Submitted:
29 July 2024
Posted:
31 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Dietary Components
2.2. Animals
2.3. Animal Monitoring and Sample Collection
2.4. Measurements of Circulatory Glucose, Triglycerides and Cholesterol
2.5. Measurements of Plasma Insulin and Pro-Inflammatory Cytokines
2.6. Monocyte Adhesion Assay
2.7. Fecal Bacteria DNA Extraction and 16S rRNA Gene Sequencing
2.8. Bioinformatics Analyses of Gut Microbiota
2.9. Analysis of Fecal SCFAs
2.10. Extraction and Metabolomics Sample Analysis of WR
2.11. Metabolomics Data Acquisition
2.12. Statistics
3. Results
3.1. Metabolites in WLD versus WHR
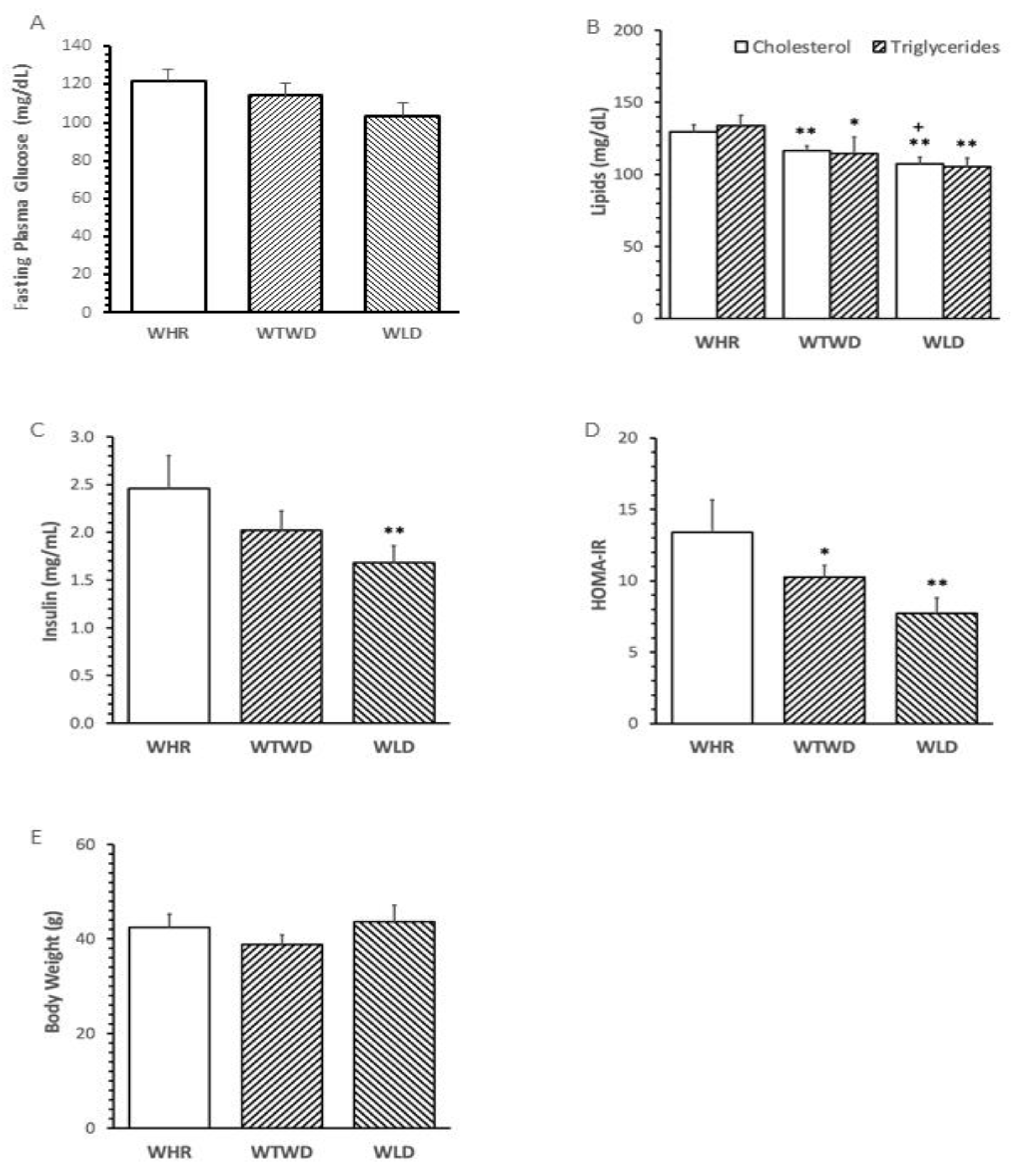
3.2. Effects of WLD on Glucose, Lipids and Insulin Resistance in HF Diet-Fed Mice
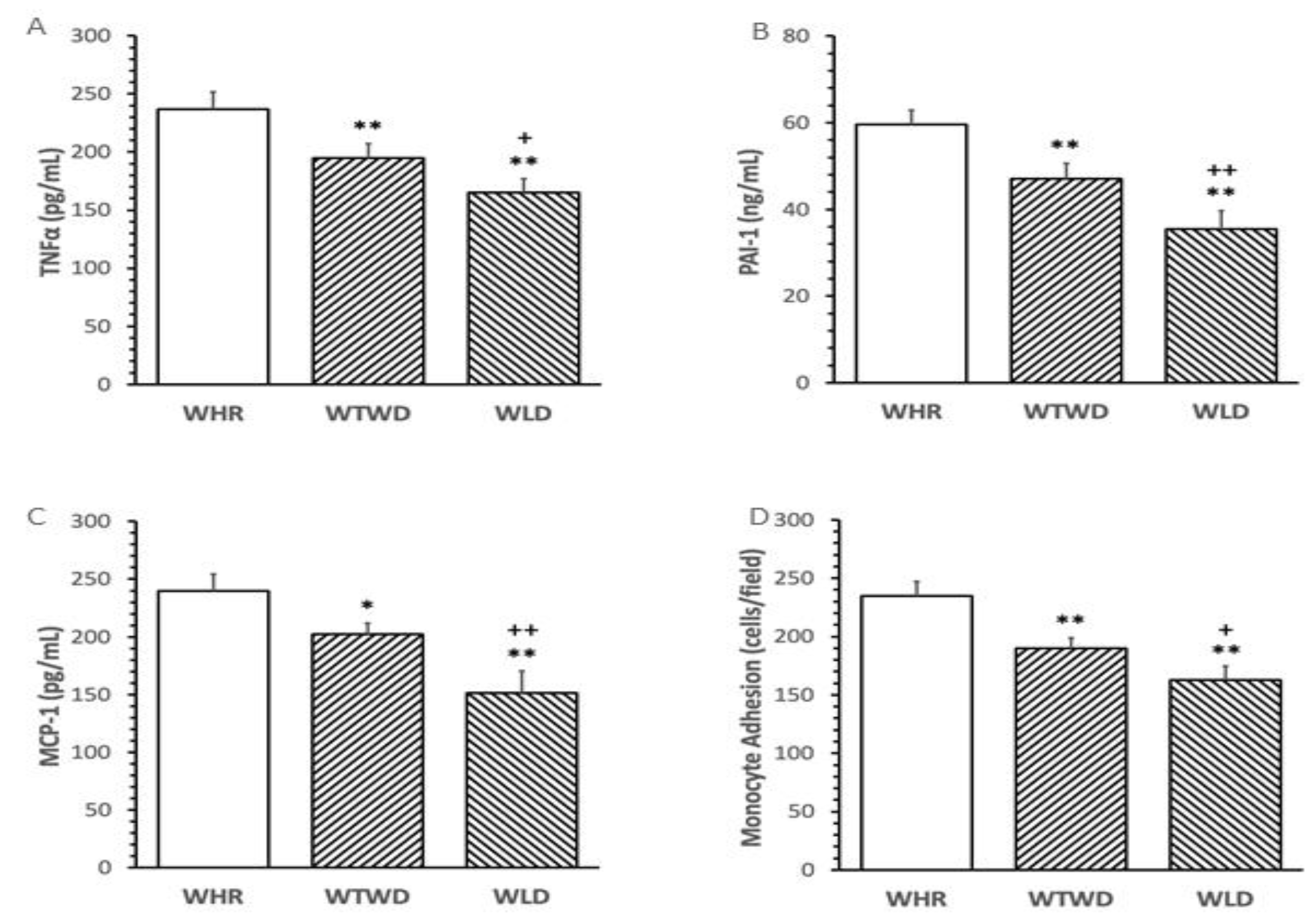
