1. Introduction
Interstitial lung diseases (ILD), are debilitating conditions characterized by thickening of the alveolar walls, airway epithelial cell hyperplasia, and lung stiffness 2] induced by chronic extracellular matrix (ECM) remodeling of lung parenchyma and loss of lung function. Idiopathic Pulmonary Fibrosis (IPF) is a subset of fibrotic ILD diseases with no known cause [
1,
2,
3]. IPF had a high mortality rate, with a median prognosis of 2.5-3.5 years post-diagnosis [
4,
5]. IPF is driven by epithelial damage, fibroblast activation, and progressive collagen production in lung fibroblasts [
6] leading to the accumulation of α-SMA positive myofibroblasts [
5] which irreversibly hinders pulmonary function [
7]. Mechanistic studies of IPF implicate TGF-β activity [
8] through activation of its canonical SMAD2/3 signaling pathway [
9,
10]. However, TGF-β-independent pathways, such as over-activation of STAT3 signaling, may also contribute to lung ECM remodeling [
11,
12]. Clinical trials of Pirfenidone (mechanisms include action on fibroblasts and TGF-β activity) have shown limited efficacy in patients and side effects [
13]. Similarly, trials with the tyrosine kinase-targeting drug Nintedanib have shown slowed progression but not cessation of the disease, along with severe side effects [
14,
15,
16]. The inadequate replication of drug effectiveness in humans compared to animal models is one notable reason why effective treatment options are scarce [
17]. Thus, alternative interventions are urgently merited, but the development of such treatments is challenged by the lack of persistent fibrosis in preclinical models.
The single administration of Bleomycin (Bleo) into the lungs of rodents is the most commonly used standard for preclinical models of fibrosis in mice and rats where there are advantages and disadvantages of various models of lung fibrosis as reviewed by us and others [
18,
19,
20]. Although the Bleo model in C57Bl/6 mice recapitulates aspects of IPF fibrogenesis (changes in lung architecture, collagen deposition, α-SMA and airway physiology) the fibrotic response resolves over time (by 50 days), unlike human IPF, which does not resolve. This discrepancy limits the translational relevance of the Bleo model, as the mechanism of disease progression and chronicity in human IPF are likely heterogeneous and not fully replicated in the murine model. This Bleo model is also strain-sensitive as C57Bl/6 mice are more susceptible to fibrotic stimuli, such as Bleo [
21,
22] or pulmonary over-expression of TGF-β, compared to BALB/c mice [
23]. We have recently shown that transient overexpression of the gp130 cytokine IL-6 exacerbates Bleo-induced fibrosis in C57Bl/6 mice [
24]. Here, we explore a new mouse model in Bleomycin-treated BALB/c mice with concurrent overexpression of IL-6. This new model exhibits unique characteristics and a transcriptome signature that more closely resembles human IPF than other rodent models.
IL-6 is a member of the gp130 cytokine family, which plays critical roles in inflammation, immunity, development, and hematopoiesis [reviewed in [
25,
26,
27]]. These cytokines act on cell surface receptors that include subunits gp130 and cytokine-specific receptor chains upon which ligation prominently activates the cell-signaling molecule STAT3. Activation of STAT3 is seen up-regulated in IPF, and STAT3 over-activation by genetic manipulation in mice can robustly exacerbate Bleo-induced lung fibrosis independently of TGF-β/SMAD signaling [
11,
12]. Transient IL-6 over-expression alone has minimal effects on lung ECM remodeling [
28], implying concomitant stimuli causing tissue damage are required. IL-6 can directly regulate macrophage populations in certain systems [
29] and plays pivotal roles (reviewed in [
25,
26,
27,
30,
31]) in adaptive immunity. Often characterized as an innate immune cell and Th2 T cell product, IL-6 regulates B cells, macrophages, can also activate naïve T cells [
32] and drives T cell expansion in inflammatory conditions but not during normal homeostasis [
33]. IL-6 (expressed by various cells stimulated with endogenous cytokines, DAMPs or exogenous PAMPs) is markedly elevated at local sites of infections and other chronic conditions.
Transient elevations of IL-6 due to acute tissue damage may integrate adaptive immune responses and drive chronic disease. Indeed, elevated IL-6 levels have been associated with clinical decline and mortality in patients diagnosed with ILD [
34] and those experiencing acute exacerbations of IPF [
35]. However, the putative role of IL-6 and underlying mechanisms in the progression of fibrosis remain unclear. Here, we characterized the molecular and cellular characteristics of a novel model of lung fibrosis in mice generated by transient overexpression of IL-6 combined with Bleomycin treatment, which is ablated by T-cell depletion, induces prolonged fibrosis, is associated with a phenotype of activated macrophages (CD16
+/MHC
hi/Clec7a+/NOS
-/Arg1
-/CD206
-) and displays a transcriptome signature closer to human IPF transcriptome than other murine models. This model provides deeper insights into the role of IL-6 in fibrosis and offers a potentially more accurate preclinical tool for studying IPF mechanisms and evaluation therapeutic interventions.
2. Materials and Methods
2.1. Animals
Female BALB/c mice, aged 8-12 weeks, were obtained from Charles River (Ottawa, ON, Canada). All animals were housed in a specific pathogen-free conditions within the McMaster University Central Animal Facility (Hamilton, ON, Canada). The mice underwent a one-week acclimatization period before the commencement of any experimental procedures. All experimental procedures were conducted in accordance with the guidelines approved by the McMaster University Animal Research Ethics Board under protocol #160414.
2.2. Administration of Adenoviral Vectors and Bleomycin
The construction and validation of the adenovirus vectors were performed in-house and characterized previously [
36,
37]. Adenovirus vectors AdDL70 (negative control vector), AdOSM, and AdIL-6 were thawed on ice, and diluted in PBS to the required concentrations. Briefly, mice were anaesthetized under gaseous isoflurane while AdDL70 (2x10
7 PFU/mouse), AdOSM (2x10
7 pfu/mouse) and AdIL-6 (1x10
7 PFU/mouse) were intratracheally administered in a volume of 50μl of sterile saline. To induce pulmonary fibrosis, mice underwent intratracheal intubation of bleomycin (Hospira Healthcare Corp., NDC 61703-332-18) at a dose of 0.03 U/mouse in a volume of 50µl sterile saline. Bleomycin was either administered alone or in combination with AdOSM, AdIL-6 or AdDL70. Following the treatments, the mice were sacrificed at timepoints: 7-, 14-, 21- and 50-days post-administration and lungs were analyzed as previously described [
37] for inflammatory and fibrotic responses.
For T cell depletion, mice were injected
ip with 200μg of anti-CD4 (clone GK1.5), and anti-CD8 (clone 2.43) or IgG isotype control (Sigma-Aldrich). A second 100μg dose was administered
ip after 2 days, with a maintenance dose every 7 days as needed to maintain depletion (CD4
+ and CD8
+ cells are depleted by >99%, verified using flow cytometry [
38]).
2.3. Collection of Mouse Specimens and Analysis of Lung Tissues
Body weights were monitored regularly throughout the study and tissue harvest was conducted at the specified timepoints. BALF was collected for cytokine and inflammatory cell analyses as previously described [
36,
37,
39]. Cytokines were analyzed by Eve technologies (Calgary, Alberta, Canada). The right lung lobes were tied off with a surgical thread, excised, and either placed in cold media or snap-frozen in liquid nitrogen. The snap-frozen right lung lobes were placed into a stainless-steel mortar and crushed into a fine powder with a stainless-steel pestle while submerged in liquid nitrogen. The powdered lungs were then placed in RIPA lysis buffer or TRIzol solutions and mechanically sheared using a homogenizer (Ultra-Turrax
® T25). The samples were subsequently processed for protein and RNA analyses, respectively. The left lung was excised and inflated with 10% neutral buffered formalin solution under a pressure of 30 cmH2O. The left lungs were fixed for 48 hours, transferred to 70% ethanol and prepared for histology and immunohistochemistry analyses. Histochemical and immunohistochemical staining was completed at the John Mayberry Histology Facility at McMaster Immunology Research Centre. 5µm sections were cut and stained with Masson’s Trichrome and Picrosirius Red for assessment of extracellular matrix and collagen, and anti-αSMA (Agilent Dako, M0851) for assessment of myofibroblasts. Airway physiology measurements used Quasi-static lung elastance (stiffness) measured at the specified time points using a rodent mechanical ventilator (flexiVent
®, SCIREQ, Montreal, PQ, Canada), following previously described protocols as we have published [
37].
2.4. Acquisition and Analysis
Full slide images were acquired with an Olympus VS120 slide and Quantified as previously described previously [
40]. Microscope slides were digitalized using an Olympus VS120-L100-W slide scanner microscope at a 20× magnification. Subsequent images were acquired to illustrate representative areas. HALO
TM Image Analysis Software (Halo Plus 3.2, Indica Labs) was used to quantify a-SMA and Masson’s trichrome stain in the lung parenchyma, excluding airways and blood vessels. A semi-quantitative assessment of fibrosis severity was conducted using the Ashcroft grading procedure based on Masson’s trichrome staining, as described previously [
40]. For α-SMA quantification, the main bronchus and larger airways were excluded.
2.5. Flow Cytometry:
Lung mononuclear cell suspensions were generated by standard methods (mechanical mincing and collagenase digestion), debris removed, and cells re-suspended in PBS/0.3% BSA or RPMI/10% FBS). Methods were as previously published [
39,
41]. Briefly, lung mononuclear cells (at least 10
7 per mouse) were washed and stained with primary antibodies directly conjugated to fluorochromes for 30 minutes at 4°C. 1x10
6 live events were acquired on an LSR II (BD Biosciences) flow cytometer and the data analyzed with FlowJo analysis. Side scatter and forward scatter parameters, as well as Zombie staining were used to define live cell and lymphocyte gates. All antibodies were purchased from BD Biosciences, eBiosciences or Biolegend, and include PE-conjugated anti-CLEC7A, PE-Dazzle-conjugated anti-CD64, PE-cy5-conjugated anti-CD11b, PE-cy7-conjugated anti-iNOS, Brilliant Violet (BV) 421-conjugated anti-F4/80, Pacific Orange-conjugated anti-Ly6G(Gr-1), BV605-conjugated anti-Ly6C, BV650-conjugated anti-CD206, APC-conjugated anti-Arginase-1, Alexa Fluor700-conjugated anti-MHC class II, APC-cy7-conjugated anti-CD45, V500-conjugated anti-CD8. Whole lung cells were detected by first surface staining cells for 30 minutes at 4°C (with antibodies to CLEC7A, CD206, CD64, CD11b, CD11c, F4/80, Gr-1, Ly6C, MHC class II and CD45), followed by intracellular staining with antibodies to iNOS, and IFNγ for 30 minutes at 4°C. Cells were then washed (PBS/0.3% BSA) prior to analysis by flow cytometry.
2.6. Statistical Analysis
Data were analyzed using GraphPad Prism version 10, with results expressed as mean ± standard error of the mean (SEM). Statistical significance was determined using one-way ANOVA or as noted in the figure legends, with p < 0.05 threshold.
