Submitted:
30 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Bioactive Compounds and Plant Extracts
2.1. Definition and Classification of Bioactive Compounds and Plant Extracts
2.2. Mechanisms of Action of Bioactive Compounds and Plant Extracts in the Treatment of Pancreatic Cancer
2.2.1. Polyphenols
2.2.1.1. Polyphenolic compounds and pancreatic cancer
2.2.1.2. Flavonoid compounds and pancreatic cancer
2.2.1.3. Phenolic acid compounds and pancreatic cancer
2.2.1.4. Lignan compounds and pancreatic cancer
2.2.2. Saponins
2.2.3. Alkaloids
2.2.4. Quinones
2.2.5. Terpenes
2.2.6. Polysaccharides
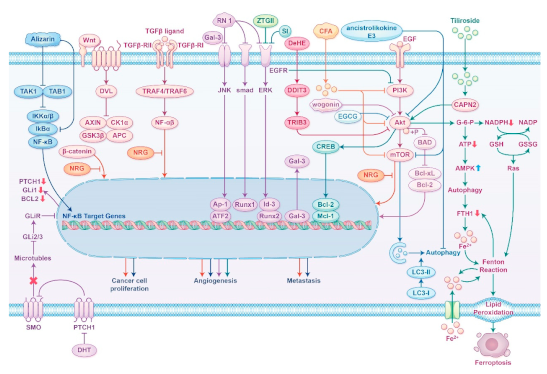
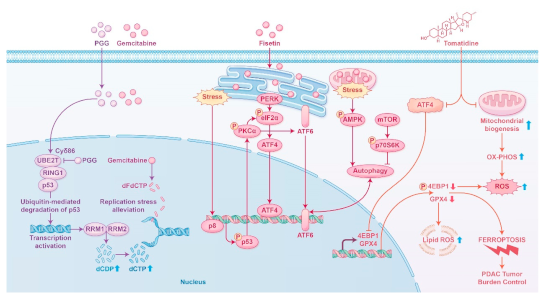
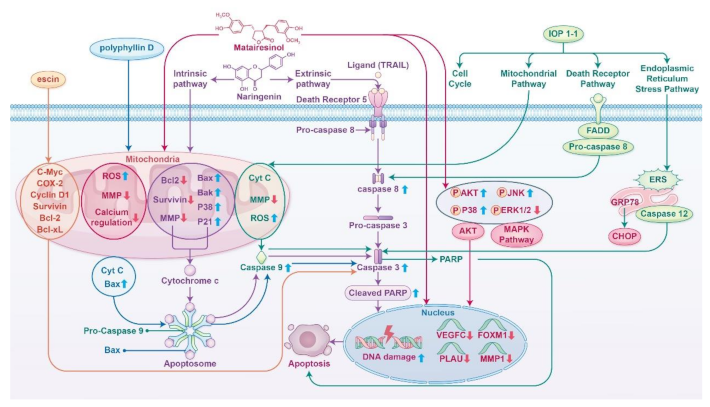
2.3. Clinical Applications and Safety of Bioactive Compounds and Plant Extracts.
2.4. Research Trends and Challenges in the Development of Bioactive Compounds and Plant Extracts
2.5. Summary and Outlook
References
- Kim, J., et al., Pancreatic Cancer Treatment Targeting the HGF/c-MET Pathway: The MEK Inhibitor Trametinib. Cancers (Basel), 2024. 16(5). [CrossRef]
- Ramalhete, L., et al., Proteomics-Driven Biomarkers in Pancreatic Cancer. Proteomes, 2023. 11(3). [CrossRef]
- Huang, X., et al., A Self-Sustained Nanoplatform Reverses TRAIL-Resistance of Pancreatic Cancer Through Coactivating of Exogenous and Endogenous Apoptotic Pathway. Biomaterials, 2021. 272: p. 120795. [CrossRef]
- Cao, Y., et al., Targeting Survivin With Tanshinone IIA Inhibits Tumor Growth and Overcomes Chemoresistance in Colorectal Cancer. Cell Death Discov, 2023. 9(1): p. 351.
- Khwaza, V., et al., Pentacyclic Triterpenoids With Nitrogen-Containing Heterocyclic Moiety, Privileged Hybrids in Anticancer Drug Discovery. Molecules, 2021. 26(9).
- Bouyahya, A., et al., Chemical Compounds of Berry-Derived Polyphenols and Their Effects on Gut Microbiota, Inflammation, and Cancer. Molecules, 2022. 27(10).
- Banik K., et al., Wogonin and its Analogs for the Prevention and Treatment of Cancer: A Systematic Review. Phytother Res, 2022. 36(5): p. 1854-1883. [CrossRef]
- Akter, M. , et al., Anti-Tumor and Antioxidant Activity of Kaempferol-3-O-Alpha-L-Rhamnoside (Afzelin) Isolated from Pithecellobium dulce Leaves. BMC Complement Med Ther, 2022. 22(1): p. 169.
- Ziemlewska, A. , et al., Assessment of Cosmetic and Dermatological Properties and Safety of Use of Model Skin Tonics With Kombucha-Fermented Red Berry Extracts. Int J Mol Sci, 2022. 23(23).
- Peng, B.Y. , et al., AGA Induces Sub-G1 Cell Cycle Arrest and Apoptosis in Human Colon Cancer Cells Through p53-Independent/p53-Dependent Pathway. BMC Cancer, 2023. 23(1): p. 1.
- Tiwary, B.K. , et al., The In Vitro Cytotoxic Activity of Ethno-Pharmacological Important Plants of Darjeeling District of West Bengal Against Different Human Cancer Cell Lines. BMC Complement Altern Med, 2015. 15: p. 22.
- Gururajanna, B. , et al., Molecular Effects of Taxol and Caffeine on Pancreatic Cancer Cells. Int J Mol Med, 1999. 4(5): p. 501-507. [CrossRef]
- Qin, T. , et al., NAF-1 Inhibition by Resveratrol Suppresses Cancer Stem Cell-Like Properties and the Invasion of Pancreatic Cancer. Front Oncol, 2020. 10: p. 1038.
