Submitted:
31 July 2024
Posted:
31 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Cell Culture
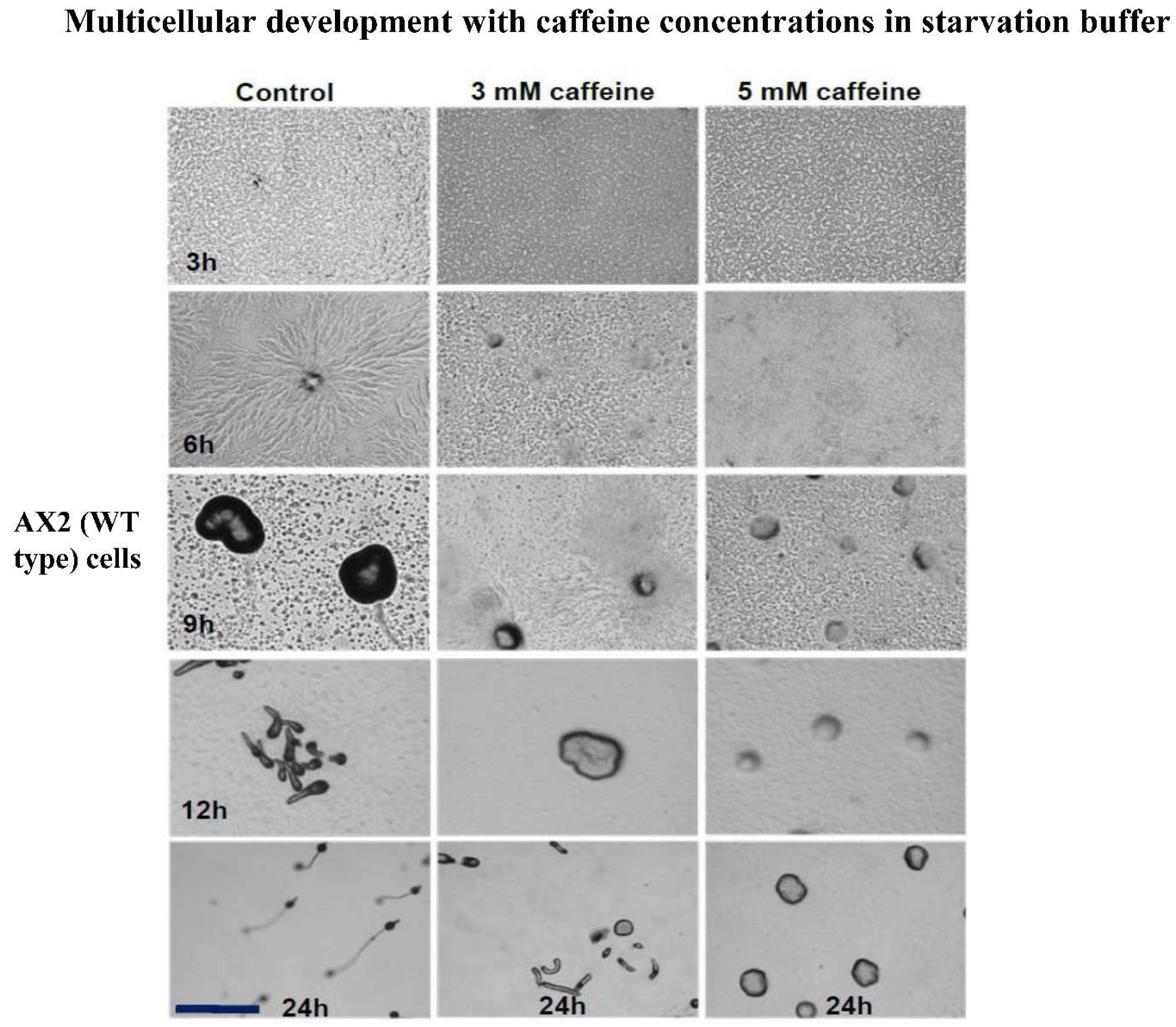
Assay for the Aggregation Pattern with Caffeine
Immunofluorescence Detection of Cellular Proteins
Western Blotting
Result
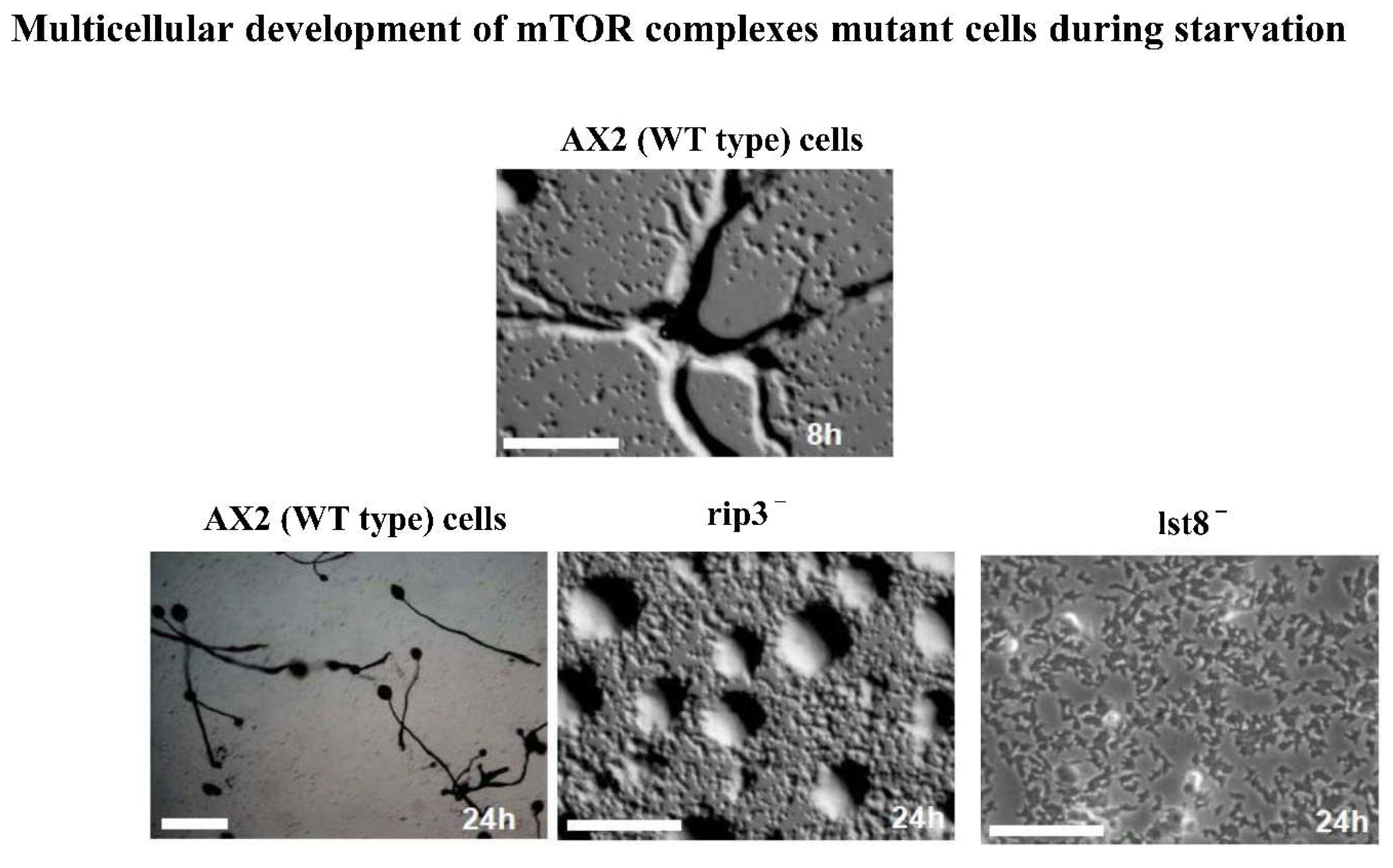
Defective Multi-Cell Aggregates Formation in lst-8- and rip-3- Cells
Reduced Aggregate Size of Mutants in mTOR Complexes I and II Components Resembles Aggregates Formed in the Presence of Caffeine
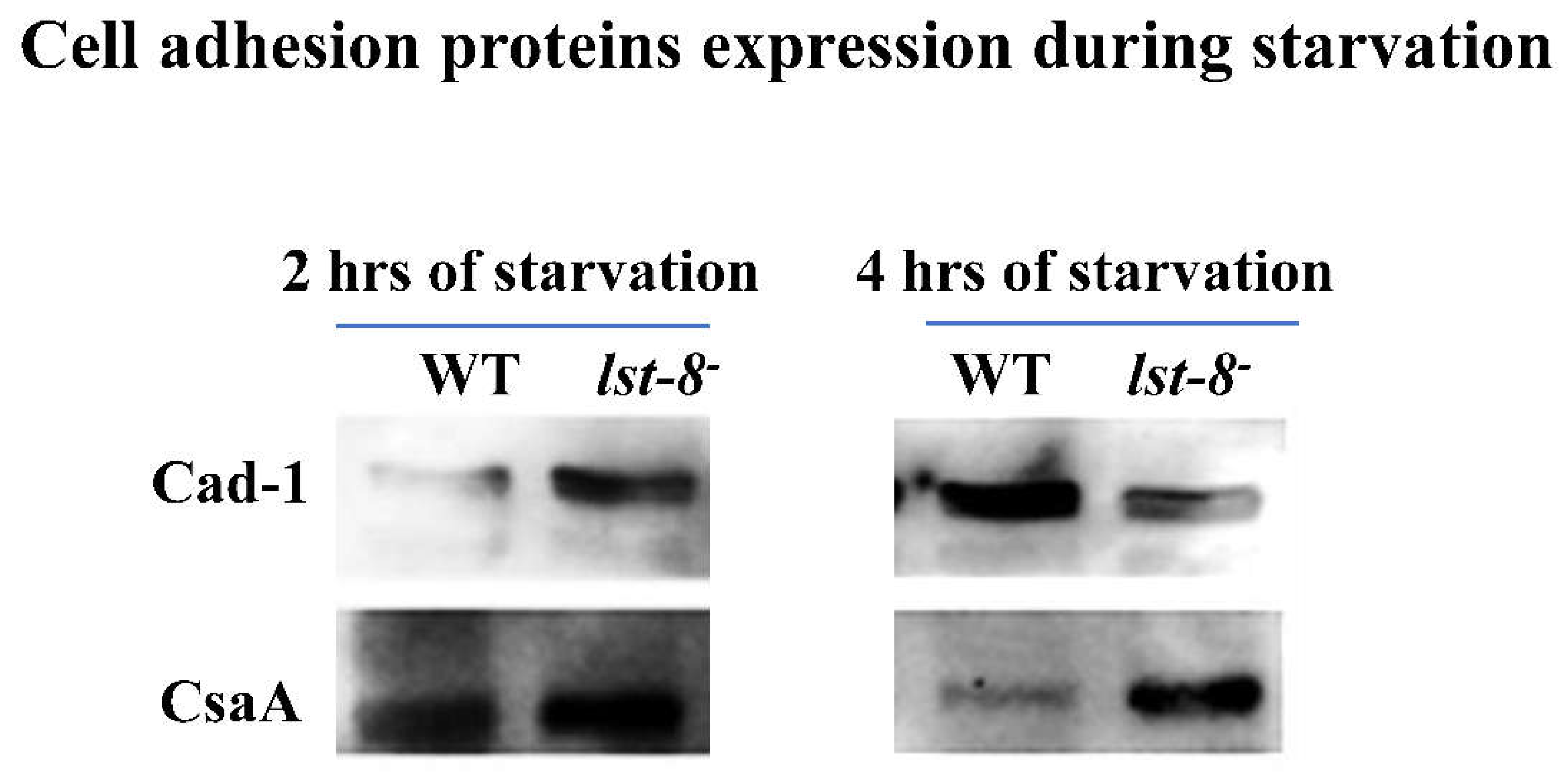
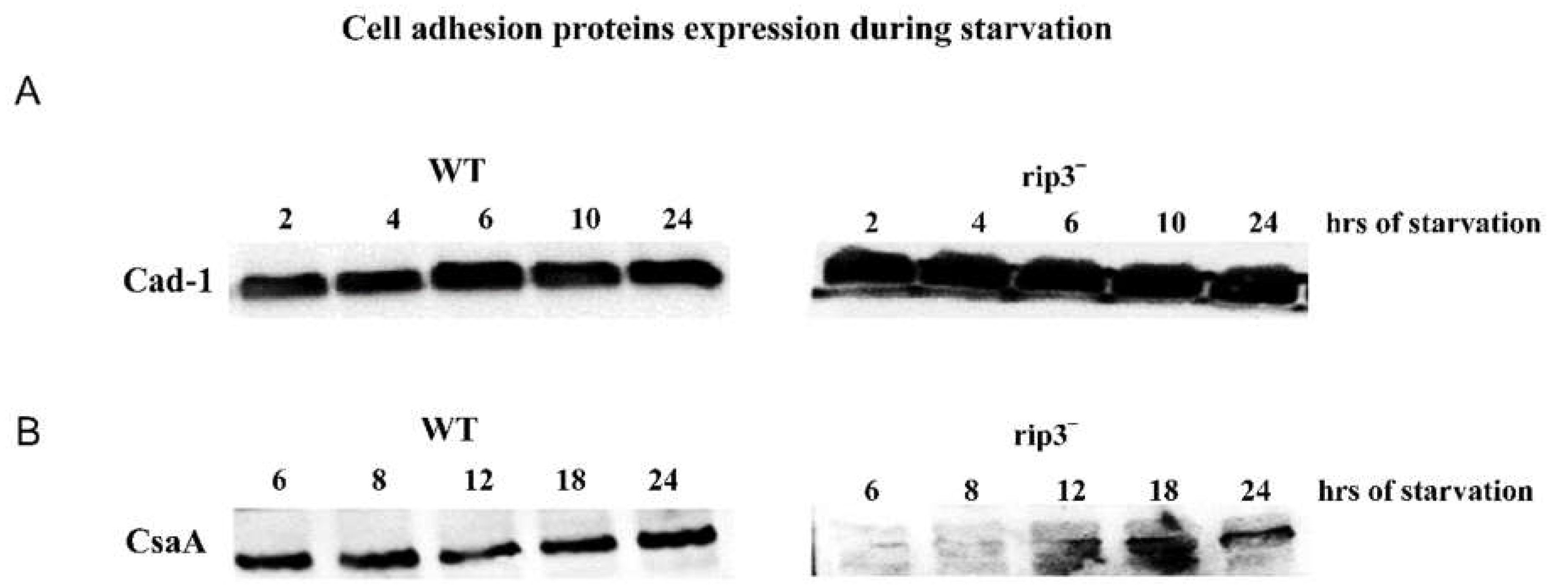
