Submitted:
29 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Ethics and Animal Welfare Considerations
Crossbreeding to Generate F1 Offspring
Animal Housing and Nutritional Care
Extraction of Genomic DNA
F1 Mouse Genotyping Protocol
Tissue Harvesting
Data Analysis
3. Results
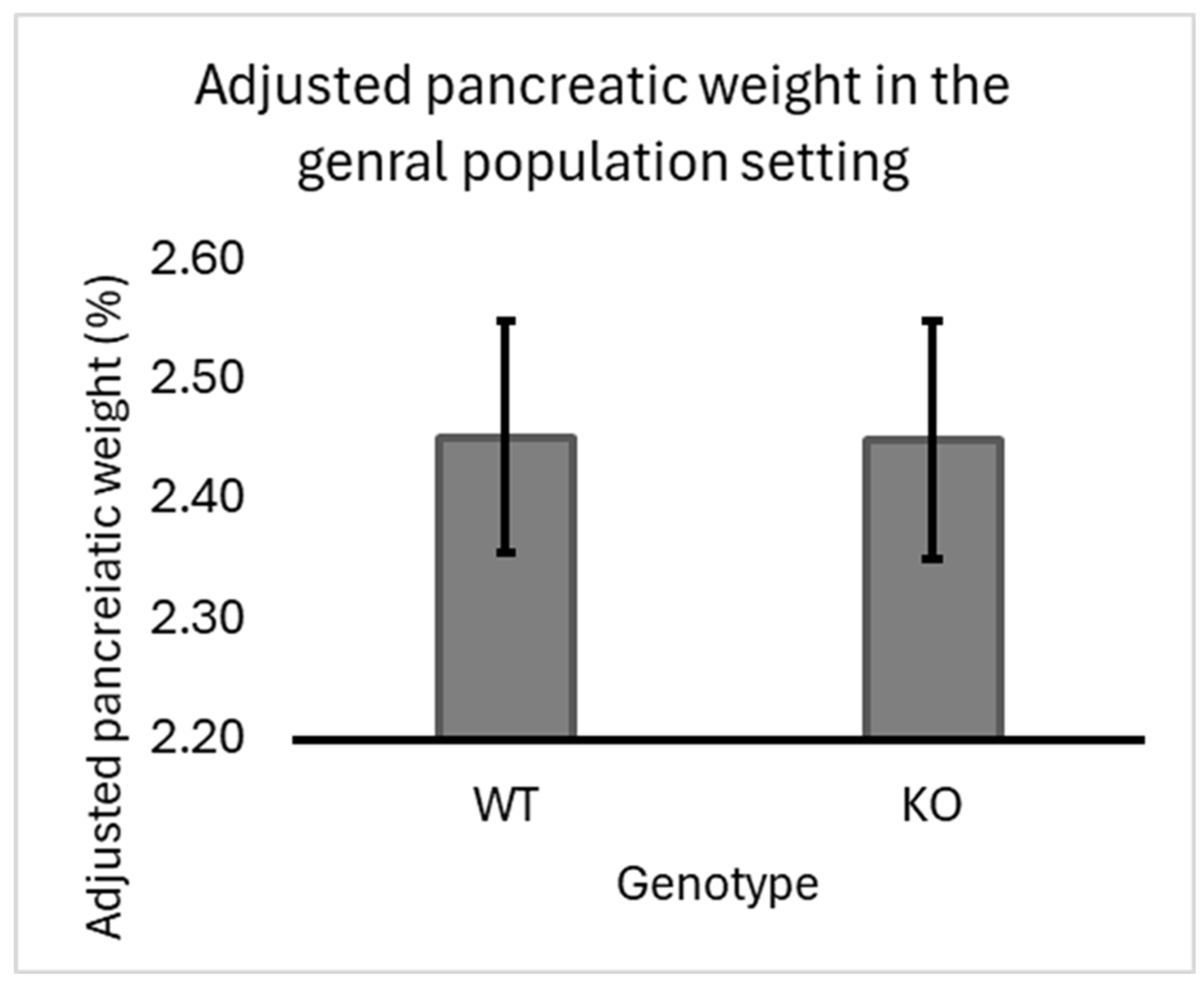
The Effect of Smad4 Kock out in the General Population of F1 Mice
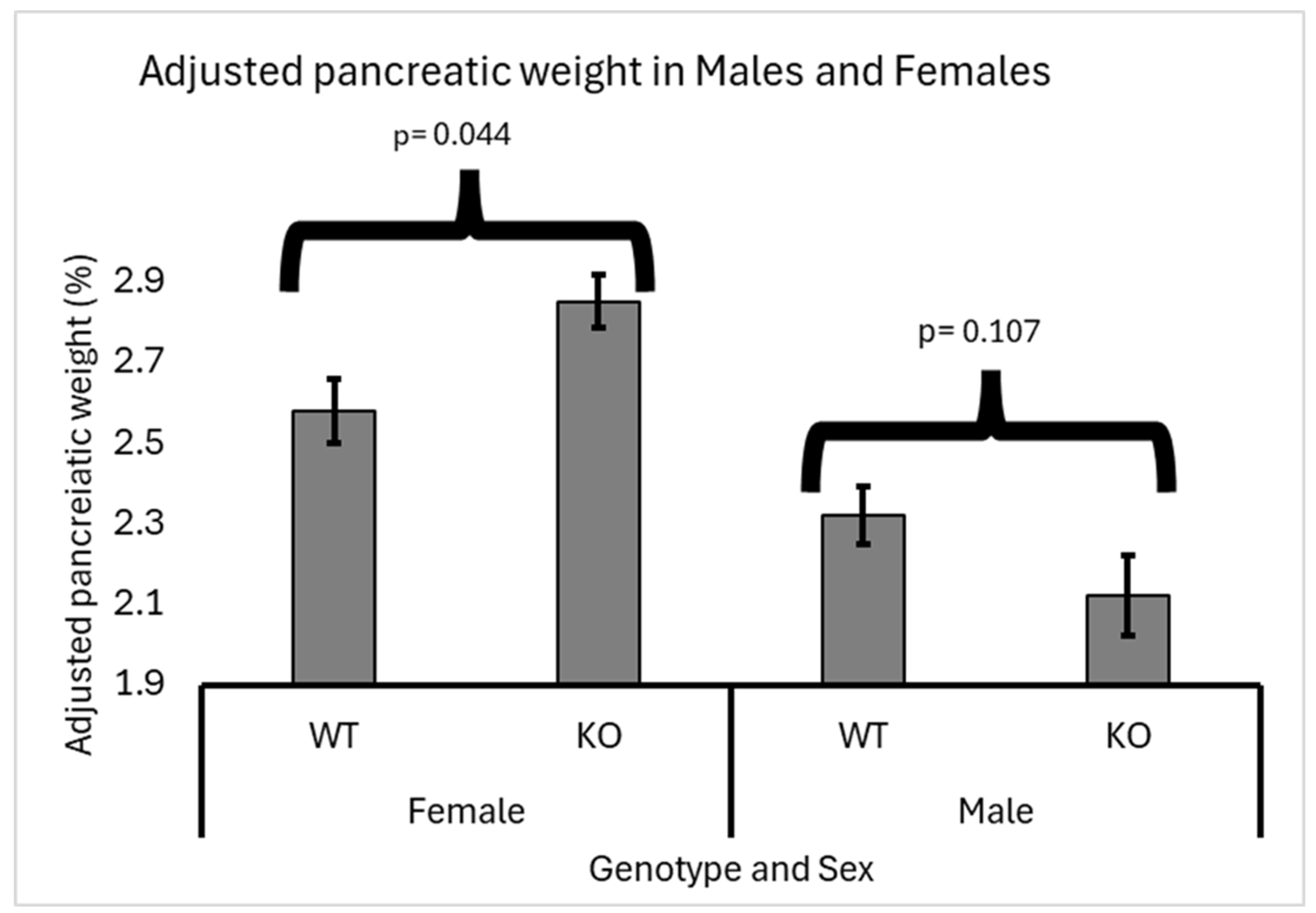
Sex Effect
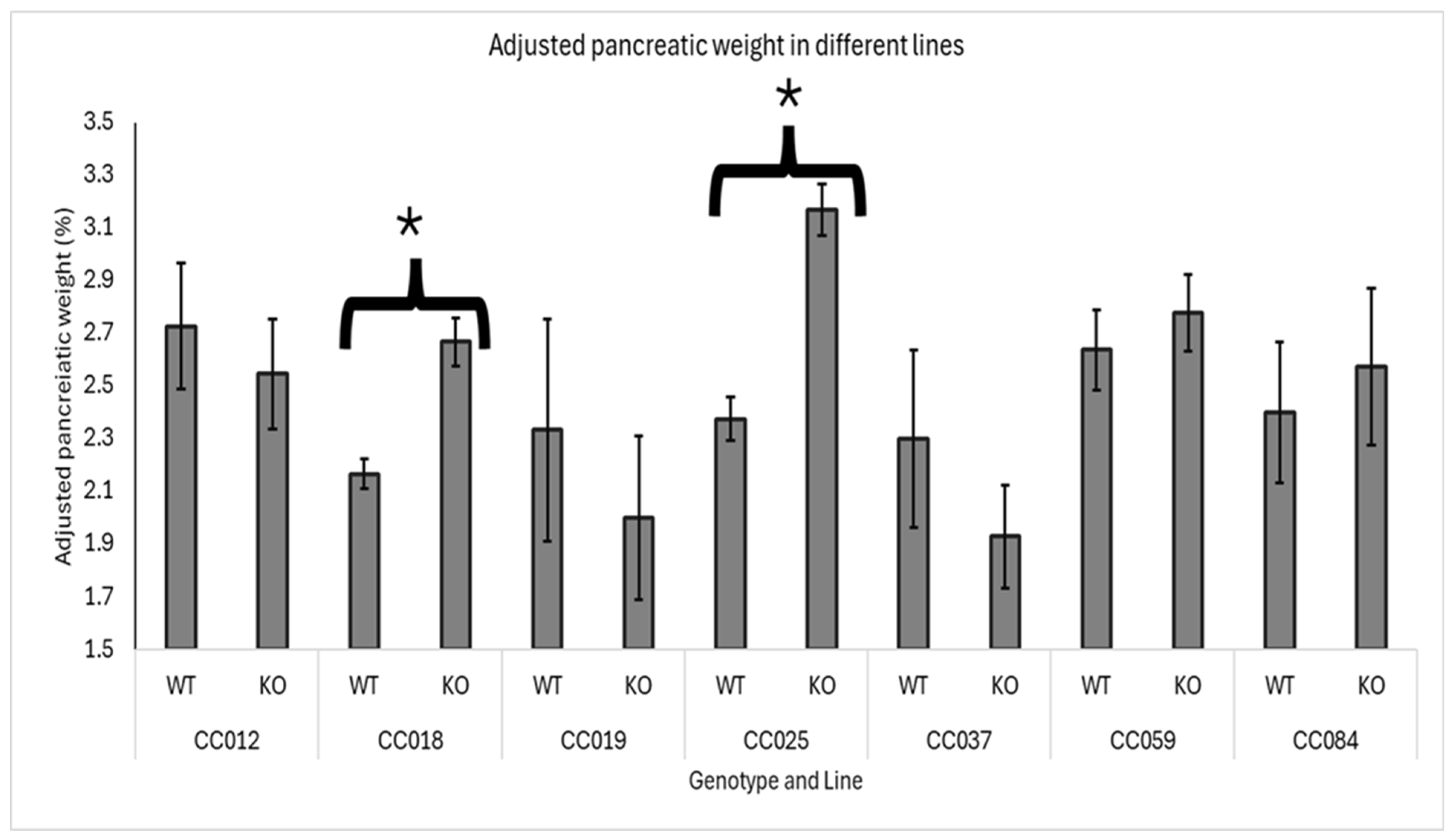
Line Genetic Effect
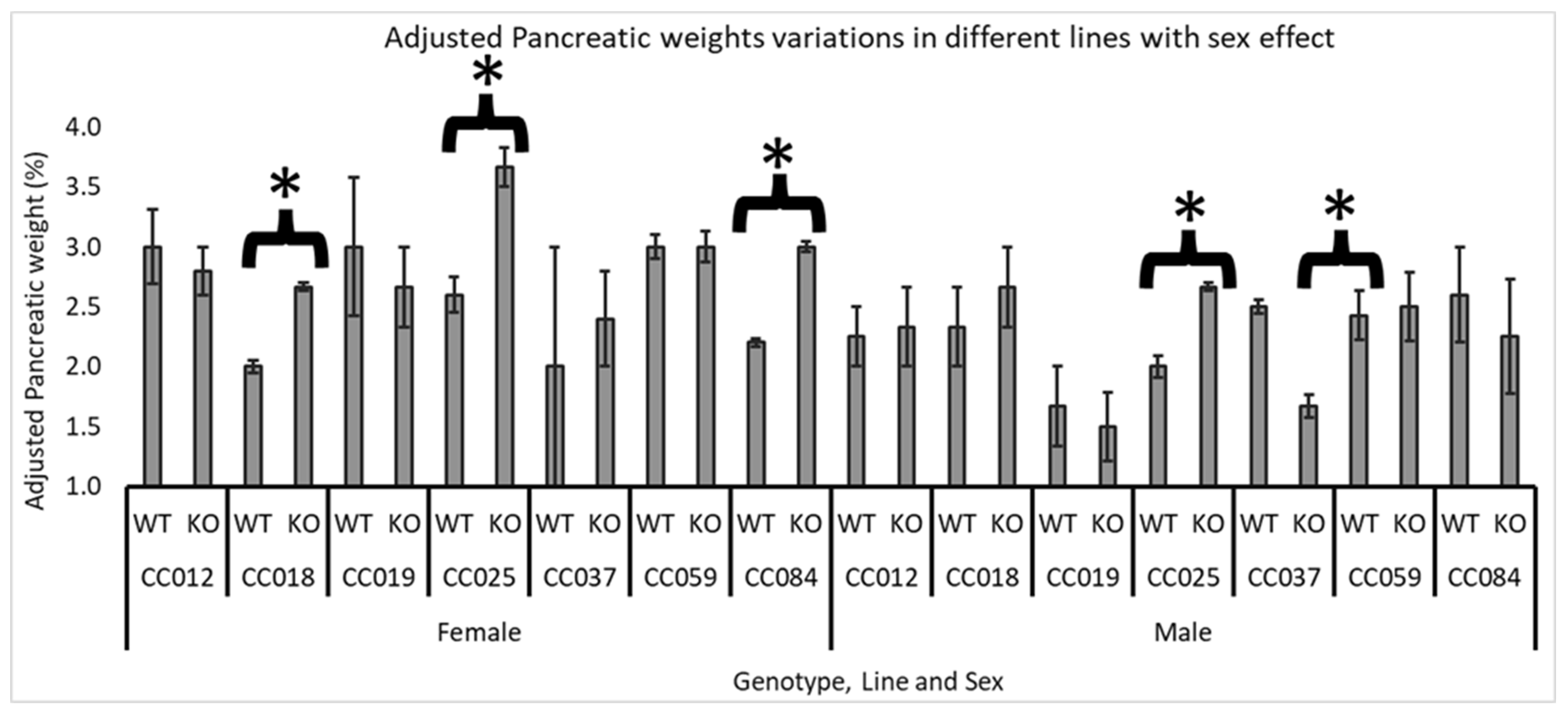
Line and Sex Effect
Heritability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paredes, J.L.; Orabi, A.I.; Ahmad, T.; Benbourenane, I.; Tobita, K.; Tadros, S.; Bae, K.T.; Husain, S.Z. A Non-Invasive Method of Quantifying Pancreatic Volume in Mice Using Micro-MRI. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Nathan, J.D.; Romac, J.; Peng, R.Y.; Peyton, M.; Macdonald, R.J.; Liddle, R.A. Transgenic Expression of Pancreatic Secretory Trypsin Inhibitor-I Ameliorates Secretagogue-Induced Pancreatitis in Mice. Gastroenterology 2005, 128. [Google Scholar] [CrossRef]
- Miyasaka, K.; Fujimoto, T.; Kawanami, T.; Takiguchi, S.; Jimi, A.; Funakoshi, A.; Shirasawa, S. Pancreatic Hypertrophy in Ki-Ras-Induced Actin-Interacting Protein Gene Knockout Mice. Pancreas 2011, 40. [Google Scholar] [CrossRef]
