1. Introduction
Antimicrobial resistance (AMR) is an increasing global health challenge, ranked fifth among the ten WHO highlighted threats affecting the public health [
1]. AMR caused an estimated 1.27 million deaths globally in 2019, and it is projected to increase to 10 million deaths per year by 2050 if no effective interventions are implemented in response to the current situation [
2,
3]. Existing evidence revealed that inappropriate prescription and use of antimicrobials are major drivers for the development and spread of AMR [
4]. In 2015, the WHO set a global action plan for AMR, with antimicrobial stewardship (AMS) in human health, defined as the promotion of rational usage of antimicrobials, as one of its cornerstones [
5].
Healthcare providers may contribute to the emergence of AMR through irrational antimicrobial prescription practices, hospitalizing severely ill and/or immunosuppressed patients prone to multi-drug resistant organisms (MDRO)-infections which may speed up the spread of infections together, and poor infection prevention and control practices at the healthcare facilities [
6,
7,
8,
9,
10,
11,
12].
In addition, low-and-middle-income countries (LMICs) including African countries such as Rwanda, are challenged with the lack of highly trained personnel, particular in infection prevention and control in healthcare facilities, limited awareness among healthcare providers about the importance and best practices in regulating the prescription and use of antibiotics. Furthermore, the high burden of a wide range of infectious diseases, limited hygiene and sanitation, limitations in the quality, transportation, and storage of medicinal products, as well as misuse of drugs, are heavily contributing to the rapidly growing burden of AMR [
13].
Despite the growing evidence about AMR at the national level in Rwanda, there is still little to no up-to-date knowledge at hospital level in the country [
14,
15,
16,
17]. At the time of this study, no data on antimicrobial use in Rwandan hospital settings, particularly a tertiary and teaching hospitals like CHUK were available. Therefore, this report provides baseline information on the prescription and usage of antimicrobials at hospital level to guide the implementation of evidence-based antimicrobial stewardship program in the country.
