Submitted:
29 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. State-of-the-Art Cooperative Sensors
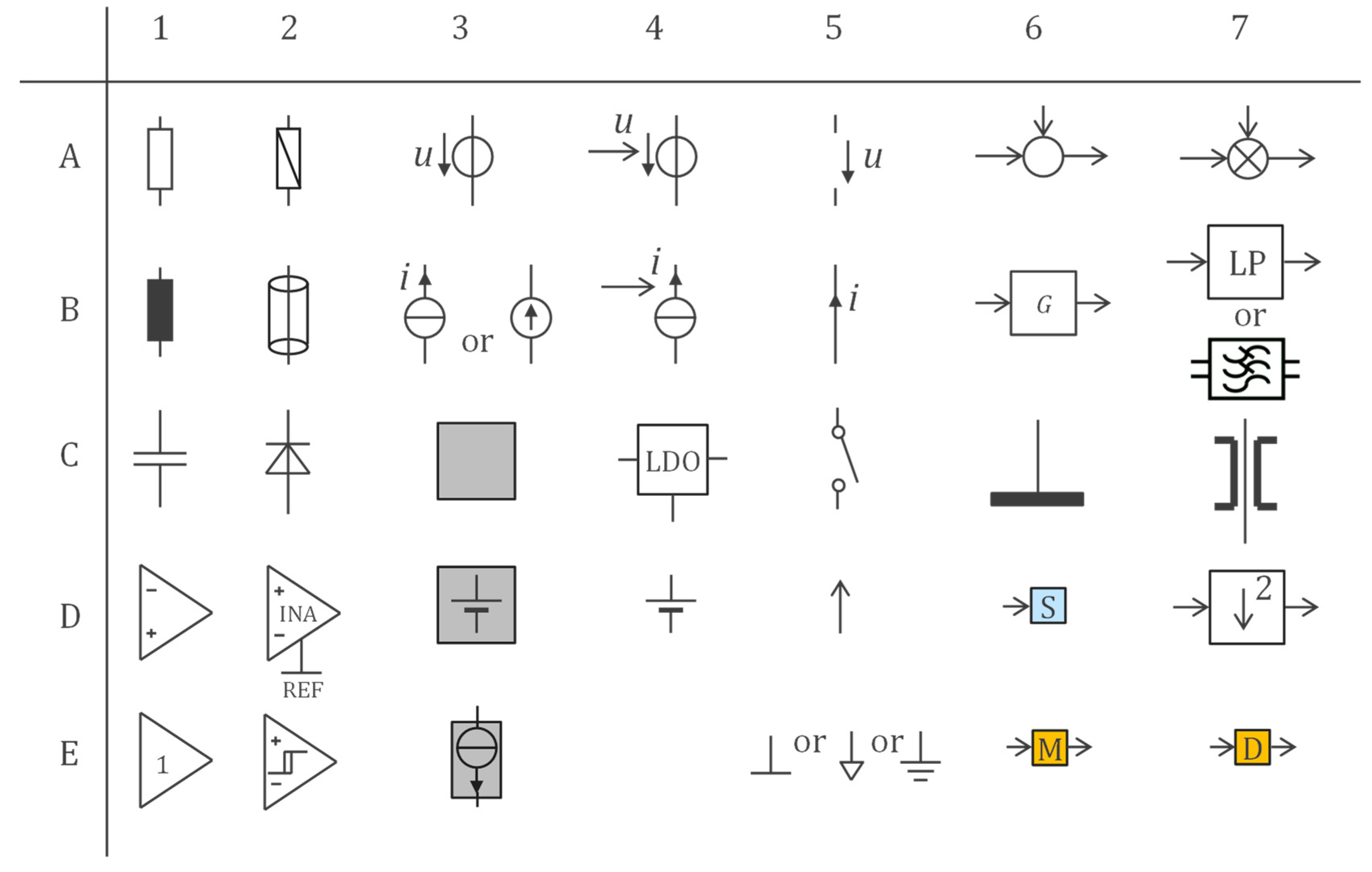
2.1. Basic Circuits and Their Interconnections
2.2. Floating Supply, Bootstrapping, Current Source, and Current/Potential Wire Separation
3. Method
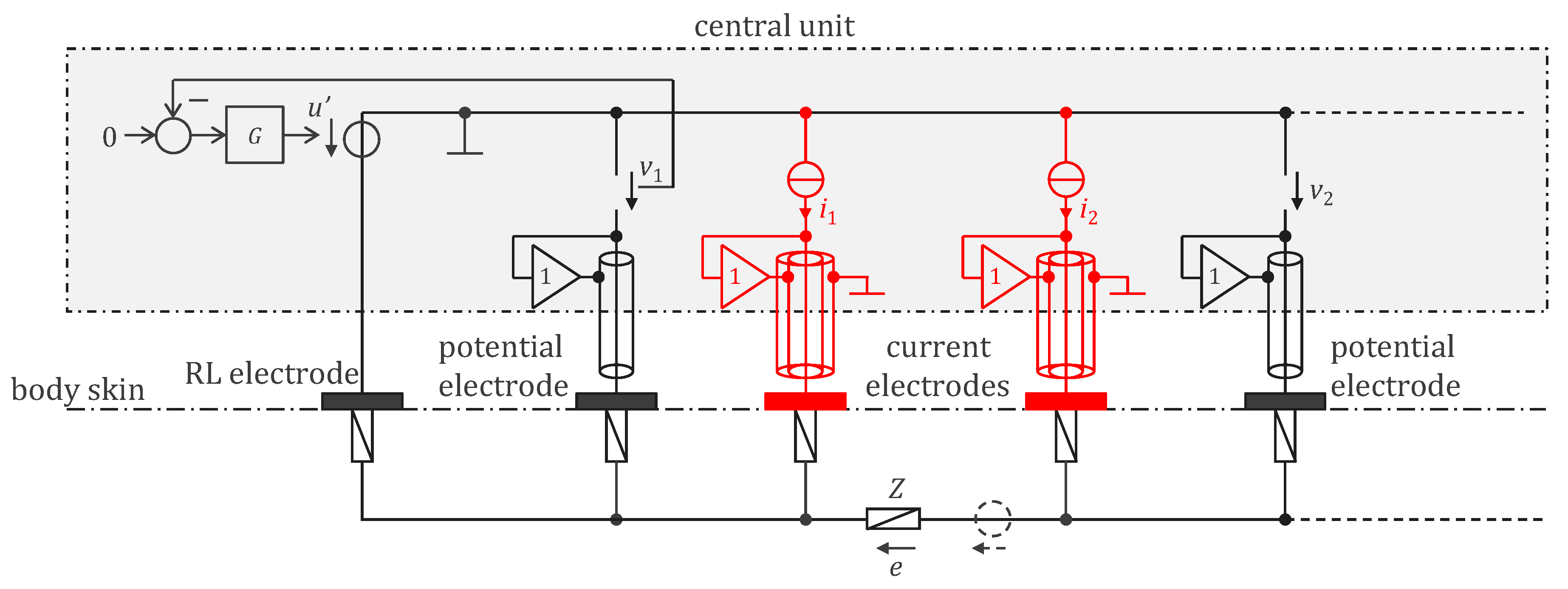
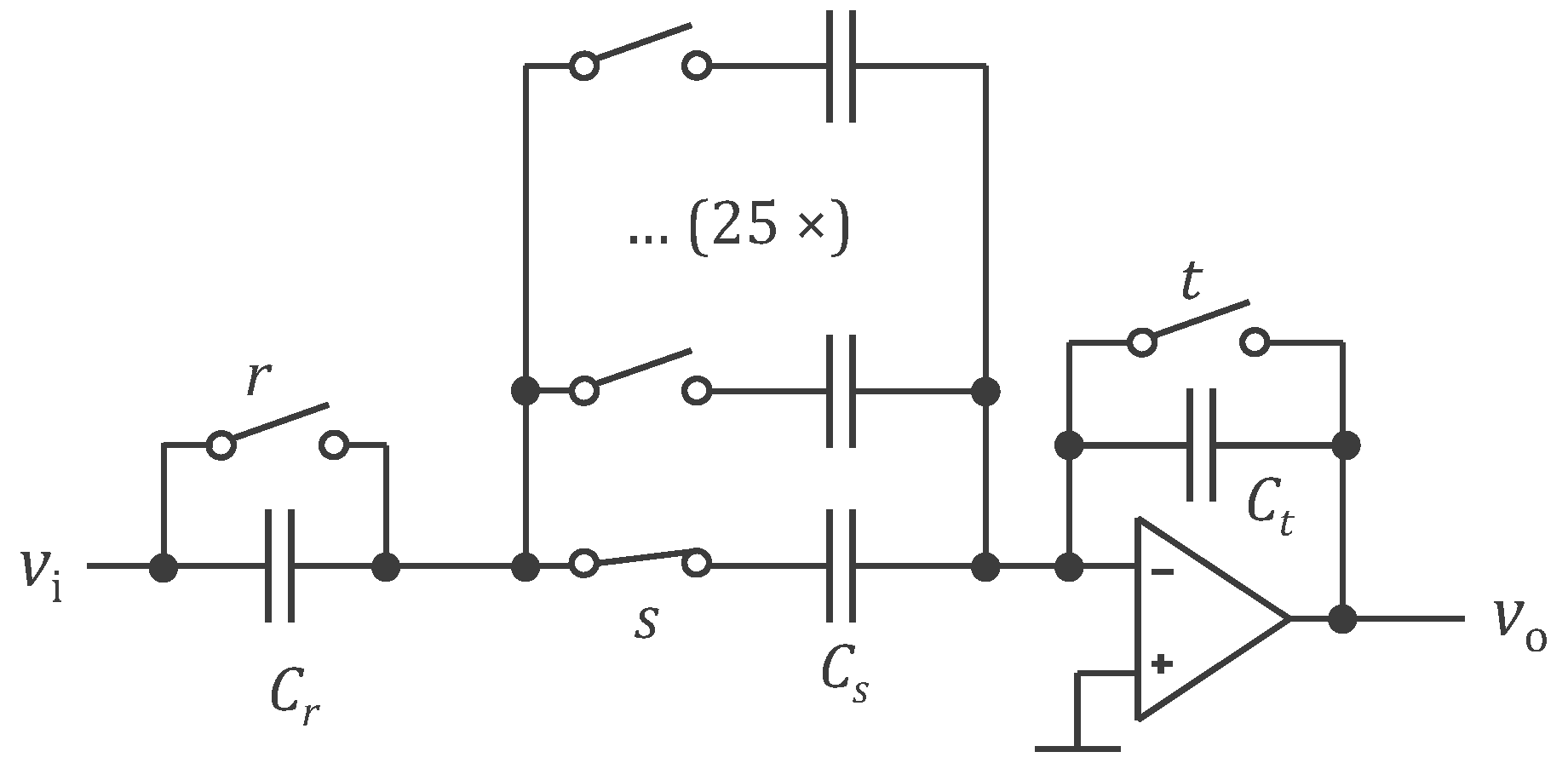
3.1. Legacy Approach with 500 Hz Powering and Off-the-Shelf Components
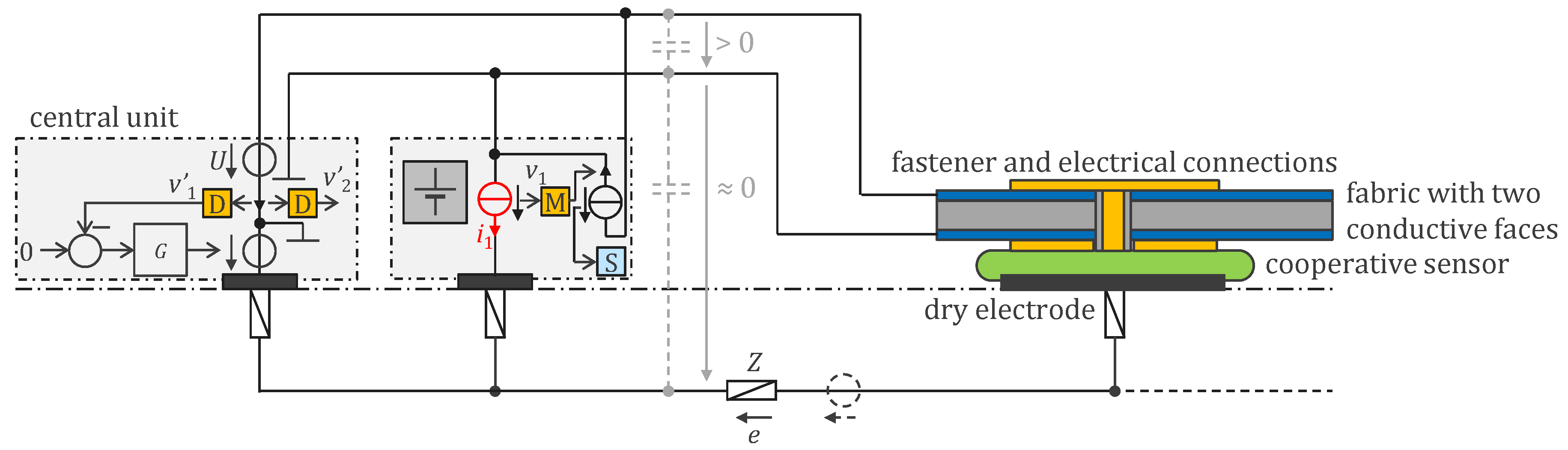
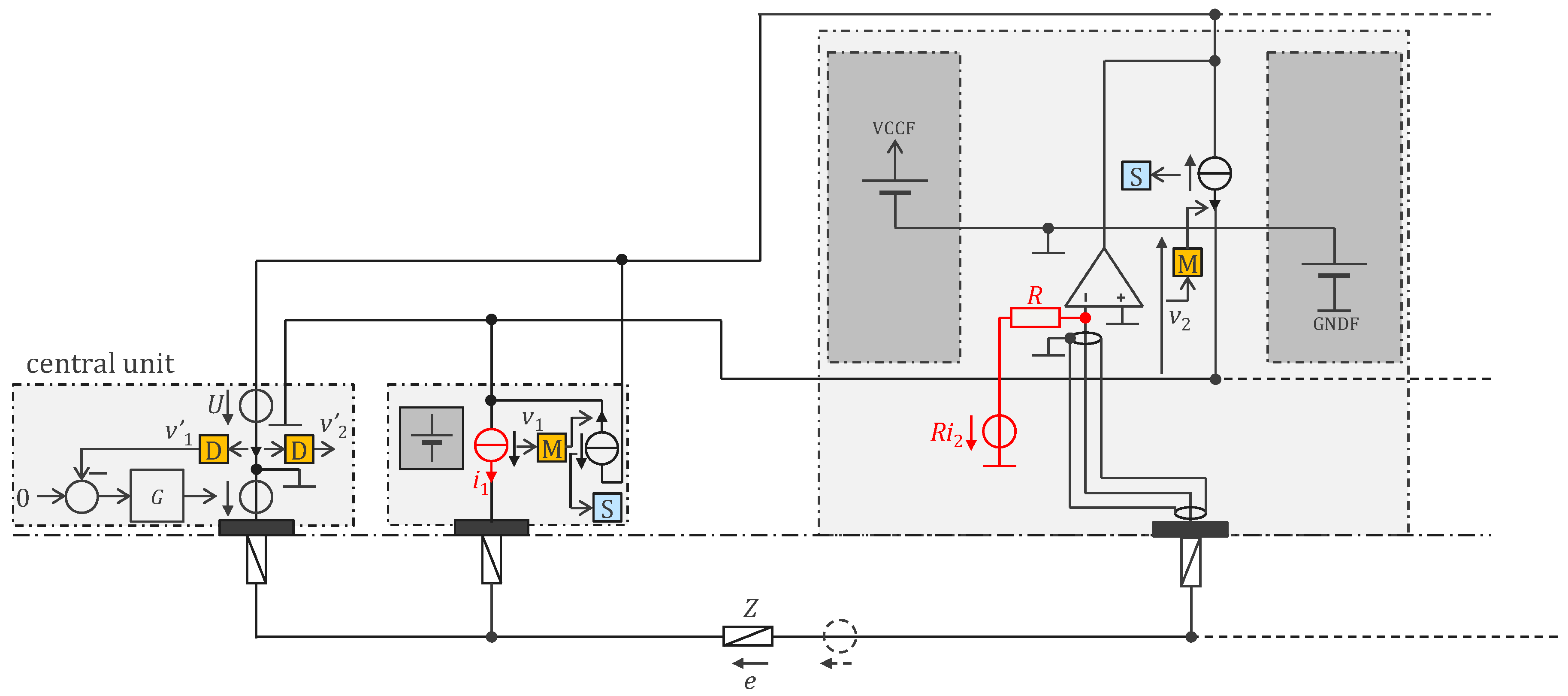
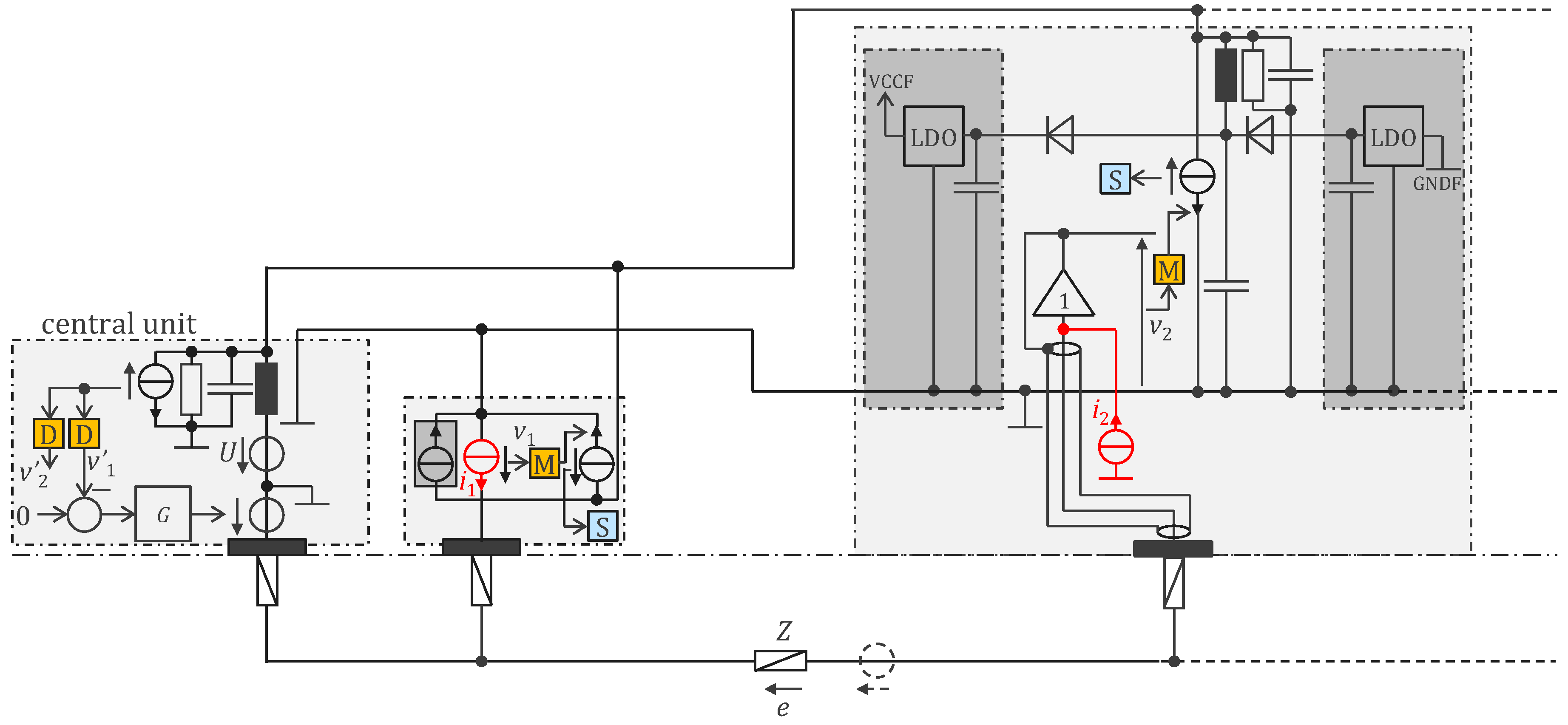
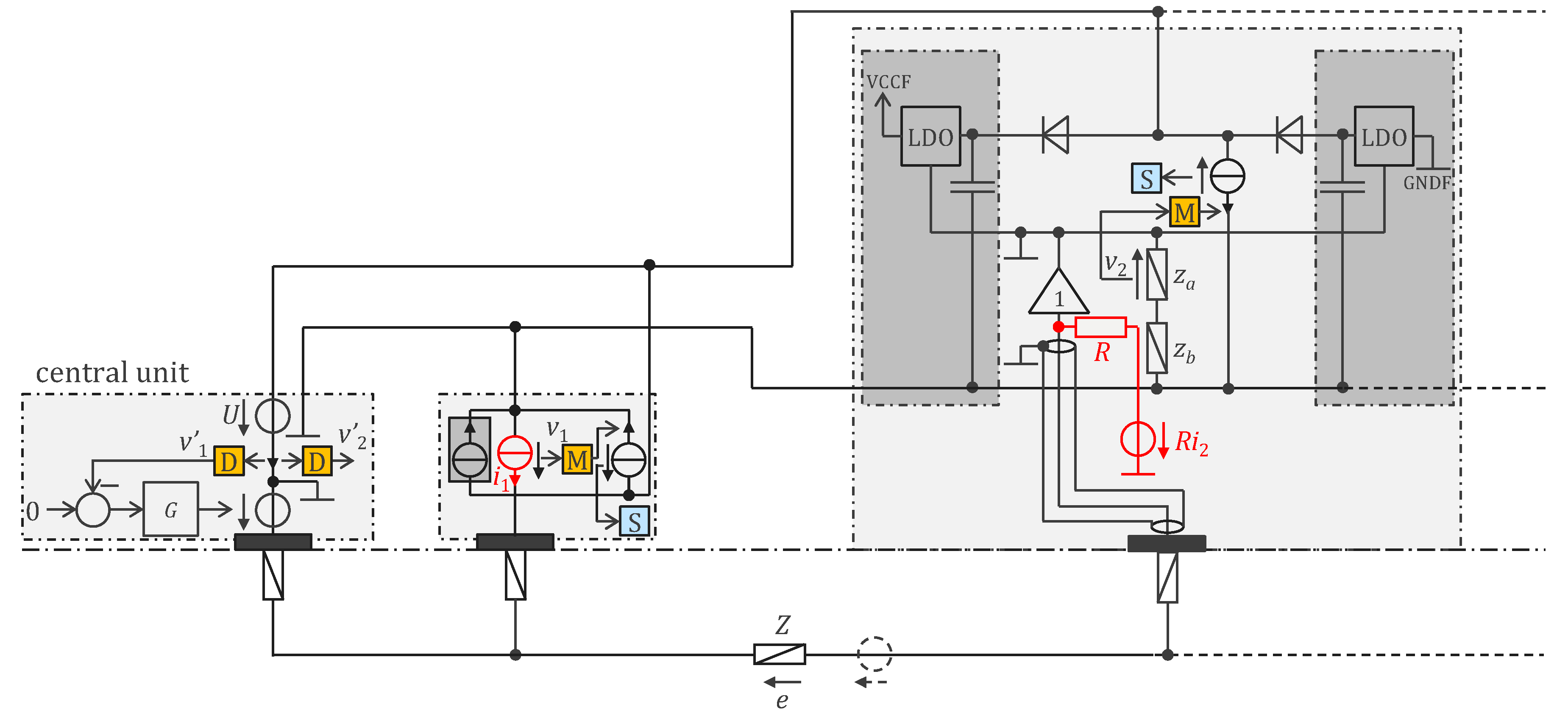
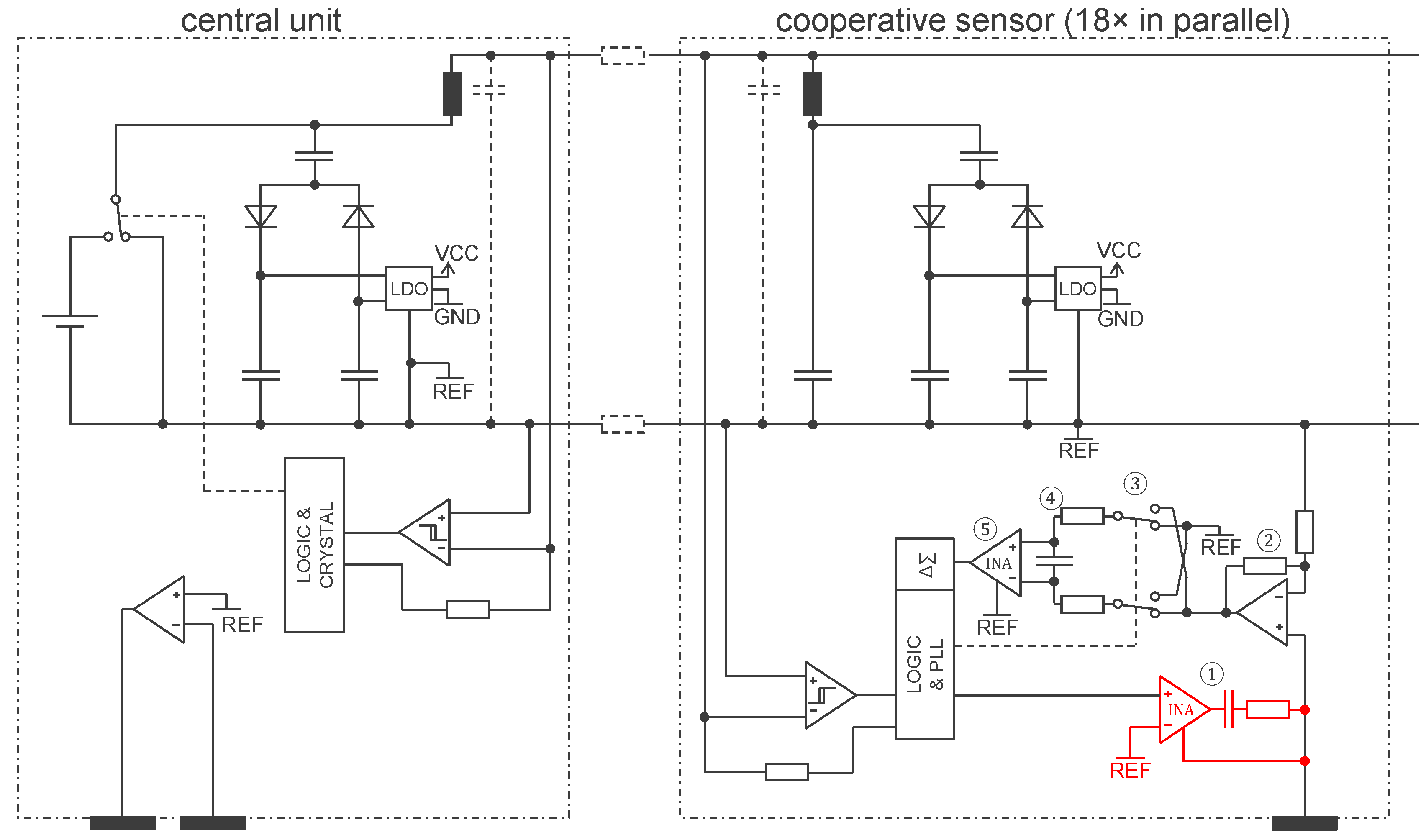
3.2. Approach Addressing the Safety Issue with Powering at 1 MHz and ASIC
3.4. Comparison to Existing Work
4. Results
4.1. Legacy Approach with 500 Hz Powering and Off-the-Shelf Components
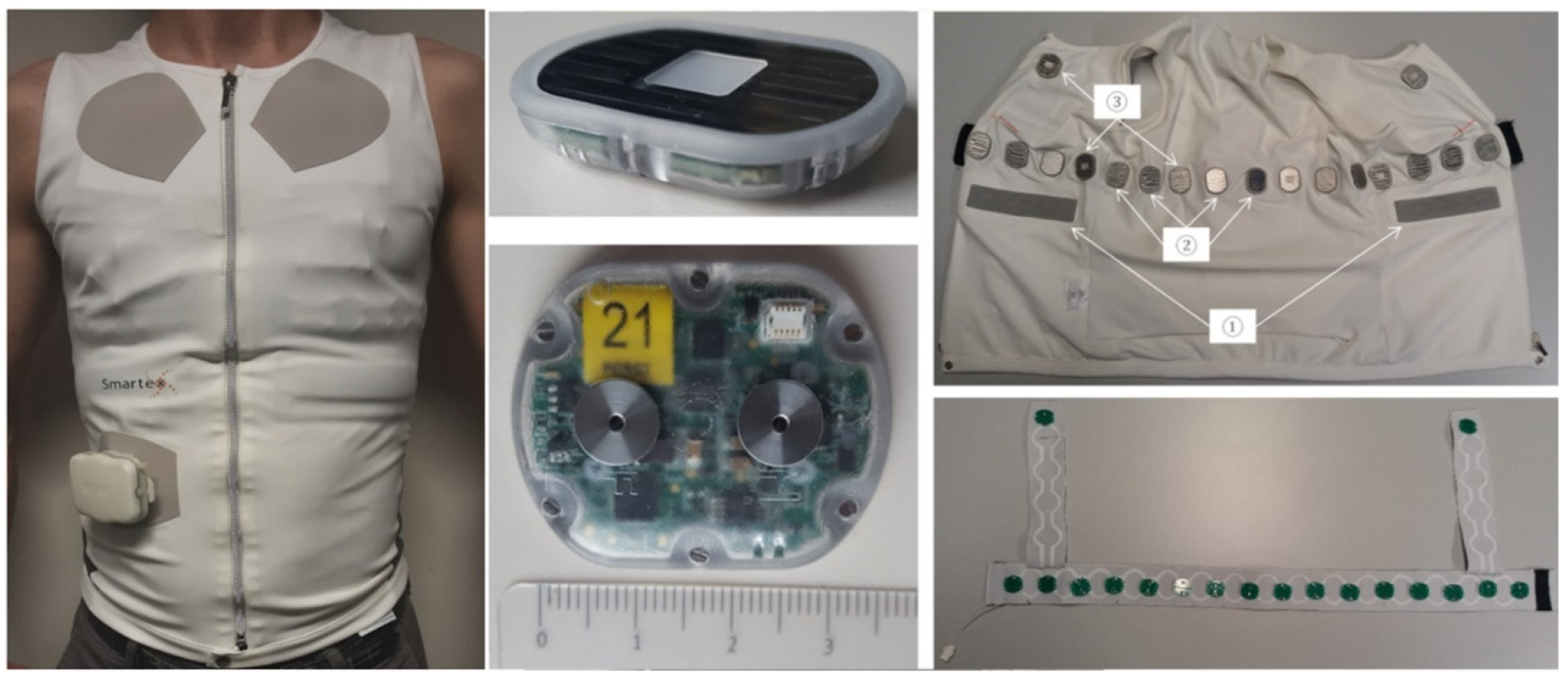
4.1.1. WELMO Vest
4.1.2. Calibration
4.2. Approach Addressing the Safety Issue with Powering at 1 MHz and ASIC
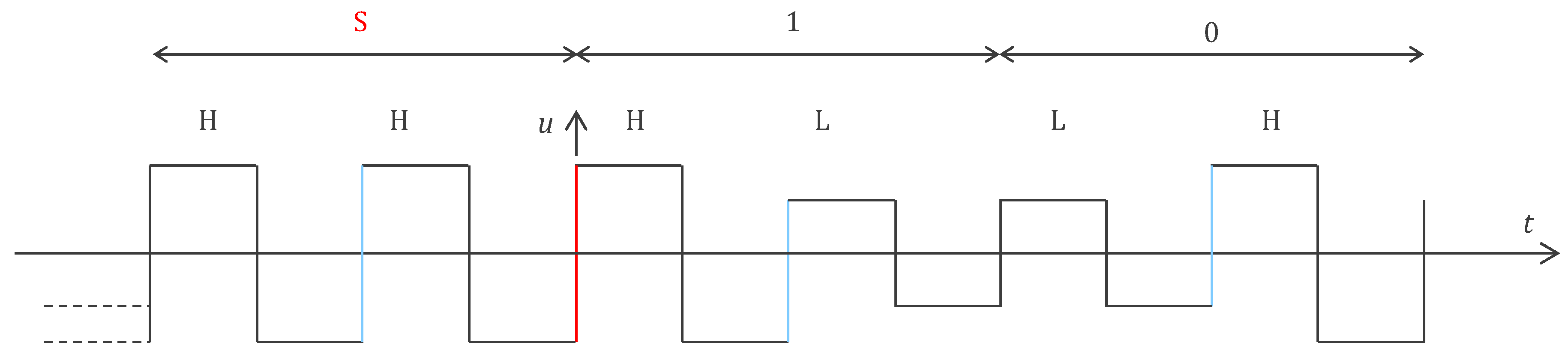
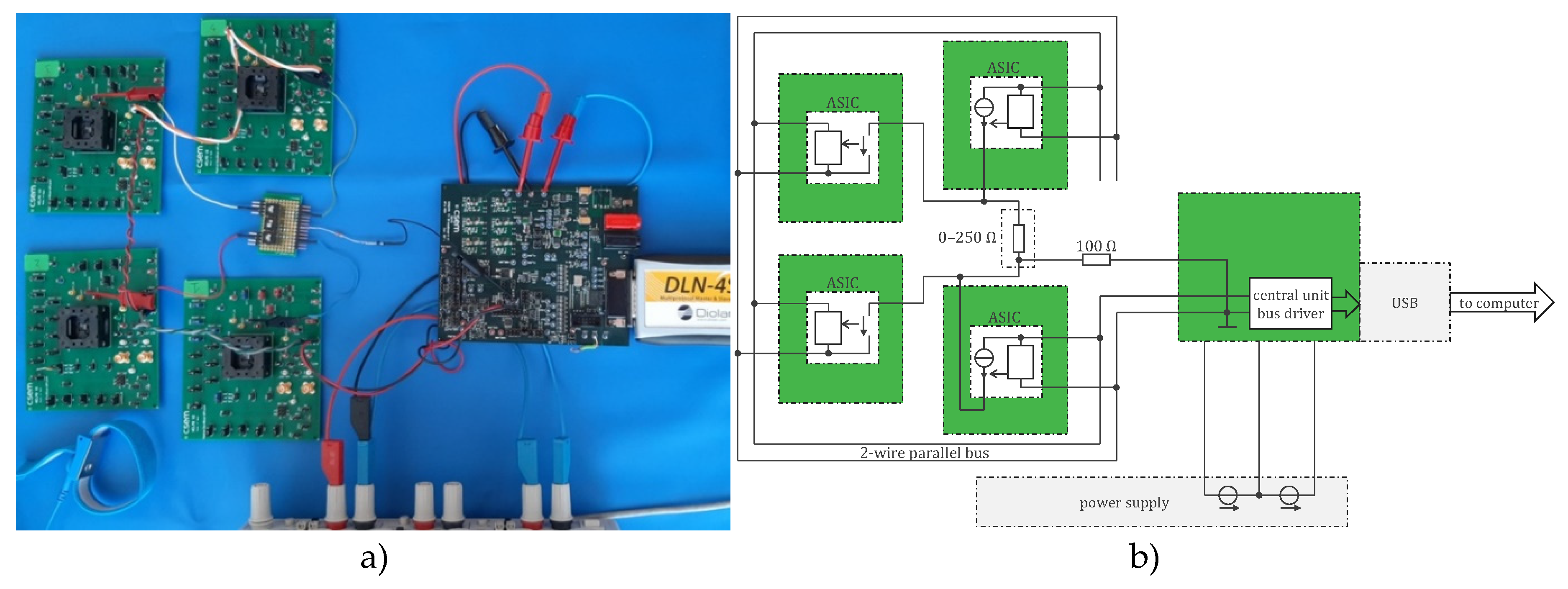
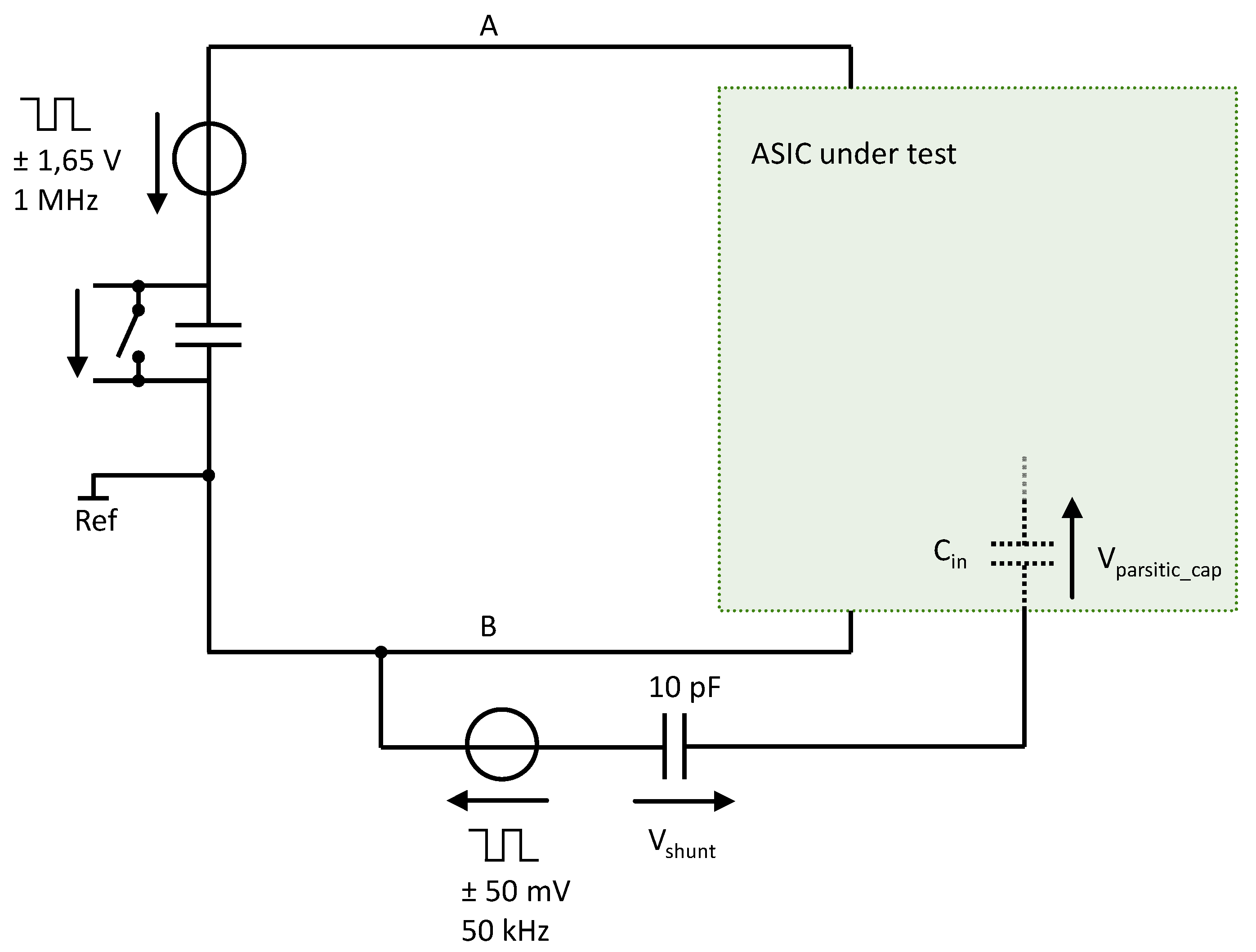
4.2.1. ASIC Architecture
