Submitted:
29 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
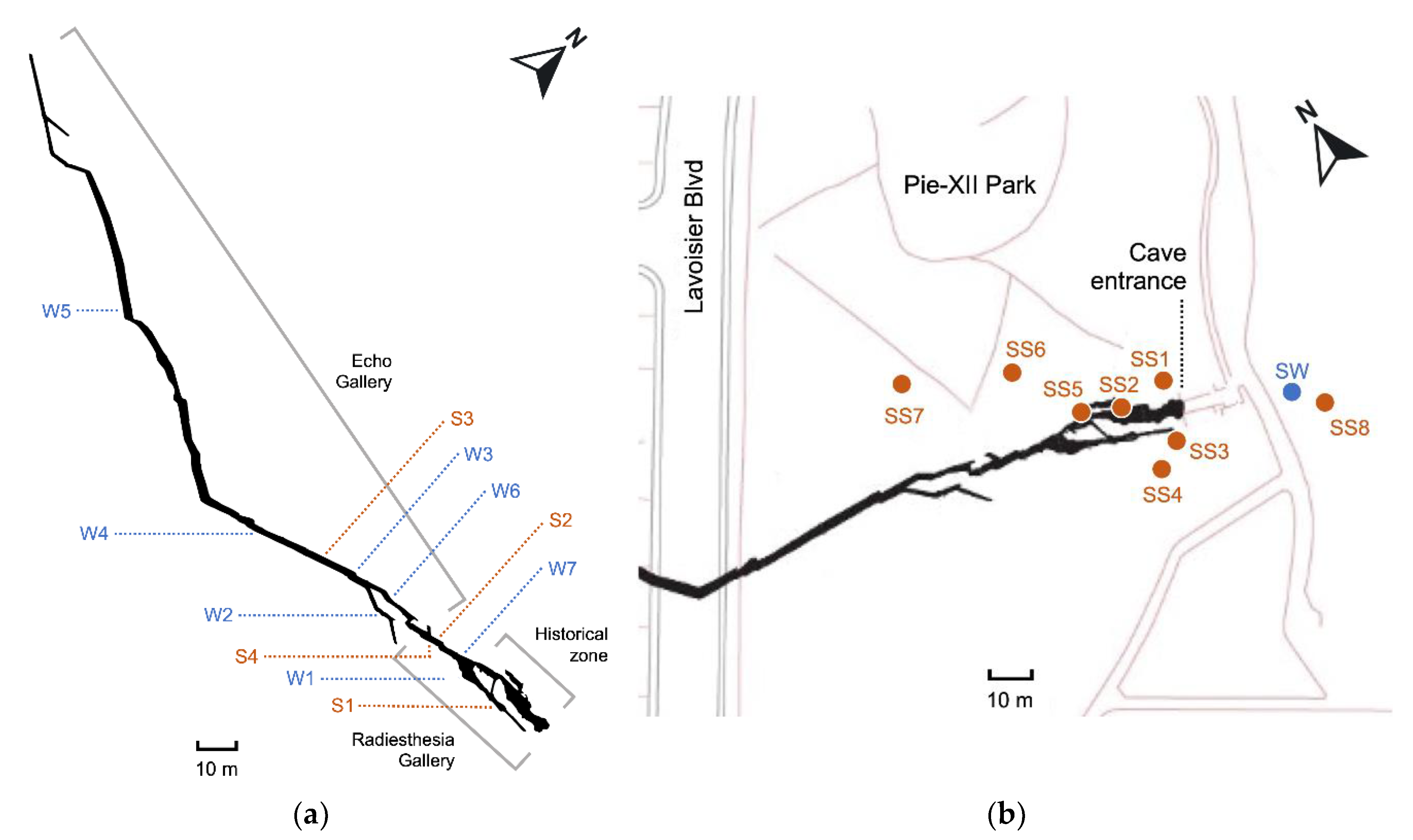
2.1. Study Site Description
2.2. Sampling and Water Filtration
2.3. Water Geochemical and Physicochemical Analyses
2.4. Sediment Characteristics
2.5. DNA Extraction
2.6. PCR, Library Preparation and Sequencing
2.7. Sequence Analysis
2.8. Statistical Analysis
3. Results
3.1. Water, Sediment and Soil Characteristics
3.2. Alpha-Diversity of the Microbial Communities
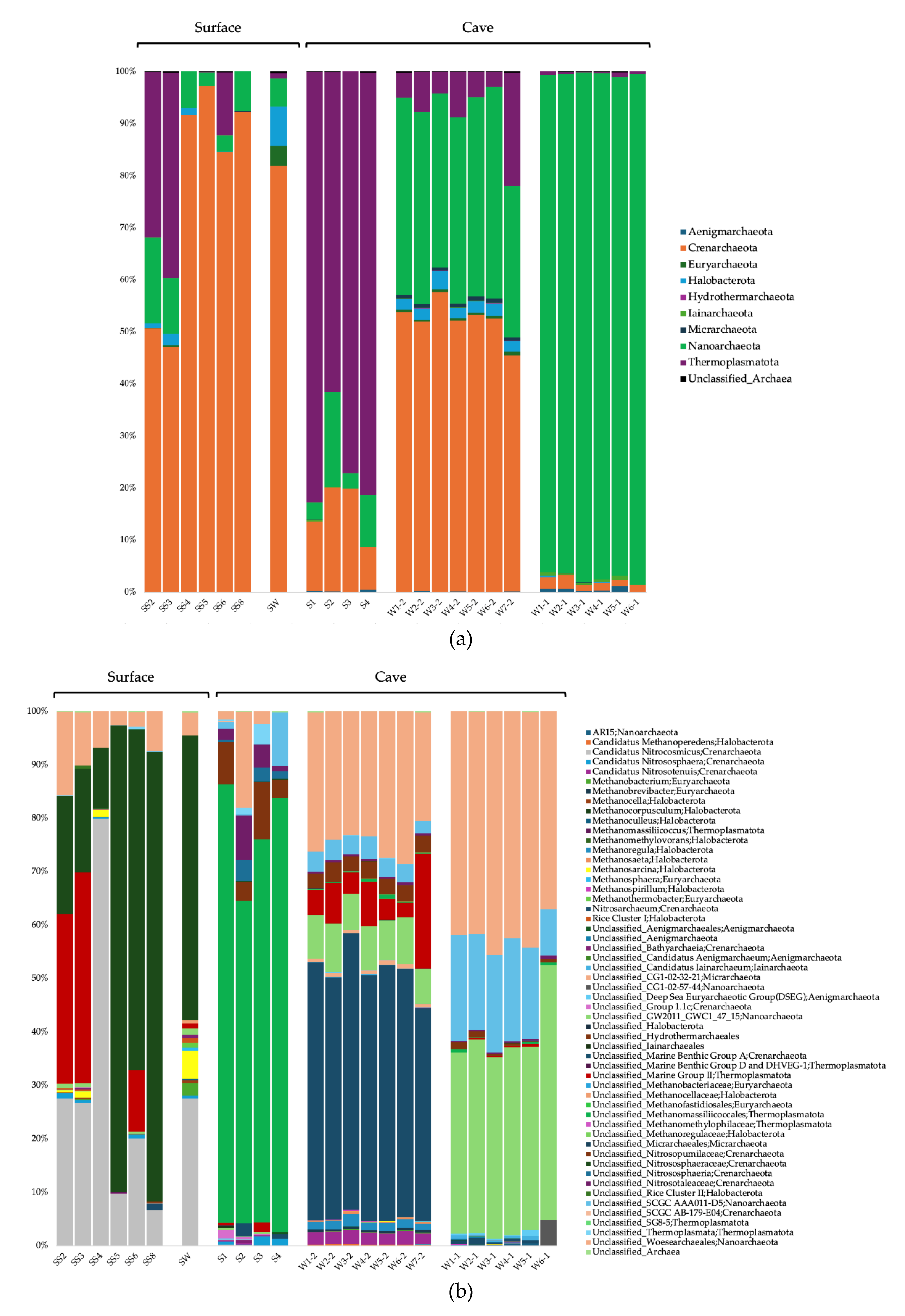
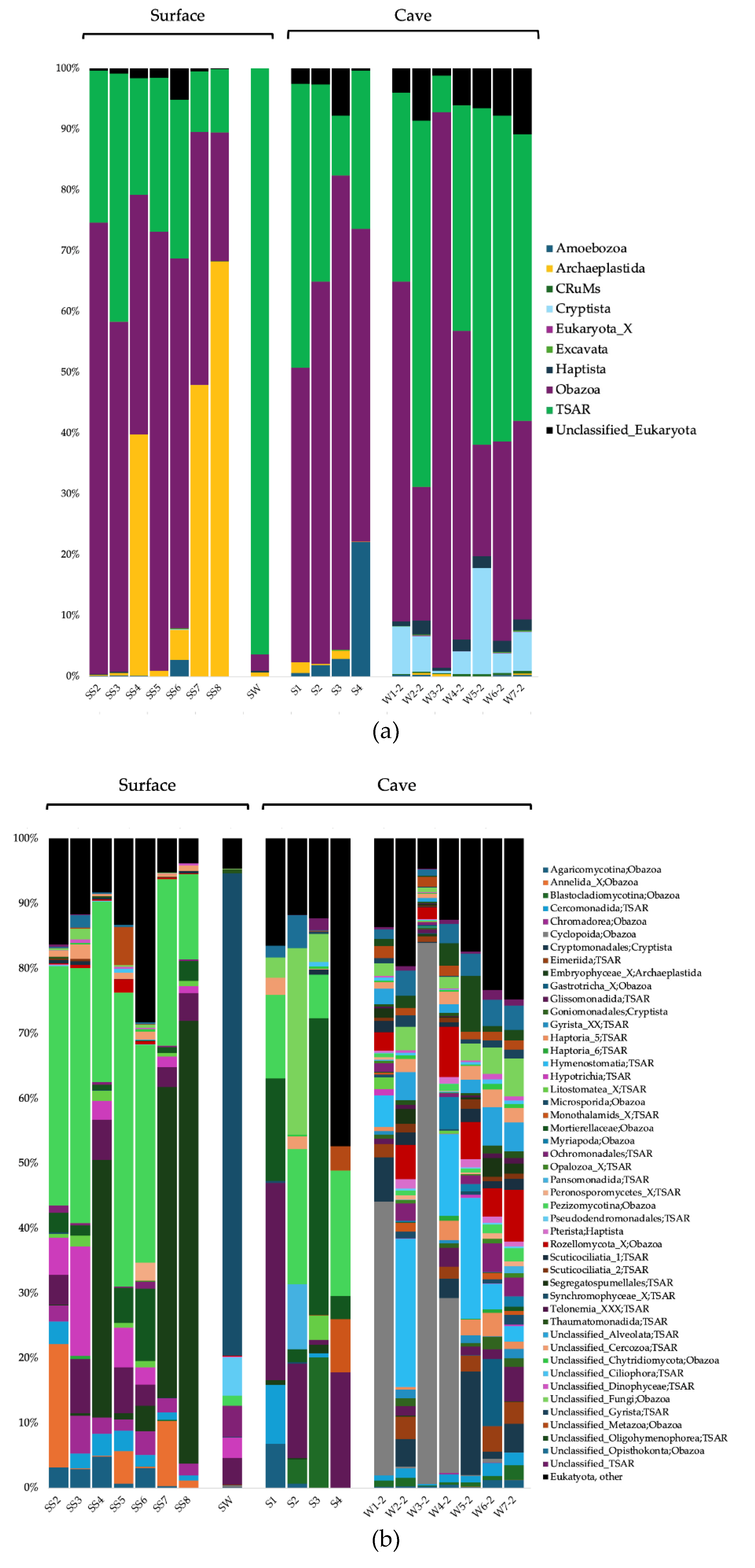
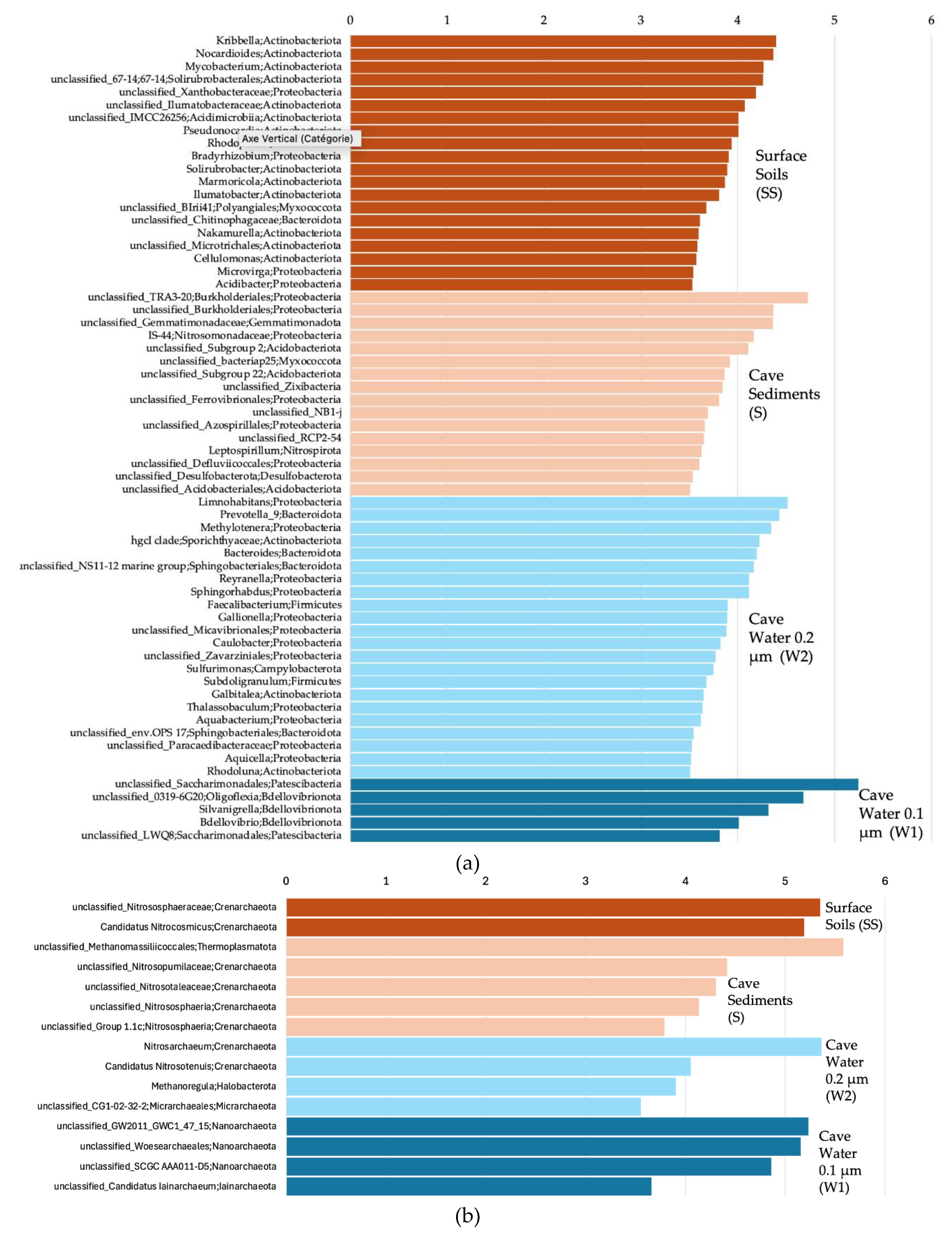
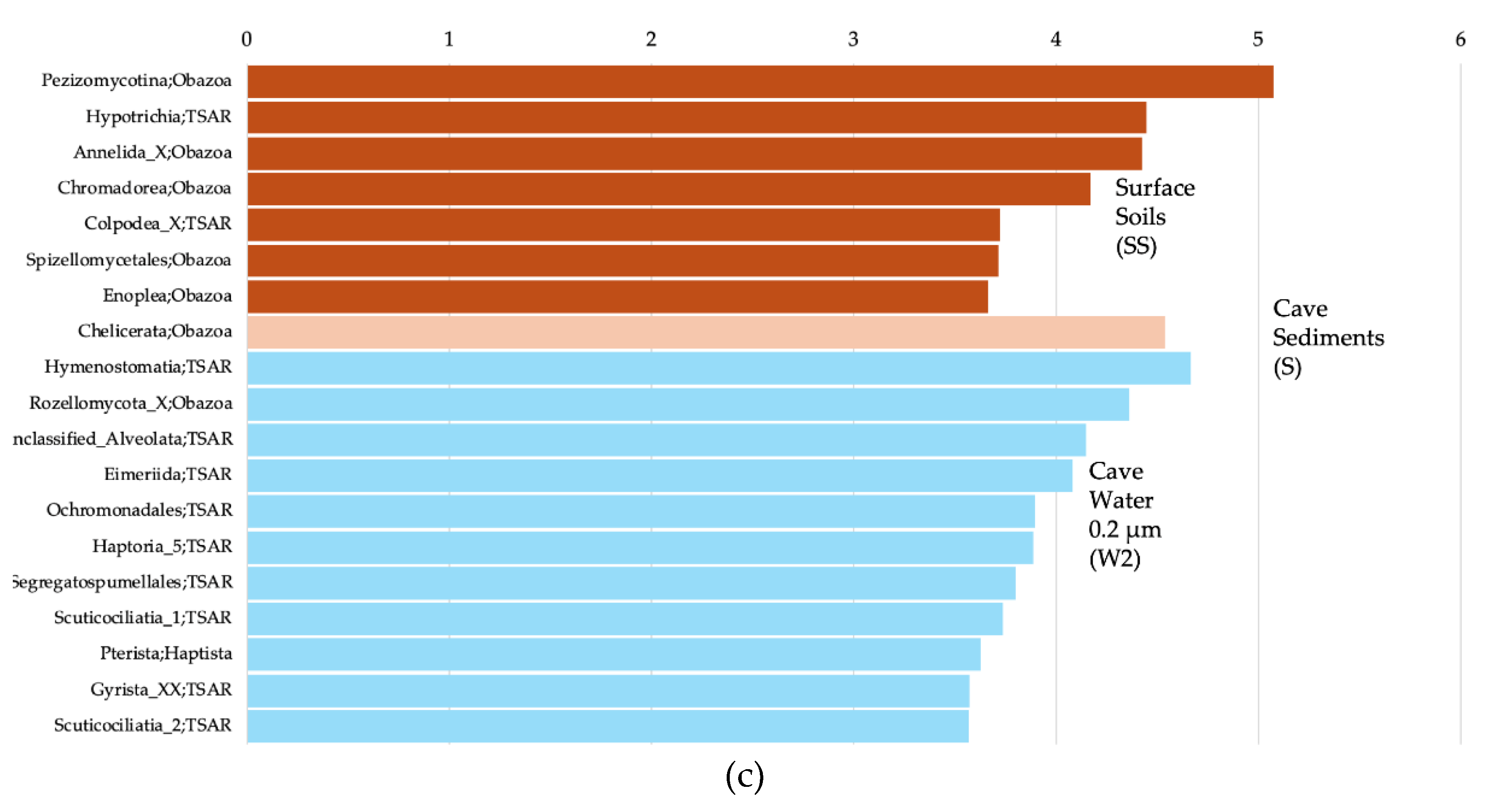
3.3. Taxonomic Composition of the Microbial Communities
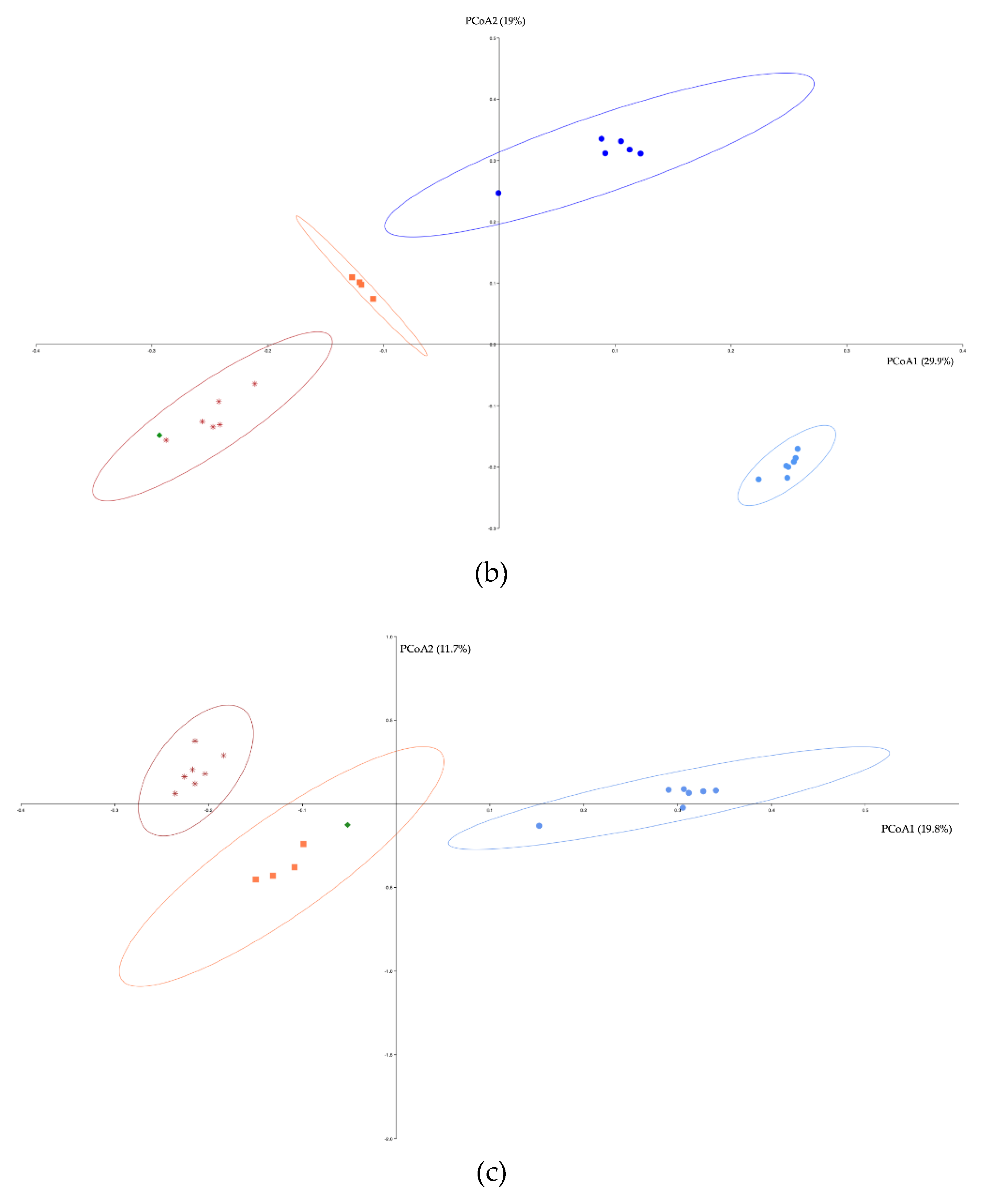
3.4. Beta-Diversity
3.5. Beta-Diversity and Correlation with Environmental Parameters
3.6. Discriminative Microbial Taxa between Sample Groups
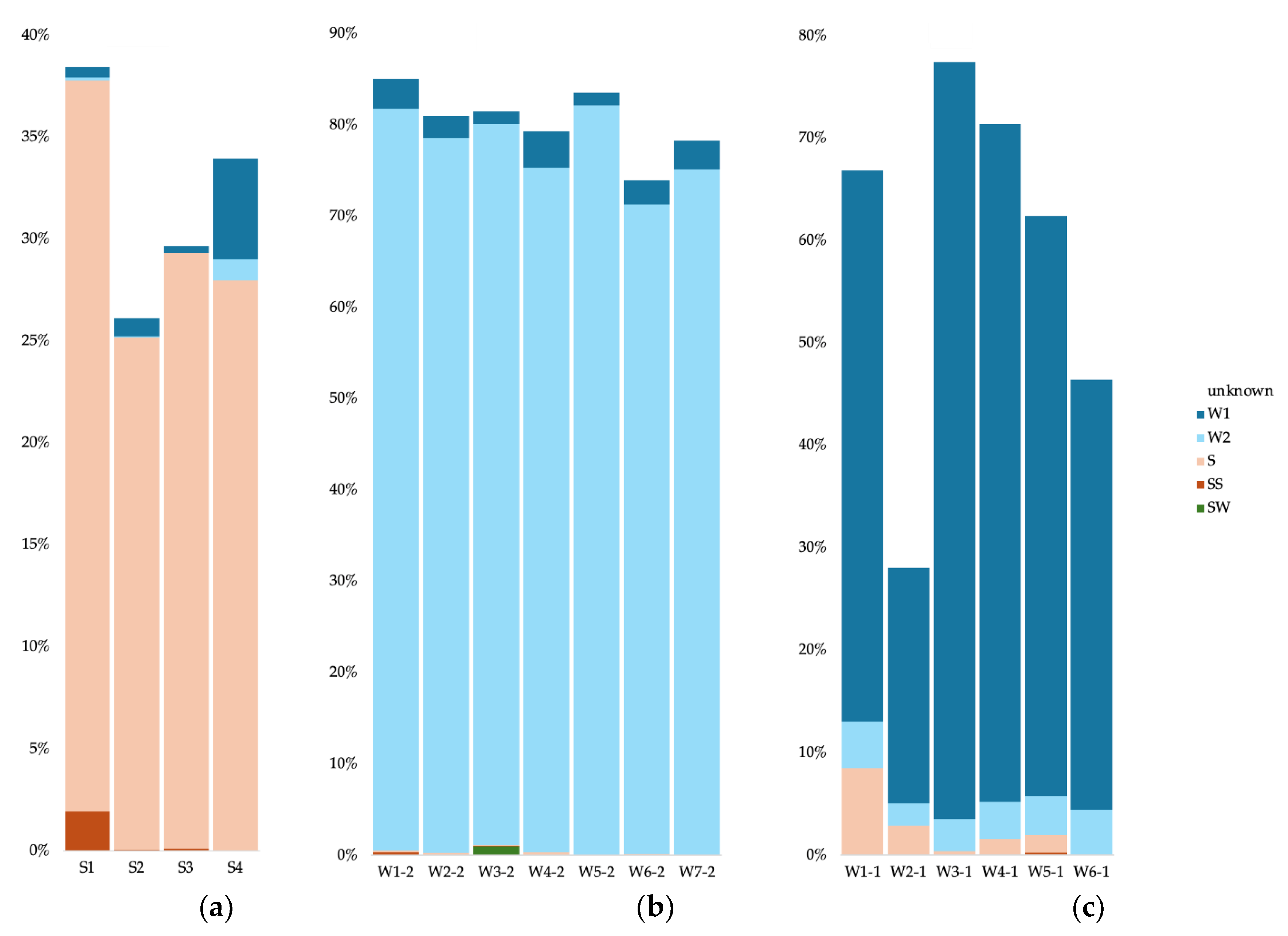
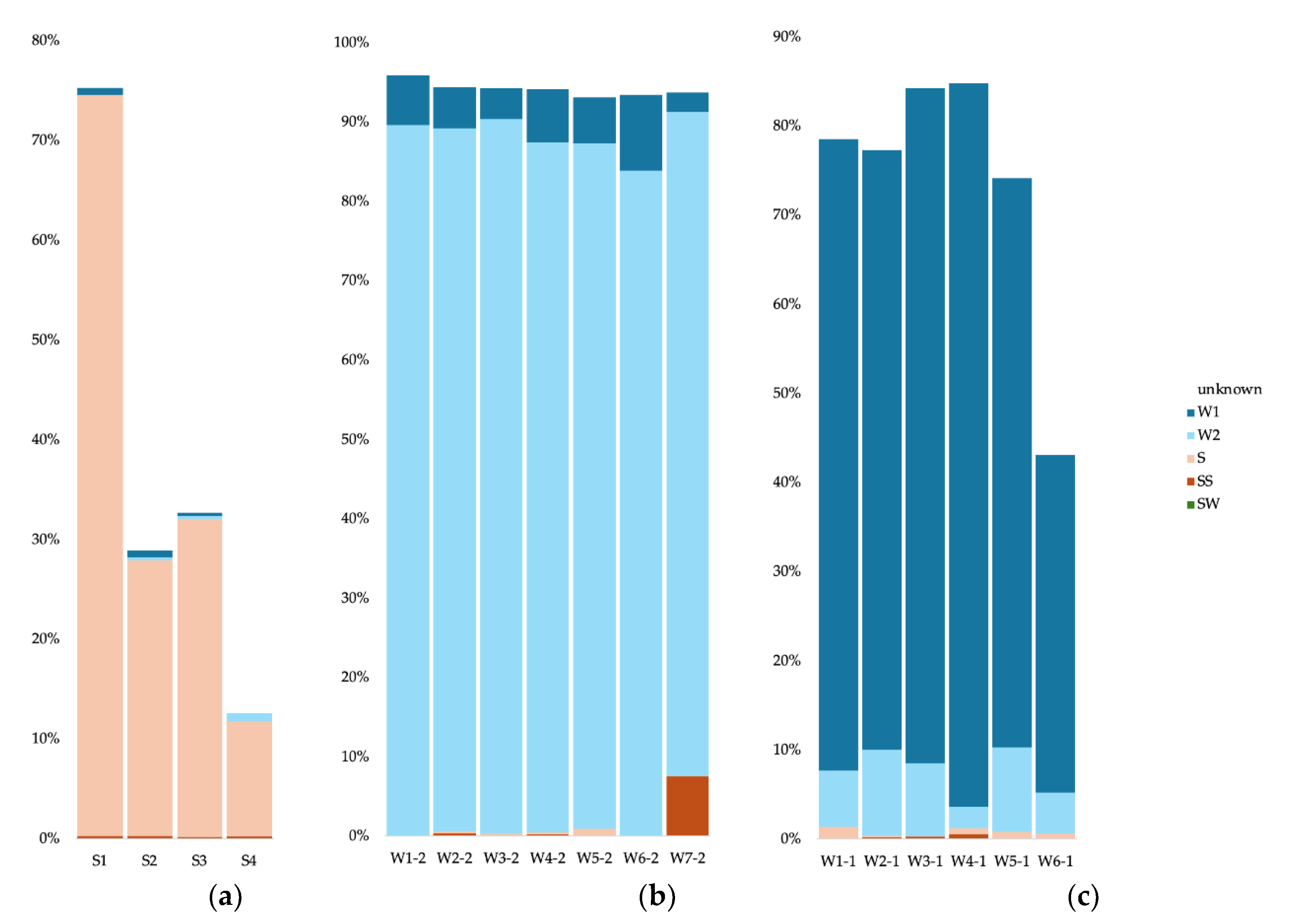
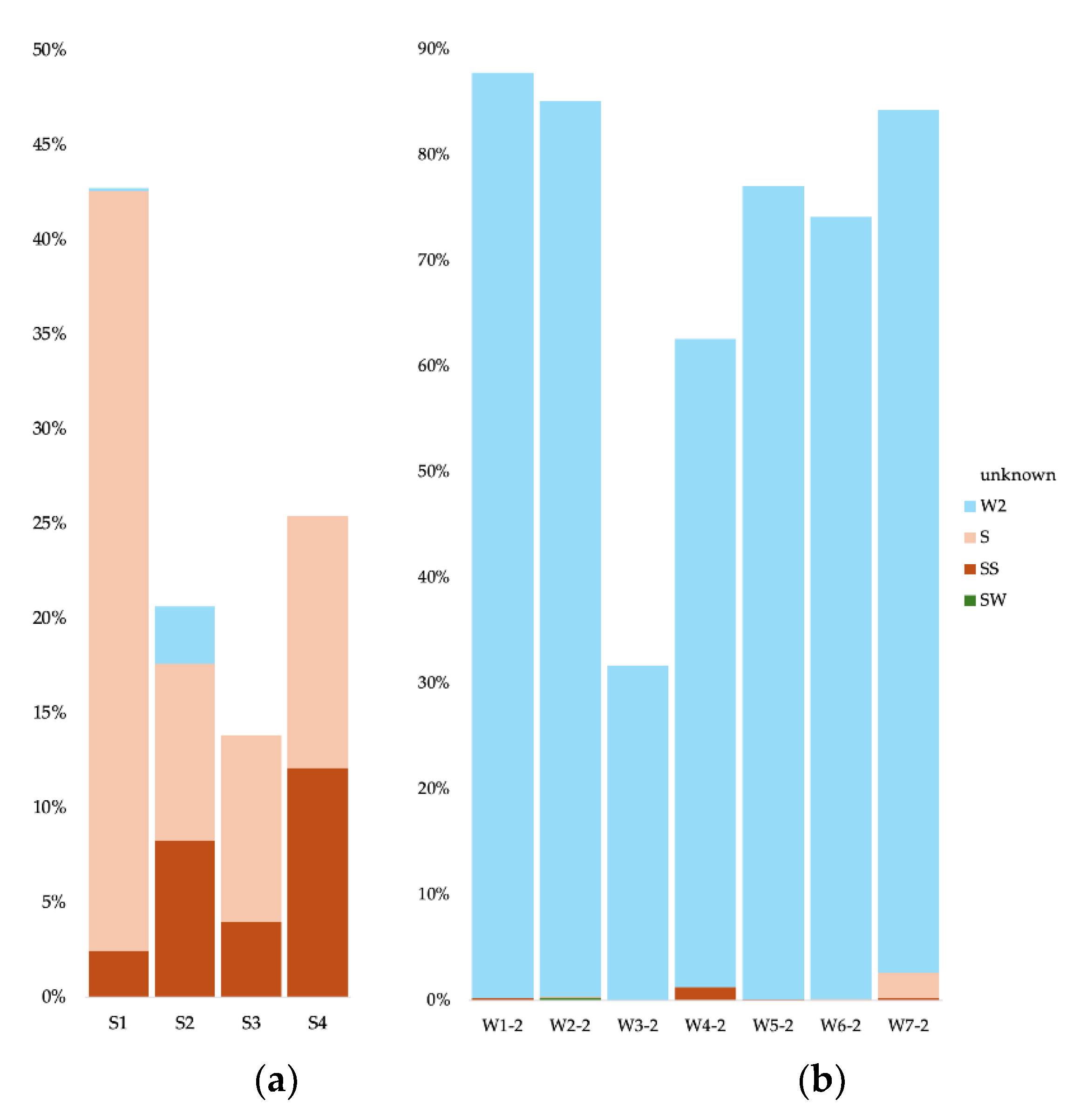
3.7. Microbial Source Tracking
4. Discussion
4.1. Environmental Properties and Geochemical Connectivity of the Saint-Leonard Cave
4.2. Potential Biological Links between Surface Soils and the Cave Sediments and Water
4.3. Sediment and Water Microbial Communities Inside the Saint-Leonard Cave
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin-Pozas, T.; Gonzalez-Pimentel, J.L.; Jurado, V.; Cuezva, S.; Dominguez-Moñino, I.; Fernandez-Cortes, A.; Cañaveras, J.C.; Sanchez-Moral, S.; Saiz-Jimenez, C. Microbial Activity in Subterranean Ecosystems: Recent Advances. Appl. Sci. 2020, 10, 8130. [Google Scholar] [CrossRef]
- Escudero, C.; Oggerin, M.; Amils, R. The Deep Continental Subsurface: The Dark Biosphere. Int. Microbiol. 2018, 21, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, L.R. Microbial Communities in the Deep Subsurface. Hydrogeol. J. 2000, 8, 4–10. [Google Scholar] [CrossRef]
- McMahon, S.; Parnell, J. Weighing the Deep Continental Biosphere. FEMS Microbiol. Ecol. 2014, 87, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Griebler, C.; Lueders, T. Microbial Biodiversity in Groundwater Ecosystems. Freshw. Biol. 2009, 54, 649–677. [Google Scholar] [CrossRef]
- Lazar, C.S.; Lehmann, R.; Stoll, W.; Rosenberger, J.; Totsche, K.U.; Küsel, K. The Endolithic Bacterial Diversity of Shallow Bedrock Ecosystems. Sci. Total Environ. 2019, 679, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Hershey, O.S.; Barton, H.A. The Microbial Diversity of Caves. In Cave Ecology; Moldovan, O.T., Kováč, Ľ., Halse, S., Eds.; Ecological Studies; Springer International Publishing: Cham, 2018; Vol. 235, pp. 69–90; ISBN 978-3-319-98850-4. [Google Scholar]
- Sprocati, A.R.; Alisi, C.; Segre, L.; Tasso, F.; Galletti, M.; Cremisini, C. Investigating Heavy Metal Resistance, Bioaccumulation and Metabolic Profile of a Metallophile Microbial Consortium Native to an Abandoned Mine. Sci. Total Environ. 2006, 366, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Lhoste, E.; Comte, F.; Brown, K.; Delisle, A.; Jaclin, D.; Ponsin, V.; Rosabal, M.; Lazar, C.S. Bacterial, Archaeal, and Eukaryote Diversity in Planktonic and Sessile Communities Inside an Abandoned and Flooded Iron Mine (Quebec, Canada). Appl. Microbiol. 2023, 3, 45–63. [Google Scholar] [CrossRef]
- Röling, W.F.M.; Head, I.M.; Larter, S.R. The Microbiology of Hydrocarbon Degradation in Subsurface Petroleum Reservoirs: Perspectives and Prospects. Res. Microbiol. 2003, 154, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.S. Microbial Diversity of Cave Ecosystems. In Geomicrobiology: Molecular and Environmental Perspective; Barton, L.L., Mandl, M., Loy, A., Eds.; Springer Netherlands: Dordrecht, 2010; pp. 219–238; ISBN 978-90-481-9203-8. [Google Scholar]
