Submitted:
29 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Characterization of the Potent ACAT1 Inhibitor F26
2.2. NP F26 Inhibits ACAT1 More Effectively and More Durably than NP F12511 in Cell Culture
2.3. NP F26 Efficacy In Vivo Assessed via IV Administration
2.4. NP F26 Efficacy In Vivo Assessed via IP Administration
2.5. F26 Is Retained Longer than F12511 in the Brain after a Single IV Injection of NP F26
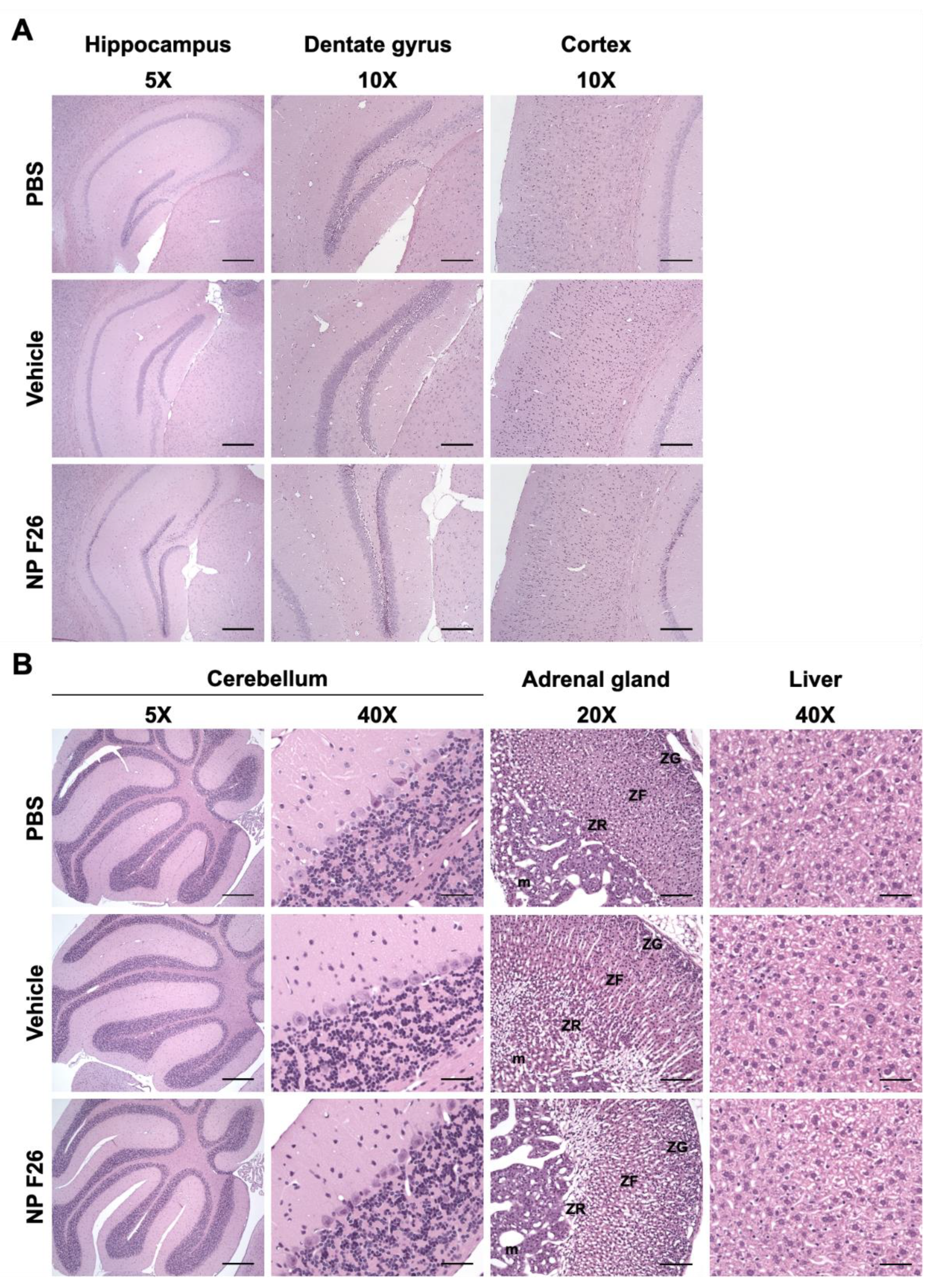
2.6. A 2-Week Administration of NP F26 Showed No Overt Systemic or Neurotoxicities In Vivo
3. Discussion
4. Materials and Methods
4.1. Ethical Handling of Animals
4.2. Materials
4.3. Nanoparticle Formation
4.4. Nanoparticle Characterization
4.5. Cell Culture
4.6. XTT Assay
4.7. Measuring ACAT Activity by [3H] Oleate Pulse Assay
4.8. Measuring ACAT Activity by Mixed Micelle Assay
4.9. LC-MS/MS
4.10. Histology
4.11. Data Analysis and Visualization
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest 2002, 110, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics. Signal Transduct Target Ther 2022, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. A receptor-mediated pathway for cholesterol homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Huh, H.Y.; Cadigan, K.M.; Chang, T.Y. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J Biol Chem 1993, 268, 20747–20755. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.Y.; Chang, C.C.; Lin, S.; Yu, C.; Li, B.L.; Miyazaki, A. Roles of acyl-coenzyme A:cholesterol acyltransferase-1 and -2. Curr Opin Lipidol 2001, 12, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Joyce, C.; Davis, M.; Reagan, J.W.; Clark, M.; Shelness, G.S.; Rudel, L.L. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J Biol Chem 1998, 273, 26747–26754. [Google Scholar] [CrossRef]
- Cases, S.; Novak, S.; Zheng, Y.W.; Myers, H.M.; Lear, S.R.; Sande, E.; Welch, C.B.; Lusis, A.J.; Spencer, T.A.; Krause, B.R.; et al. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J Biol Chem 1998, 273, 26755–26764. [Google Scholar] [CrossRef]
- Oelkers, P.; Behari, A.; Cromley, D.; Billheimer, J.T.; Sturley, S.L. Characterization of two human genes encoding acyl coenzyme A:cholesterol acyltransferase-related enzymes. J Biol Chem 1998, 273, 26765–26771. [Google Scholar] [CrossRef]
