Submitted:
30 July 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Reagents
Animal Protocols
Isolation of RNA
Time Course of Midkine Expression
Measurement of mRNA
Measurement of Inflammatory Cytokine Levels
Pathological Evaluation of Lung Sections
Cell Culture
Knockdown of Midkine
Statistical Analysis
Results
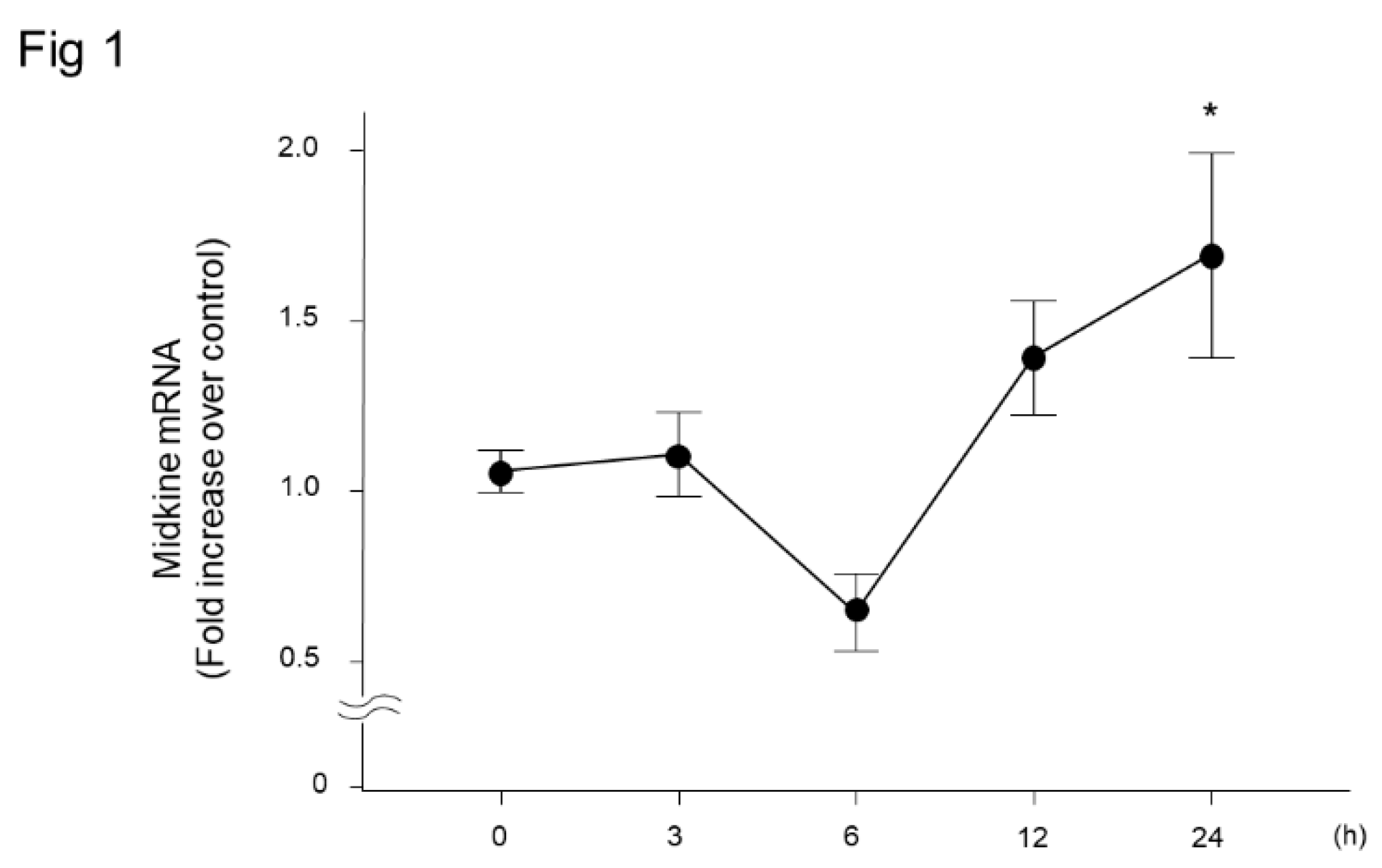
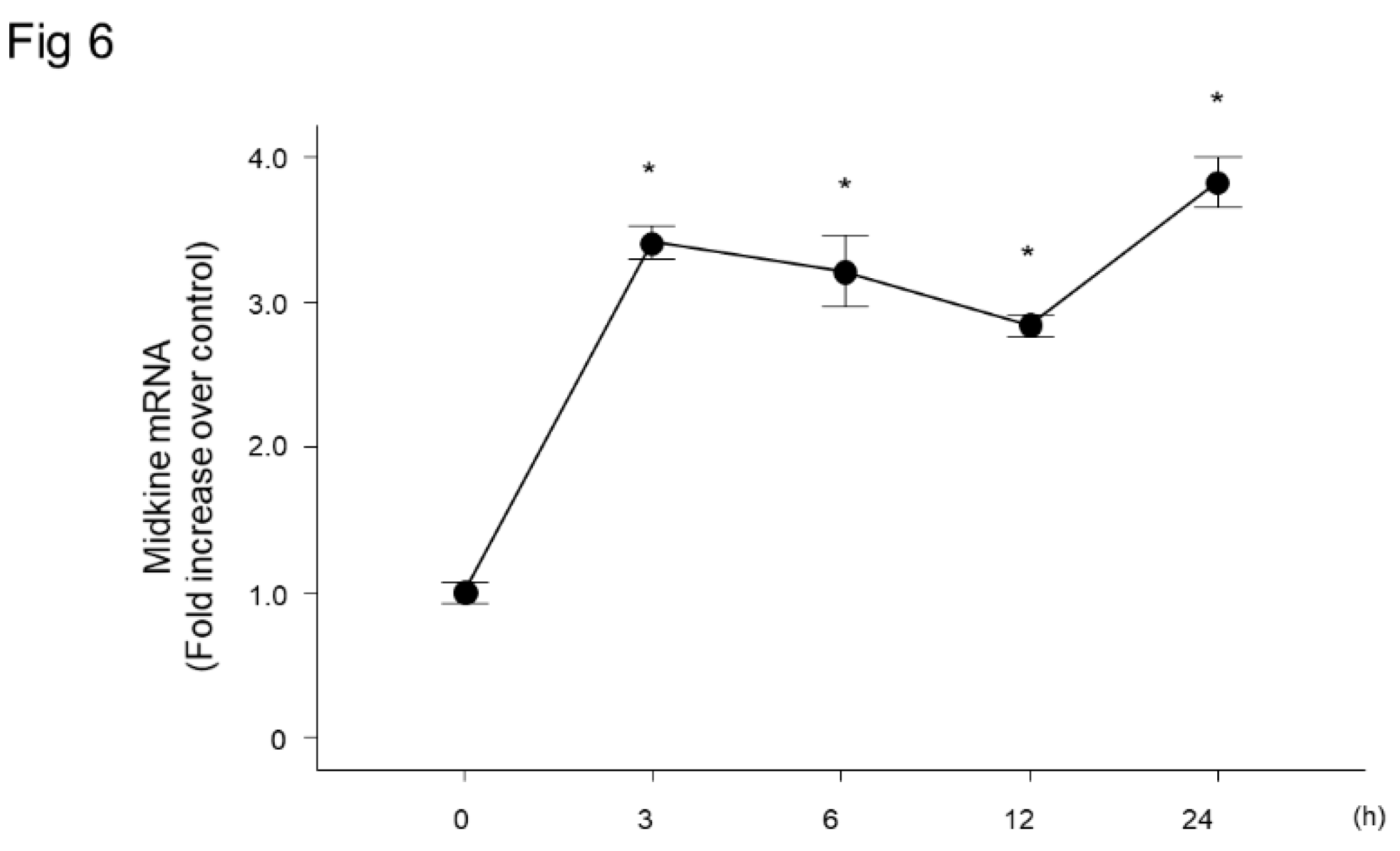
Change in Midkine Expression in Lung Tissues after LPS Administration
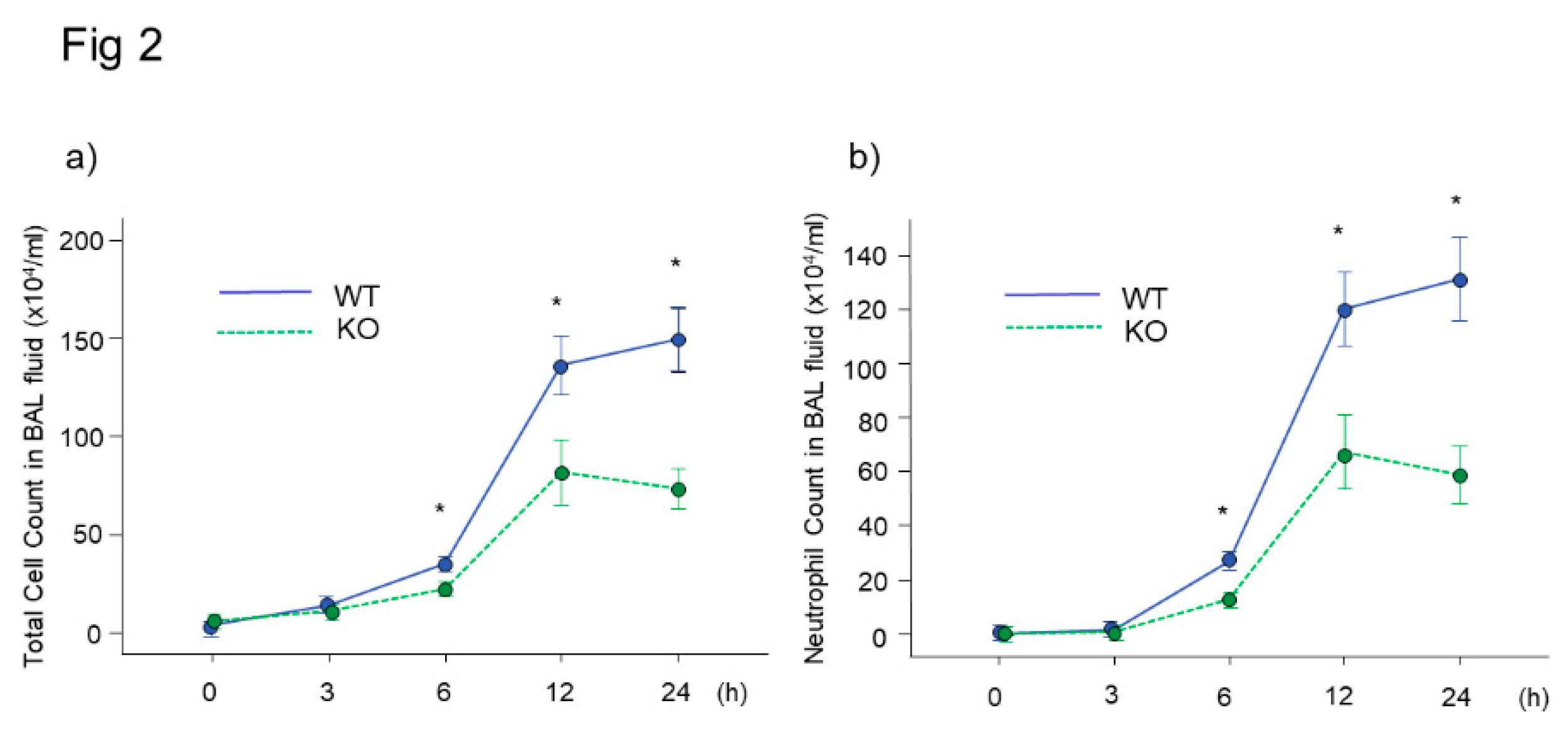
Bronchoalveolar Lavage Findings in Midkine-Deficient Mice
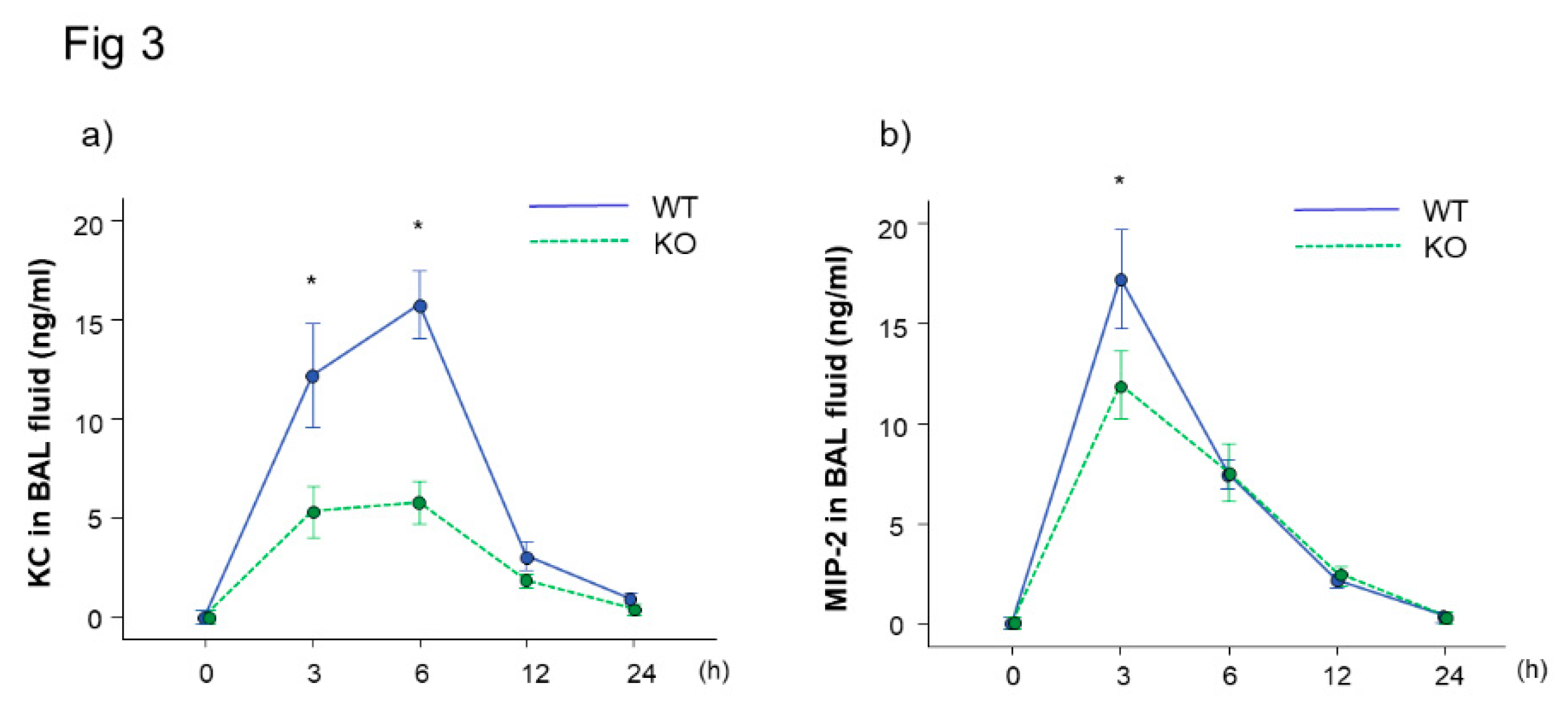
Inflammatory Cytokine Concentration in Bronchoalveolar Lavage Fluid of Midkine-Deficient Mice
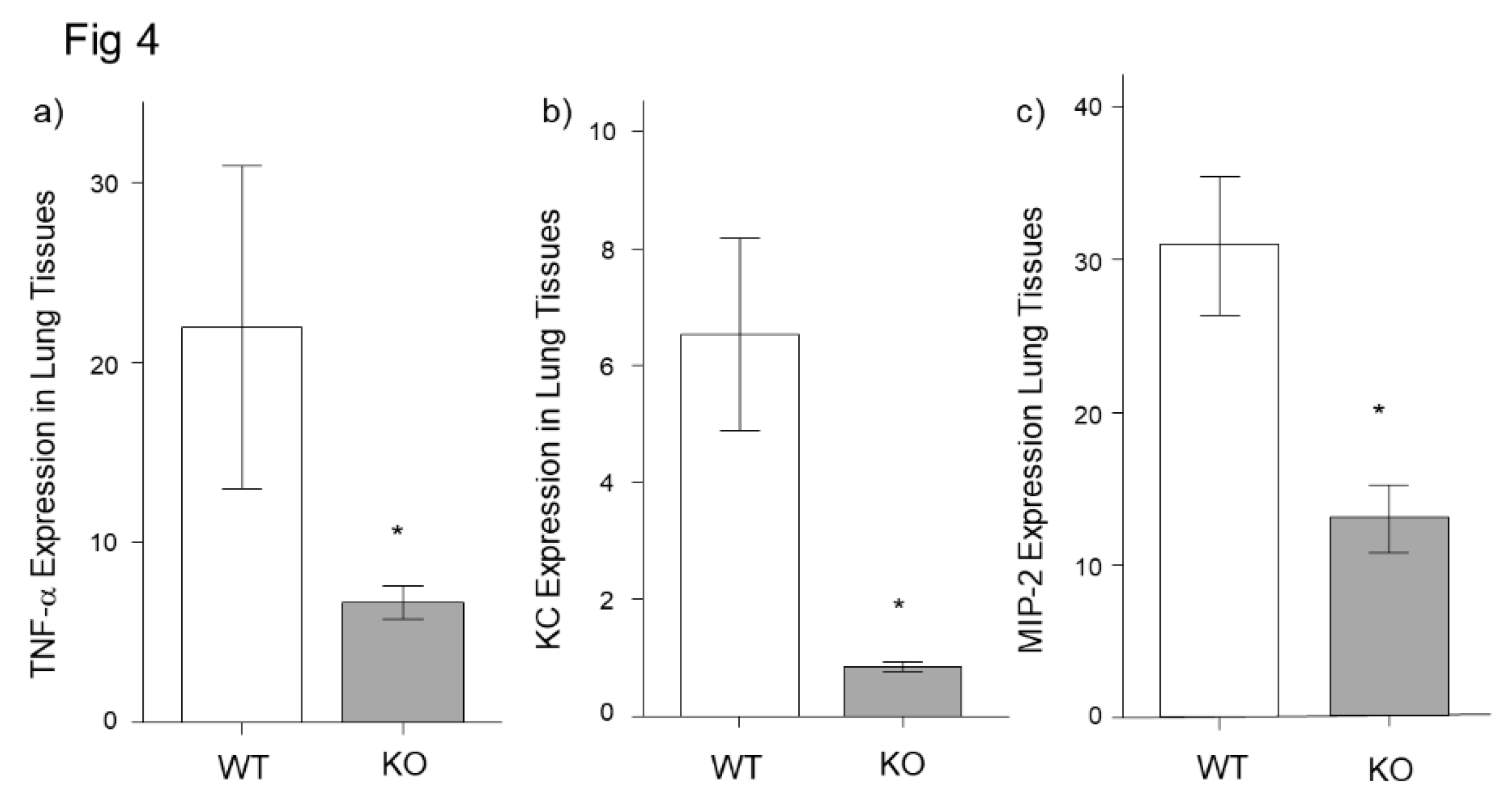
Inflammatory Cytokine Expression in Lung Tissues of Midkine-deficient Mice
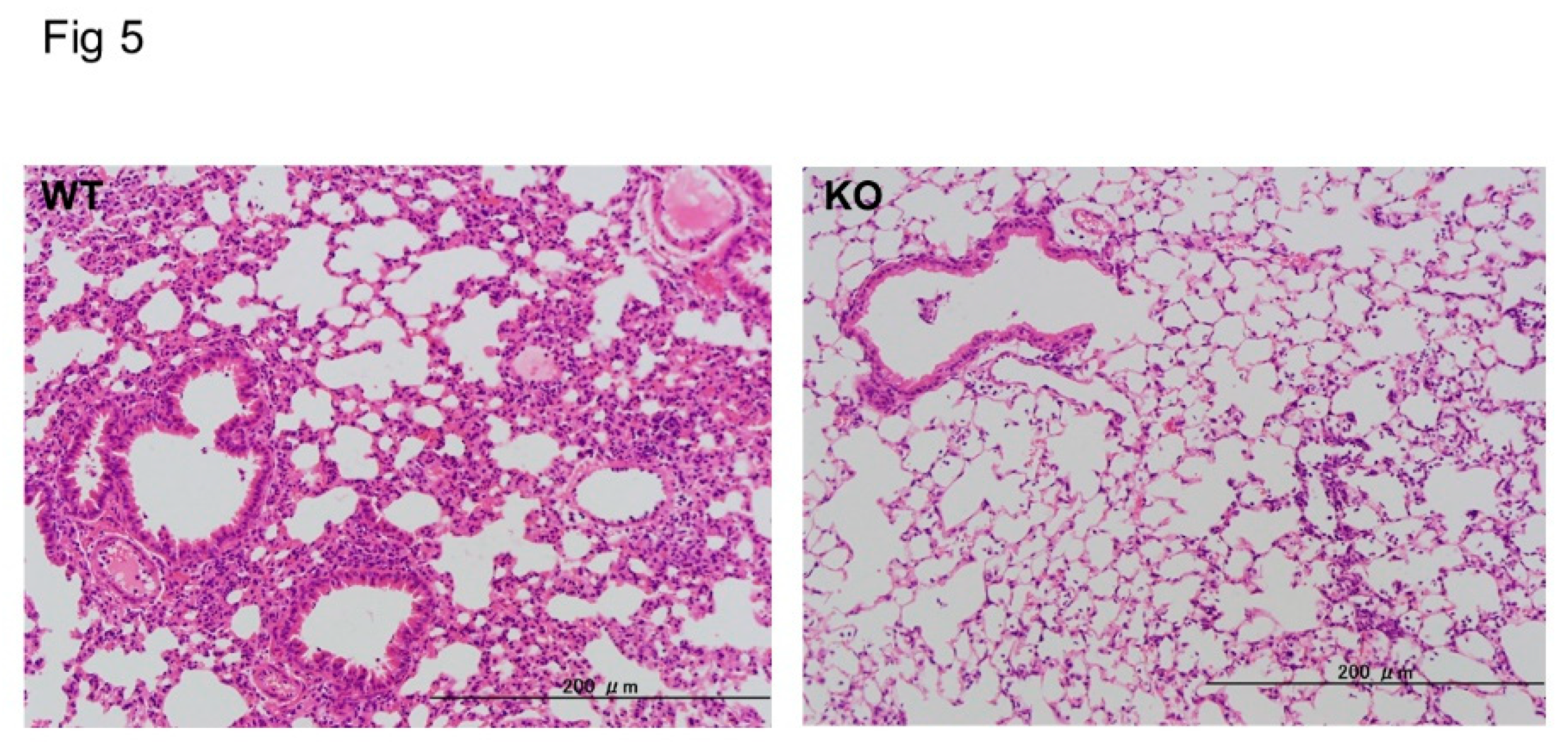
Histopathological Analysis of Midkine-Deficient Mice
Change in Midkine mRNA Expression in Bronchial Epithelial Cells after LPS Stimulation
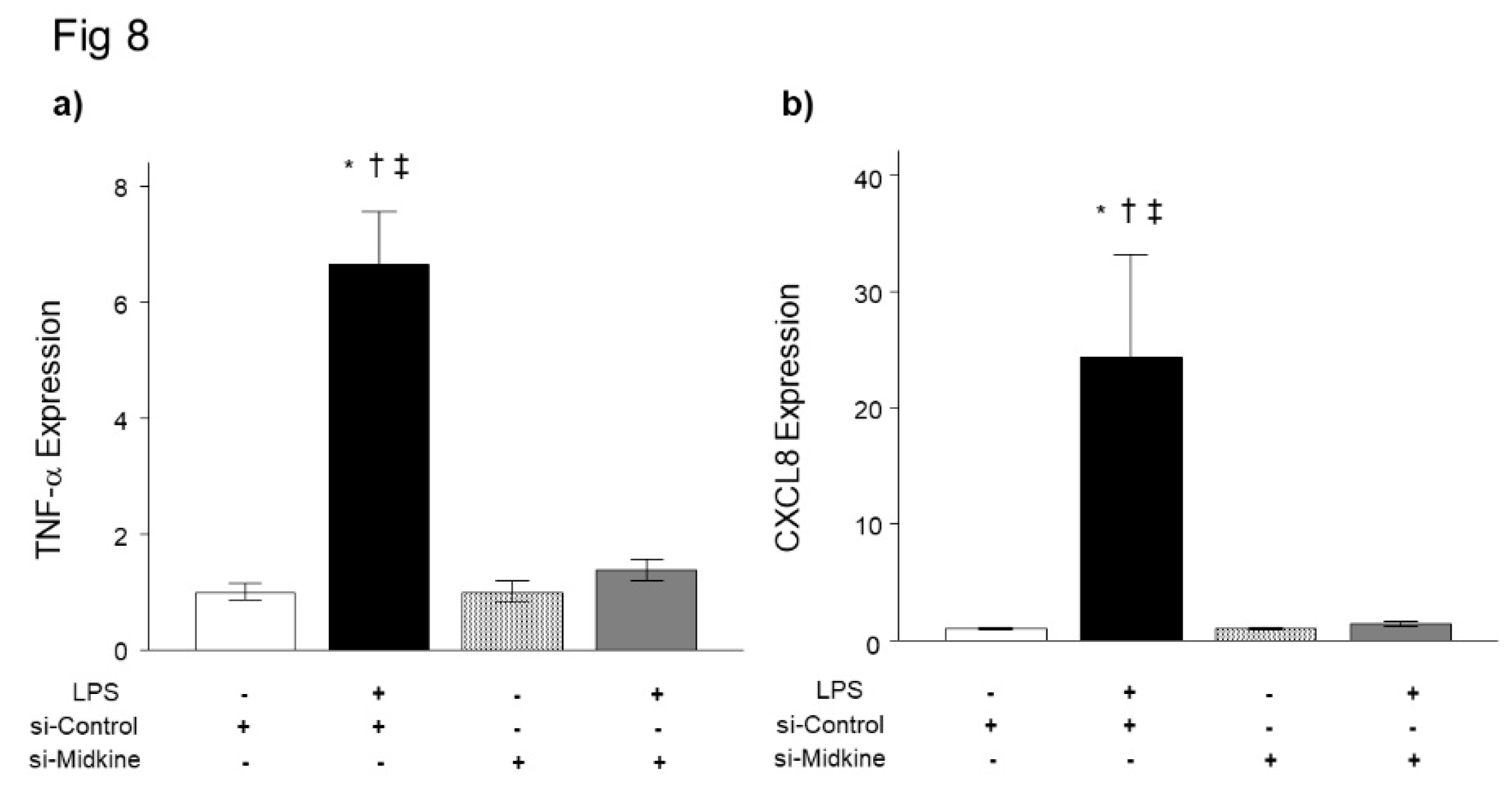
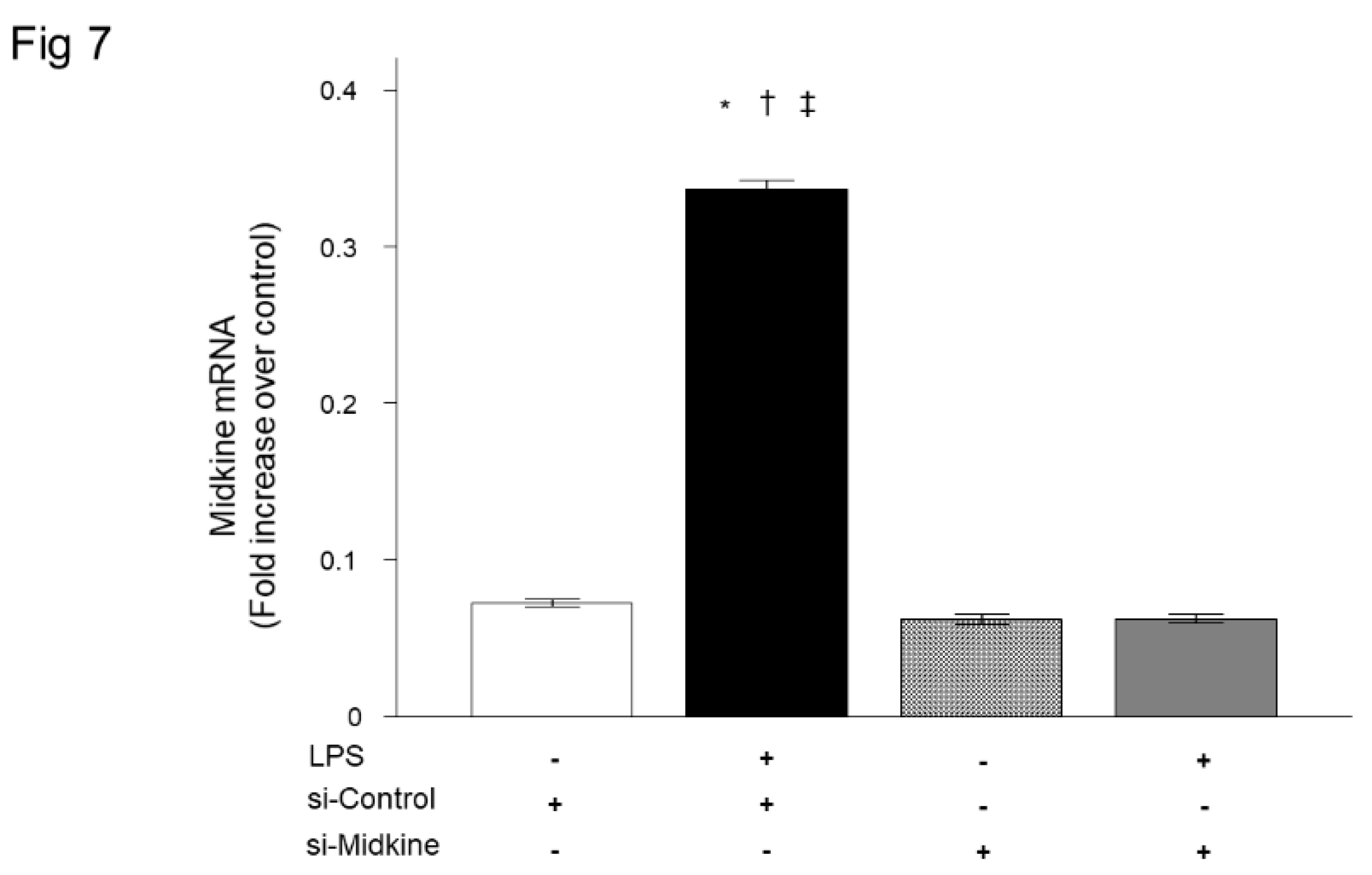
Effect of Midkine Knockdown on LPS-induced TNF-α and CXCL8 Expressions in Bronchial Epithelial Cells
Discussion
Authors Contributions
Conflicts of Interest
References
- Matthay, MA.; Zemans, RL.; Zimmerman, GA.; Yaseen M Arabi, YM.; Beitler, JR.; Mercat, A.; Margaret Herridge, M.; Adrienne G Randolph, AG.; Carolyn S Calfee, CS. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Laffey, JG.