1-Ketosteroids
Steroids skeleton containing a carbonyl group at position 1 are found in nature in the amount of over 200 compounds, mainly in plants [
28,
29]. The carbonyl group at position 1 of steroids plays a crucial role in determining the chemical and biological properties of these compounds. The carbonyl group adds rigidity to the steroid structure, which can influence the overall shape and conformation of the molecule. This can have a significant impact on the interaction of the steroid with biological receptors and enzymes. The presence of a carbonyl group introduces a polar character to the steroid molecule, which can influence its solubility in water and other solvents. This can affect the distribution and transport of the steroid within biological systems. The carbonyl group is a reactive functional group that can undergo various chemical reactions, such as reduction, oxidation, and nucleophilic addition. These reactions can be utilized in the synthesis and modification of steroid compounds. The presence and position of the carbonyl group can have a significant impact on the biological activity of the steroid. The carbonyl group can engage in hydrogen bonding and other intermolecular interactions, which can influence the binding of the steroid to receptors, proteins, and other biological molecules. Overall, the carbonyl group at position 1 of steroids is an important structural and functional element that influences the chemical reactivity, physical properties, and biological activities of these compounds.
For example, withanolides and related steroids which has a carbonyl group at position 1, are acnistins, physalins, sativolides, spiranoid-δ-lactones, subtriflora-δ-lactones, trechonolides, withametelins, withajardins, withaphysalins, ring-D aromatic withanolides, and norbornane-type withanolides, which have been found in extracts of plants belonging to the families
Datura, Dunalia, Hyoscyamus, Iochroma, Jaborosa, Larnax, Lycium, Solanum, Withania, and
Witheringia [
28,
29,
30,
31]. As an example, we present steroids with different structures containing a carbonyl group in position 1 isolated from plants.
Steroids with a carbonyl group at position 1 of the steroid skeleton in marine invertebrates are found only in Coelenterata, Formosan soft coral
Clavularia viridis and
C. violacea. It appears that these steroids could be found in corals that feed on plant remains, marine mangrove plants, algae, or phytoplankton [
32]. The following marine steroids, structurally akin to the steroids from the
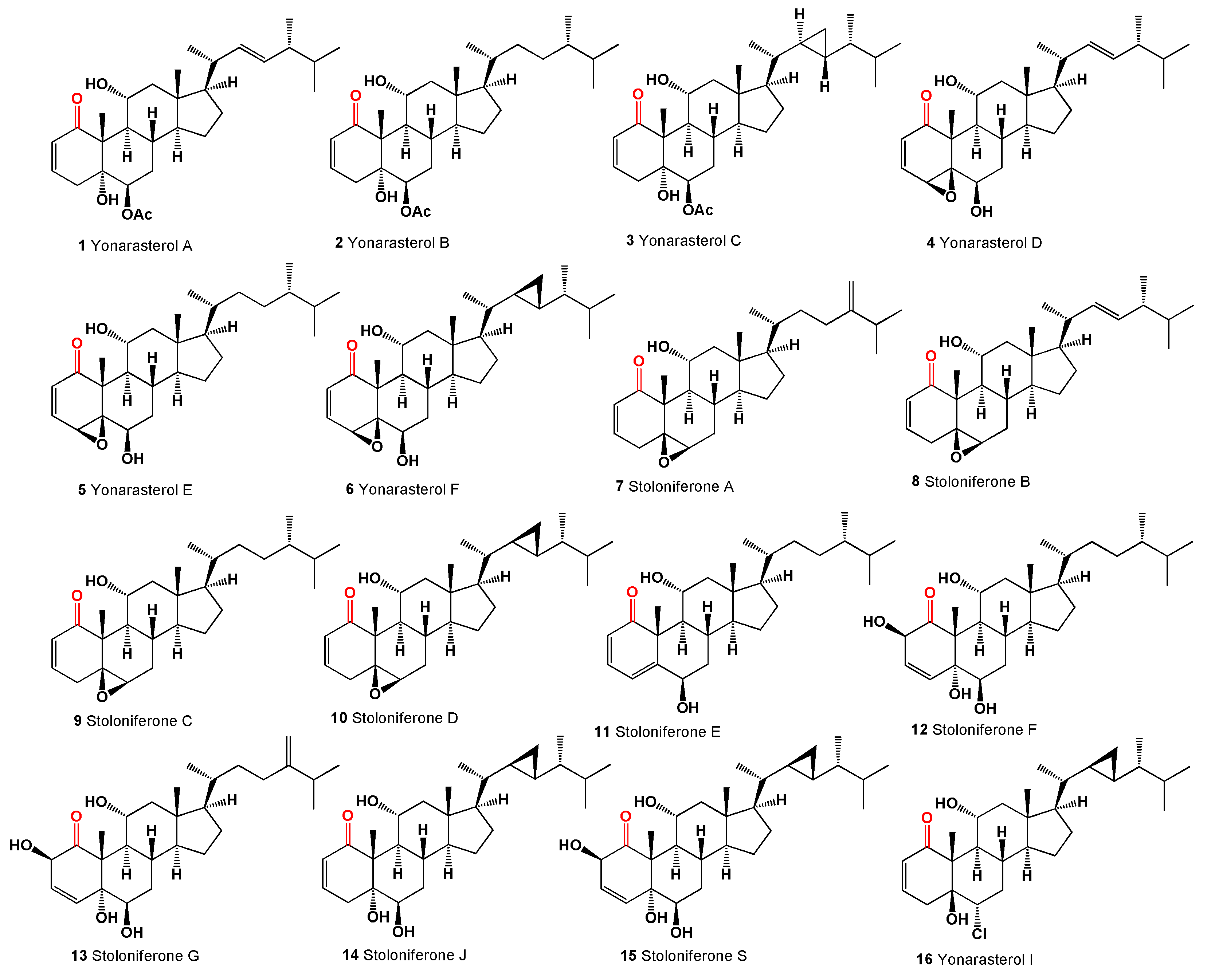
Physalis genus, have been identified. Yonarasterols A (
1), B (
2), C (
3), D (
4), E (
5), and F (
6, structures see in
Figure 2), resembling witanolides, were extracted from the Okinawan soft coral
Clavularia viridis in the Japan Sea [
33]. Additionally, several marine steroids named stoloniferones A (
7), B (
8), C (
9), and D (
10) have been isolated from
Clavularia viridis, as identified by Quoy and Gaimard in Okinawa, and steroids C and D demonstrated growth inhibitory effects on HeLa S3 cells and human diploid cells
in vitro [
34]. Moreover, cytotoxic steroids stoloniferone E-G (
11-
13) were obtained from the methylene chloride extract of the Formosan soft coral
Clavularia viridis and
C. violacea [
35]. Notably, stoloniferone Q, D, J, S, yonarasterol C (
14), F (
15), and I (
16) feature a carbonyl group at C-1 with a double bond between C-2 and C-3, except for stoloniferone S, which exhibits a double bond between C-3 and C-4. Additionally, stoloniferone Q includes an extra bond between C-4 and C-5 [
32,
33,
34,
35,
36,
37].
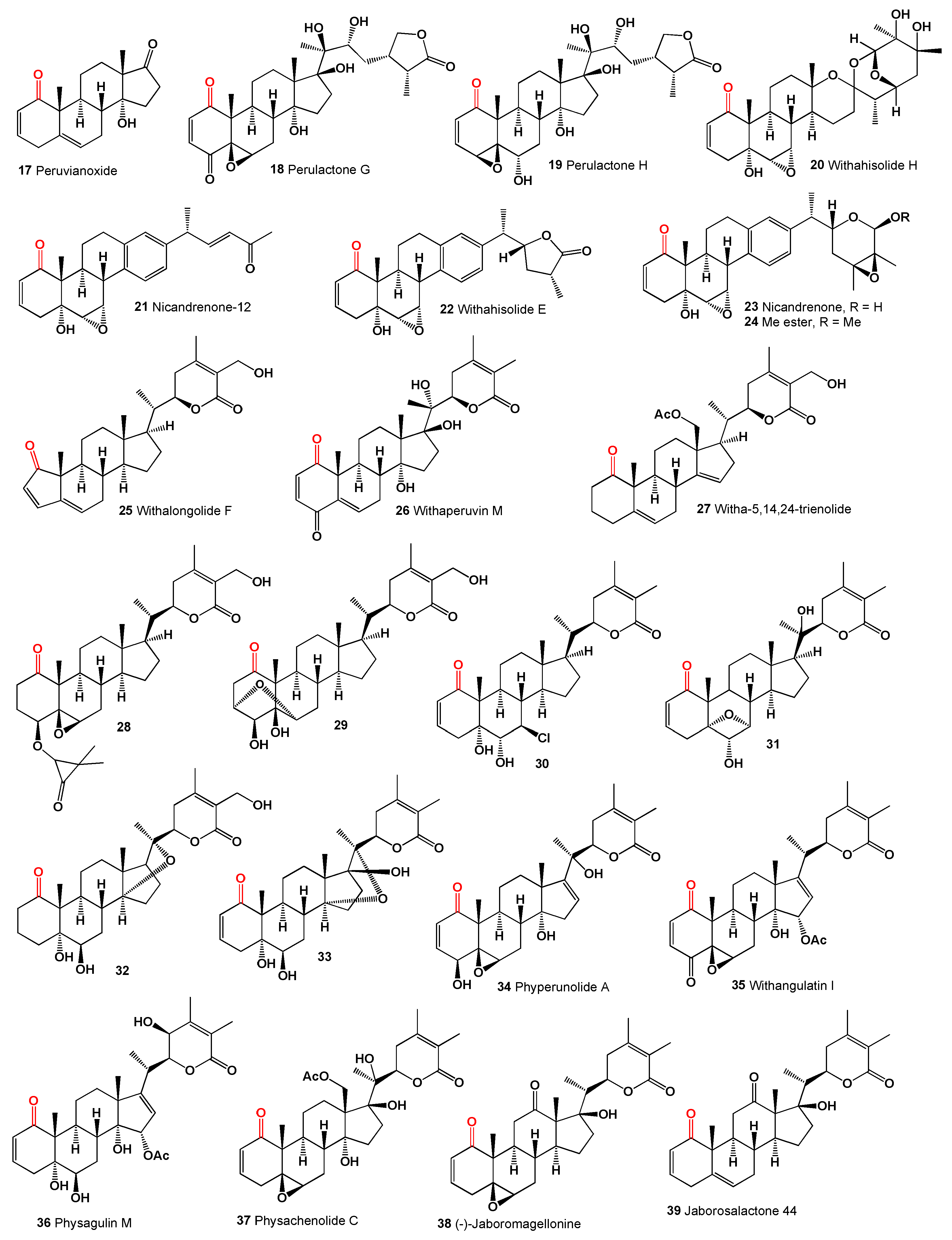
The genus Physalis (Solanaceae), which includes over 100 species primarily found in North and South America, produces cytotoxic peruvianoxide (
17), C28 steroidal lactones, and physalins (
18-
27) [
38]. Notably, the cytotoxic peruvianoxide (
17) was detected in extracts from
Physalis peruviana [
39].
The plant
Withania somnifera, commonly known as ashwagandha or winter cherry, contains a steroid (
28) [
40] and an unusual variant, compound (
29), featuring a 3α,6α-epoxy bridge. This was found in an extract from the same plant [
41]. Chlorinated withanolide Z (
30) [
42], arising from
trans-diaxial cleavage of the 6,7-epoxide, and compound (
31) with a unique 5α,7α-epoxy bridge resulting from rearrangement of the 5α-hydroxy-6α,7α-epoxide, were identified in the leaves and roots of
Withania somnifera [
43]. Additionally, two 14α,20-epoxywithanolides, including the diol coagulin M (
32) and coagulin I (
33), were isolated from
W. coagulans [
44,
45].
Figure 3.
1-Ketosteroids derived from microorganisms and plants.
Figure 3.
1-Ketosteroids derived from microorganisms and plants.
Several 14α-hydroxy withanolides have been reported from
Physalis species, including phyperunolide A (
34) [
39] from
P. peruviana and 15-acetyloxy withanolides from
P. angulata, notably withangulatin I (
35) [
46] and physagulin M (
36) featuring a free hydroxy group at C-23 [
47]. A study on Mexican
Physalis species revealed that physachenolide C (
37) was isolated from the leaves, flowers, and stems of
Physalis chenopodifolia [
48]. Two withanolides with unmodified skeletons containing a free ketone at C-12 were reported from various plants, namely (-)-jaboromagellonine (
38) from
Jaborosa magellanica [
49] and jaborosalactone 44 (
39) from
J. kurtzii [
50]. A 12-oxygenated withanolide, daturalactone 7 (
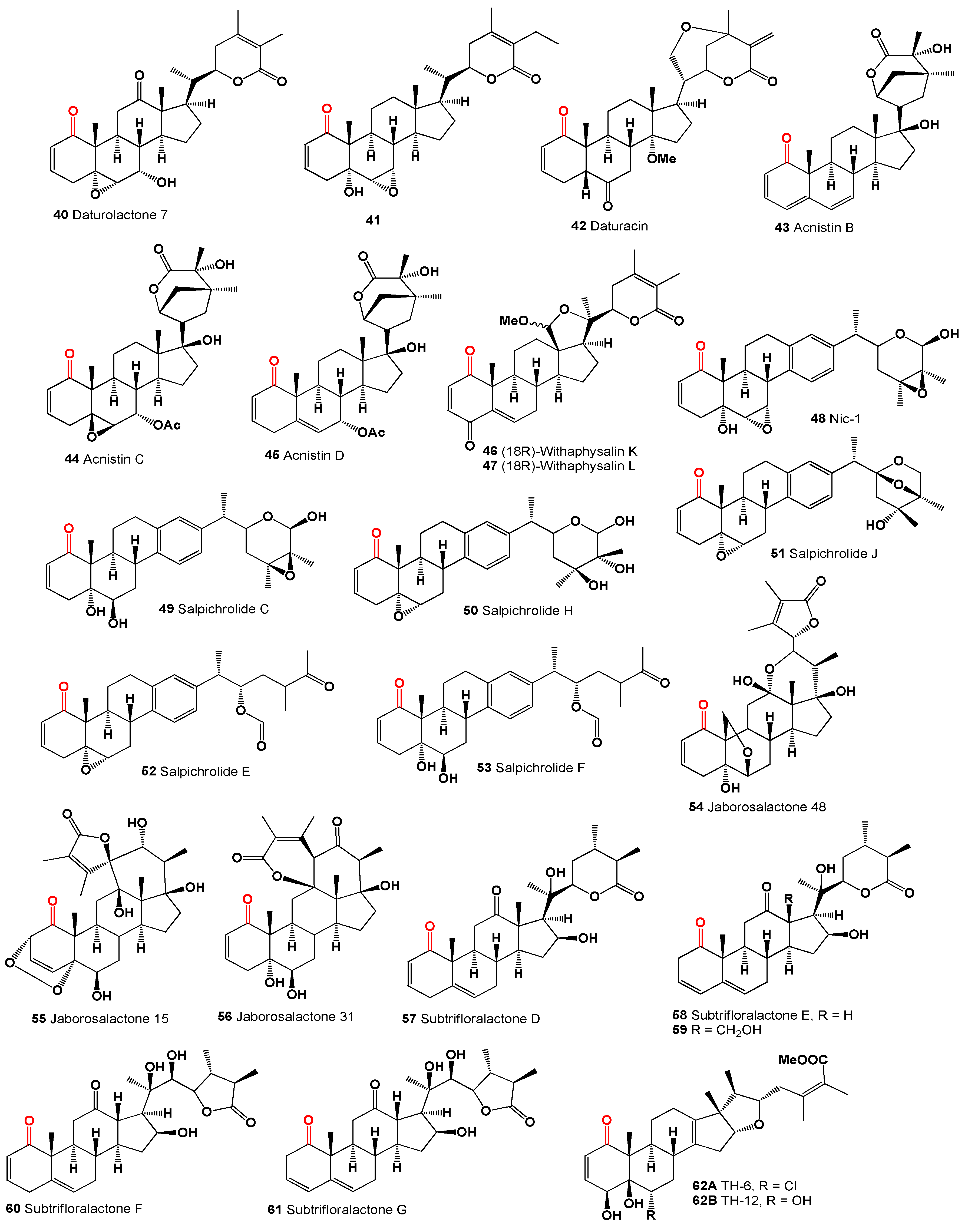
40), was isolated from
Datura ferox collected in Argentina [
51]. An intriguing example includes a C29 withanolide from the bark of
Eucalyptus globulus (
41) with an ethyl substituent at C-25 instead of the usual methyl group [
52]. Daturacin (
42), containing a 20,24 epoxide, was found in
D. inoxia extract [
53], and acnistins B (
43), C (
44), and D (
45) were isolated from the leaves of
Dunalia solanacea collected in Medellin, Colombia [
54,
55]. Withaphysalins K (
46) and L (
47) were isolated from
Eriolarynx lorentzii (sub nom.
Vassobia lorentzii) collected in Argentina [
28].
