Submitted:
30 July 2024
Posted:
31 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Market and Consumers
2.2. Risks
2.3. Health-Based-Guidance-Value (HBGV)
3. Results
3.1. Market and Consumers
3.2. Health Risks
3.3. Health-Based-Guidance-Value (HBGV)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Website | Low dosage (micro dosage) |
Medium dosage (standard dosage) |
High dosage (macro dosage) |
Recommended dosage or Maximum dosage |
|---|---|---|---|---|
| Algeacare.com | - | - | - | 20-40 mg/day, increase by 5 mg every week until the desired effect is achieved |
| Hanfgeflüster.de | - | - | - | 20-50 mg, increase until the desired effect is achieved |
| Cbd-vital.de | 1-20 mg/day (stress, sleep problems, balance) |
10-100 mg/day (severe problems) |
50-800 mg/day (very severe problems) |
- |
| Canatura.com (page on dosage according to symptoms) | 0.2 mg/kg/day = 14 mg/day 2 (general health, nausea, migraine) |
0.6 mg/kg/day = 42 mg/day 2 (nausea, chronic pain, sleep disorder, anxiety, migraine) |
1.2 mg/kg/day = 84 mg/day 2 (chronic pain, sleep disorder, anxiety, epilepsy, cancer) |
10-150 mg/day, adjust dosage individually |
| Canatura.com (page on dosage according to body weight) |
14 mg/day 2 | 49 mg/day 2 | 91 mg/day 2 | - |
| Sueddeutsche.de | - | - | - | Start with 7.5 mg/day, slowly increase until the desired effect is achieved |
| Alpinols.de |
14 mg/day 2 | 49 mg/day 2 | 70 mg/day 2 | - |
| Cbd-deal24.de |
0.5-25 mg/day | 10-100 mg/day | 50-800 mg/day | - |
| Benetui.de | 14 mg/day 2 | 49 mg/day 2 | 91 mg/day 2 |
pain: 2.5-20 mg; anxiety/stress: 5-30 mg; sleep disorder: 40-160 mg; epilepsy: 200-300 mg |
| 321cbd.com | 0.25 mg/kg/day = 17.5 mg/day 2 (mild sleep disorders) |
0.5 mg/kg/day = 35 mg/day 2 (moderate sleep disorders) |
- | Usual dosage: 25 mg/day |
| Biobloom-cbd.de | - | - | - | Dosage according to individual comfort dose |
| Naturecan.de | - | - | - | Start with 20 mg/day, increase gradually |
| Praktischarzt.de | 0.5-20 mg/day (sleep disorder, stress, imbalance, malaise, nausea, mild pain, mild mental problems) |
20-100 mg/day (depression, arthritis, MS, medium pain, medium mental problems) |
400 mg/day (epilepsy, cancer, tumor, severe pain, severe mental problems) |
Start with 2-5 mg/day, individual increase according to well-being |