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, DF.; Ranieri, M.; Rubenfeld, G.; Thompson, BT.; Wrigge, H.; Slutsky, AS.; Pesenti, A.; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Kadomatsu, K.; Tomomura, M.; Muramatsu, T. cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem. Biophys. Res. Commun. 1988, 151, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Kadomatsu, K.; Huang, RP.; Suganuma, T.; Murata, F.; Muramatsu, T. A retinoic acid responsive gene MK found in the teratocarcinoma system is expressed in spatially and temporally controlled manner during mouse embryogenesis. J. Cell Biol. 1990, 110, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Tomomura, M.; Kadomatsu, K.; Muramatsu, T. Structure of a retinoic acid-responsive gene, MK, which is transiently activated during the differentiation of embryonal carcinoma cells and the mid-gestation period of mouse embryogenesis. J. Biol. Chem. 1990, 265, 9441–9443. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, J.; Kadomatsu, K.; Matsubara, S.; Nakagawara, A.; Hamanoue, M.; Takao, S.; Shimazu, H.; Ohi, Y.; Muramatsu, T. A new family of heparin-binding growth/differentiation factors: increased midkine expression in Wilms’ tumor and other human carcinomas. Cancer Res. 1993, 53, 1281–1285. [Google Scholar] [PubMed]
- Ohta, S.; Muramatsu, H.; Senda, T.; Zou, K.; Iwata, H.; Muramatsu, T. Midkine is expressed during repair of bone fracture and promotes chondrogenesis. J. Bone Miner. Res. 1999, 14, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Misa, K.; Tanino, Y.; Wang, X.; Nikaido, T.; Kikuchi, M.; Sato, Y.; Togawa, R.; Tanino, M.; Tanaka, S.; Kadomatsu, K.; Munakata, M. Involvement of midkine in the development of pulmonary fibrosis. Physiol. Rep. 2017, 5, e13383. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J. Biochem. 2002, 132, 359–371. [Google Scholar] [CrossRef]