2. Results
2.1. Prevalence of Antimicrobial Usage at Hospital and Departmental Levels
A total of 369 patients were enrolled in this study; of which 282 (76.5%) were adults, 75 (20.3%) were children and 12 (3.2%) were neonates. Sixty-one patients (16.5%, n=369), were referred from other healthcare centers and 41 (67.2%) of them were already on antimicrobials before their current hospitalization at CHUK. Hospital-wide the prevalence of patients on antimicrobials was 39.3% (145/369), and 97% (141/145) of the patients on antibiotics were receiving at least one antibiotic. A total of 81 patients (58.9%) were receiving more than one antimicrobial.
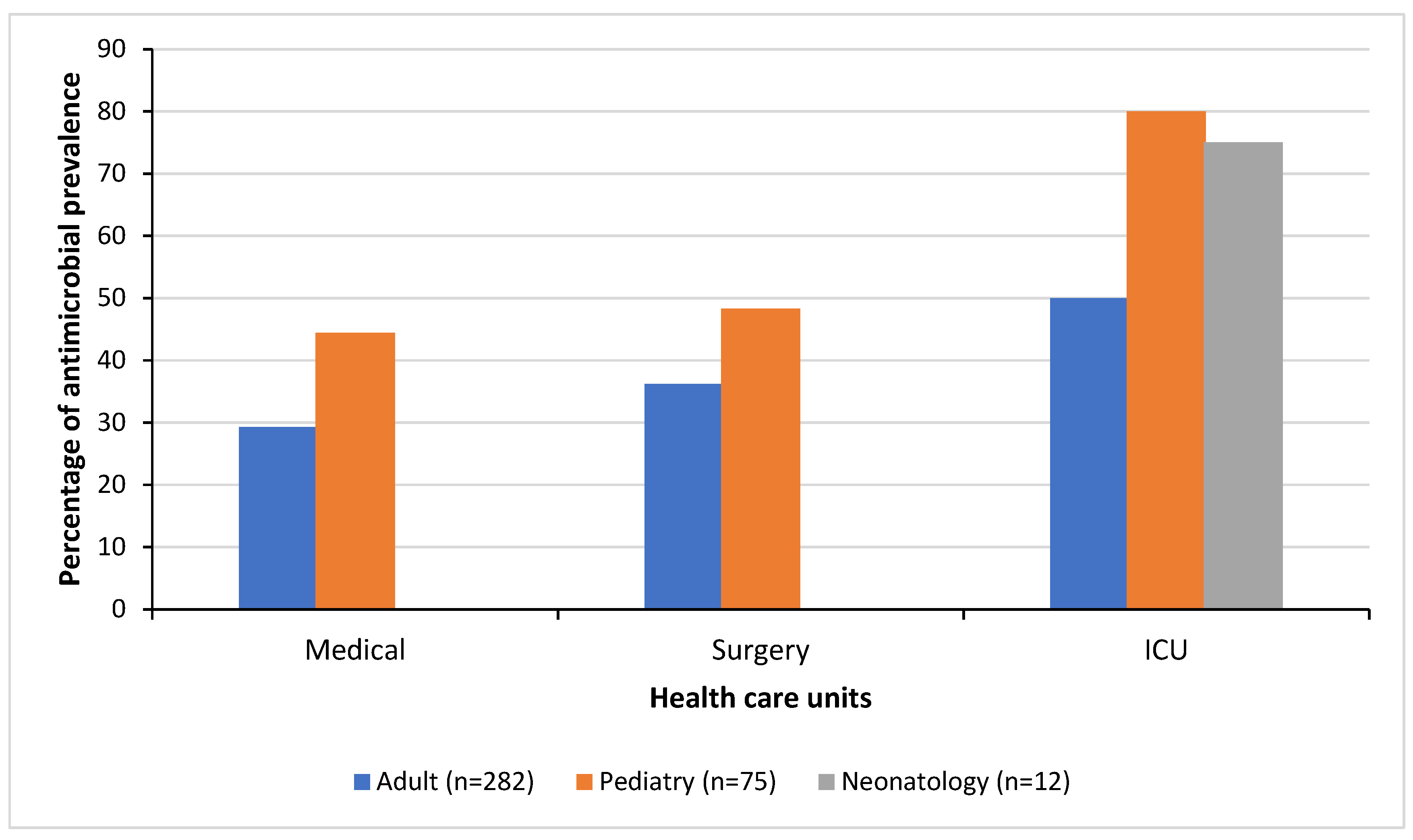
Figure 1 illustrates the antimicrobial prevalence by the healthcare units in which patients were hospitalized including medical treatment, surgical care, and intensive care for adults, pediatric and neonatology (
Figure 1). High antimicrobial prevalences were noted in patients in the pediatric surgical ward (PSW), pediatric intensive care unit (PICU), and neonatology intensive care unit (NICU) with prevalences of 68.8% (11/16), 80% (8/10) and 75% (9/12), respectively. The lowest antimicrobial prevalence was observed in the adult medical ward (AMW) with 28.3% (41/145) but increased to 40.5% (51/126) in the adult surgical ward (ASW) and to 54.5 % (6/11) in the adult intensive care unit (AICU). The remaining pediatric wards had prevalences of 30% (3/10) at the hematology-oncology ward (HOPMW) and 41% (16/39) at the medical ward (PMW).
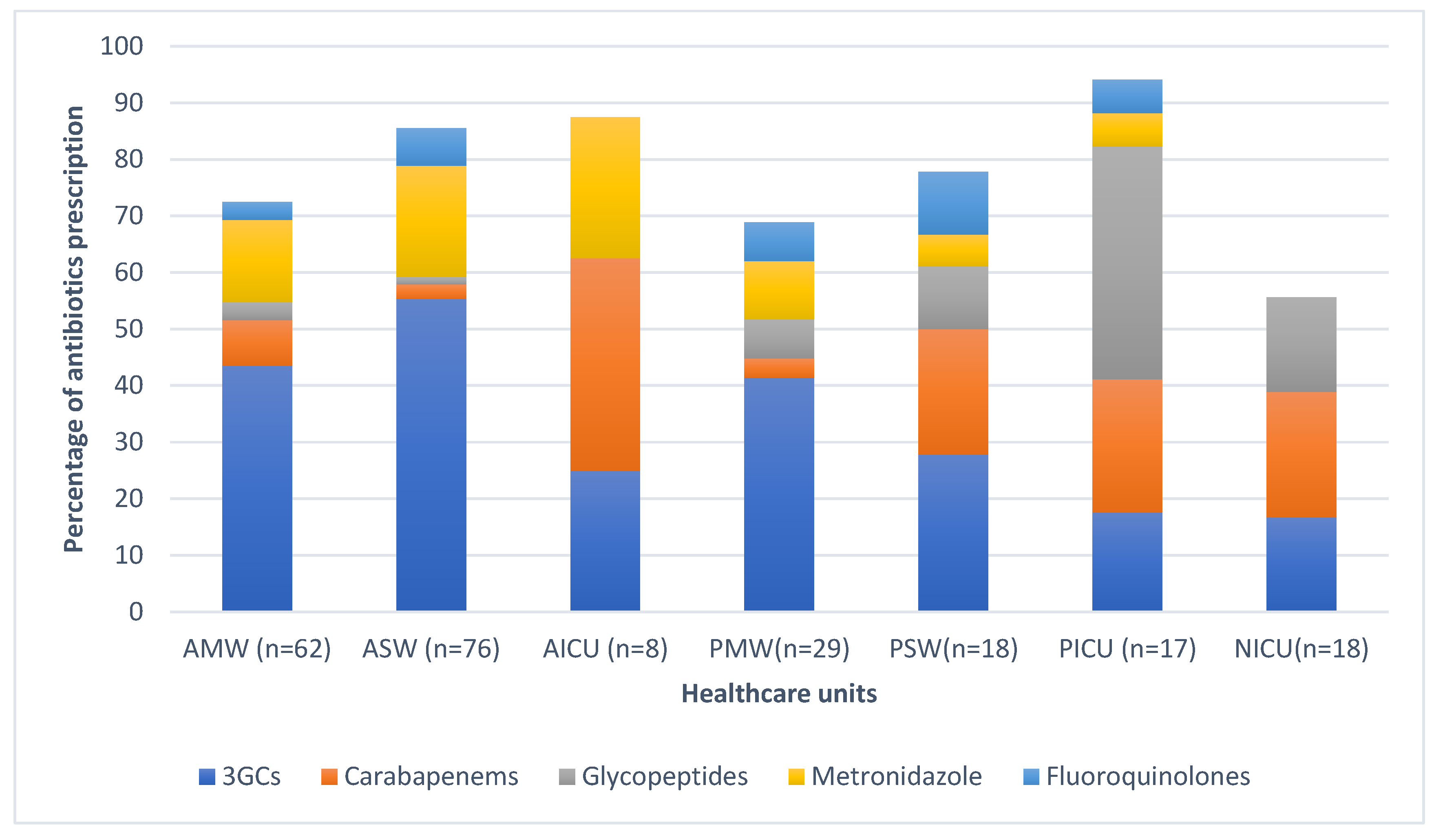
Overall, the most prescribed antibiotic classes were beta-lactams (147/232, 63.4%), followed by imidazole derivates (31/232, 13.4%,) and glycopeptides (GP) (17/232, 7.3%,). Sixty-four percent (95/147) of the prescribed beta-lactams consisted of 3rd generation cephalosporins (3GCS), mainly cefotaxime (n=87), and ceftriaxone (n=8), followed by carbapenems (23/147, 15.6%), namely meropenem. The distribution of the five most prescribed antibiotics at ward level are illustrated in
Figure 2. Carbapenem prevalence was 22.2% (4/18) in the PSW, 37.5% (3/8) in the AICU and 23.5% (4/17) in the PICU. At the PICU, glycopeptide prevalence was 41.2% (7/17).