Expression of the Cell Adhesion Proteins Cad-1 and CsaA Is Impaired in lst-8-and rip-3- Cells
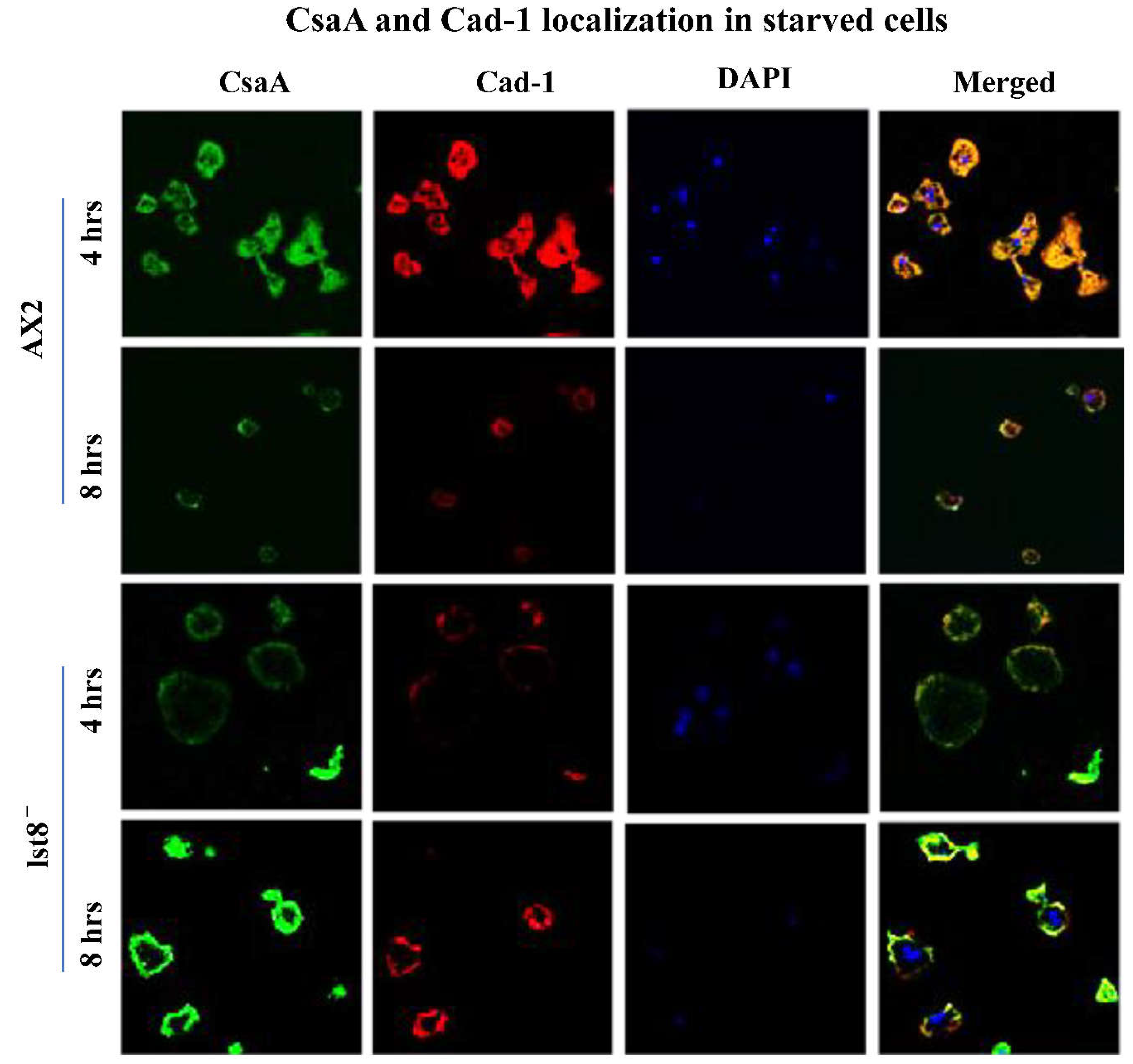
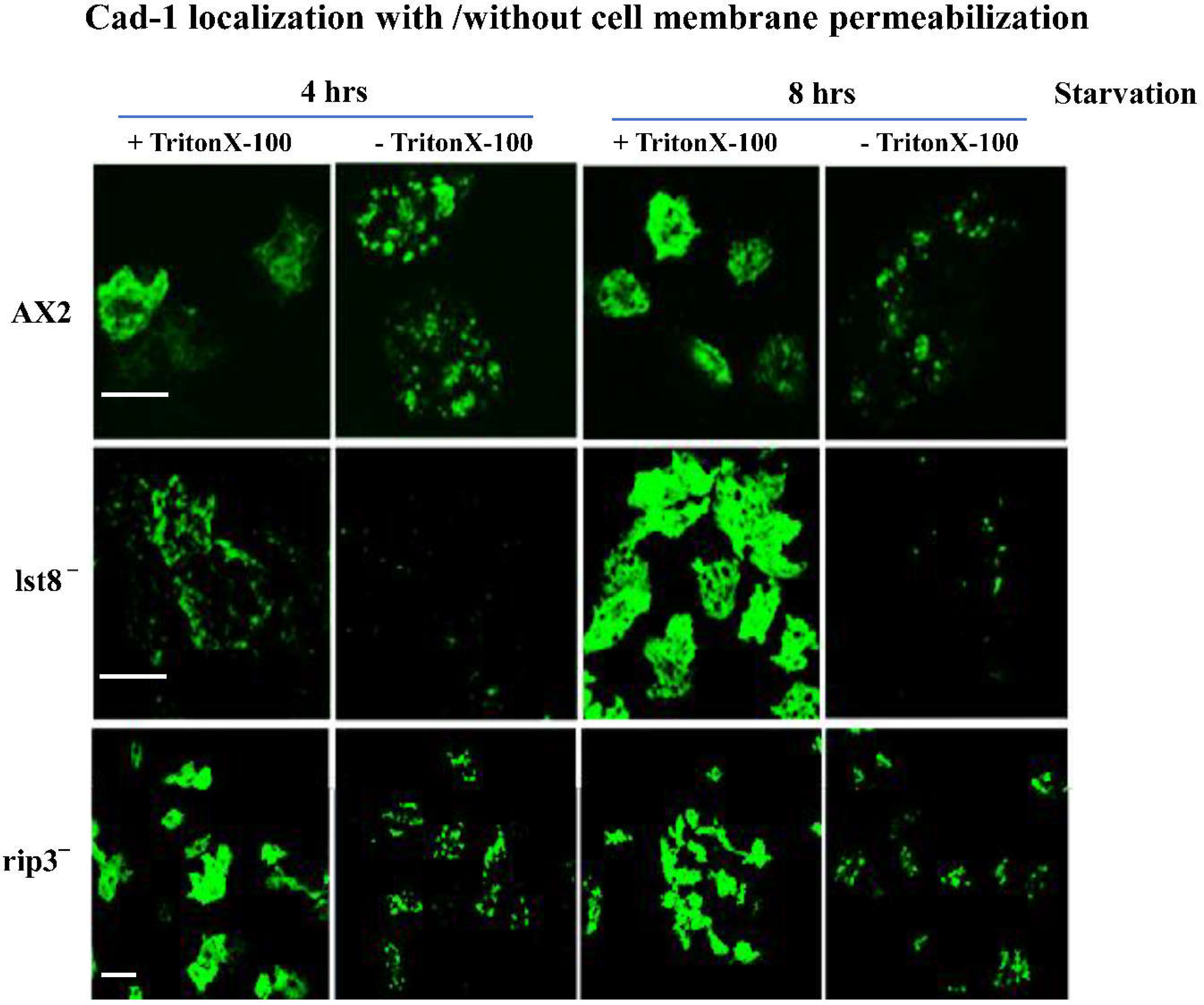
Localization of Cell Adhesion Proteins Cad-1 and CsaA Is Defective in lst-8- Cells
Discussion
Conclusions
References
- Beug, H.; Katz, F.E.; Gerisch, G. DYNAMICS OF ANTIGENIC MEMBRANE SITES RELATING TO CELL AGGREGATION IN DICTYOSTELIUM DISCOIDEUM. Journal of Cell Biology 1973, 56, 647–658. [Google Scholar] [CrossRef]