- Kinoshita, D.; Shishido, T.; Takahashi, T.; Yokoyama, M.; Sugai, T.; Watanabe, K.; Tamura, H.; Nishiyama, S.; Takahashi, H.; Arimoto, T.; Miyamoto, T.; Watanabe, T.; Kishida, S.; Kadomatsu, K.; Abe, J.; Takeishi, Y.; Konta, T.; Kubota, I.; Watanabe, M. Growth factor midkine aggravates pulmonary arterial hypertension via surface nucleolin. Sci. Rep. 2020, 10, 10345. [Google Scholar] [CrossRef]
- Nordin, SL.; Jovic, S.; Kurut, A.; Andersson, C.; Gela, A.; Bjartell, A.; Morgelin, M.; Olin, AI.; Lund, M.; Egesten, A. High expression of midkine in the airways of patients with cystic fibrosis. Am. J. Respir. Cell. Mol. Biol. 2013, 49, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Linge, HM.; Andersson, C.; Nordin, SL.; Olin, AI.; Petersson, AC.; Mörgelin, M.; Welin, A.; Bylund, J.; Bjermer, L.; Erjefält, J.; Egesten, A. Midkine is expressed and differentially processed during chronic obstructive pulmonary disease exacerbations and ventilator-associated pneumonia associated with Staphylococcus aureus infection. Mol. Med. 2013, 19, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Netsu, S.; Shishido, T.; Kitahara, T.; Honda, Y.; Funayama, A.; Narumi, T.; Kadowaki, S.; Takahashi, H.; Miyamoto, T.; Arimoto, T.; Nishiyama, S.; Watanabe, T.; Woo, CH.; Takeishi, Y.; Kubota, I. Midkine exacerbates pressure overload-induced cardiac remodeling. Biochem. Biophys. Res. Commun. 