt Ltd., a Viatris Company, TH is employee of Viatris Inc. and holds stocks. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
References
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Ferlak J, Guzenda W, Osmałek, T. Orodispersible films—Current state of the art, limitations, advances and future perspectives. Pharmaceutics. 2023, 15, 361. [CrossRef] [PubMed]
- Lau, E.T.; Steadman, K.J.; Cichero, J.A.; Nissen, L.M. Dosage form modification and oral drug delivery in older people. Adv. Drug Deliv. Rev. 2018, 135, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Khanna, S.; Pawar, P.; Arora, S. Orally dissolving strips: A new approach to oral drug delivery system. Int. J. Pharm. Investig. 2013, 3, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Boddu, S.H.S.; Gorain, B.; Sreeharsha, N.; Shah, J. An Updated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems. Pharmaceutics 2021, 13, 1206. [Google Scholar] [CrossRef]
- Salawi, A. An Insight into Preparatory Methods and Characterization of Orodispersible Film—A Review. Pharmaceuticals 2022, 15, 844. [Google Scholar] [CrossRef] [PubMed]
- Morath, B.; Sauer, S.; Zaradzki, M.; Wagner, A. Orodispersible films – Recent developments and new applications in drug delivery and therapy. Biochem. Pharmacol. 2022, 200, 115036. [Google Scholar] [CrossRef] [PubMed]
- Jannini, E.A.; Droupy, S. Needs and Expectations of Patients with Erectile Dysfunction: An Update on Pharmacological Innovations in Phosphodiesterase Type 5 Inhibition with Focus on Sildenafil. Sex. Med. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.S.; Kumar, T.P.; Gowda, D.V. Orodispersible Thin Film: A new patient-centered innovation. J. Drug Deliv. Sci. Technol. 2020, 59, 101843. [Google Scholar] [CrossRef]
- zakar RS, Özakar, E. Current overview of oral thin films. Turkish journal of pharmaceutical sciences. 2021, 18, 111.
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian, J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. 2015, 24, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Kathpalia, H.; Gupte, A. An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: A Review. Curr. Drug Deliv. 2013, 10, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Borges AF, Silva C, Coelho JF, Simões, S. Oral films: current status and future perspectives: I—galenical development and quality attributes. Journal of Controlled Release. 2015, 206, 1–19.
- Cilurzo, F.; Musazzi, U.M.; Franzé, S.; Selmin, F.; Minghetti, P. Orodispersible dosage forms: biopharmaceutical improvements and regulatory requirements. Drug Discov. Today 2018, 23, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Mahboob MBH, Riaz T, Jamshaid M, Bashir, I., Zulfiqar, S. Oral films: A comprehensive review. International Current Pharmaceutical Journal 2016, 5, 111–117. [Google Scholar] [CrossRef]
- Saini P, Kumar A, Sharma P, Visht, S. Fast disintegrating oral films: A recent trend of drug delivery. Int J Drug Dev Res. 2012, 4, 80–94.
- Desai, P.P.; Date, A.A.; Patravale, V.B. Overcoming poor oral bioavailability using nanoparticle formulations – opportunities and limitations. Drug Discov. Today: Technol. 2012, 9, e87–e95. [Google Scholar] [CrossRef] [PubMed]
- Food, U., Administration, D. Quality by design for ANDAs: an example for immediate-release dosage forms. US Department of Health and Human Service (FDA, Rockville, MD, 2012). 2012.
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J.; Pharm, D.; D., P. Advances in orodispersible films for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Gijare, C.; Deshpande, A. Orodispersible Films: A Systematic Patent Review. Recent Patents Drug Deliv. Formul. 2018, 12, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Turković, E.; Vasiljević, I.; Drašković, M.; Parojčić, J. Orodispersible films — Pharmaceutical development for improved performance: A review. J. Drug Deliv. Sci. Technol. 2022, 75, 103708. [Google Scholar] [CrossRef]
- Goel, H.; Rai, P.; Rana, V.; Tiwary, A.K. Orally Disintegrating Systems: Innovations in Formulation and Technology. Recent Patents Drug Deliv. Formul. 2008, 2, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Nagar P, Singh K, Chauhan I, Verma M, Yasir M, Khan A, et al. Orally disintegrating tablets: formulation, preparation techniques and evaluation. Journal of Applied Pharmaceutical Science. 2011:35-45.
- Shaw A, Lawrence TE, Yan T, Liu M, Summers N, Daggumati, V., et al. Bioequivalence Studies of Sildenafil Citrate Orodispersible Film Administered With and Without Water versus Viagra® Film-coated Tablets in Healthy Male Subjects. Current Therapeutic Research. 2023:100708.