- Sriskanthadevan, S.; Zhu, Y.; Manoharan, K.; Yang, C.; Siu, C.-H. The cell adhesion molecule DdCAD-1 regulates morphogenesis through differential spatiotemporal expression in Dictyostelium discoideum. Development 2011, 138, 2487–2497. [Google Scholar] [CrossRef]
- Sesaki, H.; Siu, C.-H. Novel Redistribution of the Ca2+-Dependent Cell Adhesion Molecule DdCAD-1 during Development ofDictyostelium discoideum. Developmental Biology 1996, 177, 504–516. [Google Scholar] [CrossRef]
- Jaiswal, P.; Soldati, T.; Thewes, S.; Baskar, R. Regulation of aggregate size and pattern by adenosine and caffeine in cellular slime molds. BMC developmental biology 2012, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Siu, C.H.; Cho, A.; Choi, A.H. The contact site A glycoprotein mediates cell-cell adhesion by homophilic binding in Dictyostelium discoideum. J Cell Biol 1987, 105, 2523–2533. [Google Scholar] [CrossRef] [PubMed]
- Faix, J.; Gerisch, G.; Noegel, A.A. Overexpression of the csA cell adhesion molecule under its own cAMP-regulated promoter impairs morphogenesis in Dictyostelium. J Cell Sci 1992, 102 ( Pt 2), 203–214. [Google Scholar] [CrossRef]
