Submitted:
30 July 2024
Posted:
01 August 2024
You are already at the latest version
Abstract
Keywords:
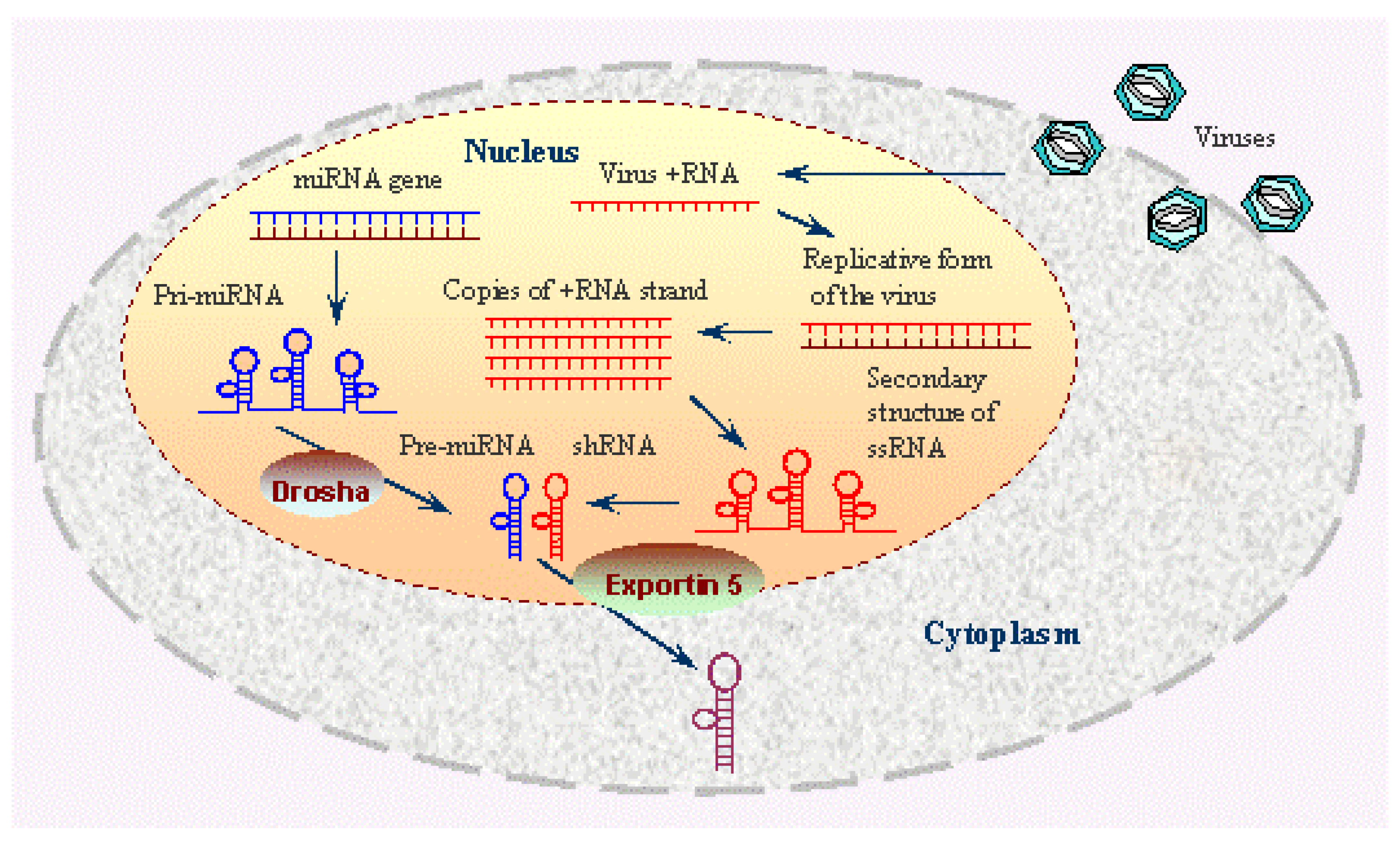
A Brief Idea about RNAi Pathway
Mammalian Dicer’s Long dsRNA Inefficiency in Processing
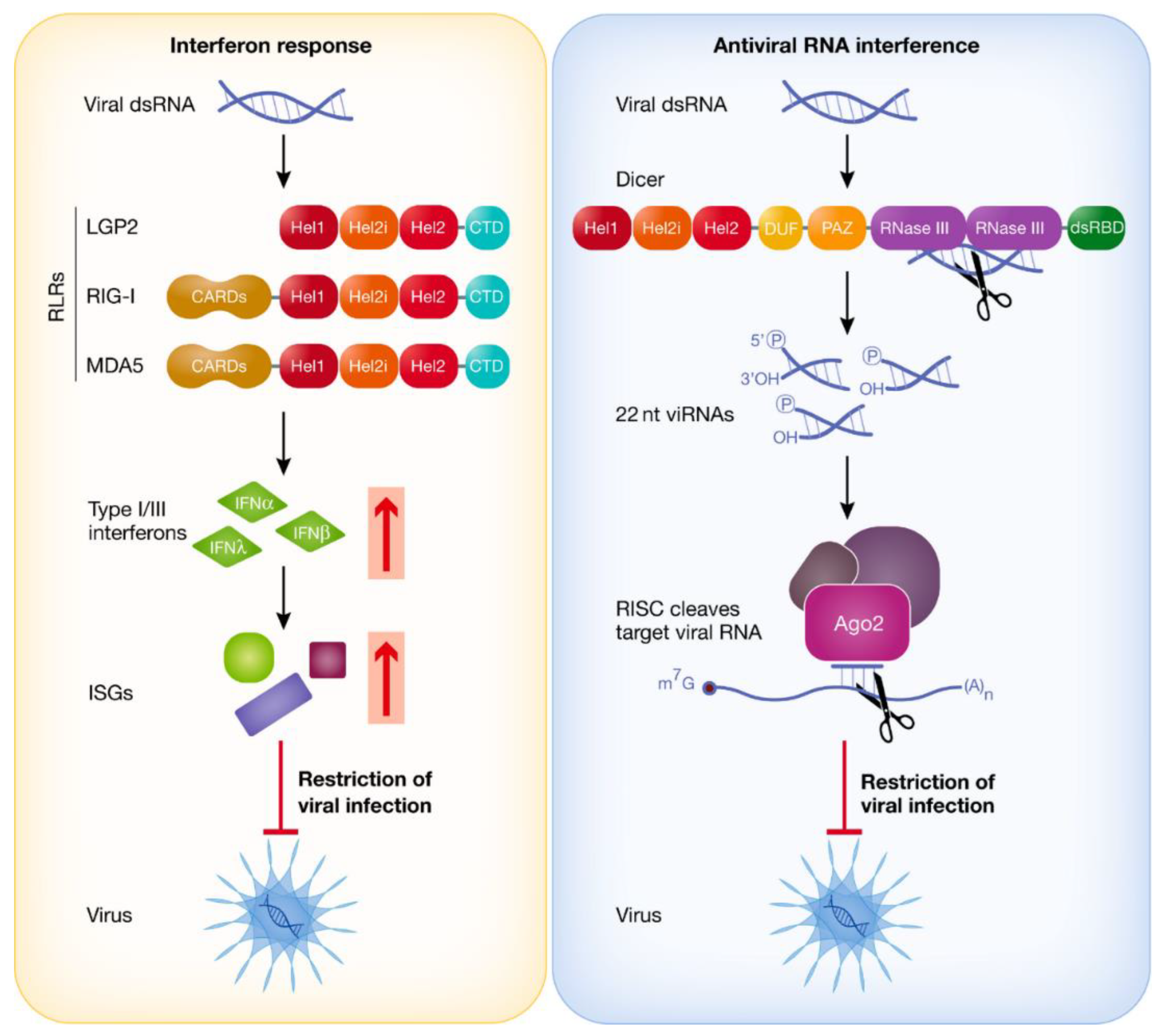
IFN Response vs. dsRNAi
Absence of Stable Mammalian vsiRNAs Detection During Viral Infection
Full-Length Dicer also Processes dsRNA
Conclusion
References
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a chimeric Chalcone synthase gene into petunia results in reversible Co-suppression of homologous genes in trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y. Current advances in antiviral RNA interference in mammals. The FEBS journal 2024, 291, 208–216. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, B. RNAi-mediated antiviral immunity in mammals. Curr Opin Virol 2018, 32, 9–14. [Google Scholar] [CrossRef]
- Song, M.S.; Rossi, J.J. Molecular mechanisms of Dicer: endonuclease and enzymatic activity. The Biochemical journal 2017, 474, 1603–1618. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K. Are Argonaute-Associated Tiny RNAs Junk, Inferior miRNAs, or a New Type of Functional RNAs? . Frontiers in molecular biosciences 2021, 8, 795356. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Ui-Tei, K. Mutual regulation of RNA silencing and the IFN response as an antiviral defense system in mammalian cells. Int J Mol Sci 2020, 21, 1348. [Google Scholar] [CrossRef]
- Hur, S. Double-stranded RNA sensors and modulators in innate immunity. Annu Rev Immunol 2019, 37, 349–375. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.; Courtney, D.G.; Kennedy, E.M.; Cullen, B.R. Influenza a virus-derived siRNAs increase in the absence of NS1 yet fail to inhibit virus replication. RNA 2018, 24, 1172–1182. [Google Scholar] [CrossRef]
- tenOever, B.R. Questioning antiviral RNAi in mammals. Nat Microbiol 2017, 2, 17052. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Dai, Y.; Li, Z.; Wang, J.; Ye, Z.; Ren, Y.; Wang, H.; Li, W.X.; Lu, J.; et al. Efficient dicer processing of virus-derived double-stranded RNAs and its modulation by RIG-I-like receptor LGP2. PLoS Pathog 2021, 17, e1009790. [Google Scholar] [CrossRef]