- Kieft, T.L. Sampling the Deep Sub-Surface Using Drilling and Coring Techniques. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2010; pp. 3427–3441; ISBN 978-3-540-77584-3. [Google Scholar]
- Barton, H.A.; Northup, D.E. Geomicrobiology in Cave Environments: Past, Current and Future Perspectives. J. Cave Karst Stud. 2007, 69, 163–178. [Google Scholar]
- Dong, H.; Yu, B. Geomicrobiological Processes in Extreme Environments: A Review. Episodes 2007, 30, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Ghuneim, L.-A.J.; Jones, D.L.; Golyshin, P.N.; Golyshina, O.V. Nano-Sized and Filterable Bacteria and Archaea: Biodiversity and Function. Front. Microbiol. 2018, 9, 1971. [Google Scholar] [CrossRef] [PubMed]
- Luef, B.; Frischkorn, K.R.; Wrighton, K.C.; Holman, H.-Y.N.; Birarda, G.; Thomas, B.C.; Singh, A.; Williams, K.H.; Siegerist, C.E.; Tringe, S.G.; et al. Diverse Uncultivated Ultra-Small Bacterial Cells in Groundwater. Nat. Commun. 2015, 6, 6372. [Google Scholar] [CrossRef]
- He, C.; Keren, R.; Whittaker, M.L.; Farag, I.F.; Doudna, J.A.; Cate, J.H.D.; Banfield, J.F. Genome-Resolved Metagenomics Reveals Site-Specific Diversity of Episymbiotic CPR Bacteria and DPANN Archaea in Groundwater Ecosystems. Nat. Microbiol. 2021, 6, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Duda, V.I.; Suzina, N.E.; Polivtseva, V.N.; Boronin, A.M. Ultramicrobacteria: Formation of the Concept and Contribution of Ultramicrobacteria to Biology. Microbiology 2012, 81, 379–390. [Google Scholar] [CrossRef]
- Kuroda, K.; Yamamoto, K.; Nakai, R.; Hirakata, Y.; Kubota, K.; Nobu, M.K.; Narihiro, T. Symbiosis between Candidatus Patescibacteria and Archaea Discovered in Wastewater-Treating Bioreactors. mBio 2022, 13, e01711–22. [Google Scholar] [CrossRef] [PubMed]
- Dey, S. Indigenous Microbial Populations of Abandoned Mining Sites and Their Role in Natural Attenuation. Arch. Microbiol. 2022, 204, 251. [Google Scholar] [CrossRef] [PubMed]
- Newsome, L.; Falagán, C. The Microbiology of Metal Mine Waste: Bioremediation Applications and Implications for Planetary Health. GeoHealth 2021, 5, e2020GH000380. [Google Scholar] [CrossRef] [PubMed]
- Meckenstock, R.U.; Elsner, M.; Griebler, C.; Lueders, T.; Stumpp, C.; Aamand, J.; Agathos, S.N.; Albrechtsen, H.-J.; Bastiaens, L.; Bjerg, P.L.; et al. Biodegradation: Updating the Concepts of Control for Microbial Cleanup in Contaminated Aquifers. Environ. Sci. Technol. 2015, 49, 7073–7081. [Google Scholar] [CrossRef] [PubMed]
- Thullner, M.; Regnier, P. Microbial Controls on the Biogeochemical Dynamics in the Subsurface. Rev. Mineral. Geochem. 2019, 85, 265–302. [Google Scholar] [CrossRef]
- Trevors, J.T.; Bej, A.K.; Van Elsas, J.D. Hypothesized Microenvironments for the Origin of Microbial Life on Earth. In Genesis - In The Beginning; Seckbach, J., Ed.; Cellular Origin, Life in Extreme Habitats and Astrobiology; Springer Netherlands: Dordrecht, 2012; Vol. 22, pp. 775–795; ISBN 978-94-007-2940-7. [Google Scholar]
- Mizuno, K.; Maree, M.; Nagamura, T.; Koga, A.; Hirayama, S.; Furukawa, S.; Tanaka, K.; Morikawa, K. Novel Multicellular Prokaryote Discovered next to an Underground Stream. eLife 2022, 11, e71920. [Google Scholar] [CrossRef] [PubMed]
- Warren-Rhodes, K.A.; Lee, K.C.; Archer, S.D.J.; Cabrol, N.; Ng-Boyle, L.; Wettergreen, D.; Zacny, K.; Pointing, S.B. ; NASA Life in the Atacama Project Team Corrigendum: Subsurface Microbial Habitats in an Extreme Desert Mars-Analog Environment. Front. Microbiol. 2019, 10, 2129. [Google Scholar] [CrossRef]