4.2.2. Central Unit Architecture
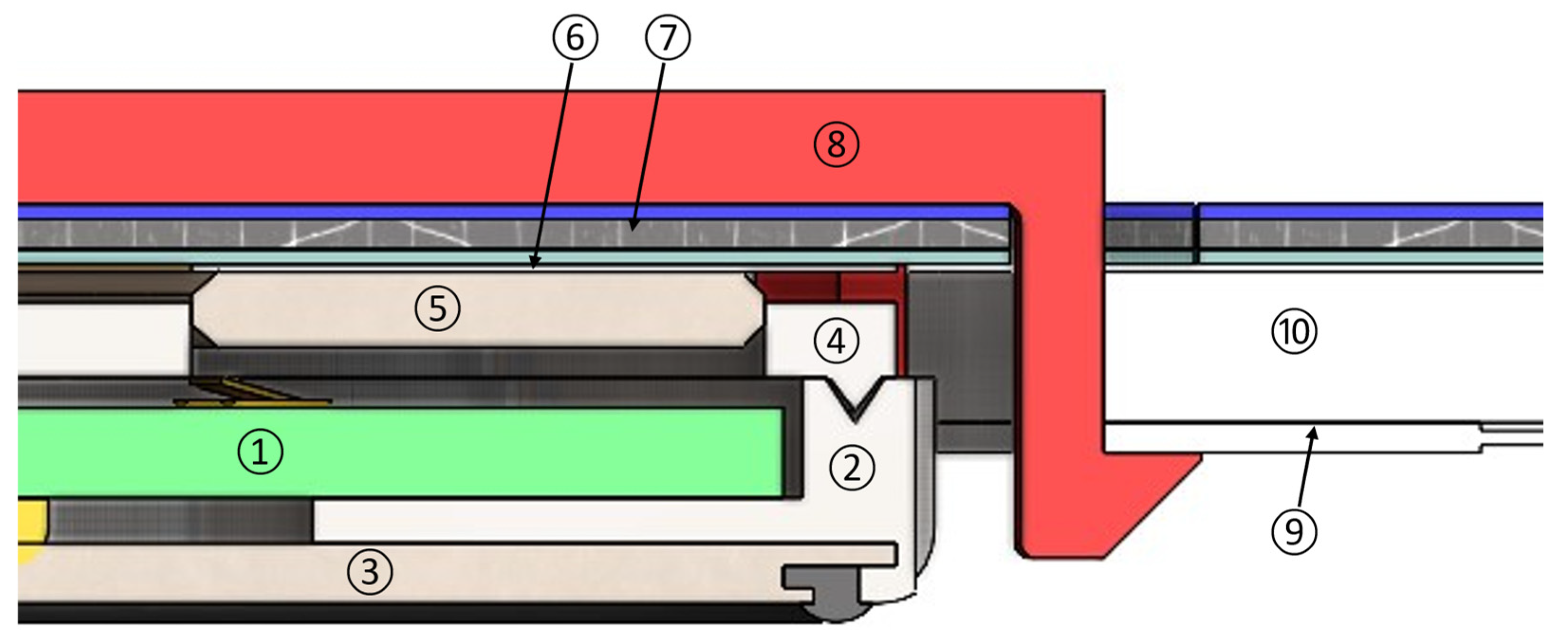
4.2.3. Sensor Housings
4.2.3. Implementation Results
5. Conclusion
6. Patents
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Adler, A.; Holder, D. , (Eds.) Electrical Impedance Tomography—Methods, History and Applications, 2nd ed.; CRC Press. ISBN 9780367023782. 2022.
- Yang J. The investigation and implementation of electrical impedance tomography hardware system, PhD Thesis of De Montfort University. dora.dmu.ac.uk/bitstream/handle/2086/19303/Jieqiu Yang.pdf. 2006.
- Lee, MH. et-al, Portable multi-parameter electrical impedance tomography for sleep apnea and hypoventilation monitoring: feasibility study, Physiol. Meas. 39 (2018),. [CrossRef]
- Gaggero, P.O. ,Miniaturization and distinguishability limits of electrical impedence tomography for biomedical application, phD thesis, https://doc.rero.ch/record/24734/files/Gaggero_Pascal_Olivier_-_Miniaturization_and_Distinguishability_Limits_UNINE_THESE_2207_2011.pdf.
- Qin Shaojie, Yao Yulong, Xu Yuqing, Xu Danling, Gao Yuan, Xing Shunpeng, Li Z, Characteristics and topic trends on electrical impedance tomography hardware publications, Frontiers in Physiology, 13, 2022,. [CrossRef]
