Introduction
Dendritic spines are small, specialized membrane organelles that protrude from neuronal dendrites. They are found on the dendrites of mainly neurons in the brain and receive input usually from the excitatory synapses. Spines contain neurotransmitter receptors and signaling systems essential for synaptic function and plasticity. During synaptic plasticity the number and shapes of dendritic spines undergo radical reorganizations; dendritic spines initially form rapidly in the early postnatal period, then they undergo experience-dependent pruning during development, where the initial overproduction of synapses are dramatically reduced leading to the maturation of refined neural circuits.
Spinal formation is initiated when the BAR-domain proteins create membrane curvatures – presumably by binding with lipids – allowing the dendritic plasma to undergo local outward protrusion. The -BAR family proteins – Inverse-BAR (I-BAR), inverse-Fes-CIP4 homology BAR (IF-BAR), Fes-CIP4 homology Bar (F-BAR) N-terminal amphipathic helix-containing BAR (N-BAR) domain proteins – promote dendritic membrane protrusions and invagination. The I-BAR domain proteins, IRSp53, Mtss1L, and the IF-BAR domain protein SrGAP2 are localized in dendritic spines, where they mediate spine formation (3). Deafferentation causes the loss of dendritic spines which suggest that spines are maintained by afferent input. Large spines are generally more stable and more resistant to modification by varying synaptic activity. Rho and Ras family small GTPases pathways are molecular signaling pathways that converge on the actin cytoskeleton, which regulate spinal formation and bi-directional activities. The Rho family of GTPases contributes to the generation of different spines, varying in the size and shape due to the process that stimulates actin polymerization.
Although the quantity of dendritic spines formed seem to be regulated, it is unclear whether the positions of these spines are selected based on spatial information about the location of pre-existing spines or are selected randomly. Through measuring the distance between dendrite spines, it can be determined whether these spines form randomly. If spines formed randomly, this distance between those spines should follow a normal distribution, while non-random formation would lead to spacing that is more uniform than random chance would predict.
Methods
Microscopy
The images from Prada et. al, discussing the transfer of miRNAs via extracellular vesicles from glia to neurons, neurons were imaged using a 63x objective using an Axiovert 200 M (Zeiss) confocal system equipped with a spinning disk (UltraVIEW acquisition system, Perkin Elmer) keeping acquisition parameters constant. Focal planes were stacked together in a projection, and RFP-positive spines were counted on 20-40 μm segments of secondary dendrites.
Image Analysis
The images from the original paper were transferred into keynote and the scale bar from Figure 4A was superimposed on the image to create a standard measurement by determining the number of pixels in the image that corresponded to the length of the scale bar (8.2 pixels per 1 μm). Spines that protruded more than 1 μm on any side of the dendrite were counted as valid. Measurements of spine length were collected by placing a 1 μm by 1 μm circle over the spine to determine if it met the length requirement. The distance between each spine was measured based on pixels shown in Keynote then converted to μm based on the scale bar of the original image.
Randomly Generated Simulation Control
Random values were generated at a 3:10 ratio of values generated to total values possible because there was an average of 3 spines per 10 micrometers for each dendritic surface in the original data of Prada et al. (6). Thus, 30 random values were generated by Google’s computer random number generator between the range of 1-100. Random values for the experimental data group were done at a 1:4 ratio where there was an average of 2.5 spines per 10 micrometers. So, 50 random samples were taken from the computer generator at a range from 1-200. Values were collected and sorted from least to greatest, where then the absolute value of the difference of the two values were calculated (|A-B|). The differences between values were sorted from least to greatest. The average and standard deviation of the 50 random values were then calculated using Microsoft excel. Using the average and standard deviation the normal distribution was calculated for each value and then used to create a distribution graph.
Statistics
The data collected was transferred onto Microsoft Excel where the data of the control spine measurements were combined and all the experimental spine measurements were combined. All values were ordered from greatest to smallest. The mean and standard deviation of the control and experimental groups were found, using the values found the normal distribution of all data values were calculated. A “Scatter with Smooth Lines” graph was made which showed the standard deviation graph for the experimental and the control groups. Outliers that exceed the largest values by 29 times or larger were removed; two values in experimental (the values 27.3170732 and 653) and one value in the control were removed (the value 27.195122).
Results
In Prada et al. (6), the density of spines were measured (spine density/10μm) to determine whether inhibiting EV-neuron contact would play a role in the transfer of miRNA. Reduced spine density signaled the loss of immature (thin) spines compared to the control which confirmed that the experimental group treated with MVs (6) which were then released from the inflammatory microglia to the neuron.