- Ma, D. , et al., Baicalein Induces Apoptosis of Pancreatic Cancer Cells by Regulating the Expression of miR-139-3p and miR-196b-5p. Front Oncol, 2021. 11: p. 653061.
- Kaur, A. , et al., In-Vitro Antiproliferative Efficacy of Abrus precatorius Seed Extracts on Cervical Carcinoma. Sci Rep, 2022. 12(1): p. 10226.
- Duan, H. , et al., Exploring the Therapeutic Mechanisms of Gleditsiae Spina Acting on Pancreatic Cancer via Network Pharmacology, Molecular Docking and Molecular Dynamics Simulation. RSC Adv, 2023. 13(20): p. 13971-13984.
- Shukla, S.K. , et al., Molecular and Physiological Evaluation of Pancreatic Cancer-Induced Cachexia. Methods Mol Biol, 2019. 1882: p. 321-333.
- Goyal, S., M. Sharma, and R. Sharma, Bioactive Compound from Lagerstroemia speciosa: Activating Apoptotic Machinery in Pancreatic Cancer Cells. 3 Biotech, 2022. 12(4): p. 96.
- Mousa, D.S. , et al., Nanoformulated Bioactive Compounds Derived from Different Natural Products Combat Pancreatic Cancer Cell Proliferation. Int J Nanomedicine, 2020. 15: p. 2259-2268.
- Ng, C.X. , et al., The Potential of Plant-Derived Extracts and Compounds to Augment Anticancer Effects of Chemotherapeutic Drugs. Nutr Cancer, 2022. 74(9): p. 3058-3076.
- Guha, B. , et al., Unveiling Pharmacological Studies Provide New Insights on Mangifera longipes and Quercus gomeziana. Saudi J Biol Sci, 2021. 28(1): p. 183-190.
- Khater, S.I. , et al., Autophagy Characteristics of Phytoestrogens in Management and Prevention of Diseases: A Narrative Review of In-Vivo and In-Vitro Studies. J Adv Vet Anim Res, 2023. 10(2): p. 308-320.
- Munir, N. , et al., Phytochemical Constituents and In vitro Pharmacological Response of Cnidium monnieri; A Natural Ancient Medicinal Herb. Dose Response, 2022. 20(3): p. 15593258221115543.
- Figueiredo, L.C. , et al., Propolis, Aloe Vera, Green Tea, Cranberry, Calendula, Myrrha and Salvia Properties Against Periodontal Microorganisms. Microorganisms, 2022. 10(11).
- Ekiert, H.M. and A. Szopa, Biological Activities of Natural Products. Molecules, 2020. 25(23).
- Chamcheu, J.C. , et al., Graviola (Annona muricata) Exerts Anti-Proliferative, Anti-Clonogenic and Pro-Apoptotic Effects in Human Non-Melanoma Skin Cancer UW-BCC1 and A431 Cells In Vitro: Involvement of Hedgehog Signaling. Int J Mol Sci, 2018. 19(6).
- Kubra Sasmaz, H. , et al., Antioxidant Capacity, Sugar Content, and Tandem HPLC-DAD-ESI/MS Profiling of Phenolic Compounds from Aronia melanocarpa Fruits and Leaves (Nero and Viking Cultivars). ACS Omega, 2024. 9(13): p. 14963-14976.
- Mei, X. , et al., Necroptosis in Pneumonia: Therapeutic Strategies and Future Perspectives. Viruses, 2024. 16(1).
- Liu, H. , et al., Rutin is a Potent Senomorphic Agent to Target Senescent Cells and Can Improve Chemotherapeutic Efficacy. Aging Cell, 2024. 23(1): p. e13921.
- Rutkowska, M. and M.A. Olszewska, Anti-Diabetic Potential of Polyphenol-Rich Fruits from the Maleae Tribe-A Review of In Vitro and In Vivo Animal and Human Trials. Nutrients, 2023. 15(17).
- Rybak, M. and A. Wojdylo, Inhibition of Alpha-Amylase, Alpha-Glucosidase, Pancreatic Lipase, 15-Lipooxygenase and Acetylcholinesterase Modulated by Polyphenolic Compounds, Organic Acids, and Carbohydrates of Prunus domestica Fruit. Antioxidants (Basel), 2023. 12(7).
- Jang, W.Y., M. Y. Kim, and J.Y. Cho, Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. Int J Mol Sci, 2022. 23(24).
- Mikhaevich, E.I., D. V. Sorokin, and A.M. Scherbakov, Honokiol Inhibits the Growth of Hormone-Resistant Breast Cancer Cells: Its Promising Effect in Combination With Metformin. Res Pharm Sci, 2023. 18(5): p. 580-591.
- Teodor, E.D., V. Moroeanu, and G.L. Radu, Lignans From Medicinal Plants and Their Anticancer Effect. Mini Rev Med Chem, 2020. 20(12): p. 1083-1090.
- Xu, X.H. , et al., Saponins From Chinese Medicines as Anticancer Agents. Molecules, 2016. 21(10).
- Sun, L.R. , et al., Modulation of Multiple Signaling Pathways of the Plant-Derived Natural Products in Cancer. Front Oncol, 2019. 9: p. 1153.
- Elizalde-Romero, C.A. , et al., Solanum Fruits: Phytochemicals, Bioaccessibility and Bioavailability, and Their Relationship With Their Health-Promoting Effects. Front Nutr, 2021. 8: p. 790582.
- Van Chen, T. , et al., Antioxidant Activity and Alpha-Glucosidase Inhibitability of Distichochlamys citrea M.F. Newman Rhizome Fractionated Extracts: In Vitro and In Silico Screenings. Chem Zvesti, 2022. 76(9): p. 5655-5675.
- Xu, Y. , et al., Alkaloids From the Roots of Sophora flavescens and Their Anti-Tumor Activity. Fitoterapia, 2023. 171: p. 105685.
- Ma, W. , et al., In-Vitro and In-Vivo Anti-Breast Cancer Activity of Synergistic Effect of Berberine and Exercise Through Promoting the Apoptosis and Immunomodulatory Effects. Int Immunopharmacol, 2020. 87: p. 106787.