2.7. Bulk RNAseq and Bioinformatic Analysis
RNA was extracted using an RNeasy RNA extraction kit (Qiagen) and treated with DNAase (Ambion. cDNA libraries were created and were sequenced using an Illumina HiSeq machine (1 x 50 bp sequence reads) at the Farncombe Metagenomics Facility (McMaster University). The reads were filtered by quality (at least 90% of the bases must have a quality score of 20 and higher) and then the remaining reads were aligned with mm10 (UCSC) reference using HISAT2 [
42]. Next, the reads were counted by using HTSeq count [
43]. Genes, showing less than 10 counts in more than 30% of the samples per group were removed using filterByExpr function in EdgeR package [
44,
45] in R, resulting in 15,030 genes. Counts for these remaining genes were normalized with TMM normalization method [
46] and then transformed using voom transformation [
47]. Differential expression between the groups of interest was examined using limma package [
48] in R. P-values were corrected with BH correction for multiple testing [
49]; corrected values <0.05 were considered to be significant. Gene Set Enrichment Analysis (GSEA) was performed using GSEA desktop application [
50] and pathway analyses were performed using STRING database [
51].
2.8. Publicly Available RNA Datasets
Human transcriptomic datasets containing profiles of lung tissues from IPF patients and corresponding controls [GSE53845 (DePianto et al., 2015) and GSE92592 (Schafer et al., 2017)] were obtained from GEO [
52] and were processed similarly to the methods described in the source publications [
53] and [
54], respectively. GSE53845 contains data obtained using microarray technology and, therefore, is being referred to as an “array dataset” or a “microarray dataset”, while GSE92592 contains data obtained using RNAseq technology and, therefore, is being referred to as an “RNAseq dataset”. Mouse Bleo model in C57Bl/6 dataset was obtained from GSE485 and the rat model from GSE48455. Differential expression analysis was performed using limma package in R. P-values were corrected with BH correction for multiple testing; corrected values <0.05 were considered to be significant.
3. Results
3.1.1. AdIL-6 Combined with Bleomycin Induces Robust Fibrosis at Day 21 in BALB/c Mice
We have previously published that concomitant transient pulmonary expression of gp130 cytokines IL-6 or Oncostatin M (OSM), using Adenovirus constructs, causes exacerbation of fibrosis and ECM deposition in Bleomycin-treated C57Bl/6 mice [
24]. In contrast to the C57Bl/6 strain, we find that Bleomycin alone does not produce changes in the BALB/c mouse strain in baseline parameters of measurements of lung fibrosis (
Supplemental Figure S1B), consistent with previous reports [
21,
22]. We found that Bleo-treated lungs with concomitant transient pulmonary over-expression with either OSM (AdOSM+Bleo) or IL-6 (AdIL-6+Bleo) results in robust elevation of collagen, α-SMA, and airway elastance (Est) shown in
Figure 1. Additionally, control Adenovirus vector (AdDL70) combined with Bleo treatment showed no statistical differences from control groups (
Figure 1B–E). Overall, the combination of AdIL-6+Bleo tended to induce greater changes in fibrosis scoring than the AdOSM+Bleo group. We did not observe elevated staining for CD206 (an alternatively activated macrophage marker) (
Supplemental Figure S1A). In groups treated with AdOSM or AdIL-6 vector alone, none of the treatments resulted in detectable change in these parameters (
supplementary Figure S1B), indicating that transient over-expression of IL-6 or OSM alone is insufficient to induce the fibrotic response in BALB/c mice at the Day 21 time point.
3.1.2. AdIL-6+Bleo Induces Sustained Fibrosis out to Day 50
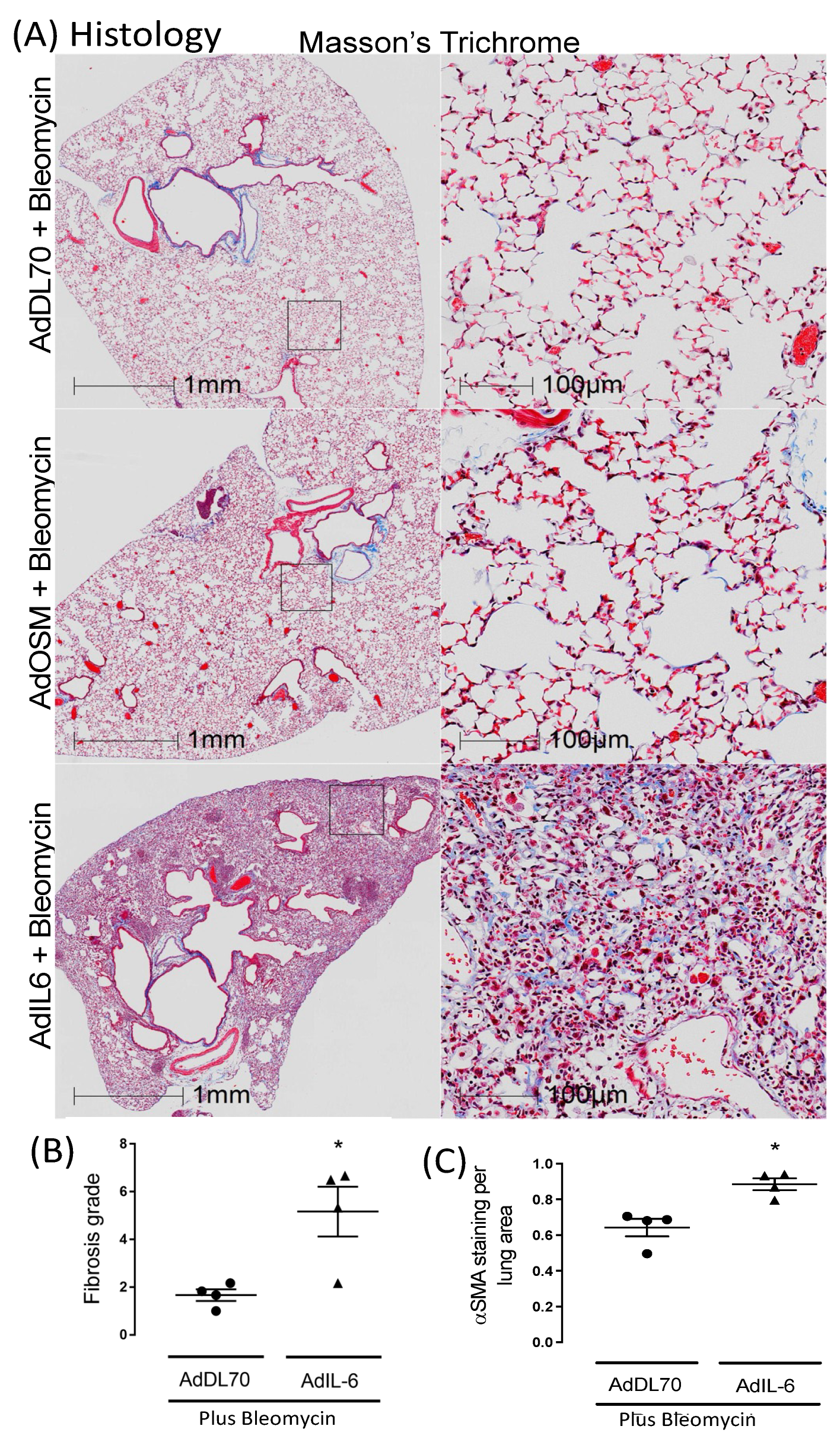
We further assessed whether sustained fibrosis was evident at a later time point as shown in
Figure 2. IL-6 expression concomitant to Bleomycin (AdIL-6+Bleo) results in persistent matrix accumulation, which was detectable until day 50 in BALB/c as shown by representative images (
Figure 2A). This was not evident in any of the mice treated with concomitant control vector AdDl70+Bleo, or with AdOSM+Bleo. Quantitative analysis of fibrosis score (
Figure 2B) and αSMA staining (
Figure 2C) show significant elevation of AdIL-6+Bleo group over AdDL70+Bleo group. Collectively, these results suggest this model of lung fibrosis in BALB/c mice is IL-6-dependent and shows a more persistent ECM accumulation, unlike other mouse models. This indicates novel actions of early IL-6 overexpression in this model. Consequently, we examined an earlier time point in more depth.
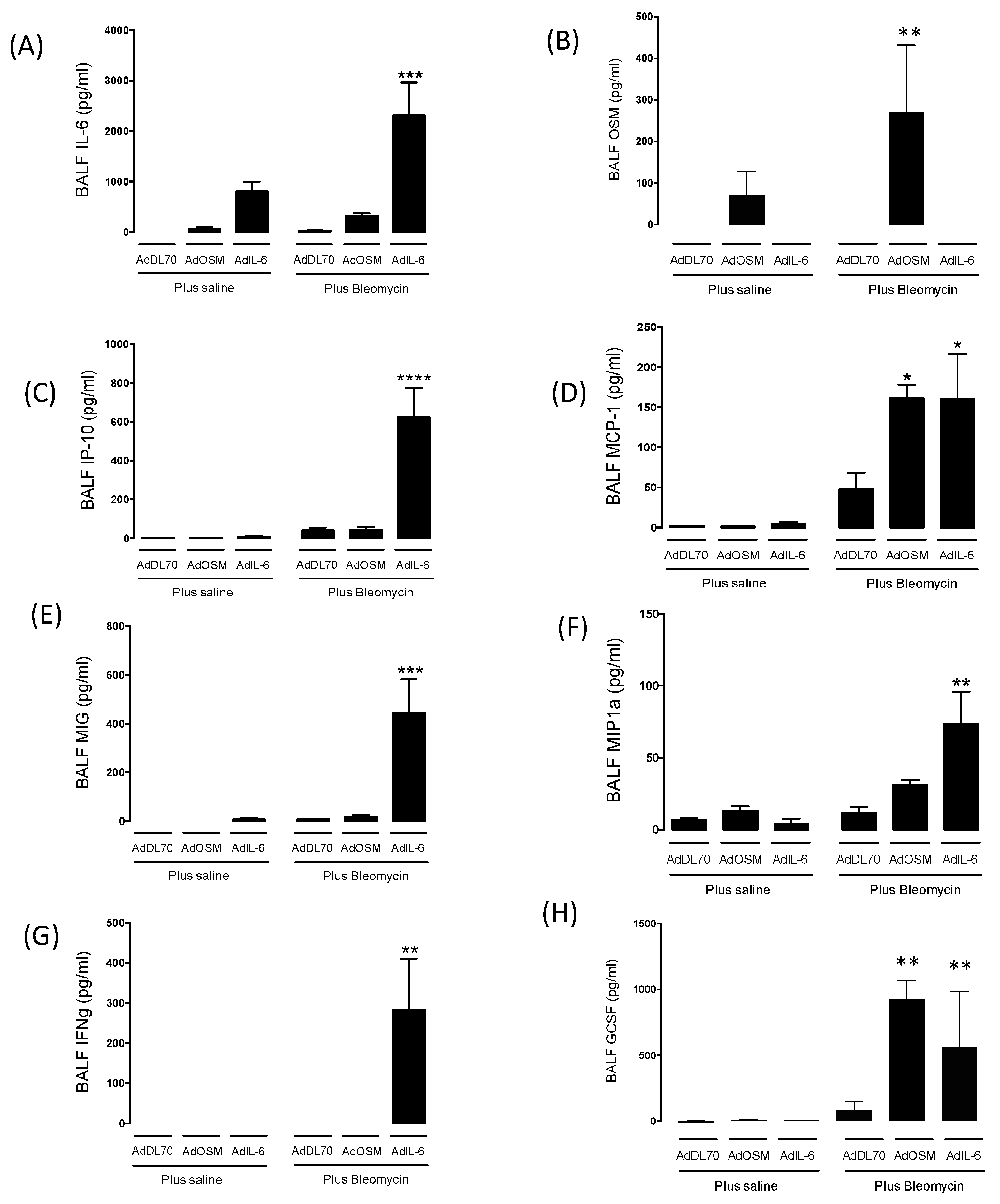
3.2. Cytokine Levels in Broncho-Alveolar Lavage and Flow Cytometry at Day 7
Since there was an effect of early IL-6 over-expression (gene inserts are expressed over 2-10 days in the Advector models) on subsequent fibrosis sequalae, we examined the cytokine levels in BALF at Day 7. As shown in
Figure 3, BALF from AdIL-6 and AdOSM treatments showed elevated levels of IL-6 or OSM (respectively) as expected from the vector-encoded cytokines). BALF from AdIL-6+Bleo treated mice showed markedly elevated levels of IP-10, MIG, MIP1a, in comparison to AdDL70+Bleo or AdOSM+Bleo. In addition to these chemokines, AdIL-6+Bleo markedly induced IFNγ (
Figure 3G) but no detectable IL-4 or IL-13 as typical Th2 cytokines (Supplemental
Figure S2). These results indicated a selective cytokine signature induced by AdIL-6+Bleo.