Antibiotics belonging to the WHO Access group were representing 35% (81/232) while the Watch group was representing 65% of all prescriptions. The ICU departments had the highest proportion of Watch antibiotics 72% (31/43), followed by the surgical wards 64.3% (63/98), and the medical wards 59% (56/95). Sixty-four percent (96/151) of all prescribed Watch antibiotics were 3GCS, with 57.6% (87/151) of them being cefotaxime, followed by carbapenems (meropenem) with 15.2% (23/151) and glycopeptides (vancomycin) with 11.2% (17/151). The most prescribed antibiotics in the Access group was metronidazole (IV) at 43.2% (35/81) (
Figure 2).
2.2. Indications for Antimicrobial Usage
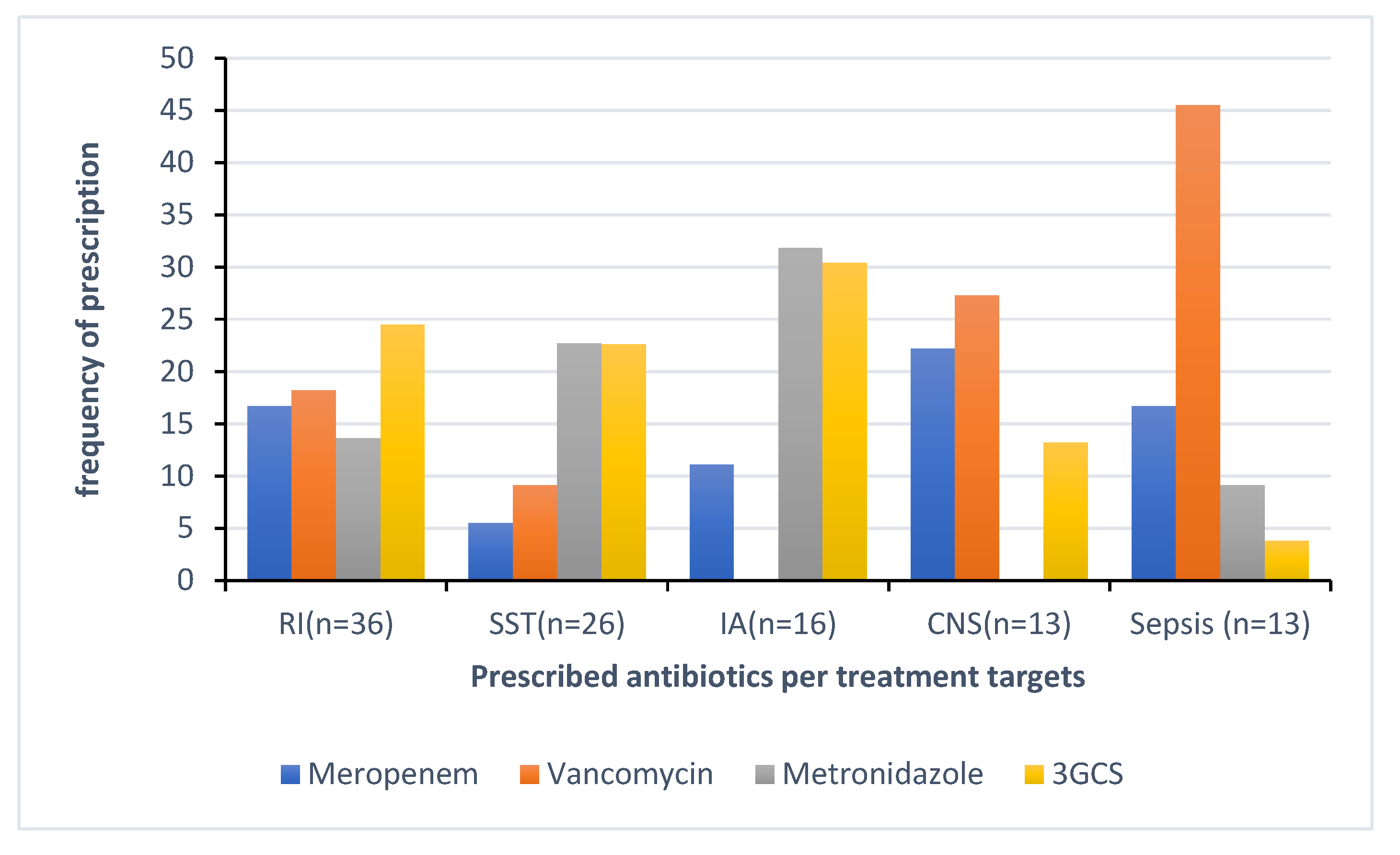
A 178 out of 259 (68.7%) antimicrobials were prescribed for treatment of infections, whereas 81 out of 259 (31.3%) for prophylaxis, of which 61 (75.3%) for surgical prophylaxis. The top five indications for antimicrobial treatment were respiratory infections (RI), including bronchitis and pneumonia counting 20.2% (36/178), followed by skin and soft tissue infections (SSTI) with 14.6% (26/178), tuberculosis 10.7 % (19/178), intra-abdominal infections (IA) with 9 % (16/178), and central nervous system infections (CNS) and sepsis with 7.3% each (13/178).