- Mosquera, Víctor & Rengifo, Carlos. (2020). Electrical Impedance Tomography: Hardware Fundamentals And Medical Applications. Ingeniería solidaria. 16. 1-29. [CrossRef]
- M. Kim, J. Bae, and H.-J. Yoo. Wearable 3D lung ventilation monitoring system with multi frequency electrical impedance tomography, in 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS), 2017, pp. 1–4. [Online]. [CrossRef]
- M. Proença, F. Braun, M. Lemay, J. Solà, A. Adler, T. Riedel, F.H. Messerli, J.-P. Thiran, S.F. Rimoldi, E. Rexhaj. Non-invasive pulmonary artery pressure estimation by electrical impedance tomography in a controlled hypoxemia study in healthy subjects. Sci Rep (2020). 10(1):21462. [CrossRef]
- Webster, J.G.; Clark, J.W. (Eds.) Medical Instrumentation: Application and Design, 4th ed.John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- E. Wade and H. H. Asada, “Cable-free wearable sensor system using a DC powerline body network in a conductive fabric vest,” The 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 2004, pp. 5376-5379. [CrossRef]
- J. Akita, T. Shinmura and M. Toda, “Flexible Network Infrastructure for Wearable Computing Using Conductive Fabric and Its Evaluation,” 26th IEEE International Conference on Distributed Computing Systems Workshops (ICDCSW’06), Lisboa, Portugal, 2006, pp. 65-65. [CrossRef]
- A. Noda and H. Shinoda, “Inter-IC for Wearables (I2We): Power and Data Transfer Over Double-Sided Conductive Textile,” in IEEE Transactions on Biomedical Circuits and Systems, vol. 13, no. 1, pp. 80-90, Feb. 2019. [CrossRef]
- Ya Zhu, Akihito Noda, Masahiro Fujiwara, Yasutoshi Makino, Hiroyuki Shinoda, Fast half-duplex communication on e-textile based wearable networks, IEICE Communications Express, 2020, Volume 9, Issue 9, Pages 426-432. [CrossRef]
- Chételat, O.; Rapin, M.; Bonnal, B.; Fivaz, A.; Wacker, J.; Sporrer, B. Remotely Powered Two-Wire Cooperative Sensors for Biopotential Imaging Wearables. Sensors 2022, 22, 8219. [Google Scholar] [CrossRef] [PubMed]
- IEC 60601-1; Medical Electrical Equipment—Part 1 : General Requirements for Basic Safety and Essential Performance. IEC: Geneva, Switzerland, 2020.