- Xu, Y.P.; Qiu, Y.; Zhang, B.; Chen, G.; Chen, Q.; Wang, M.; Mo, F.; Xu, J.; Wu, J.; Zhang, R.R.; et al. Zika virus infection induces RNAi-mediated antiviral immunity in human neural progenitors and brain organoids. Cell Res 2019, 29, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xu, Y.P.; Wang, M.; Miao, M.; Zhou, H.; Xu, J.; Kong, J.; Zheng, D.; Li, R.T.; Zhang, R.R.; et al. Flavivirus induces and antagonizes antiviral RNA interference in both mammals and mosquitoes. Sci Adv 2020, 6, eaax7989. [Google Scholar] [CrossRef]
- Han, Q.; Chen, G.; Wang, J.; Jee, D.; Li, W.X.; Lai, E.C.; Ding, S.W. Mechanism and function of antiviral RNA interference in mice. mBio 2020, 11, e03278–19. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Nakano, Y.; Onomoto, K.; Yoneyama, M.; Ui-Tei, K. LGP2 virus sensor enhances apoptosis by upregulating apoptosis regulatory genes through TRBP-bound miRNAs during viral infection. Nucleic Acids Res 2020, 48, 1494–1507. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Miesen, P.; van Rij, R.P. Antiviral RNAi in insects and mammals: parallels and differences. Viruses 2019, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Maillard, P.V.; van der Veen, A.G.; Poirier, E.Z.; Reis e Sousa, C. Slicing and dicing viruses: antiviral RNA interference in mammals. The EMBO journal 2019, 38, e100941. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; MacRae, I.J.; Kirsch, J.F.; Doudna, J.A. Autoinhibition of human dicer by its internal helicase domain. J Mol Biol 2008, 380, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, S.; Sternberg, S.H.; Kellenberger, C.A.; Doudna, J.A. Substrate-specific kinetics of Dicer-catalyzed RNA processing. J Mol Biol 2010, 404, 392–402. [Google Scholar] [CrossRef]
- Kennedy, E.M.; Whisnant, A.W.; Kornepati, A.V.R.; Marshall, J.B.; Bogerd, H.P.; Cullen, B.R. Production of functional small interfering RNAs by an amino-terminal deletion mutant of human Dicer. Proc Natl Acad Sci USA 2015, 112, E6945–E6954. [Google Scholar] [CrossRef]
- Flemr, M.; Malik, R.; Franke, V.; Nejepinska, J.; Sedlacek, R.; Vlahovicek, K.; Svoboda, P. A retrotransposon-driven dicer isoform directs endogenous small interfering RNA production in mouse oocytes. Cell 2013, 155, 807–816. [Google Scholar] [CrossRef]
