1. Introduction
In 2020, an estimated 2.2 million patients were diagnosed with breast cancer, making it the most common malignant disease among women in JAPAN [
1]. Breast cancer accounted for over 68,000 deaths that year, ranking it as the fifth leading cause of cancer related mortality worldwide [
2].
Mammography (MG) is widely utilized for breast cancer screening and has been demonstrated to reduce mortality rates [
3]. However, MG presents several challenges.
Dense breast tissue, which is prevalent among young and Asian women, can obscure cancer detection on MG and diminish its sensitivity [
4]. Additionally, the risk of developing primary contralateral breast cancer is estimated to be 2 to 6 times higher than that of developing a second breast cancer in the general population [
5,
6,
7]. Women under 50 years of age who develop contralateral breast cancer within five years of their first diagnosis face a mortality risk 3.9 times greater than those with unilateral cancer [
8]. Although the combined use of MG and Magnetic Resonance Imaging (MRI) shows a sensitivity of 67% and a specificity of 50% for detecting metachronous contralateral breast cancer post-unilateral surgery [
9], the guidelines currently recommend only physical exams and MG every 6 to 12 months for postoperative surveillance, without endorsing further imaging tests [
10,
11].
In Japan, MRI surveillance post-unilateral breast cancer surgery is generally not covered by insurance, except in cases such as hereditary breast and ovarian cancer syndrome (HBOC) [
10,
11]. Recent advancements in deep learning-based artificial intelligence (AI) have significantly outpaced traditional computer-aided detection (CAD) systems in breast imaging diagnostics [
12]. Studies have shown that AI can alleviate the workload of radiologists without compromising diagnostic quality. Despite most screening participants being cancer-free, AI systems are capable of identifying normal mammary glands and can be used for either primary or secondary readings, potentially enhancing screening efficiency [
13,
14,
15,
16]. AI also holds promise for pre-diagnostically identifying high-risk breast cancer cases, potentially reducing intermediate-stage diagnoses and false-negative rates [
16,
17,
18]. Post-unilateral breast cancer surgery, MG interpretation becomes more complex. Variations in the mammary gland background and the necessity of comparing images to the contralateral side complicate image assessments. Postoperative deformities and calcifications can preclude using the operated breast for comparative analyses, thereby increasing the diagnostic burden for radiologists and heightening patient anxiety about developing contralateral breast cancer. Bilateral breast cancer may also indicate HBOC [
19].
Currently, routine MG is the only surveillance method for early detection of contralateral breast cancer, despite its limitations. Given these challenges, this study explores the potential of the AI system FxMammo™ (FathomX, Singapore), a mammography diagnostic support AI, to surpass radiologists in the accurate and early detection of metachronous contralateral breast cancer using MG.
2. Materials and Methods
Patients
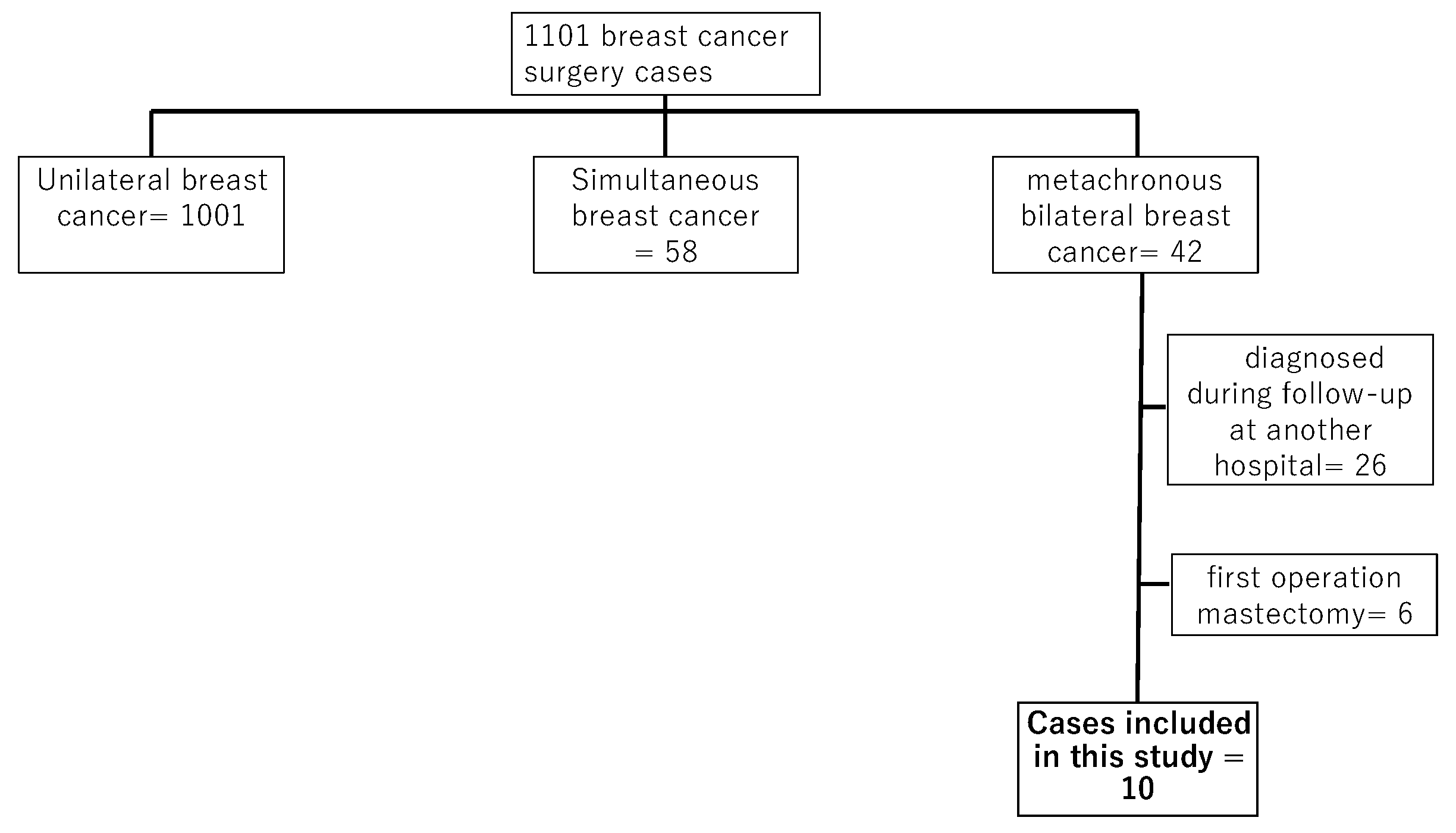
A total of 1,101 patients underwent breast cancer surgery at our hospital from January 2014 to December 2022. Among these, 42 cases of metachronous bilateral breast cancer were identified. Twenty-six patients were diagnosed with contralateral breast cancer at other institutions during follow-up, while 16 were diagnosed and underwent contralateral breast cancer surgery post-initial surgery at our hospital. Cases in which total mastectomy was performed as the initial surgery were excluded due to the inability of the AI system to align left and right images. Therefore, six patients were excluded, leaving ten cases for inclusion in this study (
Figure 1).