- Andersson, K.-E. Mechanisms of Penile Erection and Basis for Pharmacological Treatment of Erectile Dysfunction. Pharmacol. Rev. 2011, 63, 811–859. [Google Scholar] [CrossRef] [PubMed]
- Gratzke, C.; Angulo, J.; Chitaley, K.; Dai, Y.-T.; Kim, N.N.; Paick, J.-S.; Simonsen, U.; Ückert, S.; Wespes, E.; Andersson, K.E.; et al. Anatomy, Physiology, and Pathophysiology of Erectile Dysfunction. J. Sex. Med. 2010, 7, 445–475. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Cucinotta, D.; Di Lorenzo, G.; Ferlin, A.; Giagulli, V.A.; Gnessi, L.; Isidori, A.M.; Maiorino, M.I.; Miserendino, P.; Murrone, A.; et al. The Italian Society of Andrology and Sexual Medicine (SIAMS), along with ten other Italian Scientific Societies, guidelines on the diagnosis and management of erectile dysfunction. J. Endocrinol. Investig. 2023, 46, 1241–1274. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Donde, S.; Hassan, T.A.; Jannini, E.A. Phosphodiesterase Type 5 Inhibitors for the Treatment of Erectile Dysfunction: Pharmacology and Clinical Impact of the Sildenafil Citrate Orodispersible Tablet Formulation. Clin. Ther. 2017, 39, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Jannini, E.A.; Lenzi, A.; Isidori, A.; Fabbri, A. Subclinical Erectile Dysfunction: Proposal for a Novel Taxonomic Category in Sexual Medicine. J. Sex. Med. 2006, 3, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Artom, N.; Pinna, G.; Musso, N.R.; Orlandini, F.; Malasoma, P.; Uccelli, M.; Artom, A.; Rabbia, F.; Pascale, C.; Lantieri, F.; et al. Prevalence of erectile dysfunction in a cohort of Italian hypertensive subjects. Clin. Exp. Hypertens. 2015, 38, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Yafi FA, Jenkins L, Albersen M, Corona G, Isidori AM, Goldfarb, S., et al. Erectile dysfunction. Nature reviews Disease primers. 2016, 2, 1–20.
- Muneer A, Kalsi J, Nazareth I, Arya, M. Erectile dysfunction. BMJ. 2014, 348:129.
- Aytaç; McKinlay, J. B.; Krane, R.J. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999, 84, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Rastrelli, G.; Isidori, A.; Pivonello, R.; Bettocchi, C.; Reisman, Y.; Sforza, A.; Maggi, M. Erectile dysfunction and cardiovascular risk: a review of current findings. Expert Rev. Cardiovasc. Ther. 2020, 18, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Jannini, EA. SM= SM: The interface of systems medicine and sexual medicine for facing non-communicable diseases in a gender-dependent manner. Sexual Medicine Reviews. 2017, 5, 349–64. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, F.; Zhang, Y.; Wang, W.; Ran, Y.; Wu, C.; Zhu, S.; Qin, F.; Yuan, J. Insights into modifiable risk factors of erectile dysfunction, a wide-angled Mendelian Randomization study. J. Adv. Res. 2024, 58, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Terentes-Printzios, D.; Ioakeimidis, N.; Aznaouridis, K.; Rokkas, K.; Synodinos, A.; Christoforatou, E.; Aggelis, A.; Samentzas, A.; Stefanadis, C. PREDICTION OF CARDIOVASCULAR EVENTS AND ALL-CAUSE MORTALITY WITH ERECTILE DYSFUNCTION: A SYSTEMATIC REVIEW AND META-ANALYSIS OF COHORT STUDIES. Circ. 2012, 59, E2074. [Google Scholar] [CrossRef]
- Yannas D, Sansone A, Jannini EA. The canary in the coal mine. Comment on “Association between cardiometabolic index and erectile dysfunction among US adults: a cross-sectional analysis of the National Health and Nutrition Examination Survey 2001–2004”. International Journal of Impotence Research. 2024:1-2.
- Dolci, S.; Belmonte, A.; Santone, R.; Giorgi, M.; Pellegrini, M.; Carosa, E.; Piccione, E.; Lenzi, A.; Jannini, E.A. Subcellular localization and regulation of type-1C and type-5 phosphodiesterases. Biochem. Biophys. Res. Commun. 2006, 341, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Cesarini, V.; Guida, E.; Campolo, F.; Crescioli, C.; Di Baldassarre, A.; Pisano, C.; Balistreri, C.R.; Ruvolo, G.; Jannini, E.A.; Dolci, S. Type 5 phosphodiesterase (PDE5) and the vascular tree: From embryogenesis to aging and disease. Mech. Ageing Dev. 2020, 190, 111311–111311. [Google Scholar] [CrossRef] [PubMed]
- Mónica FZ, De Nucci, G. Tadalafil for the treatment of benign prostatic hyperplasia. Expert opinion on pharmacotherapy. 2019, 20, 929–37.