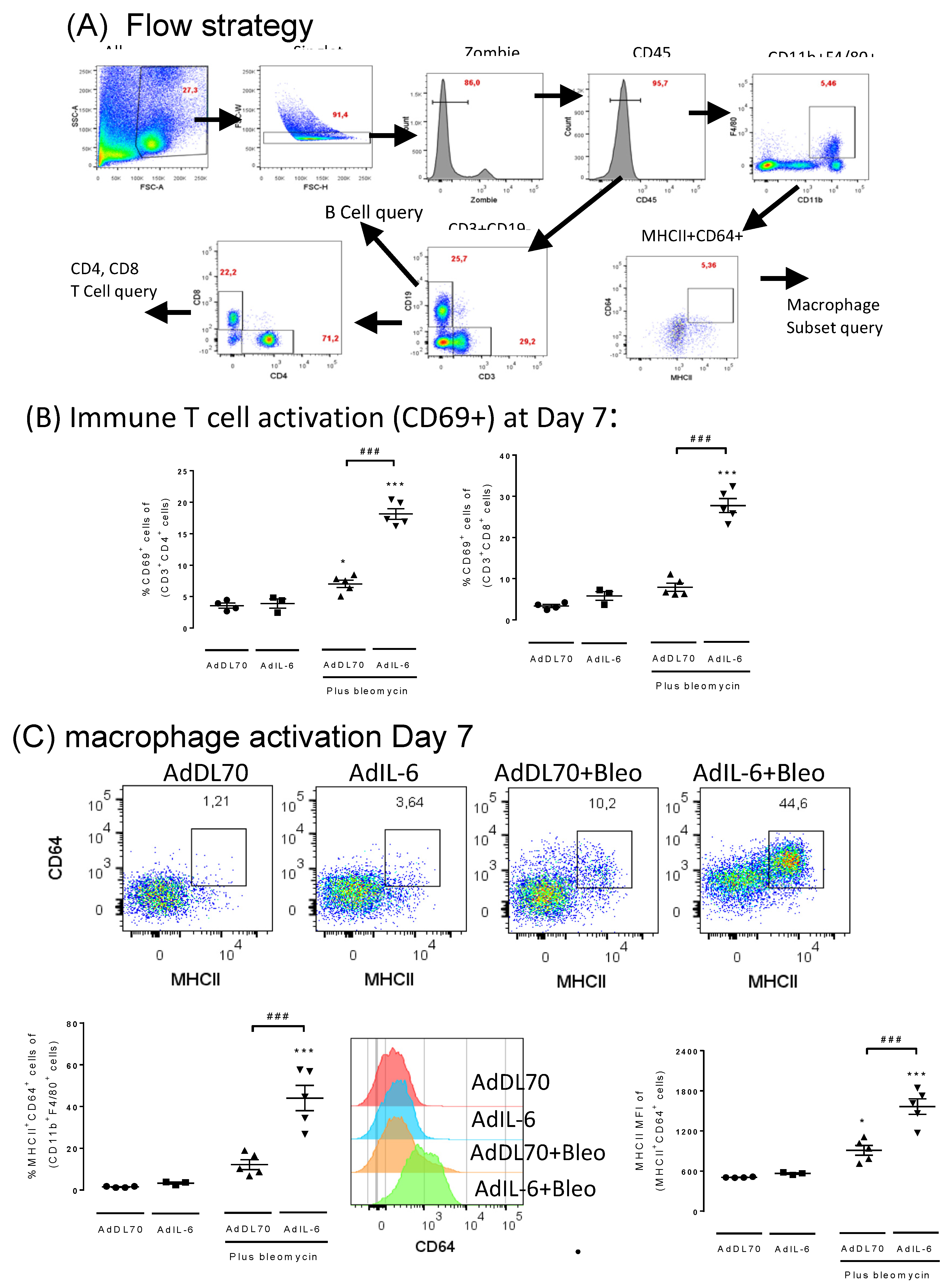
Given that IFNγ, a T cell product, is involved in regulating various immune/inflammatory responses, we further investigated T cell and macrophage activation in this system. The flow cytometry strategy used to assess immune cells is shown in
Figure 4A. In AdIL-6+Bleo BALB/c mice at Day 7, we observed elevated levels of activated (CD69
+) CD4
+ and CD8
+ T cells (
Figure 4B) and high IFNγ protein levels in BAL (
Figure 3G) but not in AdDL70+Bleo mice. This indicates activated T cells and an IFNγ signature early in the inflammatory phase (day 7).
In assessing macrophage phenotypes at Day 7 (as per flow cytometry strategy shown in
Figure 4A), we observed among macrophages (CD45
+, CD11b
+, F480
+), a selectively elevated population characterized by CD64
+/MHCII
hi/Clec7A
+, but iNOS-, Arg1-, CD206- which we term “CMC
+Macs”. AdIL-6+Bleo induced higher numbers of this macrophage phenotype but AdDL70+Bleo did not induce significantly different levels (
Figure 4C lower left panel). Furthermore, the mean fluorescence intensity (MFI) of CD64 (
Figure 4C, lower middle panel) and MHCII (
Figure 4C, lower right panel) was significantly elevated in this population of macrophages in the AdIL-6+Bleo group. In examining numbers of CD206+F480+CD11b+ (alternatively activated macrophages, which are implicated in other mouse fibrosis models), we did not observe any significant changes from saline treated mice in any of the groups at this time point (shown in supplemental
Figure S3).
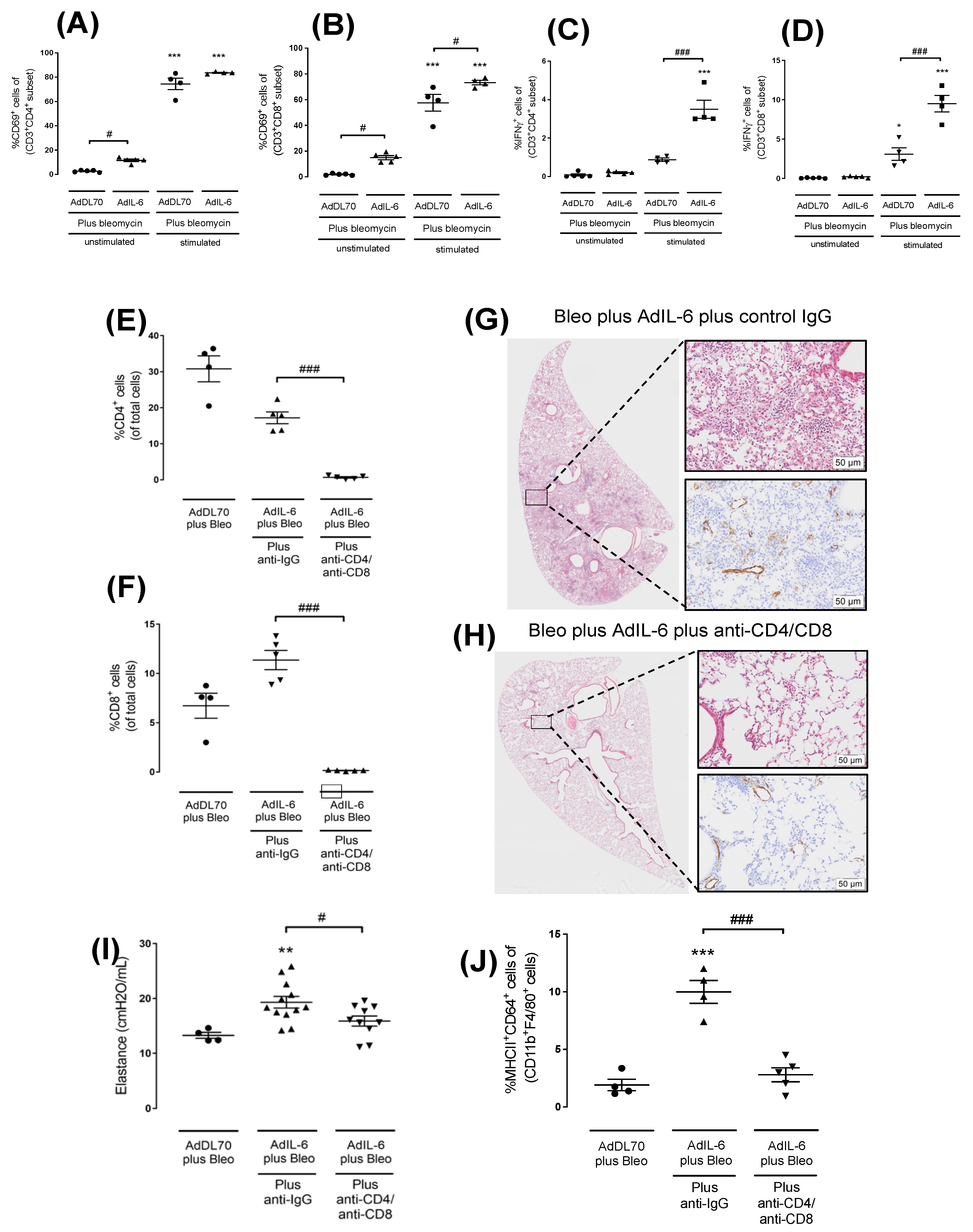
3.3. T-Cell Depleting Antibodies Inhibits Fibrotic Responses at Day 21 in AdIL-6+Bleo BALB/c Mice
We then went on to assess the requirement of T cells in this model using depletion antibodies targeting both CD4+ and CD8+ cells (
Figure 5). We first examined the ability of T cells to maintain their ability to express IFNγ at a later time point (Day 14) in the model.
Figure 5A,B show that, when assessed from lung cells at Day 14, stimulation with PMA and Ionomycin increased CD69+CD4 cells from either Addel70+Bleo or AdIL-6+Bleo groups and could elevate CD69+CD8 T cells from the AdIL-6+Bleo group at a significantly higher level than AdDL70+Bleo. Importantly, the same re-stimulation regime was able to increase percentage of IFNγ+CD4 and CD8 T cells from the AdIL-6+Bleo group markedly higher than the AdDL70+Bleo group. Thus, there are persisting populations of CD4+ and CD8+ cells that can produce IFNγ in this system, and thus we went on to assess the effects of T cell depletion in the model.
The Effects of CD4 and CD8 depletion antibodies are shown in
Figure 5E–J. Levels of CD4 and CD8 cells were measured in AdIL-6+Bleo treated mouse lungs upon injection of Anti-IgG control Ab verses anti-CD4/Anti-CD8 Antibody.