- Cantini, L.P.; Andino, L.M.; Attaway, C.C.; Butler, B.; Dumitriu, A.; Blackshaw, A.; Jakymiw, A. Identification and characterization of Dicer1e, a Dicer1 protein variant, in oral cancer cells. Mol Cancer 2014, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.W.; Ma, E.; Shigematsu, H.; Cianfrocco, M.A.; Noland, C.L.; Nagayama, K.; Nogales, E.; Doudna, J.A.; Wang, H.-W. Substrate-specific structural rearrangements of human Dicer. Nat Struct Mol Biol 2013, 20, 662–670. [Google Scholar] [CrossRef]
- Ota, H.; Sakurai, M.; Gupta, R.; Valente, L.; Wulff, B.E.; Ariyoshi, K.; Iizasa, H.; Davuluri, R.V.; Nishikura, K. ADAR1 forms a complex with Dicer to promote microRNA processing and RNA-induced gene silencing. Cell 2013, 153, 575–589. [Google Scholar] [CrossRef]
- Takahashi, T.; Heaton, S.M.; Parrish, N.F. Mammalian antiviral systems directed by small RNA. PLoS Pathog 2021, 17, e1010091. [Google Scholar] [CrossRef]
- Adiliaghdam, F.; Basavappa, M.; Saunders, T.L.; Harjanto, D.; Prior, J.T.; Cronkite, D.A.; Papavasiliou, N.; Jeffrey, K.L. A requirement for Argonaute 4 in mammalian antiviral defense. Cell Rep 2020, 30, 1690–1701e4. [Google Scholar] [CrossRef] [PubMed]
- Poirier, E.Z.; Buck, M.D.; Chakravarty, P.; Carvalho, J.; Frederico, B.; Cardoso, A.; Healy, L.; Ulferts, R.; Beale, R.; Reis e Sousa, C. An isoform of dicer protects mammalian stem cells against multiple RNA viruses. Science 2021, 373, 231–236. [PubMed]
- Jeffrey, K.L. Upping the ante on mammalian antiviral RNA interference. Cell Host Microbe 2021, 29, 1333–1335. [Google Scholar] [CrossRef] [PubMed]
- Maillard, P.V.; Van der Veen, A.G.; Deddouche Grass, S.; Rogers, N.C.; Merits, A.; Reis e Sousa, C. Inactivation of the type I interferon pathway reveals long double-stranded RNA-mediated RNA interference in mammalian cells. EMBO J 2016, 35, 2505–2518. [Google Scholar] [CrossRef]
- Van der Veen, A.G.; Maillard, P.V.; Schmidt, J.M.; Lee, S.A.; Deddouche Grass, S.; Borg, A.; Kjær, S.; Snijders, A.P.; Reis e Sousa, C. The RIG-I-like receptor LGP2 inhibits Dicer-dependent processing of long double-stranded RNA and blocks RNA interference in mammalian cells. e: EMBO J 2018, 37, 2018. [Google Scholar]
- Takahashi, T.; Nakano, Y.; Onomoto, K.; Murakami, F.; Komori, C.; Suzuki, Y.; Yoneyama, M.; Ui-Tei, K. LGP2 virus sensor regulates gene expression network mediated by TRBP-bound microRNAs. Nucleic Acids Res 2018, 42, D68–14. [Google Scholar] [CrossRef]