| Vaay.com | - | - | - | 20-100 mg/day, adjust dosage individually; start with 5 mg for 3 days, increase dosage to 10 mg for 3 days, continue with 15 mg |
| Cbd360.de | 2-20 mg/day (mild pain, mild stress, metabolic disorders, nausea, inflammation) Day 1-15: increase slowly from 0.02 mg/kg/day (= 1.4 mg/day 2) to 0.2 mg/kg/day (= 14 mg/day 2) |
20-80 mg/day (medium pain, medium stress, inflammation, autoimmune disease, anxiety disorder, lyme disease, depression, arthritis, MS, autism, weight loss) Day 16-30: If needed increase slowly from 0.3 mg/kg/day (=21 mg/day 2) to 1 mg/kg/day (=70 mg/day 2) |
80-800 mg/day (severe pain, epilepsy, cancer, liver problems) Day 31-45: If needed increase slowly from 1.5 mg/kg/day (= 105 mg/day 2) to 4 mg/kg/day (= 280 mg/day 2) |
- |
| Cbd-und-mehr.at | - | - | - | Start with low dosage and increase individually; first week: two drops of a 10% oil once a day; second week: two drops of a 10% oil twice a day; third week: two drops of a 15% oil three times a day |
| Marryjane.de | 14 mg/day 2 | 49 mg/day 2 | 91 mg/day 2 | pain: 2.5-20 mg/day anxiety: 33-49 mg/day intestinal diseases: 10 mg/day psychosis: 600 mg/day |
| Cbdwelt.de | - | - | - | ususal dosage: 20-100 mg/day Start low and increase slowly until the desired effect is achieved |
| Tomhemps.com | 1 mg/4.5 kg/day = 15.6 mg/day 2 |
3 mg/4.5 kg/day = 46.7 mg/day 2 | 6 mg/4.5 kg/day = 93.3 mg/day 2 | - |
| Cannalin.de | - | - | - | 70-100 mg, start with 5 mg and increase by 5mg every second day |
| Dutchnaturalhealing.com | - | - | - | 50-70 mg, start with 10-20mg |
| Cbdsfinest.de | 0.5-20 mg/day (headache, sleep disorders, mood swings, nausea, stress, metabolic disorder) |
10-100 mg/day (pain, inflammation, anxiety, depression, MS, IBS, arthritis, autism, fibromyalgia) |
50-800 mg/day (epilepsy, cancer, liver problems) |
- |
| Hanfgeflüster.de | - | - | - | 20-50 mg/day, individual adjustment every 2 weeks |
| Cannatrust.eu | 15 mg/day 2 | 45 mg/day 2 | 90 mg/day 2 | - |
| Magu-cbd.com | - | - | - | 20-40 mg/day |
| Pharma-hemp.com | - | - | - | Start with 0.5 mg/kg/day = 35 mg/day 2 |
References