- Kamath, A.J. , et al., Embelin: A Multifaceted Anticancer Agent With Translational Potential in Targeting Tumor Progression and Metastasis. EXCLI J, 2023. 22: p. 1311-1329.
- Cowan, J. , et al., A Novel Marine Natural Product Derived Pyrroloiminoquinone With Potent Activity Against Skin Cancer Cells. Mar Drugs, 2019. 17(8).
- Byrne, F.L. , et al., Phenotypic Screen for Oxygen Consumption Rate Identifies an Anti-Cancer Naphthoquinone That Induces Mitochondrial Oxidative Stress. Redox Biol, 2020. 28: p. 101374.
- Rui, X. , et al., Gut Microbiota Were Altered With Platelet Count and Red Blood Cell Count in Immune Thrombocytopenia Patients With Different Treatments. Front Cell Infect Microbiol, 2023. 13: p. 1168756.
- Huang, M. , et al., Terpenoids: Natural Products for Cancer Therapy. Expert Opin Investig Drugs, 2012. 21(12): p. 1801-1818.
- Bai, B. , et al., Molecular Basis of Prostate Cancer and Natural Products as Potential Chemotherapeutic and Chemopreventive Agents. Front Pharmacol, 2021. 12: p. 738235.
- Xue, J.C. , et al., Natural Products Modulate NLRP3 in Ulcerative Colitis. Front Pharmacol, 2023. 14: p. 1265825.
- Yang, S. , et al., Glycobiology in Osteoclast Differentiation and Function. Bone Res, 2023. 11(1): p. 55.
- Zhou, S. and G. Huang, Preparation, Structure and Activity of Polysaccharide Phosphate esters. Biomed Pharmacother, 2021. 144: p. 112332.
- Alves, N.M. , et al., Antioxidant Mechanisms Underlying the Gastroprotective Effect of Menthofuran on Experimentally Induced Gastric Lesions in Rodents. Evid Based Complement Alternat Med, 2023. 2023: p. 9192494.
- Ebrahimi, B. , et al., Combination of Marine Bioactive Compounds and Extracts for the Prevention and Treatment of Chronic Diseases. Front Nutr, 2022. 9: p. 1047026.
- Boy, F.R. , et al., Antifungal Effect of Autochthonous Aromatic Plant Extracts on Two Mycotoxigenic Strains of Aspergillus flavus. Foods, 2023. 12(9).
- Ziolkiewicz, A. , et al., The Effect of In Vitro Digestion on Polyphenolic Compounds and Antioxidant Properties of Sorghum (Sorghum bicolor (L.) Moench) and Sorghum-Enriched Pasta. Molecules, 2023. 28(4).
- Jia, W. , et al., Nano-Based Drug Delivery of Polyphenolic Compounds for Cancer Treatment: Progress, Opportunities, and Challenges. Pharmaceuticals (Basel), 2023. 16(1).
- Singh, N. and S.S. Yadav, A Review on Health Benefits of Phenolics Derived From Dietary Spices. Curr Res Food Sci, 2022. 5: p. 1508-1523.
- Basri, D.F., Z. A. Alamin, and K.M. Chan, Assessment of Cytotoxicity and Genotoxicity of Stem Bark Extracts From Canarium odontophyllum Miq. (dabai) Against HCT 116 Human Colorectal Cancer Cell Line. BMC Complement Altern Med, 2016. 16: p. 36.
- Rezaei, P.F. , et al., Induction of G1 Cell Cycle Arrest and Cyclin D1 Down-Regulation in Response to Pericarp Extract of Baneh in Human Breast Cancer T47D cells. Daru, 2012. 20(1): p. 101.
- Afsar, T. , et al., Growth Inhibition and Apoptosis in Cancer Cells Induced by Polyphenolic Compounds of Acacia hydaspica: Involvement of Multiple Signal Transduction Pathways. Sci Rep, 2016. 6: p. 23077.
- Fakhri, S. , et al., Modulation of TLR/NF-kappaB/NLRP Signaling by Bioactive Phytocompounds: A Promising Strategy to Augment Cancer Chemotherapy and Immunotherapy. Front Oncol, 2022. 12: p. 834072.
- Siriwaseree, J. , et al., Exploring the Apoptotic-Induced Biochemical Mechanism of Traditional Thai Herb (Kerra) Extract in HCT116 Cells Using a Label-Free Proteomics Approach. Medicina (Kaunas), 2023. 59(8).
- Xu, J. , et al., Current Advances and Future Strategies for BCL-2 Inhibitors: Potent Weapons against Cancers. Cancers (Basel), 2023. 15(20).
- Eissa, I.H. , et al., Computer-Assisted Drug Discovery of a Novel Theobromine Derivative as an EGFR Protein-Targeted Apoptosis Inducer. Evol Bioinform Online, 2023. 19: p. 11769343231217916.
- Abotaleb, M. , et al., Flavonoids in Cancer and Apoptosis. Cancers (Basel), 2018. 11(1).
- Fernandez-Cruz, E. , et al., Inhibition of VEGFR-2 Phosphorylation and Effects on Downstream Signaling Pathways in Cultivated Human Endothelial Cells by Stilbenes From Vitis Spp. J Agric Food Chem, 2019. 67(14): p. 3909-3918.
- Vengryte, M. and L. Raudone, Phytochemical Profiling and Biological Activities of Rhododendron Subsect. Ledum: Discovering the Medicinal Potential of Labrador Tea Species in the Northern Hemisphere. Plants (Basel), 2024. 13(6).
- Rizzo, A. , et al., PSMA Radioligand Uptake as a Biomarker of Neoangiogenesis in Solid Tumours: Diagnostic or Theragnostic Factor? Cancers (Basel), 2022. 14(16).
- Zhou, Z.Y. , et al., Antiangiogenesis Effect of Timosaponin AIII on HUVECs In Vitro and Zebrafish Embryos In Vivo. Acta Pharmacol Sin, 2020. 41(2): p. 260-269.
- Huang, T.T. , et al., E7050 Suppresses the Growth of Multidrug-Resistant Human Uterine Sarcoma by Inhibiting Angiogenesis via Targeting of VEGFR2-Mediated Signaling Pathways. Int J Mol Sci, 2023. 24(11).