- Derynck, R.; Gelbart, W.M.; Harland, R.M.; Heldin, C.H.; Kern, S.E.; Massagué, J.; Melton, D.A.; Mlodzik, M.; Padgett, R.W.; Roberts, A.B.; et al. Nomenclature: Vertebrate Mediators of TGFbeta Family Signals. Cell 1996, 87. [Google Scholar] [CrossRef]
- Wrana, J.L. The Secret Life of Smad4. Cell 2009, 136. [Google Scholar] [CrossRef]
- Hahn, S.A.; Schutte, M.; Shamsul Hoque, A.T.M.; Moskaluk, C.A.; Da Costa, L.T.; Rozenblum, E.; Weinstein, C.L.; Fischer, A.; Yeo, C.J.; Hruban, R.H.; et al. DPC4, A Candidate Tumor Suppressor Gene at Human Chromosome 18q21.1. Science (1979) 1996, 271, 350–353. [Google Scholar] [CrossRef]
- Xia, X.; Wu, W.; Huang, C.; Cen, G.; Jiang, T.; Cao, J.; Huang, K.; Qiu, Z. SMAD4 and Its Role in Pancreatic Cancer. Tumor Biology 2015, 36. [Google Scholar] [CrossRef]
- Liu, F.; Pouponnot, C.; Massagué, J. Dual Role of the Smad4/DPC4 Tumor Suppressor in TGF-Inducible Transcriptional Complexes. 1997. [Google Scholar]
- Miyaki, M.; Kuroki, T. Role of Smad4 (DPC4) Inactivation in Human Cancer. Biochem Biophys Res Commun 2003, 306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Mishra, L.; Deng, C.X. The Role of TGF-β/SMAD4 Signaling in Cancer. Int J Biol Sci 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Srinivasan, R.; Wig, J.D. Major Molecular Markers in Pancreatic Ductal Adenocarcinoma and Their Roles in Screening, Diagnosis, Prognosis, and Treatment. Pancreas 2011, 40. [Google Scholar] [CrossRef] [PubMed]