- Lucas, C.J.; Galettis, P.; Schneider, J. The Pharmacokinetics and the Pharmacodynamics of Cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Epidyolex | European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/epidyolex (accessed on 2 April 2024).
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef]
- Castillo-Arellano, J.; Canseco-Alba, A.; Cutler, S.J.; León, F. The Polypharmacological Effects of Cannabidiol. Molecules 2023, 28, 3271. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, G.; Gaeta, A.; Cannata, B.; Pinzaglia, C.; Aronica, E.; Morano, A.; Cifelli, P.; Palma, E. GABAergic Neurotransmission in Human Tissues Is Modulated by Cannabidiol. Life 2022, 12, 2042. [Google Scholar] [CrossRef] [PubMed]
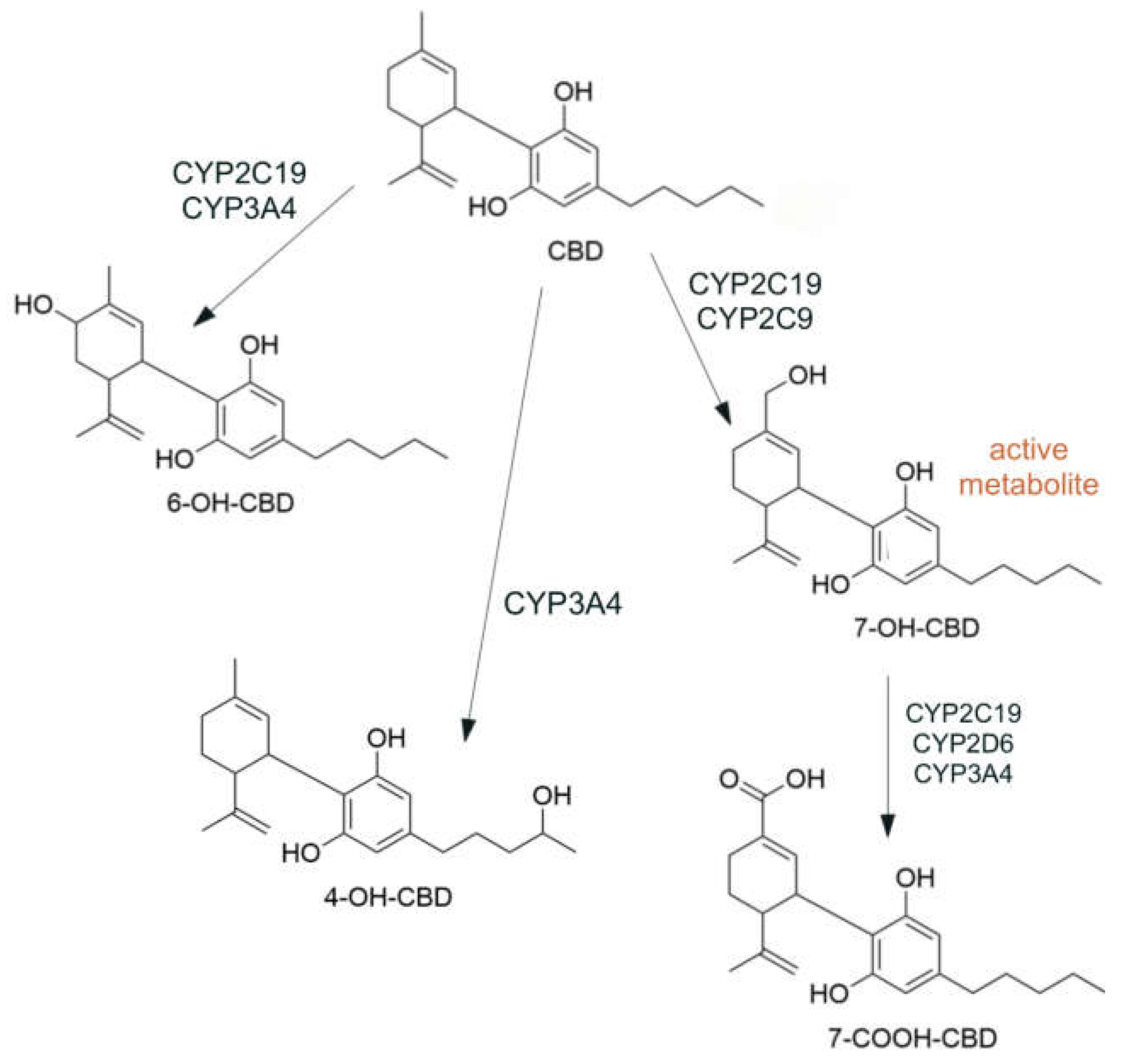
- Beers, J.L.; Fu, D.; Jackson, K.D. Cytochrome P450–Catalyzed Metabolism of Cannabidiol to the Active Metabolite 7-Hydroxy-Cannabidiol. Drug Metab. Dispos. 2021, 49, 882–891. [Google Scholar] [CrossRef]
- Jiang, R.; Yamaori, S.; Takeda, S.; Yamamoto, I.; Watanabe, K. Identification of Cytochrome P450 Enzymes Responsible for Metabolism of Cannabidiol by Human Liver Microsomes. Life Sci. 2011, 89, 165–170. [Google Scholar] [CrossRef]
- Zhang, Q.; Melchert, P.W.; Markowitz, J.S. Pharmacokinetic Variability of Oral Cannabidiol and Its Major Metabolites after Short-Term High-Dose Exposure in Healthy Subjects. Med. Cannabis Cannabinoids 2024, 7, 1–9. [Google Scholar] [CrossRef]
- Batinic, A.; Sutlovic, D.; Kuret, S.; Burcul, F.; Kalajzic, N.; Matana, A.; Dujic, G.; Vrdoljak, J.; Kumric, M.; Bozic, J.; et al. Differences in Plasma Cannabidiol Concentrations in Women and Men: A Randomized, Placebo-Controlled, Crossover Study. Int. J. Mol. Sci. 2023, 24, 10273. [Google Scholar] [CrossRef]
- Bansal, S.; Paine, M.F.; Unadkat, J.D. Comprehensive Predictions of Cytochrome P450 (P450)-Mediated In Vivo Cannabinoid-Drug Interactions Based on Reversible and Time-Dependent P450 Inhibition in Human Liver Microsomes. Drug Metab. Dispos. 2022, 50, 351–360. [Google Scholar] [CrossRef]
- Food and Feed Information Portal Database | FIP. Available online: https://ec.europa.eu/food/food-feed-portal/screen/novel-food-catalogue/search (accessed on 30 April 2024).
- European Parliament and the Council of European Union. Regulation (EU) No 2015/2283 of the European Parliament and of the Council of on Novel Foods. 25 November.