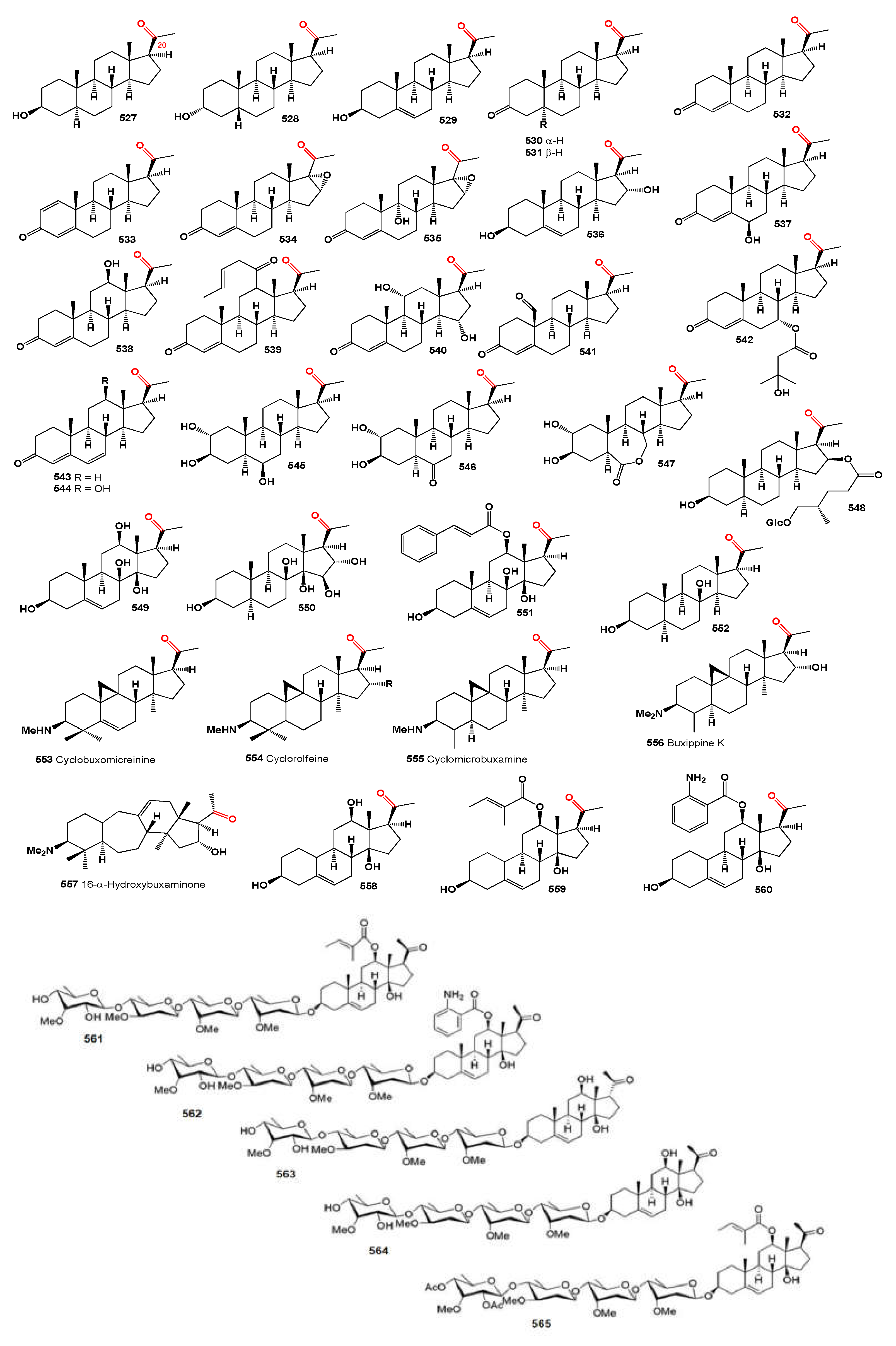
The steroid Nic-1 (
48) was obtained from
Nicandra physalodes [
30], and the minor component in
S. origanifolia plants collected in Buenos Aires and Cordoba provinces, Argentina, was salpichrolide C (
49) [
56,
57]. Salpichrolide H (
50) was isolated from plants collected in Buenos Aires during the winter [
58], and salpichrolide J (
51) from plants collected in Salta province, Argentina, during the summer. Plants collected in Buenos Aires during the winter also contained two ergostane derivatives, salpichrolides E (
52) and F (
53), likely resulting from the degradation of the lactone side chain of salpichrolides A and C [
59].
The 19-oxygenated trechonolide (
54) was isolated from
Jaborosa laciniata, with its 6,19-oxygen bridge being an unusual feature for a natural product [
60]. Notably, synthetic steroids with this moiety exhibit significant biological properties as selective glucocorticoid receptor modulators [
61], and jaborosalactone 15 (
55) was found in plants collected in the summer [
62]. Jaborosalactone 31 (
56), isolated from
J. rotacea, closely relates to the spiranoid withanolides isolated from
J. odonelliana, J. runcinata, and
J. araucana, where the C-12–C-23 bond remains intact, forming a dlactone between the C-26 carboxyl and the C-12 hydroxy group [
63].
Subtrifloralactones D (
57) and E (
58) are similar to the classic withanolide structure but notably lack the C-18 [
64]. The isolation of 13β-hydroxymethylsubtrifloralactone E (
59) from
Deprea subtriflora suggests an oxidative pathway leading to the loss of C-18, potentially resulting in a 16-formate group through the rearrangement of a 16,18-hemiketal [
65]. Additionally, subtrifloralactones F (
60) and G (
61), which exhibit a
trans fusion between rings C and D, resulting in an ixocarpalactone-type structure, were also detected in
Deprea subtriflora [
64].
Withanolides, specifically TH-6 (
62A) and TH-12 (
62B), are 17-methyl-18-nor-ergostanes that were isolated over 30 years ago by Shingu and co-workers [
66] from the acid hydrolysate of a methanolic extract of
Tubocapsicum anomalum. The researchers linked these compounds to a presumable precursor with a withanolide side chain that is thought to rearrange under acidic conditions.
Summarized on the biological activity of 1-ketosteroids is presented in
Table 1, and details are described partially in the text or a full description of the activity is written in the original articles.
Figure 3.
1-Ketosteroids derived from microorganisms and plants.
Figure 3.
1-Ketosteroids derived from microorganisms and plants.
2-Ketosteroids
2-Ketosteroids, also known as ketosteroids, are a group of steroid compounds that have a ketone group (C=O) at the second carbon atom in the steroid ring structure. This functional group distinguishes them from other steroids. These compounds are present in various biological systems and play important roles in metabolism and hormone regulation. 2-Ketosteroids can also be intermediate metabolites in the biosynthesis and metabolism of other steroids. For example, they can be precursors or breakdown products of hormones. 2-Ketosteroids are found in various tissues and organs, including the adrenal glands, gonads (ovaries and testes), and liver, depending on the specific function and type of steroid. They play crucial roles in maintaining homeostasis and regulating various physiological processes in the body [
67,
68,
69].
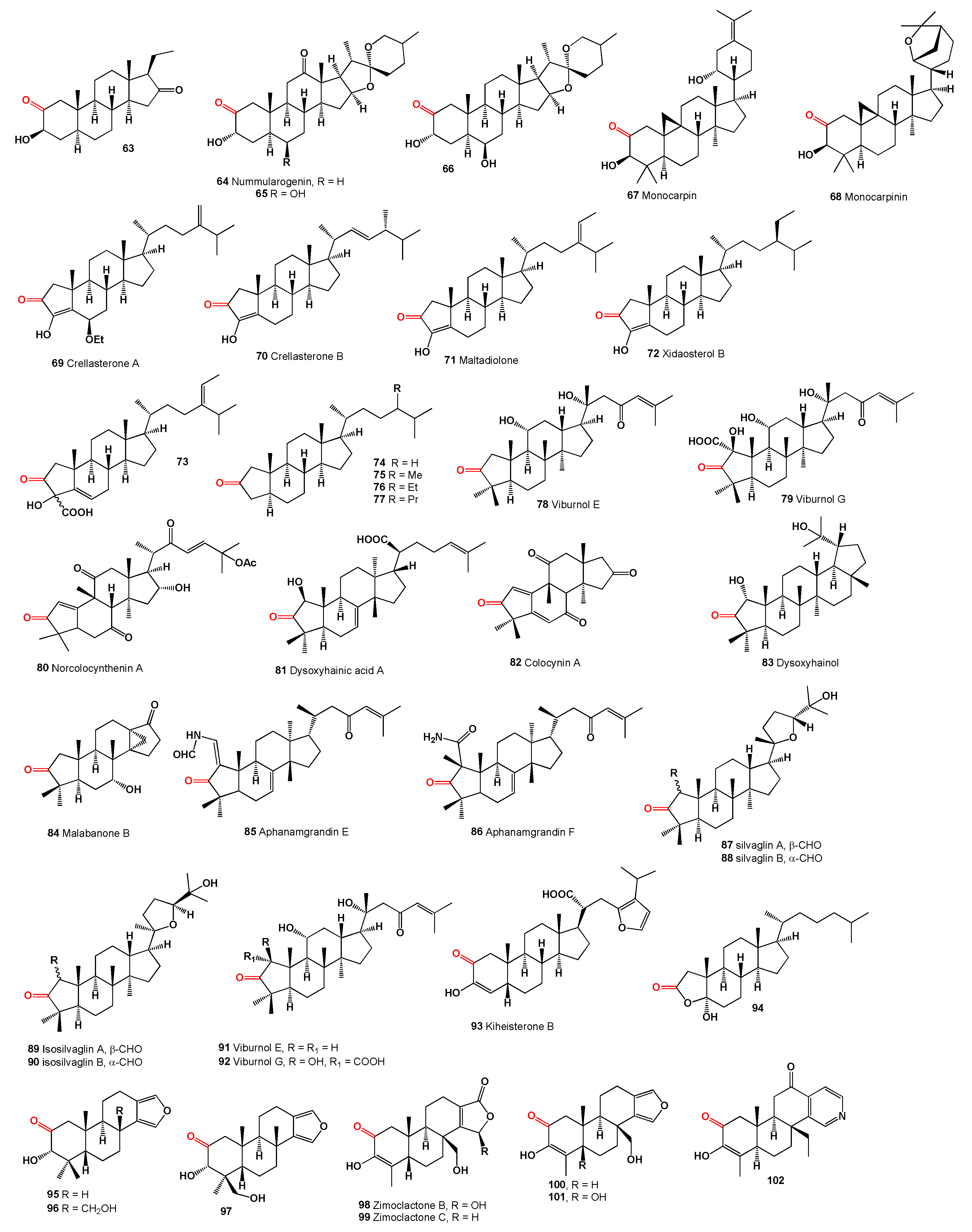
3-Hydroxypregnane-2,16-dione (
63) was isolated from ethanol extracts of the wood and bark of the Red cedar,
Trichilia hirta [
70]. The spirostane known as (25S)-3α-Hydroxy-5α-spirostane-2,12-dione, or nummularogenin (
64), along with two other spirostanes (
65 and
66), were discovered in the extract of the
Ziziphus nummularia shrub, commonly referred to as wild jujube or jhahrberi in Hindi [
71]. Monocarpine (
67), a cycloartane derivative featuring an intriguing combination of oxygen functions in ring A and a unique C-17 side chain, was isolated from the trunk bark of
Monocarpia marginalis [
72], and monocarpinin (
68) was detected in the same tree [
73].
A series of 2-ketosteroids related to A-nor steroids have been identified in marine invertebrates and various plant species. Notably, crellasterones A (
69) and B (
70) were isolated from the sponge
Crella incrustans, collected in New Caledonia [
74]. These crellasterones structurally resemble the semi-synthetic steroid maltadiolone (
71), which is medically used and exhibits calcium-binding activity comparable to cells not treated with calcium channel blockers [
75]. This category also includes xidaosterol B (
72), found in extracts from the South China Sea sponge
Neopetrosia chaliniformis [
76], and a compound from the brown alga
Sargassum carpophylum (
73), collected in the South China Sea [
77]. Additionally, A-nor steroids (
74-
77) were detected in crude oil extracts, marine sediments, and other geological and environmental sources [
67].
Viburnum dilatatum, known as linden viburnum, a deciduous shrub from the Adoxaceae family, is noted for producing clusters of red drupes when ripe. Introduced in the mid-Atlantic regions of the USA, from New York to Virginia, its berries, leaves, and stems are used in Traditional Chinese Medicine to treat snake bites, dysentery, and as an anti-helminthic. From this shrub, A-nor triterpenoids viburnols E (
78) and G (
79) have been isolated [
78,
79].
The fruits of
Citrullus colocynthis contain a cucurbitane 3-nor-triterpenoid named norcolocynthenin B (
80), with a unique 5/6/6/5-fused ring system, showing significant cytotoxic activity against human cancer cell lines HL-60 and PC-3 [
80].
Dysoxylum hainanense, known for its twigs and leaves, produces triterpenoids such as dysoxyhainic acid A (
81, structures see in
Figure 4), with a novel 2-nor-1,3-cyclotirucallane skeleton, showing moderate antibacterial activity against Gram-positive bacteria [
81].
Moreover, a phytochemical analysis of
Citrullus colocynthis fruits revealed a variety of structurally diverse nonanorcucurbitane-type triterpenoids, including colocynins, among which colocynin A (
82) displayed anti-acetylcholinesterase activity and notable cytotoxicity against various cancer cell lines [
82,
83].
In the study of
Dysoxylum hainanense, a novel triterpenoid named dysoxyhainol (
83) was identified from its twigs and leaves. This compound, with a modified ring A structure, exhibited moderate antibacterial activity against Gram-positive bacteria [
81]. Additionally, malabanone B (
84), featuring a unique tricyclo[4.3.1.01,6]decane unit, was isolated from the same plant parts [
84].
Aphanamixis grandifolia stems produced aphanamgrandins E (
85) and F (
86), which are derivatives of 2,3-seco-tirucallane triterpenoids [
85]. The rootwood of
Aglaia sylvestris yielded dammarane-type triterpenoids, including silvaglin A (
87), isosilvaglin A (
89), and their 1H-β-epimers, silvaglin B (
88) and isosilvaglin B (
90), characterized by a
Δ1(3)-bond [
86].
Viburnum dilatatum leaves yielded dammarane triterpenoids viburnols E (
91) and G (
92) [
87,
88], and the cytotoxic steroid kiheisterone B (
93) was discovered in a Maui sponge [
89]. The minor steroid (
93), isolated from the red alga
Laurencia obtusa of the Lakshadweep islands, was identified through spectroscopic data and synthesis as 2α-oxa-2-oxo-5α-hydroxy-3,4-di-norcholestane, marking the first isolation of a ring A-dinorsteroid from a natural source. This compound, named 2α-oxa-2-oxo-5α-hydroxy-3,4-dinorcholestane (
94), was also found in the Arabian Sea red alga
Laurencia obtuse [
90]. Additionally, three compounds (
95,
96, and
97) were isolated from a Western Australian sponge,
Spongia sp. [
91].
Zeng and co-workers [
92] reported the discovery of new compounds zimoclactone A (
98) and zimoclactone B (
99) from marine sponges. Furthermore, Parrish and co-workers isolated three new diterpenes: 18-nor-3,17-dihydroxy-spongia-3,13(16),14-trien-2-one (
100), 18-nor-3,5,17-trihydroxyspongia-3,13(16),14-trien-2-one (
101), and spongiapyridine (
102) from an unidentified
Spongia species collected in Sulawesi, Indonesia [
93].
Summarized on the biological activity of 2-ketosteroids is presented in
Table 2, and details are described partially in the text or a full description of the activity is written in the original articles.
Figure 4.
2-Ketosteroids derived from algae, plants, and sponges.
Figure 4.
2-Ketosteroids derived from algae, plants, and sponges.
3-Ketosteroids
3. -Ketosteroids are interesting primarily because of their biological significance and their roles in various physiological processes. Many 3-ketosteroids are key hormones, including corticosteroids and sex hormones like testosterone and progesterone. These hormones play crucial roles in regulating metabolism, immune function, salt and water balance, development of sexual characteristics, and reproductive functions. Due to their hormonal activities, 3-ketosteroids are used in a variety of therapeutic applications. For example, corticosteroids are widely used to treat inflammation, allergies, and autoimmune diseases. Similarly, synthetic derivatives of sex hormones can be used in hormone replacement therapy, contraception, and treatment of hormone-sensitive cancers. 3-ketosteroids are involved in key biochemical pathways such as steroidogenesis, where they are intermediates in the synthesis of various other steroids. This makes them critical for maintaining the balance and production of hormones in the body [
94,
95,
96,
97].
In scientific research, 3-ketosteroids are studied for their role in metabolic pathways, their impact on various health conditions, and their potential as targets for new drugs. Understanding how these compounds interact with cellular receptors and enzymes can lead to the development of new treatments for a variety of diseases [
94,
98].
The 3-keto group in these steroids affects their chemical reactivity and interaction with biological molecules, influencing how they bind to receptors and other proteins. This structural aspect is crucial for their biological function and is a key area of study in biochemistry and pharmacology. Overall, the study of 3-ketosteroids blends aspects of biochemistry, medicine, and pharmacology, making them a fascinating and important area of scientific inquiry [
98,
99,
100,
101].
Wallenone (
103, structures see in
Figure 5), a C32 tirucallane-type triterpene, was isolated from the leaves of
Gyrinops wala, a small tree from the Thymelaeaceae family grown in Ceylon [
102]. More recently, this compound was also extracted from the ethyl acetate extract of the dried leaves of
Esenbeckia stephani (Rutaceae) [
103]. A neosteroid named 24ξ-methoxy-24,25-dimethyl-lanost-9(11)-en-3-one (
104) was isolated from extracts of
Neolitsea pulchella, a tree primarily found in the subtropical biome of Hong Kong, in the early 1970s [
104,
105]. Additionally, the triterpenoid 24,25-dimethyl-9(11),23-lanostadienol (
105) was obtained from the stems of various
Quercus species (
Q. bambusaefolia, Q. championi, and
Q. myrsinaefolia) [
106].
An anticancer agent,
t-Bu 7α,12α-dihydroxy-4,4,14α-trimethyl-3,11,15-trioxo-5α-chol-8-en-24-oate (
106), known as
t-butyl lucidenate B, was isolated from the fruiting bodies of the oriental fungus
Ganoderma lucidum [
107]. Cycloeucalenone (
107) was isolated from an unidentified fungus collected in New Jersey [
108]. Akihisa and co-workers [
109] reported that the fungus
Glomerella fusarioides transformed cycloartenol into cycloartane-3,24-dione (
108), rare 4α,4β,14α-trimethyl-9β,19-cyclopregnane-3,20-dione (
109), and 24,25-dihydroxy-cycloartan-3-one (
110). An extract from
Parthenium argentatum, commonly known as guayule, contained a cytotoxic steroid named argentatin A (
111), which showed cytotoxic effects against human colon cancer cell lines and normal epidermal keratinocytes [
110].
Unique steroids named malabanone A (
112), which incorporate a unique tricyclo [4.3.1.01,6] decane unit, were isolated from the stem bark of
Ailanthus malabarica. The biosynthesis of these steroids is suggested to originate from ailanthol, also found in this plant [
111]. Three steroids with an incorporated cyclopropane unit at positions 14 and 18, named ailanthusins A (
113), B (
114), and D (
115), were isolated from the CH
2Cl
2 extracts of the Thailand rainforest tree
Ailanthus triphysa [
112].