In this experiment, the images from the original paper were taken and the distance between the dendrite spines were measured to see if spine formation occurred by random processes or in a regulated manner. Data were analyzed to determine whether an underlying molecular process dictates where spines form along a dendrite and whether the position of surrounding spines impact the location of new spine formation. Distances that were measured were analyzed by creating a distribution graph of spine distance for the experimental, control, and the random simulation group. The distribution graph would show whether the distance of the spinal formation was random in the experimental and control group. The randomly generated group’s distribution graph was used as a reference to indicate what a random distribution would be with the same ratio of spines to dendritic length to the control/experimental group. Evidence of a nonrandom distribution would indicate that spines would somehow be sharing information with one another so that spinal formation is generated on a nonrandom basis.
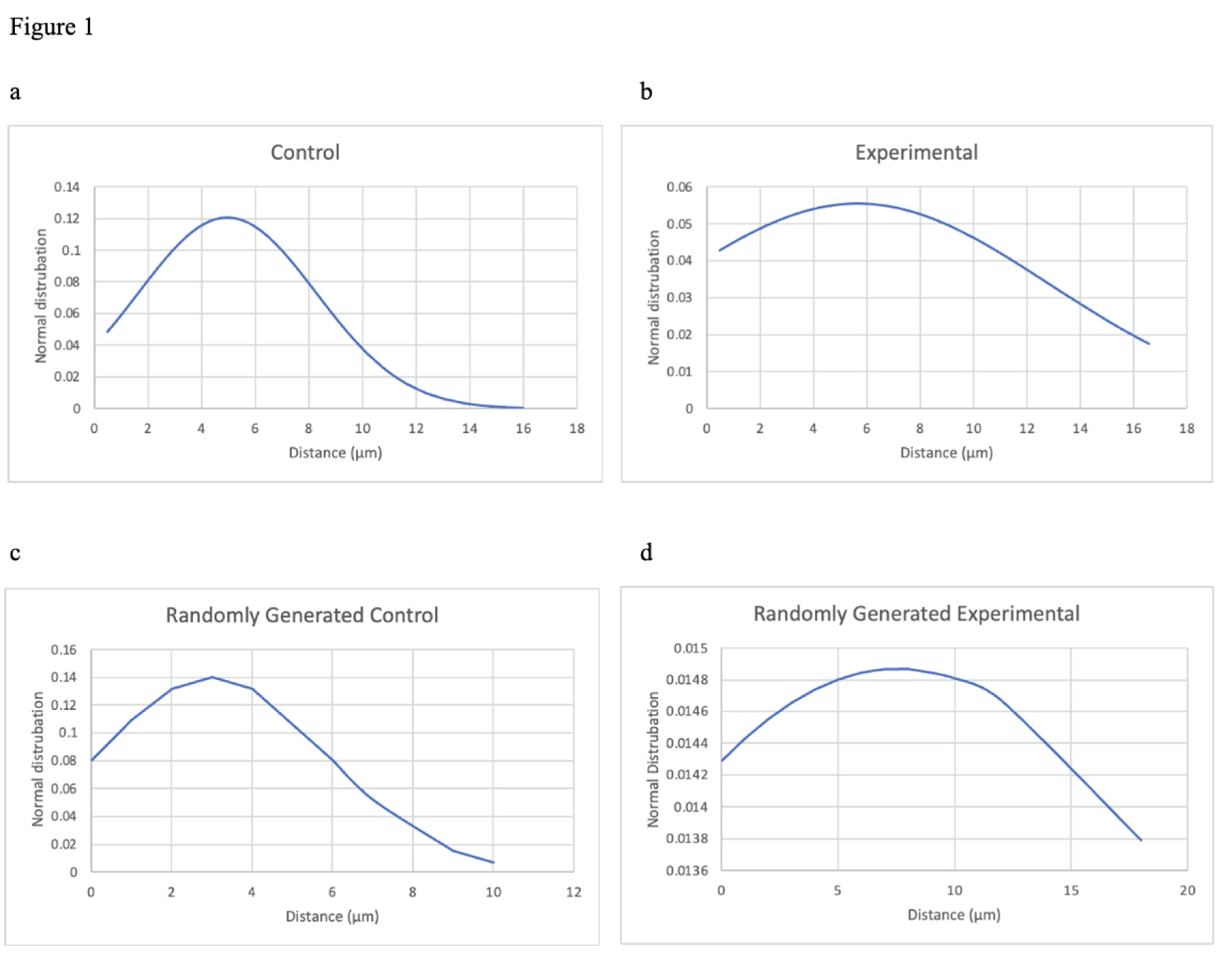
The distribution graph shows that the control group in our experiment has a standard deviation of roughly 3.31 and a mean of roughly 4.96 (see
Figure 1a). The control graph shows a left skewed bell curve distribution. The experimental group has a standard deviation of roughly 7.19 and a mean of roughly 5.67 (see
Figure 1b). The experimental graph also shows a left skewed distribution, though its standard deviation is significantly larger than the control group. The randomly generated simulation was conducted using the same ratio of spines to dendritic length as the control and experimental group; thus, two randomly generated graphs were created, one simulating the control group and one simulating the experimental group (see
Figure 1c,d).