- Wang, Y. , et al., Potential of Dietary HDAC2i in Breast Cancer Patients Receiving PD-1/PD-L1 Inhibitors. Nutrients, 2023. 15(18).
- Liu, Y. , et al., Curcumin Elevates microRNA-183-5p via Cathepsin B-Mediated Phosphatidylinositol 3-Kinase/AKT Pathway to Strengthen Lipopolysaccharide-Stimulated Immune Function of Sepsis Mice. Contrast Media Mol Imaging, 2022. 2022: p. 6217234.
- Derakhshan, A. , et al., Efficacy of Herbal Medicines on Lung Function in Asthma: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Pharmacopuncture, 2023. 26(2): p. 124-138.
- Saki, E. , et al., Chemopreventive Effects of Germinated Rough Rice Crude Extract in Inhibiting Azoxymethane-Induced Aberrant Crypt Foci Formation in Sprague-Dawley Rats. Biomed Res Int, 2017. 2017: p. 9517287.
- Pop, O.L. , et al., Polyphenols-Ensured Accessibility from Food to the Human Metabolism by Chemical and Biotechnological Treatments. Antioxidants (Basel), 2023. 12(4).
- Duan, J. , et al., The Mechanisms of Wine Phenolic Compounds for Preclinical Anticancer Therapeutics. Food Nutr Res, 2021. 65.
- Bimonte, S. , et al., An Overview of Pre-Clinical Studies on the Effects of (-)-Epigallocatechin-3-gallate, a Catechin Found in Green Tea, in Treatment of Pancreatic Cancer. Recenti Prog Med, 2017. 108(6): p. 282-287.
- Wei, R. , et al., Epigallocatechin-3-Gallate (EGCG) Suppresses Pancreatic Cancer Cell Growth, Invasion, and Migration partly through the Inhibition of Akt Pathway and Epithelial-Mesenchymal Transition: Enhanced Efficacy when Combined with Gemcitabine. Nutrients, 2019. 11(8).
- Srivani, G. , et al., Resveratrol Binds and Inhibits Transcription Factor HIF-1alpha in Pancreatic Cancer. Exp Cell Res, 2020. 394(1): p. 112126.
- Xu, Q. , et al., Resveratrol in the Treatment of Pancreatic Cancer. Ann N Y Acad Sci, 2015. 1348(1): p. 10-19.
- Gao, Y. , et al., Farnesyl Phenolic Enantiomers as Natural MTH1 Inhibitors From Ganoderma sinense. Oncotarget, 2017. 8(56): p. 95865-95879.
- Thyagarajan, A. , et al., Dietary Polyphenols in Cancer Chemoprevention: Implications in Pancreatic Cancer. Antioxidants (Basel), 2020. 9(8).
- Sobolewska, D. , et al., The Genus Cuphea P. Browne as a Source of Biologically Active Phytochemicals for Pharmaceutical Application and Beyond-A Review. Int J Mol Sci, 2023. 24(7).
- Jiang, X. , et al., Targeting UBE2T Potentiates Gemcitabine Efficacy in Pancreatic Cancer by Regulating Pyrimidine Metabolism and Replication Stress. Gastroenterology, 2023. 164(7): p. 1232-1247.
- Shankar, S. , et al., Resveratrol Inhibits Pancreatic Cancer Stem Cell Characteristics in Human and KrasG12D Transgenic Mice by Inhibiting Pluripotency Maintaining Factors and Epithelial-Mesenchymal Transition. PLoS One, 2011. 6(1): p. e16530.
- Retraction. Cancer, 2016. 122(20): p. 3247.
- Banerjee, S. , et al., Molecular Evidence for Increased Antitumor Activity of Gemcitabine by Genistein In Vitro and In Vivo Using an Orthotopic Model of Pancreatic Cancer. Cancer Res, 2005. 65(19): p. 9064-9072.
- Farhan, M. , et al., Pomegranate Juice Anthocyanidins Induce Cell Death in Human Cancer Cells by Mobilizing Intracellular Copper Ions and Producing Reactive Oxygen Species. Front Oncol, 2022. 12: p. 998346.
- Ferro, R. , et al., GPR55 Signalling Promotes Proliferation of Pancreatic Cancer Cells and Tumour Growth in Mice, and its Inhibition Increases Effects of Gemcitabine. Oncogene, 2018. 37(49): p. 6368-6382.
- Li, Y. , et al., Apoptosis-Inducing Effect of Chemotherapeutic Agents is Potentiated by Soy Isoflavone Genistein, a Natural Inhibitor of NF-kappaB in BxPC-3 Pancreatic Cancer Cell Line. Pancreas, 2004. 28(4): p. e90-e95.
- Bhaumik, S.K. , et al., Pre-Existing Dengue Immunity Drives a DENV-Biased Plasmablast Response in ZIKV-Infected Patient. Viruses, 2018. 11(1).
- Zhang, T. , et al., Wogonin Increases Gemcitabine Sensitivity in Pancreatic Cancer by Inhibiting Akt Pathway. Front Pharmacol, 2022. 13: p. 1068855.
- Jia, S. , et al., Fisetin Induces Autophagy in Pancreatic Cancer Cells via Endoplasmic Reticulum Stress- and Mitochondrial Stress-Dependent Pathways. Cell Death Dis, 2019. 10(2): p. 142.
- Motallebi, M. , et al., Naringenin: A Potential Flavonoid Phytochemical for Cancer Therapy. Life Sci, 2022. 305: p. 120752.
- Xu, M. , et al., Tiliroside Disrupted Iron Homeostasis and Induced Ferroptosis via Directly Targeting Calpain-2 in Pancreatic Cancer Cells. Phytomedicine, 2024. 127: p. 155392.
- Zaremba-Czogalla, M. , et al., Evaluation of the In Vitro Cytotoxic Activity of Caffeic Acid Derivatives and Liposomal Formulation against Pancreatic Cancer Cell Lines. Materials (Basel), 2020. 13(24).