A CHCl
3-MeOH extract from the bark of
Aglaia crassinervia collected in Indonesia led to the isolation of aglaiaglabretol A (
116), found in the stems of
Spathelia excelsa (Rutaceae), exhibiting larvicidal properties against the yellow fever mosquito,
Aedes aegypti, with an LC
50 of 4.8 µg/mL [
113]. A series of antitumor triterpene glucosides, named cumingianosides M (
117), containing a 14,18-cycloapotirucallane-type skeleton, were isolated from a cytotoxic fraction of the leaves of
Dysoxylum cumingianum, displaying significant cytotoxicity against leukemia and melanoma cell lines [
114,
115].
A cytotoxic steroid, aragusterol A (
118), possessing potent antitumor activity, was isolated from an Okinawan sponge of the genus
Xestospongia. The compound strongly inhibited cell proliferation in KB, HeLaS3, P388, and LoVo cells
in vitro and demonstrated potent
in vivo antitumor activity against P388 and L1210 in mice [
116]. Additionally, 26,27-cyclosterols aragusterols B (
119), C (
120), and D (
121) have been identified in the same Okinawan marine sponge [
116,
117]. A wide array of steroids, including aragusterol A (
118) and another compound (
122), were found in the marine sponge
Petrosia (
Strongylophora) sp. collected from the Similan Island, Thailand [
118].
A limonoid named hortiolide D (
123) was found in CH
2Cl
2 and MeOH extracts from the stem of
Hortia oreadica [
119], and a protolimonoid named capulin (
124), featuring a four-membered ring in its side chain, was isolated from the stem barks of
Capuronianthus mahafalensis, a species endemic to Madagascar [
120].
A limonoid named hortiolide D (
123) was found in CH
2Cl
2 and MeOH extracts from the stem of
Hortia oreadica [
119], and a protolimonoid named capulin (
124), featuring a four-membered ring in its side chain, was isolated from the stem barks of
Capuronianthus mahafalensis, a species endemic to Madagascar [
120].
Two unusual malabaricane-type triterpenes, (14S,17S,20S,24R)-25-hydroxy-14,17-cyclo-20,24-epoxy-malabarican-3-one (
125) and (14S,17S,20S,24R)-20,24,25-trihydroxy-14,17-cyclo-malabarican-3-one (
126), were isolated from the oleoresin of the wounded trunk of
Ailanthus malabarica [
121]. The steroid altrenogest, a progestin from the 19-nortestosterone group widely used in veterinary medicine to suppress or synchronize estrus in horses and pigs, produces two photoproducts (
127 and
128) through photolysis experiments [
122]. The steroid erectasteroid H (
129) exhibited cytotoxic activity against P-388 (leukemia) and HT-29 cells [
123], and a spirosteroid (
130) was isolated from the Formosan soft coral
Nephthea erecta [
124,
125].
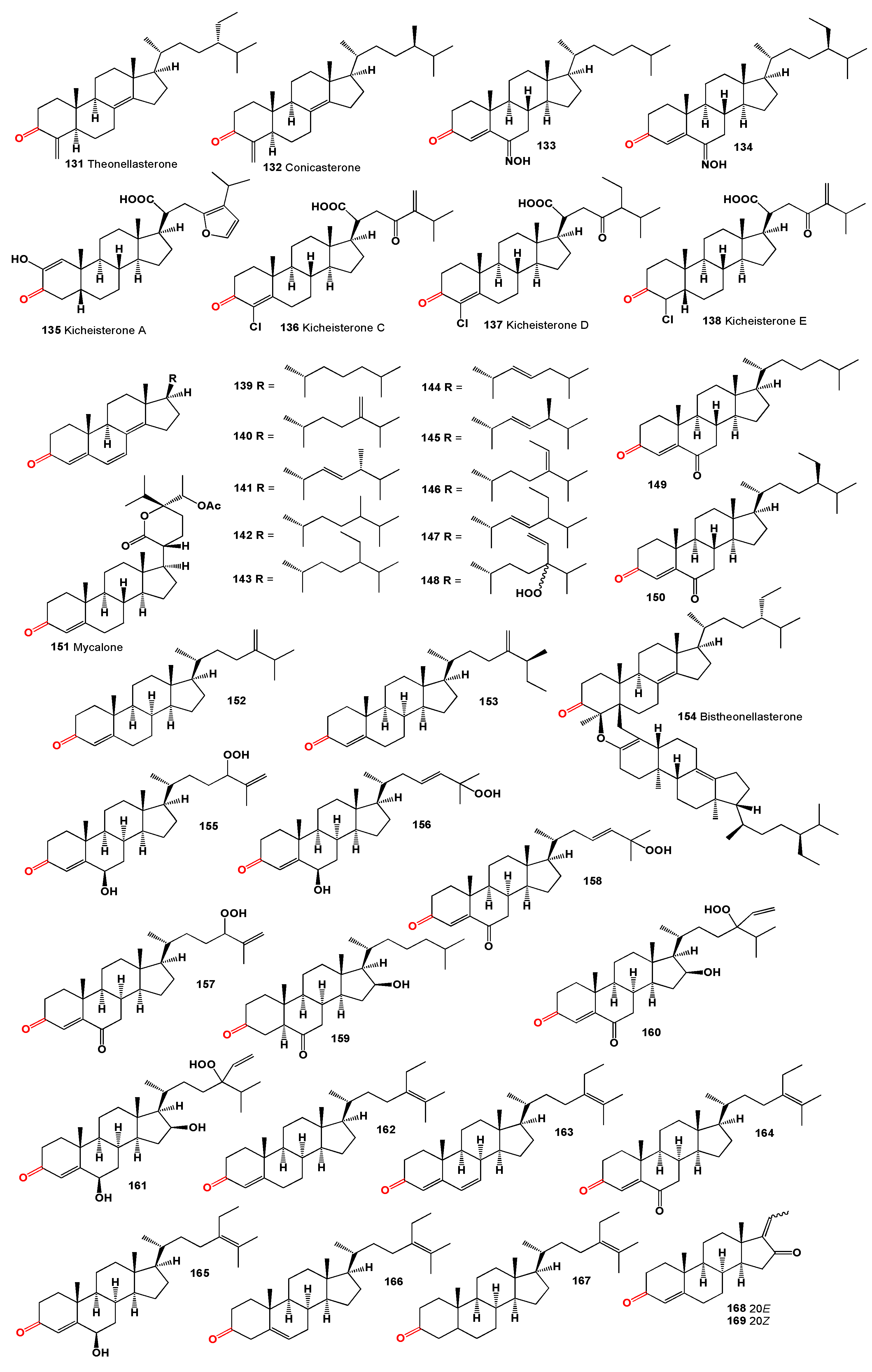
Two steroids, theonellasterone (
131) and conicasterone (
132), featuring a keto group at C-3, were identified in marine sponges from the genus
Theonella [
126]. Additionally, two steroidal oximes (
133 and
134) from the
Cinachyrella sp. sponge were found to inhibit human placental aromatase competitively, suggesting their potential as inhibitors in hormone-dependent tumors [
127]. Holland and colleagues described the synthesis of these compounds [
128]. The cytotoxic kicheisterone A (
135), distinguished by
cis-fused rings A and B with carboxy groups at position 21 and furan fragments in the side chains, was isolated from an unidentified Hawaiian sponge of the
Poecilosclerida sp. [
129]. This steroid, along with kicheisterones C-E (
136-
138) which contain a chlorine atom and are part of a rare group of halogenated steroids, was also found in the sponge
Strongylacidon sp. [
130].
A series of 3-oxo-4,6,8(14)-triunsaturated steroids with cholestane (
139 and
140), ergostane (
141–
143 and
145), and stigmastane skeletons (
144 and
146–
148) were discovered in the lipid extract of
Dysidea herbacea [
131]. Steroids
145 through
148 were isolated as C-24 epimeric mixtures. Compounds
139 through
148 represent the second instance of steroids from a marine source featuring a conjugated 3-oxo-4,6,8(14)-triene system; compound
140 had previously been isolated from
Dyctionella incisa [
132]. Cholest-4-ene-3,6-dione (
149) and (24R)-24-ethylcholest-4-ene-3,6-dione (
150) were isolated from the marine sponge
Cinachyra tarentina [
133]. Compound
149 was synthesized from cholesterol, while the structure of
150 was confirmed by synthesis from sitosterol, involving Jones oxidation to determine the R chirality at C-24.
The steroid mycalone (
151), with a six-membered lactone side chain, was isolated from a
Mycale species in southern Australia [
134]. Ergosta-4,24(28)-dien-3-one (
152) was identified in the subantarctic shallow-water sponge
Geodia cydonium [
135], and a similar compound, (25S)-26-Methyl-24-methylenecholest-4-en-3-one (
153), was isolated from the marine fossil sponge
Neosiphonia supertes [
136].
Finally, a dimeric steroid, bistheonellasterone (
154), likely biosynthesized
via a Diels-Alder cycloaddition of theonellasterone, was discovered in the Okinawan marine sponge
Theonella swinhoei [
137].
A series of steroid hydroperoxides (
155-
158) isolated from the red alga
Galaxaura marginata collected near the coasts of Taiwan demonstrated high cytotoxic activities against tumor cells P-388, KB, A-549, HT-29, with semi-inhibiting concentrations (IC
50) within the range of 0.2 μg/mL [
138]. The steroidal 3,6-diketone
159, oxygenated at C(16), was isolated from the alga
Jania rubens, showing high toxicity against some tumor cells (IC
50 = 0.5 μg/mL) [
139]. Hydroperoxides (
160 and
161) isolated from the brown alga
Turbinaria conoides also exhibited cytotoxic properties [
140].
Sterols and sterones were discovered in a sponge
Stelletta sp., collected at a depth of 700 m in the deep Coral Sea, southeast of Noumea. These compounds, the first examples of stigmastane steroids with a Δ
24,25 from a marine origin, were elucidated as stigmasta-4,24(25)-dien-3-one (
162), stigmasta-4,6,24(25)-trien-3-one (
163), stigmasta-4,24(25)-diene-3,6-dione (
164), 6β-hydroxystigmasta-4,24(25)-dien-3-one (
165), stigmasta-5,24(25)-dien-3β-ol (
166), and compound
167 [
141].
Guggulsterones (
168 and
169), phytosteroids found in the resin of the guggul plant,
Commiphora mukul, exist as two stereoisomers, (
E)-guggulsterone and (
Z)-guggulsterone [
142,
143]. In humans, they act as antagonists of the farnesoid X receptor, initially thought to decrease cholesterol synthesis in the liver, though studies indicate no overall reduction in total cholesterol, with levels of low-density lipoprotein increasing in many cases [
143,
144,
145,
146,
147].
An ethyl acetate extract from the gorgonian
Leptogorgia sp., collected from the South China Sea, contained a dihydroxy-ketosteroid (
170) [
148]. From the gorgonian coral
Euplexaura anastomosans, collected off the shore of Keomun Island, South Sea Korea, steroidal hemiacetals named anastomosacetals A (
171) and D (
172) were obtained. Another gorgonian species,
Bebryce indica, collected off the coast of Sanya (Hainan, China), was found to contain a steroidal glycoside named bebrycoside (
173) [
150]. Additionally, bebrycoside (
173) and related compounds such as 27-O-[β-D-arabino-pyranosyloxy]-20β,22α-dihydroxy-cholest-4-ene-3-one, named muricellasteroid D (
174), and the rare steroid 22α-O-acetyl-2β-O-methylene-[4β-hydroxy-phenyl]-cholest-4-ene-3-one, named muricellasteroid E (
175), were isolated from the EtOH/CH
2Cl
2 extracts of the South China Sea gorgonian coral
Muricella flexuosa. Both compounds
174 and
175 demonstrated moderate cytotoxicity against A375, K562, and A549 cancer cell lines [
151].
An unusual hemiketal steroid, 23-keto-cladiellin A (
176), was obtained from the monohydroxylated sterol fraction of the soft coral
Chromonephthea braziliensis [
152]. From the soft coral
Nephthea sp., a unique pentacyclic hemiacetal sterol named nephthoacetal (
177) and its acetyl derivative (
178) were isolated. Compound (
177) exhibited significant inhibitory effects with an EC
50 value of 2.5 μg/mL, while maintaining low toxicity with an LC
50 greater than 25.0 μg/mL. The
in vitro cytotoxic activity of both compounds (
177 and
178) showed moderate effects with IC
50 values of 12 and 10 μg/mL, respectively [
153].
Krempene A (
179, structures in Figure 6), an unprecedented pregnane-type steroid with a highly unusual hexacyclic oxadithiino unit fused to the steroidal ring A, was isolated from the marine soft coral
Cladiella krempfi [
154]. Additionally, a rare steroidal hydroperoxide, 13,14-seco-22-norergosta-4,24(28)-dien-19-hydro-peroxide-3-one (
180), has been found in the diethyl ether fraction of the Red Sea soft coral
Litophyton arboretum [
159]. Lastly, an unprecedented spinaceamine-bearing pregnane named scleronine (
181) was produced by a Chinese soft coral
Scleronephthya sp. [
156].
Two steroids (
182 and
183) along with pregna-1,4,20-trien-3-one (
184) have been isolated from the Pacific octocoral
Carijoa multiflora. Compound (
182), featuring a spiropregnane-based steroidal skeleton, has demonstrated antibacterial activity [
157]. Additional pregnane steroids (
185 and
186), similar to compounds (
183 and
184), were isolated from a gorgonian
Carijoa sp. collected from the South China Sea. These compounds, particularly (
184,
185, and
186), exhibited cytotoxicity against the human hepatoma cell line Bel-7402, with IC
50 values of 9.3, 11.0, and 18.6 µM, respectively [
158]. The Hainan soft coral
Scleronephthya gracillimum has released a pregnane analogue (
187) [
159], and two unique chloro-pregnane steroids (
188 and
189) have been isolated from the eastern Pacific octocoral
Carijoa multiflora [
160].
An unusual steroid thioester, parathiosteroid A (
190), was isolated from the 2-propanol extract of the soft coral
Paragorgia sp. collected in Madagascar. This compound displayed cytotoxicity against a panel of three human tumor cell lines at the micromolar level [
161]. The reef soft coral
S. brassica, which was cultured in an aquarium, yielded four steroids with methyl ester groups: sinubrasones A (
191), B (
192), D (
193), and C (
194). Compounds
191 and
192 showed significant cytotoxicity, while compounds
193 and
194 demonstrated notable anti-inflammatory activities [
162]. Additionally, the ethyl acetate extract of a reef soft coral
S. brassica, cultured in a tank, produced two steroids, sinubrasones A (
195) and B (
196), both of which exhibited significant cytotoxicity [
163].
The soft coral
Umbellulifera petasites produces steroid (
197), while petasiterone B (
198), and 5α-pregna-20-en-3-one (
199) have been found in the soft coral
Alcyonium gracillimum [
162,
164,
167].
Figure 6.
3-Ketosperoids derived from algae, marine invertebretes and plants.
Figure 6.
3-Ketosperoids derived from algae, marine invertebretes and plants.
Figure 6.
3-Ketosteroids derived from marine invertebretes and plants.
Figure 6.
3-Ketosteroids derived from marine invertebretes and plants.
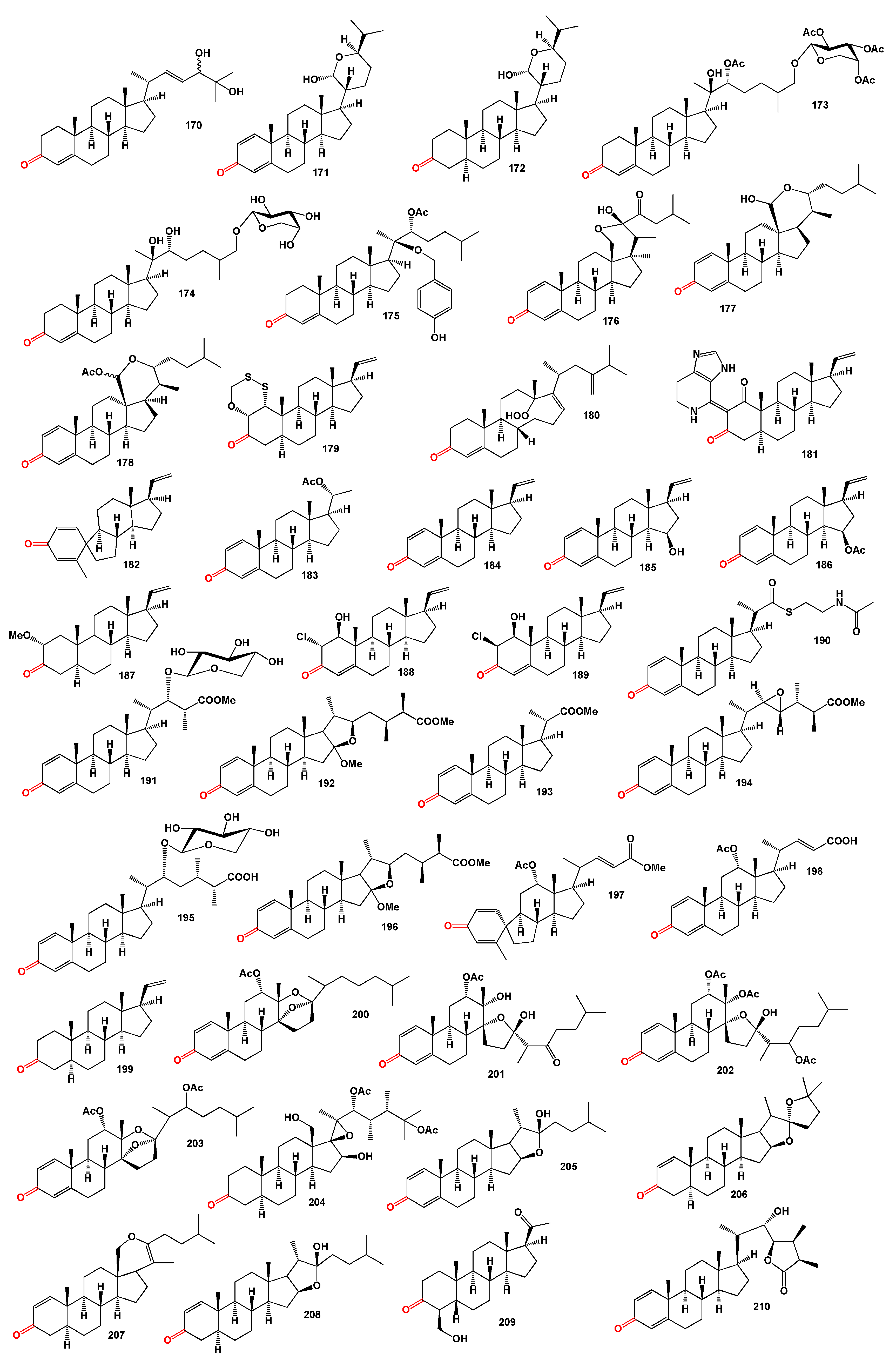
Unique highly oxygenated 13,17-secosteroids with a split D ring, named isogosterones A–D (
200–
203), were obtained from extracts of a Japanese octocoral of the genus
Dendronephthya collected off the Izu Peninsula. These steroids have demonstrated the ability to inhibit the settlement of
B. amphitrita cyprid larvae [
166]. A polyhydroxygenated steroid, hipposterone M (
204), showing cytotoxicity against human cytomegalovirus (HCMV), was derived from extracts of the Taiwanese octocoral
Isis hippuris collected at Orchid Island [
167]. Additionally, steroids (
205–
208), isolated from the crude extract of
Alcyonium gracillimum, exhibited moderate cytotoxicity (IC
50 22 µg/mL) and antiviral activity (IC
50 8 µg/mL) against P388 and HSV-I (human α-herpesvirus), respectively [
168,
169].