Figure 5E,F show that the anti-CD4/8 treatments significantly reduced CD4 and CD8 cell numbers. Supplemental
Figure S4 shows that the antibody treatments did not affect CD69+ B cells, nor CD69+NK cells. Subsequent analysis at Day 21 that examined histology (
Figure 5G,H) and elastance (
Figure 5I), showed reduced pathology (Trichrome and ASMA staining) and a statistically significant reduction in elastance by the anti-T cell antibody treatments. In addition, the percentage of CD64+MHC
hiCD11b+ macrophages was ablated by antiCD4/8 antibody depletion at Day 14 (
Figure 5J).
3.4.1. Transcriptome Analysis and Differentially Expressed Genes at Day 7 and 21 of AdIL-6+Bleo Treated BALB/c Mice
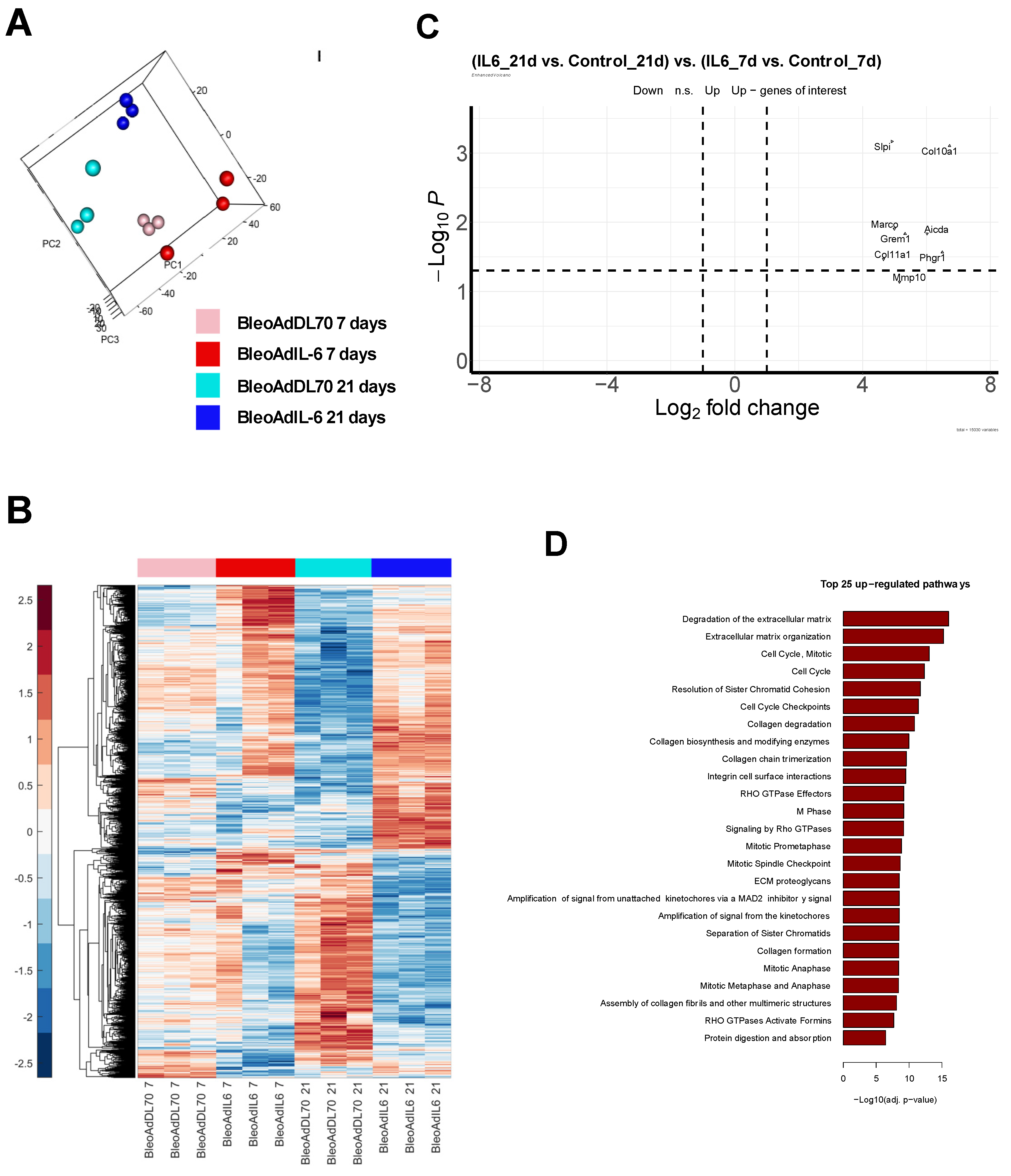
In order to examine the transcriptomic characteristics of this model we sequenced both treated (AdIL-6+Bleo) and control (AdDL70+Bleo) lung samples (n=3) collected on days 7 and 21 using bulk RNAseq. These groups are visually displayed through the PCA plot in
Figure 6A. At day 7, the AdDL70+Bleo control group (pink) exhibited a tight cluster
, while the AdIL
-6+Bleo intervention group (red) showed a bit wider dispersion. Day 21 exhibited more closely clustered samples in each group, and more pronounced separation between the AdDL70+Bleo control (cyan) and the AdIL-6+Bleo intervention (blue) groups than at day 7. This overall supports a more robust difference between the control and intervention groups at day 21. We then performed differential expression (DE) analysis comparing intervention groups to their corresponding controls (AdIL-6Bleo - AdDL70, for both day 7 and day 21), as well as examining the changes across the time course between days 21 and 7 [(IL-621d – IL-67d) - (AdDL21d - AdDL7d)]. Expression level of genes significantly regulated with an absolute fold change of at least 1.5 in at least 1 out of the 3 comparisons are shown in the heatmap in
Figure 6B, with upregulated genes shown in red and downregulated genes shown in blue. The heatmap displays more drastic differences (and thus negative correlation in the gene expression pattern) between the AdIL-6+Bleo intervention group and the AdDL70+Bleo control group at day 21 (blue bar vs. cyan bar), compared to day 7 (red bar vs. pink bar). The full list of DE genes (1515 genes in total) is shown in
Supplementary Table S1. Top up-regulated genes for AdIL
-6+Bleo at day 21, corrected against day 7 [(IL-621d – IL-67d) - (AdDL21d - AdDL7d)], included: COL10A1 (ECM remodeling), PHGR1 (Proline, Histidine and Glycine rich 1), AICDA (B cell differentiation), MARCO (phagocyte receptor), GREM1 (fibrosis), MMP10 (ECM remodeling), SLPI (secretory leukocyte protease inhibitor) and COL11A1 (ECM remodeling)
, among others. This is also visualized in the volcano plot
Figure 6C where top upregulated genes are depicted in cyan (right side of plot). Top down-regulated genes included GM5483 (cystatin A), IL-22RA2 (IL-22 receptor alpha2), and GRZB (Granzyme B), among others. The top 25 upregulated pathways are summarized in
Figure 6D, which includes pathways primarily related to extracellular matrix degradation/reorganization (8 pathways), cell cycle (12 pathways), RHO GTPase signaling (3 pathways), integrin binding (1 pathway), and protein digestion/absorption (1 pathway). The full list of pathways is shown in
Supplementary Table S2.
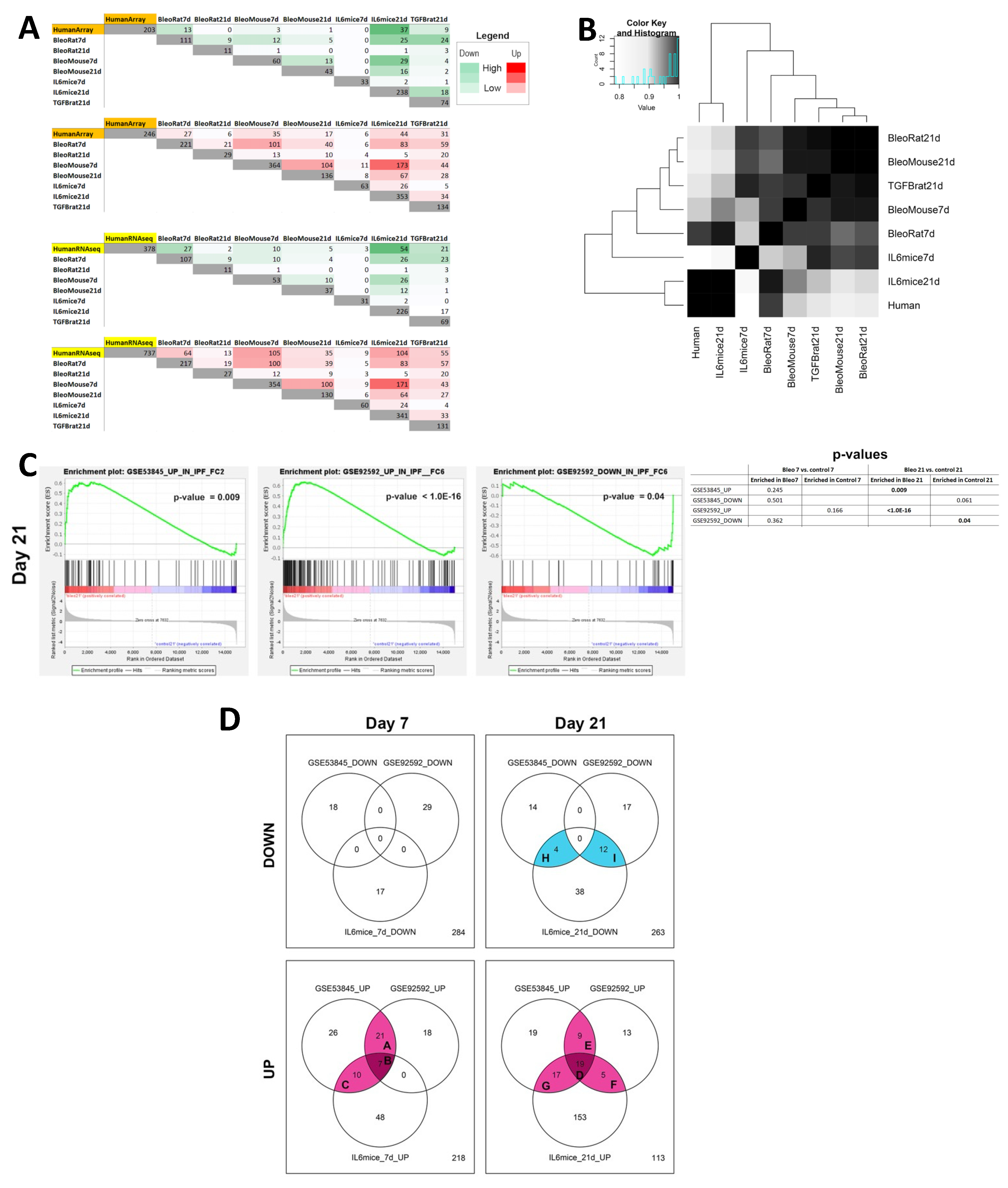
3.4.2. Comparison of Transcriptomes of Mouse Lung Fibrosis Models to Human IPF Transcriptomes
In order to examine whether the obtained DE genes are also characteristic of IPF patients, we compared our RNAseq results to signatures obtained from 2 independent human cohorts of lung tissue from IPF patients (see Methods). We also compared bulk RNAseq signatures of different rodent models of lung fibrosis to our model, including a bleomycin rat model (day 7 and 21), bleomycin C57Bl/6 mouse model (day 7 and 21), and AdTGFb rat model (day 21). Numbers of DE genes found to be shared between each of the models and each of the human datasets are shown in
Figure 7A. Day 21 AdIL-6+Bleo mice exhibit the highest numbers of genes shared with both human datasets [obtained by microarray (GSE53845), and by RNAseq GSE92592)], reflecting their higher level of similarity to the disease in human patients [sharing 37 and 54 downregulated genes, and 44 and 104 upregulated genes (IPF microarray and IPF RNAseq, respectively)]. The full set of genes is listed in Supplemental
Table S3. Based on the number of shared genes, we then proceeded to examine the similarities between the sets with a heatmap, as seen in
Figure 7B, where the human data presented is a composite of both human IPF datasets. We observed that AdIL6+Bleo at Day 21 clustered most closely with the human gene set, indicating that gene signature of our model is most biologically similar to that of the human IPF datasets. We then performed GSEA using signatures containing genes found to be differentially expressed in the human IPF datasets, as seen in
Figure 7C. We observed significant enrichment of the upregulated genes (p=0.009) in the IPF microarray dataset (GSE53845), as well as both upregulated (p<1.0E-16) and downregulated genes (p=0.04) in the IPF RNAseq dataset (GSE92592), in our AdIL-6+Bleo model at Day 21. No enrichment was found at Day 7. To further stratify the functional meaning of the observed similarities, we performed pathway analysis. We found multiple pathways to be significantly over-represented in both our model and the human IPF datasets. Venn diagrams in
Figure 7D summarize the overlap of downregulated and upregulated pathways between our model (at Day 7 and 21) and the human datasets. Quantity of overlapping pathways regulated in the AdIL-6+Bleo model are marked by various shades of magenta (upregulation) or blue (downregulation), with darker shades indicating intersections between a higher number of datasets. Letters refer to the lists of overlapping pathways, which can be found in
Supplementary Table S4. Shared pathways with human IPF and AdIL-6+Bleo are also depicted in
Supplementary Figure S5. At Day 7 AdIL-6+Bleo, these included IGF regulation, protein phosphorylation and cytokine receptor interactions among others, but not the extracellular matrix remodeling related pathways. The greatest quantity of overlapping pathways was observed with AdIL-6+Bleo at Day 21, including collagen formation (biosynthesis and modification), IGF regulation and PTK2 signaling among others, supporting the suggestion that our model exhibits the highest functional similarity to the human IPF datasets at this time point.