- Chételat, O. Synchronization and Communication Bus for Biopotential and Bioimpedance Measurement Systems. EP Patent 2567657 B1, 13 March 2013.
- Chételat, O.; Correvon, M. Measurement Device for Measuring Bio-Impedance and/or a Bio-Potential of a Human or Animal Body. US Patent 2015/0173677 B2, 25 June 2015.
- Rapin, M.; Proença, M.; Braun, F.; Meier, C.; Sola, J.; Ferrario, D.; Grossenbacher, O.; Porchet, J.-A.; Chételat, O. Cooperative dry-electrode sensors for multi-lead biopotential and bioimpedance monitoring. Physiol. Meas. 2015, 36, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Rapin, M. A Wearable Sensor Architecture for High-Quality Measurement of Multilead ECG and Frequency-Multiplexed EIT. Ph.D. Thesis, ETH, Zürich, Switzerland, 2018. [Google Scholar] [CrossRef]
- Chételat, O.; Solà, J. Floating front-end amplifier and one-wire measuring device. patent US 8427181 B2, Sep. 16, 2009.
- Chételat, O.; Gentsch, R.; Krauss, J.; Luprano, J. Getting rid of the wires and connectors in physiological monitoring. [CrossRef]
- Chételat, O.; Bonnal, B.; Fivaz, A. Remotely Powered Cooperative Sensor Device. US Patent 2021/0169543 A1, 10 June 2021.