Ethical approval and consent to participate
This study adhered to the Declaration of Helsinki, the Clinical Research Act (Act No. 16 of 2017), the Enforcement Regulations of the Clinical Research Act (Ministry of Health, Labor and Welfare Ordinance No. 17 of 2018), and relevant notices. Ethical approval was obtained from the Ethics Review Committee of our hospital (approval ID: M2019-232, approval date: 13 December 2019). Comprehensive informed consent was obtained from all patients regarding the use of their clinical data for research purposes.
Data collecting
Clinical information and pathological data were collected from medical records retrospectively. Images studies and diagnostic reports were obtained from the radiology reporting system. The imaging modalities utilized included MG
, ultrasound (US), and MRI conducted before surgery for contralateral breast cancer. MG used 2D diagnosis without tomosynthesis. Imaging diagnosis was evaluated by Japanese radiologists according to breast imaging reporting and data system (BI-RADS) categories [
20]. The BI-RADS categories were collected from the reporting system as diagnosed by the radiologists at the time of imaging. Malignancy was defined as BI-RADS category 4 or higher. Pathological diagnoses were made by physicians with a specialty in Japanese pathology.
The AI system
We used the MG AI system FxMammo🄬 (FathomX Pte Ltd, Singapore). The AI system is based on deep learning and has been put into practical use in Singapore and other countries. The mechanism of FxMammo has been described in previous papers. The AI system is based on the VGG-16 network [
23]. The VGG network is one of the most used feature-extractor in medical imaging classification [
24]. The AI was created by collecting 17,769 cases (of which 45% were malignant) from 10 institutions in Taiwan, Thailand, Singapore, Hong Kong, China, Malaysia and Japan. An analysis using validation data yielded an AUC of 0.909 (95% Confidence Interval CI [0.900, 0.918]). Although in Japan, the AI system has not been approved for clinical use and is used for research purposes. Four MG images (craniocaudal [CC], mediolateral oblique [MLO], left and right) taken before surgery for heterochronic contralateral breast cancer were transferred from the reporting system to the AI system. The AI system analyzed MG data. The threshold value was set as 40.0 %, (Sensitivity were set at 91.5 %and specificity were 82.0%). The AI system indicated the probability of malignancy for each of the four cards as a percentage. In addition, areas that the AI system was interested in were displayed in color on a heat map. (
Figure 2).
Postoperative surveillance
Postoperative breast cancer surveillance at our hospital basically includes MG and US once a year. MRI is performed when breast cancer is strongly suspected by MG or US, or after breast cancer is diagnosed by biopsy. However, the interval of surveillance may become wider or narrower depending on the patients’ reasons. MG (CC and MLO) was performed both of right and left. The US was performed by radiologists who specialize in breast imaging diagnosis. MRI of both breasts was acquired using a 3.0-T system with a breast coil and the patient in the prone position. Unenhanced and enhanced phases were acquired at 1, 2, and 6 min in the axial plane after intravenous bolus injection of gadolinium (0.1 mL/kg), using a fat-suppressed T1-weighted sequence (TR/TE = 6.5/2.4, flip angle = 10°, 2 mm thick section, 512 × 512 matrix, 360 mm field of view). The number of years until contralateral surgery was calculated in full years, omitting decimal places.
Postoperative surveillance
For each image, the possibility of malignancy was diagnosed according to BI-RADS. In cases where the AI system diagnosis differed from the radiologists' interpretation, the images and pathology were compared and examined in detail. Based on the analysis results, the breast cancer detection rate for each modality was calculated. All the analyses in were conducted using the EZR software package version 1.31 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [
25].
Postoperative breast cancer surveillance at our hospital basically includes MG and US once a year. MRI is performed when breast cancer is strongly suspected by MG or US, or after breast cancer is diagnosed by biopsy. However, the interval of surveillance may become wider or narrower depending on the patients’ reasons. MG (CC and MLO) was performed both of right and left. The US was performed by radiologists who specialize in breast imaging diagnosis. MRI of both breasts was acquired using a 3.0-T system with a breast coil and the patient in the prone position. Unenhanced and enhanced phases were acquired at 1, 2, and 6 min in the axial plane after intravenous bolus injection of gadolinium (0.1 mL/kg), using a fat-suppressed T1-weighted sequence (TR/TE = 6.5/2.4, flip angle = 10°, 2 mm thick section, 512 × 512 matrix, 360 mm field of view). The number of years until contralateral surgery was calculated in full years, omitting decimal places
Statical analysis
For each image, the possibility of malignancy was diagnosed according to BI-RADS. In cases where the AI system diagnosis differed from the radiologists' interpretation, the images and pathology were compared and examined in detail. Based on the analysis results, the breast cancer detection rate for each modality was calculated. All the analyses in were conducted using the EZR software package version 1.31 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [
25].
3. Results
Patient characteristics
The study included 10 cases, all of whom were Japanese females.
Table 1 presents the clinical and pathological characteristics at the time of the first surgery. The median age diagnosed at first breast cancer was 68 years (range: 40 -74 years). There were 3 cases (30%) at Stage 0, 4 cases (40%) at Stage I, and 3 cases (30%) at Stage II. Chemotherapy was administered to 4 cases (40%), and endocrine therapy was given to 6 cases (60%). Radiation to the preserved breasts was performed in all cases.
Table 2 describes clinical and pathological factors regarding contralateral breast cancer. The median time to contralateral breast cancer was 8 years (range: 2-10 years). The T classification ranged from Tis in 2 cases (20%), T1 in 7 cases (70%), T2 in 1 case (10%), Stage 0 was in 2 cases (20%), Stage Ⅰ in 7 cases (70%), and Stage ⅡA in 1 case (10%). Histological types were invasive ductal carcinoma (IDC) in 6 cases (60%), ductal carcinoma in situ (DCIS) in 2 cases (20%), apocrine carcinoma in 1 case (10%) and invasive lobular carcinoma (ILC) in 1 case (10%). Biology was Luminal in 4 cases (40%), human epidermal growth factor receptor (HER)2 in 1 cases (10%), Luminal-HER2 in 1 cases (10%), and triple negative breast cancer (TNBC) in 4 cases (40%).