Figure 3 illustrates the distribution of antibacterials prescribed for the top five indications for treatment. The top four indications for prophylaxis were for medical and surgical conditions of gastro-intestinal 30.8% (25/81), bone and joint with 18.5% (15/81) and medical prophylaxis for maternal risk factors (MP-MAT) and newborn risk factors (NEO-MP) such as very low birth weight representing with 13.6% (11/81) each.
Sixty-one (61) antimicrobials were prescribed to 42 patients for surgical prophylaxis. The most prescribed antimicrobials were 3GCS (62.3%,38/61) and metronidazole (13.2%, 8/61). Only 1.6% (1/42) of the patients received a 1st generation cephalosporin (1GC). Of all 42 patients for whom surgical prophylaxis was prescribed, 7 (16.6%) received a single dose, while the remaining 35 (83.3%) received multiple doses extended to more than one day.
Out of the 178 prescribed antimicrobials for treatment, 71% (127) were for community-acquired infections (CAI), while 29% (51) were for hospital-acquired infections (HAI). The overall prevalence of HAI in the hospital was 9.5% (35/369 patients). However, among patients receiving antibiotics, this prevalence of HAI increased to 24.8% (35/141 patients). The main HAI were surgical site infections (SSI) counting for 37.1% (13/35), followed by non-intervention related HAI other than BSI (31.4%), intervention related infections (20%), and blood stream infections (BSI) (11.4%). The top three antibacterials prescribed for CAI were third-generation cephalosporins (3GCs) at 38.8% (40/103), metronidazole at 16.5% (17/103), and glycopeptides at 10.7% (11/103). For hospital-acquired infections (HAI), the most frequently prescribed antibacterials were 3GCs at 28.6% (14/49), followed by carbapenems at 26.5% (13/49), and metronidazole at 12.2% (6/49).
Two-hundred and four out of 259 (78.8%) prescribed antimicrobials were administered parenterally compared to 54 (20.8%) and one (0.4%) orally and intramuscularly respectively. Parenteral antimicrobials exceeded 90% in the pediatric department and 70% in the adult department. A reason for prescription was present in the patient file for the treatments of 239 (92.3%), but only 105 (40.5%) prescriptions were based on an elevated biomarker of infection (WBC, CRP). For 62 (24%) prescriptions, a body fluid or tissue sample was sent to the microbiology lab for culture and antimicrobial sensitivity testing (AST).
In the subset of patients receiving targeted antimicrobial treatment, the commonly isolated bacteria were
Escherichia coli 12/22 (54.5%) among which 41.7% were extended-spectrum beta-lactamases producing bacteria (ESBL). Additionally, 8.3% were Third Generation Cephalosporins resistant bacteria (3GCREB), and 33.3% were both, ESBL and 3GCREB. They were followed by
Klebsiella pneumoniae and
Acinetobacter spp with 13.6% (n=3) each and their phenotypic resistance were 33.3% of ESBL and 33.3% of Carbapenem-resistant Non fermenters (CR-NF), respectively. Only two (9%)
Staphylococcus aureus were isolated and none showed resistance. Other isolated bacteria were one
Enterococcus spp. and one
Pseudomonas Aeruginosa, with resistance profile of Vancomycin-resistant enterococci (VRE) and CR-NF, respectively. Antimicrobials were prescribed for empiric use in 220 (85%) cases and only 34 (15.5%) of these prescriptions had sent samples to the microbiology laboratory for culture and AST. In all prescribed antimicrobials, patients missed some doses of antibiotics that were supposed to be taken in 17.4% (45/259) prescriptions. Guidelines were only assessed on tuberculosis cases; other prescriptions of antimicrobial were made without having guidelines.
Table 1 includes data of the different quality indicators of antimicrobial prescription in the pediatric and adult departments.