A pregnane derivative, 4-hydroxymethyl-5β-pregnan-3, 20-dione (
209), was isolated from the South China Sea gorgonian
Subergorgia suberosa [
170]. From the lipid extracts of the Formosan soft coral
Paraminabea acronocephala, a marine withanolide named paraminabeolide D (
210) was obtained [
171]. Summarized on the biological activity of 3-ketosteroids is presented in
Table 3, and details are described partially in the text or a full description of the activity is written in the original articles.
6-Ketosteroids
6-Ketosteroids are another interesting group within the steroid family, characterized by the presence of a ketone group at the sixth carbon of the steroid nucleus. Similar to other ketosteroids, 6-ketosteroids can be involved in specific metabolic pathways. They may serve as intermediates in the synthesis or breakdown of steroid hormones, although their presence and role in these pathways tend to be more specialized and less general than those of 3- or 4-ketosteroids. The specific activities of 6-ketosteroids can vary, but like other steroids, they might interact with certain receptors or enzymes, influencing various physiological processes. The exact functions and significance of these interactions can depend heavily on the particular structure and context of the 6-ketosteroid [
187,
188,
189,
190,
191].
Due to their unique position of the ketone group, they may have distinct biochemical properties that could be exploited in drug development or in studies of steroid metabolism and function. The presence of a ketone group at the sixth position can significantly affect the steroid’s ability to bind to and activate or inhibit various receptors. Structural studies, such as X-ray crystallography or NMR spectroscopy, can help elucidate how these changes impact receptor interaction and function. While not as directly utilized in therapy as some other ketosteroids, understanding the properties of 6-ketosteroids could lead to the development of new drugs, particularly if these steroids exhibit unique interactions with biological molecules that can be modulated for therapeutic benefit [
191,
192,
193]. In general, 6-ketosteroids are a more niche area of study within the broader field of steroid research. Their unique characteristics can provide valuable insights into steroid chemistry and biology, contributing to our overall understanding of steroid functions and their implications in health and disease.
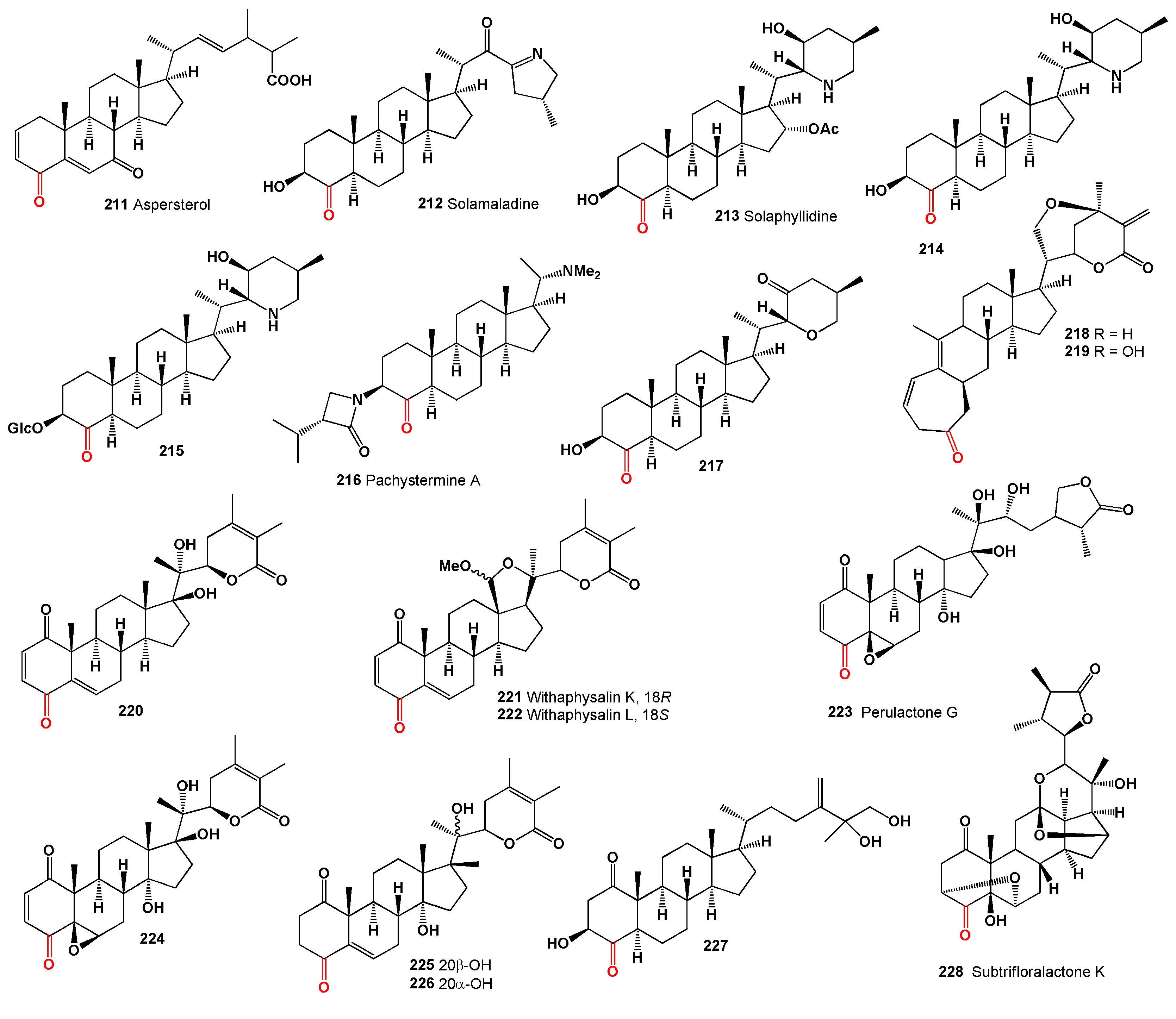
Antibiotic WF 15604A (
229, structures see
Figure 8), a bioactive secondary metabolite that inhibits cholesterol synthesis, has been found in the leaves of
Preussia minima collected in the Iberian Peninsula [
194]. Two steroids, chiograsterone (
230) and isochiograsterone (
231), have been isolated from
Chionographis japonica [
195].
Plant hormones such as 3,24-diepicastasterone (
232) were detected in the seeds of
Phaseolus vulgaris. Homoteasterone (
233) was isolated from the EtOAc-hexane extract of rice from
Oryza sativa, radish
Raphanus sativus, and “Remo” seeds. Furthermore, 25-methylcastasterone (
234) and another compound (
235) were isolated from a methanol extract of ryegrass
Lolium perenne pollen [
196].
Polyoxygenated meroterpenoids named aperterpene N (
236) and O (
237) were isolated from the marine algal-derived fungus
Aspergillus terreus EN-539 [
197]. Compound (
236) displayed neuraminidase inhibitory activity, with an IC
50 value of 18.0 μM. Meroterpenoid terretonin D1 (
238) was obtained from the marine sediment-derived fungus
Aspergillus ustus KMM 4664 and from
Aspergillus terreus EN-539, associated with the fresh gut of a Pacific oyster [
198].
The 5α,9α-endoperoxide (
239) was isolated from the fruiting bodies of
Stropharia rugosoannulata and protected neuronal cells by attenuating endoplasmic reticulum stress caused by thapsigargin, an inhibitor of Ca2+-ATPase [
199]. Additionally, two steroids containing an oxetane ring (
240 and
241) have been isolated from the endophytic fungi
Chaetomium sp. and
Colletotrichum sp., respectively. Both compounds demonstrated activity in an AChE inhibitory assay [
200,
201].
A cytotoxic steroid (
242) has been discovered in the filamentous fungus
Talaromyces stipitatus, belonging to the family Trichocomaceae. It exhibited cytotoxic activity against Hep3B (IC
50 4.75 µM), HepG2 (IC
50 8.85 µM), and Huh-7 (IC
50 13.78 µM) [
202]. A 4-ketosteroid (
243) was identified in several fungal species, including
Fomes fomentarius [
203],
Grifola frondosa [
204], and
Phellinus linteus [
205], showing activities such as β-hexosaminidase inhibition, HNE inhibitory, and NO production inhibition.
Hericium erinaceus, commonly known as the lion’s mane mushroom, contained a steroid (
244) that demonstrated inhibition of TNF-α secretion and NO production in lipopolysaccharide-induced RAW 264.7 cells [
206].
Three ergosterols, ganocalidophins A–C (
245‒
247), were isolated from the fruiting bodies of
Ganoderma sinense. These compounds exhibited inhibitory activities against NO production with IC
50 values of 17.7, 32.4, and 19.8 µM, respectively [
207]. 4-Keto-ergosterol (
248), produced by various fungi including
Colletotrichum sp. [
200],
Ganoderma sinense [
207],
Pleurotus eryngii [
208],
Psathyrella candolleana [
209], and
Volvariella volvacea [
210], demonstrated cytotoxic activity against HepG2 (IC
50 5.90 µM), SGC-7901 (IC
50 12.03 µM) [
210], and minimal activity against A549, HL-60, MCF-7, SMMC-7721, SW480 (IC
50 > 40 µM) [
209], RAW264.7 (IC
50 > 100 µM) [
208]. This compound also inhibited NO production with varying efficacy (IC
50 28.5 µM and 100 µM) [
208].
Steroid (
249), obtained from the fungus
Volvariella volvacea, exhibited cytotoxic activity against HepG2 cells with an IC
50 of 20.27 µM [
210]. Another steroid (
250) was isolated from the rare poroid fungus
Ganoderma resinaceum and showed inhibition of NO production with an IC
50 of 35.19 µM [
211]. A 3,11-diol steroid (
251), detected in the fungus
Gliomastix sp. belonging to the family Bionectriaceae, displayed antiviral activity against the EV-71 virus with an IC
50 of 17.8 µM and cytotoxic activity against several cancer cell lines including HL-60 (IC
50 1.75 µM), DU-145 (IC
50 7.37 µM), HeLa (IC
50 12.1 µM), and MOLT-4 (IC
50 6.53 µM) [
212].
Dankasterone A (
252) and B (
253) were isolated from a fungal strain of
Gymnascella dankaliensis derived from the sponge
Halichondria japonica [
213]. Dankasterone A demonstrated significant cytotoxicity against tumor cells in culture. Phomopsterone B (
254), produced by the endophytic fungus
Phomopsis sp., exhibited anti-inflammatory activity and showed promising results in iNOS inhibitory and NO production inhibition assays [
214]. Three C25 steroids, neocyclocitrinols E-G (
255–
257), were isolated from the endophytic fungus
Chaetomium sp. M453 [
200].
Makisterone A (
258), a 28-carbon ecdysteroid distinguished from the common C27 molting hormones like ecdysone and 20-hydroxy-ecdysone by an additional methyl group at the C-24 position, was isolated and characterized from the leaves of
Podocarpus macrophyllus [
215]. The steroidal alkaloid petisidine (
260) was isolated from the leaves of
Petilium raddeanum [
216]. Another alkaloid, korsevinine (
261), was detected in the total ether-soluble alkaloids of
Korolkowia sewerzowii [
217]. A unique steroidal alkaloid with a 5β-hydroxyl group, ebeietinone (
262), was isolated from the bulbs of
Fritillaria ebeiensis var.
purpurea [
218], and petisidinine (
263) was detected in the essential oil of
Petilium raddeana [
219].
Summarized on the biological activity of 6-ketosteroids is presented in
Table 5, and details are described partially in the text or a full description of the activity is written in the original articles.
7-Ketosteroids
7-Ketosteroids represent another specialized class of steroid compounds characterized by the presence of a ketone group at the seventh carbon of the steroid skeleton. While not as prominently featured in basic biological processes as some other steroid classes, 7-ketosteroids are noteworthy. The ketone group at the seventh position can significantly influence the steroid’s chemical reactivity and interaction with biological molecules, such as enzymes and receptors. This unique placement may alter the way these steroids are metabolized compared to their counterparts with ketone groups at other positions. 7-ketosteroids can occur as natural metabolites in the body, particularly as products of steroid hormone metabolism. They might play roles in various physiological processes, although these roles are often less direct than those of primary hormones like estrogens, androgens, or corticosteroids [
220,
221,
222,
223].
One of the most well studied 7-ketosteroids is 7-keto-DHEA (7-ketodehydroepi-androsterone). This compound is known for its potential in enhancing metabolism and is researched for its use in weight loss and immune function. Unlike many other steroids, 7-keto-DHEA is not converted to steroid hormones such as testosterone or estrogen, which makes it a candidate for non-hormonal applications. Overall, while 7-ketosteroids are not as central to major physiological pathways as some other steroids, they offer interesting possibilities for health and wellness applications, particularly in the realm of non-hormonal interventions. Their unique chemical structure and effects continue to be a subject of research and discussion in the scientific community [
224,
225,
226,
227,
228].
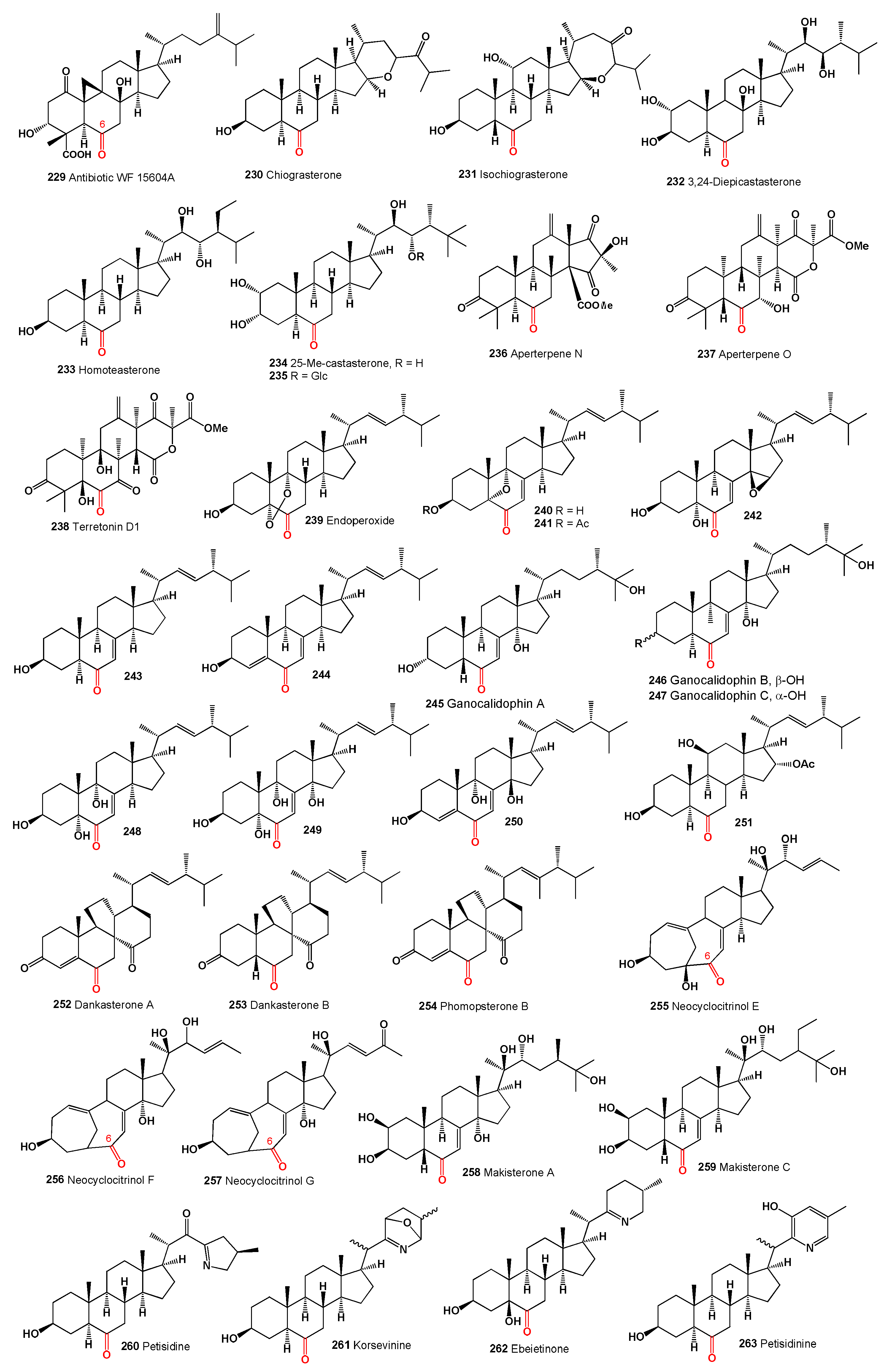
The compound 15α-hydroxy-(22E,24R)-ergosta-3,5,8(14),22-tetraen-7-one (
264, structures see in
Figure 9) was isolated from the endophytic fungus
Penicillium sp. FJ-1 associated with
Acanthus ilicifolius, commonly known as holly-leaved acanthus, sea holly, or holy mangrove. This compound demonstrated potent cytotoxicity against glioma cell lines, with IC
50 values of 3.2 µM for U251, 4.1 µM for BT-325, and 2.3 µM for SHG-44 [
229].