- Liu, J.; Chang, C.C.; Westover, E.J.; Covey, D.F.; Chang, T.Y. Investigating the allosterism of acyl-CoA:cholesterol acyltransferase (ACAT) by using various sterols: in vitro and intact cell studies. Biochem J 2005, 391, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, N.; Miyazaki, A.; Takeya, M.; Horiuchi, S.; Chang, C.C.; Chang, T.Y.; Takahashi, K. Localization of human acyl-coenzyme A: cholesterol acyltransferase-1 (ACAT-1) in macrophages and in various tissues. Am J Pathol 2000, 156, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Sakashita, N.; Ornvold, K.; Lee, O.; Chang, E.T.; Dong, R.; Lin, S.; Lee, C.Y.; Strom, S.C.; Kashyap, R.; et al. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J Biol Chem 2000, 275, 28083–28092. [Google Scholar] [CrossRef]
- Lu, M.; Hu, X.H.; Li, Q.; Xiong, Y.; Hu, G.J.; Xu, J.J.; Zhao, X.N.; Wei, X.X.; Chang, C.C.; Liu, Y.K.; et al. A specific cholesterol metabolic pathway is established in a subset of HCCs for tumor growth. J Mol Cell Biol 2013, 5, 404–415. [Google Scholar] [CrossRef]
- Lee, R.G.; Willingham, M.C.; Davis, M.A.; Skinner, K.A.; Rudel, L.L. Differential expression of ACAT1 and ACAT2 among cells within liver, intestine, kidney, and adrenal of nonhuman primates. J Lipid Res 2000, 41, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huynh, T.N.; Duong, M.T.; Gow, J.G.; Chang, C.C.Y.; Chang, T.Y. ACAT1/SOAT1 Blockade Suppresses LPS-Mediated Neuroinflammation by Modulating the Fate of Toll-like Receptor 4 in Microglia. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, A.; Sakashita, N.; Lee, O.; Takahashi, K.; Horiuchi, S.; Hakamata, H.; Morganelli, P.M.; Chang, C.C.; Chang, T.Y. Expression of ACAT-1 protein in human atherosclerotic lesions and cultured human monocytes-macrophages. Arterioscler Thromb Vasc Biol 1998, 18, 1568–1574. [Google Scholar] [CrossRef]
- Wang, Y.J.; Bian, Y.; Luo, J.; Lu, M.; Xiong, Y.; Guo, S.Y.; Yin, H.Y.; Lin, X.; Li, Q.; Chang, C.C.Y.; et al. Cholesterol and fatty acids regulate cysteine ubiquitylation of ACAT2 through competitive oxidation. Nat Cell Biol 2017, 19, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Rangaraj, K.; Simpson, R.; Maclean, D.J.; Nathanson, L.K.; Stuart, K.A.; Scott, S.P.; Ramm, G.A.; de Jersey, J. Quantitative analysis of the expression of ACAT genes in human tissues by real-time PCR. J Lipid Res 2004, 45, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Hutter-Paier, B.; Huttunen, H.J.; Puglielli, L.; Eckman, C.B.; Kim, D.Y.; Hofmeister, A.; Moir, R.D.; Domnitz, S.B.; Frosch, M.P.; Windisch, M.; et al. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer's disease. Neuron 2004, 44, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Farese, R.V., Jr. The nine lives of ACAT inhibitors. Arterioscler Thromb Vasc Biol 2006, 26, 1684–1686. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.Y.; Li, B.L.; Chang, C.C.; Urano, Y. Acyl-coenzyme A:cholesterol acyltransferases. Am J Physiol Endocrinol Metab 2009, 297, E1–9. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, Y.; Chang, C.C.; Chang, T.Y. ACAT1/SOAT1 as a therapeutic target for Alzheimer's disease. Future Med Chem 2015, 7, 2451–2467. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Cheng, X.; Wu, X.; Yoo, J.Y.; Cheng, C.; Guo, J.Y.; Mo, X.; Ru, P.; Hurwitz, B.; Kim, S.H.; et al. Inhibition of SOAT1 Suppresses Glioblastoma Growth via Blocking SREBP-1-Mediated Lipogenesis. Clin Cancer Res 2016, 22, 5337–5348. [Google Scholar] [CrossRef] [PubMed]
- Ayyagari, V.N.; Wang, X.; Diaz-Sylvester, P.L.; Groesch, K.; Brard, L. Assessment of acyl-CoA cholesterol acyltransferase (ACAT-1) role in ovarian cancer progression-An in vitro study. PLoS One 2020, 15, e0228024. [Google Scholar] [CrossRef]
- Smith, D.C.; Kroiss, M.; Kebebew, E.; Habra, M.A.; Chugh, R.; Schneider, B.J.; Fassnacht, M.; Jafarinasabian, P.; Ijzerman, M.M.; Lin, V.H.; et al. A phase 1 study of nevanimibe HCl, a novel adrenal-specific sterol O-acyltransferase 1 (SOAT1) inhibitor, in adrenocortical carcinoma. Invest New Drugs 2020, 38, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Nugent, A.A.; Lin, K.; van Lengerich, B.; Lianoglou, S.; Przybyla, L.; Davis, S.S.; Llapashtica, C.; Wang, J.; Kim, D.J.; Xia, D.; et al. TREM2 Regulates Microglial Cholesterol Metabolism upon Chronic Phagocytic Challenge. Neuron 2020, 105, 837–854. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Chang, C.C.Y.; Maue, R.A.; Melton, E.M.; Peden, A.A.; Garver, W.S.; Lee, J.; Schroen, P.; Huang, M.; Chang, T.Y. Acat1/Soat1 knockout extends the mutant Npc1 mouse lifespan and ameliorates functional deficiencies in multiple organelles of mutant cells. Proc Natl Acad Sci U S A 2022, 119, e2201646119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Luo, L.M.; Huang, T.S.; Feng, L.F. SOAT1 is a new prognostic factor of colorectal cancer. Ir J Med Sci 2022, 191, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, A.L.; Huynh, T.N.; Chang, C.C.Y.; Pooler, D.B.; Ness, D.B.; Lewis, L.D.; Pannem, S.; Feng, Y.; Samkoe, K.S.; Hickey, W.F.; et al. Stealth Liposomes Encapsulating a Potent ACAT1/SOAT1 Inhibitor F12511: Pharmacokinetic, Biodistribution, and Toxicity Studies in Wild-Type Mice and Efficacy Studies in Triple Transgenic Alzheimer's Disease Mice. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Valencia-Olvera, A.C.; Balu, D.; Faulk, N.; Amiridis, A.; Wang, Y.; Pham, C.; Avila-Munoz, E.; York, J.M.; Thatcher, G.R.J.; LaDu, M.J. Inhibition of ACAT as a Therapeutic Target for Alzheimer's Disease Is Independent of ApoE4 Lipidation. Neurotherapeutics 2023, 20, 1120–1137. [Google Scholar] [CrossRef]
- Sun, T.; Xiao, X. Targeting ACAT1 in cancer: from threat to treatment. Front Oncol 2024, 14, 1395192. [Google Scholar] [CrossRef]
- Farhat, N.Y.; Alexander, D.; McKee, K.; Iben, J.; Rodriguez-Gil, J.L.; Wassif, C.A.; Cawley, N.X.; Balch, W.E.; Porter, F.D. Sterol O-Acyltransferase 1 (SOAT1): A Genetic Modifier of Niemann-Pick Disease, Type C1. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Tardif, J.C.; Gregoire, J.; L'Allier, P.L.; Anderson, T.J.; Bertrand, O.; Reeves, F.; Title, L.M.; Alfonso, F.; Schampaert, E.; Hassan, A.; et al. Effects of the acyl coenzyme A:cholesterol acyltransferase inhibitor avasimibe on human atherosclerotic lesions. Circulation 2004, 110, 3372–3377. [Google Scholar] [CrossRef]
- Nissen, S.E.; Tuzcu, E.M.; Brewer, H.B.; Sipahi, I.; Nicholls, S.J.; Ganz, P.; Schoenhagen, P.; Waters, D.D.; Pepine, C.J.; Crowe, T.D.; et al. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med 2006, 354, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Ikenoya, M.; Yoshinaka, Y.; Kobayashi, H.; Kawamine, K.; Shibuya, K.; Sato, F.; Sawanobori, K.; Watanabe, T.; Miyazaki, A. A selective ACAT-1 inhibitor, K-604, suppresses fatty streak lesions in fat-fed hamsters without affecting plasma cholesterol levels. Atherosclerosis 2007, 191, 290–297. [Google Scholar] [CrossRef]
- Lopez-Farre, A.J.; Sacristan, D.; Zamorano-Leon, J.J.; San-Martin, N.; Macaya, C. Inhibition of acyl-CoA cholesterol acyltransferase by F12511 (Eflucimibe): could it be a new antiatherosclerotic therapeutic? Cardiovasc Ther 2008, 26, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Meuwese, M.C.; de Groot, E.; Duivenvoorden, R.; Trip, M.D.; Ose, L.; Maritz, F.J.; Basart, D.C.; Kastelein, J.J.; Habib, R.; Davidson, M.H.; et al. ACAT inhibition and progression of carotid atherosclerosis in patients with familial hypercholesterolemia: the CAPTIVATE randomized trial. JAMA 2009, 301, 1131–1139. [Google Scholar] [CrossRef]