- Müller, K.; Gerisch, G. A specific glycoprotein as the target site of adhesion blocking Fab in aggregating Dictyostelium cells. Nature 1978, 274, 445–449. [Google Scholar] [CrossRef]
- Meena, N.P.; Jaiswal, P.; Chang, F.-S.; Brzostowski, J.; Kimmel, A.R. DPF is a cell-density sensing factor, with cell-autonomous and non-autonomous functions during Dictyostelium growth and development. BMC biology 2019, 17, 1–21. [Google Scholar] [CrossRef]
- Clarke, M.; Gomer, R.H. PSF and CMF, autocrine factors that regulate gene expression during growth and early development of Dictyostelium. Experientia 1995, 51, 1124–1134. [Google Scholar] [CrossRef]
- Roisin-Bouffay, C.; Jang, W.; Caprette, D.R.; Gomer, R.H. A precise group size in Dictyostelium is generated by a cell-counting factor modulating cell-cell adhesion. Mol Cell 2000, 6, 953–959. [Google Scholar] [CrossRef]
- Jaiswal, P.; Kimmel, A.R. mTORC1/AMPK responses define a core gene set for developmental cell fate switching. BMC biology 2019, 17, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P. Caffeine and Rapamycin Impair Growth and Morphogenesis by Affecting Both TOR-Dependent Pathways in Dictyostelium. 2024.