The apolar extract of the marine sponge
Theonella swinhoei revealed a family of polyhydroxy steroids, including conicasterol G (
265) which was isolated [
230]. The compound (22E,24R)-3β-hydroxyergosta-5,8,22-trien-7-one (
266) was identified from the culture extracts of
Aspergillus oryzae, an endophytic fungus isolated from the marine red alga
Heterosiphonia japonica [
231]. Another compound, (22E)-25carboxy-8β,14β-epoxy-4α,5α-dihydroxyergosta-2,22-dien-7-one (
267), exhibited cytotoxic activity towards the A-549 cell line and was isolated from the marine-derived fungus
Aspergillus flavus collected from the Bohai Sea [
177].
Three steroids with a rearranged ring B, including eringiacetal B (
268), were isolated from the fruiting bodies of
Pleurotus eryngii [
232]. Eringiacetal B (
268) demonstrated an IC
50 of 13.0 µM and showed inhibitory effects on nitric oxide production, compared to 23.9 µM for the L-NMMA positive control.
Two sterols, gargalol B (
269) and C (
270), were isolated from the polypore fungus
Grifola gargal from the family Meripilaceae. Both compounds showed potential in inhibiting osteoclast formation, which may be relevant for the prevention of osteoporosis [
233].
A 5,6-epoxy steroid (
271) was discovered in
Hericium erinaceum, also known as the lion’s mane mushroom, and demonstrated cytotoxic activity against HGC-27 with an IC
50 of 29.34 µM [
234]. This sterol was also found in the saprophytic and parasitic fungus
Omphalia lapidescens, where it showed HNE inhibitory activity (IC
50 75 µM) and inhibited TNF-α secretion by 37% at 10 µM [
235]. A polyoxygenated ergosteroid (
272) was detected in an extract of the macrofungus
Omphalia lapidescens, displaying a structure-cytotoxicity relationship with an IC
50 of 23.41 µM against the human gastric cancer cell line HGC-27 [
235]. Another epoxy sterol (
273) was obtained from the fruiting bodies of the fungus
Amauroderma subresinosum and demonstrated 20.9% inhibition of acetylcholinesterase at 100 µM [
236].
In the family Tricholomataceae, the agaric fungus Tricholoma imbricatum contained two steroids (
274 and
275), which exhibited cytotoxic activity against various cancer cell lines: A549 (IC
50 12.4 µM), HL-60 (IC50 12.2 µM), K562 (IC50 13.8 µM), MCF-7 (IC50 17.8 µM), SMMC-7721 (IC
50 27.6 µM), and SW480 (IC
50 19.7 µM) [
237]. Additionally, steroid (
275) was found in the fungus
Phomopsis sp. [
238] and
Chaetomium globosum [
239] and demonstrated α-glucosidase inhibition (IC
50 > 100 µM). Steroid (
276) from the fruiting bodies of
Tricholoma imbricatum showed cytotoxicity against A549 (IC
50 22.8 µM) and SMMC-7721 (IC50 19.5 µM) [
237].
Two steroids (
277 and
278) were discovered in the
Ganoderma mushroom. Specifically,
277, isolated from
Ganoderma philippii, exhibited acetylcholinesterase inhibition [
240], while steroid (
278), found in Ganoderma resinaceum, demonstrated inhibition of NO production [
241].
The compound 22E-3β-hydroxy-5α,6α,8α,14α-diepoxy-ergosta-22-en-7-one (
279) was isolated from the fungus
Aspergillus awamori, which was obtained from soil surrounding the mangrove plant
Acrostichum speciosum [
242]. Compound
279 exhibited weak cytotoxic activity against A549 cancer cell lines. Two 7-keto-ergosterol derivatives, 3β,11α-dihydroxyergosta-8,24(28)-dien-7-one (
280) and 3β-hydroxyergosta-8,24(28)-dien-7-one (
281), were isolated from the fungus
Aspergillus ochraceus EN-31 obtained from the marine brown alga
Sargassum kjellmanianum [
243].
The marine-derived fungus
Rhizopus sp., isolated from the bryozoan
Bugula sp. collected in Jiaozhou Bay, China, yielded several ergosterols: 3β-hydroxy-(22E,24R)-ergosta-5,8,22-trien-7,15-dione (
282), 3β-hydroxy-(22E,24R)-ergosta-5,8,14,22-tetraen-7-one (
283), 3β,15β-dihydroxy-(22E,24R)-ergosta-5,8(14),22-trien-7-one (
284), and 3β-hydroxyl-(22E,24R)-ergosta-5,8(14),22-trien-7,15-dione (
285) [
244]. All isolated compounds demonstrated cytotoxic activity to varying degrees against four different cancer cell lines.
Incrustasterol B (
286), derived from the Mediterranean sponge
Dysidea incrustans, is distinguished from other polar sponge steroids by the presence of Δ
8(9)-7,9-diketo fragments, which are uncommon for marine steroids [
245].
Three 7-keto-phytoecdysteroids (
287-
289), cyclopentanoperhydrophenanthrene-ringed polyhydroxylated chemicals known for protecting plants from nematodes and insect pests, have been isolated from various plant extracts [
246]. Two ecdysterone-type sterol glycosides, which also act as melanogenesis inhibitors, were isolated from different sources: pfaffiaglycosides E (
287) from the roots of
Pfaffia glomerata and brainesteroside C (
288, 25-deoxycalonysterone-3-O-β-D-glucopyranoside) from the rhizomes of
Brainea insignis [
247]. A minor ecdysteroid named calonysterone (
289) was detected in extracts of
Cyanotis arachnoidea [
248].
The steroidal sapogenin, pogosterol (
290), isolated from the leaves of
Vernonia pogosperma, was characterized for its structure and stereochemistry. Pogosterol exhibited weak cytotoxic activity against L-1210 cells in vitro, with an IC
50 of 1.7 µg/mL [
250]. Additionally, an antibiotic metabolite, helvolic acid (
291), was isolated from the rice sheath rot pathogen fungus
Sarocladium oryzae [
251].
Taccalonolides are a unique class of microtubule-stabilizing agents isolated from plants of the genus
Tacca, demonstrating effectiveness against drug-resistant tumors in cellular and animal models [
252]. Taccalonolides AD (
292), I (
293), J (
294), and K (
295) are 7-keto steroids that exhibit cytotoxic effects. Taccalonolide AD (
292) was isolated from
Tacca plantaginea, increasing cellular microtubule density and microtubule bundling in HeLa cells at 17 µM, with anti-proliferative actions exhibiting an IC
50 of 3.48 µM [
253]. Taccalonolides I, J, and K, characterized by a shift of the ketone group from C6 to C7 on ring B, were detected in extracts of
T. plantaginea, with taccalonolide K (
295) also found in
T. paxiana [
254].
The methanol extract of the Mediterranean encrusting sponge Oscarella lobularis yielded small amounts of two polyoxygenated sterols (
296 and
297) featuring a unique 5α,6α-epoxy-7-keto function [
255]. The marine sponge
Polymastia sobustia from the South China Sea contained 3β-hydroxystigmast-5-en-7-one (
298) [
256], and polysterol A (
299) was obtained from a Japanese sponge,
Epipolasis sp. [
257]. A rare steroid with a cyclopropane ring at C-25 and C-26, named 7-oxopetrosterol, 26,27-cyclo-24,27-dimethyl-3β-hydroxycholest-5-en-7-one (
300), was observed in the marine sponge
Strongylophora corticata [
258]. An oxygenated sterol (
301), obtained from a collection of marine sponge
Polymastia tenax from the Caribbean coast of Colombia, exhibited significant anti-proliferative activity toward A-549, HT-29, H-116, MS-1, and PC-3 tumor cells in the range of 0.5−10 μg/mL [
259]. A specimen from the South China Sea of
Geodia japonica yielded 26-methylergosta-5,24(28)-dien-3β-ol (
302) [
260].
Acetylenic sterols, gelliusterol B (
303, 26,27-bisnorcholest-5-en-23-yn-3β-ol-7-one), gelliusterol C (
304, cholest-5-en-23-yn-3β,7-one), and D (
305, cholest-5-en-23-yn-3β,25-diol-7-one), were isolated from an unidentified species of sponge,
Gellius sp. [
261].
Summarized on the biological activity of 7-ketosteroids is presented in
Table 6, and details are described partially in the text or a full description of the activity is written in the original articles.
11-Ketosteroids
11-Ketosteroids are notable for their biological activities and their roles in human physiology. These steroids, characterized by a ketone group at the eleventh carbon of the steroid nucleus, include important physiological compounds such as 11-keto-testosterone and 11-dehydrocorticosterone. 11-Ketosteroids are biologically active and play crucial roles in the body. For example, 11-keto-testosterone is a potent androgen in fish, although it has lesser activity in mammals. However, its presence and effects in various species suggest a significant evolutionary and functional role [
262,
263,
264,
265,
266].
Corticosteroid 11-ketosteroids, like 11-dehydrocorticosterone, are involved in the body’s stress response and immune regulation. They help modulate inflammation and immune responses, acting through glucocorticoid receptors to exert their effects [
267,
268,
269]. These steroids are part of the metabolism of more commonly known corticosteroids and androgens. They are metabolized by enzymes in the liver and other tissues, influencing both the production and degradation of steroid hormones. Levels of certain 11-ketosteroids can serve as biomarkers for diagnosing and monitoring diseases that involve steroid metabolism disorders or adrenal function anomalies.
In essence, 11-ketosteroids contribute to a variety of physiological processes, and their study helps in understanding both the endocrine system’s complexity and the potential for targeted therapeutic strategies in medicine [
270,
271,
272].
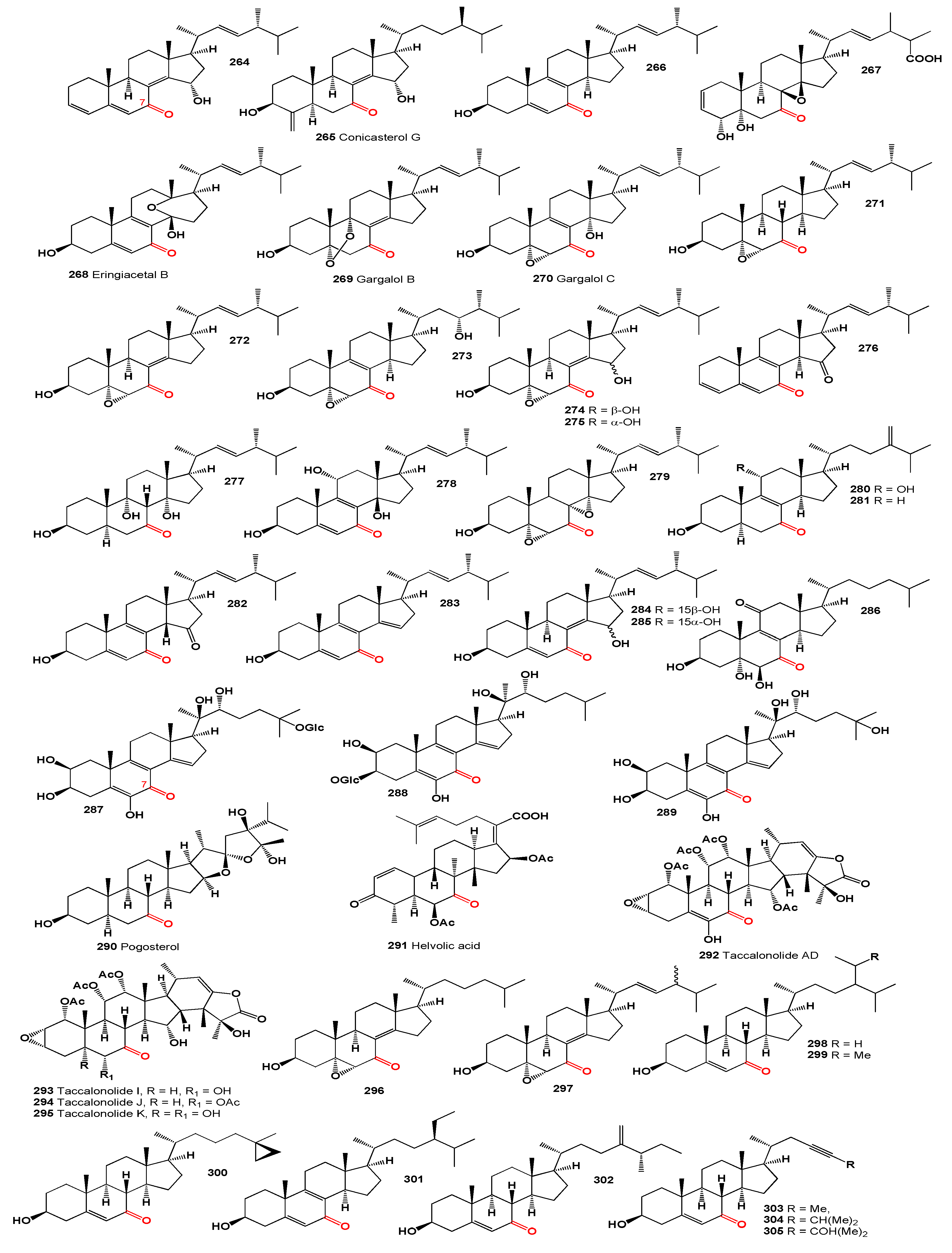
An antifungal antibiotic, 18,22-cyclosterol, Mer-NF8054X (
306), and its analog (
307) have been isolated from
Aspergillus sp. [
273]. Additionally, Mer-NF8054X was isolated from a shaken culture of
Aspergillus ustus and from the culture filtrate of
Emericella heterothallica [
274]. Three lanostanoid triterpenes, ganotropic acid (
308), 3β,7β,15α,24-tetrahydroxy-11,23-dioxo-lanost-8-en-26-oic acid (
309), and 3β,7β,15α,28-tetrahydroxy-11,23-dioxo-lanost-8,16-dien-26-oic acid (
310, structures see in
Figure 10), were isolated from the
n-BuOH extract of the fruiting bodies of the mushroom
Ganoderma tropicum. Ganotropic acid possessed a two-oxygenic five-membered ring system in the side chain of the lanostane skeleton [
275]. Both compounds demonstrated cytotoxic activity against A549, HepG2, THP-1 (with an IC
50 > 80 µM), as well as IL-6 immune-suppressive activity (with an IC
50 of 21 µM) and TNF-secretion inhibition (with an IC
50 of 28 µM) [
275].
A polyhydroxy steroid, zahramycin B (
313), has been isolated from the polar fraction of the extract of the coral
Sarcophyton trocheliophorum. This steroid showed high antibacterial activity against
Staphylococcus aureus, Bacillus subtilis, and the fungus
Pythium ultimum [
276].
Furthermore, 3β,7β-dihydroxy-5α-cholestan-11-one (
314) was found in a mixture of chloroform and methanol (1:1) extracts of the red alga
Laurencia papillosa [
277].
The male African catfish (
Clarias gariepinus) secretes 11-ketotestosterone (
315), a potent androgen, in addition to testosterone. Research indicates that 11-ketotestosterone stimulates spermatogenesis, unlike testosterone, which promotes the development of pituitary gonadotrophs [
278]. Additionally, 11-ketotestosterone (
315) has been extracted from postspawned male sockeye salmon,
Oncorhynchus nerka [
279].
Three 11-ketosteroids, namely incrustasterol A (
316), (
317), and (
318), were identified in the Mediterranean sponge
Dysidea incrustans [
280] and
Dysidea fragilis from the Venice lagoon [
281]. From the soft coral
Klyxum flaccidum, two bioactive steroids, klyflaccisteroid C (
319) and D (
320), were isolated [
282]. Furthermore, a sulfated polyhydroxy steroid (
321) was isolated from three species of brittle stars collected near Noumea (New Caledonia):
Ophiocoma dentata,
Ophiarthrum elegans, and
Ophiarachna incrassata [
283].
Asperflotone (
322), an 8(14-->15)-
abeo-ergostane derived from the sponge-associated fungus
Aspergillus flocculosus 16D-1, exhibited cytotoxic effects against A549, HepG2, and THP-1 cell lines with an IC
50 exceeding 80 µM, along with notable IL-6 immune-suppressive activity (IC
50 22 µM) [
284]. Additionally, the same fungal genus yielded three steroids featuring identical side chains, including asperflosterol (
323) [
285], compound (
324), and asperfloroid (
325) [
285,
286], both asperflosterol (
323) and asperfloroid (
325) exhibited anti-inflammatory properties. From the same fungus, an ochratoxin-ergosteroid heterodimer, ochrasperfloroid (
326), was isolated and shown to significantly inhibit IL-6 and nitric oxide (NO) production in LPS-stimulated cells [
284].