- Hai, Q.; Smith, J.D. Acyl-Coenzyme A: Cholesterol Acyltransferase (ACAT) in Cholesterol Metabolism: From Its Discovery to Clinical Trials and the Genomics Era. Metabolites 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, L.; Konopka, G.; Pack-Chung, E.; Ingano, L.A.; Berezovska, O.; Hyman, B.T.; Chang, T.Y.; Tanzi, R.E.; Kovacs, D.M. Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat Cell Biol 2001, 3, 905–912. [Google Scholar] [CrossRef]
- Bryleva, E.Y.; Rogers, M.A.; Chang, C.C.; Buen, F.; Harris, B.T.; Rousselet, E.; Seidah, N.G.; Oddo, S.; LaFerla, F.M.; Spencer, T.A.; et al. ACAT1 gene ablation increases 24(S)-hydroxycholesterol content in the brain and ameliorates amyloid pathology in mice with AD. Proc Natl Acad Sci U S A 2010, 107, 3081–3086. [Google Scholar] [CrossRef]
- Vance, J.E. Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis Model Mech 2012, 5, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.R.; Chang, C.C.; Dogbevia, G.; Bryleva, E.Y.; Bowen, Z.; Hasan, M.T.; Chang, T.Y. Acat1 knockdown gene therapy decreases amyloid-beta in a mouse model of Alzheimer's disease. Mol Ther 2013, 21, 1497–1506. [Google Scholar] [CrossRef]
- Shibuya, Y.; Chang, C.C.; Huang, L.H.; Bryleva, E.Y.; Chang, T.Y. Inhibiting ACAT1/SOAT1 in microglia stimulates autophagy-mediated lysosomal proteolysis and increases Abeta1-42 clearance. J Neurosci 2014, 34, 14484–14501. [Google Scholar] [CrossRef]
- Jin, U.; Park, S.J.; Park, S.M. Cholesterol Metabolism in the Brain and Its Association with Parkinson's Disease. Exp Neurobiol 2019, 28, 554–567. [Google Scholar] [CrossRef] [PubMed]
- van der Kant, R.; Langness, V.F.; Herrera, C.M.; Williams, D.A.; Fong, L.K.; Leestemaker, Y.; Steenvoorden, E.; Rynearson, K.D.; Brouwers, J.F.; Helms, J.B.; et al. Cholesterol Metabolism Is a Druggable Axis that Independently Regulates Tau and Amyloid-beta in iPSC-Derived Alzheimer's Disease Neurons. Cell Stem Cell 2019, 24, 363–375. [Google Scholar] [CrossRef]
- De La Torre, A.L.; Smith, C.; Granger, J.; Anderson, F.L.; Harned, T.C.; Havrda, M.C.; Chang, C.C.Y.; Chang, T.Y. Facile method to incorporate high-affinity ACAT/SOAT1 inhibitor F12511 into stealth liposome-based nanoparticle and demonstration of its efficacy in blocking cholesteryl ester biosynthesis without overt toxicity in neuronal cell culture. J Neurosci Methods 2022, 367, 109437. [Google Scholar] [CrossRef]