4. Discussion
This new model of pulmonary fibrosis in BALB/c mice, which are typically thought of as a relatively fibrosis-resistant strain, is characterized by robust fibrosis at 21 days that is maintained up to Day 50. Results showed that AdIL-6+Bleo induced a pronounced unique early cytokine protein signature featuring typical chemokines that activate T cells and macrophages, distinct from that of comparator or control treatments. Both CD4+ and CD8+ T cells were activated, and T cell depletion experiments indicated that T cells were required for the fibrotic response. Fibrosis was associated with the accumulation of activated macrophages with a particular phenotype (CD64+/MHCIIhi/Clec7A+/iNOS-/Arg1-/CD206-). Transcriptomic signature studies showed elevation of typical genes and pathways involved in extracellular matrix remodeling and cell cycle. Comparison to two publicly available datasets of human IPF transcriptomes, and to that of other models of Bleo in mice, revealed more shared genes and pathways with the Human IPF datasets than Bleo in C57Bl/6 or over-expression of TGF in rats. Thus, with more persistent fibrosis and more transcriptomic similarity, we have here characterized a model that appears closer to the human disease than other preclinical models that have been used to explore therapies for the IPF condition.
The fibrotic response in this model is dependent on early (up to Day 7) high levels of IL-6 and needs a concomitant stimulus (Bleo-induced lung damage). Since IL-6 elevation has been associated with acute exacerbations [
55], this is consistent with a potential role of IL-6 in the incipient stages of disease development and/or in exacerbation in humans. A clinical trial targeting IL-6 with tocilizumab in Systemic Sclerosis (SSC) patients did not reach endpoint in skin fibrosis effects, but did suggest it might preserve lung function in patients with early SSc-ILD, a subset of the groups studied [
56]. In the standard Bleomycin-induced model of lung fibrosis in C57Bl/6 mice, IL-6 appears to have a bidirectional role. While IL-6 blockade within the fibrotic phase (day 8- and on) reduced matrix accumulation, IL-6 blockade in the early phase (3 days) of the model exacerbated inflammation and accelerated fibrotic responses in C57Bl/6 mice [
57]. Another study demonstrated that blockade of IL-6 trans signaling attenuates pulmonary fibrosis in mice [
58]. This suggests that the production of soluble IL-6 receptor alpha (sIL-6Rα) in the diseased lung could contribute to IL-6 trans signaling that influences events crucial in pulmonary fibrosis. Whether the BALB/c AdIL-6+Bleo model shows increased sIL-6Rα requires further study. We have published that IL-6 can induce activated STAT3 and polarized alternatively activated phenotypes of macrophages
in vitro, and transient overexpression of IL-6 can augment the fibrotic response in combination with the Bleomycin model in C57Bl/6 mice [
37]. Collectively, this suggests that elevated IL-6 could play roles in separate models and may engage cell types including T cells and macrophages to produce an early switch that facilitates a progression to persistent fibrosis.
In the AdIL-6+Bleo BALB/c model, we observed elevated activated (CD69
+IFNγ
+) CD4
+ and CD8
+ T cells (
Figure 2C) and high IFNγ protein levels in BAL (prominent among 32 analytes) indicating activated T cells and an IFNγ signature early in the inflammatory response that was not evident in Bleo alone or AdOSM+Bleo treatments. We have published that IFNγ was undetectable in AdIL-6/Bleo-induced fibrosis in C57Bl/6 mice [
37] punctuating the difference in this model compared to that in C57Bl/6. Studies on the role of IFN-γ in mouse and human lung fibrosis give conflicting interpretations. In the Bleomycin-induced C57Bl/6 model, IFN-γ KO mice appear to be protected from early inflammatory responses [
59] and from collagen deposition at Day 21 [
60]. On the other hand, IFN-γ is known to inhibit fibroblast proliferation, collagen production and myofibroblast differentiation in human systems in vitro [
61]. However, placebo controlled clinical trial of 58 IPF patients showed no benefit of IFN-γ treatment [
62], although the authors note that the size and length of the trial may not have been sufficient to test true effects. Given that the intervention was provided in established disease, effectiveness at early incipient stages of progression is not known and is difficult to assess in humans.
It is not clear what the primary sources of IFNγ are in the present model, although activated T cells are certainly able to express it (
Figure 4 and
Figure 5), and others have published that gamma-delta T cells are a major source of IFNγ in the Bleomycin model in C57Bl/6 mice [
63]. NK and NKT (also potential sources of IFNγ) have been examined in other studies in which depletion of NK cells appears to have no effect on Bleomycin induced lung fibrosis in C57BL/6 mice [
64]. Thus, although roles of NK cells are unclear in the BALB/c AdIL-6+Bleo model here, T cell depleting antibody reduced the fibrosis (
Figure 5) without affecting activation (CD69+) of NK cells (supplemental
Figure S4) suggesting the mechanisms are independent of activated NK cells in this model.
Typical AA/M2 macrophages (CD206
+,Clec7a
+, Arginase
+) are thought to drive fibrosis in other mouse models. However in the BALB/c AdIL-6+Bleo model here there was a lack of AA/M2 macrophages induced, and instead, flow strategy (
Figure 4) has identified among macrophages (CD45
+, CD11b
+, F480
+) a selectively elevated population which is CD64
+/MHCII
hi/Clec7A
+/iNOS-Arg1-/CD206- that we term “CMC
+Macs”. Clec7A expression (Dectin-1, a receptor for beta glucans from fungus and plants) is typical of AA/M2 in both mouse and human systems [
65]. CD64 (FcR1), MHCII
hi and iNOS are typical in classically activated macrophage markers and thus these “CMC
+Macs” (Clec7A+/iNOS-) are not typically classically activated phenotype. CMC+Macs were present at day 7 and persisted to at least day 14 in lungs of mice treated with AdIL-6+Bleo. T cell depletion with anti-CD4/8 ablated both fibrosis induction (day 21) and these macrophages (Day 7, 14) in AdIL-6+Bleo treated mice. Furthermore, these cells were not present in the AdOSM+Bleo treatment, which generates fibrosis at Day 21 but then resolves. The difference in the detection of these populations between C57Bl/6 and BALB/c models is striking and may suggest multiple macrophage activation pathways can lead to fibrosis. Although we have not determined a cause-and-effect role of these CMC+Macs, the lack of typical alternatively activated macrophages in this BALB/c mouse model we believe provides an opportunity to explore new mechanisms of persistent ECM accumulation. Whether the CMC+Macs express sIL-6Receptor, and thus contribute to IL-6transignalling is not known at present. Alternatively, the fibrotic response in these BALB/c mice may not require either of these specific populations of macrophages, which would require further experimentation.
Although the publicly available data set on IPF in humans shows 737 genes induced and 378 genes reduced (RNAseq) compared to control lung tissue, which gene signals are critical for driving (or have driven and reduced after IPF diagnosis) the persistent fibrosis is not clear at this time. As expected, transcripts of various genes involved in ECM remodeling were elevated (examples Col1A1, Col3A1, elastin, MMP13, others). Of the prominent pathways shared in both AdIL-6+Bleo and Human IPF, IGF signaling is of interest. IGF is also up at Days 7-21 in the Bleo model, and IGF receptor 1 blockade reduces the fibrotic response [
66]. IGF also stimulates fibroblast differentiation to myofibroblasts on soft substrates in mouse systems in vitro [
67], and although there has been varied results as reviewed by Zhu et al. [
68] it has more recently been shown that IGF-II stimulates TGBbeta-1, collagen 3A1, and collagen post-translation modifying enzymes in primary human lung fibroblasts [
69]
Further transcriptome analysis at different time points in the model (AdIL-6/Bleo vs AdOSM/Bleo) might enable identification of signatures of inflammatory (early, Day 7) and fibrotic (day 21) than differentiate persistence (AdIl-6/Bleo day 50) vs. resolution (AdOSM/Bleo day 50). The human data is derived from bulk tissue RNA, as is the mouse data, and thus which cells are expressing which genes is largely undefined in this system at present. However, further exploration of this model should generate information more relevant to the human IPF condition compared to other models.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1-S6: title; Table S1-4: title;
Author Contributions
Individual Author contributions are as follows. Conceptualization: E.A., K.A., A.D., F.B. and CDR. Methodology, E.A., K.A., A.D., F.B., S.N., M.V., CDR. Validation, E.A., A.D., F.B. Formal analysis, E.A., A.D., F.B., S.N., Investigation, E.A., A.D., F.B., M.V., S.N.; Resources, C.D.R., M.K., K.A.; Data curation, A.D., S.N, F.B..; Original draft preparation, CDR, E.A. Review and editing, CDR, A.D., M.V., S.N., K.A.; Visualization, S.N., A.D., M.V. Supervision, CDR, M.K., K.A.; Funding acquisition, CDR, KA, M.K. All authors have read and agreed to the published version of the manuscript
Funding
Part of this study was funded by the Canadian Institutes of Health Research (CIHR; Grant Numbers. FRN 153084 and PJT 486882). Part of this study was performed with funding from the Ontario Thoracic Society (OTS) (Ontario Lung Association); EA was funded by the Canadian Institutes of Health Research (CIHR; Grant No. 140358); [MV: (Doctoral Award) Grant No. 170793; SN: (Doctoral Award) Grant No. 476552,
Institutional Review Board Statement
The animal study protocol was approved by the Animal Ethics Review Board of McMaster University AUP (Animal Utilization Protocol) #160414.