- Chapelle, F.H.; O’Neill, K.; Bradley, P.M.; Methé, B.A.; Ciufo, S.A.; Knobel, L.L.; Lovley, D.R. A Hydrogen-Based Subsurface Microbial Community Dominated by Methanogens. Nature 2002, 415, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.M.; Meisinger, D.B.; Aubrecht, R.; Kovacik, L.; Saiz-Jimenez, C.; Baskar, S.; Baskar, R.; Liebl, W.; Porter, M.L.; Engel, A.S. Caves and Karst Environments. In Life at extremes: environments, organisms and strategies for survival; Bell, E.M., Ed.; CABI: UK, 2012; pp. 320–344; ISBN 978-1-84593-814-7. [Google Scholar]
- Barton, H.A.; Jurado, V. What’s Up Down There? Microbial Diversity in Caves. Microbe 2007, 2, 132–138. [Google Scholar]
- Anantharaman, K.; Brown, C.T.; Hug, L.A.; Sharon, I.; Castelle, C.J.; Probst, A.J.; Thomas, B.C.; Singh, A.; Wilkins, M.J.; Karaoz, U.; et al. Thousands of Microbial Genomes Shed Light on Interconnected Biogeochemical Processes in an Aquifer System. Nat. Commun. 2016, 7, 13219. [Google Scholar] [CrossRef] [PubMed]
- Turrini, P.; Tescari, M.; Visaggio, D.; Pirolo, M.; Lugli, G.A.; Ventura, M.; Frangipani, E.; Visca, P. The Microbial Community of a Biofilm Lining the Wall of a Pristine Cave in Western New Guinea. Microbiol. Res. 2020, 241, 126584. [Google Scholar] [CrossRef] [PubMed]
- Schlatter, D.C.; Kinkel, L.L. Antibiotics: Conflict and Communication in Microbial Communities. Microbe Mag. 2014, 9, 282–288. [Google Scholar] [CrossRef]
- Davies, J. Are Antibiotics Naturally Antibiotics? J. Ind. Microbiol. Biotechnol. 2006, 33, 496–499. [Google Scholar] [CrossRef]
- Dantas, G.; Sommer, M.O.A.; Oluwasegun, R.D.; Church, G.M. Bacteria Subsisting on Antibiotics. Science 2008, 320, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; Van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Kováč, Ľ. Caves as Oligotrophic Ecosystems. In Cave Ecology; Moldovan, O.T., Kováč, Ľ., Halse, S., Eds.; Ecological Studies; Springer International Publishing: Cham, 2018; Vol. 235, pp. 297–307; ISBN 978-3-319-98850-4. [Google Scholar]
- Barton, H.A.; Taylor, M.R.; Pace, N.R. Molecular Phylogenetic Analysis of a Bacterial Community in an Oligotrophic Cave Environment. Geomicrobiol. J. 2004, 21, 11–20. [Google Scholar] [CrossRef]
- Chelius, M.K.; Moore, J.C. Molecular Phylogenetic Analysis of Archaea and Bacteria in Wind Cave, South Dakota. Geomicrobiol. J. 2004, 21, 123–134. [Google Scholar] [CrossRef]
- E. Northup, Kathleen H. Lavoie, D. Geomicrobiology of Caves: A Review. Geomicrobiol. J. 2001, 18, 199–222. [Google Scholar] [CrossRef]
- Wiseschart, A.; Mhuantong, W.; Tangphatsornruang, S.; Chantasingh, D.; Pootanakit, K. Shotgun Metagenomic Sequencing from Manao-Pee Cave, Thailand, Reveals Insight into the Microbial Community Structure and Its Metabolic Potential. BMC Microbiol. 2019, 19, 144. [Google Scholar] [CrossRef] [PubMed]
- De Mandal, S.; Chatterjee, R.; Kumar, N.S. Dominant Bacterial Phyla in Caves and Their Predicted Functional Roles in C and N Cycle. BMC Microbiol. 2017, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk-Żak, K.; Zielenkiewicz, U. Microbial Diversity in Caves. Geomicrobiol. J. 2016, 33, 20–38. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Portillo, M.C.; Saiz-Jimenez, C. Metabolically Active Crenarchaeota in Altamira Cave. Naturwissenschaften 2006, 93, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Northup, D.E.; Barns, S.M.; Yu, L.E.; Spilde, M.N.; Schelble, R.T.; Dano, K.E.; Crossey, L.J.; Connolly, C.A.; Boston, P.J.; Natvig, D.O.; et al. Diverse Microbial Communities Inhabiting Ferromanganese Deposits in Lechuguilla and Spider Caves. Environ. Microbiol. 2003, 5, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Vanderwolf, K.; Malloch, D.; McAlpine, D.; Forbes, G. A World Review of Fungi, Yeasts, and Slime Molds in Caves. Int. J. Speleol. 2013, 42, 77–96. [Google Scholar] [CrossRef]