- Duan, J. , et al., Direct Interaction Between Caffeic Acid Phenethyl Ester and Human Neutrophil Elastase Inhibits the Growth and Migration of PANC-1 Cells. Oncol Rep, 2017. 37(5): p. 3019-3025.
- Chen, X. , et al., Chlorogenic Acid Inhibits Proliferation, Migration and Invasion of Pancreatic Cancer Cells via AKT/GSK-3beta/beta-catenin Signaling Pathway. Recent Pat Anticancer Drug Discov, 2024. 19(2): p. 146-153.
- Gupta, S. , et al., Caffeic Acid, a Dietary Polyphenol, Pre-Sensitizes Pancreatic Ductal Adenocarcinoma to Chemotherapeutic Drug. J Biomol Struct Dyn, 2024: p. 1-15.
- Torres, S.M. , et al., Gallic Acid Markedly Stimulates GLUT1-Mediated Glucose Uptake by the AsPC-1 Pancreatic Cancer Cell Line. Can J Physiol Pharmacol, 2023. 101(2): p. 90-105.
- Brecht, K. , et al., Mechanistic Insights Into Selective Killing of OXPHOS-Dependent Cancer Cells by Arctigenin. Toxicol In Vitro, 2017. 40: p. 55-65.
- Sung, Y.Y., A. Y. Lee, and H.K. Kim, Forsythia suspensa Fruit Extracts and the Constituent Matairesinol Confer Anti-Allergic Effects in an Allergic Dermatitis Mouse Model. J Ethnopharmacol, 2016. 187: p. 49-56.
- Bel Mabrouk, S. , et al., The Marine Seagrass Halophila stipulacea as a Source of Bioactive Metabolites against Obesity and Biofouling. Mar Drugs, 2020. 18(2).
- Lee, W., G. Song, and H. Bae, Matairesinol Induces Mitochondrial Dysfunction and Exerts Synergistic Anticancer Effects with 5-Fluorouracil in Pancreatic Cancer Cells. Mar Drugs, 2022. 20(8).
- Arora, S. , et al., Honokiol: a Novel Natural Agent for Cancer Prevention and Therapy. Curr Mol Med, 2012. 12(10): p. 1244-1252.
- Arora, S. , et al., Honokiol Arrests Cell Cycle, Induces Apoptosis, and Potentiates the Cytotoxic Effect of Gemcitabine in Human Pancreatic Cancer Cells. PLoS One, 2011. 6(6): p. e21573.
- Averett, C. , et al., Honokiol Suppresses Pancreatic Tumor Growth, Metastasis and Desmoplasia by Interfering With Tumor-Stromal Cross-Talk. Carcinogenesis, 2016. 37(11): p. 1052-1061.
- Son, M.K. , et al., SB365, Pulsatilla saponin D Suppresses Proliferation and Induces Apoptosis of Pancreatic Cancer Cells. Oncol Rep, 2013. 30(2): p. 801-808.
- Ma, Y. , et al., Advancements and Challenges in Pharmacokinetic and Pharmacodynamic Research on the Traditional Chinese Medicine Saponins: A Comprehensive Review. Front Pharmacol, 2024. 15: p. 1393409.
- Liu, C. , et al., A Natural Food Sweetener With Anti-Pancreatic Cancer Properties. Oncogenesis, 2016. 5(4): p. e217.
- Yao, L.C. , et al., Panax notoginseng Saponins Promote Cell Death and Chemosensitivity in Pancreatic Cancer Through the Apoptosis and Autophagy Pathways. Anticancer Agents Med Chem, 2021. 21(13): p. 1680-1688.
- Tezuka, H. and S. Imai, Fine-Tuning of Mononuclear Phagocytes for Improved Inflammatory Responses: Role of Soybean-Derived Immunomodulatory Compounds. Front Nutr, 2024. 11: p. 1399687.
- Shen, L. , et al., Potential Immunoregulatory Mechanism of Plant Saponins: A Review. Molecules, 2023. 29(1).
- Timilsena, Y.P., A. Phosanam, and R. Stockmann, Perspectives on Saponins: Food Functionality and Applications. Int J Mol Sci, 2023. 24(17).
- MarElia, C.B. , et al., Anemarrhena asphodeloides Bunge and its Constituent Timosaponin-AIII Induce Cell Cycle Arrest and Apoptosis in Pancreatic Cancer Cells. FEBS Open Bio, 2018. 8(7): p. 1155-1166.
- Zou, J. , et al., Ginsenoside Rg3 Suppresses the Growth of Gemcitabine-Resistant Pancreatic Cancer Cells by Upregulating lncRNA-CASC2 and Activating PTEN Signaling. J Biochem Mol Toxicol, 2020. 34(6): p. e22480.
- Xu, X. , et al., Saikosaponin d Modulates the Polarization of Tumor-Associated Macrophages by Deactivating the PI3K/AKT/mTOR Pathway in Murine Models of Pancreatic Cancer. Int Immunopharmacol, 2023. 122: p. 110579.
- He, X. , et al., The Therapeutic Potential of Natural Products for Treating Pancreatic Cancer. Front Pharmacol, 2022. 13: p. 1051952.
- Liu, Y. , et al., Steroidal Saponins PPI/CCRIS/PSV Induce Cell Death in Pancreatic Cancer Cell Through GSDME-Dependent Pyroptosis. Biochem Biophys Res Commun, 2023. 673: p. 51-58.
- Zhong, Y. , et al., Ziyuglycoside II Inhibits the Growth of Digestive System Cancer Cells Through Multiple Mechanisms. Chin J Nat Med, 2021. 19(5): p. 351-363.
- Xiao, M.F. , [Effect of Polyphyllin D on Proliferation and Apoptosis of Human Pancreatic Cancer Cells]. Zhongguo Zhong Yao Za Zhi, 2020. 45(6): p. 1418-1422.
- Wang, Y.W. , et al., Escin Augments the Efficacy of Gemcitabine Through Down-Regulation of Nuclear Factor-kappaB and Nuclear Factor-kappaB-Regulated Gene Products in Pancreatic Cancer Both In Vitro and In Vivo. J Cancer Res Clin Oncol, 2012. 138(5): p. 785-797.