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Statement on Safety of Cannabidiol as a Novel Food: Data Gaps and Uncertainties. EFSA J. Eur. Food Saf. Auth. 2022, 20, e07322. [Google Scholar] [CrossRef]
- Commissioner, O. of the FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD). FDA 2024. [Google Scholar]
- Lo, L.A.; Christiansen, A.; Eadie, L.; Strickland, J.C.; Kim, D.D.; Boivin, M.; Barr, A.M.; MacCallum, C.A. Cannabidiol-Associated Hepatotoxicity: A Systematic Review and Meta-Analysis. J. Intern. Med. 2023, 293, 724–752. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.K.; Andersen, M.L.; Mazaro-Costa, R. The Effects of Cannabidiol on Male Reproductive System: A Literature Review. J. Appl. Toxicol. 2020, 40, 132–150. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.K.; Rocha, T.L.; Fernandes, F.H.; Gonçalves, B.B.; Souza, M.R.; Araújo, A.A.; Barbosa, C.C.; Silva, D.M.; Campos, H.M.; Tomazett, M.V.; et al. Decreasing Sperm Quality in Mice Subjected to Chronic Cannabidiol Exposure: New Insights of Cannabidiol-Mediated Male Reproductive Toxicity. Chem. Biol. Interact. 2022, 351, 109743. [Google Scholar] [CrossRef] [PubMed]
- European Parliament an the Council of the European Union Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying down Procedures in Matters of Food Safety. 2002.
- Lachenmeier, D.W.; Sproll, C.; Walch, S.G. Does Cannabidiol (CBD) in Food Supplements Pose a Serious Health Risk? Consequences of the European Food Safety Authority (EFSA) Clock Stop Regarding Novel Food Authorisation. Psychoactives 2023, 2, 66–75. [Google Scholar] [CrossRef]
- Committee, E.S.; More, S.J.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; et al. Guidance on the Use of the Benchmark Dose Approach in Risk Assessment. EFSA J. 2022, 20, e07584. [Google Scholar] [CrossRef]
- Davis, J.A.; Gift, J.S.; Zhao, Q.J. Introduction to Benchmark Dose Methods and U.S. EPA’s Benchmark Dose Software (BMDS) Version 2.1.1. Toxicol. Appl. Pharmacol. 2011, 254, 181–191. [Google Scholar] [CrossRef] [PubMed]
- US EPA, O. Benchmark Dose Technical Guidance. Available online: https://www.epa.gov/risk/benchmark-dose-technical-guidance (accessed on 21 May 2024).
- Arout, C.A.; Haney, M.; Herrmann, E.S.; Bedi, G.; Cooper, Z.D. A Placebo-Controlled Investigation of the Analgesic Effects, Abuse Liability, Safety and Tolerability of a Range of Oral Cannabidiol Doses in Healthy Humans. Br. J. Clin. Pharmacol. 2022, 88, 347–355. [Google Scholar] [CrossRef] [PubMed]
- McCartney, D.; Kevin, R.C.; Suraev, A.S.; Sahinovic, A.; Doohan, P.T.; Bedoya-Pérez, M.A.; Grunstein, R.R.; Hoyos, C.M.; McGregor, I.S. How Long Does a Single Oral Dose of Cannabidiol Persist in Plasma? Findings from Three Clinical Trials. Drug Test. Anal. 2023, 15, 334–344. [Google Scholar] [CrossRef]
- Schoedel, K.A.; Szeto, I.; Setnik, B.; Sellers, E.M.; Levy-Cooperman, N.; Mills, C.; Etges, T.; Sommerville, K. Abuse Potential Assessment of Cannabidiol (CBD) in Recreational Polydrug Users: A Randomized, Double-Blind, Controlled Trial. Epilepsy Behav. EB 2018, 88, 162–171. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Thiele, E.A.; Wong, M.H.; Appleton, R.; Harden, C.L.; Greenwood, S.; Morrison, G.; Sommerville, K. Randomized, Dose-Ranging Safety Trial of Cannabidiol in Dravet Syndrome. Neurology 2018, 90, e1204–e1211. [Google Scholar] [CrossRef] [PubMed]
- Committee, E.S. Guidance on Selected Default Values to Be Used by the EFSA Scientific Committee, Scientific Panels and Units in the Absence of Actual Measured Data. EFSA J. 2012, 10, 2579. [Google Scholar] [CrossRef]
- Sales of CBD in Europe 2022-2026 Forecast. Available online: https://www.statista.com/statistics/1306959/forecast-cbd-sales-europe/ (accessed on 26 January 2024).