Imaging diagnosis at the time of diagnosis
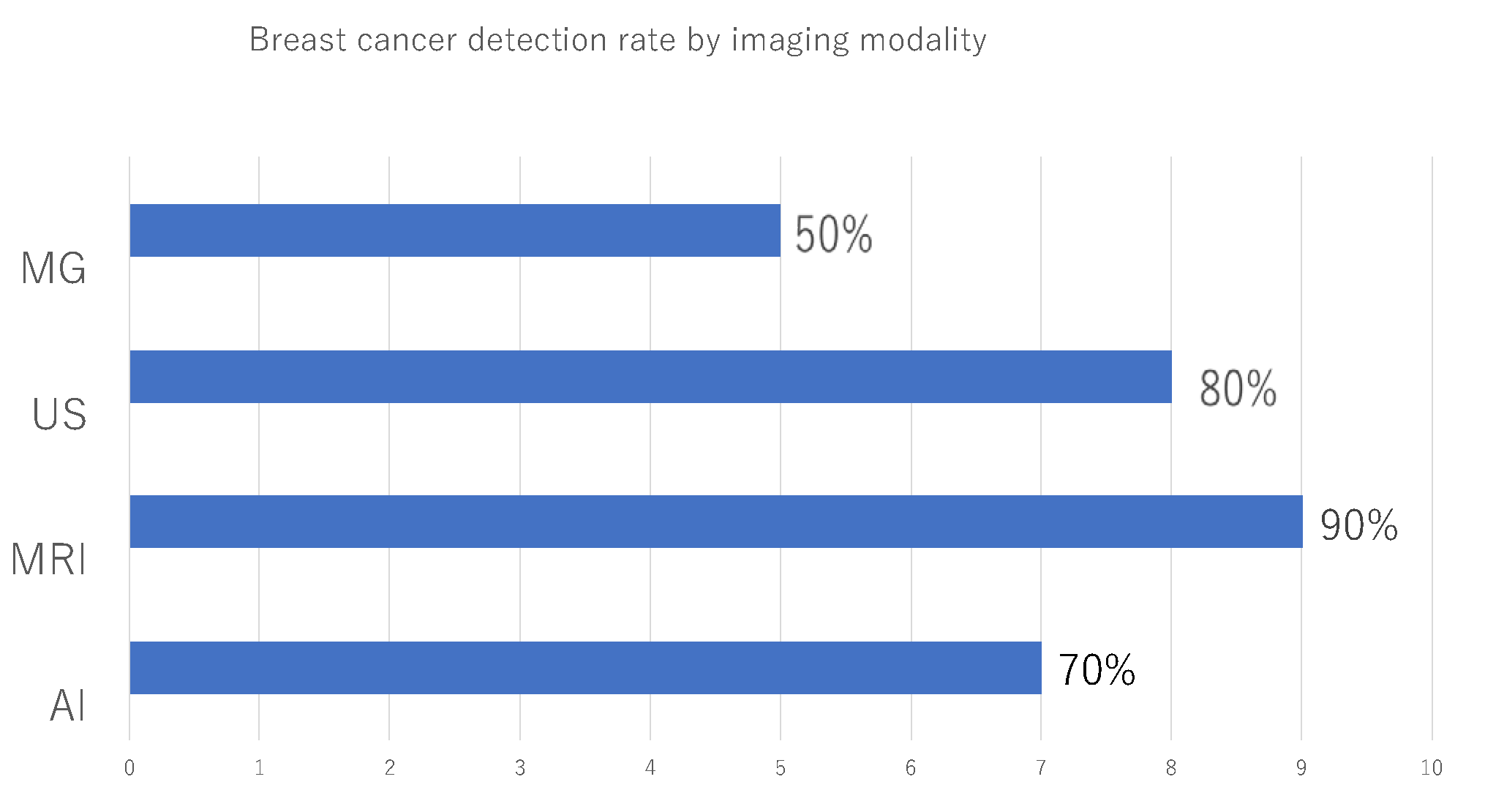
Table 3 describes the imaging findings at the time of diagnosis of contralateral breast cancer. The MG categories according to BI-RADS were 1 in 4cases (40%), 2 in 1case (10%), 4 in 3 cases (30%), and 5 in 2 cases (20%). The US categories according to BI-RADS were 2 in 2cases (20%), 4 in 5cases (50%), and 5 in 3 cases (30%). The MRI categories according to BI-RADS were 1 in 1 case (10%)and 4 in 9cases (90%). In cases 1 to 8, the lesions could be identified by US, so preoperative histological diagnosis was performed by US. In case 9, the lesion could be identified only by MRI, so MRI-guided biopsy was performed. In case 10, the preoperative diagnosis was Paget's disease based on abrasive cytology. Although no lesions were found within the breast o imaging, postoperative specimens determined that there was DCIS within the breast. Accuracy was calculated for each imaging modality and the AI system. The accuracy for each imaging modality and the AI system was calculated, with MG at 50%, the AI system at 70%, US at 80%, and MRI at 90%, with MRI having the highest accuracy, followed by US, the AI system, and radiologist readings of MG (
Figure 3).
Diagnosis by the AI system and comparison with past images
Table 4 shows the results of the analysis of the diagnosis of MG heterochronic bilateral breast cancer using the AI system. In cases 1 to 6, the diagnosis by the AI system indicated a possibility of malignancy. In cases 1 and 2, where the radiologists' readings diagnosed no possibility of malignancy, only the AI system diagnosed possible malignancy. We reviewed the MG images of these two cases. The area of interest by the AI system was identified as focal asymmetric density (FAD) with increased density compared to other areas upon viewing after the final diagnosis. In cases 3, 4, 5, and 6, both the AI and the radiologists diagnosed malignancy. Two cases involved masses and 2 cases involved calcifications (
Table 3). In case 7, only the radiologists diagnosed a mass visible only in the MLO direction as potentially malignant, which was a low-density mass. In cases 8, 9, and 10, both the radiologists and the AI system diagnosed no malignant findings. For cases 1 to 6, one MG image prior to the time of diagnosis was analyzed to verify whether the AI system could diagnose breast cancer at an earlier timing (
Table 5). In most cases, there was no difference between the AI system's diagnosis and the radiologists’ diagnosis. However, in cases 1 and 2, the AI system had previously diagnosed malignancy.
Representative case
Cases 1, where only the AI system could diagnose, were examined in detail. In case 1, the AI system diagnosed a malignant finding in the right C area, considering it as the region of interest (
Figure 4). In case 7, only the radiologists diagnosed a potentially malignant mass about MG (
Figure 5). Compared to last year, it appeared as focal asymmetric density. Radiologists diagnosed as BI-RADS category 4; AI diagnosed no malignant findingsThis section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
4. Discussion
In this study, we investigated the efficacy of an AI system for the MG diagnosis of asynchronous contralateral breast cancer. By comparing the diagnostic abilities of radiologists and the AI system, we found that in 2 out of 10 cases (20%), only the AI system identified potential malignancy, which had also been similarly assessed by the AI prior to mammographic diagnosis. Additionally, both the radiologists and AI system concurred in the absence of malignancy signs in 3 cases (30%), which were diagnosed complementarily with US, MRI, and physical examination. The primary reason for concordant diagnoses between radiologists and the AI system seems to be that the AI training data was created by humans, suggesting that the AI system may mimic human diagnostic patterns.
The significance of this study can be divided into three major aspects. Firstly, the use of an AI system could enhance diagnostic accuracy and facilitate early detection of breast cancer. According to the NCCN guidelines [
10], annual MG is recommended for post-operative breast cancer patients, who face a 2-6 times higher risk of contralateral breast cancer compared to non-cancer patients [
5,
6,
7]. Particularly in cases of contralateral surgeries, the lack of comparative images complicates diagnosis, hence the AI system could effectively supplement this limitation. Some instances where the AI system solely diagnosed malignancy on past MG highlight its potential as a diagnostic aid.
Secondly, the implementation of an AI system could reduce the workload and stress on radiologists. By undertaking efficient image analysis, AI allows radiologists to focus on more critical cases, potentially improving diagnostic accuracy. Additionally, referencing AI analysis could boost the radiologists' confidence in their diagnoses, thereby reducing psychological stress. With advancements in technology, the precision of AI diagnostics may evolve, possibly transforming the role of radiologists into a more efficient diagnostic process.