- Cahoon, A.B.; VanGundy, R.D. Alveolates (Dinoflagellates, Ciliates and Apicomplexans) and Rhizarians Are the Most Common Microbial Eukaryotes in Temperate Appalachian Karst Caves. Environ. Microbiol. Rep. 2022, 14, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Jones, B. Microbes in Caves: Agents of Calcite Corrosion and Precipitation. Geol. Soc. Lond. Spec. Publ. 2010, 336, 7–30. [Google Scholar] [CrossRef]
- Engel, A.S.; Stern, L.A.; Bennett, P.C. Microbial Contributions to Cave Formation: New Insights into Sulfuric Acid Speleogenesis. Geology 2004, 32, 369. [Google Scholar] [CrossRef]
- Maciejewska, M.; Adam, D.; Naômé, A.; Martinet, L.; Tenconi, E.; Całusińska, M.; Delfosse, P.; Hanikenne, M.; Baurain, D.; Compère, P.; et al. Assessment of the Potential Role of Streptomyces in Cave Moonmilk Formation. Front. Microbiol. 2017, 8, 1181. [Google Scholar] [CrossRef] [PubMed]
- Melim, Kristen M. Shinglman, Pen, L. Evidence for Microbial Involvement in Pool Finger Precipitation, Hidden Cave, New Mexico. Geomicrobiol. J. 2001, 18, 311–329. [CrossRef]
- Banks, E.D.; Taylor, N.M.; Gulley, J.; Lubbers, B.R.; Giarrizzo, J.G.; Bullen, H.A.; Hoehler, T.M.; Barton, H.A. Bacterial Calcium Carbonate Precipitation in Cave Environments: A Function of Calcium Homeostasis. Geomicrobiol. J. 2010, 27, 444–454. [Google Scholar] [CrossRef]
- Zhu, H.-Z.; Zhang, Z.-F.; Zhou, N.; Jiang, C.-Y.; Wang, B.-J.; Cai, L.; Wang, H.-M.; Liu, S.-J. Bacteria and Metabolic Potential in Karst Caves Revealed by Intensive Bacterial Cultivation and Genome Assembly. Appl. Environ. Microbiol. 2021, 87, e02440–20. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.W.; Auler, A.S.; Senko, J.; Sasowsky, I.D.; Piló, L.B.; Smith, M.; Barton, H. Microbial Iron Cycling and Biospeleogenesis: Cave Development in the Carajás Formation, Brazil. Presented at the 2013 ICS Proceedings - Biospeleology, Geomicrobiology and Ecology, 2013.
- Fru, E.C.; Piccinelli, P.; Fortin, D. Insights into the Global Microbial Community Structure Associated with Iron Oxyhydroxide Minerals Deposited in the Aerobic Biogeosphere. Geomicrobiol. J. 2012, 29, 587–610. [Google Scholar] [CrossRef]
- Carmichael, S.K.; Bräuer, S.L. 7. Microbial Diversity and Manganese Cycling: A Review of Manganese-Oxidizing Microbial Cave Communities. In Microbial Life of Cave Systems; Summers Engel, A., Ed.; De Gruyter, 2015; pp. 137–160 ISBN 978-3-11-033499-9.
- Paula, C.C.P.D.; Sirová, D.; Sarmento, H.; Fernandes, C.C.; Kishi, L.T.; Bichuette, M.E.; Seleghim, M.H.R. First Report of Halobacteria Dominance in a Tropical Cave Microbiome. bioRxiv 2021. [Google Scholar] [CrossRef]
- Palmer, A.N. Origin and Morphology of Limestone Caves. Geol. Soc. Am. Bull. 1991, 103, 1–21. [Google Scholar] [CrossRef]
- Klimchouk, A. The Karst Paradigm: Changes, Trends and Perspectives. Acta Carsologica 2015, 44, 289–313. [Google Scholar] [CrossRef]
- White, W.B.; Culver, D.C. Cave, Definition Of. In Encyclopedia of Caves; Elsevier, 2012; pp. 103–107 ISBN 978-0-12-383832-2.
- Schroeder, J.; Beaupré, M.; Cloutier, M. Ice-Push Caves in Platform Limestones of the Montréal Area. Can. J. Earth Sci. 1986, 23, 1842–1851. [Google Scholar] [CrossRef]
- Schroeder, J.; Beaupré, M.; Caron, D. Glacitectonic Caves in the St. Lawrence Lowlands of Québec. In Landscapes and Landforms of Eastern Canada; Slaymaker, O., Catto, N., Eds.; World Geomorphological Landscapes; Springer International Publishing: Cham, 2020; pp. 509–523; ISBN 978-3-030-35135-9. [Google Scholar]
