Submitted:
26 July 2024
Posted:
29 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
3. Results
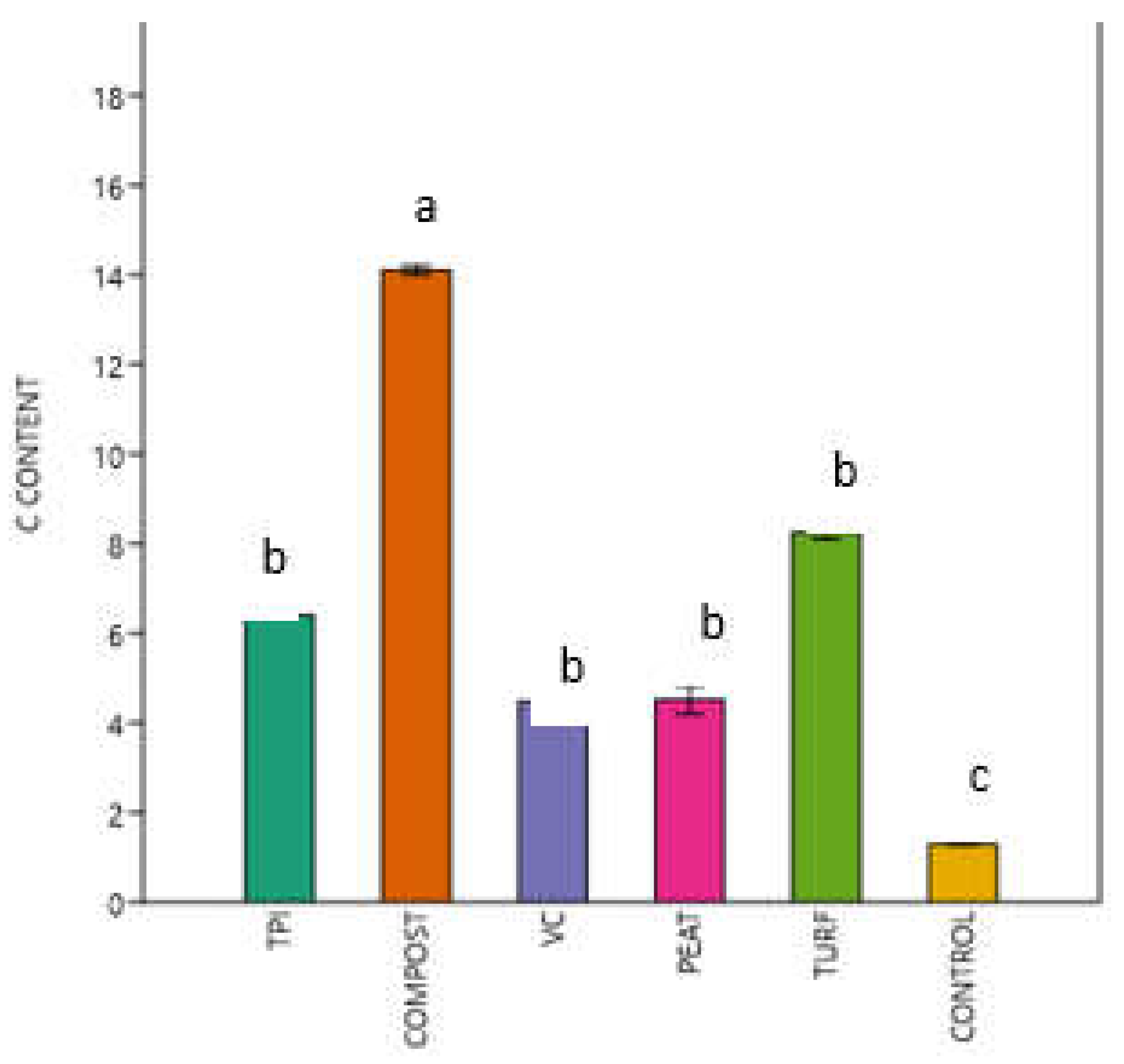
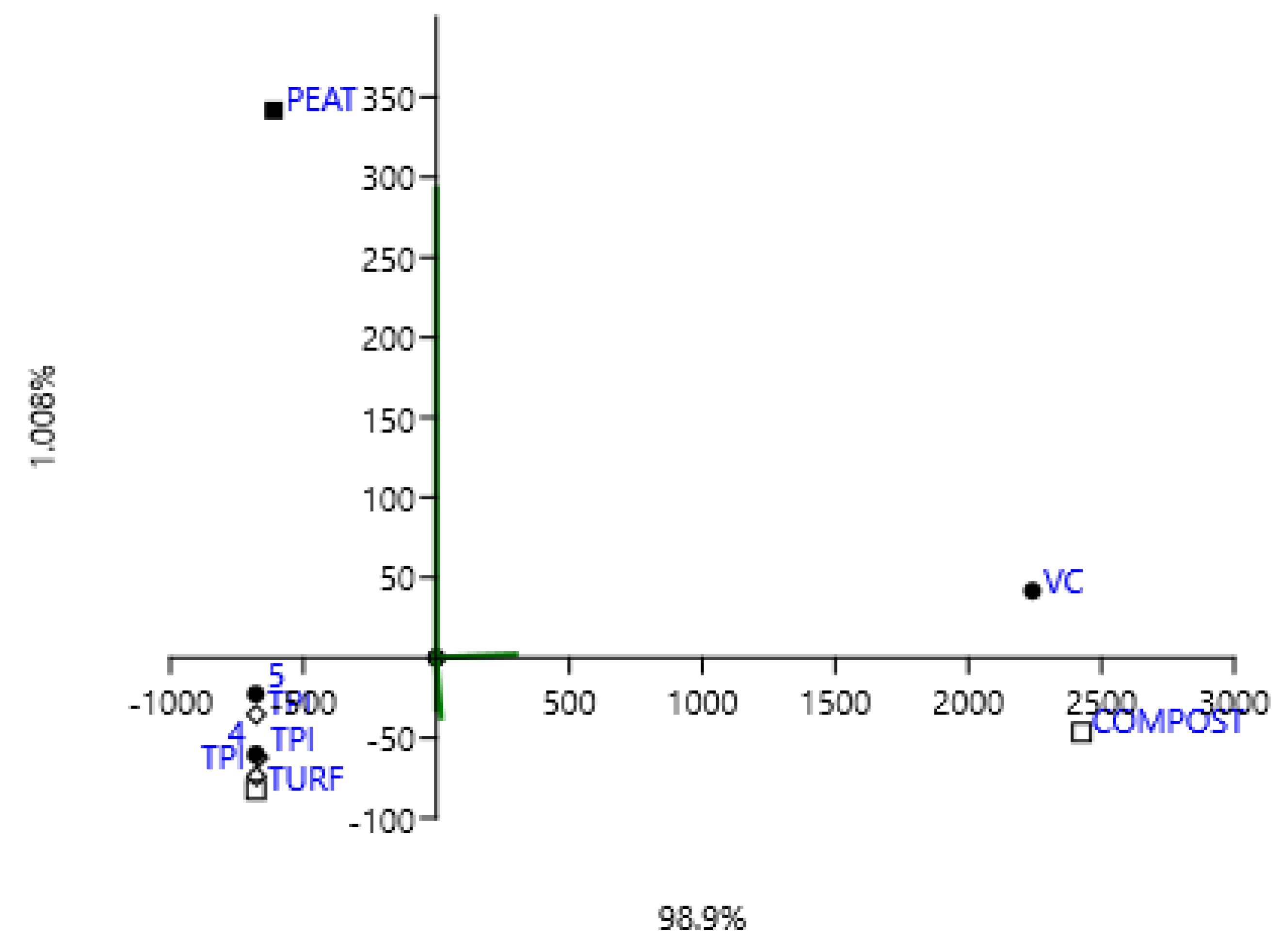
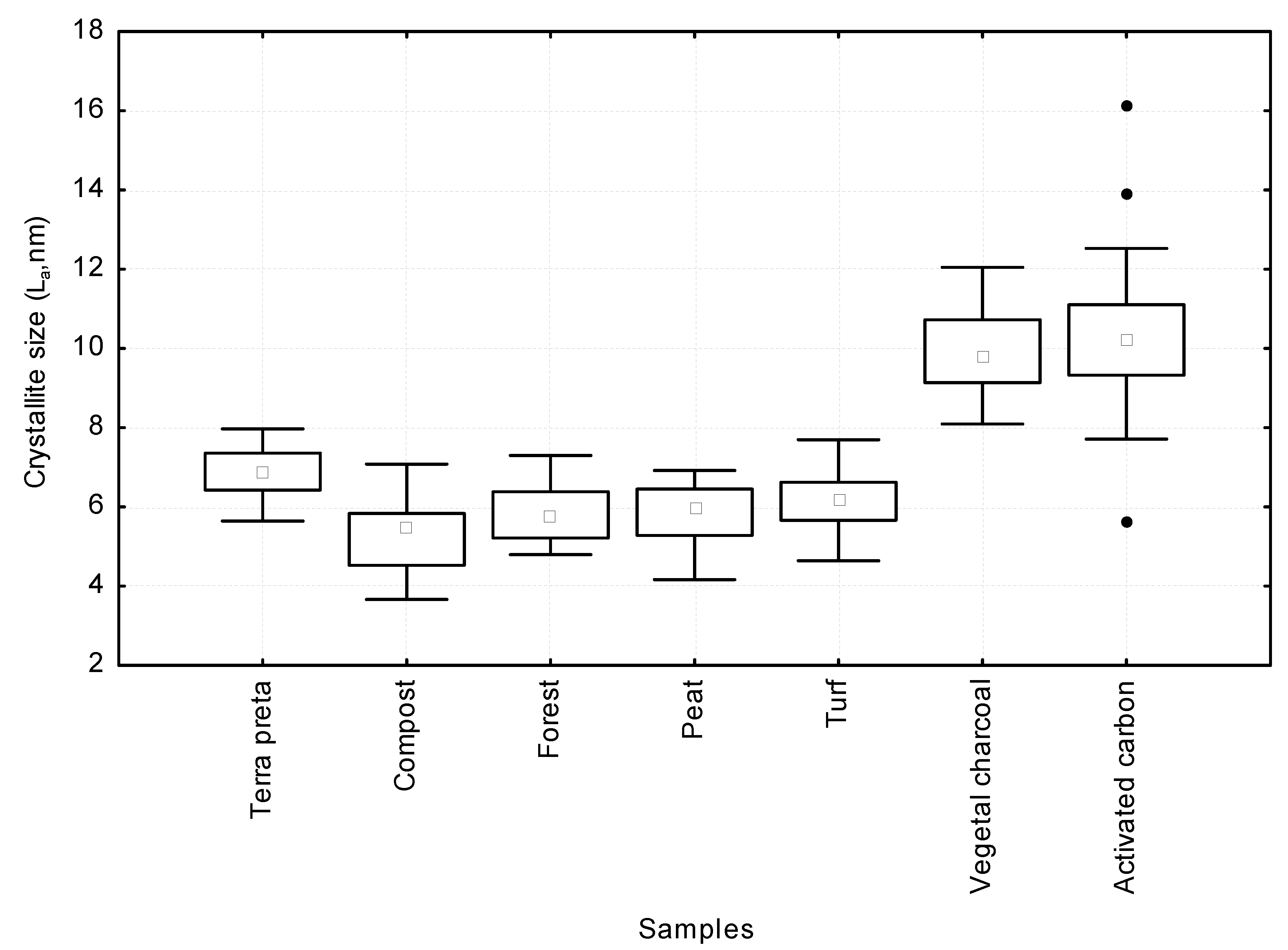
3.2. Spectroscopic Analysis of Carbon
4. Discussion
5. Conclusions
Author Contributions
Funding
Data availability
Acknowledgements
Conflict of interest
References
- Gabhane, J.W., Bhange, V.P., Patil, P.D. et al. Recent trends in biochar production methods and its application as a soil health conditioner: a review. SN Appl. Sci2020, 2, 1307. https://doi.org/10.1007/s42452-020-3121-5. [CrossRef]
- Knicker, H. 2007. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochem. 85, 91–118. https://doi.org/10.1007/s10533-007-9104-4. [CrossRef]
- Kavitha, B., Venkata P. L Reddy. L., Kim, B., Lee SS, Pandey, SK, Kim, KH. Benefits and limitations of biochar amendment in agricultural soils: A review. J Environ. Manage2018, 227, 146-154. [CrossRef]
- Pagano, MC, Ribeiro-Soares, J, Cançado, LG, Falcão, NPS. Gonçalves, V. N., Rosa, L., H, Takahashi, JA., Achete, CA, Jorio, A. Depth dependence of black carbon structure, elemental and microbiological composition in anthropic Amazonian dark soil. Soil Till.Res.,2016, 155, 298-307.
- Glaser, B. McKey, D. Giani, L. Teixeira, W., di Rauso Simeone, G, Schneeweiß, J., Nguyen Hien, Homburg, J. 2024. Historical accumulation of biochar as a soil amendment 2024. In: Biochar for Environmental Management,3rd Ed., Routledge, ISBN9781003297673.