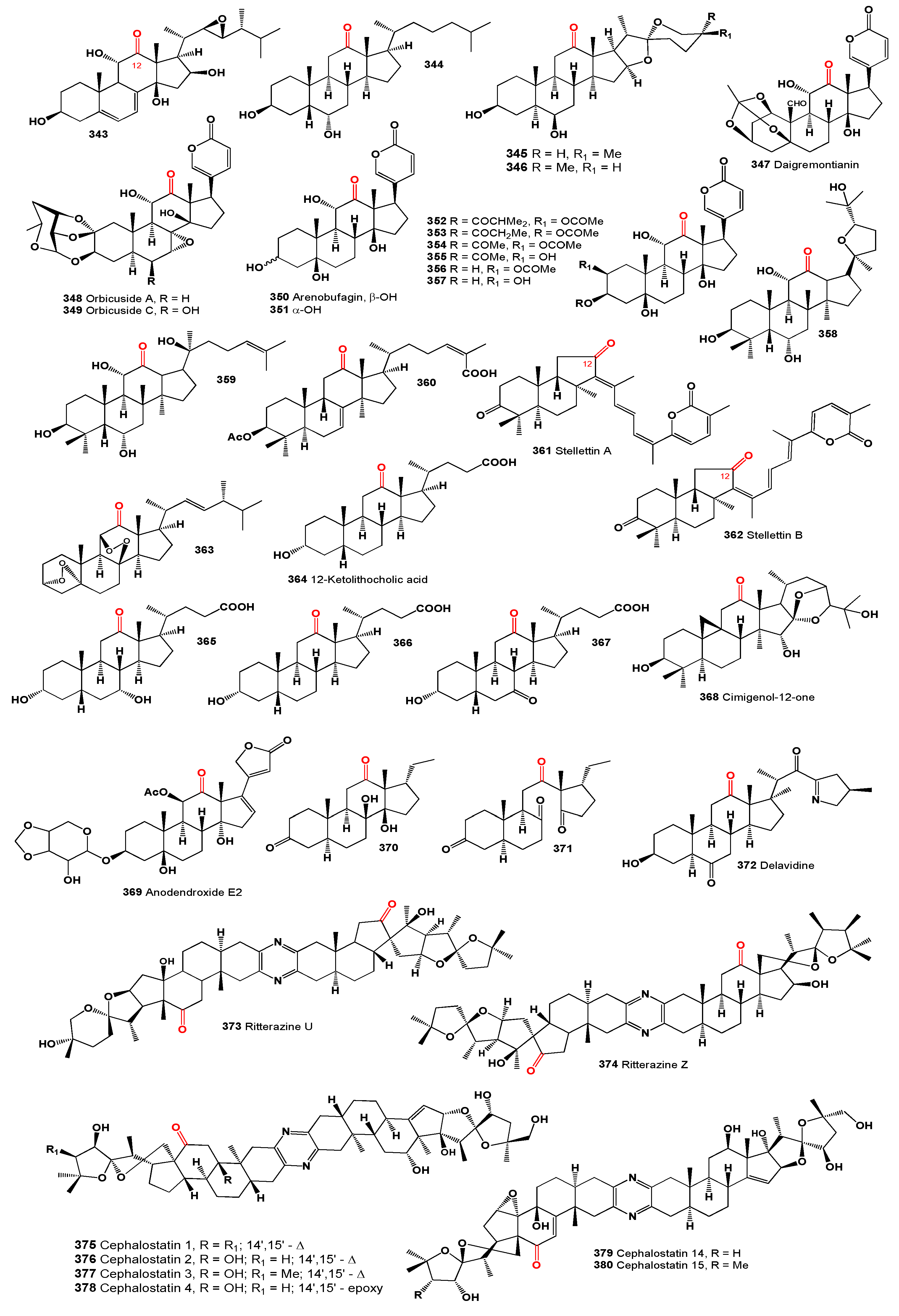
Major cardenolide glycosides, affinosides La-Le (
327-
331), featuring oxygen functionalities at C-11 of the aglycone, were isolated from the leaves of
Anodendron affine [
288]. The unique steroid inertogenin (
332), characterized by a rare 7,15-tetrahydrofuran group, was found in the leaves of
Strophanthus amboensis, a deciduous shrub used medicinally in Southwest Africa [
289]. Furthermore, the fusidic acid-related antibiotics, including 11-keto-fusidic acid (
333) produced by
Fusidium coccineum, effectively inhibit protein synthesis both
in vivo and
in vitro in prokaryotic and eukaryotic cells [
290,
291,
292].
The sex hormone 1,3,20-trihydroxypregnan-11-one (
334) was identified in the urine of a patient diagnosed with 17α-hydroxylase deficiency syndrome [
293]. Similarly, β-cortolone (3α,17α,20β,21-tetrahydroxypregnane-11-one,
335), a consistent metabolite, was detected in the urine of 20 young and elderly individuals of both genders [
294].
Steroidal alkaloids (
336-
342), characterized by a basic steroidal structure with a nitrogen atom integrated into the rings or side chains, represent a significant group of natural compounds [
295]. Among these, the rare 11-keto
Buxus alkaloids (
336-
338), a type of triterpenoid alkaloids derived from the cycloartenol skeleton with a modified C-20 side chain and nitrogen-containing groups at C-3 and/or C-20, have shown diverse biological effects [
296,
297,
298]. These alkaloids are notable secondary metabolites found in various plant families, including Solanaceae, Liliaceae, Apocynaceae, and Buxaceae [
295]. Notably, steroidal alkaloids (
339-
342) featuring a carbonyl group at position 11 were extracted from
Veratrum album and
V. taliense, exhibiting antihypertensive properties [
299,
300,
301].
Summarized on the biological activity of 11-ketosteroids is presented in
Table 7, and details are described partially in the text or a full description of the activity is written in the original articles.
12-Ketosteroids
12-Ketosteroids are indeed rare and interesting compounds, primarily found in plants and algae, as opposed to the more commonly studied animal-derived steroids. 12-ketosteroids are predominantly found in plants and algae. Their presence indicates specialized roles in the biochemistry of these organisms, potentially involved in plant and algal growth, development, or defense mechanisms against environmental stressors. Steroids in plants, including 12-ketosteroids, contribute to the chemical diversity found in the plant kingdom. These compounds can vary greatly in structure and function, reflecting the wide array of evolutionary adaptations plants have developed to thrive in diverse environments. In plants, steroids, including those with a ketone group at the 12th position, are thought to play roles in cell membrane integrity, signaling, and possibly as precursors to other important biochemicals. Their exact functions, however, can be quite specific to the species and the environmental context [
302,
303,
304].
A halotolerant fungus,
Aspergillus flocculosus PT05-1, was discovered in the sediment of the Putian saltern in Fujian Province, China, cultivated in a hypersaline medium. It produced (22R,23S)-epoxy-3β,11α,14β,16β-tetrahydroxyergosta-5,7-dien-12-one (
343), which exhibited cytotoxic effects against HL-60 and BEL-7402 cells with IC
50 values between 12-18 μM, and demonstrated antimicrobial activity against
Enterobacter aerogenes, Pseudomonas aeruginosa, and
Candida albicans with MIC values ranging from 1.6-15 μM [
305].
The chloroform/methanol extract of the red alga
Laurencia papillosa, sourced from the Red Sea in Saudi Arabia, was found to contain a cholestane derivative: 3α,6α-dihydroxy-5β-cholestan-12-one (
344) [
306].
From the organic extract of
Allium porrum, sapogenins named 12-keto-porrigenin ((25R)-5α-spirostan-3β,6β-diol-12-one,
345 and
346) were isolated and shown to possess antiproliferative activity against four tumor cell lines
in vitro [
307].
The toxic 12-keto-bufadienolide, daigremontianin (
347), was isolated from the plant
Kalanchoe daigremontiana, formerly known as
Bryophyllum daigremontianum [
308]. Additionally, two bufadienolide glycosides, orbicusides A (
348) and C (
349), were identified as toxic constituents in
Cotyledon orbiculata var.
orbiculata [
309].
Bufadienolides and their more polar conjugates, bufotoxins, are present in toads from the genus
Bufo. These compounds are found not only in their unconjugated forms but also as several C-3 conjugates [
310]. Two bufodienolides, arenobufagin (3β,11α,14-trihydroxy-12-oxo-5β,14β-bufa-20,22-dienolide,
350), and a second compound (
351) were isolated from the Central Asian green toad,
Bufo viridis [
311]. Arenobufagin, a primary bufadienolide from
Bufo viridis toad venom, has been shown to inhibit growth in various cancer cell lines [
312].
Research into the defensive mechanisms of fireflies, specifically
Photinus pyralis, P. ignitus, and
P.marginellus (Coleoptera: Lampyridae), resulted in the isolation of certain compounds (
352-
357) that make the fireflies distasteful to birds [
313].
Additionally, microbial transformation of 20(S)-protopanaxatriol by cell suspension cultures of
Aspergillus niger AS3.1858 produced two steroids (
358 and
359), which exhibited anticancer activity against multiple cancer cell lines including Du-145, Hela, K562, K562/ADR, SH-SY5Y, HepG2, and MCF-7 [
314].
Figure 11.
12-Ketosteroids derived from algae, plants, and fungi.
Figure 11.
12-Ketosteroids derived from algae, plants, and fungi.
Hu and colleagues [
315] identified a triterpenoid named kadcoccinone F (
360) from the plant
Kadsura coccinea. Additionally, a bioactive triterpenoid pigment, stellettin A (
361), was detected in the marine sponge
Stelletta tenuis [
316], while the cytotoxic triterpenoid pigment stellettin B (
362) was isolated from the marine sponge
Jaspis stellifera [
317]. Stellettin B demonstrated significant inhibitory effects on the growth of human glioblastoma cancer SF295 cells.
An unusual steroidal compound, (3α,5α),(8β,11β)-diepidioxy-ergost-22E-en-12-one (
363), was isolated and characterized from the dried fruit bodies of
Trametes orientalis [
318].
Regarding bile acids, most naturally occurring varieties are part of the 5β-series, featuring hydroxyl groups in the A, B, and C rings of the steroid system, commonly located at positions C3, C6, C7, C12, and C23, and predominantly oriented in the α configuration. Typically, the A/B ring junction is
cis, whereas both the B/C and C/D ring junctions are
trans [
319]. 12-Ketolithocholic acid (
364) was first identified in cattle bile by Wieland and Kishi over 90 years ago [
320] and has also been detected in the feces of some animal species [
321]. Furthermore, 3α,7α-dihydroxy-12-keto-5β-cholanic acid (
365) has been identified in human feces [
322]. This acid and its esters are crucial intermediates in the synthesis of chenodeoxycholic acid from cholic acid. 3α-Hydroxy-12-keto-5β-cholanoic acid (
366), 3α-hydroxy-7,12-diketo-5β-cholanoic acids (
367), and
365 were found in the urine of male patients with liver cirrhosis [
323].
A 9,19-cycloartane triterpene, cimigenol-12-one (
368), was isolated from the aerial parts of
Cimicifuga foetida [
324], and anodendroxide E2 (
369) was detected in the volatile oil from matured leaves of
Calotropis procera [
325]. Additionally, a steroidal sapogenin (
370) and its oxidized analogue (
371) were isolated from the roots of
Cynanchum otophyllum, demonstrating cytotoxic activities [
326]. An isosteroidal alkaloid, delavidine (
372), was isolated from
Fritillaria delavayi collected from Sichuan, China [
327].
Ritterazines, a class of complex natural compounds, have been isolated from ascidians, commonly known as sea squirts [
328]. These compounds were first discovered in the 1990s in the ascidian
Ritterella tokioka [
329]. Characterized by their unique and diverse structures, which include macrocyclic lactams and multiple fused rings, ritterazines have garnered attention for their potent biological activities, particularly their cytotoxic properties against various cancer cell lines. Notably, ritterazines U (
373) and Z (
374), which are 12-keto
bis-steroidal pyrazine alkaloids produced by
Ritterella tokioka, have demonstrated significant anticancer properties [
328].
FABCephalostatins are a group of steroidal pyrazine alkaloids that were first isolated from the marine worm
Cephalodiscus gilchristi [
331,
332]. They are known for their potent cytotoxicity and have been the subject of extensive research due to their potential as anticancer agents. The cephalostatins exhibit remarkable biological activity, with some compounds in the series showing nanomolar or even picomolar potency against various cancer cell lines. Their mechanism of action is believed to involve the inhibition of tubulin polymerization, which disrupts the formation of the mitotic spindle, leading to cell cycle arrest and apoptosis in cancer cells. The chemical structure of cephalostatins is characterized by a unique dimeric steroid framework, with two steroid units linked by a pyrazine bridge. This complex structure has made the total synthesis of cephalostatins a challenging and significant achievement in synthetic organic chemistry. Due to their potent biological activity and complex structure, cephalostatins continue to be a focus of research in the fields of natural products chemistry, medicinal chemistry, and cancer pharmacology [
333]. Cephalostatins 1-4 (
375-
378), 14 (
379) and 15 (
380) containing unique dimeric 12-keto steroids were found, isolated and their biological activity was established [
334,
335].
12-Ketosteroids in plants and algae may exhibit unique biological activities, such as antifungal, antibacterial, or anti-inflammatory properties. The rarity and specific occurrence of 12-ketosteroids in plants and algae make them fascinating subjects for chemical ecology and natural product chemistry. Overall, while not as extensively studied as their animal-derived counterparts, 12-ketosteroids in plants and algae represent an intriguing area of natural product research, with potential implications for both ecological studies and biotechnological applications. Summarized on the biological activity of 12-ketosteroids is presented in
Table 8, and details are described partially in the text or a full description of the activity is written in the original articles.
15-Ketosteroids
15-Ketosteroids are a class of steroids characterized by the presence of a ketone group at the 15th carbon atom of the steroid nucleus. These compounds are found across a diverse range of organisms, including fungi, sea sponges, and some plants, reflecting their broad biological importance and diversity. In fungi, 15-ketosteroids may play roles in their development, reproduction, and secondary metabolism. Fungi are known for their capability to produce a variety of bioactive secondary metabolites, and ketosteroids can be crucial components of these pathways.
In marine sponges, these steroids are part of a complex chemical arsenal used for defense against predators and microbial infection, as well as for communication. Sponges are known to house a rich array of chemical compounds that serve ecological functions, and steroids such as 15-ketosteroids contribute to these roles [
336,
337,
338].
In plants, while less commonly reported, steroids including 15-ketosteroids can influence growth, development, and defense mechanisms. They are part of the broader group of plant steroids that regulate various physiological processes [
339].
Additionally, the biochemical pathways leading to the synthesis of 15-ketosteroids involve modifications of standard steroidal structures, which can alter their biological activity significantly. These modifications can impact receptor binding and biological outcomes, influencing processes such as hormone signaling and cellular regulation. Research into 15-ketosteroids often focuses on their potential pharmacological applications, given their structural diversity and biological activity. These studies might look into anti-inflammatory, anti-cancer, or antimicrobial properties, which are common areas of interest in natural product drug discovery.
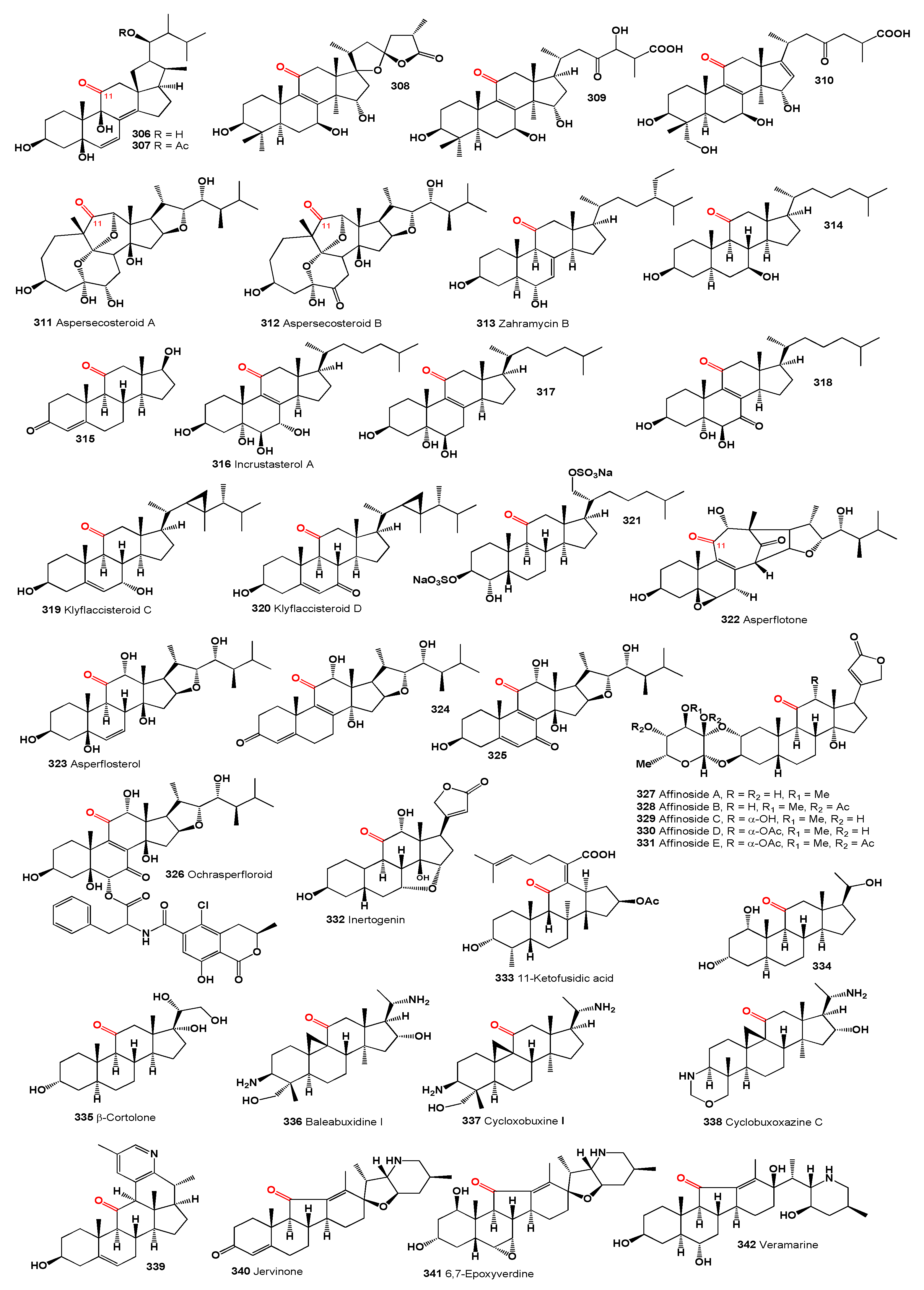
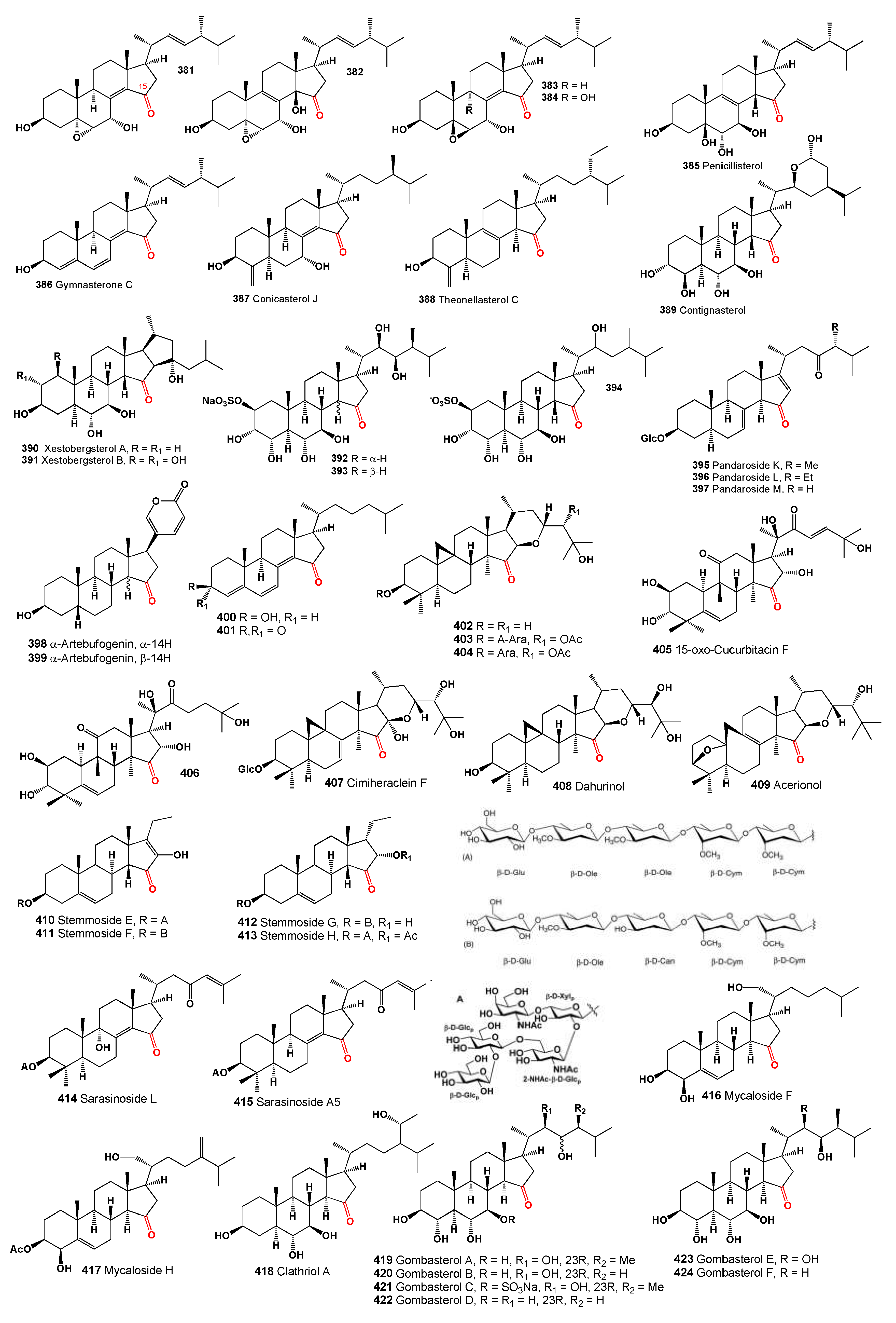
A 15-ketosteroid (
381, structures see in
Figure 12) discovered in the fruiting bodies of the fungus
Amauroderma subresinosum exhibited cytotoxic activity against the HL-60 cell line with an IC
50 of 32.1 µM and demonstrated less potency (IC
50 > 40 µM) against SMMC-7721, A549, MCF-7, and SW480 cell lines [
340]. This compound was also detected in the culture of the basidiomycete
Polyporus ellisii [
341]. Additionally, a cytotoxic steroid (
382) isolated from extracts of the king trumpet mushroom,
Pleurotus eryngii, showed anti-proliferative activity toward RAW264.7 cells with an IC
50 over 30 µM and inhibited NO production with an IC
50 of 13.2 µM [
342].