Thirdly, the AI system could offer psychological reassurance to patients. Patients who have experienced breast cancer often fear recurrence or new cancer development; detailed image analysis by AI could help them better understand their health status and gain reassurance[
26]. Particularly, heatmaps generated by AI could visually demonstrate suspicious areas, potentially alleviating patient anxiety.
In recent years, AI technology has advanced and is being applied to various medical imaging modalities [
27,
28,
29,
30,
31,
32]. In breast cancer imaging diagnosis, its usefulness has been demonstrated in multiple modalities, including MG, US, MRI, and positron emission tomography [
33,
34,
35,
36,
37].
To date, only one study has reported the use of AI for postoperative surveillance of breast cancer. Out of 314 cases, three were heterochronic contralateral breast cancers. The recall rate for contralateral breast cancer was significantly lower with the combination of mammography and AI system (1.5%) compared to mammography alone (6.6%, p<0.01). The accuracy was significantly higher with the combination of mammography and AI system (97.1%) compared to mammography alone (92.5%, p<0.001) [
21]. The use of AI resulted in a decreased recall rate and improved accuracy, which mirrors the trend observed in this study. We only considered cases of heterochronic contralateral breast cancer; however, a larger-scale validation including non-recurrent cases is necessary to further verify the utility of the AI system.
There are several limitations to this study. Firstly, it is a retrospective, single-center study with a small sample size. Additionally, while the AI system is approved for mammographic screening in other countries, it is still not approved for clinical use in Japan and is only available for research purposes. There is little evidence on its use in post-operative patients. Future studies should collect more mammographic data from multiple facilities to conduct prospective validations of the AI system.
5. Conclusions
In the mammographic diagnosis of asynchronous contralateral breast cancer, the AI system demonstrated the ability to identify signs of malignancy that might be overlooked by radiologists. These results suggest that the AI system could contribute to the early detection and enhanced accuracy of breast cancer diagnosis.
Author Contributions
All authors contributed to the study conception and design. Conceptualization M. A. and T. I.; methodology and data curation .M.S., and K.H.; software, D. H., M. H., F. M.; validation, M.N.; formal analysis, E.Y., and L.K.; investigation, M.A.; resources, Y.K. writing—original draft preparation, M.A..; writing—review and editing, T. F; visualization, M.A.; supervision ; G.O., and K. K.; and project administration ; T. U.; All authors have read and agreed to the published version.
Funding
This study was supported by the Japan-Singapore Bilateral Joint Research Projects 2023, funded by the Japan Society for the Promotion of Science.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Tokyo Medical and Dental University Hospital, (approval ID: M2019-232, approval date: 13 December 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Raw data were generated at Tokyo Medical and Dental University Hospital. Derived data supporting the findings of this study are available from the corresponding author M.A. on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdoli, G.; Bottai, M.; Sandelin, K.; Moradi, T. Breast Cancer Diagnosis and Mortality by Tumor Stage and Migration Background in a Nationwide Cohort Study in Sweden. Breast 2017, 31, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Badve, S.S.; Beitsch, P.D.; Bose, S.; Byrd, D.R.; Chen, V.W.; Mayer, I.A.; Mccormick, B.; Mittendorf, E.A.; Recht, A.; Reis-Filho, J.S.; et al. Members of the Breast Expert Panel. 2017. [CrossRef]
- Løberg, M.; Lousdal, M.L.; Bretthauer, M.; Kalager, M. Benefits and Harms of Mammography Screening. Breast Cancer Research 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M.; Kim, E.H. Breast Density and Risk of Breast Cancer in Asian Women: A Meta-Analysis of Observational Studies. Journal of Preventive Medicine and Public Health 2016, 49, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Schaapveld, M.; Jansen, L.; Bagherzadegan, E.; Sahinovic, M.M.; Baas, P.C.; Hanssen, L.M.H.C.; van der Mijle, H.C.J.; Brandenburg, J.D.; Wiggers, T.; et al. The Value of Surveillance Mammography of the Contralateral Breast in Patients with a History of Breast Cancer. Eur J Cancer 2009, 45, 3000–3007. [Google Scholar] [CrossRef] [PubMed]
- Soerjomataram, I.; Louwman, W.J.; Lemmens, V.E.P.P.; De Vries, E.; Klokman, W.J.; Coebergh, J.W.W. Risks of Second Primary Breast and Urogenital Cancer Following Female Breast Cancer in the South of The Netherlands, 1972-2001. Eur J Cancer 2005, 41, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Chaudary MA, M.R.H.E.H.M.B.R.C.J.H.JL. Bilateral Primary Breast Cancer: A Prospective Study of Disease Incidence. Br J Surg. 1984, 71, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.; Czene, K.; Reilly, M.; Adolfsson, J.; Bergh, J.; Adami, H.O.; Dickman, P.W.; Hall, P. Incidence and Prognosis of Synchronous and Metachronous Bilateral Breast Cancer. Journal of Clinical Oncology 2007, 25, 4210–4216. [Google Scholar] [CrossRef]
- Robertson, C.; Ragupathy, S.K.A.; Boachie, C.; Fraser, C.; Heys, S.D.; MacLennan, G.; Mowatt, G.; Thomas, R.E.; Gilbert, F.J. Surveillance Mammography for Detecting Ipsilateral Breast Tumour Recurrence and Metachronous Contralateral Breast Cancer: A Systematic Review. Eur Radiol 2011, 21, 2484–2491. [Google Scholar] [CrossRef]
- Smith TJ, D.N.S.D.G.E.M.H.V.V. American Society of Clinical Oncology 1998 Update of Recommended Breast Cancer Surveillance Guidelines. American Society of Clinical Oncology 1999, 3, 1080–1082. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Masuda, K.; Hiraswa, A.; Takehara, K.; Tsuda, H.; Watanabe, Y.; Oda, K.; Nagase, S.; Mandai, M.; Okamoto, A.; et al. Current Status of Hereditary Breast and Ovarian Cancer Practice among Gynecologic Oncologists in Japan: A Nationwide Survey by the Japan Society of Gynecologic Oncology (JSGO). J Gynecol Oncol 2022, 33. [Google Scholar] [CrossRef]
- Lehman, C.D.; Wellman, R.D.; Buist, D.S.M.; Kerlikowske, K.; Tosteson, A.N.A.; Miglioretti, D.L. Diagnostic Accuracy of Digital Screening Mammography with and without Computer-Aided Detection. JAMA Intern Med 2015, 175, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, A.D.; Rodríguez-Ruiz, A.; von Euler-Chelpin, M.C.; Lynge, E.; Vejborg, I.; Nielsen, M.; Karssemeijer, N.; Lillholm, M. An Artificial Intelligence–Based Mammography Screening Protocol for Breast Cancer: Outcome and Radiologist Workload. Radiology 2022, 304, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Dembrower, K.; Wåhlin, E.; Liu, Y.; Salim, M.; Smith, K.; Lindholm, P.; Eklund, M.; Strand, F. Effect of Artificial Intelligence-Based Triaging of Breast Cancer Screening Mammograms on Cancer Detection and Radiologist Workload: A Retrospective Simulation Study. Lancet Digit Health 2020, 2, e468–e474. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, A.; Lång, K.; Gubern-Merida, A.; Teuwen, J.; Broeders, M.; Gennaro, G.; Clauser, P.; Helbich, T.H.; Chevalier, M.; Mertelmeier, T.; et al. Can We Reduce the Workload of Mammographic Screening by Automatic Identification of Normal Exams with Artificial Intelligence? A Feasibility Study. Eur Radiol 2019, 29, 4825–4832. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Aglen, C.F.; Lee, C.I.; Hoff, S.R.; Lund-Hanssen, H.; Lång, K.; Nygård, J.F.; Ursin, G.; Hofvind, S. Artificial Intelligence Evaluation of 122969 Mammography Examinations from a Population-Based Screening Program. Radiology 2022, 303, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Lång, K.; Hofvind, S.; Rodríguez-Ruiz, A.; Andersson, I. Can Artificial Intelligence Reduce the Interval Cancer Rate in Mammography Screening? [CrossRef]
- Byng, D.; Strauch, B.; Gnas, L.; Leibig, C.; Stephan, O.; Bunk, S.; Hecht, G. AI-Based Prevention of Interval Cancers in a National Mammography Screening Program. Eur J Radiol 2022, 152. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.P.; Feldman, J.; Mirzaa, G.M. Associated Hereditary Breast and Ovarian Cancer; 1998;
- BIRADS Atlas Preface.