- Chang, C.C.; Lee, C.Y.; Chang, E.T.; Cruz, J.C.; Levesque, M.C.; Chang, T.Y. Recombinant acyl-CoA:cholesterol acyltransferase-1 (ACAT-1) purified to essential homogeneity utilizes cholesterol in mixed micelles or in vesicles in a highly cooperative manner. J Biol Chem 1998, 273, 35132–35141. [Google Scholar] [CrossRef] [PubMed]
- Junquero, D.; Oms, P.; Carilla-Durand, E.; Autin, J.; Tarayre, J.; Degryse, A.; Patoiseau, J.; Colpaert, F.C.; Delhon, A. Pharmacological profile of F 12511, (S)-2',3', 5'-trimethyl-4'-hydroxy-alpha-dodecylthioacetanilide a powerful and systemic acylcoenzyme A: cholesterol acyltransferase inhibitor. Biochem Pharmacol 2001, 61, 97–108. [Google Scholar] [CrossRef]
- Junquero, D.; Bruniquel, F.; N'Guyen, X.; Autin, J.M.; Patoiseau, J.F.; Degryse, A.D.; Colpaert, F.C.; Delhon, A. F 12511, a novel ACAT inhibitor, and atorvastatin regulate endogenous hypercholesterolemia in a synergistic manner in New Zealand rabbits fed a casein-enriched diet. Atherosclerosis 2001, 155, 131–142. [Google Scholar] [CrossRef]
- Junquero, D.; Pilon, A.; Carilla-Durand, E.; Patoiseau, J.F.; Tarayre, J.P.; Torpier, G.; Staels, B.; Fruchart, J.C.; Colpaert, F.C.; Clavey, V.; et al. Lack of toxic effects of F 12511, a novel potent inhibitor of acyl-coenzyme A: cholesterol O-acyltransferase, on human adrenocortical cells in culture. Biochem Pharmacol 2001, 61, 387–398. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine 2006, 1, 297–315. [Google Scholar] [PubMed]
- Gref, R.; Luck, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Muller, R.H. 'Stealth' corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Miteva, M.; Kirkbride, K.C.; Kilchrist, K.V.; Werfel, T.A.; Li, H.; Nelson, C.E.; Gupta, M.K.; Giorgio, T.D.; Duvall, C.L. Tuning PEGylation of mixed micelles to overcome intracellular and systemic siRNA delivery barriers. Biomaterials 2015, 38, 97–107. [Google Scholar] [CrossRef]
- Mesplet, N.; Morin, P.; Ribet, J.P. Development of a method for simultaneous determination of eflucimibe and its three major metabolites in rat plasma by liquid chromatography/electrospray tandem mass spectrometry: a preliminary study. Rapid Commun Mass Spectrom 2005, 19, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Patoiseau, J.-f.C. , FR), Autin, Jean-marie (Labruguiere, FR), Delhon, Andre (Castres, FR), Junquero, Didier (Burlats, FR). Alpha-phenyl acetanilide derivatives having an acat inhibiting activity and the therapeutic application thereof. US20060135785A1, 2006. [Google Scholar]