3.3. Effects of WLD on Circulatory Inflammatory Cytokines and Monocyte Adhesion
3.4. Influence of WLD on Diversities of Gut Microbiota
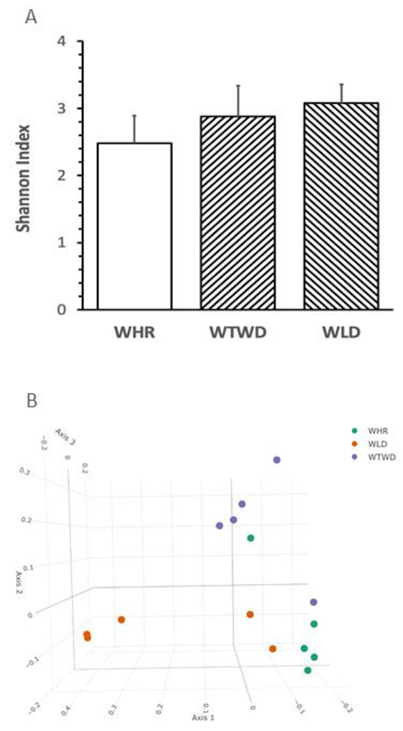
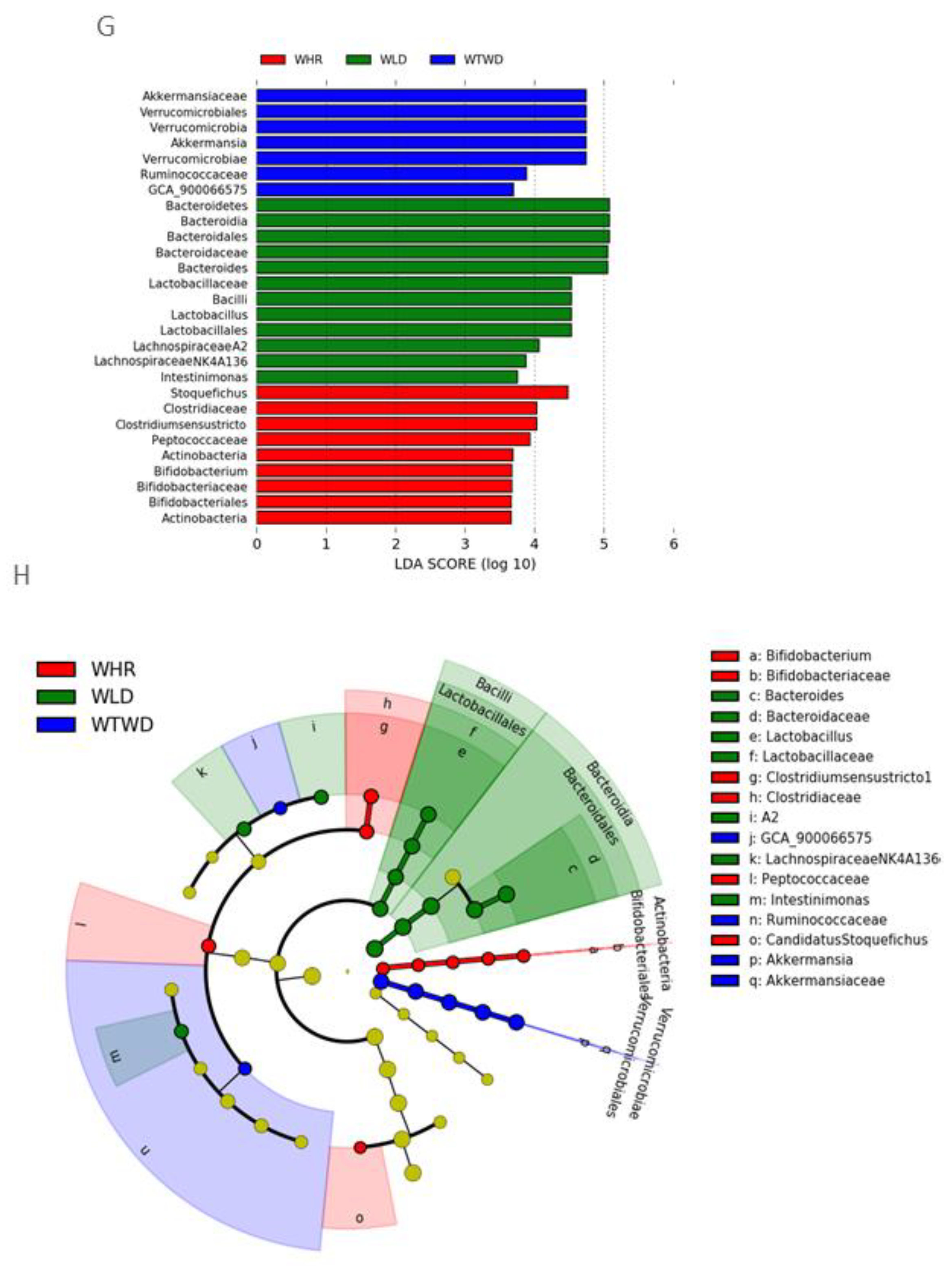
3.5. Influence of WLD on Taxonomic Composition of Gut Microbiota

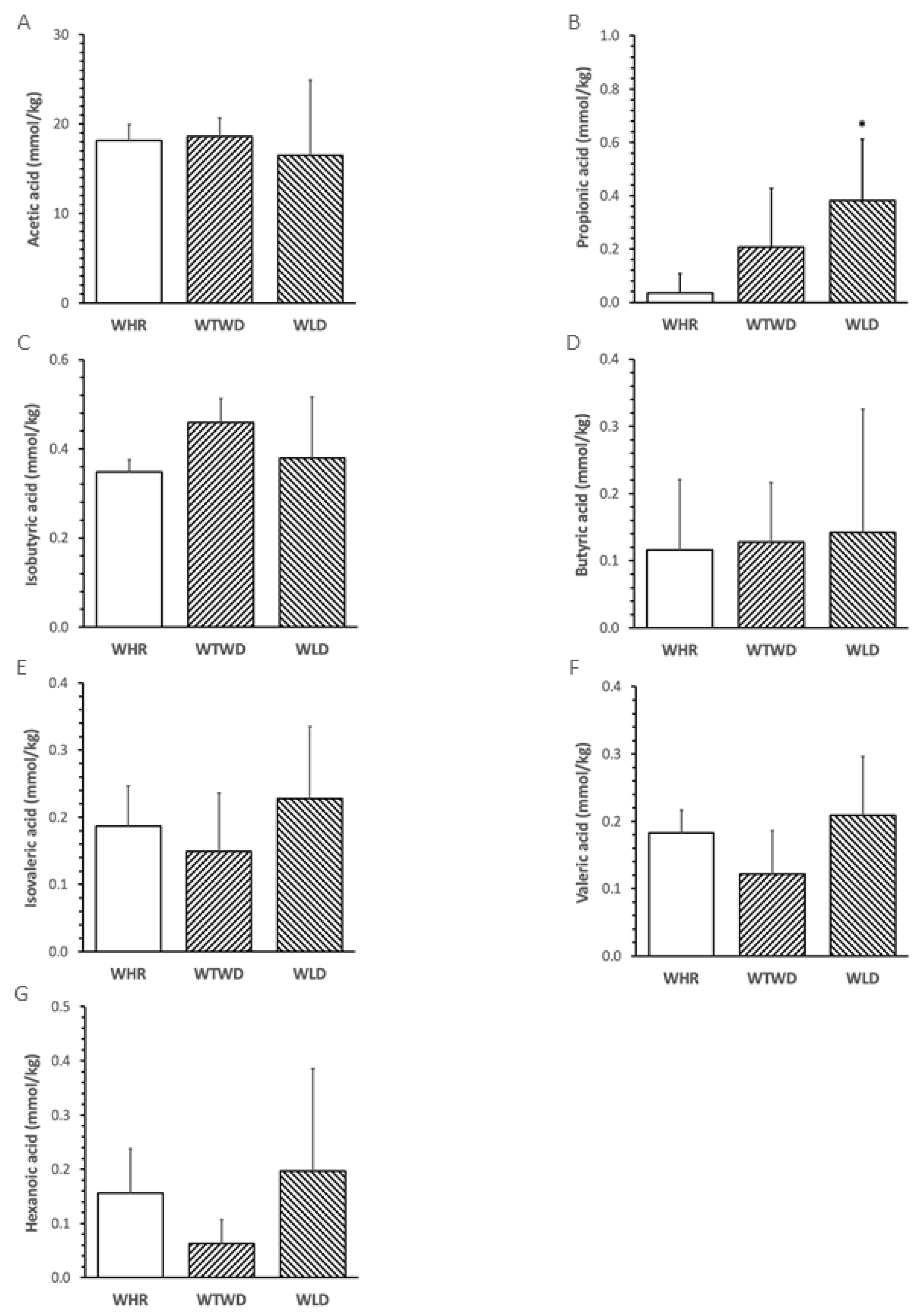
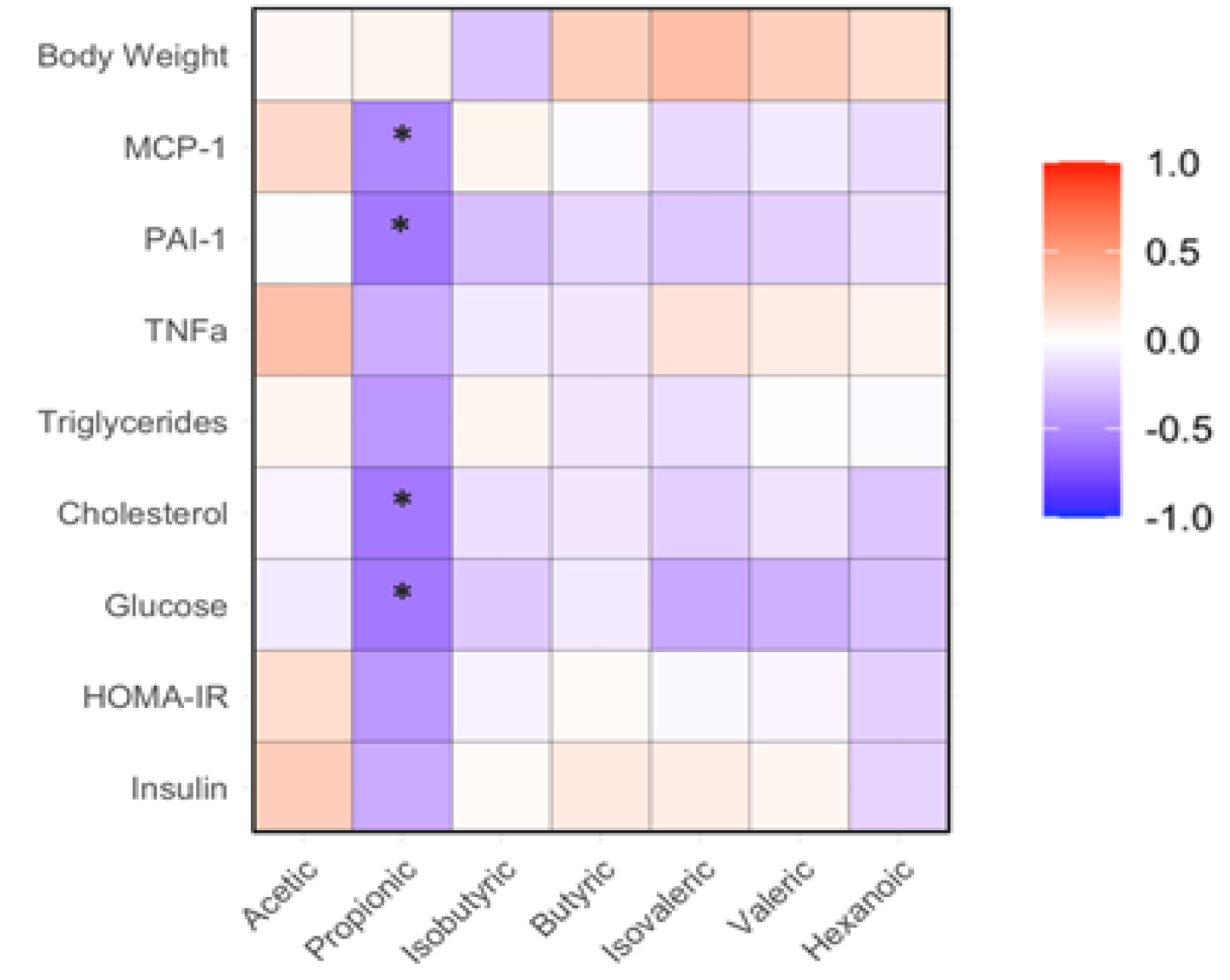