- Sánchez, OJ., Ospina, DA, Montoya, S. 2017. Compost supplementation with nutrients and microorganisms in composting process. Waste Manage, 69, 2023136-153. [CrossRef]
- Pilla, N., Tranchida-Lombardo, V., Gabrielli P., Aguzzi A., Caputo, M, Lucarini M., Durazzo, A., Zaccardelli, M. Effect of Compost Tea in Horticulture. Horticulturae, 9, 984. https://doi.org/10.3390/horticulturae9090984 https://www.mdpi.com/journal/horticulturae. [CrossRef]
- Sultan, H., Khan, M.N. Biochar and nano biochar: Enhancing salt resilience in plants and soil while mitigating greenhouse gas emissions: A comprehensive review. J Environ Manage. 2024; 355,120448. [CrossRef]
- Singh, P. Tomar, B. Patle, T., Gupta, S.( Biochar in Sustainable Agriculture In: Approach Towards Sustainable Agriculture2024, 231-243. In: Emerging Advancement In: Agriculture, Chapter: 12, AkiNik Publications.
- Parmar, A., Prabhat K., Nema, Agarwal, T. Biochar production from agro-food industry residues: a suitable approach for soil and environmental management., Current Science, 2014., 107(10, 1673-1682.
- Hammer, E. C. et al. A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol. Biochem, 2014, 77, .252-260. [CrossRef]
- Tsolis V., Barouchas, P. Biochar as Soil Amendment: The Effect of Biochar on Soil Properties Using VIS-NIR Diffuse Reflectance Spectroscopy, Biochar Aging and Soil Microbiology-A Review. Land 2023, 12,8, 1580. [CrossRef]
- Parihar, M., Rakshit, A, Meena, VS., Gupta, V K. Rana, K., Choudhary, M. Gopal, G. ·Mishra, PK., Pattanayak, A, Kumar. “The Potential of Arbuscular Mycorrhizal Fungi in C Cycling: A Review, Archives of Microbiology, 2020, 202 1581–1596, https://doi.org/10.1007/s00203-020-01915-x. [CrossRef]
- Gerdemann JW, Nicolson TH Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc,1963, 46: 235–244.
- Wright, S.F., and Upadhyaya, A. 1998. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil, 198, 97-107. [CrossRef]
- Glaser, B. McKey, D. Giani, L. Teixeira, W., di Rauso Simeone, G, Schneeweiß, J., Nguyen Hien, Homburg, J. 2024. Historical accumulation of biochar as a soil amendment 2024. In: Biochar for Environmental Management,3rd Edition. Routledge, ISBN9781003297673.
- Rillig, M., Ramsey, P.W., Morris, S., Paul, E.A. Glomalin, an arbuscular mycorrhizal fungal soil protein, responds to soil-use change. Plant Soil,2003, 253:293-299.
- Glaser, B., Birk, J.J., 2012. State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (terra preta de Índio). Geochim. Cosmochim. Ac. 82, 39–51. [CrossRef]
- Borie F., R Rubio, JL Rouanet, Morales, A., Borie, G. Effects of tillage systems on soil characteristics, glomalin and mycorrhizal propagules in a Chilean Ultisol, Soil Till. Res, 2006.88, 1–2, 253-261. [CrossRef]
- Hammer, E, C, Balogh-Brunstad, Z., Jakobsen, I. Olsson, PA, Stipp, S., Rillig, MC. A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol. Biochem, 2014, 77 252-260.
- Hammer, O., Harper, D. A. T., Ryan, P. D., 2001. PAST: paleontological statistics software package for education and data analysis. Paleontologica Electronica 4, 9. art 4, 178 kb.
- Taiz, L. Moller, IM. plant physiology, 2022.