The compound 5β,6β-epoxide (
383) was found in
Polyporus ellisii and a
Phomopsis species, exhibiting antibacterial activity (MIC of 28.2 µM against
Micrococcus tenuis) and cytotoxic activity against cancer cell lines HL-60, SMMC-7721, A549, MCF-7, and SW480 (IC50 > 40 µM) [
17,
342]. A similar steroid (
384) detected in fungi
Ganoderma resinaceum,
Polyporus ellisii, and
Phomopsis sp. also showed cytotoxic activity, particularly against HL-60 with an IC
50 of 18.8 µM and other mentioned cell lines with IC
50 values over 40 µM [
342].
The fungus
Penicillium purpurogenum, known as a steroid producer (
385), has demonstrated cytotoxic activity against A549, HepG2, and MCF-7 cancer cell lines, with IC
50 values exceeding 100 µM [
343]. Furthermore, a fungus derived from the
Halichondria sponge,
Gymnacella dankaliensis, was cultured resulting in the isolation of gymnasterone C (
386) from the original malt extract medium [
344,
345].
Marine sponge
Theonella swinhoei, sourced from various locations in the Solomon Islands (Malaita and Vangunu Is.), has produced conicasterol J (
387) [
230], and theonellasterol C (
388) was also detected in the same sponge [
346]. Contignasterol (
389), a highly oxygenated steroid with an unusual 14β-configuration, was obtained from the Papua New Guinea sponge
Petrosia contignata [
347]. Additionally, two unique pentacyclic steroids (
390 and
391) with a cis C/D ring junction were isolated from
Xestospongia bergquistia and have been noted as powerful inhibitors of histamine release [
348].
Marine sponges from the genus
Oceanapia, which includes over 50 species found in tropical and subtropical seas, contain tamasterone sulfates (
392) and (
393), a C-14 epimeric pair of polyhydroxylated sterols [
349,
350]. Similarly, 15-keto-haliclostanone sulfate (
394) has been isolated from a
Haliclona sponge [
351].
Three saponins, pandaroside K–M (
395–
397), were identified from the marine sponge
Pandaros acanthifolium (Martinique Is., Caribbean) and exhibited antiprotozoal activity [
352]. Over 50 years ago, 14α-artebufogenin (
398) and 14β-artebufogenin (
399) were isolated from traditional Chinese medicine known as Chan Su [
353].
Two structurally unusual steroids, compounds
400 and
401, were metabolized by a marine strain of
Gymnasella dankaliensis isolated from the sponge
Halichondria japonica (Osaka Bay, Japan). These compounds exhibited significant and marginal growth inhibition against the lymphocytic leukemia P388 cell line with ED50 values of 0.9, and 2.5 µg/mL, respectively [
344,
345].
Three cycloartane triterpenoids, isodahurinol (
402), 20,24-di-O-acetylisodahurinol-3-O-α-L-arabinopyranoside (
403), and 24-O-acetylisodahurinol-3-O-α-L-arabinopyranoside (
404) were isolated from the aerial parts of
Cimicifuga foetida [
324], and isodahurinol (
402) was also detected in other
Cimicifuga species [
354]. Additionally, 15-oxo-cucurbitacin F (
405) and 15-oxo-23,24-dihydrocucurbitacin F (
406), isolated from
Cowania mexicana, exhibited inhibitory activity against HIV-1 replication in H9 cells, with EC
50 values of 0.3 and 2.5 μg/mL, respectively, and therapeutic index values of 17.0 and 15.2, respectively [
355].
A cycloartane triterpene glycoside, 16α-hydroxyl-7(8)-en-dahurinol-3-O-[4′-O-acetyl]-α-L-arabinopyranoside, named cimiheraclein F (
407), was isolated from the aerial parts of
Actaea heracleifolia collected from Yichun County, Heilongjiang Province, China [
356]. Dahurinol (
408, (24R)-24,25-dihydroxy-15-oxoacta-(16R,23R)-16,23-monoxol) and acerionol (
409, 3-deoxy-8,9-didehydro-(24S)-24,25-dihydroxy-(3S,10S)-3,10-epoxy-15-oxo-9,10-secoacta-(16R,23R)-16,23-monoxol) were found in extracts of
Actaea racemosa [
357].
Additionally, several 15-keto pregnane glycosides, namely stemmosides E, F, G, and H (
410-
413), were isolated from
Solenostemma argel. These compounds, characterized by a unique 14 proton configuration, with stemmosides E and F additionally featuring a rare enolic function at C-16 and stemmosides G-J displaying an unusual C-17 side chain, effectively reduced cell proliferation in a dose-dependent manner [
358].
The sponge
Melophlus sarasinorum, belonging to the family Geodiidae and subfamily Erylinae, commonly found in the Indo-West Pacific tropical region, contains glycosides known as sarasinosides [
359]. Dai and colleagues [
360] isolated a glycoside, sarasinoside L (
414), from specimens harvested near Sulawesi, Indonesia, while Santalova and coworkers [
361] isolated a similar triterpene glycoside named sarasinoside A5 (
415). Both steroids feature a keto group at position 15. Additionally, other steroidal oligoglycosides, mycalosides F (
416) and H (
417), were isolated from the polar extract of the Caribbean sponge
Mycale laxissima [
362].
From the Korean marine sponge
Clathria gombawuiensis, a series of polyoxygenated steroids (
418 -
424) were isolated. The structures of gombasterols A–F (
419–
424) were elucidated through combined spectroscopic analyses. These compounds are characterized as highly oxygenated steroids with a 3β,4α,6α,7β-tetrahydroxy substitution pattern or an equivalent structure (7β-sodium O-sulfonato for
421) and a common structural motif of a C-15 keto group [
363]. Summarized on the biological activity of 15-ketosteroids is presented in
Table 9, and details are described partially in the text or a full description of the activity is written in the original articles.
16-Ketosteroids
16-Ketosteroids are another interesting class of steroid compounds, defined by the presence of a ketone group at the carbon-16 position in the steroid nucleus. These compounds are primarily known for their presence in various plant species but are also found in some marine organisms such as sea cucumbers and sponges.
In plants, 16-ketosteroids are typically found in the leaves, bark, roots, seeds, pollen, and fruits. Similar to other phytochemicals, ketosteroids can help protect plants from herbivores and pathogens. Their bitter taste or toxic properties can deter predators, while their antimicrobial properties can prevent fungal and bacterial infections. Steroids are crucial in plant growth and development, influencing cell division, elongation, and differentiation. The specific roles of 16-ketosteroids in these processes might be less well-defined but are likely important due to the widespread presence of related compounds in the plant kingdom. Plants produce certain steroids in response to environmental stresses such as drought, salinity, and extreme temperatures. These compounds can help the plant manage the stress by modulating biochemical pathways [
364,
365].
In marine organisms like sea cucumbers and sponges, the presence of 16-ketosteroids, though less common, suggests a potential role in similar biological functions such as defense against predators or infections. The rarity of these compounds in marine environments compared to plants might indicate specialized roles or specific ecological adaptations [
15].
Pharmacologically, steroids including 16-ketosteroids are of interest for their potential anti-inflammatory, anticancer, and other bioactive properties. The modification at the 16th carbon could influence how these steroids interact with biological receptors, potentially leading to unique activities not seen in more common steroids [
366].
Aglaia lawii, a species in the Meliaceae family, contains three 16-ketosteroids in its leaves: 3-
epi-dyscusin C (
425, structures see in
Figure 13), 3-
epi-lansisterone E (
426), and (
Z)-2α-hydroxyaglawone (
427). These C-21 pregnane steroids feature a highly oxygenated ring A and have shown significant anti-inflammatory activities, with IC
50 values for NO inhibition ranging from 4.47 to 7.67 µM [
367].
From the wood of the Costa Rican tree
Trichilia hirta, two steroids, trichiliasterone A (3β-hydroxypregnan-2,16-dione,
428) and trichiliasterone B (2-hydroxyandrost-1,4-diene-3,16-dione,
429), were isolated. Both compounds inhibited the growth of the European corn borer,
Ostrinia nubilalis, and the variegated cutworm,
Peridroma saucia. Steroid
428 was also isolated from
Trichilia americana [
368].
The fruits of
Artocarpus heterophyllus led to the isolation of a steroid named artoheterophoid (
430), which exhibited remarkable inhibitory effects on NO production with an IC
50 value of 0.72 µM [
369]. Additionally, three pregnanes (
431-
433) were isolated from the leaves of
Aglaia grandis [
370], and several pregnane-type steroids, including 2β,3β-dihydroxy-5α-pregn-17(20)-(Z)-en-16-one (
434) and aglatomin A (
435), were detected in the leaves of
Aglaia tomentosa [
371]. Lansisterone E (
436) and 2β,3β,4β-trihydroxypregnan-16-one (
437) were obtained from the branches of
A. perviridis [
372].
Ampelozizyphus amazonicus, a medicinal climbing shrub native to the Amazonian region, used traditionally to prevent malaria, contains aglycone structures of saponins in its crude extract, specifically 16-keto-tetrahydroxydammar-23-ene (
438) and 16-keto-tetrahydroxy-dammar-24-methylene (
439) [
373].
Bacopa monnieri, known as brahmi in Ayurveda, is celebrated for enhancing intelligence, memory, and revitalizing sensory organs. Its nootropic activity has been extensively studied, with several researchers confirming that alcoholic/hydroalcoholic extracts of the whole plant exhibit varied activities. These extracts contain two steroids, bacoside A (
440) and B (
441), which contribute to its therapeutic properties [
374].
Several 9,10-seco-9,19-cyclolanostane arabinosides, named podocarpasides A–D (
442-
445), F (
447), and G (
448), were isolated from the roots of
Actaea podocarpa, a species closely related to black cohosh, a well-known dietary supplement. Specifically, podocarpaside C (
444) displayed modest complement activity inhibition with an IC
50 value of 200 µM [
375].
The structure of podocarpaside E (
446), also from
Actaea podocarpa, was revised to 3β,15α,25-trihydroxy-16,23-dioxo-6α,19α-epidioxy-9,10-seco-9,19-cyclolanost-5(10),9(11)-diene 3α-O-L-arabinopyranoside [
375]. Another compound, podocarpaside (
449), an arabinoside with a unique triterpene skeleton isolated from the same species, exhibited anticomplement activity [
376].
A phytochemical study on the rhizomes of
Cimicifuga foetida led to the isolation of two cycloartane triterpenoids (
450 and
451), which are part of a seven-membered-ring variant of 9,10-seco-9,19-cycloartane triterpenoids. Additionally, compounds
452 and
453 were identified as 3-O-β-D-xylopyranosides [
377]. Further, from the roots of
Actaea podocarpa, three cycloartane-type triterpene arabinosides, podocarpasides H-J (
454-
456), were isolated, with podocarpaside I (
455) showing moderate anticomplement activity with an IC
50 value of 250 µM [
375].
A glycoside named acanthifolioside A (
457), a minor component, was isolated from the marine sponge
Pandarosa canthifolium. Acanthifoliosides are distinct for having a rare oxidation pattern at C-15 and C-16 on the D ring [
352].
From the Far Eastern sea cucumber
Psolus chitonoides, collected near Bering Island (Commander Islands) at depths of 100–150 m, triterpene pentasides named chitonoidosides A (
458) and A1 (
459), which contain one or two sulfate groups, were isolated [
378]. Additionally, from the sea cucumber
Actinopyga flammea, 16-keto-holothurinogenin (
460) was obtained through acid hydrolysis of the crude extract [
379].
Steroidal alkaloids, cortistatins C (
461) and D (
462), featuring a 9(10−19)-abeo-androstane and isoquinoline skeleton, were isolated from the marine sponge
Corticium simplex. These compounds demonstrated high selectivity in inhibiting the proliferation of human umbilical vein endothelial cells (HUVEC) [
380].
Two toxic triterpenoid alkaloids, cyclobuxophylline O (
463) and cyclobuxophylline M (
464), have been discovered in the box tree moth
Cydalima perspectalis [
381]. Additionally, cyclobuxophylline M (
464) and cyclobuxophylline K (
465) were isolated from
Buxus sp. and
Buxus sempervirens [
382,
383]. An interesting feature of these alkaloids is the increase in the number of methyl groups on the amino group located at the 3rd position. Another alkaloid, sempervirone (
466), was isolated from the leaves of
Buxus sempervirens [
384].
Pregnane alkaloids such as terminamine S (
467), 3β-methylamino-16-oxo-5,17(20)-cis-pregnadiene (
468) [
385], (Z)-salignone (
469) [
386], terminamine H (
470) [
387], 3β-methylamino-16-oxo-5,17(20)-
trans-pregnadiene (
471) [
385], and (
E)-salignone (
472) [
386] were isolated from the whole herb of
Pachysandra terminalis [
388].
A phytochemical study on the stem of
Ecdysanthera rosea resulted in the isolation of C-21 pregnane glycosides ecdysosides A–D (
473–
476). Notably, compound
473 exhibited moderate antibacterial activity against
Enterococcus faecalis and
Providencia smartii [
389]. Summarized on the biological activity of 16-ketosteroids is presented in
Table 10, and details are described partially in the text or a full description of the activity is written in the original articles.
17-Ketosteroids
17-ketosteroids (17-KS) are metabolites derived primarily from the catabolism of androgens and, to a lesser extent, estrogens. These compounds are significant markers in the body, providing insights into adrenal and gonadal function. 17-KS are key byproducts found in the urine, originating from the metabolism of androgenic hormones like testosterone and dehydroepiandrosterone (DHEA). The presence and levels of these ketosteroids are indicators of androgenic activity in the body, reflecting the functioning of the adrenal cortex and gonads. The measurement of 17-KS in urine is a valuable diagnostic tool. It helps in assessing adrenal gland function and diagnosing disorders like adrenal hyperplasia, adrenal tumors, and conditions affecting the gonads. This is particularly useful in clinical settings to monitor and diagnose endocrine disorders [
9,
100,
390,
391].
Though 17-KS themselves are not hormonally active, their formation and excretion reflect the body’s metabolic pathways in breaking down sex hormones. Thus, they indirectly influence the regulatory mechanisms associated with hormonal balance, impacting physiological processes such as growth, reproduction, and stress responses. In research, the levels of 17-KS can be studied to understand variations in hormonal activity due to various diseases, lifestyle factors, or environmental influences. This research helps in developing treatments for hormonal imbalances and related health issues [
392,
393]. In non-human animals, the specific functions of 17-KS can vary, but generally, they serve as markers of similar hormonal and metabolic processes. For example, in veterinary medicine, measuring these ketosteroids can assist in diagnosing health conditions in domestic and wild animals, particularly those related to reproductive and adrenal functions [
393].
17-Ketosteroids are vital as both metabolic intermediates and diagnostic markers across various species, playing a significant role in understanding and managing health issues related to hormone regulation and adrenal gland function [
393,
394,
395,
396,
397].