- Schaffter, T.; Buist, D.S.M.; Lee, C.I.; Nikulin, Y.; Ribli, D.; Guan, Y.; Lotter, W.; Jie, Z.; Du, H.; Wang, S.; et al. Evaluation of Combined Artificial Intelligence and Radiologist Assessment to Interpret Screening Mammograms. JAMA Netw Open 2020, 3, e200265. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Kim, E.K.; Kim, G.R.; Han, K.; Moon, H.J. Mammographic Surveillance After Breast-Conserving Therapy: Impact of Digital Breast Tomosynthesis and Artificial Intelligence-Based Computer-Aided Detection. American Journal of Roentgenology 2022, 218, 42–51. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. 2014.
- Kim, H.E.; Cosa-Linan, A.; Santhanam, N.; Jannesari, M.; Maros, M.E.; Ganslandt, T. Transfer Learning for Medical Image Classification: A Literature Review. BMC Med Imaging 2022, 22. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transplant 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Ranieri, J.; Guerra, F.; Di Giacomo, D. Role of Metacognition Thinking and Psychological Traits in Breast Cancer Survivorship. Behavioral Sciences 2020, 10. [Google Scholar] [CrossRef]
- Barat, M.; Pellat, A.; Hoeffel, C.; Dohan, A.; Coriat, R.; Fishman, E.K.; Nougaret, S.; Chu, L.; Soyer, P. CT and MRI of Abdominal Cancers: Current Trends and Perspectives in the Era of Radiomics and Artificial Intelligence. Jpn J Radiol 2024, 42, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Tatsugami, F.; Nakaura, T.; Yanagawa, M.; Fujita, S.; Kamagata, K.; Ito, R.; Kawamura, M.; Fushimi, Y.; Ueda, D.; Matsui, Y.; et al. Recent Advances in Artificial Intelligence for Cardiac CT: Enhancing Diagnosis and Prognosis Prediction. Diagn Interv Imaging 2023, 104, 521–528. [Google Scholar] [CrossRef]
- Yardimci, A.H.; Kocak, B.; Sel, I.; Bulut, H.; Bektas, C.T.; Cin, M.; Dursun, N.; Bektas, H.; Mermut, O.; Yardimci, V.H.; et al. Radiomics of Locally Advanced Rectal Cancer: Machine Learning-Based Prediction of Response to Neoadjuvant Chemoradiotherapy Using Pre-Treatment Sagittal T2-Weighted MRI. Jpn J Radiol 2023, 41, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Fujima, N.; Kamagata, K.; Ueda, D.; Fujita, S.; Fushimi, Y.; Yanagawa, M.; Ito, R.; Tsuboyama, T.; Kawamura, M.; Nakaura, T.; et al. Current State of Artificial Intelligence in Clinical Applications for Head and Neck MR Imaging. Magnetic Resonance in Medical Sciences 2023, 22, 401–414. [Google Scholar] [CrossRef]
- Du, G.; Zeng, Y.; Chen, D.; Zhan, W.; Zhan, Y. Application of Radiomics in Precision Prediction of Diagnosis and Treatment of Gastric Cancer. Jpn J Radiol 2023, 41, 245–257. [Google Scholar] [CrossRef]
- Hirata, K.; Kamagata, K.; Ueda, D.; Yanagawa, M.; Kawamura, M.; Nakaura, T.; Ito, R.; Tatsugami, F.; Matsui, Y.; Yamada, A.; et al. From FDG and beyond: The Evolving Potential of Nuclear Medicine. Ann Nucl Med 2023, 37, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, J.; Fujioka, T.; Yamaga, E.; Hayashi, A.; Kujiraoka, Y.; Imokawa, T.; Takahashi, K.; Okawa, S.; Yashima, Y.; Mori, M.; et al. Deep Learning Method with a Convolutional Neural Network for Image Classification of Normal and Metastatic Axillary Lymph Nodes on Breast Ultrasonography. Jpn J Radiol 2022, 40, 814–822. [Google Scholar] [CrossRef]
- Goto, M.; Sakai, K.; Toyama, Y.; Nakai, Y.; Yamada, K. Use of a Deep Learning Algorithm for Non-Mass Enhancement on Breast MRI: Comparison with Radiologists’ Interpretations at Various Levels. Jpn J Radiol 2023, 41, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Satake, H.; Ishigaki, S.; Ito, R.; Naganawa, S. Radiomics in Breast MRI: Current Progress toward Clinical Application in the Era of Artificial Intelligence. Radiologia Medica 2022, 127, 39–56. [Google Scholar] [CrossRef]
- Nara, M.; Fujioka, T.; Mori, M.; Aruga, T.; Tateishi, U. Prediction of Breast Cancer Risk by Automated Volumetric Breast Density Measurement. Jpn J Radiol 2023, 41, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, T.; Nakashima, K.; Harada, T.L.; Nasu, H.; Igarashi, T. Comparisons between Artificial Intelligence Computer-Aided Detection Synthesized Mammograms and Digital Mammograms When Used Alone and in Combination with Tomosynthesis Images in a Virtual Screening Setting. Jpn J Radiol 2023, 41, 63–70. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Algorithm for the cases included in this study. The number of cases operated on at the hospital was 1,101. There were 1,101 cases of unilateral breast cancer, 58 cases of simultaneous bilateral breast cancer, and 42 cases of heterochronic bilateral breast cancer. Twenty-six cases of contralateral breast cancer were noted during follow-up at other hospitals, and 6 cases of total resection were performed as the initial surgery, making 10 cases eligible for this study.
Figure 1.
Algorithm for the cases included in this study. The number of cases operated on at the hospital was 1,101. There were 1,101 cases of unilateral breast cancer, 58 cases of simultaneous bilateral breast cancer, and 42 cases of heterochronic bilateral breast cancer. Twenty-six cases of contralateral breast cancer were noted during follow-up at other hospitals, and 6 cases of total resection were performed as the initial surgery, making 10 cases eligible for this study.