- Komuro, A.; Homma, Y.; Negoro, T.; Barber, G.N.; Horvath, C.M. The TAR-RNA binding protein is required for immunoresponses triggered by Cardiovirus infection. Biochem Biophys Res Commun 2016, 480, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Girardi, E.; Lefèvre, M.; Chane-Woon-Ming, B.; Paro, S.; Claydon, B.; Imler, J.-L.; Meignin, C.; Pfeffer, S. Cross-species comparative analysis of Dicer proteins during Sindbis virus infection. Sci Rep 2015, 5, 10693–12. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.J.; Kincaid, R.P.; Phanaksri, T.; Burke, J.M.; Pare, J.M.; Cox, J.E.; Hsiang, T.-Y.; Krug, R.M.; Sullivan, C.S. Reciprocal inhibition between intracellular antiviral signaling and the RNAi machinery in mammalian cells. Cell Host Microbe 2013, 14, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Husain, B.; Mukerji, I.; Cole, J.L. Analysis of high-affinity binding of protein kinase R to double-stranded RNA. Biochemistry 2012, 51, 8764–8770. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kong, J.; Lyu, B.; Wang, X.; Qian, Q.; Zhou, X.; Qiu, Y. The capsid protein of rubella virus antagonizes RNA interference in mammalian cells. Viruses 2021, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Zhou, H.; Shu, T.; Mu, J.; Fang, Y.; Xu, J.; Li, T.; Kong, J.; Qiu, Y.; Zhou, X. The capsid protein of Semliki Forest virus antagonizes RNA interference in mammalian cells. J Virol 2020, 94, e01233–19. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-X.; Ding, S.-W. Mammalian viral suppressors of RNA interference. Trends Biochem Sci 2022, 47, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liu, Z.; Qiu, Y.; Kong, J.; Fu, Y.; Liu, Y.; Wang, C.; Quan, J.; Wang, Q.; Xu, W.; et al. Inhibition of viral suppressor of RNAi proteins by designer peptides protects from enteroviral infection in vivo. Immunity 2021, 54, 2231–2244e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Rechavi, O. Plant and animal small RNA communications between cells and organisms. Nat Rev Mol Cell Biol 2022, 23, 185–203. [Google Scholar] [CrossRef]
- Goic, B.; Vodovar, N.; Mondotte, J.A.; Monot, C.; Frangeul, L.; Blanc, H.; Gausson, V.; Vera-Otarola, J.; Cristofari, G.; Saleh, M.C. RNA-mediated interference and reverse transcription control the persistence of RNA viruses in the insect model drosophila. Nat Immunol 2013, 14, 396–403. [Google Scholar] [CrossRef]