Informed Consent Statement
Publicly available Human transcriptome datasets were used in this study so informed consent is not applicable.
Data Availability Statement
Bioinformatic datasets generated by this manuscript work will be deposited in GEO, and is currently in progress.
Acknowledgments
We thank Christine Mader (Farncombe Metagenomics Facility, McMaster University, ON, Canada) for assistance with bulk RNA-seq experiments. We thank Dr Z Xing for his expertise and advice on the T cell depletion protocol, and provision of the anti-CD4 and anti-CD8 antibodies.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study’s design; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- King, T.E.; Pardo, A.; Selman, M. Idiopathic Pulmonary Fibrosis. The Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef]
- Sgalla, G.; Biffi, A.; Richeldi, L. Idiopathic Pulmonary Fibrosis: Diagnosis, Epidemiology and Natural History. Respirology 2016, 21, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, E.B.; Noble, P.W. Idiopathic Pulmonary Fibrosis. Orphanet J Rare Dis 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Ley, B.; Collard, H.R.; King, T.E. Clinical Course and Prediction of Survival in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2011, 183, 431–440. [Google Scholar] [CrossRef] [PubMed]
- du Bois, R.M. An Earlier and More Confident Diagnosis of Idiopathic Pulmonary Fibrosis. European Respiratory Review 2012, 21, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G, Masta S, Meyers D, N. A. American Review of Respiratory Disease. Am Rev Respir Dis 1989, 140, 95–100. [Google Scholar]
- Pardo, A.; Selman, M. Idiopathic Pulmonary Fibrosis: New Insights in Its Pathogenesis; 2002; Vol. 34;
- King, T.E.; Pardo, A.; Selman, M. Idiopathic Pulmonary Fibrosis. The Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.C.; Liebler, J.M.; Luby-Phelps, K.; Nicholson, A.G.; Crandall, E.D.; du Bois, R.M.; Borok, Z. Induction of Epithelial-Mesenchymal Transition in Alveolar Epithelial Cells by Transforming Growth Factor-Beta1: Potential Role in Idiopathic Pulmonary Fibrosis. Am J Pathol 2005, 166, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.A. Epithelial-Mesenchymal Interactions in Pulmonary Fibrosis. Annu Rev Physiol 2011, 73, 413–435. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, R.J.J.; Knight, D.A.; Richards, C.D.; Prêle, C.M.; Lau, H.L.; Jarnicki, A.G.; Jones, J.; Bozinovski, S.; Vlahos, R.; Thiem, S.; et al. Genetic Partitioning of Interleukin-6 Signalling in Mice Dissociates Stat3 from Smad3-Mediated Lung Fibrosis. EMBO Mol Med 2012, 4, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Botelho, F.M.; Rodrigues, R.M.; Richards, C.D. Oncostatin M Overexpression Induces Matrix Deposition, STAT3 Activation, and SMAD1 Dysregulation in Lungs of Fibrosis-Resistant BALB/c Mice. Laboratory Investigation 2014, 94, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, L.; Albera, C.; Bradford, W.Z.; Costabel, U.; du Bois, R.M.; Fagan, E.A.; Fishman, R.S.; Glaspole, I.; Glassberg, M.K.; King Jr, T.E.; et al. Safety of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis: Integrated Analysis of Cumulative Data from 5 Clinical Trials. BMJ Open Respir Res 2016, 3, e000105–e000105. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. New England Journal of Medicine 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Wollin, L.; Wex, E.; Pautsch, A.; Schnapp, G.; Hostettler, K.E.; Stowasser, S.; Kolb, M. Mode of Action of Nintedanib in the Treatment of Idiopathic Pulmonary Fibrosis. Eur Respir J 2015, 45, 1434–1445. [Google Scholar] [CrossRef]
- Raghu, G.; Selman, M. Nintedanib and Pirfenidone-New Antifibrotic Treatments Indicated for Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2015, 191, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, J.; Rubio, G.A.; Limper, A.H.; Williams, K.; Elliot, S.J.; Ninou, I.; Aidinis, V.; Tzouvelekis, A.; Glassberg, M.K. Exploring Animal Models That Resemble Idiopathic Pulmonary Fibrosis. Front Med (Lausanne) 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.B.; Hogaboam, C.M. Murine Models of Pulmonary Fibrosis. American Journal of Physiology-Lung Cellular and Molecular Physiology 2008, 294, L152–L160. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.; Ask, K.; Warburton, D.; Gauldie, J.; Kolb, M. The Bleomycin Animal Model: A Useful Tool to Investigate Treatment Options for Idiopathic Pulmonary Fibrosis? Int J Biochem Cell Biol 2008, 40, 362–382. [Google Scholar] [CrossRef] [PubMed]
- Kolb, P.; Upagupta, C.; Vierhout, M.; Ayaub, E.; Bellaye, P.S.; Gauldie, J.; Shimbori, C.; Inman, M.; Ask, K.; Kolb, M.R.J. The Importance of Interventional Timing in the Bleomycin Model of Pulmonary Fibrosis. Eur Respir J 2020, 55. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.H.; Lazo, J.S. High Dose Continuous Infusion of Bleomycin in Mice: A New Model for Drug-Induced Pulmonary Fibrosis. Journal of Pharmacology and Experimental Therapeutics 1987, 243, 1185, LP – 1194. [Google Scholar] [PubMed]
- Schrier, D.J.; Kunkel, R.G.; Phan, S.H. The Role of Strain Variation in Murine Bleomycin-Lnduced Pulmonary Fibrosis. American Review of Respiratory Disease 1983, 127, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Ask, K.; Labiris, R.; Farkas, L.; Moeller, A.; Froese, A.; Farncombe, T.; McClelland, G.B.; Inman, M.; Gauldie, J.; Kolb, M.R.J. Comparison between Conventional and “Clinical” Assessment of Experimental Lung Fibrosis. J Transl Med 2008, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Ayaub, E.A.; Dubey, A.; Imani, J.; Botelho, F.; Kolb, M.R.J.; Richards, C.D.; Ask, K. Overexpression of OSM and IL-6 Impacts the Polarization of pro-Fibrotic Macrophages and the Development of Bleomycin-Induced Lung Fibrosis. Sci Rep 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Taga, T.; Kishimoto, T. Gp130 AND THE INTERLEUKIN-6 FAMILY OF CYTOKINES. Annu Rev Immunol 1997, 15, 797–819. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Therapeutic Targeting of the Interleukin-6 Receptor. Annu Rev Pharmacol Toxicol 2012, 52, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.S.; Hunter, C.A. Gp130 at the Nexus of Inflammation, Autoimmunity, and Cancer. J Leukoc Biol 2010, 88, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Braciak, T.; Jordana, M.; Croitoru, K.; Graham, F.L.; Gauldie, J. Adenovirus-Mediated Cytokine Gene Transfer at Tissue Sites. Overexpression of IL-6 Induces Lymphocytic Hyperplasia in the Lung. The Journal of Immunology 1994, 153, 4059, LP – 4069. [Google Scholar] [CrossRef]
- Mauer, J.; Chaurasia, B.; Goldau, J.; Vogt, M.C.; Ruud, J.; Nguyen, K.D.; Theurich, S.; Hausen, A.C.; Schmitz, J.; Brönneke, H.S.; et al. Signaling by IL-6 Promotes Alternative Activation of Macrophages to Limit Endotoxemia and Obesity-Associated Resistance to Insulin. Nat Immunol 2014, 15, 423–430. [Google Scholar] [CrossRef] [PubMed]
- West, N.R. Coordination of Immune-Stroma Crosstalk by IL-6 Family Cytokines. Front Immunol 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Dienz, O.; Rincon, M. The Effects of IL-6 on CD4 T Cell Responses. Clin Immunol 2009, 130, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ridgley, L.A.; Anderson, A.E.; Maney, N.J.; Naamane, N.; Skelton, A.J.; Lawson, C.A.; Emery, P.; Isaacs, J.D.; Carmody, R.J.; Pratt, A.G. IL-6 Mediated Transcriptional Programming of Naïve CD4+ T Cells in Early Rheumatoid Arthritis Drives Dysregulated Effector Function. Front Immunol 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jones, L.L.; Geiger, T.L. IL-6 Promotes T Cell Proliferation and Expansion under Inflammatory Conditions in Association with Low-Level RORγt Expression. The Journal of Immunology 2018, 201, 2934–2946. [Google Scholar] [CrossRef] [PubMed]
- De Lauretis, A.; Sestini, P.; Pantelidis, P.; Hoyles, R.; Hansell, D.M.; Goh, N.S.L.; Zappala, C.J.; Visca, D.; Maher, T.M.; Denton, C.P.; et al. Serum Interleukin 6 Is Predictive of Early Functional Decline and Mortality in Interstitial Lung Disease Associated with Systemic Sclerosis. J Rheumatol 2013, 40, 435, LP – 446. [Google Scholar] [CrossRef] [PubMed]
- Collard, H.R.; Calfee, C.S.; Wolters, P.J.; Song, J.W.; Hong, S.-B.; Brady, S.; Ishizaka, A.; Jones, K.D.; King Jr, T.E.; Matthay, M.A.; et al. Plasma Biomarker Profiles in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Am J Physiol Lung Cell Mol Physiol 2010, 299, L3–L7. [Google Scholar] [CrossRef]
- Wong, S.; Botelho, F.M.; Rodrigues, R.M.; Richards, C.D. Oncostatin M Overexpression Induces Matrix Deposition, STAT3 Activation, and SMAD1 Dysregulation in Lungs of Fibrosis-Resistant BALB/c Mice. Laboratory Investigation 2014, 94, 1003–1016. [Google Scholar] [CrossRef]
- Ayaub, E.A.; Dubey, A.; Imani, J.; Botelho, F.; Kolb, M.R.J.; Richards, C.D.; Ask, K. Overexpression of OSM and IL-6 Impacts the Polarization of pro-Fibrotic Macrophages and the Development of Bleomycin-Induced Lung Fibrosis. Sci Rep 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Yao, Y.; Jeyanathan, M.; Haddadi, S.; Barra, N.G.; Vaseghi-Shanjani, M.; Damjanovic, D.; Lai, R.; Afkhami, S.; Chen, Y.; Dvorkin-Gheva, A.; et al. Induction of Autonomous Memory Alveolar Macrophages Requires T Cell Help and Is Critical to Trained Immunity. Cell 2018, 175, 1634–1650.e17. [Google Scholar] [CrossRef]
- Botelho, F.M.; Rangel-Moreno, J.; Fritz, D.; Randall, T.D.; Xing, Z.; Richards, C.D. Pulmonary Expression of Oncostatin M (OSM) Promotes Inducible BALT Formation Independently of IL-6, Despite a Role for IL-6 in OSM-Driven Pulmonary Inflammation. The Journal of Immunology 2013, 191, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Mekhael O; Naiel S; Vierhout M; Hayat AI; Revill SD; Abed S; Inman MD; Kolb MRJ; Ask K. Mouse Models of Lung Fibrosis. Methods Mol Biol. 2021, 2299, 291–321. [Google Scholar]
- Botelho, F.M.; Rodrigues, R.; Guerette, J.; Wong, S.; Fritz, D.K.; Richards, C.D. Extracellular Matrix and Fibrocyte Accumulation in BALB/c Mouse Lung upon Transient Overexpression of Oncostatin M. Cells 2019, 8, 126. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate Alignment of Transcriptomes in the Presence of Insertions, Deletions and Gene Fusions. Genome Biol 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq-A Python Framework to Work with High-Throughput Sequencing Data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.J.; Chen, Y.; Smyth, G.K. Differential Expression Analysis of Multifactor RNA-Seq Experiments with Respect to Biological Variation. Nucleic Acids Res 2012, 40, 4288–4297. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Oshlack, A. A Scaling Normalization Method for Differential Expression Analysis of RNA-Seq Data; 2010;
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision Weights Unlock Linear Model Analysis Tools for RNA-Seq Read Counts; 2014; Vol. 15;
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Benjamini_and_Hochberg_1995.