- Brant, W. , Lue, T. , Smith, J. Evaluation and management of erectile dysfunction in clinical practice. Journal of Clinical Outcomes Management. 2009, 16, 83–96. [Google Scholar]
- Seftel, A.D. Phosphodiesterase type 5 inhibitor differentiation based on selectivity, pharmacokinetic, and efficacy profiles. Clin. Cardiol. 2004, 27, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Hill, S. A comparison of the available phosphodie sterase-5 inhibitors in the treatment of erectile dysfunction: a focus on avanafil. Patient Preference Adherence 2015, ume 9, 1159–1164. [Google Scholar] [CrossRef]
- Hatzimouratidis, K.; Amar, E.; Eardley, I.; Giuliano, F.; Hatzichristou, D.; Montorsi, F.; Vardi, Y.; Wespes, E. Guidelines on Male Sexual Dysfunction: Erectile Dysfunction and Premature Ejaculation. Eur. Urol. 2010, 57, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Medicines.org.uk. Viagra 50 mg film-coated tablets - Summary of Product Characteristics (SmPC) - (emc). [updated 04 May 2023. Available from: https://www.medicines.org.uk/emc/product/7980/smpc#about-medicine.
- Corona, G.; Rastrelli, G.; Burri, A.; Serra, E.; Gianfrilli, D.; Mannucci, E.; Jannini, E.A.; Maggi, M. First-generation phosphodiesterase type 5 inhibitors dropout: a comprehensive review and meta-analysis. Andrology 2016, 4, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, H.; Sasaki, H.; Ogushi, Y.; Niikura, A.; Ota, T.; Ichimura, Y.; Hshimoto, Y.; Kurokawa, I.; Sugishita, H.; Tanifuji, S.; et al. 167 Clinical Analysis on the Pharmaceutical Formulation of VIAGRA OD Film. J. Sex. Med. 2022, 19, S190–S190. [Google Scholar] [CrossRef]
- Radicioni, M.; Castiglioni, C.; Giori, A.; Cupone, I.; Frangione, V.; Rovati, S. Bioequivalence study of a new sildenafil 100 mg orodispersible film compared to the conventional film-coated 100 mg tablet administered to healthy male volunteers. Drug Des. Dev. Ther. 2017, 11, 1183–1192. [Google Scholar] [CrossRef]
- Roh, H.; Son, H.; Lee, D.; Yeon, K.J.; Kim, H.S.; Kim, H.; Park, K. Pharmacokinetic Comparison of an Orally Disintegrating Film Formulation With a Film-Coated Tablet Formulation of Sildenafil in Healthy Korean Subjects: A Randomized, Open-Label, Single-Dose, 2-Period Crossover Study. Clin. Ther. 2013, 35, 205–214. [Google Scholar] [CrossRef]
- Lv, Y.; Luo, B.; LaBadie, R.R.; Zhu, H.; Feng, Y.; Ernst, C.; Crownover, P.H.; Liang, Y.; Zhao, Q. Bioequivalence and Bioavailability of an Orodispersible Tablet of Sildenafil Citrate in Healthy Chinese Male Subjects. Clin. Pharmacol. Drug Dev. 2020, 9, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Maggi, M.; Jannini, E.A. EDEUS, a Real-Life Study on the Users of Phosphodiesterase Type 5 Inhibitors: Prevalence, Perceptions, and Health Care-Seeking Behavior Among European Men With a Focus on 2nd-Generation Avanafil. Sex. Med. 2018, 6, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Jannini, E.A.; Sternbach, N.; Limoncin, E.; Ciocca, G.; Gravina, G.L.; Tripodi, F.; Petruccelli, I.; Keijzer, S.; Isherwood, G.; Wiedemann, B.; et al. Health-Related Characteristics and Unmet Needs of Men with Erectile Dysfunction: A Survey in Five European Countries. J. Sex. Med. 2014, 11, 40–50. [Google Scholar] [CrossRef]
- Jannini, E.A.; Isidori, A.M.; Gravina, G.L.; Aversa, A.; Balercia, G.; Bocchio, M.; Boscaro, M.; Carani, C.; Corona, G.; Fabbri, A.; et al. The ENDOTRIAL Study: A Spontaneous, Open-Label, Randomized, Multicenter, Crossover Study on the Efficacy of Sildenafil, Tadalafil, and Vardenafil in the Treatment of Erectile Dysfunction. J. Sex. Med. 2009, 6, 2547–2560. [Google Scholar] [CrossRef]
- Zucchi, A.; Costantini, E.; Scroppo, F.I.; Silvani, M.; Kopa, Z.; Illiano, E.; Petrillo, M.G.; Cari, L.; Nocentini, G. The first-generation phosphodiesterase 5 inhibitors and their pharmacokinetic issue. Andrology 2019, 7, 804–817. [Google Scholar] [CrossRef]
- Tapolsky GH, Osborne DW. Bioerodable film for delivery of pharmaceutical compounds of mucosal surfaces. Google Patents; 2000.
- Shimoda, H.; Taniguchi, K.; Nishimura, M.; Matsuura, K.; Tsukioka, T.; Yamashita, H.; Inagaki, N.; Hirano, K.; Yamamoto, M.; Kinosada, Y.; et al. Preparation of a fast dissolving oral thin film containing dexamethasone: A possible application to antiemesis during cancer chemotherapy. Eur. J. Pharm. Biopharm. 2009, 73, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Efremov E, Kasatonova E, Mel’nik YI, Nikushina, A. PDE-5 inhibitors: patients’ preferences. Urologiia. 2017,120-6.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).