Figure 2.
Displaying images in FxMammo. An image of the mediolateral oblique of mammography is shown on the left. Spiculated mass is seen in the left upper area. On the right is the result of the AI system analysis, with the areas of interest to the AI system indicated by the colors in the heatmap. The percentage of malignancy is shown on the left and right sides, respectively (right 3.3%, left 94.2%).
Figure 2.
Displaying images in FxMammo. An image of the mediolateral oblique of mammography is shown on the left. Spiculated mass is seen in the left upper area. On the right is the result of the AI system analysis, with the areas of interest to the AI system indicated by the colors in the heatmap. The percentage of malignancy is shown on the left and right sides, respectively (right 3.3%, left 94.2%).
Figure 3.
Breast cancer detection rate by imaging modality.Mammography (MG), ultrasonography (US), Magnetic Resonance Imaging (MRI), and the artificial intelligence (AI) system, respectively, to diagnose the degree of malignancy. The highest diagnostic accuracy was 90% for MRI, followed by US, AI systems, and MG read by radiologists, in that order.
Figure 3.
Breast cancer detection rate by imaging modality.Mammography (MG), ultrasonography (US), Magnetic Resonance Imaging (MRI), and the artificial intelligence (AI) system, respectively, to diagnose the degree of malignancy. The highest diagnostic accuracy was 90% for MRI, followed by US, AI systems, and MG read by radiologists, in that order.
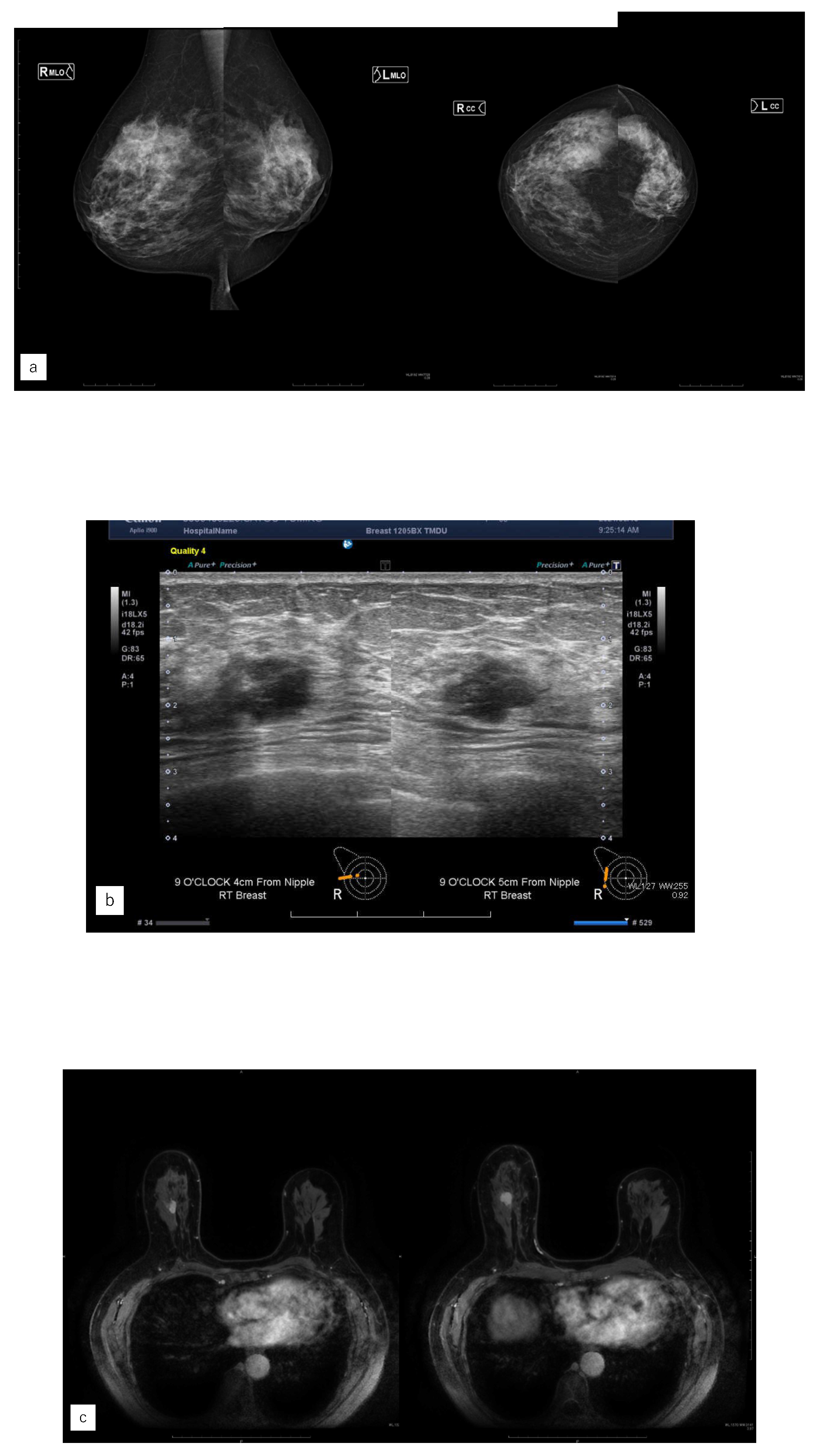
Figure 4.