2014, 443, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Pan, Y.; Fanelli, V.; Wu, S.; Luo, AA.; Islam, D.; Han, B.; Mao, P.; Ghazarian, M.; Zeng, W.; Spieth, PM.; Wang, D.; Khang, J.; Mo, H.; Liu, X.; Uhlig, S.; Liu, M.; Laffey, J.; Slutsky, AS.; Li, Y.; Zhang, H. Mechanical Stress and the Induction of Lung Fibrosis via the Midkine Signaling Pathway. Am. J. Respir. Crit. Care. Med. 2015, 192, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, E.; Kadomatsu, K.; Yuasa, S.; Muramatsu, H.; Mamiya, T.; Nabeshima, T.; Fan, QW.; Ishiguro, K.; Matsubara, S.; Kaname, T.; Horiba, M.; Saito, H.; Muramatsu, T. Disruption of the midkine gene (Mdk) results in altered expression of calcium binding protein in the hippocampus of infant mice and their abnormal behaviour. Genes Cells 1998, 3, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, T.; Tanino, Y.; Wang, X.; Sato, S.; Misa, K.; Fukuhara, N.; Sato, Y.; Fukuhara, A.; Uematsu, M.; Suzuki, Y.; Kojima, T.; Tanino, M.; Endo, Y.; Tsuchiya, K.; Kawamura, I.; Frevert, CW.; Munakata, M. Serum syndecan-4 as a possible biomarker in patients with acute pneumonia. J. Infect. Dis. 2015, 212, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Tanino, Y.; Makita, H.; Miyamoto, K.; Betsuyaku, T.; Ohtsuka, Y.; Nishihira, J.; Nishimura, M. Role of macrophage migration inhibitory factor in bleomycin-induced lung injury and fibrosis in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 283, L156–62. [Google Scholar] [CrossRef] [PubMed]
- Tanino, Y.; Chang, MY.; Wang, X.; Gill, SE.; Skerrett, S.; McGuire, JK.; Sato, S.; Nikaido, T.; Kojima, T.; Munakata, M.; Mongovin, S.; Park, WC.; Martin, TR.; Wight, TN.; Frevert, CW. Syndecan-4 regulates early neutrophil migration and pulmonary inflammation