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles; 2005;
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING Database in 2021: Customizable Protein-Protein Networks, and Functional Characterization of User-Uploaded Gene/Measurement Sets. Nucleic Acids Res 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI Gene Expression and Hybridization Array Data Repository; 2002; Vol. 30;
- DePianto, D.J.; Chandriani, S.; Abbas, A.R.; Jia, G.; N’Diaye, E.N.; Caplazi, P.; Kauder, S.E.; Biswas, S.; Karnik, S.K.; Ha, C.; et al. Heterogeneous Gene Expression Signatures Correspond to Distinct Lung Pathologies and Biomarkers of Disease Severity in Idiopathic Pulmonary Fibrosis. Thorax 2015, 70, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular Senescence Mediates Fibrotic Pulmonary Disease. Nat Commun 2017, 8. [Google Scholar] [CrossRef]
- De Lauretis, A.; Sestini, P.; Pantelidis, P.; Hoyles, R.; Hansell, D.M.; Goh, N.S.L.; Zappala, C.J.; Visca, D.; Maher, T.M.; Denton, C.P.; et al. Serum Interleukin 6 Is Predictive of Early Functional Decline and Mortality in Interstitial Lung Disease Associated with Systemic Sclerosis. J Rheumatol 2013, 40, 435, LP – 446. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Lin, C.J.F.; Furst, D.E.; Goldin, J.; Kim, G.; Kuwana, M.; Allanore, Y.; Matucci-Cerinic, M.; Distler, O.; Shima, Y.; et al. Tocilizumab in Systemic Sclerosis: A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Respir Med 2020, 8, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Tanaka, K.; Fujita, T.; Umezawa, H.; Amano, H.; Yoshioka, K.; Naito, Y.; Hatano, M.; Kimura, S.; Tatsumi, K.; et al. Bidirectional Role of IL-6 Signal in Pathogenesis of Lung Fibrosis. Respir Res 2015, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-T.T.; Karmouty-Quintana, H.; Melicoff, E.; Le, T.-T.T.; Weng, T.; Chen, N.-Y.; Pedroza, M.; Zhou, Y.; Davies, J.; Philip, K.; et al. Blockade of IL-6 Trans Signaling Attenuates Pulmonary Fibrosis. The Journal of Immunology 2014, 193, 3755–3768. [Google Scholar] [CrossRef] [PubMed]
- Segel, M.J.; Izbicki, G.; Cohen, P.Y.; Or, R.; Christensen, T.G.; Wallach-Dayan, S.B.; Breuer, R. Role of Interferon-in the Evolution of Murine Bleomycin Lung Fibrosis. Am J Physiol Lung Cell Mol Physiol 2003, 285, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.S.; Greenlee, B.M.; Wills-Karp, M.; Moller, D.R. Attenuation of Lung Inflammation and Fibrosis in Interferon-Deficient Mice after Intratracheal Bleomycin; 2001; Vol. 24;
- Vu, T.N.; Chen, X.; Foda, H.D.; Smaldone, G.C.; Hasaneen, N.A. Interferon-Γenhances the Antifibrotic Effects of Pirfenidone by Attenuating IPF Lung Fibroblast Activation and Differentiation. Respir Res 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Brown, K.K.; Bradford, W.Z.; Starko, K.; Noble, P.W.; Schwartz, D.A.; King, T.E. A Placebo-Controlled Trial of Interferon Gamma-1b in Patients with Idiopathic Pulmonary Fibrosis; 2004; Vol. 350;
- Segawa, S.; Goto, D.; Iizuka, A.; Kaneko, S.; Yokosawa, M.; Kondo, Y.; Matsumoto, I.; Sumida, T. The Regulatory Role of Interferon-γ Producing Gamma Delta T Cells via the Suppression of T Helper 17 Cell Activity in Bleomycin-Induced Pulmonary Fibrosis. Clin Exp Immunol 2016, 185, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Monnier, J.; Zabel, B.A. Anti-Asialo GM1 NK Cell Depleting Antibody Does Not Alter the Development of Bleomycin Induced Pulmonary Fibrosis. PLoS One 2014, 9, e99350–e99350. [Google Scholar] [CrossRef] [PubMed]
- El Taweel, M.; Walker, H.; Mekhael, O.; Vierhout, M.; Padwal, M.; Naiel, S.; Patel, H.; Parthasarathy, P.; Imani, J.; Ayaub, E.; et al. Dectin-1 Is a Marker of Alternatively Activated Macrophages and a Therapeutic Target in Interstitial Fibrotic Lung Diseases. In C64. PULMONARY FIBROSIS MODELS AND MECHANISTIC INSIGHTS; American Thoracic Society International Conference Abstracts; American Thoracic Society, 2019; pp. A5403–A5403.
- Choi, J.E.; Lee, S.S.; Sunde, D.A.; Huizar, I.; Haugk, K.L.; Thannickal, V.J.; Vittal, R.; Plymate, S.R.; Schnapp, L.M. Insulin-Like Growth Factor-i Receptor Blockade Improves Outcome in Mouse Model of Lung Injury. Am J Respir Crit Care Med 2009, 179, 212–219. [Google Scholar] [CrossRef]
- Hung, C.F.; Rohani, M.G.; Lee, S. soon; Chen, P.; Schnapp, L.M. Role of IGF-1 Pathway in Lung Fibroblast Activation. Respir Res 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, L.; Song, B.; Cui, Z.; Chen, G.; Yu, Z.; Song, B. Insulin-like Growth Factor-2 (IGF-2) in Fibrosis. Biomolecules 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Waldrep, K.M.; Rodgers, J.I.; Garrett, S.M.; Wolf, B.J.; Feghali-Bostwick, C.A. The Role of SOX9 in IGF-II-Mediated Pulmonary Fibrosis. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Mice treated with Advectors expressing OSM or IL-6 in combination with Bleomycin induces fibrosis at Day 21 in BALB/c mice. Female BALB/c mice received 2x107PFU/ mouse of either control adenoviral vector (AdDL70), Advector expressing OSM (AdOSM) or IL-6 (AdIL6) alone or each of the 3 vectors in combination with 0.03 Units of bleomycin by intratracheal administration. Lung function and histological assessment were performed at 21 days. A) Representative images of histological serial sections of lung parenchyma stained with Masson’s Trichrome (Upper and middle panels) or αSMA (Lower panels). B) Quantification of fibrotic score (Ashcroft) and αSMA and (C) Quasistatic Elastance (Est) derived on PV-loops (flexiVent), n = 5 mice per group, results expressed as mean ± SEM. Difference between groups were compared using Dunnett’s non-parametric ANOVA. *p<0.05, **p<0.01, ***p<0.001. D) Quantification of Hydroxyproline in whole right lungs and (E) Quantification of ASMA imunohisto-staining using HALO image analysis.
Figure 1.
Mice treated with Advectors expressing OSM or IL-6 in combination with Bleomycin induces fibrosis at Day 21 in BALB/c mice. Female BALB/c mice received 2x107PFU/ mouse of either control adenoviral vector (AdDL70), Advector expressing OSM (AdOSM) or IL-6 (AdIL6) alone or each of the 3 vectors in combination with 0.03 Units of bleomycin by intratracheal administration. Lung function and histological assessment were performed at 21 days. A) Representative images of histological serial sections of lung parenchyma stained with Masson’s Trichrome (Upper and middle panels) or αSMA (Lower panels). B) Quantification of fibrotic score (Ashcroft) and αSMA and (C) Quasistatic Elastance (Est) derived on PV-loops (flexiVent), n = 5 mice per group, results expressed as mean ± SEM. Difference between groups were compared using Dunnett’s non-parametric ANOVA. *p<0.05, **p<0.01, ***p<0.001. D) Quantification of Hydroxyproline in whole right lungs and (E) Quantification of ASMA imunohisto-staining using HALO image analysis.
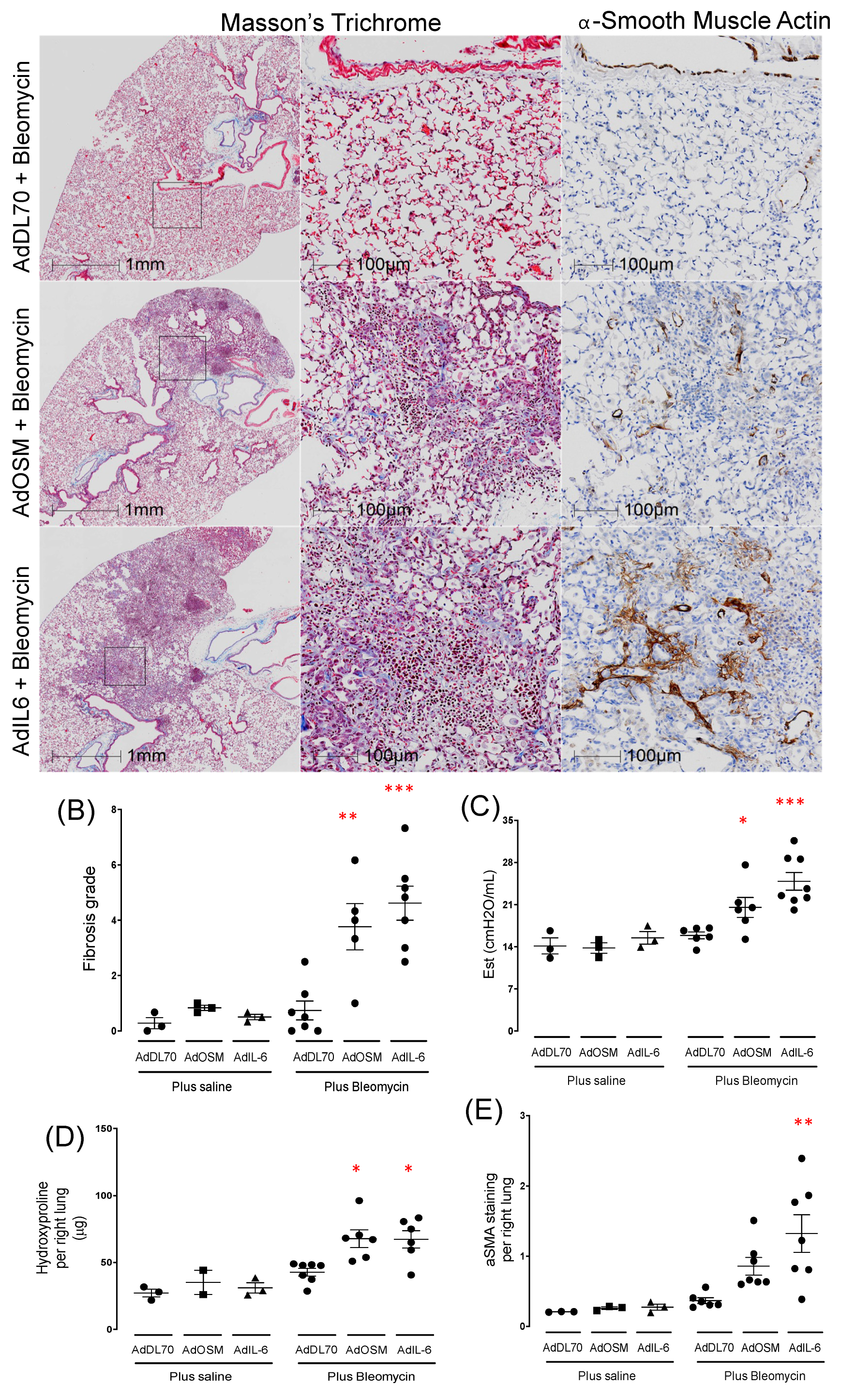
Figure 2.