- Rocha, B.M. , Filos D., Mendes L., Serbes G., Ulukaya S., Kahya Y.P., Jakovljevic N., Turukalo T.L., Vogiatzis I.M., Perantoni E., Kaimakamis E., Natsiavas P., Oliveira A., Jácome C., Marques A., Maglaveras N., Pedro Paiva R., Chouvarda I., de Carvalho P. An open access database for the evaluation of respiratory sound classification algorithms. Physiol Meas. 2019 Mar 22;40(3):035001. [CrossRef] [PubMed]
- Frerichs, I. , Vogt, B., Wacker, J., Paradiso, R., Braun, F., Rapin, M., Caldani, L., Chételat, O., Weiler, N. Multimodal remote chest monitoring system with wearable sensors: a validation study in healthy subjects. Physiol Meas. 2020 Feb 5;41(1):015006. [CrossRef] [PubMed]
- Yilmaz, G. , Rapin, M., Pessoa, D., Rocha, B.M., de Sousa, A.M., Rusconi, R., Carvalho, P., Wacker, J., Paiva, R.P., Chételat, O. A Wearable Stethoscope for Long-Term Ambulatory Respiratory Health Monitoring. Sensors 2020, 20, 5124. [Google Scholar] [CrossRef] [PubMed]
- Lasarow, L. , Vogt, B., Zhao, Z., Balke, L., Weiler, N., Frerichs, I.: Regional lung function measures determined by electrical impedance tomography during repetitive ventilation manoeuvres in patients with COPD. Physiol Meas. 2021, 42, 015008. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.M. , Pessoa, D., Marques, A., Carvalho, P., Paiva, R.P. Automatic Classification of Adventitious Respiratory Sounds: A (Un)Solved Problem? Sensors 2021, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, I.: EIT for Measurement of Lung Function. In: Electrical Impedance Tomography: Methods, History and Applications, 2nd Edition. Eds.: A. Adler and D. Holder. CRC Press, Taylor & Francis Group, Boca Raton, USA. pp. 171-190, ISBN 978-0-7503-0952-3, 2022. [CrossRef]
- Haris, K., Vogt, B., Strodthoff, C., Pessoa, D., Cheimariotis, G.A., Rocha, B., Petmezas, G.., Weiler, N., Paiva, R.P., de Carvalho, P., Maglaveras, N., Frerichs, I. Identification and analysis of stable breathing periods in electrical impedance tomography recordings. Physiol Meas 42, doi: 10.1088/1361-6579/ac08e5, 2021.