- Jaiswal, P.; Majithia, A.R.; Rosel, D.; Liao, X.-H.; Khurana, T.; Kimmel, A.R. Integrated actions of mTOR complexes 1 and 2 for growth and development of Dictyostelium. International Journal of Developmental Biology 2019, 63, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal Pundrik, K.A.R. Nutrient/Starvation sensing for Reciprocal mTORC1/AMPK response in Dictyostelium, at the junction between Growth and Development. In Proceedings of the The FASEB Journal; 2018; p. 141. [Google Scholar]
- Jaiswal, P.; Meena, N.P.; Chang, F.-S.; Liao, X.-H.; Kim, L.; Kimmel, A. An Integrated, Cross-Regulation Pathway Model Involving Activating/Adaptive and Feed-Forward/Feed-Back Loops for Directed Oscillatory cAMP Signal-Relay/Response during the Development of Dictyostelium. Frontiers in Cell and Developmental Biology 2024, 11, 1263316. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Comer, F.I.; Sasaki, A.; McLeod, I.X.; Duong, Y.; Okumura, K.; Yates, J.R.; Parent, C.A.; Firtel, R.A. TOR Complex 2 Integrates Cell Movement during Chemotaxis and Signal Relay in Dictyostelium. Molecular Biology of the Cell 2005, 16, 4572–4583. [Google Scholar] [CrossRef] [PubMed]
- Rosel, D.; Khurana, T.; Majithia, A.; Huang, X.; Bhandari, R.; Kimmel, A.R. TOR complex 2 (TORC2) in Dictyostelium suppresses phagocytic nutrient capture independently of TORC1-mediated nutrient sensing. J Cell Sci 2012, 125, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Parent, C.A.; Insall, R.; Firtel, R.A. A Novel Ras-interacting Protein Required for Chemotaxis and Cyclic Adenosine Monophosphate Signal Relay inDictyostelium. Molecular Biology of the Cell 1999, 10, 2829–2845. [Google Scholar] [CrossRef]
- Singh, S.P.; Dhakshinamoorthy, R.; Jaiswal, P.; Schmidt, S.; Thewes, S.; Baskar, R. The thyroxine inactivating gene, type III deiodinase, suppresses multiple signaling centers in Dictyostelium discoideum. Developmental biology 2014, 396, 256–268. [Google Scholar] [CrossRef]
- Hagedorn, M.; Rohde, K.H.; Russell, D.G.; Soldati, T. Infection by Tubercular Mycobacteria Is Spread by Nonlytic Ejection from Their Amoeba Hosts. Science 2009, 323, 1729–1733. [Google Scholar] [CrossRef]
- Jaiswal, P.; Singh, S.P.; Aiyar, P.; Akkali, R.; Baskar, R. Regulation of multiple tip formation by caffeine in cellular slime molds. BMC developmental biology 2012, 12, 1–14. [Google Scholar] [CrossRef]
- Senoo, H.; Kamimura, Y.; Kimura, R.; Nakajima, A.; Sawai, S.; Sesaki, H.; Iijima, M. Phosphorylated Rho–GDP directly activates mTORC2 kinase towards AKT through dimerization with Ras–GTP to regulate cell migration. Nature Cell Biology 2019, 21, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Katoh-Kurasawa, M.; Muramoto, T.; Santhanam, B.; Long, Y.; Li, L.; Ueda, M.; Iglesias, P.A.; Shaulsky, G.; Devreotes, P.N. Nucleocytoplasmic Shuttling of a GATA Transcription Factor Functions as a Development Timer. Science 2014, 343, 1249531. [Google Scholar] [CrossRef]
- Smith, S.F.; Collins, S.E.; Charest, P.G. Ras, PI3K and mTORC2 - three’s a crowd? J Cell Sci 2020, 133. [Google Scholar] [CrossRef]
- Fu, W.; Hall, M.N. Regulation of mTORC2 Signaling. Genes 2020, 11, 1045. [Google Scholar] [CrossRef]
- Secko, D.M.; Siu, C.H.; Spiegelman, G.B.; Weeks, G. An activated Ras protein alters cell adhesion by dephosphorylating Dictyostelium DdCAD-1. Microbiology (Reading) 2006, 152, 1497–1505. [Google Scholar] [CrossRef]