- Tassetto, M.; Kunitomi, M.; Andino, R. Circulating immune cells mediate a systemic RNAi-based adaptive antiviral response in drosophila. Cell 2017, 169, 314–325.e13. [Google Scholar] [CrossRef]
- Schierhorn, K.L.; Sanchez-David, R.Y.; Maillard, P.V. Mammalian antiviral RNAi is on the move. EMBO J 2022, 41, e111210. [Google Scholar] [CrossRef]
- Ding, S.W.; Han, Q.; Wang, J.; Li, W.X. Antiviral RNA interference in mammals. Curr Opin Immunol 1210 2018, 54, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, J.; Xu, Y.; Li, Z.; Wang, B.; Li, Y. The interaction of influenza a NS1 and cellular TRBP protein modulates the function of RNA interference machinery. Front Microbiol 2022, 13, 859420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Ye, Z.; Xu, Y.; Wang, B.; Wang, C.; Dai, Y.; Lu, J.; Lu, B.; Zhang, W.; et al. The activation of antiviral RNA interference not only exists in neural progenitor cells but also in somatic cells in mammals. Emerg Microbes Infect 2020, 9, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Overheul, G.J.; Bauer, L.; van Kuppeveld, F.J.M.; van Rij, R.P. No evidence for viral small RNA production and antiviral function of Argonaute 2 in human cells. Sci Rep 2019, 9, 13752. [Google Scholar] [CrossRef]
- Parameswaran, P.; Sklan, E.; Wilkins, C.; Burgon, T.; Samuel, M.A.; Lu, R.; Ansel, K.M.; Heissmeyer, V.; Einav, S.; Jackson, W.; et al. Six RNA viruses and forty-one hosts: viral small RNAs and modulation of small RNA repertoires in vertebrate and invertebrate systems. PLoS Pathog 2010, 6, e1000764. [Google Scholar] [CrossRef]
- Girardi, E.; Chane-Woon-Ming, B.; Messmer, M.; Kaukinen, P.; Pfeffer, S. Identification of RNase L-dependent, 3′-end-modified, viral small RNAs in Sindbis virus-infected mammalian cells. MBio 2013, 4, e00698–13. [Google Scholar] [CrossRef]
- Kennedy, E.M.; Whisnant, A.W.; Kornepati, A.V.; Marshall, J.B.; Bogerd, H.P.; Cullen, B.R. Production of functional small interfering RNAs by an amino-terminal deletion mutant of human dicer. Proc Natl Acad Sci USA 2015, 112, E6945–E6954. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Dai, Y.; Li, Z.; Wang, J.; Ye, Z.; Ren, Y.; Wang, H.; Li, W.X.; Lu, J.; et al. Efficient dicer processing of virus-derived double-stranded RNAs and its modulation by RIG-I-like receptor LGP2. PLoS Pathog 2021, 17, e1009790.