3. Discussion
To the best of our knowledge, this PPS was the first to be conducted in a Rwandan hospital and it gives baseline information on antimicrobial prescription and use at a tertiary care facility in a LMIC settings. This PPS showed several areas for improvement, gaps in antimicrobial prescription and use at CHUK. Hospital-wide, prevalence of patients on antimicrobials was 38% with a wide variation between the different healthcare units. This overall prevalence is comparable to observations in other tertiary hospitals from the East-African region such as a tertiary-care referral hospital in Tanzania with 38%, [
18] but lower than the observed prevalence in certain hospitals in other African countries [
19,
20]. However, prevalence of antimicrobial use in patients varied between 55 to 80% in certain healthcare units such as the adult ICU, pediatric ICU, and pediatric surgical ward. This could be attributed to several factors including higher rate of bacterial infections among specific population groups, very small number of patients in certain subgroups, prevalence of healthcare acquired infections (such as intravascular device- related and surgical site infections) in these healthcare units, as well as capacity and practices in implementing effective infection prevention and control measures in the different healthcare units.
Sixty-five percent (65%) of all prescribed antimicrobials belonged to the Watch group of the WHO AWaRe classification, such as third generation cephalosporins, carbapenems and glycopeptides. This rate is comparable to some Sub-Saharan African countries such as Tanzania and Democratic Republic of Congo [
18,
21]. However, compared to other LMICs, this is relatively high [
22,
23,
24,
25]. This may be due to different factors such as limited knowledge of the WHO AWaRe Classification and AWaRe antibiotic guideline and the large antibacterial spectrum of Watch antibiotics. Others contributing factors might be the limited choice and/or availability of certain Access antibiotics, the lack of local treatment guidelines and microbiological diagnosis as well as lack of stewardship program in many LMICs [
26,
27,
28,
29]. At CHUK and in particular at the ICUs, besides the forementioned reasons, the presence of many severely ill patients and long hospital stays may have contributed to this high Watch prescription level [
30].
As in many other WHO regions, 3GCS (e.g., ceftriaxone and cefotaxime) were the most prescribed antimicrobials, followed by metronidazole [
20,
31,
32]. This was the case for treatment as well as prophylaxis of infection. This finding is a concern given the role of 3GCS in the selection of extended-spectrum beta-lactamase (ESBL)-producing bacteria [
33,
34]. The highest rate of 3GCS prescription was recorded in adult surgical wards (55.3%). These findings are comparable to reports from countries such as Kenya (55%) and Bangladesh (44.6%) [
31]. One of the reasons is their high usage for surgical prophylaxis, although international guidelines recommend using first generation cephalosporins (with or without metronidazole, depending on the type of surgery), which cover most bacteria involved in surgical site infections [
35]. The high prescription level of 3GCs might also be due to their use in the treatment of postoperative infections, because of their broad-spectrum activity in both Gram-positive and Gram-negative bacteria [
36].
Besides the high level of 3GCS prescription for surgical prophylaxis, the duration of surgical prophylaxis exceeded one day in 84% of the patients. This finding is lower compared to findings from Tanzania, Uganda and Zambia reporting prolonged SP in more than 96% of the cases [
37]. International guidelines as well as the WHO antibiotic guide recommend for most cases a single dose of surgical antibiotic prophylaxis with cefazolin for surgical interventions not exceeding 4 hours [
35,
38] The prolonged SP at CHUK could be explained by different factors such as the prescription behavior of healthcare providers, fear of post operation SSI, inadequate Infection prevention control (IPC) measures the lack of a local SP guideline, little awareness of the WHO SP guideline, and poor recording of stop and review dates.
In addition, 3GCS and other Watch antibiotics were prescribed for community-acquired infections in 56% of the cases, whereas the WHO antibiotic guide and AWaRe classification often recommends otherwise [
39]. This prescription behavior can be explained by several reasons, such as the lack of local guidelines, inadequate microbiological diagnostics and AMR surveillance, which could tailor adequate and locally adapted empiric antimicrobial prescription if present.
Eighty-five percent of all antimicrobials were prescribed empirically with 62% of the antibiotics being of Watch class. A possible explanation lies in the fact that CHUK is a tertiary care and referral hospital, treating patients already having received first-line (Access) antimicrobials. This finding is comparable to the finding from studies done in Northern & Southern Europe and other African Countries [
40,
41,
42,
43]. Surprisingly, in only 14.3% of the empirical treatments samples of body fluids were sent to the microbiology laboratory for culture and AST. This is comparable to findings from other PPS reports [
40,
41]. The low number of samples sent to the laboratory may be due to low diagnostic prescription of the treating physicians; confidence of physicians in their empirical prescription practices [
30]; little expectations of getting AST results on time due to limited technical expertise and overall limited laboratory capacities. In addition, the microbiology lab at CHUK is often confronted with supply issues regarding blood culture bottles, antibiotic disks, and reagents required for culture and AST, hampering even more the adequate diagnosis of infectious diseases.
The samples sent for culture showed a worrying amount of multi drug resistant organisms, such as ESBL producing and 3GCS resistant enterobacterales, CPE and carbapenem resistant non fermenters. However, because of the low number of samples sent, no robust conclusions on the hospital epidemiology at CHUK can be drawn for the moment.
The antimicrobial prescribing quality was suboptimal, illustrated by low to very low levels of oral administration, absence of guidelines, and limited documentation of stop or review date in the patient files. However, the reason for antimicrobial prescription was noted in the patient files in more than 90% of the cases. This is in line with many other African countries, having performed a PPS [
37,
43]. The potential drivers are diverse and need further exploration to be elucidated. However, possible explanations for these observations may include the poor documentation habits of healthcare providers, the availability of drugs for parenteral routes comparatively to oral drugs, limited awareness of the international guidelines, inadequate evidence-based information to create local guidelines, limited awareness of health care providers on the advantage of usings oral antibiotics.