- Cardenas, M.E.; Cutler, N.S.; Lorenz, M.C.; Di Como, C.J.; Heitman, J. The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev 1999, 13, 3271–3279. [Google Scholar] [CrossRef]
- Tariqul Islam, A.F.M.; Scavello, M.; Lotfi, P.; Daniel, D.; Haldeman, P.; Charest, P.G. Caffeine inhibits PI3K and mTORC2 in Dictyostelium and differentially affects multiple other cAMP chemoattractant signaling effectors. Molecular and Cellular Biochemistry 2019, 457, 157–168. [Google Scholar] [CrossRef]
- Soulard, A.; Cohen, A.; Hall, M.N. TOR signaling in invertebrates. Curr Opin Cell Biol 2009, 21, 825–836. [Google Scholar] [CrossRef]
- Brenner, M.; Thoms, S.D. Caffeine blocks activation of cyclic AMP synthesis in Dictyostelium discoideum. Dev Biol 1984, 101, 136–146. [Google Scholar] [CrossRef]
- Powers, T.; Walter, P. Regulation of ribosome biogenesis by the rapamycin-sensitive TOR-signaling pathway in Saccharomyces cerevisiae. Mol Biol Cell 1999, 10, 987–1000. [Google Scholar] [CrossRef]
- Mayer, C.; Grummt, I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 2006, 25, 6384–6391. [Google Scholar] [CrossRef]
- Jiao, L.; Liu, Y.; Yu, X.-Y.; Pan, X.; Zhang, Y.; Tu, J.; Song, Y.-H.; Li, Y. Ribosome biogenesis in disease: new players and therapeutic targets. Signal Transduction and Targeted Therapy 2023, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Iadevaia, V.; Liu, R.; Proud, C.G. mTORC1 signaling controls multiple steps in ribosome biogenesis. Semin Cell Dev Biol 2014, 36, 113–120. [Google Scholar] [CrossRef]
- Papadopoli, D.; Boulay, K.; Kazak, L.; Pollak, M.; Mallette, F.A.; Topisirovic, I.; Hulea, L. mTOR as a central regulator of lifespan and aging. F1000Res 2019, 8. [Google Scholar] [CrossRef]
- Shor, B.; Wu, J.; Shakey, Q.; Toral-Barza, L.; Shi, C.; Follettie, M.; Yu, K. Requirement of the mTOR kinase for the regulation of Maf1 phosphorylation and control of RNA polymerase III-dependent transcription in cancer cells. J Biol Chem 2010, 285, 15380–15392. [Google Scholar] [CrossRef]
- Kantidakis, T.; Ramsbottom, B.A.; Birch, J.L.; Dowding, S.N.; White, R.J. mTOR associates with TFIIIC, is found at tRNA and 5S rRNA genes, and targets their repressor Maf1. Proc Natl Acad Sci U S A 2010, 107, 11823–11828. [Google Scholar] [CrossRef]
- Chauhan, S.; Jena, K.K.; Mehto, S.; Chauhan, N.R.; Sahu, R.; Dhar, K.; Yadav, R.; Krishna, S.; Jaiswal, P.; Chauhan, S. Innate immunity and inflammophagy: balancing the defence and immune homeostasis. The FEBS Journal 2022, 289, 4112–4131. [Google Scholar] [CrossRef]
- Bai, J.; Liu, F. The cGAS-cGAMP-STING Pathway: A Molecular Link Between Immunity and Metabolism. Diabetes 2019, 68, 1099–1108. [Google Scholar] [CrossRef]
- Mehto, S.; Jena, K.K.; Yadav, R.; Priyadarsini, S.; Samal, P.; Krishna, S.; Dhar, K.; Jain, A.; Chauhan, N.R.; Murmu, K.C. Selective autophagy of RIPosomes maintains innate immune homeostasis during bacterial infection. The EMBO Journal 2022, 41, e111289. [Google Scholar] [CrossRef]
- Tattoli, I.; Philpott, D.J.; Girardin, S.E. The bacterial and cellular determinants controlling the recruitment of mTOR to the Salmonella-containing vacuole. Biol Open 2012, 1, 1215–1225. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