- Beaupré, M.; Caron, D. Cavernes Du Québec, Guide de Spéléologie; Éditions Michel Quintin: Montréal, 2021. [Google Scholar]
- Jean-François Hélie (Université du Québec à Montréal, Montréal, Québec, Canada). Personal Communication, 2022.
- Patton, C.J.; Kryskalla, J.R. Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory-Evaluation of Alkaline Persulfate Digestion as an Alternative to Kjeldahl Digestion for Determination of Total and Dissolved Nitrogen and Phosphorus in Water. Water-Resour. Investig. Rep. 03-4174 2003. [CrossRef]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A.G. Profiling of Complex Microbial Populations by Denaturing Gradient Gel Electrophoresis Analysis of Polymerase Chain Reaction-Amplified Genes Coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Gantner, S.; Andersson, A.F.; Alonso-Sáez, L.; Bertilsson, S. Novel Primers for 16S rRNA-Based Archaeal Community Analyses in Environmental Samples. J. Microbiol. Methods 2011, 84, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Stahl, D.A.; Amann, R. Development and Application of Nucleic Acid Probes in Bacterial Systematics. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley & Sons Ltd.: Chichester, 1991; pp. 205–248. [Google Scholar]
- Van De Peer, Y. The European Small Subunit Ribosomal RNA Database. Nucleic Acids Res. 2000, 28, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Gast, R.J.; Dennett, M.R.; Caron, D.A. Characterization of Protistan Assemblages in the Ross Sea, Antarctica, by Denaturing Gradient Gel Electrophoresis. Appl. Environ. Microbiol. 2004, 70. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, 2022.
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Pan, J.; Wang, F.; Gu, J.-D.; Li, M. Bathyarchaeota: Globally Distributed Metabolic Generalists in Anoxic Environments. FEMS Microbiol. Rev. 2018, 42, 639–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, M.; Castelle, C.J.; Probst, A.J.; Zhou, Z.; Pan, J.; Liu, Y.; Banfield, J.F.; Gu, J.-D. Insights into the Ecology, Evolution, and Metabolism of the Widespread Woesearchaeotal Lineages. Microbiome 2018, 6, 102. [Google Scholar] [CrossRef]
- Vaulot, D.; Schönle, A.; Sandin, M.; Morard, R.; Mahé, F.; Fiore-Donno, A.M.; del Campo, J. Pr2database/Pr2database: PR2 Version 4.14.0, Zenodo, 2021.
- Davis, N.M.; Proctor, D.M.; Holmes, S.P.; Relman, D.A.; Callahan, B.J. Simple Statistical Identification and Removal of Contaminant Sequences in Marker-Gene and Metagenomics Data. Microbiome 2018, 6, 226. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.B.; Wallroth, M.; Jonsson, V.; Kristiansson, E. Comparison of Normalization Methods for the Analysis of Metagenomic Gene Abundance Data. BMC Genomics 2018, 19, 274. [Google Scholar] [CrossRef] [PubMed]
- Ogle, D.H.; Doll, J.C.; Wheeler, A.P.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods (v.0.9.5), 2023.
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4. [Google Scholar]
- Anderson, M.J. A New Method for Non-parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package 2022.
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Shenhav, L.; Thompson, M.; Joseph, T.A.; Briscoe, L.; Furman, O.; Bogumil, D.; Mizrahi, I.; Pe’er, I.; Halperin, E. FEAST: Fast Expectation-Maximization for Microbial Source Tracking. Nat. Methods 2019, 16, 627–632. [Google Scholar] [CrossRef]
- Barton, H.A. Starving Artists: Bacterial Oligotrophic Heterotrophy in Caves. In Microbial Life of Cave Systems; Summers Engel, A., Ed.; De Gruyter, 2015; pp. 79–104 ISBN 978-3-11-033499-9.