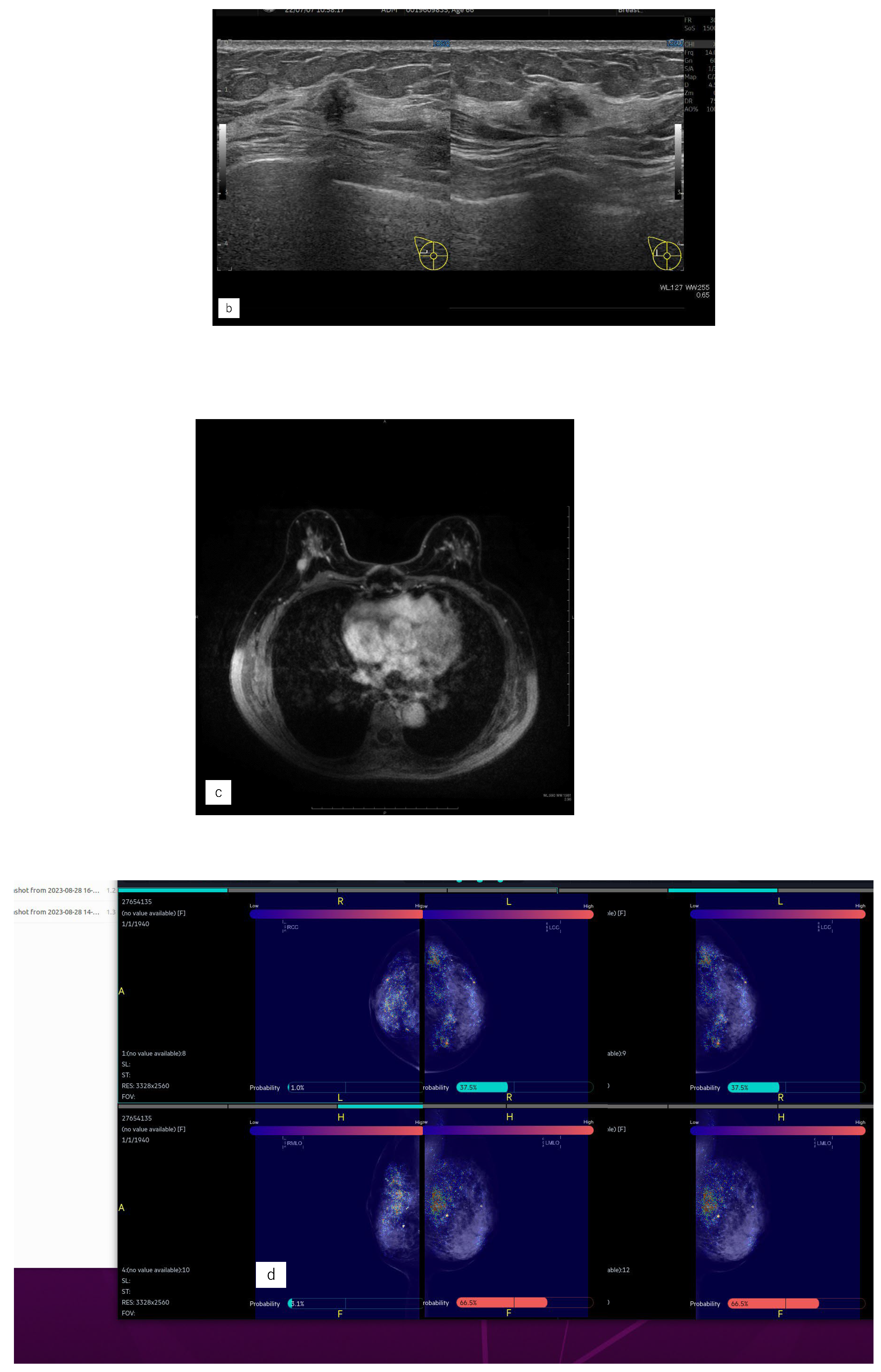
Representative case (case1). (a) MG. (b) US. (c) MRI. (d) AI diagnosis. (e) previous AI diagnosis.A 55 years old woman had left side breast cancer. Eight years later, she was diagnosed with right breast cancer. It was Lumina human epidermal growth factor receptor2 with 15 mm of invasive cancer and 15 mm of non-invasive cancer.(a) There were no malignant findings in the right side of mammography (MG).(b) Ultrasonography revealed a hypoechoic mass in the right outer area.(c) Magnetic Resonance Imaging revealed a mass with contrast enhancement of a total size of 37 mm in the right outer area.(d) The Artificial Intelligence (AI) system diagnosed malignant findings in the right breast based on MG at the time of diagnosis.(e) The AI system also showed areas of interest in MG prior to diagnosis and diagnosed as possibly malignant.
Figure 4.
Representative case (case1). (a) MG. (b) US. (c) MRI. (d) AI diagnosis. (e) previous AI diagnosis.A 55 years old woman had left side breast cancer. Eight years later, she was diagnosed with right breast cancer. It was Lumina human epidermal growth factor receptor2 with 15 mm of invasive cancer and 15 mm of non-invasive cancer.(a) There were no malignant findings in the right side of mammography (MG).(b) Ultrasonography revealed a hypoechoic mass in the right outer area.(c) Magnetic Resonance Imaging revealed a mass with contrast enhancement of a total size of 37 mm in the right outer area.(d) The Artificial Intelligence (AI) system diagnosed malignant findings in the right breast based on MG at the time of diagnosis.(e) The AI system also showed areas of interest in MG prior to diagnosis and diagnosed as possibly malignant.
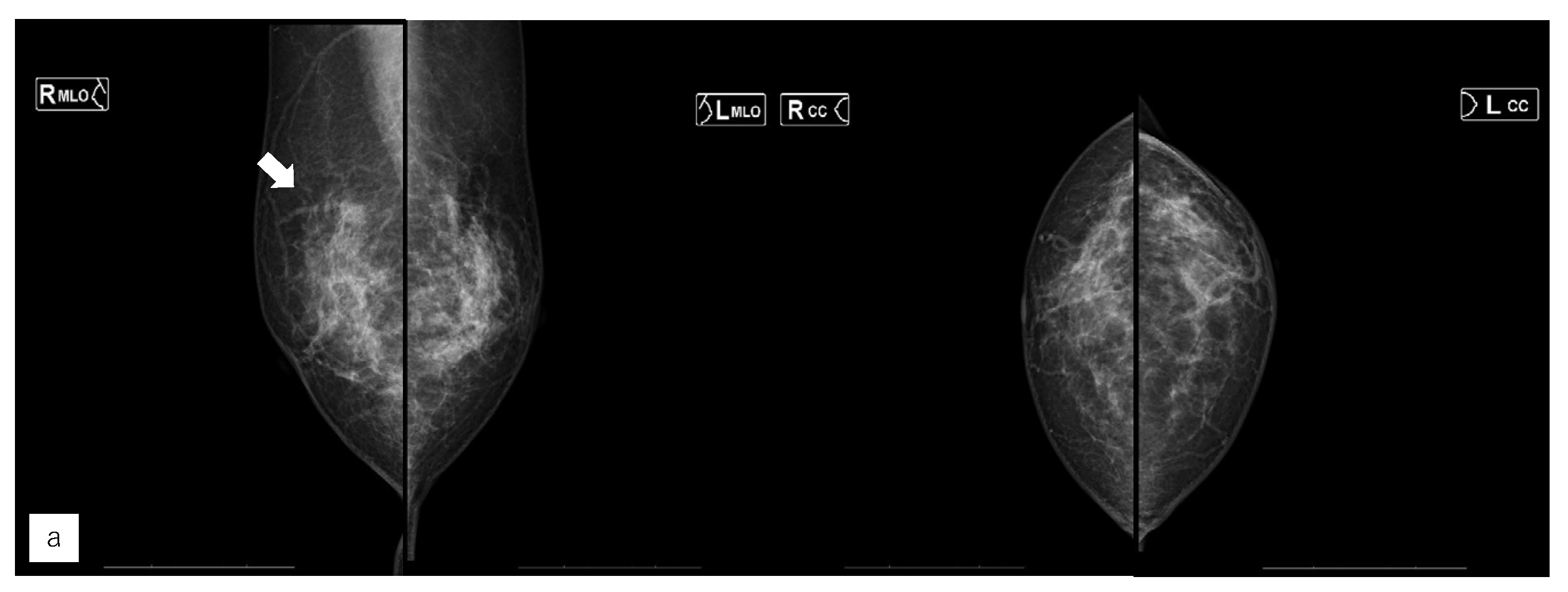
Figure 5.
Representative case (case 7). (a) MG. (b) US. (c) MRI. (d) AI diagnosis.A 63 yearsold woman had left side breast cancer. Two years later, she was diagnosed with right breast cancer. It was T1c, triple negative breast cancer. (a) A mass was found in the right upper area in mammography (MG) and diagnosed as breast imaging reporting and data system (BI-RADS) Category 4. (b) Ultrasonography revealed a hypoechoic masse in the right upper outer area.(c) Magnetic Resonance Imaging revealed a mass with contrast enhancement in the left upper outer area.(d) The Artificial Intelligence system diagnosed no malignancy. The mass visible on MG was not seen on MG a year earlier. Although the mass was of the same density as the background mammary gland, the radiologists diagnosed it to be possibly malignant upon comparison and reading.