3.6. Impact of WLD on Fecal SCFAs and Correlation with Metabolic or Inflammatory Variables
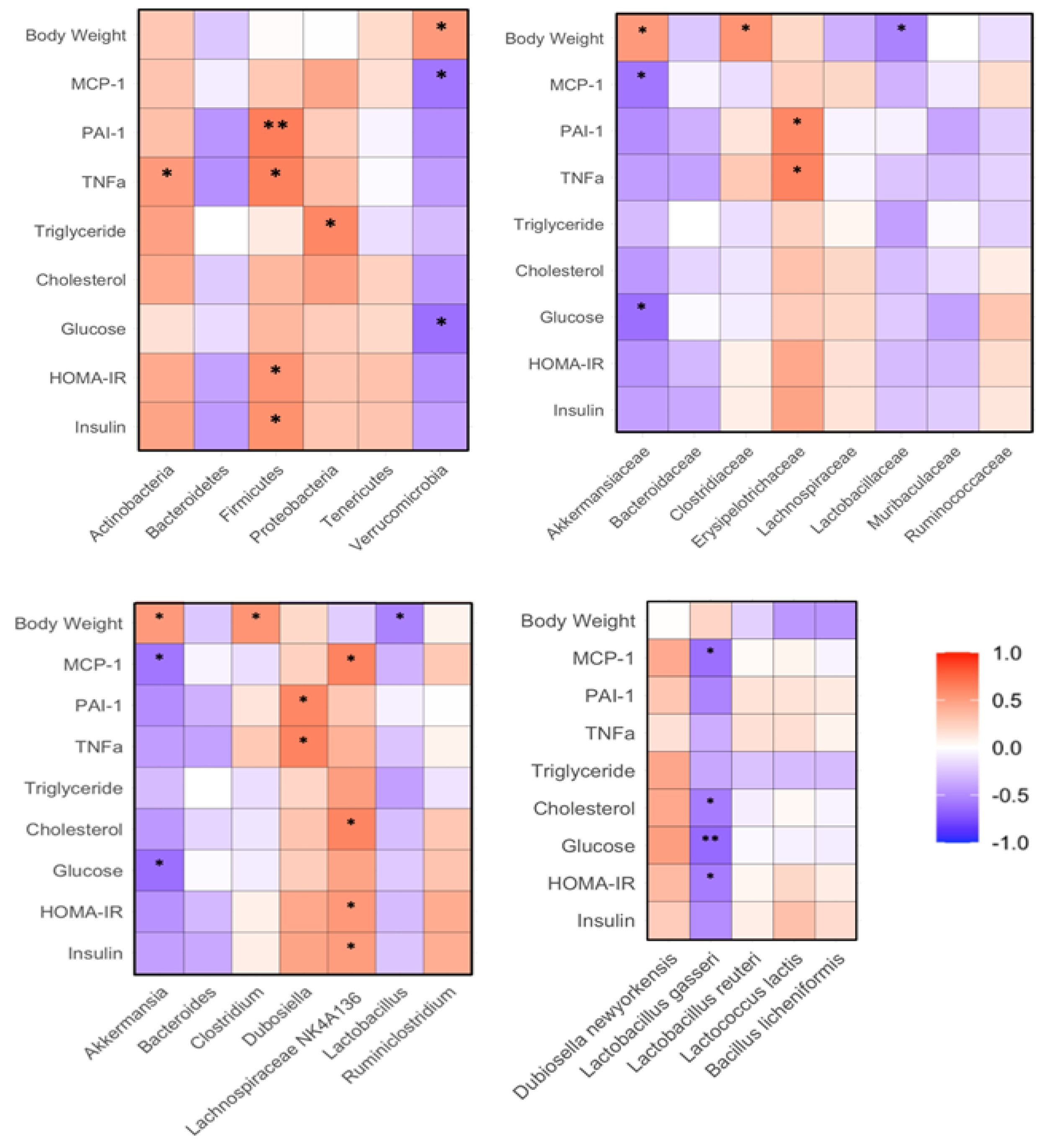
3.7. Correlation between SCFAs and Gut Microbiota
3.8. Correlation between Fecal and Body Weights, Metabolic or Inflammatory Variables
4. Discussion
Conflicts of Interests
Acknowledgments
Abbreviations Used
References
- Stack Whitney, K. Manoomin: The Taming of Wild Rice in the Great Lakes Region. Environment & Society Portal, Arcadia, no.2. Rachel Carson Center for Environment and Society. [CrossRef]
- Kellogg, E.A. The Evolutionary History of Ehrhartoideae, Oryzeae, and Oryza. Rice 2009, 2, 1–14. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Wild rice, cooked. https://fdc.nal.usda.gov/fdc-app.html#/food-details/168897/nutrients. Accessed May 10, 2024.