- Hansen, J.M.; Piorczynski, T.B. Use of Primary Mouse Embryonic Fibroblasts in Developmental Toxicity Assessments. Methods Mol Biol 2019, 1965, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Harned, T.C.; Stan, R.V.; Cao, Z.; Chakrabarti, R.; Higgs, H.N.; Chang, C.C.Y.; Chang, T.Y. Acute ACAT1/SOAT1 Blockade Increases MAM Cholesterol and Strengthens ER-Mitochondria Connectivity. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Garman, R.H. Histology of the central nervous system. Toxicol Pathol 2011, 39, 22–35. [Google Scholar] [CrossRef]
- Harvey, P.W.; Everett, D.J. The adrenal cortex and steroidogenesis as cellular and molecular targets for toxicity: critical omissions from regulatory endocrine disrupter screening strategies for human health? J Appl Toxicol 2003, 23, 81–87. [Google Scholar] [CrossRef]
- Harvey, P.W.; Everett, D.J.; Springall, C.J. Adrenal toxicology: a strategy for assessment of functional toxicity to the adrenal cortex and steroidogenesis. J Appl Toxicol 2007, 27, 103–115. [Google Scholar] [CrossRef]
- Sliskovic, D.R.; Picard, J.A.; Krause, B.R. ACAT inhibitors: the search for a novel and effective treatment of hypercholesterolemia and atherosclerosis. Prog Med Chem 2002, 39, 121–171. [Google Scholar] [CrossRef]
- Shibuya, K.; Morikawa, S.; Miyamoto, M.; Ogawa, S.I.; Tsunenari, Y.; Urano, Y.; Noguchi, N. Brain Targeting of Acyl-CoA:Cholesterol O-Acyltransferase-1 Inhibitor K-604 via the Intranasal Route Using a Hydroxycarboxylic Acid Solution. ACS Omega 2019, 4, 16943–16955. [Google Scholar] [CrossRef]
- Namiot, E.D.; Sokolov, A.V.; Chubarev, V.N.; Tarasov, V.V.; Schioth, H.B. Nanoparticles in Clinical Trials: Analysis of Clinical Trials, FDA Approvals and Use for COVID-19 Vaccines. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Safra, T.; Muggia, F.; Jeffers, S.; Tsao-Wei, D.D.; Groshen, S.; Lyass, O.; Henderson, R.; Berry, G.; Gabizon, A. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol 2000, 11, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.; Nag, O.K.; Rogers, K.E.; Delehanty, J.B. Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Juhairiyah, F.; de Lange, E.C.M. Understanding Drug Delivery to the Brain Using Liposome-Based Strategies: Studies that Provide Mechanistic Insights Are Essential. AAPS J 2021, 23, 114. [Google Scholar] [CrossRef]