- Geppert, J.; Lietzow, J.; Hessel-Pras, S.; Kirsch, F.; Schäfer, B.; Sachse, B. Usage and Health Perception of Cannabidiol-Containing Products among the Population in Germany: A Descriptive Study Conducted in 2020 and 2021. BMC Public Health 2023, 23, 2318. [Google Scholar] [CrossRef] [PubMed]
- Bhamra, S.K.; Desai, A.; Imani-Berendjestanki, P.; Horgan, M. The Emerging Role of Cannabidiol (CBD) Products; a Survey Exploring the Public’s Use and Perceptions of CBD. Phytother. Res. PTR 2021, 35, 5734–5740. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Moeller, K.E.; McGuire, M.; Melton, B.L. Consumer Perception, Knowledge, and Uses of Cannabidiol. Ment. Health Clin. 2023, 13, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.; Wadsworth, E.; Schauer, G.; Hammond, D. Use and Perceptions of Cannabidiol Products in Canada and in the United States. Cannabis Cannabinoid Res. 2022, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.; Merten, J.W.; Gordon, B.T.; Hamadi, H. CBD (Cannabidiol) Product Attitudes, Knowledge, and Use Among Young Adults. Subst. Use Misuse 2020, 55, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Mathur, K.; Vuppalanchi, V.; Gelow, K.; Vuppalanchi, R.; Lammert, C. Cannabidiol (CBD) Consumption and Perceived Impact on Extrahepatic Symptoms in Patients with Autoimmune Hepatitis. Dig. Dis. Sci. 2020, 65, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Corroon, J.; Phillips, J.A. A Cross-Sectional Study of Cannabidiol Users. Cannabis Cannabinoid Res. 2018, 3, 152–161. [Google Scholar] [CrossRef]
- Soleymanpour, M.; Saderholm, S.; Kavuluru, R. Therapeutic Claims in Cannabidiol (CBD) Marketing Messages on Twitter. Proc. IEEE Int. Conf. Bioinforma. Biomed. 2021, 2021, 3083–3088. [Google Scholar] [CrossRef]
- Merten, J.W.; Gordon, B.T.; King, J.L.; Pappas, C. Cannabidiol (CBD): Perspectives from Pinterest. Subst. Use Misuse 2020, 55, 2213–2220. [Google Scholar] [CrossRef]
- Kaufmann, R.; Bozer, A.H.; Jotte, A.K.; Aqua, K. Long-Term, Self-Dosing CBD Users: Indications, Dosage, and Self-Perceptions on General Health/Symptoms and Drug Use. Med. Cannabis Cannabinoids 2023, 6, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Melchert, D.; Schaare, F.; Winterhalter, P.; Beuerle, T. CBD Products: Labeling Accuracy of an Obscure Niche Market. Food Control 2024, 160, 110375. [Google Scholar] [CrossRef]
- Bonn-Miller, M.O.; Loflin, M.J.E.; Thomas, B.F.; Marcu, J.P.; Hyke, T.; Vandrey, R. Labeling Accuracy of Cannabidiol Extracts Sold Online. JAMA 2017, 318, 1708–1709. [Google Scholar] [CrossRef]
- Johnson, E.; Kilgore, M.; Babalonis, S. Label Accuracy of Unregulated Cannabidiol (CBD) Products: Measured Concentration vs. Label Claim. J. Cannabis Res. 2022, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Vandrey, R.; Raber, J.C.; Raber, M.E.; Douglass, B.; Miller, C.; Bonn-Miller, M.O. Cannabinoid Dose and Label Accuracy in Edible Medical Cannabis Products. JAMA 2015, 313, 2491–2493. [Google Scholar] [CrossRef]
- Lindekamp, N.; Weigel, S.; Sachse, B.; Schäfer, B.; Rohn, S.; Triesch, N. Comprehensive Analysis of 19 Cannabinoids in Commercial CBD Oils: Concentrations, Profiles, and Safety Implications. J. Consum. Prot. Food Saf. 2024. [Google Scholar] [CrossRef]
- Kaufmann, R.; Harris Bozer, A.; Jotte, A.R.K.; Aqua, K. The Effects of Long-Term Self-Dosing of Cannabidiol on Drowsiness, Testosterone Levels, and Liver Function. Med. Cannabis Cannabinoids 2023, 6, 32–40. [Google Scholar] [CrossRef]
- Kaufmann, R.; Aqua, K.; Lombardo, J.; Lee, M. Observed Impact of Long-Term Consumption of Oral Cannabidiol on Liver Function in Healthy Adults. Cannabis Cannabinoid Res. 2023, 8, 148–154. [Google Scholar] [CrossRef]