- Health Canada. Guidance on Natural Organic Matter in Drinking Water; Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, 2020;
- McDonough, L.K.; Santos, I.R.; Andersen, M.S.; O’Carroll, D.M.; Rutlidge, H.; Meredith, K.; Oudone, P.; Bridgeman, J.; Gooddy, D.C.; Sorensen, J.P.R.; et al. Changes in Global Groundwater Organic Carbon Driven by Climate Change and Urbanization. Nat. Commun. 2020, 11, 1279. [Google Scholar] [CrossRef] [PubMed]
- Downing, J.A.; Striegl, R.G. Size, Age, Renewal, and Discharge of Groundwater Carbon. Inland Waters 2018, 8, 122–127. [Google Scholar] [CrossRef]
- Kratzer, C.R.; Brezonik, P.L. A Carlson-Type Trophic State Index for Nitrogen in Florida Lakes. JAWRA J. Am. Water Resour. Assoc. 1981, 17, 713–715. [Google Scholar] [CrossRef]
- Nürnberg, G.K. Trophic State of Clear and Colored, Soft- and Hardwater Lakes with Special Consideration of Nutrients, Anoxia, Phytoplankton and Fish. Lake Reserv. Manag. 1996, 12, 432–447. [Google Scholar] [CrossRef]
- Różkowski, J.; Różkowski, K.; Rahmonov, O.; Rubin, H. Nitrates and Phosphates in Cave Waters of Kraków-Częstochowa Upland, Southern Poland. Environ. Sci. Pollut. Res. 2017, 24, 25870–25880. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, L.; Boden, R.; Hillebrand, A.; Kumaresan, D.; Moussard, H.; Baciu, M.; Lu, Y.; Colin Murrell, J. Life without Light: Microbial Diversity and Evidence of Sulfur- and Ammonium-Based Chemolithotrophy in Movile Cave. ISME J. 2009, 3, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.; Legatzki, A.; Neilson, J.W.; Fryslie, B.; Nelson, W.M.; Wing, R.A.; Soderlund, C.A.; Pryor, B.M.; Maier, R.M. Making a Living While Starving in the Dark: Metagenomic Insights into the Energy Dynamics of a Carbonate Cave. ISME J. 2014, 8, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Macalady, J.L.; Lyon, E.H.; Koffman, B.; Albertson, L.K.; Meyer, K.; Galdenzi, S.; Mariani, S. Dominant Microbial Populations in Limestone-Corroding Stream Biofilms, Frasassi Cave System, Italy. Appl. Environ. Microbiol. 2006, 72, 5596–5609. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.S.; Porter, M.L.; Stern, L.A.; Quinlan, S.; Bennett, P.C. Bacterial Diversity and Ecosystem Function of Filamentous Microbial Mats from Aphotic (Cave) Sulfidic Springs Dominated by Chemolithoautotrophic “Epsilonproteobacteria. ” FEMS Microbiol. Ecol. 2004, 51, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Gibert, J.; Deharveng, L. Subterranean Ecosystems: A Truncated Functional Biodiversity. BioScience 2002, 52, 473. [Google Scholar] [CrossRef]
- Meyers, P.A. Organic Geochemical Proxies of Paleoceanographic, Paleolimnologic, and Paleoclimatic Processes. Org. Geochem. 1997, 27, 213–250. [Google Scholar] [CrossRef]
- Peterson, B.J.; Howarth, R.W. Sulfur, Carbon, and Nitrogen Isotopes Used to Trace Organic Matter Flow in the Salt-Marsh Estuaries of Sapelo Island, Georgia. Limnol. Oceanogr. 1987, 32, 1195–1213. [Google Scholar] [CrossRef]
- Cole, M.L.; Kroeger, K.D.; Mcclelland, J.W.; Valiela, I. Effects of Watershed Land Use on Nitrogen Concentrations and Δ15 Nitrogen in Groundwater. Biogeochemistry 2006, 77, 199–215. [Google Scholar] [CrossRef]
- Groult, B.; Bredin, P.; Lazar, C.S. Ecological Processes Differ in Community Assembly of Archaea, Bacteria and Eukaryotes in a Biogeographical Survey of Groundwater Habitats in the Quebec Region (Canada). Environ. Microbiol. 2022, 24, 5898–5910. [Google Scholar] [CrossRef]
- Kurtbӧke, D.İ. Ecology and Habitat Distribution of Actinobacteria. In Biology and Biotechnology of Actinobacteria; Wink, J., Mohammadipanah, F., Hamedi, J., Eds.; Springer International Publishing: Cham, 2017; pp. 123–149; ISBN 978-3-319-60339-1. [Google Scholar]
- Sun, J.-Q.; Xu, L.; Guo, Y.; Li, W.-L.; Shao, Z.-Q.; Yang, Y.-L.; Wu, X.-L. Kribbella Deserti Sp. Nov., Isolated from Rhizosphere Soil of Ammopiptanthus Mongolicus. Int. J. Syst. Evol. Microbiol. 2017, 67, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir-Kocak, F.; Isik, K.; Saricaoglu, S.; Saygin, H.; Inan-Bektas, K.; Cetin, D.; Guven, K.; Sahin, N. Kribbella Sindirgiensis Sp. Nov. Isolated from Soil. Arch. Microbiol. 2017, 199, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir-Kocak, F.; Saygin, H.; Saricaoglu, S.; Cetin, D.; Guven, K.; Spröer, C.; Schumann, P.; Klenk, H.-P.; Sahin, N.; Isik, K. Kribbella Soli Sp. Nov., Isolated from Soil. Antonie Van Leeuwenhoek 2017, 110, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Liu, Q.; Maeng, S.; Choi, W.J.; Chang, Y.; Im, W.-T. Nocardioides Convexus Sp. Nov. and Nocardioides Anomalus Sp. Nov., Isolated from Soil and Mineral Water. Int. J. Syst. Evol. Microbiol. 2020, 70, 6402–6407. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.R. Kribbella. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons, Ltd, 2021; pp. 1–41 ISBN 978-1-118-96060-8.
- Yoon, J.-H.; Park, Y.-H. The Genus Nocardioides. In The Prokaryotes: Volume 3: Archaea. Bacteria: Firmicutes, Actinomycetes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, 2006; pp. 1099–1113; ISBN 978-0-387-30743-5. [Google Scholar]
- Walsh, C.M.; Gebert, M.J.; Delgado-Baquerizo, M.; Maestre, F.T.; Fierer, N. A Global Survey of Mycobacterial Diversity in Soil. Appl. Environ. Microbiol. 2019, 85, e01180–19. [Google Scholar] [CrossRef] [PubMed]
- Schleper, C.; Nicol, G.W. Ammonia-Oxidising Archaea – Physiology, Ecology and Evolution. In Advances in Microbial Physiology; Elsevier, 2010; Vol. 57, pp. 1–41 ISBN 978-0-12-381045-8.
- Ochsenreiter, T.; Selezi, D.; Quaiser, A.; Bonch-Osmolovskaya, L.; Schleper, C. Diversity and Abundance of Crenarchaeota in Terrestrial Habitats Studied by 16S RNA Surveys and Real Time PCR. Environ. Microbiol. 2003, 5, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Lazar, C.S.; Stoll, W.; Lehmann, R.; Herrmann, M.; Schwab, V.F.; Akob, D.M.; Nawaz, A.; Wubet, T.; Buscot, F.; Totsche, K.-U.; et al. Archaeal Diversity and CO2 Fixers in Carbonate-/Siliciclastic-Rock Groundwater Ecosystems. Archaea 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brochier-Armanet, C.; Boussau, B.; Gribaldo, S.; Forterre, P. Mesophilic Crenarchaeota: Proposal for a Third Archaeal Phylum, the Thaumarchaeota. Nat. Rev. Microbiol. 2008, 6, 245–252. [Google Scholar] [CrossRef]
- Könneke, M.; Schubert, D.M.; Brown, P.C.; Hügler, M.; Standfest, S.; Schwander, T.; Schada Von Borzyskowski, L.; Erb, T.J.; Stahl, D.A.; Berg, I.A. Ammonia-Oxidizing Archaea Use the Most Energy-Efficient Aerobic Pathway for CO2 Fixation. Proc. Natl. Acad. Sci. 2014, 111, 8239–8244. [Google Scholar] [CrossRef]
- Kozlowski, J.A.; Stieglmeier, M.; Schleper, C.; Klotz, M.G.; Stein, L.Y. Pathways and Key Intermediates Required for Obligate Aerobic Ammonia-Dependent Chemolithotrophy in Bacteria and Thaumarchaeota. ISME J. 2016, 10, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-M.; Offre, P.R.; He, J.-Z.; Verhamme, D.T.; Nicol, G.W.; Prosser, J.I. Autotrophic Ammonia Oxidation by Soil Thaumarchaea. Proc. Natl. Acad. Sci. 2010, 107, 17240–17245. [Google Scholar] [CrossRef] [PubMed]
- Kerou, M.; Schleper, C. Nitrososphaeraceae. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons, Ltd, 2016; pp. 1–2 ISBN 978-1-118-96060-8.
- Cheng, X.; Xiang, X.; Yun, Y.; Wang, W.; Wang, H.; Bodelier, P.L.E. Archaea and Their Interactions with Bacteria in a Karst Ecosystem. Front. Microbiol. 2023, 14, 1068595. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, D.; Wang, B. Soil Bacterial Diversity and Its Determinants in the Riparian Zone of the Lijiang River, China. Curr. Sci. 2019, 117, 1324–1332. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, Y.; He, D.; Gu, J.-D.; Guo, Q.; Liu, X.; Duan, Y.; Zhao, J.; Wang, W.; Feng, H. Community Structures of Bacteria and Archaea Associated with the Biodeterioration of Sandstone Sculptures at the Beishiku Temple. Int. Biodeterior. Biodegrad. 2021, 164, 105290. [Google Scholar] [CrossRef]
- Sauder, L.A.; Albertsen, M.; Engel, K.; Schwarz, J.; Nielsen, P.H.; Wagner, M.; Neufeld, J.D. Cultivation and Characterization of Candidatus Nitrosocosmicus Exaquare, an Ammonia-Oxidizing Archaeon from a Municipal Wastewater Treatment System. ISME J. 2017, 11, 1142–1157. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, M.; Jiang, Y.; Lin, W.; Luo, J. Production and Excretion of Polyamines To Tolerate High Ammonia, a Case Study on Soil Ammonia-Oxidizing Archaeon “Candidatus Nitrosocosmicus Agrestis. ” mSystems 2021, 6. [Google Scholar] [CrossRef]
- Lehtovirta-Morley, L.E.; Ross, J.; Hink, L.; Weber, E.B.; Gubry-Rangin, C.; Thion, C.; Prosser, J.I.; Nicol, G.W. Isolation of “Candidatus Nitrosocosmicus Franklandus”, a Novel Ureolytic Soil Archaeal Ammonia Oxidiser with Tolerance to High Ammonia Concentration. FEMS Microbiol. Ecol. 2016, 92, fiw057. [Google Scholar] [CrossRef]
- Medina, M.; Collins, A.G.; Taylor, J.W.; Valentine, J.W.; Lipps, J.H.; Amaral-Zettler, L.; Sogin, M.L. Phylogeny of Opisthokonta and the Evolution of Multicellularity and Complexity in Fungi and Metazoa. Int. J. Astrobiol. 2003, 2, 203–211. [Google Scholar] [CrossRef]
- Bik, H.M. Microbial Metazoa Are Microbes Too. mSystems 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Burki, F.; Shalchian-Tabrizi, K.; Minge, M.; Skjæveland, Å.; Nikolaev, S.I.; Jakobsen, K.S.; Pawlowski, J. Phylogenomics Reshuffles the Eukaryotic Supergroups. PLoS ONE 2007, 2, e790. [Google Scholar] [CrossRef] [PubMed]
- Strassert, J.F.H.; Jamy, M.; Mylnikov, A.P.; Tikhonenkov, D.V.; Burki, F. New Phylogenomic Analysis of the Enigmatic Phylum Telonemia Further Resolves the Eukaryote Tree of Life. Mol. Biol. Evol. 2019, 36, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Grattepanche, J.; Walker, L.M.; Ott, B.M.; Paim Pinto, D.L.; Delwiche, C.F.; Lane, C.E.; Katz, L.A. Microbial Diversity in the Eukaryotic SAR Clade: Illuminating the Darkness Between Morphology and Molecular Data. BioEssays 2018, 40, 1700198. [Google Scholar] [CrossRef] [PubMed]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A Few Ascomycota Taxa Dominate Soil Fungal Communities Worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global Diversity and Geography of Soil Fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.H.; Healy, R. Pezizomycetes. In Encyclopedia of Mycology; Zaragoza, Ó., Casadevall, A., Eds.; Elsevier, 2021; pp. 295–309 ISBN 978-0-323-85180-0.