Holarrhena pubescens, an Indian medicinal tree indigenous to the tropical Himalaya and Assam, is known for its stem bark, commercially referred to as kurchi. This bark has astringent, antidysenteric, anthelmintic, stomachic, febrifugal, and tonic properties. A unique compound, puboestrene [3-acetoxy-17-oxo-1,3,5(10)-estratriene] (
474, structures see in
Figure 14), has been isolated from the bark of
Holarrhena pubescens [
398].
Estra-1,3,5(10)-triene-3-ol-17-one (
475), commonly known as estrone, is a naturally occurring estrane steroid produced
in vivo from androstenedione and/or testosterone via estradiol. This estrogen demonstrates estrogenic activity, with variations depending on its structure, some of which may also exhibit anticancer properties [
399,
400,
401]. Butenandt and Jacobi [
402] isolated estrone (
475) from seeds and pollen of
Phoenix dactylifera, Punica granatus, Malus pumila, Hyphaene thebaica, Salix caprea, and
Glossostemon bruguieri. In 1926, Dohrn and co-workers first detected estrone (
475), equilin (
476), and hippulin (
477) as female sex hormonal steroids in plants [
403]. This discovery was followed by further identifications in the 1930s by Butenandt and Jacobi [
402] and Skarzynski [
404]. Additionally, extracts from the bark of the main wooden rod of ketapang
Terminalia catappa (Combretaceae) contained estrone (
474), equilin (
475), and equilin sulfate (
478) [
405].
6,8-Didehydroestrone (
479) and estra-1,3,5(10),6,8-pentaen-3-ol-17-one, known as equilenin, were first isolated from the urine of pregnant mares in 1936 by Desmond Beall [
406]. Later, equilenin sulfate (
480) was also extracted from the urine of pregnant mares by Schachter and Marrian in 1938, and subsequently synthesized by Bachmann and Wilds in 1939 [
407,
408]. Two naphthalene-containing steroids (
481 and
482) have been found in the bark of
Terminalia catappa, although it’s speculated that these compounds are naphthalenic steroids produced by the tree itself [
409].
Terminalia catappa and
Terminalia mantaly (Combretaceae) are recognized medicinal plants used in Cameroon to treat various diseases.
In the lipophilic fractions of
Loranthus micranthus, a medicinal plant known in eastern Nigeria as a species of the African mistletoe and commonly used in traditional medicine, two notable steroids were identified: 5α,16,16-dimethyl-androstan-17-one (
483) and 6β-hydroxy-17-oxo-4,5-secoandrostan-4-oic acid (
484) [
410].
Additionally, 3β-Hydroxy-5α-androstan-17-one (epiandrosterone,
485), 5α-androstan-3,17-dione (
486), and 3α-hydroxy-5α-androstan-17-one (androsterone,
487), have been identified in the solvent-soluble fraction of peat from Bolton Fell Moss (Cumbria, U.K.). The structures and δ
13C values of these androstane derivatives suggest a diagenetic origin from sterols also present in the peat, providing the first evidence that cleavage of the C-17 side-chain can occur during early diagenesis, likely through microbial oxidation [
411].
Comamonas testosteroni is recognized as a key model in the study of bacterial aerobic steroid degradation, capable of degrading cholic acid. Three major steroids (
488-
490) have been identified as byproducts of cholic acid degradation [
412].
From the stem bark of
Ailanthus malabarica, rare octanor- and nonanor-triterpenoids, malabanones A (
491) and B (
492), featuring a unique tricyclo[4.3.1.0.1,6]decane unit, were isolated [
413]. Additionally, a strain of the fungus
Fusarium oxysporum SC1301, isolated from soil samples, is known as a steroid producer (
493) [
414].
Pinus nigra, also known as the Austrian pine or black pine, is found across Southern Europe from the Iberian Peninsula to the eastern Mediterranean, the Anatolian peninsula of Turkey, Corsica, Cyprus, and the high mountains of Northwest Africa. Various steroid hormones have been detected in the pollen of this pine species. These include epiandrosterone (
485), androsterone (3α-hydroxy-5α-androstan-17-one,
487), dehydroepiandrosterone (3β-hydroxyandrost-5-en-17-one,
493), 2α,3α-dihydroxy-5β-androstan-17-one (
494), 11β-hydroxy-etiocholanolone (3α,11β-dihydroxy-5β-androstan-17-one,
495), 3α-hydroxy-5β-androstane-11,17-dione (
496), and 3α-hydroxy-5α-androstane-11,17-dione (
497) [
415]. Additionally, androstane-3,17-dione (
486) was identified in the sugar pine (
Pinus lambertiana) [
416].
Steroid modifications
via selected wild and engineered microbial strains have become a crucial method for producing valuable steroid drugs and their precursors for the pharmaceutical industry. These microorganisms excel in sterol side chain degradation, oxyfunctionalization of the steroid core, and redox reactions at various positions of the steroid molecule. Over ten 17-ketosteroids (
498-
516) have been isolated and identified, demonstrating the diverse capabilities of these microorganisms [
9,
99,
417,
418,
419,
420,
421].
Phytochemical studies on an ethanol-soluble extract of the roots of
Buxus sempervirens of Turkish origin have led to the isolation of (+)-17-oxocycloprotobuxine (
517) [
422].
Urinary steroids, metabolites of steroid hormones excreted in urine, are pivotal in assessing an individual’s metabolic state, endocrine function, and overall health [
423]. The measurement of these steroids plays a critical role in diagnosing and monitoring various endocrine disorders. For example, elevated levels of certain urinary steroids may indicate adrenal gland disorders such as Cushing’s syndrome or adrenal tumors [
425].
Urinary steroid profiles are invaluable for understanding an individual’s metabolic processes. The ratios of specific steroids can reveal the activity of enzymes involved in steroid metabolism. These steroids are end products of steroid hormone metabolism, formed in the liver and other tissues through various chemical modifications before being excreted in the urine [
426].
Common urinary steroids include metabolites derived from steroid hormones such as cortisol, testosterone, estrogen (
475), and progesterone. Notable among these are etiocholanolone (
485), androsterone (
487), and a range of 17-ketosteroids (
485,
487,
518-
526) [
427].
Summarized on the biological activity of 17-ketosteroids is presented in
Table 11, and details are described partially in the text or a full description of the activity is written in the original articles.
20-Ketosteroids
20-Ketosteroids are a category of steroid hormones characterized by the presence of a ketone group at the 20th carbon of the steroid structure. These compounds include important hormones such as cortisone, which is a significant glucocorticoid. The most well-known 20-ketosteroid, cortisone, plays a crucial role in the body’s stress response. It is involved in regulating metabolism, immune response, and inflammatory processes. Cortisone is produced in the adrenal cortex, specifically in the zona fasciculata and zona reticularis [
428,
429,
430,
431].
As a glucocorticoid, cortisone helps in the regulation of glucose metabolism. It stimulates gluconeogenesis, the process by which the liver generates glucose from amino acids and other substrates, which is vital during periods of fasting or stress [
432,
433,
434]. Cortisone and similar 20-ketosteroids have potent anti-inflammatory and immunosuppressive effects. They inhibit the activities of white blood cells and other components of the immune system, thus reducing inflammation and the immune response. This makes them useful in treating a variety of inflammatory and autoimmune conditions, such as asthma, allergies, and rheumatoid arthritis. Cortisone is also part of the body’s response to stress. It is released in response to ACTH (adrenocorticotropic hormone) from the pituitary gland, which is stimulated by physical, emotional, or chemical stressors [
435]. Due to their powerful anti-inflammatory and immunosuppressive actions, synthetic derivatives of 20-ketosteroids (such as prednisone) are commonly used in medical treatments. These are designed to mimic the effects of naturally occurring cortisone but may be more potent or have longer-lasting effects [
435,
436].
Overall, 20-ketosteroids like cortisone are crucial for maintaining homeostasis in the body, especially in response to stress. They play significant roles in metabolism, immune regulation, and inflammation control, making them vital both as natural hormones and as pharmacological agents [
437,
438].
The gorgonian
Menella spinifera, collected off the South China Sea, was found to contain 3β-hydroxy-5α-pregnane-20-one (
527) [
439]. Additionally, several pregnane steroids—3α-hydroxy-5β-pregnan-20-one (
528), 3β-hydroxy-pregnan-5-en-20-one (
529), 5β-pregnan-3,20-dione (
530), 5α-pregnan-3,20-dione (
531), pregnan-4-en-3,20-dione (
532), and pregnan-1,4-dien-3,20-dione (
533) - were isolated from another species of the gorgonian
Menella, collected off Meishan Island, Sanya Bay, Hainan province, China [
440]. A strain of the fungus
Fusarium oxysporum SC1301, isolated from soil samples, is known to produce pregnenolone (
534) [
414]. Moreover, extracts of
Rhodococcus sp. cells have been shown to produce two oxygenated pregnane steroids (
535 and
536) [
441]. Phytochemical analysis of the methanolic extract of
Euonymus alatus twigs led to the isolation of a sterol identified as (3,16)-3,16-dihydroxypregn-5-en-20-one (
537) [
442]. The sponge
Psammaplysilla purpurea contains 3β-hydroxy-5α-pregnan-20-one (
528) and 3β-hydroxy-pregn-5-en-20-one (
529) as minor constituents in its free sterol mixture [
443].
Recent findings highlight that only a few insect taxa are known to produce steroids essential for insects, including several chrysomelids (Chrysomelidae), carrion beetles (Silphidae, Staphylinidae), lampyrid beetles (Lampyridae), and giant aquatic bedbugs (Belostomatidae) [
444,
445,
446]. The prothoracic protective glands of dytiscids are noted for producing a remarkable array of known vertebrate steroid hormones along with new and unusual steroids (
530,
531,
532,
537–
544), found also in predaceous diving beetles and belostomatid bugs. Some of these molecules are believed to be synthesized from cholesterol acquired from their prey [
445].
Brassinosteroid metabolites, known as plant hormones (
545-
547), have been detected in extracts of the flowering plant
Ornithopus sativus from the family Fabaceae [
447]. Several pregnane glycosides and their aglycones (
548-
550) were identified in the roots of
Asclepias tuberosa [
448]. Chemical investigations into the roots of
Dysoxylum densiflorum identified two known steroids, (
527 and
528) [
449], and from the leaves of oleander (
Nerium indicum), pregnane 3β,14-dihydroxy-5α,14β-pregnan-20-one (
551) has been identified [
450].
A bioactive steroid (
552) was isolated from an alcohol extraction of
Solanum nigrum, demonstrating significant cytotoxic activities against SW480 and Hep3B cells [
451].
Steroidal alkaloids, which possess a basic steroidal skeleton with a nitrogen atom integrated into rings or side chains, showcase a broad range of biological activities. Some steroidal alkaloids, such as abiraterone acetate—a widely used treatment for prostate cancer—exemplify how these compounds can be developed into therapeutic drugs. The structural diversity of natural steroidal alkaloids presents a spectrum of biological activities, making them highly valuable to both natural product chemistry and medicinal chemistry communities [
295]. Examples include two steroidal alkaloids, cyclobuxomicreinine (
553) and cyclorolfeine (
554), found in extracts of
Buxus hildebrandtii [
452], cyclobuxomicreinine (
555) identified from
Buxus species [
453], and buxippine K (
556) which would be isolated from the leaves of
Buxus hyrcana [
454]. An antiprotozoal nor-triterpene alkaloid, 16-α-hydroxybuxaminone (
557), was isolated from
Buxus sempervirens and
Buxus sp. [
455,
456].
Several steroidal glycosides (
558-
565) were isolated from the aerial parts of Ceropegia fusca (Asclepiadaceae), a crassulacean acid metabolism plant endemic to the Canary Islands, traditionally used as a cicatrizant, vulnerary, and disinfectant. The dichloromethane extract exhibited significant cytostatic activity against HL-60, A-431, and SK-MEL-1 cells—models for human leukemic, epidermoid carcinoma, and melanoma cells respectively [
457]. Summarized on the biological activity of 20-ketosteroids is presented in
Table 12, and details are described partially in the text or a full description of the activity is written in the original articles.
Figure 15.
20-Ketosteroids derived from insects, and plants.
Figure 15.
20-Ketosteroids derived from insects, and plants.
Miscellaneous Ketosteroids Derived from Natural Sources
A rearranged sterol with an unusual tetracycle core skeleton, penicillitone (
566), was obtained from the culture of the fungus
Penicillium purpurogenum SC0070. This compound demonstrated potent inhibitory effects on tumor cell growth and key pro-inflammatory cytokine production in macrophages [
458]. The mushroom
Stropharia rugosoannulata, known as saketsubatake in Japanese and wine-cap stropharia in English, produces an unusual steroid, strophasterol A (
567), which exhibited an inhibitory effect on TG toxicity in a dose-dependent manner [
459].
An unusual steroid-type compound, dankasterone A (
568), is produced by the fungus
Gymnascella dankaliensis, which was isolated from the Japanese sponge
Halichondria japonica [
344,
345]. Citreoanthrasteroid A (
569) was isolated from the mycelia of a hybrid strain, KO 0231, prepared by a cell fusion technique using
Penicillium citreo-viride IFO 6200 and 4692 [
460].
Ergosteroid, gloeophyllin J (
570), has been isolated from the solid cultures of fungus
Gloeophyllum abietinum. This compound represents the first ergosteroid featuring the cleavage of a C8−C14 bond [
461]. Steroids (234R)-3β-hydroxy-24-methyl-4-methylene-8,14-secocholestane-8,14-dione (
571) and swinhosterol A (
572) were isolated from an Okinawan sponge
Theonella swinhoei. An undescribed 9,11-secosteroid, cyclosecosteroid A (
573), was isolated from the mangrove endophytic fungus
Talaromyces sp. SCNU-F0041, and it showed moderate inhibitory activity against AChE, with an IC
50 value of 46 µM [
462].
An oxygenated 4-exo-methylene sterol, 28-homoswinhoeisterol (
574), was discovered in the marine sponge of
Theonella swinhoei collected from the Bohol province in the Philippines [
463].
The 9,11-secosteroids, pinnigorgiols A (
575) and E (
576) with a rare carbon skeleton, a tricyclo[
5,
2,
1]decane ring, were isolated from a gorgonian coral identified as
Pinnigorgia sp. These compounds displayed inhibitory effects on the generation of superoxide anions and the release of elastase by human neutrophils [
464,
465]. Two secosteroids, 3β,11-dihydroxy-5β,6β-epoxy-9,11-secocholestan-9-one (
577) and 3β,11-dihydroxy-5β,6β-epoxy-9,11-secogorgostan-9-one (
578), have been identified from extracts of the Taiwanese soft coral
Cespitularia taeniata [
466,
467,
468].
Steroid, 8αH-3β,11-dihydroxy-5α,6α-epoxy-24-methylene-9,11-secocholestan-9-one (
579) was obtained from
Sinularia granosa and
S. crassa soft coral extracts [
469]. Two unusual steroidal derivatives, erectsterates A (
580) and B (
581), epimers at C-10, were isolated from the South China Sea soft coral
S. erecta. These compounds are characterized by a high degree of degradation in ring B and an ester linkage between the A and C/D rings, similar to the compounds chaxines B and D from the edible mushroom
Agrocybe chaxingu [
470]. Compound (
581) displayed cytotoxic activity against several cancer cell lines including A549 (human adenocarcinoma), HT-29 (human colorectal adenocarcinoma), SNU-398 (hepatocellular carcinoma), and Capan-1 (human pancreatic ductal adenocarcinoma) [
471].
A series of cytotoxic steroids called stereonsteroids B (
582) and F (
583) were isolated from the methylene chloride extract of the Formosan soft coral
Stereonephthya crystalliana. This coral extract showed significant cytotoxicity against A549, HT-29, and P-388 cancer cells
in vitro [
472].
Several neotecleanin-type limonoids, walrobsin C (
584), I (
585), Q (
586), R (
587), and U (
588), were detected in methanol (MeOH) extracts of the root barks of
Walsura robusta [
473].
Belamchinanes A–D (
589-
592), four triterpenoids with a novel skeleton, were isolated from the seeds of
Belamcanda chinensis. These structures feature a unique 4/6/6/6/5 polycyclic system where a four-membered carbocyclic ring bridges the C-1 and C-11 positions of a classical triterpenoid framework [
474].
Strophasterols E and F (
594 and
595) were isolated from
Pleurotus eryngii, showing interesting structural properties [
342], and tricholumin A (
595), an ergosterol derivative from
Trichoderma asperellum, exhibited antimicrobial activity [
475].
In marine pharmacology, stellettins Q (
596) from
Stelletta sp. is a D-nor steroid with a cyclopentane unit linked to different positions of side chains [
476,
477], and stelliferins L (
597) and M (
598) from
Rhabdastrella cf.
globostellata exhibited antimicrobial activity [
478].
Two C-nor-D-homo-estrones (
599 and
600) were discovered in the Solanum family [
479]. These compounds underscore the diverse potential of C-nor steroids in various therapeutic applications.
C-nor derivatives such as 14-hydroxy-7-methoxy-11,16-diketo-apian-8-en-(22,6)-olide (
601) and 7-methoxy-11,16-diketo-apian-8,14-dien-(22,6)-olide (
602) have been identified in
Salvia officinalis, along with three other complex apianane terpenoids (
603,
604, and
605) [
480,
481].
An unusual steroid-like metabolite, asterogynin B (
606), with potential antimalarial properties, was produced by an endophytic fungus from the small palm
Asterogyne martiana [
482].
From the aerial parts of
Premna fulva, a plant used in Zhuang medicine, a unique metabolite, premnafulvol A (
607), was isolated. This compound features a distinct 6/5/7/3-fused tetracyclic carbon skeleton [
483]. Spiroseoflosterol (
608), an unusual ergostane steroid, was isolated from
Butyriboletus roseoflavus and demonstrated cytotoxicity against liver cancer cell lines [
484].
Figure 16.
Miscellaneous ketosteroids derived from different speices.
Figure 16.
Miscellaneous ketosteroids derived from different speices.
Additionally, two steroids, urceoloids A (
609) and B (
610), characterized by a rearranged carbon skeleton with a unique spiro[4.4]nona-3,6,8-triene system, were identified. Both compounds exhibited immune-suppressive activities [
485]. Summarized on the biological activity of 20-ketosteroids is presented in
Table 12, and details are described partially in the text or a full description of the activity is written in the original articles.