- Surendiran, G. , Goh, C., Le, K., Zhao, Z., Askarian, F., Othman, R., Nicholson, T., Moghadasian, P., Wang, Y.J., Aliani. M, Shen, G.X., Beta, T., Moghadasian, M.H. Wild rice (Zizania palustris L.) prevents atherogenesis in LDL receptor knockout mice. Atherosclerosis 2013, 230, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Moghadasian, M.H. , Alsaif, M., Le, K., Gangadaran, S., Masisi, K., Beta, T., Shen, G.X. (2016). Combination effects of wild rice and phytosterols on prevention of atherosclerosis in LDL receptor knockout mice. J Nutr Bioch 2016, 33, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Moghadasian, M.H. , Zhao, R., Ghazawwi, N., Le, K., Apea-Bah, F.B., Beta, T., Shen, G.X. Inhibitory Effects of North American Wild Rice on Monocyte Adhesion and Inflammatory Modulators in Low-Density Lipoprotein Receptor-Knockout Mice. J Agric Food Chem 2017, 65, 9054–9060. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.A. , Pan, A., Malik, V., Sun, Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. Br Med J 2012, 344, e1454. [Google Scholar] [CrossRef] [PubMed]
- Jannasch, F. , Kröger, J., Schulze, M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J Nutr 2017, 147, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E. , Herrero, L., Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Zhao, R. , Wan, P., Shariati-Ievari, S., Michel, A., Shen, G.X. North American Wild Rice Attenuated Hyperglycemia in High Fat-Induced Obese Mice: Involvement of AMP-Activated Protein Kinase. J Agri Food Chem 2020, 68, 8855–8862. [Google Scholar] [CrossRef]
- Netto Candido, T.L. , Bressan, J., Alfenas, R.C.G. Dysbiosis and metabolic endotoxemia induced by high-fat diet. Nutr Hosp 2018, 35, 1432–1440. [Google Scholar]
- Turnbaugh, P.J. , Backhed, F., Fulton, L., Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, N. , Gasbarrini, A., Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int J Environ Res Public Health 2021, 18, 12836. [Google Scholar] [CrossRef] [PubMed]
- He, J. , Zhang, P., Shen, L., Niu, L., Tan, Y., Chen, L., Zhao, Y., Bai, L., Hao, X., Li, X., Zhang, S., Zhu, L. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int J Mol S 21, 6356. [CrossRef] [PubMed]
- Zhao, R. , Ren, S., Moghadasain, M.H., Rempel, J.D., Shen, G.X. Involvement of Fibrinolytic Regulators in Adhesion of Monocytes to Vascular Endothelial Cells Induced by Glycated LDL and to Aorta from Diabetic Mice. J Leukocy Biol 2014, 95, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R. , Le, K., Li, W., Ren, S., Moghadasian, M.H., Beta, T., Shen, G.X. Effects of Saskatoon Berry Powder on Monocyte Adhesion to Vascular Wall of Leptin Receptor-Deficient Diabetic Mice. J Nutr Biochem 2014, 25, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Huang, F. , Zhao, R., Xia, M., Shen, G.X. Impact of Cyanidin-3-Glucoside on Gut Microbiota and Relationship with Metabolism and Inflammation in High Fat-High Sucrose Diet-Induced Insulin Resistant Mice. Microorganisms 2020, 8, 1238. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X. , Qiu, Y., Zhong, W., Baxter, S., Su, M., Li, Q., Xie, G., Ore, B.M., Qiao, S., Spencer, M.D., Zeisel, S.H., Zhou, Z., Zhao, A., Jia, W.A. A targeted metabolomic protocol for short-chain fatty acids and branched-chain amino acids. Metabolomics 2013, 9, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Moghadasian, M.H. , Kaur, R., Kostal, K., Joshi, A.A., Molaei, M., Le, K., Fischer, G., Bonomini, F., Favero, G., Rezzani, R., Gregorchuk, B.S.J., Leung-Shing, V., Wuzinski, M., Seo, A.I., Bay, D.C. Anti-Atherosclerotic Properties of Wild Rice in Low-Density Lipoprotein Receptor Knockout Mice: The Gut Microbiome, Cytokines, and Metabolomics Study. Nutrients 2019, 11, 2894. [Google Scholar] [PubMed]
- Hou, X.D. , Yan, N., Du, Y.M., Liang, H., Zhang, Z.F., Yuan, X.L. Consumption of Wild Rice (Zizania latifolia) Prevents Metabolic Associated Fatty Liver Disease through the Modulation of the Gut Microbiota in Mice Model. Int J Mol Sci 2020, 21, 5375. [Google Scholar] [CrossRef]
- Łagowska, K. , Malinowska, A.M., Zawieja, B., Zawieja, E. Improvement of glucose metabolism in pregnant women through probiotic supplementation depends on gestational diabetes status: meta-analysis. Sci Rep 2020, 10, 17796. [Google Scholar] [CrossRef]
- Álvarez-Arraño, V. , Martín-Peláez, S. Effects of Probiotics and Synbiotics on Weight Loss in Subjects with Overweight or Obesity: A Systematic Review. Nutrients 2021, 13, 3627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L. , Zhang, F., Ding, X., Wu, G., Lam, Y.Y., Wang, X., Fu, H., Xue, X., Lu, C., Ma, J., Yu, L., Xu, C., Ren, Z., Xu, Y., Xu, S., Shen, H., Zhu X, Shi, Y., Shen, Q., Dong, W., Liu, R., Ling, Y., Zeng, Y., Wang, X., Zhang, Q., Wang, J., Wang, L., Wu, Y., Zeng, B., Wei, H., Zhang, M., Peng, Y., Zhang, C. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [PubMed]
- Fu, J. , Xu, K., Ni, X., Li, X., Zhu, X., Xu, W. Habitual Dietary Fiber Intake, Fecal Microbiota, and Hemoglobin A1c Level in Chinese Patients with Type 2 Diabetes. Nutrients 2022, 14, 1003. [Google Scholar] [CrossRef]
- Lazyplant. How much fiber in brown, white & wild rice? https://lazyplant.com/fiber-rice/. Accessed May 3, 2024.