in response to lipopolysaccharide. Am. J. Respir. Cell Mol. Biol. 2012, 47, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Tanino, Y.; Wang, X.; Nikaido, T.; Misa, K.; Sato, Y.; Togawa, R.; Kawamata, T.; Kikuchi, M.; Frevert, CW.; Tanino, M.; Kojima, T.; Shibata, Y. Syndecan-4 inhibits the development of pulmonary fibrosis by attenuating TGF-β signaling. Int. J. Mol. Sci. 2019, 20, 4989. [Google Scholar] [CrossRef]
- Tang, SE.; Wu, SY.; Chu, SJ.; Tzeng, YS.; Peng, CK.; Lan, CC.; Perng, WC.; Wu, CP.; Huang, KL. Pre-treatment with ten-minute carbon dioxide inhalation prevents lipopolysaccharide-induced lung injury in mice via down-regulation of toll-like receptor 4 expression. Int. J. Mol. Sci. 2019, 20, 6293. [Google Scholar] [CrossRef]
- Sato, W.; Kadomatsu, K.; Yuzawa, Y.; Muramatsu, H.; Hotta, N.; Matsuo, S.; Muramatsu, T. Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J. Immunol. 2001, 167, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, T.; Yuzawa, Y.; Sato, W.; Arata-Kawai, H.; Suzuki, N.; Kato, N.; Matsuo, S.; Kadomatsu, K. Midkine is involved in tubulointerstitial inflammation associated with diabetic nephropathy. Lab. Invest. 2007, 87, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Sato, W.; Takei, Y.; Yuzawa, Y.; Matsuo, S.; Kadomatsu, K.; Muramatsu, T. Midkine antisense oligodeoxyribonucleotide inhibits renal damage induced by ischemic reperfusion. Kidney Int. 2005, 67, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Sato, W.; Yuzawa, Y.; Kosugi, T.; Matsuo, S.; Takei, Y.; Kadomatsu, K.; Muramatsu, T. Lack of the growth factor midkine enhances survival against cisplatin-induced renal damage. Am. J. Pathol. 