- Zohud, O.; Lone, I.M.; Nashef, A.; Iraqi, F.A.; Fuad Iraqi, C.A. Towards System Genetics Analysis of Head and Neck Squamous Cell Carcinoma Using the Mouse Model, Cellular Platform, and Clinical Human Data. Anim Models Exp Med 2023, 6, 537–558. [Google Scholar] [CrossRef]
- Dorman, A.; Binenbaum, I.; Abu-Toamih Atamni, H.J.; Chatziioannou, A.; Tomlinson, I.; Mott, R.; Iraqi, F.A. Genetic Mapping of Novel Modifiers for Apc Min Induced Intestinal Polyps’ Development Using the Genetic Architecture Power of the Collaborative Cross Mice. BMC Genomics 2021, 22. [Google Scholar] [CrossRef]
- Zohud, O.; Midlej, K.; Lone, I.M.; Nashef, A.; Abu-Elnaaj, I.; Iraqi, F.A. Studying the Effect of the Host Genetic Background of Juvenile Polyposis Development Using Collaborative Cross and Smad4 Knock-Out Mouse Models. International Journal of Molecular Sciences 2024, 25, 5812. [Google Scholar] [CrossRef]
- Churchill, G.A.; Airey, D.C.; Allayee, H.; Angel, J.M.; Attie, A.D.; Beatty, J.; Beavis, W.D.; Belknap, J.K.; Bennett, B.; Berrettini, W.; et al. The Collaborative Cross, a Community Resource for the Genetic Analysis of Complex Traits. Nat Genet 2004, 36, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Atamni, H.J.A.T.; Botzman, M.; Mott, R.; Gat-Viks, I.; Iraqi, F.A. Mapping Liver Fat Female-Dependent Quantitative Trait Loci in Collaborative Cross Mice. Mammalian Genome 2016, 27. [Google Scholar] [CrossRef]