- Chen, S.; Kim, J.-K. The Role of Cannabidiol in Liver Disease: A Systemic Review. Int. J. Mol. Sci. 2024, 25, 2370. [Google Scholar] [CrossRef]
- Caputi, T.L. Re: “Observed Impact of Long-Term Consumption of Oral Cannabidiol on Liver Function in Healthy Adults” and a Recent Announcement of a New Cannabidiol Safety Study. Cannabis Cannabinoid Res. 2022, 7, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Sarrafpour, S.; Urits, I.; Powell, J.; Nguyen, D.; Callan, J.; Orhurhu, V.; Simopoulos, T.; Viswanath, O.; Kaye, A.D.; Kaye, R.J.; et al. Considerations and Implications of Cannabidiol Use During Pregnancy. Curr. Pain Headache Rep. 2020, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Feinshtein, V.; Erez, O.; Ben-Zvi, Z.; Erez, N.; Eshkoli, T.; Sheizaf, B.; Sheiner, E.; Huleihel, M.; Holcberg, G. Cannabidiol Changes P-Gp and BCRP Expression in Trophoblast Cell Lines. PeerJ 2013, 1, e153. [Google Scholar] [CrossRef] [PubMed]
- Feinshtein, V.; Erez, O.; Ben-Zvi, Z.; Eshkoli, T.; Sheizaf, B.; Sheiner, E.; Holcberg, G. Cannabidiol Enhances Xenobiotic Permeability through the Human Placental Barrier by Direct Inhibition of Breast Cancer Resistance Protein: An Ex Vivo Study. Am. J. Obstet. Gynecol. 2013, 209, 573–e1. [Google Scholar] [CrossRef] [PubMed]
- Guth, S.; Roth, A.; Engeli, B.; Lachenmeier, D.W.; Cartus, A.T.; Hüser, S.; Baum, M.; Diel, P.; Eisenbrand, G.; Hengstler, J.G.; et al. Comparison of Points of Departure between Subchronic and Chronic Toxicity Studies on Food Additives, Food Contaminants and Natural Food Constituents. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 146, 111784. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.G.; Lefever, T.W.; Heintz, M.M.; Trexler, K.R.; Borghoff, S.J.; Bonn-Miller, M.O. Oral Toxicity Evaluation of Cannabidiol. Food Chem. Toxicol. 2023, 176, 113778. [Google Scholar] [CrossRef] [PubMed]
- Tallon, M.J.; Child, R. Subchronic Oral Toxicity Assessment of a Cannabis Extract. Regul. Toxicol. Pharmacol. 2023, 144, 105496. [Google Scholar] [CrossRef]
- Dziwenka, M.; Dolan, L.C.; Rao, M. Safety of Elixinol Hemp Extract: In Vitro Genetic Toxicity and Subchronic Toxicity in Rats. J. Toxicol. 2023, 2023, 5982883. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.S.; Zuardi, A.W.; Guimarães, F.S.; Campos, A.C.; de Lima Osório, F.; Loureiro, S.R.; dos Santos, R.G.; Souza, J.D.S.; Ushirohira, J.M.; Pacheco, J.C.; et al. Efficacy and Safety of Cannabidiol Plus Standard Care vs Standard Care Alone for the Treatment of Emotional Exhaustion and Burnout Among Frontline Health Care Workers During the COVID-19 Pandemic: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2120603. [Google Scholar] [CrossRef]
- Arcella, D.; Cascio, C.; Mackay, K. Acute Human Exposure Assessment to Tetrahydrocannabinol (Δ9-THC). EFSA J. 2020, 18, e05953. [Google Scholar] [CrossRef]
- FDA. Drug Approval Package: Epidiolex (Cannabidiol). Company: GW Research Ltd. Application Number: 210365 Orig 1. FDA Application Review Files. Pharmacology Review(s). 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210365Orig1s000PharmR.pdf.
- Henderson, R.G.; Vincent, M.; Rivera, B.N.; Bonn-Miller, M.O.; Doepker, C. Cannabidiol Safety Considerations: Development of a Potential Acceptable Daily Intake Value and Recommended Upper Intake Limits for Dietary Supplement Use. Regul. Toxicol. Pharmacol. 2023, 144, 105482. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F. Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- BLV (Bundesamt für Lebensmittelsicherheit und Veterinärwesen) Briefing Letter Cannabidiol (CBD) in Lebensmitteln und Lebereffekte. Available online: https://www.blv.admin.ch/blv/de/home/lebensmittel-und-ernaehrung/rechts-und-vollzugsgrundlagen/bewilligung-und-meldung/bewilligung/cannabis-cannabidiol.html (accessed on 11 June 2024).