2004, 165, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Takeuchi, H.; Sonobe, Y.; Jin, S.; Mizuno, T.; Miyakawa, S.; Fujiwara, M.; Nakamura, Y.; Kato, T.; Muramatsu, H.; Muramatsu, T.; Suzumura, A. Inhibition of midkine alleviates experimental autoimmune encephalomyelitis through the expansion of regulatory t cell population. Proc. Natl. Acad. Sci. USA 2008, 105, 3915–3920. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Kosugi, T.; Sato, W.; Sato, Y.; Maeda, K.; Kato, N.; Kato, K.; Inaba, S.; Ishimoto, T.; Tsuboi, N.; Matsuo, S.; Maruyama, S.; Yuzawa, Y.; Kadomatsu, K. Deficiency of growth factor midkine exacerbates necrotizing glomerular injuries in progressive glomerulonephritis. Am. J. Pathol. 2013, 182, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Ohuchida, T.; Okamoto, K.; Akahane, K.; Higure, A.; Todoroki, H.; Abe, Y.; Kikuchi, M.; Ikematsu, S.; Muramatsu, T.; Itoh, H. Midkine protects hepatocellular carcinoma cells against trail-mediated apoptosis through down-regulation of caspase-3 activity. Cancer 2004, 100, 2430–2436. [Google Scholar] [CrossRef] [PubMed]
- Horiba, M.; Kadomatsu, K.; Yasui, K.; Lee, JK.; Takenaka, H.; Sumida, A.; Kamiya, K.; Chen, S.; Sakuma, S.; Muramatsu, T.; Kodama, I. Midkine plays a protective role against cardiac ischemia/reperfusion injury through a reduction of apoptotic reaction. Circulation 2006, 114, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, H.; Horiba, M.; Ishiguro, H.; Sumida, A.; Hojo, M.; Usui, A.; Akita, T.; Sakuma, S.; Ueda, Y.; Kodama, I.; Kadomatsu, K. Midkine prevents ventricular remodeling and improves long-term survival after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H462–469. [Google Scholar] [CrossRef]