- Foissner, W.; Agatha, S.; Berger, H. Soil Ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with Emphasis on Two Contrasting Environments, the Etosha Region and the Namib Desert. Part I: Text and Line Drawings. Part II: Photographs. PLUS Res. Denisia 2002, 5. [Google Scholar]
- Li, B.; Song, Y.; Hao, T.; Wang, L.; Zheng, W.; Lyu, Z.; Chen, Y.; Pan, X. Insights into the Phylogeny of the Ciliate of Class Colpodea Based on Multigene Data. Ecol. Evol. 2022, 12, e9380. [Google Scholar] [CrossRef] [PubMed]
- Amossé, J.; Dózsa-Farkas, K.; Boros, G.; Rochat, G.; Sandoz, G.; Fournier, B.; Mitchell, E.A.D.; Le Bayon, R.-C. Patterns of Earthworm, Enchytraeid and Nematode Diversity and Community Structure in Urban Soils of Different Ages. Eur. J. Soil Biol. 2016, 73, 46–58. [Google Scholar] [CrossRef]
- Yan, L.; Herrmann, M.; Kampe, B.; Lehmann, R.; Totsche, K.U.; Küsel, K. Environmental Selection Shapes the Formation of Near-Surface Groundwater Microbiomes. Water Res. 2020, 170, 115341. [Google Scholar] [CrossRef] [PubMed]
- Lehosmaa, K.; Muotka, T.; Pirttilä, A.M.; Jaakola, I.; Rossi, P.M.; Jyväsjärvi, J. Bacterial Communities at a Groundwater-surface Water Ecotone: Gradual Change or Abrupt Transition Points along a Contamination Gradient? Environ. Microbiol. 2021, 23, 6694–6706. [Google Scholar] [CrossRef] [PubMed]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobet, A.; Kurm, V.; Küsel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F.; et al. Where Less May Be More: How the Rare Biosphere Pulls Ecosystems Strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Huang, L.; Yu, F.-H. Increasing Soil Configurational Heterogeneity Promotes Plant Community Evenness through Equalizing Differences in Competitive Ability. Sci. Total Environ. 2021, 750, 142308. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, H.; Bennett, D.M.; Cadotte, M.W. Consequences of Dominance: A Review of Evenness Effects on Local and Regional Ecosystem Processes. Ecology 2008, 89, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-Z.; Zhang, Z.-F.; Zhou, N.; Jiang, C.-Y.; Wang, B.-J.; Cai, L.; Liu, S.-J. Diversity, Distribution and Co-Occurrence Patterns of Bacterial Communities in a Karst Cave System. Front. Microbiol. 2019, 10, 1726. [Google Scholar] [CrossRef] [PubMed]
- Vellend, M. Conceptual Synthesis in Community Ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Nemergut, D.R.; Schmidt, S.K.; Fukami, T.; O’Neill, S.P.; Bilinski, T.M.; Stanish, L.F.; Knelman, J.E.; Darcy, J.L.; Lynch, R.C.; Wickey, P.; et al. Patterns and Processes of Microbial Community Assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, J.; Wu, Y.; Tu, C.; Soininen, J.; Stegen, J.C.; He, J.; Liu, X.; Zhang, L.; Zhang, E. Phylogenetic Beta Diversity in Bacterial Assemblages across Ecosystems: Deterministic versus Stochastic Processes. ISME J. 2013, 7, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and Qualitative β Diversity Measures Lead to Different Insights into Factors That Structure Microbial Communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Auguet, J.-C.; Barberan, A.; Casamayor, E.O. Global Ecological Patterns in Uncultured Archaea. ISME J. 2010, 4, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, L.; Hug, K.; Griebler, C. Selection Imposed by Local Environmental Conditions Drives Differences in Microbial Community Composition across Geographically Distinct Groundwater Aquifers. FEMS Microbiol. Ecol. 2019, 95, fiz160. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Konopka, A.E. Estimating and Mapping Ecological Processes Influencing Microbial Community Assembly. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Dini-Andreote, F.; Stegen, J.C.; Van Elsas, J.D.; Salles, J.F. Disentangling Mechanisms That Mediate the Balance between Stochastic and Deterministic Processes in Microbial Succession. Proc. Natl. Acad. Sci. 2015, 112. [Google Scholar] [CrossRef]
- Allen, R.; Hoffmann, L.J.; Larcombe, M.J.; Louisson, Z.; Summerfield, T.C. Homogeneous Environmental Selection Dominates Microbial Community Assembly in the Oligotrophic South Pacific Gyre. Mol. Ecol. 2020, 29, 4680–4691. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Zhang, W.; Wang, C.; Wang, P.; Niu, L.; Wu, H. Homogeneous Selection Dominates the Microbial Community Assembly in the Sediment of the Three Gorges Reservoir. Sci. Total Environ. 2019, 690, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Barns, S.M.; Cain, E.C.; Sommerville, L.; Kuske, C.R. Acidobacteria Phylum Sequences in Uranium-Contaminated Subsurface Sediments Greatly Expand the Known Diversity within the Phylum. Appl. Environ. Microbiol. 2007, 73, 3113–3116. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Huang, X.; Wang, H.; Yun, Y.; Cheng, X.; Liu, D.; Lu, X.; Qiu, X. Microbial Interactions Drive Distinct Taxonomic and Potential Metabolic Responses to Habitats in Karst Cave Ecosystem. Microbiol. Spectr. 2021, 9, e01152–21. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Wang, X.; Bao, X.; Zhu, L.; Xie, Z.; Che, Z.; Sun, B. Exogenous Substrate Quality Determines the Dominant Keystone Taxa Linked to Carbon Mineralization: Evidence from a 30-Year Experiment. Soil Biol. Biochem. 2022, 169, 108683. [Google Scholar] [CrossRef]
- Bar-Or, I.; Ben-Dov, E.; Kushmaro, A.; Eckert, W.; Sivan, O. Methane-Related Changes in Prokaryotes along Geochemical Profiles in Sediments of Lake Kinneret (Israel). Biogeosciences 2015, 12, 2847–2860. [Google Scholar] [CrossRef]
- Grenier, V.; Laur, J.; Gonzalez, E.; Pitre, F.E. Glyphosate Has a Negligible Impact on Bacterial Diversity and Dynamics during Composting. Environ. Microbiol. 2023, 25, 2897–2912. [Google Scholar] [CrossRef] [PubMed]
- Nkamga, V.D.; Drancourt, M. Methanomassiliicoccales. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons, Ltd, 2016; pp. 1–2 ISBN 978-1-118-96060-8.
- Ai, J.; Guo, J.; Li, Y.; Zhong, X.; Lv, Y.; Li, J.; Yang, A. The Diversity of Microbes and Prediction of Their Functions in Karst Caves under the Influence of Human Tourism Activities—a Case Study of Zhijin Cave in Southwest China. Environ. Sci. Pollut. Res. 2022, 29, 25858–25868. [Google Scholar] [CrossRef]
- Mammola, S.; Isaia, M. Spiders in Caves. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170193. [Google Scholar] [CrossRef]
- Parimuchová, A.; Dušátková, L.P.; Kováč, Ľ.; Macháčková, T.; Slabý, O.; Pekár, S. The Food Web in a Subterranean Ecosystem Is Driven by Intraguild Predation. Sci. Rep. 2021, 11, 4994. [Google Scholar] [CrossRef]
- Thomas, F.; Hehemann, J.-H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and Gut Bacteroidetes: The Food Connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Mao, G.; Gao, G.; Bartlam, M.; Wang, Y. Structural and Functional Changes of Groundwater Bacterial Community During Temperature and pH Disturbances. Microb. Ecol. 2019, 78, 428–445. [Google Scholar] [CrossRef] [PubMed]
- Rangseekaew, P.; Pathom-aree, W. Cave Actinobacteria as Producers of Bioactive Metabolites. Front. Microbiol. 2019, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Montano, E.T.; Henderson, L.O. Studies of Antibiotic Production by Cave Bacteria. In Cave Microbiomes: A Novel Resource for Drug Discovery; Cheeptham, N., Ed.; SpringerBriefs in Microbiology; Springer: New York, NY, 2013; Vol. 1 ; ISBN 978-1-4614-5205-8. [Google Scholar]
- Bernhard, A.E.; Field, K.G. A PCR Assay To Discriminate Human and Ruminant Feces on the Basis of Host Differences in Bacteroides-Prevotella Genes Encoding 16S rRNA. Appl. Environ. Microbiol. 2000, 66, 4571–4574. [Google Scholar] [CrossRef] [PubMed]
- Allsop, K.; Stickler, D. j. An Assessment of Bacteroides Fragilis Group Organisms as Indicators of Human Faecal Pollution. J. Appl. Bacteriol. 1985, 58, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.W.; Kasalický, V.; Jezbera, J.; Brandt, U.; Jezberová, J.; Šimek, K. Limnohabitans Curvus Gen. Nov., Sp. Nov., a Planktonic Bacterium Isolated from a Freshwater Lake. Int. J. Syst. Evol. Microbiol. 2010, 60, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Jezberová, J.; Jezbera, J.; Znachor, P.; Nedoma, J.; Kasalický, V.; Šimek, K. The Limnohabitans Genus Harbors Generalistic and Opportunistic Subtypes: Evidence from Spatiotemporal Succession in a Canyon-Shaped Reservoir. Appl. Environ. Microbiol. 2017, 83, e01530–17. [Google Scholar] [CrossRef] [PubMed]
- Pearman, J.K.; Thomson-Laing, G.; Thomson-Laing, J.; Thompson, L.; Waters, S.; Reyes, L.; Howarth, J.D.; Vandergoes, M.J.; Wood, S.A. The Role of Environmental Processes and Geographic Distance in Regulating Local and Regionally Abundant and Rare Bacterioplankton in Lakes. Front. Microbiol. 2022, 12, 793441. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Kim, S.; Islam, Md.R.; Cho, J.-C. The First Complete Genome Sequences of the acI Lineage, the Most Abundant Freshwater Actinobacteria, Obtained by Whole-Genome-Amplification of Dilution-to-Extinction Cultures. Sci. Rep. 2017, 7, 42252. [Google Scholar] [CrossRef]
- Chen, F.; Koh, X.P.; Tang, M.L.Y.; Gan, J.; Lau, S.C.K. Microbiological Assessment of Ecological Status in the Pearl River Estuary, China. Ecol. Indic. 2021, 130, 108084. [Google Scholar] [CrossRef]
- Van Grinsven, S.; Sinninghe Damsté, J.S.; Harrison, J.; Polerecky, L.; Villanueva, L. Nitrate Promotes the Transfer of Methane-derived Carbon from the Methanotroph Methylobacter Sp. to the Methylotroph Methylotenera Sp. in Eutrophic Lake Water. Limnol. Oceanogr. 2021, 66, 878–891. [Google Scholar] [CrossRef]
- Chen, L.-X.; Méndez-García, C.; Dombrowski, N.; Servín-Garcidueñas, L.E.; Eloe-Fadrosh, E.A.; Fang, B.-Z.; Luo, Z.-H.; Tan, S.; Zhi, X.-Y.; Hua, Z.-S.; et al. Metabolic Versatility of Small Archaea Micrarchaeota and Parvarchaeota. ISME J. 2018, 12, 756–775. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Vanteeva, A.; Merkel, A. Microbiological Activity during Co-Composting of Food and Agricultural Waste for Soil Amendment. Agronomy 2021, 11, 928. [Google Scholar] [CrossRef]
- Corsaro, D.; Walochnik, J.; Venditti, D.; Hauröder, B.; Michel, R. Solving an Old Enigma: Morellospora Saccamoebae Gen. Nov., Sp. Nov. (Rozellomycota), a Sphaerita-like Parasite of Free-Living Amoebae. Parasitol. Res. 2020, 119, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Sisson, C.; Gulla-Devaney, B.; Katz, L.A.; Grattepanche, J.-D. Seed Bank and Seasonal Patterns of the Eukaryotic SAR (Stramenopila, Alveolata and Rhizaria) Clade in a New England Vernal Pool. J. Plankton Res. 2018, 40, 376–390. [Google Scholar] [CrossRef]
- Herrmann, M.; Wegner, C.-E.; Taubert, M.; Geesink, P.; Lehmann, K.; Yan, L.; Lehmann, R.; Totsche, K.U.; Küsel, K. Predominance of Cand. Patescibacteria in Groundwater Is Caused by Their Preferential Mobilization From Soils and Flourishing Under Oligotrophic Conditions. Front. Microbiol. 2019, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Ning, D.; He, Z.; Zhang, P.; Spencer, S.J.; Gao, S.; Shi, W.; Wu, L.; Zhang, Y.; Yang, Y.; et al. Small and Mighty: Adaptation of Superphylum Patescibacteria to Groundwater Environment Drives Their Genome Simplicity. Microbiome 2020, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Gios, E.; Mosley, O.E.; Takeuchi, N.; Handley, K.M. Genetic Exchange Shapes Ultra-Small Patescibacteria Metabolic Capacities in the Terrestrial Subsurface 2022.