- Liu, Q. , et al., Pulsatilla saponin A, an Active Molecule From Pulsatilla chinensis, Induces Cancer Cell Death and Inhibits Tumor Growth in Mouse Xenograft Models. J Surg Res, 2014. 188(2): p. 387-395.
- Zhou, Z.G. , et al., Phenolic Alkaloids From Menispermum dauricum Inhibits BxPC-3 Pancreatic Cancer Cells by Blocking of Hedgehog Signaling Pathway. Pharmacogn Mag, 2015. 11(44): p. 690-697.
- Prabhu, K.S. , et al., Sanguinarine Mediated Apoptosis in Non-Small Cell Lung Cancer via Generation of Reactive Oxygen Species and Suppression of JAK/STAT Pathway. Biomed Pharmacother, 2021. 144: p. 112358.
- Jang, H.J. , et al., Chelidonine Induces Apoptosis via GADD45a-p53 Regulation in Human Pancreatic Cancer Cells. Integr Cancer Ther, 2021. 20: p. 15347354211006191.
- Zhao, J. , et al., Total Alkaloids of Rubus alceifolius Poir Inhibit Tumor Angiogenesis Through Suppression of the Notch Signaling Pathway in a Mouse Model of Hepatocellular Carcinoma. Mol Med Rep, 2015. 11(1): p. 357-361.
- Wang, H. , et al., Effects of Compound Kushen Injection on Pathology and Angiogenesis of Tumor Tissues. Oncol Lett, 2019. 17(2): p. 2278-2282.
- Qin, R. , et al., Naturally Derived Indole Alkaloids Targeting Regulated Cell Death (RCD) for Cancer Therapy: From Molecular Mechanisms to Potential Therapeutic Targets. J Hematol Oncol, 2022. 15(1): p. 133.
- Ishii, N. , et al., Conophylline Suppresses Pancreatic Cancer Desmoplasia and Cancer-Promoting Cytokines Produced by Cancer-Associated Fibroblasts. Cancer Sci, 2019. 110(1): p. 334-344.
- Wang, X. , et al., The Recent Developments of Camptothecin and its Derivatives as Potential Anti-Tumor Agents. Eur J Med Chem, 2023. 260: p. 115710.
- Wang, W. , et al., Structure-Activity Relationship of FL118 Platform Position 7 Versus Position 9-Derived Compounds and Their Mechanism of Action and Antitumor Activity. J Med Chem, 2023. 66(24): p. 16888-16916.
- Zhu, S.L. , et al., A Novel DDIT3 Activator Dehydroevodiamine Effectively Inhibits Tumor Growth and Tumor Cell Stemness in Pancreatic Cancer. Phytomedicine, 2024. 128: p. 155377.
- Awale, S. , et al., Ancistrolikokine E(3), a 5,8'-Coupled Naphthylisoquinoline Alkaloid, Eliminates the Tolerance of Cancer Cells to Nutrition Starvation by Inhibition of the Akt/mTOR/Autophagy Signaling Pathway. J Nat Prod, 2018. 81(10): p. 2282-2291.
- Mukherjee, D. , et al., Tomatidine Targets ATF4-Dependent Signaling and Induces Ferroptosis to Limit Pancreatic Cancer Progression. iScience, 2023. 26(8): p. 107408.
- Liu, A. , et al., Antiproliferative and Antimetastatic Effects of Emodin on Human Pancreatic Cancer. Oncol Rep, 2011. 26(1): p. 81-89.
- Awale, S. , et al., Targeting Pancreatic Cancer with Novel Plumbagin Derivatives: Design, Synthesis, Molecular Mechanism, In Vitro and In Vivo Evaluation. J Med Chem, 2023. 66(12): p. 8054-8065.
- Colucci, M.A., C. J. Moody, and G.D. Couch, Natural and Synthetic Quinones and Their Reduction by the Quinone Reductase Enzyme NQO1: From Synthetic Organic Chemistry to Compounds With Anticancer Potential. Org Biomol Chem, 2008. 6(4): p. 637-656.
- Zhou, H. , et al., Research Progress on the Synergistic Anti-Tumor Effect of Natural Anti-Tumor Components of Chinese Herbal Medicine Combined with Chemotherapy Drugs. Pharmaceuticals (Basel), 2023. 16(12).
- Zhao, Z. , et al., Advances in Research on the Relationship Between Thymoquinone and Pancreatic Cancer. Front Oncol, 2022. 12: p. 1092020.
- Lin, S.Z. , et al., Emodin Inhibits Angiogenesis in Pancreatic Cancer by Regulating the Transforming Growth Factor-Beta/Drosophila Mothers Against Decapentaplegic Pathway and Angiogenesis-Associated microRNAs. Mol Med Rep, 2015. 12(4): p. 5865-5871.
- Yamamoto, K. , et al., Autophagy Promotes Immune Evasion of Pancreatic Cancer by Degrading MHC-I. Nature, 2020. 581(7806): p. 100-105.
- Wei, W.T. , et al., Antitumor and Apoptosis-Promoting Properties of Emodin, an Anthraquinone Derivative From Rheum officinale Baill, Against Pancreatic Cancer in Mice via Inhibition of Akt Activation. Int J Oncol, 2011. 39(6): p. 1381-1390.
- Yagublu, V. , et al., Treatment of Experimental Pancreatic Cancer by Doxorubicin-, Mitoxantrone-, and Irinotecan-Drug Eluting Beads. Pancreatology, 2013. 13(1): p. 79-87.
- Virtanen, P. and K. Isotupa, Staining Properties of Alizarin Red S for Growing Bone In Vitro. Acta Anat (Basel), 1980. 108(2): p. 202-207.
- Huang, W. , et al., Dihydrotanshinone I Inhibits Pancreatic Cancer Progression via Hedgehog/Gli Signal Pathway. Curr Cancer Drug Targets, 2023. 23(9): p. 731-741.
- Enchev, P. , et al., Terpenes From Cecropia Species and Their Pharmacological Potential. Pharmaceuticals (Basel), 2024. 17(3).