- Frerichs, I., Zhao, Z., Dai, M., Braun, F., Proença, M., Rapin, M., Wacker, J., Lemay, M., Haris, K., Petmezas, G., Cheimariotis, A., Lekka, I., Maglaveras, N., Strodthoff, C., Vogt, B., Lasarow, L., Weiler, N., Pessoa, D., Rocha, B.M., de Carvalho, P., Paiva, R.P., Adler, A. Respiratory image analysis. In: Paiva R.P., de Carvalho P, Kilintzis V. Wearable sensing and intelligent data analysis for respiratory management. Academic Press, Elsevier, London, UK. pp. 169-212, ISBN 978-0-12-823447-1, 2022.
- Frerichs, I., Vogt, B., Weiler, N. Elektrische Impedanztomographie zur Untersuchung der regionalen Lungenfunktion. Atemwegs- und Lungenkrankheiten 47: 401-411, 2021. [CrossRef]
- Frerichs, I., Lasarow, L., Strodthoff, C., Vogt, B., Zhao, Z., Weiler, N. Spatial ventilation inhomogeneity determined by electrical impedance tomography in patients with COPD. Front Physiol 12: 762791, doi: 10.3389/fphys.2021.762791, 2021.
- Pessoa, D., Rocha, B.M., Cheimariotis, G.A., Haris, K., Strodthoff, C., Kaimakamis, E., Maglaveras, N., Frerichs, I., de Carvalho, P., Paiva, R.P. Classification of electrical impedance tomography data using machine learning. IEEE Eng Med Biol Soc 2021: 349-353, doi: 10.1109/EMBC46164.2021.9629961, 2021.
- Paradiso, R. , Caldani L., Textiles and Smart Materials for Wearable Monitoring Systems, In: Paiva R.P., de Carvalho P, Kilintzis V. Wearable sensing and intelligent data analysis for respiratory management. Academic Press, Elsevier, London, UK. pp. 169-212, ISBN 978-0-12-823447-1, 2022.
- Wacker, J. , Bonnal, B., Braun, F., Chételat, O., Ferrario, D., Lemay, M., Rapin, M., Renevey, P., Yilmaz, G., In: Paiva R.P., de Carvalho P, Kilintzis V. Wearable sensing and intelligent data analysis for respiratory management. Academic Press, Elsevier, London, UK. pp. 59-93, ISBN 978-0-12-823447-1, 2022.
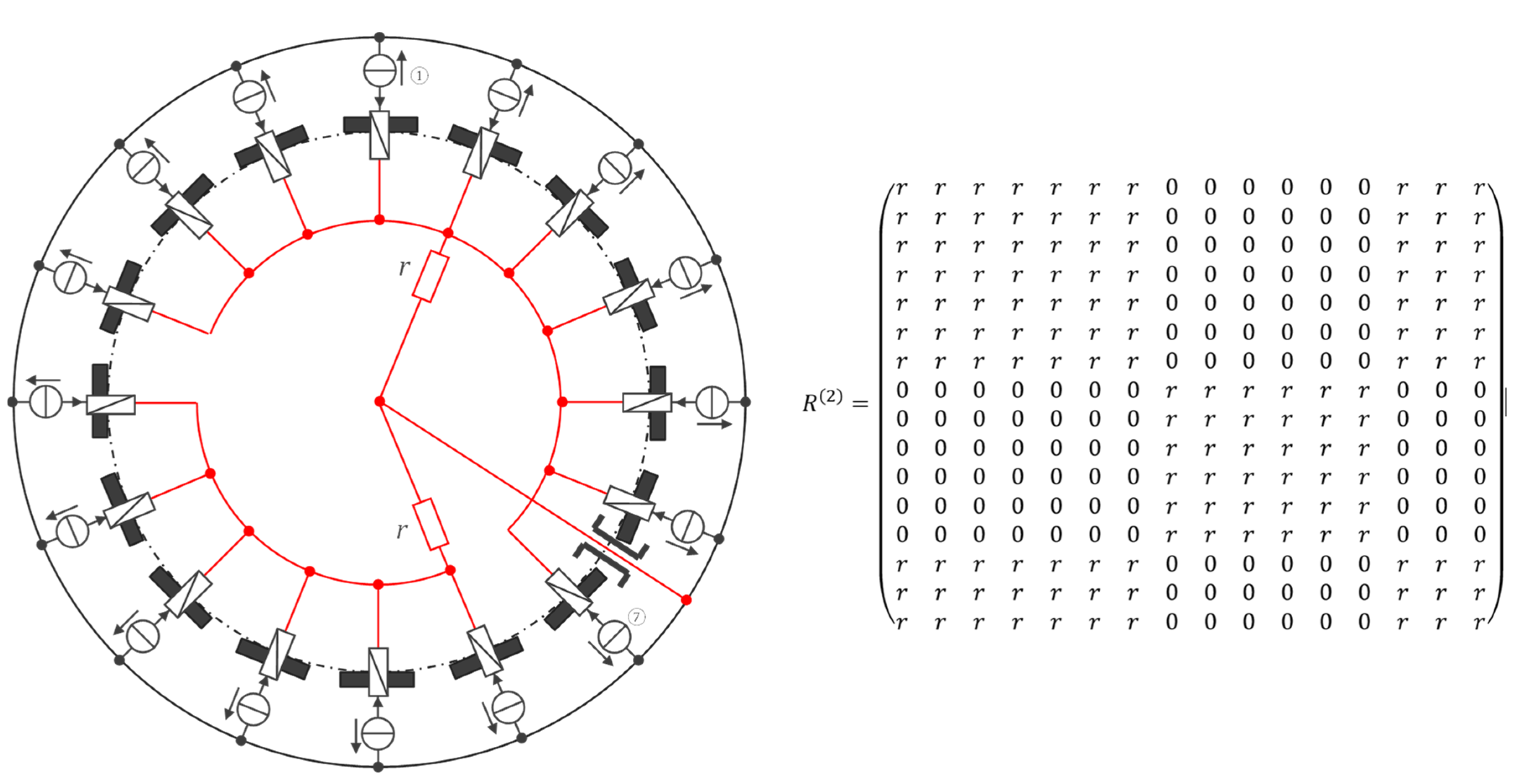
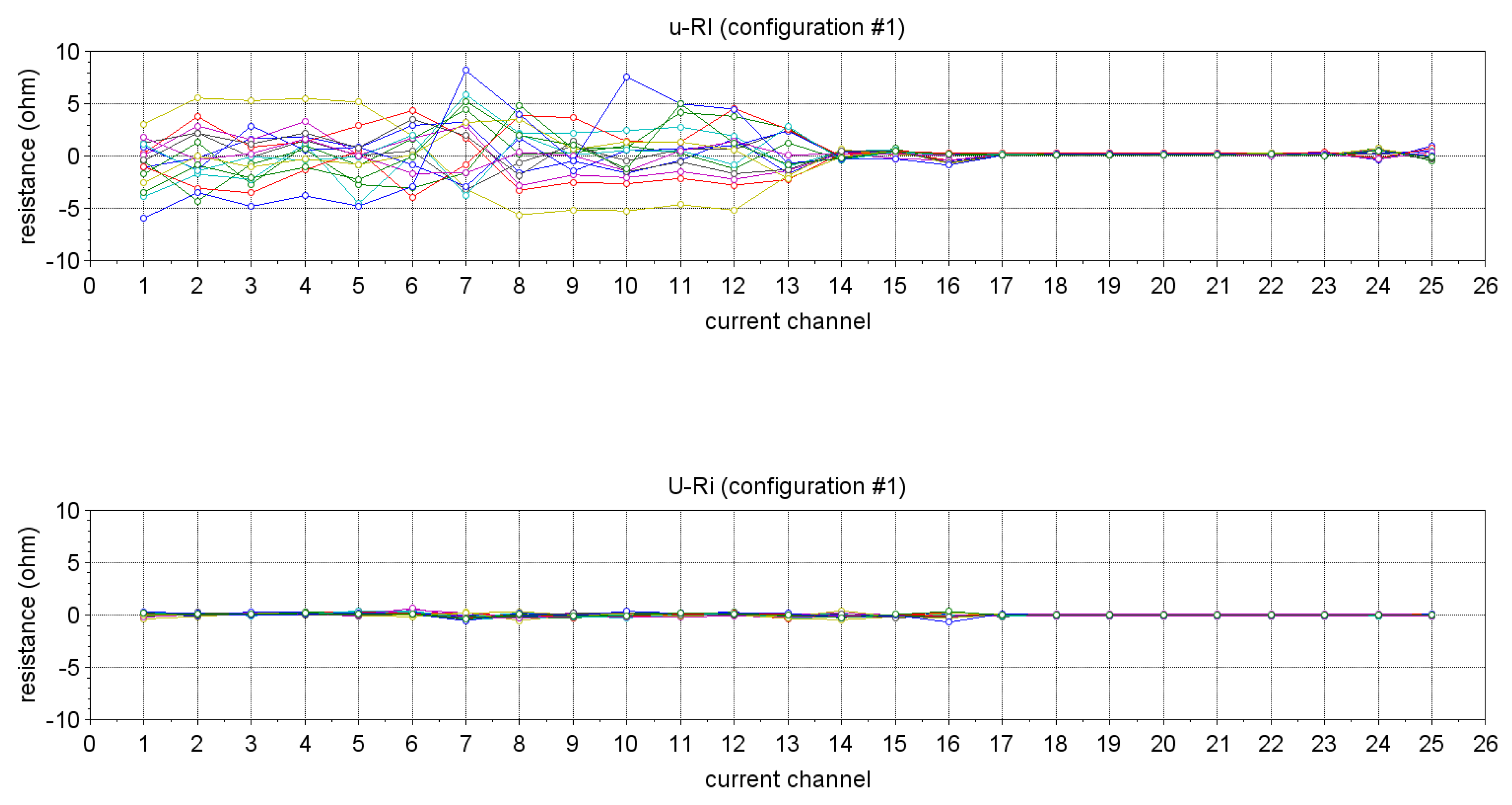
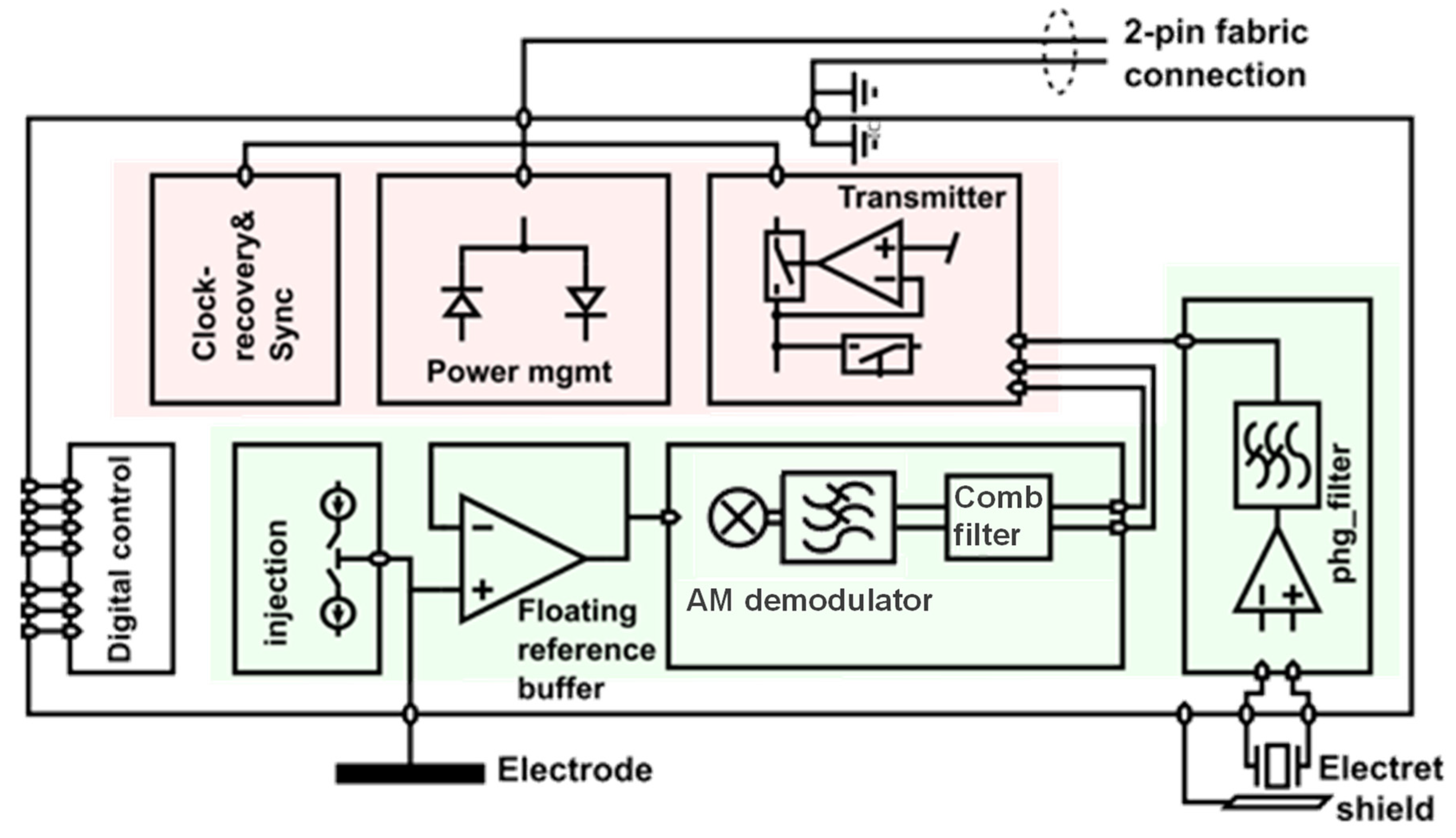
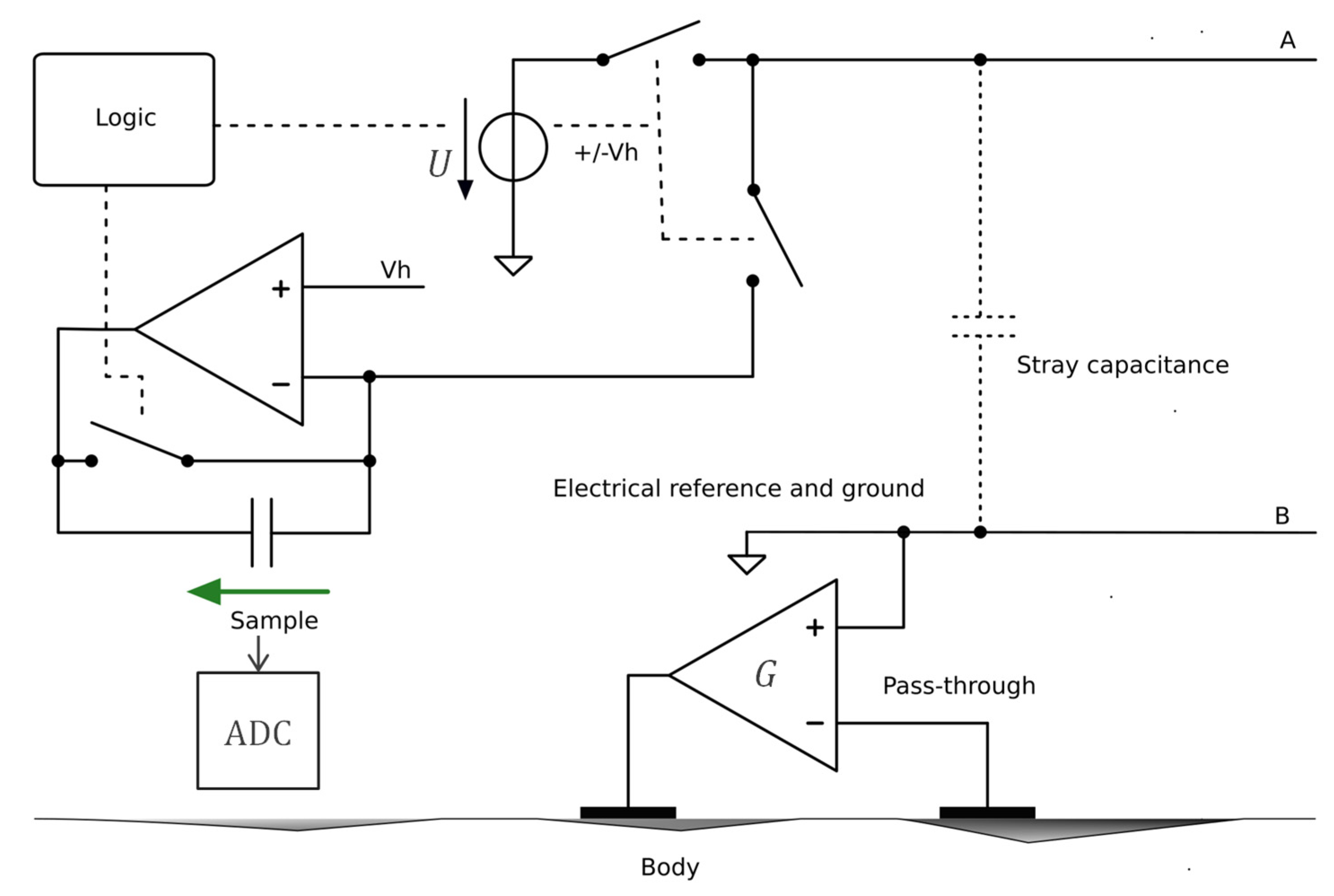