This is the first PPS conducted at the University Teaching Hospital of Kigali, implemented by the healthcare providers who have been trained using the G-PPS tool. This study provides a baseline data for CHUK and serves as reference to other tertiary hospitals in Rwanda and regional LMIC settings. Our survey has a couple of limitations. First, the PPS was conducted on different days however each ward was entirely surveyed on a single day as per the protocol. Second, the PPS provides a snapshot of antimicrobial prescription data at a single time point, which does not capture the overall hospital antimicrobial prescription practices, over time. Therefore, additional measurements and interventions will be performed such as auditing SP practices in order to confirm the findings of the PPS.
4. Materials and Methods
This is a descriptive study that was conducted at CHUK, a tertiary-care and teaching hospital, located in Nyarugenge district, Rwanda. CHUK has a bed capacity of 519 beds for hospitalization and an average occupancy level of 81%. The PPS was conducted as part of the Antimicrobial Stewardship program (ASP), following the protocol of the Global PPS of Antimicrobial Consumption and Resistance (Global-PPS) project (
https://www.global-pps.com/) from 27th -30th March, 2023. The Global-PPS data collection was performed using the basic PPS protocol to survey inpatient antimicrobial use, and the additional healthcare-associated infections (HAI) PPS module (Global-PPS protocol version 2023) (Ref: Supplementary document).
Prior to the conduct of the PPS, multidisciplinary teams consist of nurses, physicians, pharmacists, microbiologists, quality assurance personnels were trained on the usage of the G-PPS tool and a pilot survey was conducted. The ethical approval to conduct PPS was obtained from CHUK division of research, education and Training.
All data were collected from the patient files and included all inpatients occupying a bed at 8:00 AM on the day of the PPS. In addition to the data on the use of antimicrobials, we also collected patient demographics and medical information including healthcare unit of admission. Collected data for all patients on antimicrobials at the time of the study included age, sex, biomarker of inflammation (white blood cell count, CRP, PCT), information on the type and results of culture(s) of body fluids and/or tissue. Additionally, we collected information on the name of prescribed antimicrobial, start date, dosage, and administration route, diagnosis and type of indication (healthcare-associated versus community-acquired infection, surgical or medical prophylaxis). Furthermore, we collected information about documentation of stop/review date of antimicrobial treatment and the reason for prescribing in patient notes; presence/absence of as well as compliance to (local) guidelines, and the number of missed doses. If the antimicrobial treatment was targeted based on microbiological results, the type of micro-organism(s) and their resistance profile(s) were noted. For the HAI module, additional data were collected for all patients on antimicrobials, including the presence of invasive devices and underlying morbidities.
Data analysis: Data were entered into the web-based application for Global-PPS data entry, an in-house built application for data-entry, validation and reporting, developed by the University of Antwerp. Data were extracted in Microsoft Excel (2021), analyzed descriptively and results were expressed as frequencies and percentages. Antibiotics were categorized as Access, Watch, Reserve, or Not Recommended following the 2023 WHO AWaRE classification (
https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04).The HAI prevalence was calculated by dividing the number of patients receiving at least one antimicrobial for an HAI by the total number of admitted patients at the time of the survey.
5. Conclusions
This survey highlights different gaps that are associated with irrational prescription and usage of antibiotics, and it calls for urgent need to establish and implement a local and national antimicrobial stewardship program to optimize the usage of antibiotics and further exploring the drivers of the prescribing behaviors.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Acsa Igizeneza, Caroline Theunissen, Leopold Bitunguhari, Innocent Hahirwa, Florence Masaisa, and Jean Claude S Ngabonziza, Methodology, Ines Pauwels, Ann Versporten, Erika Vlieghe, Acsa Igizeneza, Caroline Theunissen, Leopold Bitunguhari; Formal Analysis, Ines Pauwels, Ann Versporten, Caroline Theunissen and Acsa Igizeneza.; Investigation, Acsa Igizeneza, Leopold Bitunguhari, Innocent Hahirwa Florence Masaisa, Lorette D Uwamahoro, Osee Sebatunzi, Nathalie Umugwaneza; Data Curation: Ines Pauwels and Ann Versporten; Writing—Original Draft Preparation, Acsa Igizeneza and Caroline Theunissen; Writing—Review & Editing, Claude S Ngabonziza, Ayman Ahmed, Leopold Bitunguhari, Innocent Hahirwa, Ines Pauwels and Ann Versporten; Supervision, Caroline Theunissen and Jean Claude S Ngabonziza; Visualization, Acsa Igizeneza, Caroline Theunissen, Project Administration, Acsa Igizeneza, Leopold Bitunguhari and Caroline Theunissen; Funding Acquisition, Jean Claude S Ngabonziza.
Funding
This research was funded by The Belgian Federal Public Service of Development Cooperation (DGD) under FA5-Rwanda Project, through a partnership between the University of Rwanda, the Rwanda Biomedical Center, the Kigali University Teaching Hospital and the Institute of Tropical Medicine, Antwerp/Belgium, grant number [BE-BCE_KBO-0410057701-prg2022-13-RW] and The APC was funded by DGD.