- Durrant, C.; Tayem, H.; Yalcin, B.; Cleak, J.; Goodstadt, L.; Pardo-Manuel De Villena, F.; Mott, R.; Iraqi, F.A. Collaborative Cross Mice and Their Power to Map Host Susceptibility to Aspergillus Fumigatus Infection. Genome Res 2011, 21, 1239–1248. [Google Scholar] [CrossRef]
- Dorman, A.; Baer, D.; Tomlinson, I.; Mott, R.; Iraqi, F.A. Genetic Analysis of Intestinal Polyp Development in Collaborative Cross Mice Carrying the Apc Min/+ Mutation. BMC Genet 2016, 17, 1–11. [Google Scholar] [CrossRef]
- Lorè, N.I.; Sipione, B.; He, G.; Strug, L.J.; Atamni, H.J.; Dorman, A.; Mott, R.; Iraqi, F.A.; Bragonzia, A. Collaborative Cross Mice Yield Genetic Modifiers for Pseudomonas Aeruginosa Infection in Human Lung Disease. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Abu-Toamih-Atamni, H.J.; Lone, I.M.; Binenbaum, I.; Mott, R.; Pilalis, E.; Chatziioannou, A.; Iraqi, F.A. Mapping Novel QTL and Fine Mapping of Previously Identified QTL Associated with Glucose Tolerance Using the Collaborative Cross Mice. Mammalian Genome 2023, 35, 31–55. [Google Scholar] [CrossRef]
- Iraqi, F.A.; Churchill, G.; Mott, R. The Collaborative Cross, Developing a Resource for Mammalian Systems Genetics: A Status Report of the Wellcome Trust Cohort. Mammalian Genome 2008, 19. [Google Scholar] [CrossRef]