- Pinheiro, R.G.R.; Coutinho, A.J.; Pinheiro, M.; Neves, A.R. Nanoparticles for Targeted Brain Drug Delivery: What Do We Know? Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Gulcur, E.; Thaqi, M.; Khaja, F.; Kuzmis, A.; Onyuksel, H. Curcumin in VIP-targeted sterically stabilized phospholipid nanomicelles: a novel therapeutic approach for breast cancer and breast cancer stem cells. Drug Deliv Transl Res 2013, 3. [Google Scholar] [CrossRef]
- Jhaveri, A.M.; Torchilin, V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front Pharmacol 2014, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Ashok, B.; Arleth, L.; Hjelm, R.P.; Rubinstein, I.; Onyuksel, H. In vitro characterization of PEGylated phospholipid micelles for improved drug solubilization: effects of PEG chain length and PC incorporation. J Pharm Sci 2004, 93, 2476–2487. [Google Scholar] [CrossRef] [PubMed]
- Willnow, T.E.; Herz, J. Genetic deficiency in low density lipoprotein receptor-related protein confers cellular resistance to Pseudomonas exotoxin A. Evidence that this protein is required for uptake and degradation of multiple ligands. J Cell Sci 1994, 107 Pt 3, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Doolittle, G.M.; Chang, T.Y. Cycloheximide sensitivity in regulation of acyl coenzyme A:cholesterol acyltransferase activity in Chinese hamster ovary cells. 1. Effect of exogenous sterols. Biochemistry 1986, 25, 1693–1699. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Dana, S.E.; Brown, M.S. Esterification of low density lipoprotein cholesterol in human fibroblasts and its absence in homozygous familial hypercholesterolemia. Proc Natl Acad Sci U S A 1974, 71, 4288–4292. [Google Scholar] [CrossRef]
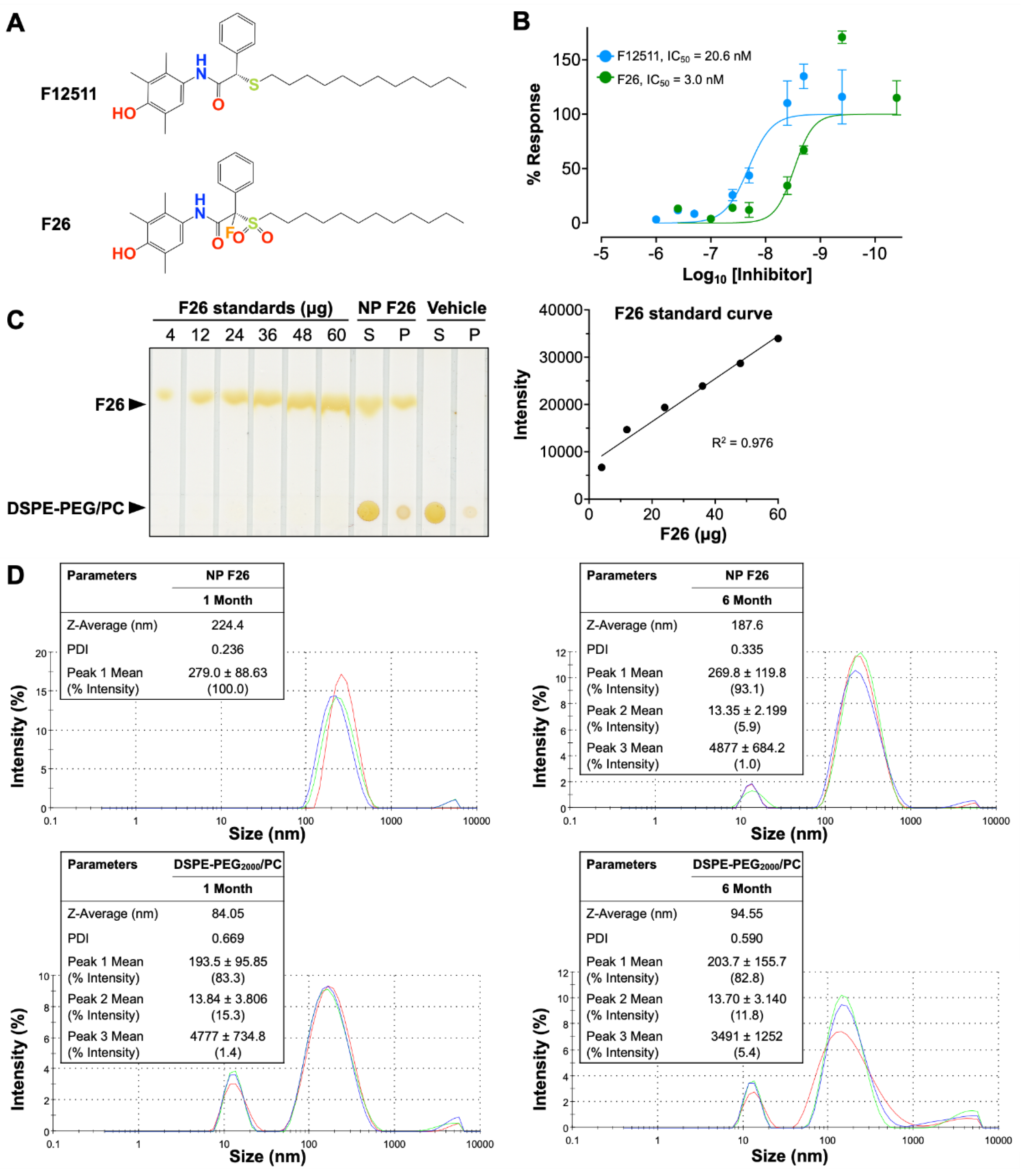
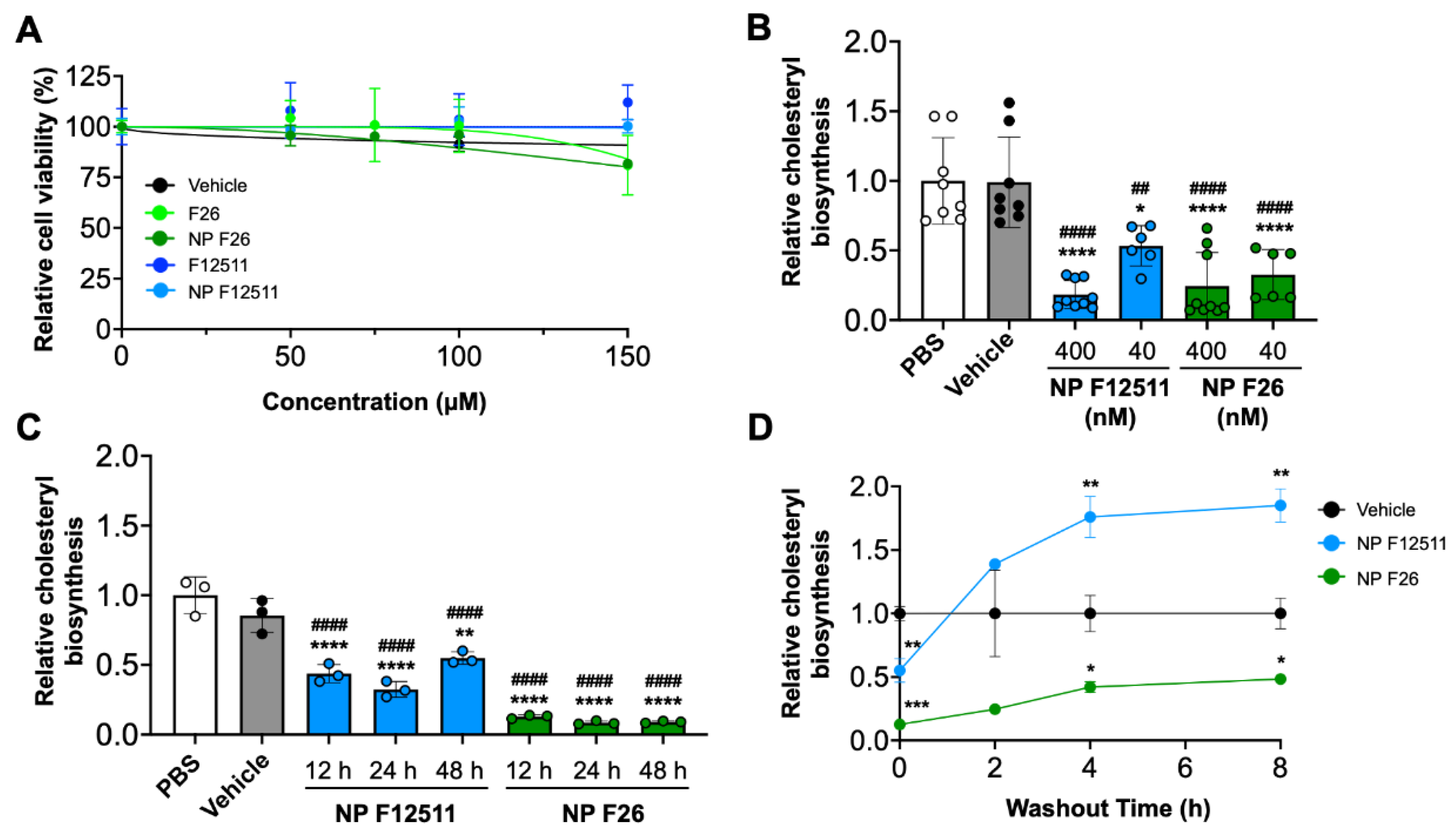
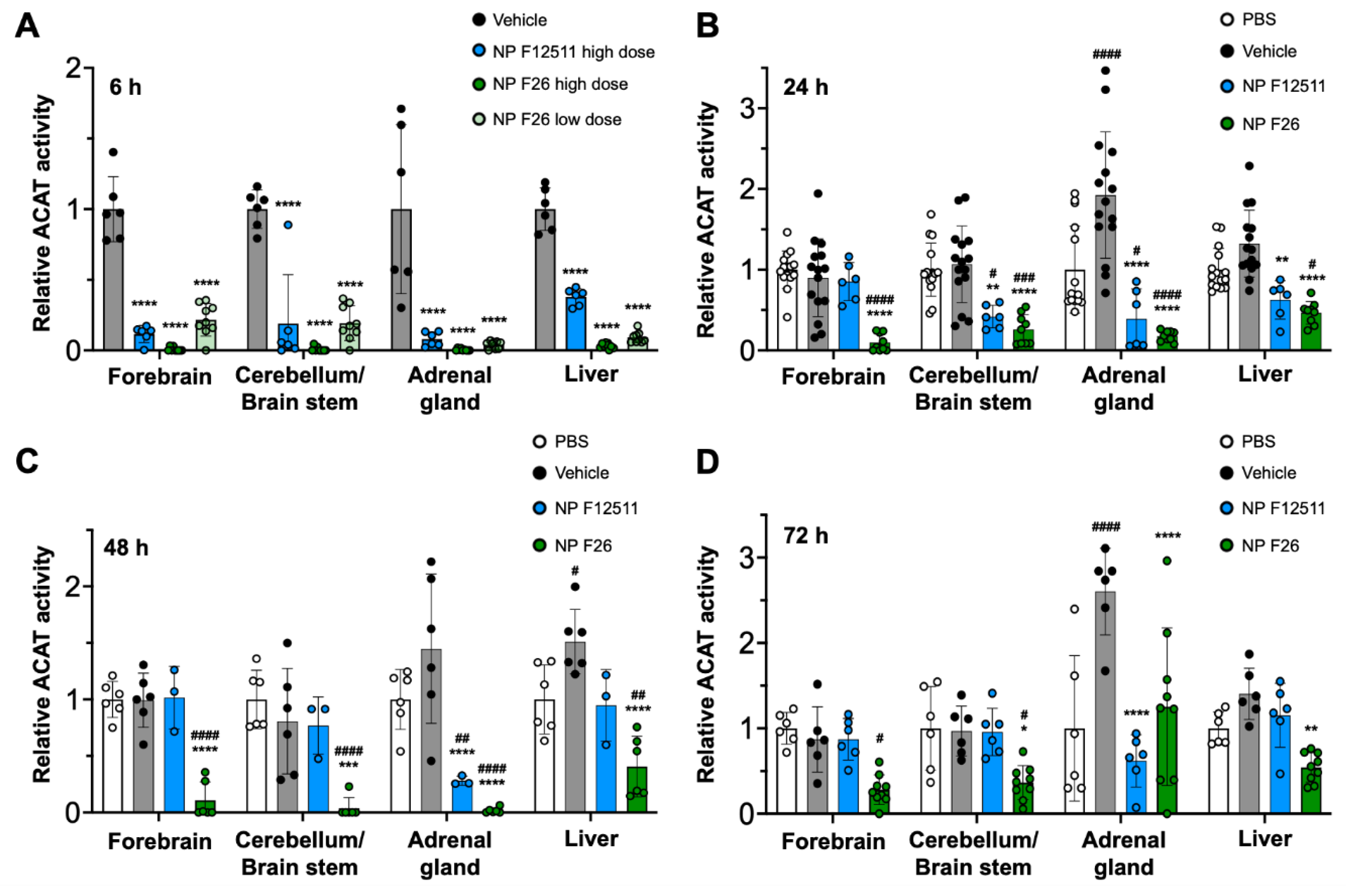
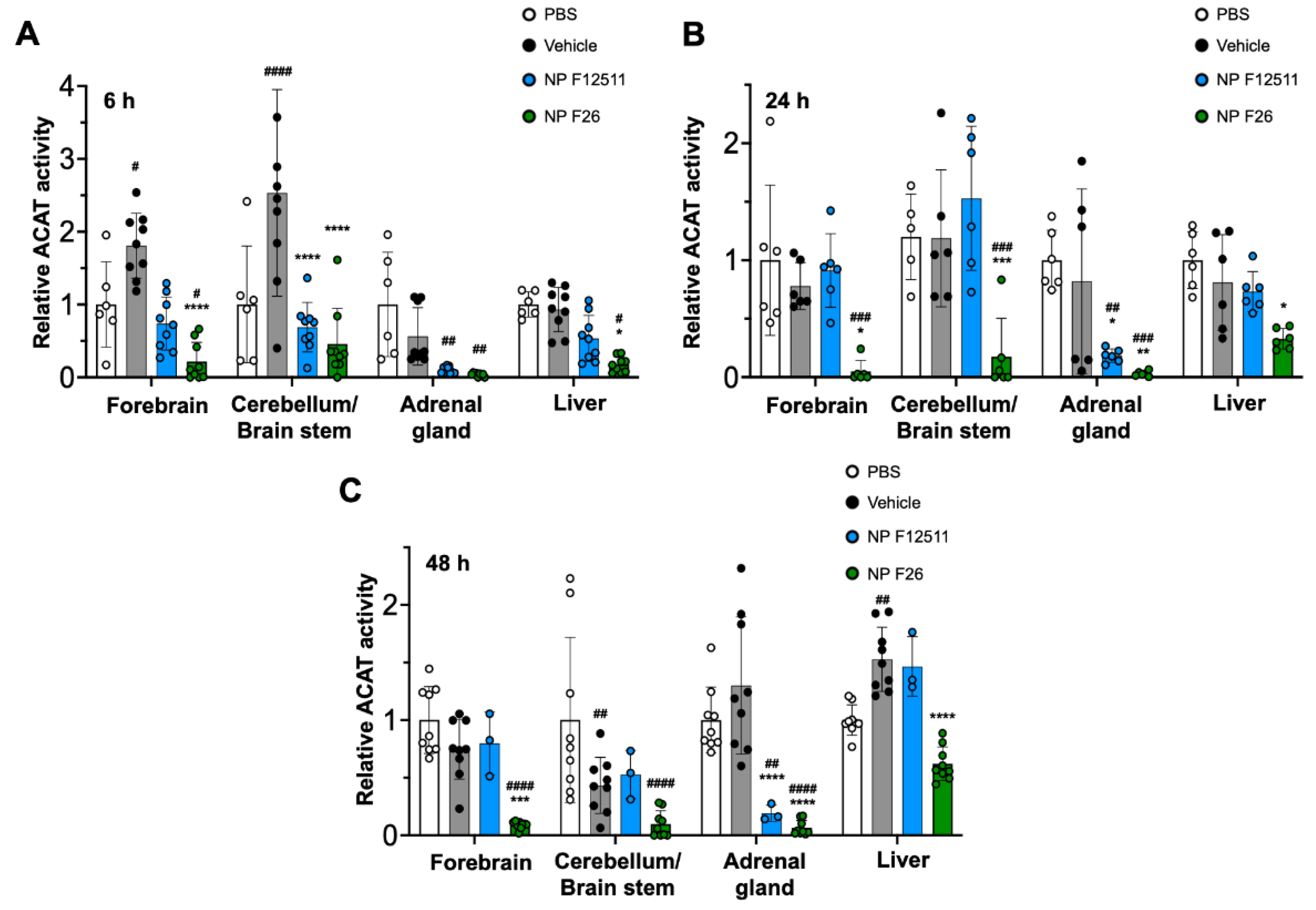
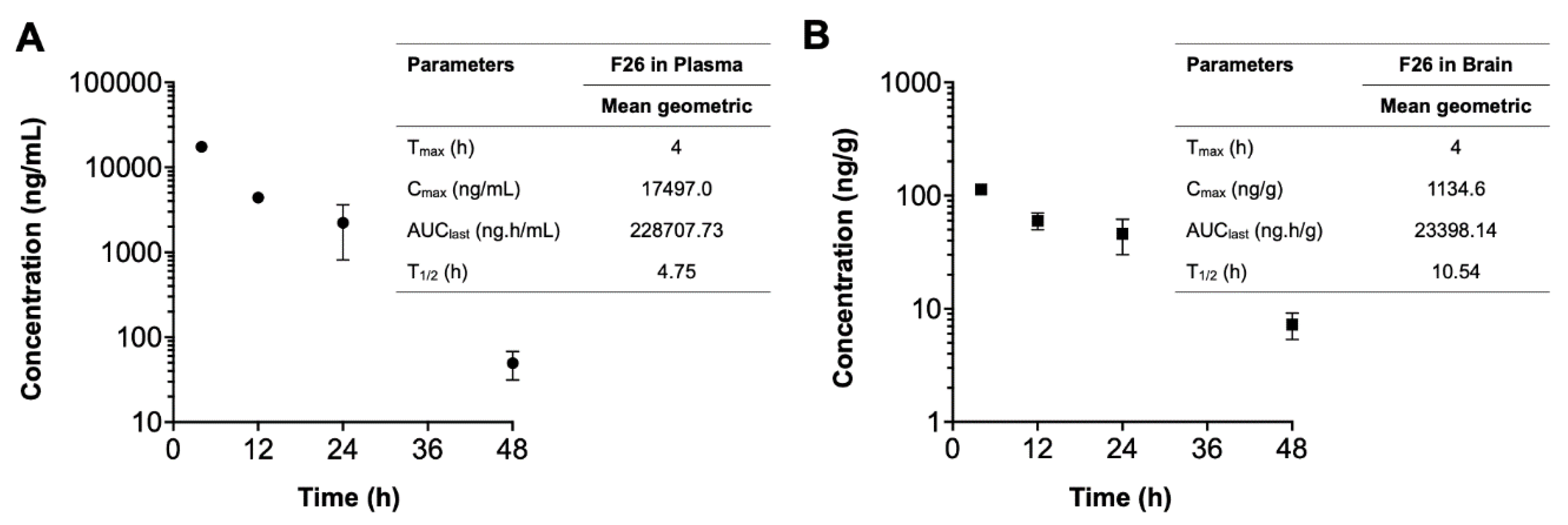
| Compounds | Time | Sample number |
Plasma concentration (ng/mL) |
Brain concentration (ng/g) |
Brain-to-Plasma ratio |
|---|---|---|---|---|---|
| F26 | 4 h | 1 | 18454 | 1121 | 0.061 |
| 2 | 16540 | 1148 | 0.069 | ||
| 12 h | 1 | 4572 | 671 | 0.147 | |
| 2 | 4243 | 529 | 0.125 | ||
| 24 h | 1 | 3218 | 571 | 0.178 | |
| 2 | 1226 | 347 | 0.283 | ||
| 48 h | 1 | 62 | 86 | 1.377 | |
| 2 | 37 | 59 | 1.604 | ||
| F12511 | 4 h | 1 | 11185 | 56 | 0.005 |
| 2 | 13184 | 83 | 0.006 | ||
| 3 | 12883 | 98 | 0.008 | ||
| 4 | 31224 | 95 | 0.003 | ||
| 12 h | 1 | 345 | 25 | 0.072 | |
| 2 | 487 | 26 | 0.053 | ||
| 24 h | 1 | 101 | 7 | 0.069 | |
| 2 | 21 | 9 | 0.429 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