Institutional Review Board Statement
Ethical approval for this study was obtained from the research ethics committee of the University Teaching Hospital of Kigali (Ref: EC/CHUK/055/2023).
Informed Consent Statement
Patient consent was waived due to the methodology used which did not need patients’ approval.
Data Availability Statement
The supporting data of this manuscript can be made available on request from the corresponding author.
Acknowledgments
We would like to acknowledge the Global point prevalence network for their support in providing the data collection form, and training on its usage. We thank the CHUK Antimicrobial Stewardship team for their help in data collection. We would also like to acknowledge the CHUK administration assistance in this study. We also thank BioMérieux the sole private partner of the Global Point Prevalence Survey. BioMérieux had no role nor responsibility in the study design, data collection, data analysis, data interpretation, or writing the report, which was done under the sole responsibility of the University of Antwerp. Data are strictly confidential and stored anonymously at the coordinating center of the University of Antwerp.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qayum, I. Top ten global health threats for 2019: the WHO list. J Rehman Med Inst 2019, 5, 1–2. [Google Scholar]
- Murray, C.; Kevin, S. I; Fablina, S.; Lucien, S.; Aguilar, G. R. , et al., “Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet, 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Tang, K. W. K.; Millar, B. C.; Moore, J. E. Antimicrobial Resistance (AMR). Br J Biomed Sci, 2023, 11387. [CrossRef]
- Chokshi, A.; Sifri, Z. , Cennimo D.; and Horng H. Global contributors to antibiotic resistance. J Glob Infect Dis, 2019, 11, 36–42. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Action Plan on Antimicrobial Resistance Geneva, Switzerland, 15. Available on https://iris.who.int/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1, accessed on 14th April, 2024.
- Chang, Y.; Chusri, S.; Sangthong, R.; McNeil, E.; Hu, J.; Du, W.; Li, D.; Fan, X.; Zhou, H.; Chongsuvivatwong, V.; et al. Clinical pattern of antibiotic overuse and misuse in primary healthcare hospitals in the southwest of China. PLoS One, 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Ibrahim, O. M.; Saber-ayad, M. Antibiotic Misuse in Different Hospital Wards (A Pilot Study in an Egyptian Academic Sciences). Asian J Pharm Clin Res 2012, 5, 5–8. [Google Scholar]
- Vinh, D. C.; Embil, J. M. Device-Related Infections: A Review. J Long Term Eff Med Implants, 2005, 15, 467–488. [Google Scholar] [CrossRef]
- Percival, S. L.; Suleman, L.; Vuotto, C.; Donelli, G.; Percival, S. L. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J. Med. Microbiol, 2015, 64, 323–334. [Google Scholar] [CrossRef]
- Cornejo-Juárez, P.; Vilar-Compte, D.; Pérez-Jiménez, C.; Ñamendys-Silva, S. A.; Sandoval-Hernández, S.; Volkow-Fernández, P. The impact of hospital-acquired infections with multidrug-resistant bacteria in an oncology intensive care unit. Int J Infect Dis. [CrossRef]
- Bostik, P.; Kolar, M. Hospital Acquired Infections, Multidrug Resistant (MDR) Bacteria, Alternative Approaches to Antibiotic Therapy.” MDPI, 2022. [Online]. Available: www.mdpi.com/journal/antibiotics.
- Fernando, S. A.; Gray, T. J; Gottlieb, T. Healthcare-acquired infections: prevention strategies. Intern Med J, 2017, 47, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, B.; Gray, A.; Davis, W. N.; Robles, A. G.; Swetschinski, L.; Ikuta, K.; Mestrovic, T.; Chung, E.; Wool, E.; Han, C.; et al. The burden of bacterial antimicrobial resistance in the WHO African region in 2019: a cross-country systematic analysis. Lancet Glob Health, 2024, 12, e201–e216. [Google Scholar] [CrossRef]
- Munyemana, J. B.; Gatare, B.; Kabanyana, P.; Ivang, A.; Mbarushimana,D.; Itangishaka, I.; Niringiyumukiza,J.; Musoni, E. Antimicrobial Resistance Profile of Bacteria Causing Pediatric Infections at the University Teaching Hospital in Rwanda. Am J Trop Med Hyg, 2022, 107, 1308–1314. [CrossRef]
- Habyarimana, T.; Murenzi, D.; Musoni,E.; Yadufashije, C.; and Niyonzima, F. N. Bacteriological profile and antimicrobial susceptibility patterns of bloodstream infection at Kigali University Teaching Hospital. Infect Drug Resist, 2021,14, 699–707. [CrossRef]