- Iraqi, F.A.; Mahajne, M.; Salaymah, Y.; Sandovski, H.; Tayem, H.; Vered, K.; Balmer, L.; Hall, M.; Manship, G.; Morahan, G.; et al. The Genome Architecture of the Collaborative Cross Mouse Genetic Reference Population. Genetics 2012, 190. [Google Scholar] [CrossRef]
- Truett, G.E.; Heeger, P.; Mynatt, R.L.; Truett, A.A.; Walker, J.A.; Warman, M.L. Preparation of PCR-Quality Mouse Genomic Dna with Hot Sodium Hydroxide and Tris (HotSHOT). Biotechniques 2000, 29. [Google Scholar] [CrossRef] [PubMed]
- Veite-Schmahl, M.J.; Regan, D.P.; Rivers, A.C.; Nowatzke, J.F.; Kennedy, M.A. Dissection of the Mouse Pancreas for Histological Analysis and Metabolic Profiling. J. Vis. Exp 2017, 55647. [Google Scholar] [CrossRef]
- Chang, W.; Renaut, P.; Pretorius, C. SMAD4 Juvenile Polyposis Syndrome and Hereditary Haemorrhagic Telangiectasia Presenting in a Middle-Aged Man as a Large Fungating Gastric Mass, Polyposis in Both Upper and Lower GI Tract and Iron Deficiency Anaemia, with No Known Family History. BMJ Case Rep 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Bardeesy, N.; Cheng, K.H.; Berger, J.H.; Chu, G.C.; Pahler, J.; Olson, P.; Hezel, A.F.; Horner, J.; Lauwers, G.Y.; Hanahan, D.; et al. Smad4 Is Dispensable for Normal Pancreas Development yet Critical in Progression and Tumor Biology of Pancreas Cancer. Genes Dev 2006, 20. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Tanaka, S.; Umemori, H.; Minowa, O.; Usui, M.; Ikematsu, N.; Hosoda, E.; Imamura, T.; Kuno, J.; Yamashita, T.; et al. Negative Regulation of BMP/Smad Signaling by Tob in Osteoblasts. Cell 2000, 103. [Google Scholar] [CrossRef] [PubMed]