- Guo, Y.-L.; Gurung, C.; Fendereski, M.; Huang, F. Dicer and PKR as novel regulators of embryonic stem cell fate and antiviral innate immunity. J Immunol 2022, 208, 2259–2266. [Google Scholar] [CrossRef]
- Girardi, E.; Messmer, M.; Lopez, P.; Fender, A.; Chicher, J.; Chane-Woon-Ming, B.; Hammann, P.; Pfeffer, S. Proteomics-based determination of double stranded RNA interactome reveals known and new factors involved in Sindbis virus infection. bioRxiv 2022. [CrossRef]
- Cullen, B.R. Viruses and RNA interference: issues and controversies. J Virol 2014, 88, 12934–12936. [Google Scholar] [CrossRef]
- Witteveldt, J.; Knol, L.I.; Macias, S. MicroRNA-deficient mouse embryonic stem cells acquire a functional interferon response. Elife 2019, 8, e44171. [Google Scholar] [CrossRef]
- Gurung, C.; Fendereski, M.; Sapkota, K.; Guo, J.; Huang, F.; Guo, Y.L. Dicer represses the interferon response and the double-stranded RNA-activated protein kinase pathway in mouse embryonic stem cells. J Biol Chem 2021, 296, 100264. [Google Scholar] [CrossRef] [PubMed]
- Shiromoto, Y.; Sakurai, M.; Qu, H.; Kossenkov, A.V.; Nishikura, K. Processing of Alu small RNAs by DICER/ADAR1 complexes and their RNAi targets. RNA 2020, 26, 1801–1814. [Google Scholar] [CrossRef] [PubMed]
- Sadeq, S.; Al-Hashimi, S.; Cusack, C.M.; Werner, A. Endogenous double-stranded RNA. Noncoding RNA 2021, 7, 15. [Google Scholar] [CrossRef]
- Kim, S.; Ku, Y.; Ku, J.; Kim, Y. Evidence of aberrant immune response by endogenous double-stranded RNAs: attack from within. Bioessays 2019, 41, e1900023. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xu, Y.; Zhang, Y.; Zhou, H.; Deng, Y.Q.; Li, X.F.; Miao, M.; Zhang, Q.; Zhong, B.; Hu, Y.; et al. Human virus-derived small RNAs can confer antiviral immunity in mammals. Immunity 2017, 46, 992–1004e5. [Google Scholar] [CrossRef]
- Chen, G.R.; Sive, H.; Bartel, D.P. A seed mismatch enhances Argonaute2-catalyzed cleavage and partially rescues severely impaired cleavage found in fish. Mol Cell 2017, 68, 1095–1107e5. [Google Scholar] [CrossRef]
- Price, B.D.; Eckerle, L.D.; Ball, L.A.; Johnson, K.L. Nodamura virus RNA replication in Saccharomyces cerevisiae: heterologous gene expression allows replication-dependent colony formation. Journal of virology 2005, 79, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.S.; Ganem, D. A virus-encoded inhibitor that blocks RNA interference in mammalian cells. Journal of virology 2005, 79, 7371–7379. [Google Scholar] [CrossRef] [PubMed]
- Aliyari, R.; Wu, Q.; Li, H.W.; Wang, X.H.; Li, F.; Green, L.D.; Han, C.S.; Li, W.X.; Ding, S.W. (2008). Mechanism of induction and suppression of antiviral immunity directed by virus-derived small RNAs in Drosophila. Cell host.; microbe, 4, 387–397.
- Li, Y.; Lu, J.; Han, Y.; Fan, X.; Ding, S.W. RNA interference functions as an antiviral immunity mechanism in mammals. Science (New York, N.Y.) 2013, 342, 231–234. [Google Scholar]
- Bogerd, H.P.; Skalsky, R.L.; Kennedy, E.M.; Furuse, Y.; Whisnant, A.W.; Flores, O.; Schultz, K.L.; Putnam, N.; Barrows, N.J.; Sherry, B.; Scholle, F.; Garcia-Blanco, M.A.; Griffin, D.E.; Cullen, B.R. Replication of many human viruses is refractory to inhibition by endogenous cellular microRNAs. Journal of virology 2014, 88, 8065–8076. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