| Technique/Features | Ref | Comment |
|---|---|---|
| Conventional star arrangement | Not suitable for wearables with many electrodes | |
| Passive electrodes, shielded cables | [1] | Widespread |
| Active electrodes, multi-wire cables | [2,3] | Well-known, but not often used |
| Parallel bus arrangement | Scalable (connector size independent of nb. of electr.) | |
| Bus with more than 2 wires | [4] | Not easily flexible, stretchable, breathable, washable |
| Two-wire bus (cooperative sensors) | §2 | Simplest connection |
| Locally powered Bootstrapping Separate potential/impedance wires |
§2.1 §2.2 §2.2 |
Easy to comply with safety (medical standards) Suitable for dry electrodes, easy current source Wire impedance is not part of measured bioimpedance |
| Remotely powered (biopotential only) | [14] | |
| Remotely powered (+bioimpedance) | §3/§4 | Sensors can be miniaturized |
| No monitoring of leakage currents No bootstrapping No separate potential/impedance wires |
§3.1/ §4.1 |
Requires reliable waterproof double insulation Not ideal for dry electrodes, complex current source Measured bioimpedance include wire impedance |
| Monitorable leakage currents Bootstrapping Separate potential/impedance wires |
§3.2/ §4.2 |
Suitably flexible, stretchable, breathable, washable Suitable for dry electrodes, high-end current source Wire impedance is not part of measured bioimpedance |
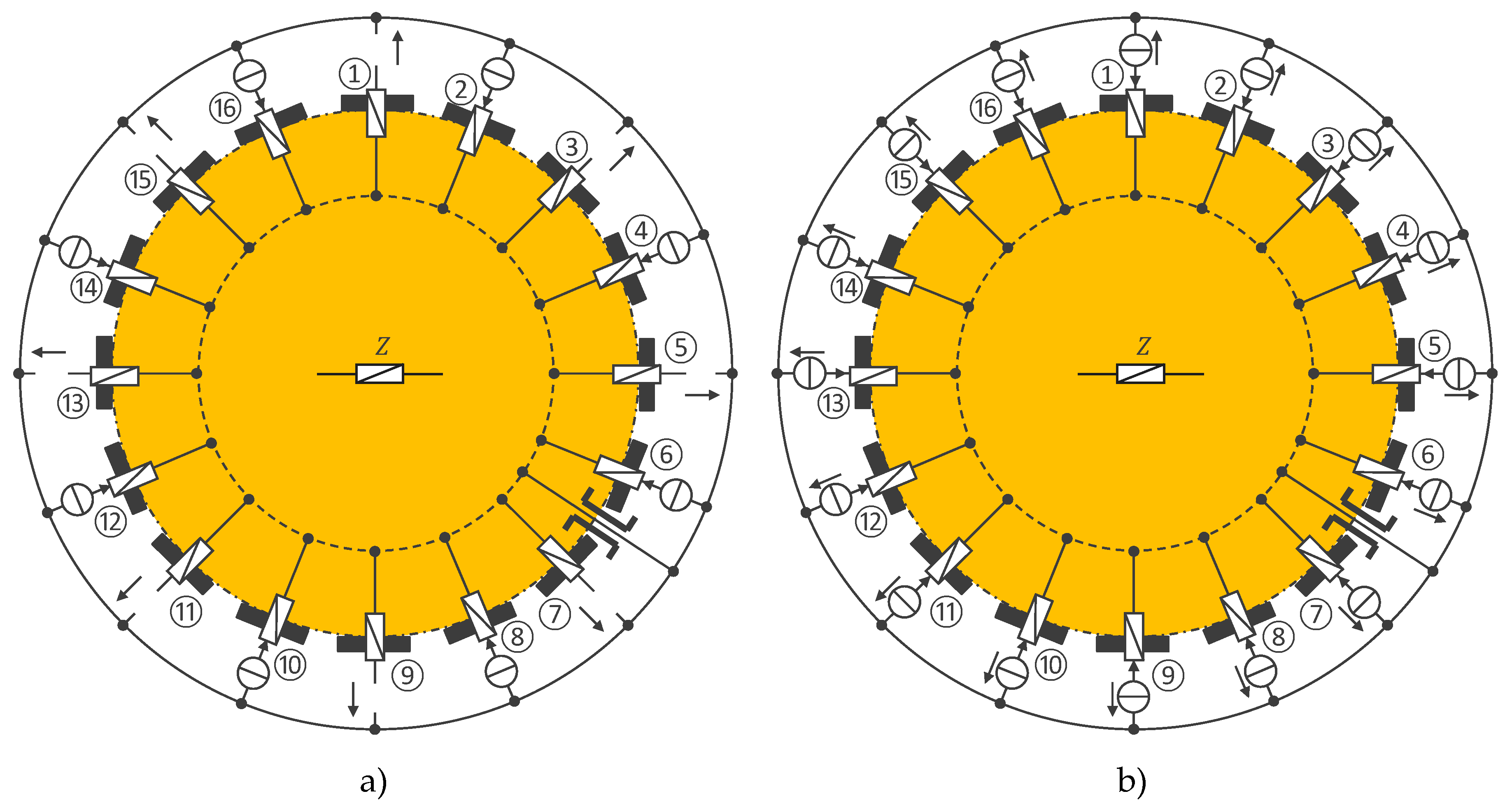
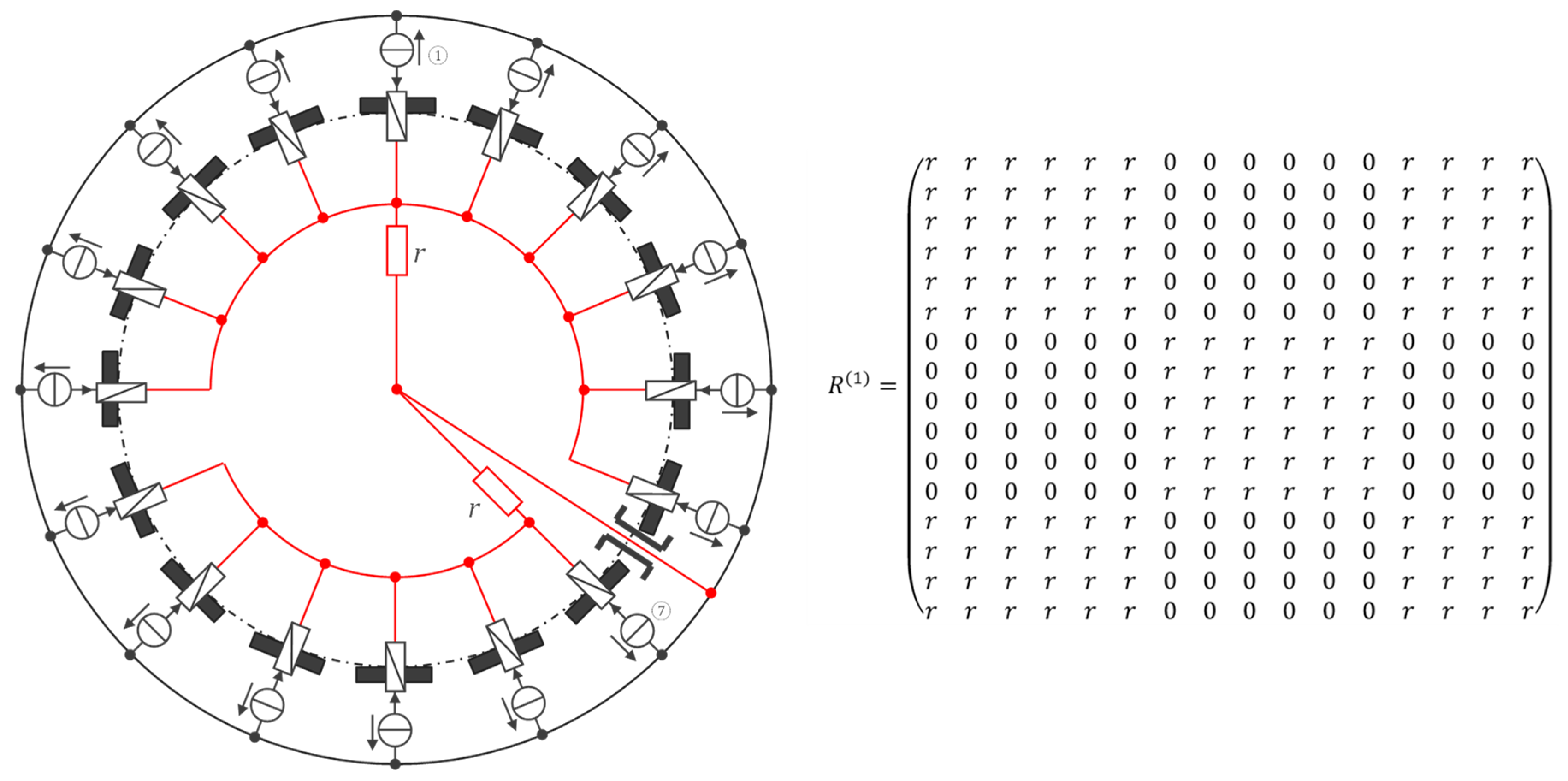
| Channel | Injected current (100 µA rms, 40 kHz square wave) |
|---|---|
| 1 | ① → ⑦ |
| 2 | ② → ⑧ |
| … | … |
| 10 | ⑩ → ⑯ |
| 11 | ⑪ → ① |
| … | … |
| 16 | ⑯ → ⑥ |
| 17–25 | unused (yet) |
|
R (Ω) ±0.02% |
Measurement (Ω) |
Linearity(% FS) | Noise in 0–2.5 Hz (mΩ rms) |
|---|---|---|---|
| 250 | 249.95 | −0.02 | 32.49 |
| 200 | 200.75 | 0.30 | 37.38 |
| 150 | 149.54 | −0.18 | 39.79 |
| 100 | 99.32 | −0.27 | 33.19 |
| 50 | 49.97 | −0.01 | 32.95 |
| 0 | 0.47 | 0.19 | 30.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