- Mamot, C.; Mild, G.; Reuter, J.; Laffer, U.; Metzger, U.; Terracciano, L.; Boulay, J.L.; Herrmann, R.; Rochlitz, C. Infrequent Mutation of the Tumour-Suppressor Gene Smad4 in Early-Stage Colorectal Cancer. Br J Cancer 2003, 88. [Google Scholar] [CrossRef] [PubMed]
- Buchou, T.; Vernet, M.; Blond, O.; Jensen, H.H.; Pointu, H.; Olsen, B.B.; Cochet, C.; Issinger, O.-G.; Boldyreff, B. Disruption of the Regulatory β Subunit of Protein Kinase CK2 in Mice Leads to a Cell-Autonomous Defect and Early Embryonic Lethality. Mol Cell Biol 2003, 23. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Yoshizawa, T.; Takahashi, Y.; Nitahara-Kasahara, Y.; Okada, T.; Nomura, Y.; Yamanaka, H.; Kosho, T.; Matsumoto, K. Backcrossing to an Appropriate Genetic Background Improves the Birth Rate of Carbohydrate Sulfotransferase 14 Gene-Deleted Mice. Exp Anim 2020, 69. [Google Scholar] [CrossRef]
- Garcia-Carracedo, D.; Yu, C.-C.; Akhavan, N.; Fine, S.A.; Schönleben, F.; Maehara, N.; Karg, D.C.; Xie, C.; Qiu, W.; Fine, R.L.; et al. Smad4 Loss Synergizes with TGFα Overexpression in Promoting Pancreatic Metaplasia, PanIN Development, and Fibrosis. 2015. [Google Scholar] [CrossRef]