- Linge, HM.; Andersson, C.; Nordin, SL.; Olin, AI.; Petersson, AC.; Mörgelin, M.; Welin, A.; Bylund, J.; Bjermer, L.; Erjefält, J.; Egesten, A. Midkine is expressed and differentially processed during chronic obstructive pulmonary disease exacerbations and ventilator-associated pneumonia associated with staphylococcus aureus infection. Mol. Med. 2013, 19, 314–323. [Google Scholar] [CrossRef]
- Reynolds, PR.; Mucenski, ML.; Le Cras, TD.; Nichols, WC.; Whitsett, JA. Midkine is regulated by hypoxia and causes pulmonary vascular remodeling. J. Biol. Chem. 2004, 279, 37124–37132. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Peng, F.; Sun, Q.; Meng, SS.; Qiu, HB.; Xu, JY. Plasma midkine is associated with 28-day mortality and organ function in sepsis. J. Intensive. Care Med. 2020, 35, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Ketenci, S.; Kalayci, MU.; Dündar, B.; Duranay, R.; Aynacıoğlu, AS. Elevated serum midkine levels in severe acute respiratory syndrome coronavirus 2 (sars-cov-2) infected patients. Int. Immunopharmacol. 2022, 110, 108939. [Google Scholar] [CrossRef] [PubMed]
- Xu, JY.; Chang, W.; Sun, Q.; Peng, F.; Yang, Y. Pulmonary midkine inhibition ameliorates sepsis induced lung injury. J. Transl. Med. 2021, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Weckbach, LT.; Groesser, L.; Borgolte, J.; Pagel, JI.; Pogoda, F.; Schymeinsky, J.; Müller-Höcker, J.; Shakibaei, M.; Muramatsu, T.; Deindl, E.; Walzog, B. Midkine acts as proangiogenic cytokine in hypoxia-induced angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H429–438. [Google Scholar] [CrossRef] [PubMed]