- Dr. Axe. Is wild rice is the best rice of all? Learn how to cook. https://draxe.com/nutrition/wild-rice/. Accessed May 3, 2024.
- Mohseni, R. , Teimouri, M., Safaei, M., Arab Sadeghabadi Z. AMP-activated protein kinase is a key regulator of obesity-associated factors. Cell Biochem Funct 2023, 41, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, T. , Song, X., An, Y., Wu, X., Zhang, W., Li, J., Sun, Y., Jin, G., Liu, X., Guo, Z., Wang, B., Lei, P., Cao, H. Lactobacillus rhamnosus GG Colonization in Early Life Ameliorates Inflammaging of Offspring by Activating SIRT1/AMPK/PGC-1α Pathway. Oxid Med Cell Longev 2021, 2021, 3328505. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H. , Ishii, M., Akagawa, M. Propionate suppresses hepatic gluconeogenesis via GPR43/AMPK signaling pathway. Arch Biochem Biophys 2019, 672, 108057. [Google Scholar] [CrossRef] [PubMed]
- Heimann, E. , Nyman, M., Degerman, E. Propionic acid and butyric acid inhibit lipolysis and de novo lipogenesis and increase insulin-stimulated glucose uptake in primary rat adipocytes. Adipocyte 2014, 4, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Al-Lahham, S. , Roelofsen, H., Rezaee, F., Weening, D., Hoek, A., Vonk, R., Venema, K. Propionic acid affects immune status and metabolism in adipose tissue from overweight subjects. Eur J Clin Invest 2012, 42, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T. , Di, K. M., Filip Vlavcheski, Quadrilatero, J., Evangelia Litsa Tsiani, Mourtzakis, M. Glutamate increases glucose uptake in L6 myotubes in a concentration- and time-dependent manner that is mediated by AMPK. Applied Physiology, Nutrition and Metabolism/Applied Physiology, Nutrition, and Metabolism 2018, 43, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Sadanandan Jayanarayanan, Anju, T. R., Soman Smijin, Cheramadathikudiyil Skaria Paulose. Vitamin D3 supplementation increases insulin level by regulating altered IP3 and AMPA receptor expression in the pancreatic islets of streptozotocin-induced diabetic rat. ˜the œJournal of Nutritional Biochemistry 2015, 26, 1041–1049.