- Simeone, D.M.; Zhang, L.; Treutelaar, M.K.; Zhang, L.; Graziano, K.; Logsdon, C.D.; Burant, C.F. Islet Hypertrophy Following Pancreatic Disruption of Smad4 Signaling. Am J Physiol Endocrinol Metab 2006, 291, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Qahaz, N.; Lone, I.M.; Khadija, A.; Ghnaim, A.; Zohud, O.; Nun, N.B.; Nashef, A.; Abu El-Naaj, I.; Iraqi, F.A. Host Genetic Background Effect on Body Weight Changes Influenced by Heterozygous Smad4 Knockout Using Collaborative Cross Mouse Population. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
| Line | ||||||||
|---|---|---|---|---|---|---|---|---|
| CC012 | CC018 | CC019 | CC025 | CC037 | CC059 | CC084 | ||
| Female | WT | 7 | 3 | 3 | 5 | 4 | 4 | 5 |
| KO | 5 | 3 | 3 | 3 | 5 | 5 | 3 | |
| Male | WT | 4 | 3 | 3 | 3 | 6 | 7 | 5 |
| KO | 6 | 3 | 4 | 3 | 9 | 4 | 4 | |
| Sex | Genotype | Trait | VG | H2 | CVg |
|---|---|---|---|---|---|
| Female | WT | Adjusted Pan Weight % | 0.284903 | 0.477302 | 0.214363 |
| Female | WT | Delta_BW | 14.5507 | 0.153954 | 0.062006 |
| Female | KO | Adjusted Pan Weight % | 0.257129 | 0.603407 | 0.201222 |
| Female | KO | Delta_BW | 4.166905 | 0.036585 | 0.032808 |
| Male | WT | Adjusted Pan Weight % | 0.536923 | 0.456597 | 0.319978 |
| Male | WT | Delta_BW | 6.331883 | 0.04312 | 0.04313 |
| Male | KO | Adjusted Pan Weight % | 0.243643 | 0.412505 | 0.196654 |
| Male | KO | Delta_BW | 4.073576 | 0.030492 | 0.03395 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