- Wang, Y. , et al., The Multifaceted Mechanisms of Pristimerin in the Treatment of Tumors State-of-the-Art. Biomed Pharmacother, 2022. 154: p. 113575.
- Li, D. , et al., Analysis of Anti-Cancer and Anti-Inflammatory Properties of 25 High-THC Cannabis Extracts. Molecules, 2022. 27(18).
- Wroblewska-Luczka, P. , et al., Anticancer Effect of Terpenes: Focus on Malignant Melanoma. Pharmacol Rep, 2023. 75(5): p. 1115-1125.
- Amrutkar, M. and I.P. Gladhaug, Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers (Basel), 2017. 9(11).
- Markowski, A. , et al., Design and Development of a New Type of Hybrid PLGA/Lipid Nanoparticle as an Ursolic Acid Delivery System against Pancreatic Ductal Adenocarcinoma Cells. Int J Mol Sci, 2022. 23(10).
- Asgharian, P. , et al., Potential Mechanisms of Quercetin in Cancer Prevention: Focus on Cellular and Molecular Targets. Cancer Cell Int, 2022. 22(1): p. 257.
- Kui, L. , et al., High-Throughput In Vitro Gene Expression Profile to Screen of Natural Herbals for Breast Cancer Treatment. Front Oncol, 2021. 11: p. 684351.
- Li, R. , et al., Natural Products: A Promising Therapeutics for Targeting Tumor Angiogenesis. Front Oncol, 2021. 11: p. 772915.
- Singh, R.P. and R. Agarwal, Tumor Angiogenesis: A Potential Target in Cancer Control by Phytochemicals. Curr Cancer Drug Targets, 2003. 3(3): p. 205-217.
- Panda, S.K. , et al., Anticancer Activities of Mushrooms: A Neglected Source for Drug Discovery. Pharmaceuticals (Basel), 2022. 15(2).
- Wu, X. , et al., Nano-Herb Medicine and PDT Induced Synergistic Immunotherapy for Colon Cancer Treatment. Biomaterials, 2021. 269: p. 120654.
- Xu, L. , et al., Inhibition of NLRP3 Inflammasome Activation in Myeloid-Derived Suppressor Cells by Andrographolide Sulfonate Contributes to 5-FU Sensitization in Mice. Toxicol Appl Pharmacol, 2021. 428: p. 115672.
- Laurella, L.C. , et al., Sesquiterpene Lactones as Promising Candidates for Cancer Therapy: Focus on Pancreatic Cancer. Molecules, 2022. 27(11).
- Wang, Y., P. Camateros, and W.Y. Cheung, A Real-World Comparison of FOLFIRINOX, Gemcitabine Plus nab-Paclitaxel, and Gemcitabine in Advanced Pancreatic Cancers. J Gastrointest Cancer, 2019. 50(1): p. 62-68.
- Long, J., Z. Liu, and L. Hui, Anti-Tumor Effect and Mechanistic Study of Elemene on Pancreatic Carcinoma. BMC Complement Altern Med, 2019. 19(1): p. 133.
- Geng, Y. , et al., Xanthatin Suppresses Pancreatic Cancer Cell Growth via the ROS/RBL1 Signaling Pathway: In Vitro and In Vivo Insights. Phytomedicine, 2023. 119: p. 155004.
- Wang, H. , et al., Umbelliprenin Induces Autophagy and Apoptosis While Inhibits Cancer Cell Stemness in Pancreatic Cancer Cells. Cancer Med, 2023. 12(14): p. 15277-15288.
- Ma, L.M. , et al., Terpenoids From Nardostachys jatamansi and Their Cytotoxic Activity Against Human Pancreatic Cancer Cell Lines. Phytochemistry, 2022. 200: p. 113228.
- Wu, J.Y., K. C. Siu, and P. Geng, Bioactive Ingredients and Medicinal Values of Grifola frondosa (Maitake). Foods, 2021. 10(1).
- Liu, Y., J. Wu, and H. Hao, Antitumor Immunostimulatory Activity of the Traditional Chinese Medicine Polysaccharide on Hepatocellular Carcinoma. Front Immunol, 2024. 15: p. 1369110.
- Murakami, T. , et al., Role of the Tumor Microenvironment in Pancreatic Cancer. Ann Gastroenterol Surg, 2019. 3(2): p. 130-137.
- Ji, C.F., Y. B. Ji, and D.Y. Meng, Sulfated Modification and Anti-tumor Activity of Laminarin. Exp Ther Med, 2013. 6(5): p. 1259-1264.
- Liu, D., H. Zhu, and C. Li, Galectins and Galectin-Mediated Autophagy Regulation: New Insights Into Targeted Cancer Therapy. Biomark Res, 2023. 11(1): p. 22.
- Yuan, P. , et al., Structure and Anti-Tumor Activities of Exopolysaccharides from Alternaria mali Roberts. Molecules, 2019. 24(7).
- Bian, Y. , et al., A Pectin-Like Polysaccharide From Polygala tenuifolia Inhibits Pancreatic Cancer Cell Growth In Vitro and In Vivo by Inducing Apoptosis and Suppressing Autophagy. Int J Biol Macromol, 2020. 162: p. 107-115.
- Rui, X. , et al., Anti-Tumor and Anti-Angiogenic Effects of Fucoidan on Prostate Cancer: Possible JAK-STAT3 Pathway. BMC Complement Altern Med, 2017. 17(1): p. 378.
- Zong, S. , et al., Synergistic Antitumor Effect of Polysaccharide From Lachnum sp. in Combination With Cyclophosphamide in Hepatocellular Carcinoma. Carbohydr Polym, 2018. 196: p. 33-46.
- Liu, X. , et al., CRIP1 Fosters MDSC Trafficking and Rsets Tumour Microenvironment via Facilitating NF-kappaB/p65 Nuclear Translocation in Pancreatic Ductal Adenocarcinoma. Gut, 2023. 72(12): p. 2329-2343.
- Tao, H. , et al., Corn Silk Crude Polysaccharide Exerts Anti-Pancreatic Cancer Activity by Blocking the EGFR/PI3K/AKT/CREB Signaling Pathway. Food Funct, 2020. 11(8): p. 6961-6970.