- Fan, S. , De Feng Li, Da Cheng Wang, Fleming, J., Zhang, H., Zhou, Y., Zhou, L., Zhou, J., Chen, T., Chen, G., Xian En Zhang, Bi, L. Structure and function of Mycobacterium smegmatis 7-keto-8-aminopelargonic acid (KAPA) synthase. Int J Biochem Cell Biol 2015, 58, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Romero-Navarro, G. , Cabrera-Valladares, G., German, M. S., Matschinsky, F. M., Velazquez, A., Wang, J., & Fernandez-Mejia, C. Biotin Regulation of Pancreatic Glucokinase and Insulin in Primary Cultured Rat Islets and in Biotin- Deficient Rats1. Endocrinology 2019, 140, 4595–4600. [Google Scholar]
- Henningsson, R. , Lundquist, I. Arginine-induced insulin release is decreased and glucagon increased in parallel with islet NO production. Am Physiol-Endocr aMetabolism 1998, 275, E500–E506. [Google Scholar]
- Pilz, S. , Meinitzer, A., Gaksch, M., Grübler, M., Verheyen, N., Drechsler, C., Hartaigh, B. ó, Lang, F., Alesutan, I., Voelkl, J., März, W., Tomaschitz, A. Homoarginine in the renal and cardiovascular systems. Amino Acids 2015, 47, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Katz, A. , Bogardus, C. Relationship between carbohydrate oxidation and G-1,6-P2 in human skeletal muscle during euglycemic hyperinsulinemia. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology/American Journal of Physiology. Regulatory, Integrative, and Comparative Physiology 1991, 260, R113–R119. [Google Scholar] [CrossRef] [PubMed]
- Katz, A. , Bogardus, C. Insulin-mediated increase in glucose 1,6-bisphosphate is attenuated in skeletal muscle of insulin-resistant man. Metabolism, 1990, 39, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Maessen, D. E. , Brouwers, O., Gaens, K. H., Wouters, K., Cleutjens, J. P., Janssen, B. J., Miyata, T., Stehouwer, C. D., Schalkwijk, C. G. Delayed Intervention With Pyridoxamine Improves Metabolic Function and Prevents Adipose Tissue Inflammation and Insulin Resistance in High-Fat Diet–Induced Obese Mice. Diabetes 2015, 65, 956–966. [Google Scholar] [PubMed]
- Dakshinamurti, K. Vitamins and their derivatives in the prevention and treatment of metabolic syndrome diseases (diabetes). Can J Physiol and Pharmac. 2015, 93, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Salib, J. , Michael, H., Eskande, E. Anti-diabetic properties of flavonoid compounds isolated from Hyphaene thebaica epicarp on alloxan induced diabetic rats. Pharmacognosy Research 2013, 5, 22. [Google Scholar] [CrossRef]
- Litwiniuk, M. , Krejner, A., Speyrer, M.S., Gauto, A.R., Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar] [PubMed]
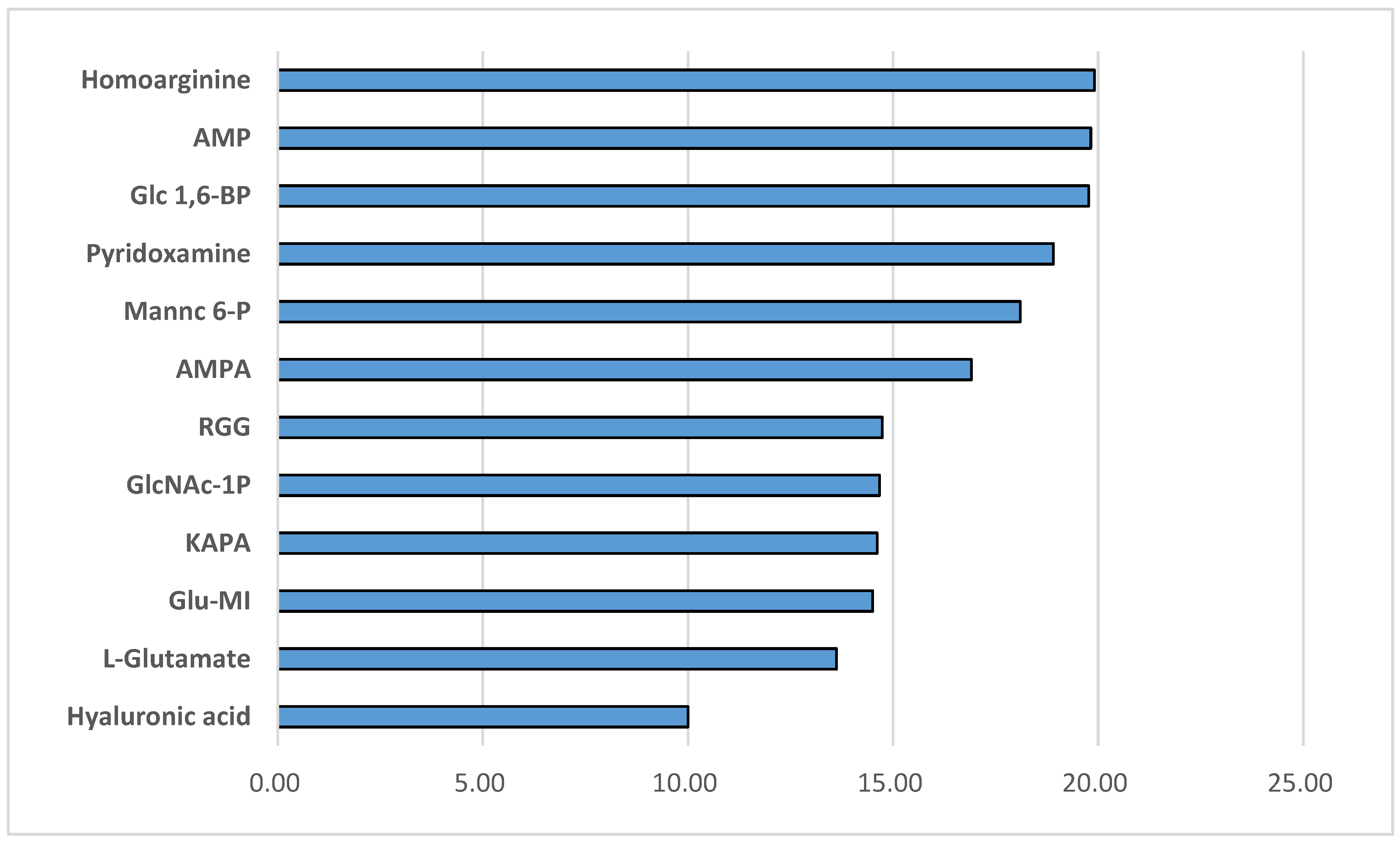

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