Sustained ECM deposition at Day 50 in BALB/c mice treated with bleomycin plus AdIL-6. BALB/c mice were treated with AdDL70 control vector, or AdIL-6 (1x107 PFU/mouse), alone or in combination with bleomycin (0.03U) (n=4/group). (A) Representative images of lung tissue (AdDL70+Bleo compared to AdIL-6+Bleo) stained for Masson’s Trichrome at Day 50. (B) Representative images of Picrosirius red staining at Day 21 and 50 from AdDL70+Bleo and AdIL-6+Bleo treated animals. (B) Fibrotic grade observed at Day 50 and (C)) ASMA staining at Day 50 of AdDel70+Bleo and AdIL-6+Bleo treated mice. Results expressed as mean ± SEM and differences between groups were compared using Dunnett’s non-parametric ANOVA. *p<0.05.
Figure 2.
Sustained ECM deposition at Day 50 in BALB/c mice treated with bleomycin plus AdIL-6. BALB/c mice were treated with AdDL70 control vector, or AdIL-6 (1x107 PFU/mouse), alone or in combination with bleomycin (0.03U) (n=4/group). (A) Representative images of lung tissue (AdDL70+Bleo compared to AdIL-6+Bleo) stained for Masson’s Trichrome at Day 50. (B) Representative images of Picrosirius red staining at Day 21 and 50 from AdDL70+Bleo and AdIL-6+Bleo treated animals. (B) Fibrotic grade observed at Day 50 and (C)) ASMA staining at Day 50 of AdDel70+Bleo and AdIL-6+Bleo treated mice. Results expressed as mean ± SEM and differences between groups were compared using Dunnett’s non-parametric ANOVA. *p<0.05.
Figure 3.
Cytokine profile in the bronchoalveolar lavage fluid of BALB/c mice following overexpression of OSM or IL-6 with concomitant bleomycin-induced lung injury. BALB/c mice were intubated with AdDL70, AdOSM or AdIL-6, alone or in combination with bleomycin (0.03U) for 7 days (n=3-6 mice /group). Levels of (A) IL-6, (B) OSM, (C) IP-10, (D) MCP-1, (E) MIG, (F) MIP1a, (G) IFNγ and (H) GCSF in the BALF as measured by ELISA are shown. Statistics were completed using One-way ANOVA and Newman-Keuls post-hoc analysis, where p*<0.05, p**<0.01, p***<0.001, p****<0.0001 compared to AdDL70+Bleo control.
Figure 3.
Cytokine profile in the bronchoalveolar lavage fluid of BALB/c mice following overexpression of OSM or IL-6 with concomitant bleomycin-induced lung injury. BALB/c mice were intubated with AdDL70, AdOSM or AdIL-6, alone or in combination with bleomycin (0.03U) for 7 days (n=3-6 mice /group). Levels of (A) IL-6, (B) OSM, (C) IP-10, (D) MCP-1, (E) MIG, (F) MIP1a, (G) IFNγ and (H) GCSF in the BALF as measured by ELISA are shown. Statistics were completed using One-way ANOVA and Newman-Keuls post-hoc analysis, where p*<0.05, p**<0.01, p***<0.001, p****<0.0001 compared to AdDL70+Bleo control.
Figure 4.
AdIL-6+Bleo induces T cell activation and CD64+MHChi Macrophage activation at Day 7 BALB/c mice were intubated with AdDL70 or AdIL-6 alone or in combination with bleomycin (0.03U) for 7 days (n=3-6 mice /group). A) Flow cytometry strategy is summarized. B) Levels of CD69+ CD4 and CD8+ T cells are shown. C) Upper panels show representative Flow plots of the four groups which are quantified on lower left panel. The lower middle panel shows the signal distribution of CD64 of the four groups and lower right panel quantifies the MFI of the MHCII of the CD64+MHChi macrophages.
Figure 4.
AdIL-6+Bleo induces T cell activation and CD64+MHChi Macrophage activation at Day 7 BALB/c mice were intubated with AdDL70 or AdIL-6 alone or in combination with bleomycin (0.03U) for 7 days (n=3-6 mice /group). A) Flow cytometry strategy is summarized. B) Levels of CD69+ CD4 and CD8+ T cells are shown. C) Upper panels show representative Flow plots of the four groups which are quantified on lower left panel. The lower middle panel shows the signal distribution of CD64 of the four groups and lower right panel quantifies the MFI of the MHCII of the CD64+MHChi macrophages.
Figure 5.
CD4/CD8 T cell depletion reduces fibrosis at Day 21. (A-D) BALB/c mice were treated with AdIL-6+Bleo and flow cytometry was used at day 14 to examine T cell activation at Day 14. (E-J) BALB/C mice were treated with AdIL-6+Bleo and ip injections of anti-CD4 and anti-CD8 or isotype control Ab as described in Methods. E) Flow cytometry of % of CD4 and CD8 T cells in lung. (G) Representative images of lung sections at Day 21 stained with Trichrome (upper right panel) and SMA (lower right panel) of control antibody treated mice and H) the same representative images of Anti-CD4/8 treated mice. I) Elastance at Day 21 (J) Flow cytometry showing the % of MHCIIhiCD64+ population at Day 14. # p<0.05, ### <,0.01 compared to Control Antibody.
Figure 5.
CD4/CD8 T cell depletion reduces fibrosis at Day 21. (A-D) BALB/c mice were treated with AdIL-6+Bleo and flow cytometry was used at day 14 to examine T cell activation at Day 14. (E-J) BALB/C mice were treated with AdIL-6+Bleo and ip injections of anti-CD4 and anti-CD8 or isotype control Ab as described in Methods. E) Flow cytometry of % of CD4 and CD8 T cells in lung. (G) Representative images of lung sections at Day 21 stained with Trichrome (upper right panel) and SMA (lower right panel) of control antibody treated mice and H) the same representative images of Anti-CD4/8 treated mice. I) Elastance at Day 21 (J) Flow cytometry showing the % of MHCIIhiCD64+ population at Day 14. # p<0.05, ### <,0.01 compared to Control Antibody.
Figure 6.
Transcriptomic profile of bulk RNAseq of AdIL-6+Bleo model at Day 7 and Day 21. BALB/c mice (n=3 per group) were treated as described previously in four groups: (i) AdDL70+Bleo Day 7; (ii) AdIL-6+Bleo at day 7; (iii) AdDL70+Bleo at Day 21; (iv) AdIL-6+Bleo at Day 21. Total lung RNA was extracted and bulk RNAseq data was processed as described in the methods. A) shows a PCA plot including each sample using all detectable genes analyzed of the four different groups. B) shows a heatmap of expression level of DE genes with an absolute fold change of at least 1.5 in at least 1 out of the 3 comparisons [(AdIL-6Bleo - AdDL70, for both day 7 and day 21), and (IL-621d – IL-67d) - (AdDL21d - AdDL7d)], in each of the samples of the four groups. C) shows a volcano plot visualizing DE genes for comparison of (AdIL-6 21 days vs. AdDL70 21 days) vs. (AdIL-6 7 days vs. AdDL70 7 days). D) Pathway analysis showing top 25 upregulated pathways (many relating to collagen/ECM reorganization).
Figure 6.
Transcriptomic profile of bulk RNAseq of AdIL-6+Bleo model at Day 7 and Day 21. BALB/c mice (n=3 per group) were treated as described previously in four groups: (i) AdDL70+Bleo Day 7; (ii) AdIL-6+Bleo at day 7; (iii) AdDL70+Bleo at Day 21; (iv) AdIL-6+Bleo at Day 21. Total lung RNA was extracted and bulk RNAseq data was processed as described in the methods. A) shows a PCA plot including each sample using all detectable genes analyzed of the four different groups. B) shows a heatmap of expression level of DE genes with an absolute fold change of at least 1.5 in at least 1 out of the 3 comparisons [(AdIL-6Bleo - AdDL70, for both day 7 and day 21), and (IL-621d – IL-67d) - (AdDL21d - AdDL7d)], in each of the samples of the four groups. C) shows a volcano plot visualizing DE genes for comparison of (AdIL-6 21 days vs. AdDL70 21 days) vs. (AdIL-6 7 days vs. AdDL70 7 days). D) Pathway analysis showing top 25 upregulated pathways (many relating to collagen/ECM reorganization).
Figure 7.
Similarity of the BALB/c AdIL-6+Bleo transcriptome to Human IPF transcriptome. A) Numbers of DE genes found to be shared between each of the models and each of the human datasets. B) Heatmap exhibiting correlation between each of the animal datasets and the human datasets. “Human” set presented in the heatmap is a composite of both human IPF datasets. C) GSEA showing significant enrichment of human IPF datasets in our AdIL-6+Bleo model (Day 7 and 21). No enrichment was found at Day 7. Adjusted p-values for enrichment are presented. Only plots for significant results are shown. D) Venn diagrams showing the numbers of pathways found to be significantly regulated in the AdIL6+Bleo model (Day 7 and 21) and in the IPF cohorts. Quantity of overlapping pathways regulated in the AdIL-6+Bleo model are marked by various shades of magenta (upregulation) or blue (downregulation), with darker shades indicating intersections between a higher number of datasets (from 1 to 4). Letters refer to the lists of overlapping pathways, which can be found in
Supplementary Table S4.
Figure 7.
Similarity of the BALB/c AdIL-6+Bleo transcriptome to Human IPF transcriptome. A) Numbers of DE genes found to be shared between each of the models and each of the human datasets. B) Heatmap exhibiting correlation between each of the animal datasets and the human datasets. “Human” set presented in the heatmap is a composite of both human IPF datasets. C) GSEA showing significant enrichment of human IPF datasets in our AdIL-6+Bleo model (Day 7 and 21). No enrichment was found at Day 7. Adjusted p-values for enrichment are presented. Only plots for significant results are shown. D) Venn diagrams showing the numbers of pathways found to be significantly regulated in the AdIL6+Bleo model (Day 7 and 21) and in the IPF cohorts. Quantity of overlapping pathways regulated in the AdIL-6+Bleo model are marked by various shades of magenta (upregulation) or blue (downregulation), with darker shades indicating intersections between a higher number of datasets (from 1 to 4). Letters refer to the lists of overlapping pathways, which can be found in
Supplementary Table S4.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).