- Takada, T.; Toriyama, K.; Muramatsu, H.; Song, XJ.; Torii, S.; Muramatsu, T. Midkine, a retinoic acid-inducible heparin-binding cytokine in inflammatory responses: chemotactic activity to neutrophils and association with inflammatory synovitis. J. Biochem. 1997, 122, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Weckbach, LT.; Gola, A.; Winkelmann, M.; Jakob, SM.; Groesser, L.; Borgolte, J.; Pogoda, F.; Pick, R.; Pruenster, M.; Müller-Höcker, J.; Deindl, E.; Sperandio, M.; Walzog, B. The cytokine midkine supports neutrophil trafficking during acute inflammation by promoting adhesion via β2 integrins (CD11/CD18). Blood 2014, 123, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Herter, JM.; Mayadas, TN. Midkine, a middle manager of β2 integrins. Blood 2014, 123, 1777–1779. [Google Scholar] [CrossRef] [PubMed]
- Weckbach, LT.; Grabmaier, U.; Uhl, A.; Gess, S.; Boehm, F.; Zehrer, A.; Pick, R.; Salvermoser, M.; Czermak, T.; Pircher, J.; Sorrelle, N.; Migliorini, M.; Strickland, DK.; Klingel, K.; Brinkmann, V.; Abed, UA.; Eriksson, U.; Massberg, S.; Brunner, S.; Walzog, B. Midkine drives cardiac inflammation by promoting neutrophil trafficking and netosis in myocarditis. J. Exp. Med. 2019, 216, 350–368. [Google Scholar] [CrossRef]
- Liu, G.; Ren, X.; Li, Y.; Li, H. Midkine promotes kidney injury in diabetic kidney disease by increasing neutrophil extracellular traps formation. Ann. Transl. Med. 2022, 10, 693. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