- Ding, M. , et al., Structural Characterization of the Polysaccharide From the Black Crystal Region of Inonotus obliquus and its Effect on AsPC-1 and SW1990 Pancreatic Cancer Cell Apoptosis. Int J Biol Macromol, 2024. 268(Pt 2): p. 131891.
- Zhang, L. , et al., RN1, a Novel Galectin-3 Inhibitor, Inhibits Pancreatic Cancer Cell Growth In Vitro and In Vivo via Blocking Galectin-3 Associated Signaling Pathways. Oncogene, 2017. 36(9): p. 1297-1308.
- Wu, P.P. , et al., Triptolide Induces Apoptosis in Human Adrenal Cancer NCI-H295 Cells Through a Mitochondrial-Dependent Pathway. Oncol Rep, 2011. 25(2): p. 551-557.
- Clawson, K.A. , et al., Triptolide and TRAIL Combination Enhances Apoptosis in Cholangiocarcinoma. J Surg Res, 2010. 163(2): p. 244-249.
- Giri, B. , et al., Pre-Clinical Evaluation of Minnelide as a Therapy for Acute Myeloid Leukemia. J Transl Med, 2019. 17(1): p. 163.
- Borja-Cacho, D. , et al., TRAIL and Triptolide: An Effective Combination That Iduces Apoptosis in Pancreatic Cancer Cells. J Gastrointest Surg, 2010. 14(2): p. 252-60.
- Chugh, R. , et al., A Preclinical Evaluation of Minnelide as a Therapeutic Agent Against Pancreatic Cancer. Sci Transl Med, 2012. 4(156): p. 156ra139.
- Zhao, X. , et al., Triptolide Inhibits Pancreatic Cancer Cell Proliferation and Migration via Down-Regulating PLAU Based on Network Pharmacology of Tripterygium wilfordii Hook F. Eur J Pharmacol, 2020. 880: p. 173225.
- Noel, P. , et al., Triptolide Targets Super-Enhancer Networks in Pancreatic Cancer Cells and Cancer-Associated Fibroblasts. Oncogenesis, 2020. 9(11): p. 100.
- Hu, J. , et al., Clinical Efficacy and Safety of Traditional Medicine Preparations Combined With Chemotherapy for Advanced Pancreatic Cancer: A Systematic Review and Meta-Analysis. Front Oncol, 2022. 12: p. 828450.
- Kuo, Y.T. , et al., Complementary Chinese Herbal Medicine Therapy Improves Survival of Patients With Pancreatic Cancer in Taiwan: A Nationwide Population-Based Cohort Study. Integr Cancer Ther, 2018. 17(2): p. 411-422.
- Hashem, S. , et al., Targeting Cancer Signaling Pathways by Natural Products: Exploring Promising Anti-Cancer Agents. Biomed Pharmacother, 2022. 150: p. 113054.
- Xu, Z. , et al., Alizarin, a Nature Compound, Inhibits the Growth of Pancreatic Cancer Cells by Abrogating NF-kappaB Activation. Int J Biol Sci, 2022. 18(7): p. 2759-2774.
- Suhail, M. , et al., Targeting a Transcription Factor NF-kappaB by Green Tea Catechins Using In Silico and In Vitro Studies in Pancreatic Cancer. Front Nutr, 2022. 9: p. 1078642.
- Kingston, D.G. , A Natural Love of Natural Products. J Org Chem, 2008. 73(11): p. 3975-3984.
- Dai, H. , et al., PUM1 Knockdown Prevents Tumor Progression by Activating the PERK/eIF2/ATF4 Signaling Pathway in Pancreatic Adenocarcinoma Cells. Cell Death Dis, 2019. 10(8): p. 595.
- Fathi, F. , et al., Exploring Gunnera tinctoria: From Nutritional and Anti-Tumoral Properties to Phytosome Development Following Structural Arrangement Based on Molecular Docking. Molecules, 2021. 26(19).
- Alhakamy, N.A. , et al., Apamin-Conjugated Alendronate Sodium Nanocomplex for Management of Pancreatic Cancer. Pharmaceuticals (Basel), 2021. 14(8).
- Duan, H., L. Li, and S. He, Advances and Prospects in the Treatment of Pancreatic Cancer. Int J Nanomedicine, 2023. 18: p. 3973-3988.
- Najm, A. , et al., Chitosan and Cyclodextrins-Versatile Materials Used to Create Drug Delivery Systems for Gastrointestinal Cancers. Pharmaceutics, 2023. 16(1).
- Shakeel, F. , et al., Hepatoprotective Effects of Bioflavonoid Luteolin Using Self-Nanoemulsifying Drug Delivery System. Molecules, 2021. 26(24).
- Dasari, N., G. S. Guntuku, and S. Pindiprolu, Targeting Triple Negative Breast Cancer Stem Cells Using Nanocarriers. Discov Nano, 2024. 19(1): p. 41.
- Wei, Y. , et al., The Emergence of TRP Channels Interactome as a Potential Therapeutic Target in Pancreatic Ductal Adenocarcinoma. Biomedicines, 2023. 11(4).
- Han, X. , et al., Overexpression of miR-135b-5p Promotes Unfavorable Clinical Characteristics and Poor Prognosis via the Repression of SFRP4 in Pancreatic Cancer. Oncotarget, 2017. 8(37): p. 62195-62207.
- Hu, Y. , et al., The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules, 2020. 10(2).
- Xia, Y. , et al., Drug Repurposing for Cancer Therapy. Signal Transduct Target Ther, 2024. 9(1): p. 92.
- Liu, T. , et al., Management of Advanced Pancreatic Cancer through Stromal Depletion and Immune Modulation. Medicina (Kaunas), 2022. 58(9).
- Oloulade, B.M. , et al., Cancer Drug Response Prediction With Surrogate Modeling-Based Graph Neural Architecture Search. Bioinformatics, 2023. 39(8).
- van der Wijst, M.G.P. , et al., An Integrative Approach for Building Personalized Gene Regulatory Networks for Precision Medicine. Genome Med, 2018. 10(1): p. 96.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