| Sample | pH (H2O) | K | Fe | Ca | Mg | Al | P | C | Cu | Mn | Zn | GC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # TPI | 5.8 | 14 | 61.26 | 2.90 | 3.1 | 0.74 | 15.5 | 6.42 | 0.001 | 22.12 | 3.72 | 0.6 |
| Compost | 7.8 | 3100 | 60.15 | 14.225 | 4.3 | 0.01 | 41 | 14.2 | 0.85 | 81.3 | 12.1 | NE |
| VC | 2900 | 4.5 | - | 162.9 | 4.5 | 1 | 10.2 | 1.1 | NE | |||
| Peat | 2.3 | 72 | 4.33 | 4.835 | 2.6 | 1.75 | 3 | 4.83 | 1.9 | 2.65 | 1.45 | NE |
| Turf | 5.3 | 3.5 | 8.4 | 8.095 | 0.65 | 0.6 | 20.95 | 8.4 | 0.001 | 18.55 | 1.45 | NE |
| Control | 4.6 | 6 | 316.6 | 6 | 0.01 | 0.2 | 1 | 1.3 | 0.001 | 0.1 | 1.1 | NE |
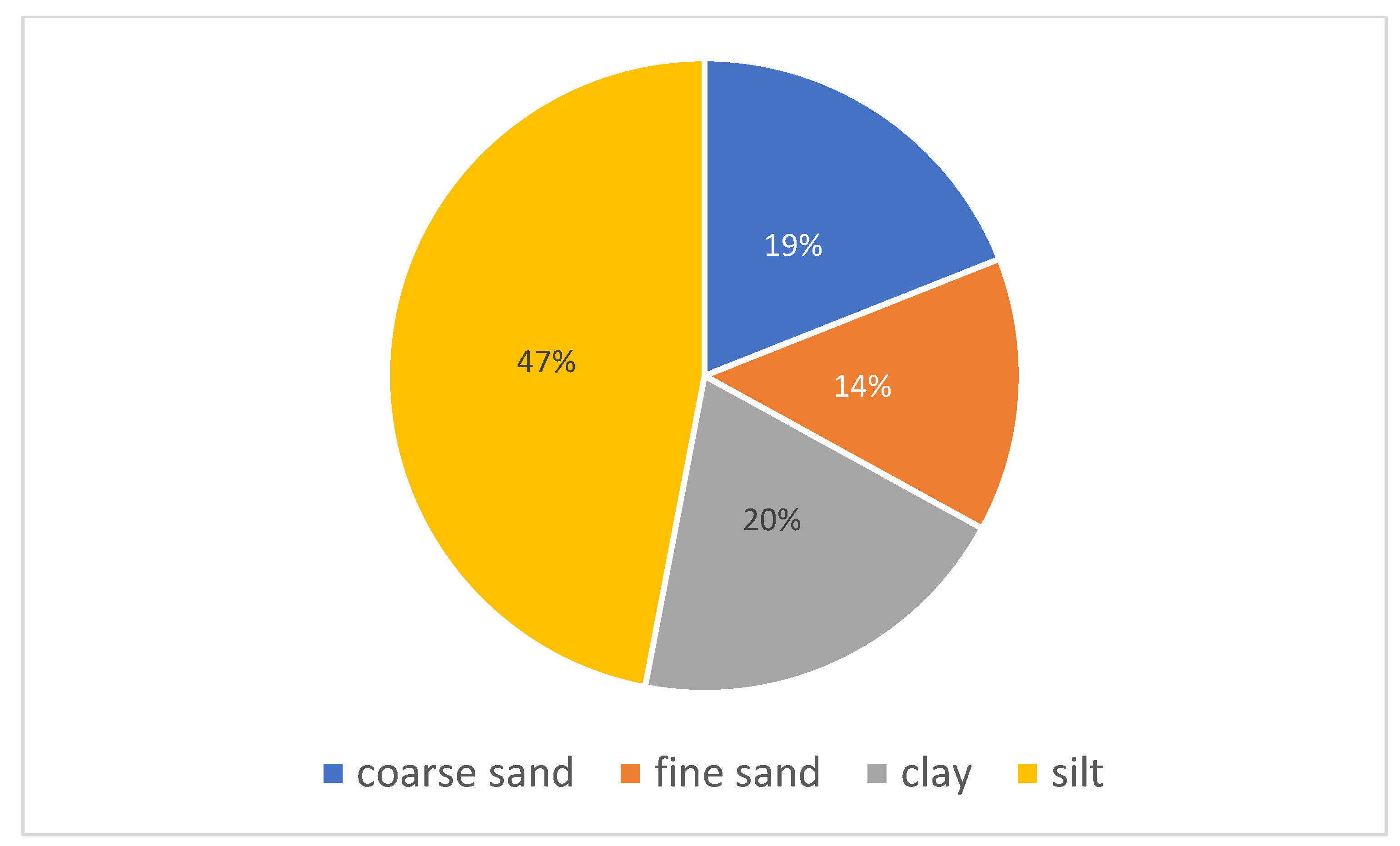
| Sample | Coarse sand | Fine sand | Clay | Silt (%) |
|---|---|---|---|---|
| TPI | 19 | 14 | 20 | 47 |
| AMF species | ||||||||
| Acaulospora bireticulata | ||||||||
| Acaulospora mellea | ||||||||
| Acaulospora rhemii | ||||||||
| Acaulospora scrobiculata | ||||||||
| Ambispora appendicula | ||||||||
| Claroideoglomus etunicatum | ||||||||
| Glomus crenatum | ||||||||
| Funneliformes geosporus | ||||||||
| Glomus rubiforme | ||||||||
| Pacispora franciscana | ||||||||
| Species richness (number of species): | 10 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