- Nakai, R. Size Matters: Ultra-Small and Filterable Microorganisms in the Environment. Microbes Environ. 2020, 35, n. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-M.; Zhou, Y.-L.; Wei, Z.-F.; Wang, Y. Phylogenomic Insights into Distribution and Adaptation of Bdellovibrionota in Marine Waters. Microorganisms 2021, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Sockett, R.E. Predatory Lifestyle of Bdellovibrio Bacteriovorus. Annu. Rev. Microbiol. 2009, 63, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.N.; Chen, H. Environmental Regulation of the Distribution and Ecology of Bdellovibrio and Like Organisms. Front. Microbiol. 2020, 11, 545070. [Google Scholar] [CrossRef] [PubMed]
- Paix, B.; Ezzedine, J.A.; Jacquet, S. Diversity, Dynamics, and Distribution of Bdellovibrio and Like Organisms in Perialpine Lakes. Appl. Environ. Microbiol. 2019, 85, e02494–18. [Google Scholar] [CrossRef] [PubMed]
- Mehrshad, M.; Lopez-Fernandez, M.; Sundh, J.; Bell, E.; Simone, D.; Buck, M.; Bernier-Latmani, R.; Bertilsson, S.; Dopson, M. Energy Efficiency and Biological Interactions Define the Core Microbiome of Deep Oligotrophic Groundwater. Nat. Commun. 2021, 12, 4253. [Google Scholar] [CrossRef]
- Wurch, L.; Giannone, R.J.; Belisle, B.S.; Swift, C.; Utturkar, S.; Hettich, R.L.; Reysenbach, A.-L.; Podar, M. Genomics-Informed Isolation and Characterization of a Symbiotic Nanoarchaeota System from a Terrestrial Geothermal Environment. Nat. Commun. 2016, 7, 12115. [Google Scholar] [CrossRef] [PubMed]
- Huber, H.; Hohn, M.J.; Rachel, R.; Fuchs, T.; Wimmer, V.C.; Stetter, K.O. A New Phylum of Archaea Represented by a Nanosized Hyperthermophilic Symbiont. Nature 2002, 417, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Casanueva, A.; Galada, N.; Baker, G.C.; Grant, W.D.; Heaphy, S.; Jones, B.; Yanhe, M.; Ventosa, A.; Blamey, J.; Cowan, D.A. Nanoarchaeal 16S rRNA Gene Sequences Are Widely Dispersed in Hyperthermophilic and Mesophilic Halophilic Environments. Extremophiles 2008, 12, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Mardanov, A.V.; Gumerov, V.M.; Beletsky, A.V.; Ravin, N.V. Microbial Diversity in Acidic Thermal Pools in the Uzon Caldera, Kamchatka. Antonie Van Leeuwenhoek 2018, 111, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Jarett, J.K.; Nayfach, S.; Podar, M.; Inskeep, W.; Ivanova, N.N.; Munson-McGee, J.; Schulz, F.; Young, M.; Jay, Z.J.; Beam, J.P.; et al. Single-Cell Genomics of Co-Sorted Nanoarchaeota Suggests Novel Putative Host Associations and Diversification of Proteins Involved in Symbiosis. Microbiome 2018, 6, 161. [Google Scholar] [CrossRef] [PubMed]


| Water samples | pH | DOC | DIC | NHx | NO2- | NO3- |
|---|---|---|---|---|---|---|
| Radiesthesia | ||||||
| W1 | 7.8 | 1.7 | 46.81 | 0.0715 | 0.01 | 2.43 |
| W7 | 7.4 | 1.6 | 43.89 | 0.0235 | 0.01 | 2.42 |
| Echo | ||||||
| W2 | 7.5 | 1.67 | 46.11 | 0.0325 | 0.01 | 2.43 |
| W3 | 7.6 | 1.59 | 48.22 | 0.0275 | 0.01 | 2.44 |
| W4 | 7.5 | 1.82 | 45.09 | 0.0275 | 0.01 | 2.42 |
| W5 | 7.5 | 1.57 | 46.72 | 0.022 | 0.01 | 2.43 |
| W6 | 7.5 | 1.53 | 45.13 | 0.027 | 0.01 | 2.45 |
| Samples | pH | Ctot | Corg | Cinorg | Ntot | δ13C | δ15N |
|---|---|---|---|---|---|---|---|
| Surface Soil | |||||||
| SS1 | 5.8 | 28.05 | 27.88 | 0.17 | 0.95 | -24.4 | 0.5 |
| SS2 | 7.5 | 9.68 | 9.68 | 0 | 0.51 | -28.1 | 2.0 |
| SS3 | 7.1 | 11.18 | 11.18 | 0 | 0.49 | -27.3 | 1.9 |
| SS4 | 7.3 | 17.96 | 17.76 | 0.2 | 0.68 | -25.9 | 0.6 |
| SS5 | 7.5 | 10.64 | 10.09 | 0.55 | 0.88 | -27.0 | 4.0 |
| SS6 | 7.8 | 4.87 | 4.87 | 0 | 0.36 | -27.9 | 3.9 |
| Sediment | |||||||
| S1 (Rad) | 7.9 | 0.39 | 0.23 | 0.17 | 0.03 | -24.8 | na |
| S2 (Rad) | 8.1 | 1.21 | 1.11 | 0.10 | 0.15 | -24.9 | 7.0 |
| S3 (Echo) | 8.1 | 0.96 | 0.17 | 0.79 | 0.03 | -24.7 | na |
| S4 (Rad) | 8.3 | 0.36 | 0.18 | 0.18 | 0.05 | -26.3 | 3.8 |
| BACTERIA | ARCHAEA | EUKARYOTE | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Df | SoS | F | Pr(>F) | Df | SoS | F | Pr(>F) | Df | SoS | F | Pr(>F) | ||
| Soil/Sediments | |||||||||||||
| pH | 1 | 0.69251 | 2.1159 | 0.003 | 1 | 1.03584 | 3.9466 | 0.008 | 1 | 0.70173 | 2.4631 | 0.012 | |
| Ctot | 1 | 0.40209 | 1.2285 | 0.213 | 1 | 0.29826 | 1.1364 | 0.325 | 1 | 0.24526 | 0.8609 | 0.619 | |
| Corg | 1 | 0.38685 | 1.1820 | 0.255 | 1 | 0.24336 | 0.9272 | 0.484 | 1 | 0.30758 | 1.0796 | 0.332 | |
| Cinorg | 1 | 0.30256 | 0.9244 | 0.547 | 1 | 0.23710 | 0.9034 | 0.539 | 1 | 0.34873 | 1.2240 | 0.227 | |
| Ntot | 1 | 0.55689 | 1.7015 | 0.031 | 1 | 0.34423 | 1.3115 | 0.253 | 1 | 0.27023 | 0.9485 | 0.536 | |
| δ13C | 1 | 0.48418 | 1.4793 | 0.082 | 1 | 0.31459 | 1.1986 | 0.292 | 1 | 0.36436 | 1.2789 | 0.189 | |
| Residual | 3 | 0.98189 | 2 | 0.52492 | 2 | 0.56980 | |||||||
| Water 0.2 µm | |||||||||||||
| DIC | 1 | 0.15371 | 1.2129 | 0.086 | 1 | 0.083487 | 1.1955 | 0.035 | 1 | 0.225233 | 1.7533 | 0.142 | |
| DOC | 1 | 0.17432 | 1.3756 | 0.014 | 1 | 0.074546 | 1.0675 | 0.286 | 1 | 0.092212 | 0.7178 | 0.688 | |
| NHx | 1 | 0.12353 | 0.9748 | 0.508 | 1 | 0.065149 | 0.9329 | 0.668 | 1 | 0.094777 | 0.7378 | 0.658 | |
| NO3- | 1 | 0.12813 | 1.0111 | 0.435 | 1 | 0.088699 | 1.2701 | 0.023 | 1 | 0.078628 | 0.6121 | 0.789 | |
| Residual | 2 | 0.25345 | 2 | 0.139670 | 2 | 0.256930 | |||||||
| Water 0.1 µm | |||||||||||||
| DIC | 1 | 0.28232 | 0.7755 | 0.8750 | 1 | 0.21246 | 1.1640 | 0.4708 | |||||
| DOC | 1 | 0.32200 | 0.8845 | 0.7042 | 1 | 0.34974 | 1.9161 | 0.0875 | |||||
| NHx | 1 | 0.29307 | 0.8050 | 0.7931 | 1 | 0.18416 | 1.0089 | 0.4944 | |||||
| NO3- | 1 | 0.26997 | 0.7416 | 0.9722 | 1 | 0.22242 | 1.2185 | 0.3361 | |||||
| Residual | 1 | 0.36405 | 1 | 0.18253 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