Figure 5.
Representative case (case 7). (a) MG. (b) US. (c) MRI. (d) AI diagnosis.A 63 yearsold woman had left side breast cancer. Two years later, she was diagnosed with right breast cancer. It was T1c, triple negative breast cancer. (a) A mass was found in the right upper area in mammography (MG) and diagnosed as breast imaging reporting and data system (BI-RADS) Category 4. (b) Ultrasonography revealed a hypoechoic masse in the right upper outer area.(c) Magnetic Resonance Imaging revealed a mass with contrast enhancement in the left upper outer area.(d) The Artificial Intelligence system diagnosed no malignancy. The mass visible on MG was not seen on MG a year earlier. Although the mass was of the same density as the background mammary gland, the radiologists diagnosed it to be possibly malignant upon comparison and reading.
Table 1.
Clinical and pathological characteristics at the time of the first surgery.
Table 1.
Clinical and pathological characteristics at the time of the first surgery.
| No of case |
Age |
TNM |
Stage |
Procedure |
Axillary lymph node |
Histology |
Biology |
Chemotherapy |
Endocrine therapy |
RT
|
| 1 |
55 |
T2N1M0 |
0 |
Bp |
Ax |
IDC |
HER2 |
○ |
× |
○ |
| 2 |
71 |
T1micN0M0 |
Ⅰ |
Bp |
None |
A
pocrine |
HER2 |
× |
× |
○ |
| 3 |
68 |
T1cN0M0 |
Ⅰ |
Bp |
SNB |
IDC |
Luminal |
× |
○ |
○ |
| 4 |
70 |
T1bN1M0 |
Ⅰ |
Bp |
None |
IDC |
Luminal |
× |
○ |
○ |
| 5 |
68 |
T2M0M0 |
ⅡA |
Bp |
Ax |
IDC |
Luminal |
○ |
○ |
○ |
| 6 |
60 |
T1cN0M0 |
Ⅰ |
Bp |
SNB |
IDC |
Luminal |
○ |
○ |
○ |
| 7 |
63 |
TisN0M0 |
0 |
Bp |
SNB |
DCIS |
Luminal |
× |
× |
○ |
| 8 |
40 |
TisN0M0 |
0 |
Bp |
SNB |
DCIS |
Luminal |
× |
× |
○ |
| 9 |
66 |
T1cN0M0 |
Ⅰ |
Bp |
Ax |
IDC |
Luminal |
× |
○ |
○ |
| 10 |
74 |
T1bN1M0 |
ⅡA |
Bp |
SNB |
IDC |
Luminal |
○ |
○ |
○ |
Table 2.
Age at diagnosis of contralateral breast cancer, surgical method, and pathological examination.
Table 2.
Age at diagnosis of contralateral breast cancer, surgical method, and pathological examination.
| No of case |
Years to contralateral breast cancer (years) |
Age at diagnosis of contralateral breast cancer |
TNM |
Stage |
Procedure |
Axillary lymph node |
Histology |
Subtype |
| 1 |
8 |
63 |
T1cN0 |
Ⅰ |
Bt |
SNB |
IDC |
LuminalHER2 |
| 2 |
3 |
74 |
T1micN0 |
Ⅰ |
Bp |
SNB |
Apocrine |
TNBC |
| 3 |
10 |
78 |
T1cN0 |
Ⅰ |
Bp |
SNB |
ILC |
Luminal |
| 4 |
9 |
79 |
TisN0 |
0 |
Bt |
SNB |
DCIS |
TNBC |
| 5 |
8 |
76 |
T1micN0 |
Ⅰ |
Bt |
SNB |
IDC |
Luminal |
| 6 |
9 |
69 |
T2N0 |
ⅡA |
Bp |
SNB |
IDC |
Luminal |
| 7 |
2 |
65 |
T1cN0 |
Ⅰ |
Bp |
SNB |
IDC |
TNBC |
| 8 |
6 |
46 |
T1cN0 |
Ⅰ |
Bt |
SNB |
IDC |
Luminal |
| 9 |
8 |
74 |
T1aN0 |
Ⅰ |
Bt |
SNB |
IDC |
TNBC |
| 10 |
8 |
82 |
TisN0 |
0 |
Bt |
SNB |
DCIS |
HER2 |
Table 3.
BI-RADS categories by image and mammary gland density in MG at the time of diagnosis of contralateral breast cancer.
Table 3.
BI-RADS categories by image and mammary gland density in MG at the time of diagnosis of contralateral breast cancer.
| No of case |
Mammographic density |
MG BI-RADS |
MG findings |
US BI-RADS |
MRI BI-RADS |
| 1 |
Heterogeneous |
2 |
Calcification(benign) |
5 |
4 |
| 2 |
Scattered |
1 |
No |
4 |
4 |
| 3 |
Scattered |
5 |
Mass |
5 |
4 |
| 4 |
Scattered |
4 |
Calcification |
4 |
4 |
| 5 |
Heterogeneous |
4 |
Calcification |
4 |
4 |
| 6 |
Heterogeneous |
5 |
Mass |
4 |
4 |
| 7 |
Heterogeneous |
4 |
Mass |
5 |
4 |
| 8 |
Heterogeneous |
1 |
No |
4 |
4 |
| 9 |
Heterogeneous |
1 |
No |
1 |
4 |
| 10 |
Heterogeneous |
1 |
No |
1 |
1 |
Table 4.
Malignant possibility and diagnosis using the AI system when diagnosing breast cancer.
Table 4.
Malignant possibility and diagnosis using the AI system when diagnosing breast cancer.
| No of case |
MLO, % |
CC, % |
AI diagnosis |
| 1 |
44.6 |
68.9 |
Malignancy |
| 2 |
6.9 |
77.0 |
Malignancy |
| 3 |
3.4 |
50.2 |
Malignancy |
| 4 |
68.5 |
65.9 |
Malignancy |
| 5 |
66.5 |
37.5 |
Malignancy |
| 6 |
30.2 |
42.5 |
Malignancy |
| 7 |
2.6 |
4.0 |
No |
| 8 |
9.9 |
2.2 |
No |
| 9 |
13.8 |
27.5 |
No |
| 10 |
14.0 |
17.4 |
No |
Table 5.
Analysis of MG by AI in the year before the time of diagnosis.
Table 5.
Analysis of MG by AI in the year before the time of diagnosis.
| No of case |
Duration since diagnosing MG |
Previous MLO, % |
Previous CC, % |
AI diagnosis |
| 1 |
1Y3M |
88.5 |
77.0 |
Malignancy |
| 2 |
1Y6M |
0.1 |
60.9 |
Malignancy |
| 3 |
7Y0M |
5.4 |
1.8 |
No |
| 4 |
1Y10M |
17.6 |
5.5 |
No |
| 5 |
3Y7M |
19.4 |
19.3 |
No |
| 6 |
1Y5M |
0.6 |
2.3 |
No |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).