Discussion
This study shows that there was no process which dictates where spines form along a dendrite; however when EV-neuron contact is inhibited blocking the transfer of miRNA, part of the randomness contributing to the formation of dendritic spines are taken away. In the original paper the experimental groups (MVs) were shown to have about a 1-3 spine density/10μm decrease from the control group, demonstrating that when EV neuron contact was inhibited that microglia-to-neuron transfer of miRNAs decreased; this shows that EVs plays a role in miRNA transfer between neurons. In our experiment, using the randomly generated simulation graphs, it can be seen that the control group is similar to the randomly generated control simulation group as the control graph has a mean of roughly 4.96 and the randomly generated control simulation group has a mean of 3; both graphs also have a left skewed distribution graph with similar standard deviations – the control group had a standard deviation of roughly 3.31 and the randomly generated control simulation group had a standard deviation of roughly 2.84. However, the experimental group and the randomly generated experimental group differ as the experimental group has a mean of roughly 5.67 and the randomly generated experimental simulation group has a mean of roughly 3.816; while the experimental group’s graph has significantly larger standard deviation of roughly 7.188 compared to the randomly generated experimental simulation group, which has a standard deviation of roughly 3.45.
Experimentally some element of randomness was lost when compared with the random variable control group as the distribution of the experimental is flatter than in the randomly generated experimental group, indicating that there is a increase variation in spine distance than what was expected; thus, when the EV-neuron contact was inhibited blocking the transfer of miRNA a element of randomness was lost. If spinal formation is random then individual spines are likely not sharing positional information through the signaling molecules that are forming new spines which means the proteins and molecules initiating spinal formation can form randomly.
Future research focusing on how the signaling molecules initiate spine formation of the membrane, especially in the absence of spatial information from preexisting spines. Investigating the intricate processes involved in the initiation of dendritic spine formation, despite the lack of spatial cues, could provide valuable insights into the positional development of dendritic spines and their intricate signaling interactions with one another, which holds potential in developing therapies that initiate dendritic spine formation to combat neurodegenerative diseases that causes the depletion of dendritic spines.
Acknowledgements
I would like to express my deepest gratitude to Dr. Justin Nussbaum, Ph.D. from Lakeland Community College, for his invaluable mentorship throughout the development of this paper.
References
- Chidambaram, S. B., Rathipriya, A. G., Bolla, S. R., Bhat, A., Ray, B., Mahalakshmi, A. M., … Sakharkar, M. K. (2019). Dendritic spines: Revisiting the physiological role. Progress in Neuro-Psychopharmacology and Biological Psychiatry. [CrossRef]
- Gamper, N., & Shapiro, M. S. (2007). Target-specific PIP(2) signaling: how might it work?. The Journal of physiology, 582(Pt 3), 967–975. [CrossRef]
- Khanal, P., & Hotulainen, P. (2021). Dendritic Spine Initiation in Brain Development, Learning and Diseases and Impact of BAR-Domain Proteins. Cells, 10(9), 2392. [CrossRef]
- Minegishi, T., Kastian, R. F., & Inagaki, N. (2023). Mechanical regulation of synapse formation and plasticity. Seminars in Cell & Developmental Biology, Volume 140, 82-89, ISSN 1084-9521. [CrossRef]
- Nimchinsky, E. A., Sabatini, B. L., & Svoboda, K. (2002). Structure and Function of Dendritic Spines. Annual Review of Physiology, 64(1), 313–353. [CrossRef]
- Prada, I., Gabrielli, M., Turola, E. et al. Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol 135, 529–550 (2018). [CrossRef]
- Stein, I. S., & Zito, K. (2019). Dendritic Spine Elimination: Molecular Mechanisms and Implications. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry, 25(1), 27–47. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).