- GW Pharmaceuticals Submission on Scientific Data and Information about Products Containing Cannabis or Cannabis-Derived Compounds (FDA-2019-N-1482-4257 Attachment 1) Available online: https://downloads.regulations.gov/FDA-2019-N-1482-4257/attachment_1.pdf.
- Committee on Toxicity Updated Position Paper on the Potential Risk of CBD in CBD Food Products Available online: https://cot.food.gov.uk/sites/default/files/2021-08/CBD%20Position%20Paper%20updated%20July%202021.pdf.
- Joint Position Paper from ACNFP & COT on Establishing Provisional ADI for Pure Form CBD in Foods | Advisory Committee on Novel Foods and Processes. Available online: https://acnfp.food.gov.uk/JointpositionpaperfromACNFP%26COTonestablishingprovisionalADIforpureformCBDinfoods (accessed on 17 June 2024).
- O’Sullivan, S.E.; Jensen, S.S.; Kolli, A.R.; Nikolajsen, G.N.; Bruun, H.Z.; Hoeng, J. Strategies to Improve Cannabidiol Bioavailability and Drug Delivery. Pharmaceuticals 2024, 17, 244. [Google Scholar] [CrossRef] [PubMed]

| Dosage 1 | Number of investigated websites (n= 26) | Dosage mean value (and range) [mg/day] |
|---|---|---|
| Low dosage (micro dosage) |
13 | 15 (0.5 – 25) |
| Medium dosage (standard dosage) |
13 | 47 (10 – 100) |
| High dosage (macro dosage) |
12 | 227 (50 – 800) |
| Other dosage information | 10 | 62 (2.5 – 300) |
| Medicinal indication statements 2 | 8 | - |
| Study, Tested Substance |
Species, Sex1 |
Study design, CBD Doses 2 | Endpoint | BMDL | BMD | p-Value | Model 3 |
|---|---|---|---|---|---|---|---|
| Henderson et al. [52] CBD-isolate |
Rats, female | 90 day oral toxicity study 0, 50, 80, 120, 140 mg/kg bw/day |
liver weight | 43 mg/kg bw/day | 64 mg/kg bw/day | 0.242 | Power |
| Tallon and Child [53] Hemp extract (6.27% CBD) |
Rats, female | 90 day oral toxicity study 0, 30, 115.13, 230.25, 460.5 mg/kg bw/day |
liver weight | (80 mg/kg bw/day) 3 |
(94 mg/kg bw/day) 3 | 0.948 | Linear |
| Dziwenka et al. [54] Elixinol Hemp Extract (around 65% CBD) |
Rats, male | 90 day oral toxicity study 0, 18.95, 33.16, 56.84 mg/kg bw/day |
liver weight | (30 mg/kg bw/day)4 | (41 mg/kg bw/day)4 | 0.644 | Exponential 3 |
| Human study | Animal study | |
|---|---|---|
| Study | Crippa et al. [55] | Henderson et al. [52] |
| Reference Point (RP) | LOAEL of 300 mg/day (= 4.29 mg/kg bw/day) |
BMDL of 43 mg/kg bw/day |
| Uncertainty factor (UF) | 30 1 | 200 2 |
| HBGV | 0.14 mg/kg bw/day (10 mg/day) |
0.21 mg/kg bw/day (15 mg/day) |
| Study | Health-based guidance value/ acceptable daily intake |
Rationale |
|---|---|---|
| COT FSA [62] (2021) | 70 mg/day | 1 mg/kg bw/day for inhibitory interactions in humans |
| BLV [60] (2021) | 12 mg/day | 5 mg/kg bw/day for DILI in humans |
| Lachenmeier et al. [19] (2023) | 10 mg/day | 300 mg/day for DILI in humans |
| Henderson et al. [58] (2023) | 30 mg/day | 300 mg/day for DILI in humans |
| COT FSA [63] (2023) | 10 mg/day | 72, 50 and 25 mg/kg bw/day for adverse liver effects in rodents |
| This study | 10 mg/day | 300 mg/day for DILI in humans |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