- Ntirenganya, C.; Manzi, O.; Muvunyi, C. M.; Ogbuagu, O. High prevalence of antimicrobial resistance among common bacterial isolates in a tertiary healthcare facility in Rwanda. Am J Trop Med Hyg, 2015, 92, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.; Mpirimbanyi, C.; Nziyomaze, E.; Niyomugabo, J.; Niyonsenga, Z.; Muvunyi, C.; Mueller, A.; Bebell, L.; Nkubana, T.; Musoni, E.; et al. Widespread antimicrobial resistance among bacterial infections in a Rwandan referral hospital. PLoS One, 2019, 14, e0221121. [Google Scholar] [CrossRef] [PubMed]
- Horumpende, P. G.; Mshana, S. E.; Mouw, E. F.; Mmbaga, B. T.; Chilongola, J. O. Point prevalence survey of antimicrobial use in three hospitals in North-Eastern Tanzania. Antimicrob Resist Infect Control, 2020, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Seni, J.; Mapunjo, S.; Wittenauer, R.; Valimba, R.; Stergachis, A.; Werth, B.; Saitoti, S.; Mhadu, N.; Lusaya, E.; Konduri, N. Antimicrobial use across six referral hospitals in Tanzania: a point prevalence survey. BMJ Open, 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Okoth, C.; Opanga, S.; Okalebo, F.; Oluka, M.; Baker, A.; Godman, B. Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: findings and implications. Hosp Pract. [CrossRef]
- Kakumba, J. M.; Kindenge, J. M.; Kapepula, P. M.; Iyamba, J. M. L.; Mashi, M. L.; Mulwahali, J. W.; Kialengila, D. M. Evaluation of Antibiotic Prescribing Pattern Using WHO Access, Watch and Reserve Classification in Kinshasa, Democratic Republic of Congo. Antibiotics, 2023, 12, 1239. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, I.; Versporten, A.; Drapier, N.; Vlieghe, E.; Goossens, H. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): results from a worldwide point prevalence survey in 69 countries. J Antimicrob Chemother, 1614; 76. [Google Scholar] [CrossRef]
- Kiggundu, R.; Wittenauer, R.; Waswa, J.; Nakambale, H.; Kitutu, F.; Murungi, M.; Okuna, N.; Morries, S.; Lawry, L.; Joshi, M.; et al. Point Prevalence Survey of Antibiotic Use across 13 Hospitals in Uganda. Antibiotics, 2022, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Kamita, M.; Maina, M.; Kimani, R.; Mwangi, R.; Mureithi, D.; Nduta, C.; Gitaka, J.M. Point prevalence survey to assess antibiotic prescribing pattern among hospitalized patients in a county referral hospital in Kenya. Front Antibiot., 2022, 1, 993271. [Google Scholar] [CrossRef]
- Costantine, J.; Bwire, G.; Myemba, D.; Sambayi, G.; Njiro, B.; Kilipamwambu, A.; Ching’oro, N.; Shungu, R.; Mganga, M.; Majigo, M. WHO/INRUD prescribing indicators among tertiary regional referral hospitals in Dar es Salaam, Tanzania: A call to strengthen antibiotic stewardship programmes. JAC Antimicrob Resist, 2023; 5, dlad093. [Google Scholar] [CrossRef]
- Abu-Ajaleh, S.; Darwish, E.; lhajji, F.; Al-Bsoul, S.; AbuFarha, R. , Al-Hammouri, F.; Amer, A.; Al Rusasi, A.; Al-Azzam, S.; Araydah, M.; et al. An Evaluation of the Impact of Increasing the Awareness of the WHO Access, Watch, and Reserve (AWaRe) Antibiotics Classification on Knowledge, Attitudes, and Hospital Antibiotic Prescribing Practices. Antibiotics, 2023; 12, 951. [Google Scholar] [CrossRef]
- Nepal, A.; Hendrie, D.; Selvey, L. A.; Robinson, S. Factors influencing the inappropriate use of antibiotics in the Rupandehi district of Nepal. Int J Health Plann Manage, 2021; 36, 42–59. [Google Scholar] [CrossRef]
- Lum, E. P. M.; Page, K.; Whitty, J. A.; Doust, J.; Graves, N. Antibiotic prescribing in primary healthcare: Dominant factors and trade-offs in decision-making. Infect Dis Health, 2018, 23, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.; Rego, C.; Semedo, G.; Gomes, D.; Figueiras, A.; Roque, F.; Herdeiro, M. Systematic review on the impact of guidelines adherence on antibiotic prescription in respiratory infections., Antibiotics. 2020,9,546. [CrossRef]
- Md Rezal, R. S.; Hassali, M. A.; Alrasheedy, A. A.; Saleem, F.; Md Yusof, F. A.; Godman, B. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: A systematic review of the literature. Expert Rev Anti Infect Ther. 2015, 13, 665–680. [Google Scholar] [CrossRef]
- Rashid, M.; Akhtar, Z.; Chowdhury, S.; Islam, A.; Parveen, S. Pattern of Antibiotic Use among Hospitalized Patients according to WHO Access, Watch, Reserve (AWaRe) Classification: Findings from a Point Prevalence Survey. Antibiotics, 2022, 11, 1–14. [Google Scholar] [CrossRef]
- Mudenda, S.; Nsofu, E.; Chisha, P.; Daka, V.; Chabalenge, B.; Mufwambi, W.; Kainga, H.; Kanaan, M.; Mfune, R.; Mwaba, F.; et al. Prescribing Patterns of Antibiotics According to the WHO AWaRe Classification during the COVID-19 Pandemic at a Teaching Hospital in Lusaka, Zambia: Implications for Strengthening of Antimicrobial Stewardship Programmes. Pharmacoepidemiology 2023, 2, 42–53. [Google Scholar] [CrossRef]
- Colodner, R.; Rock, W.; Chazan, B.; Keller, N.; Guy, N.; Sakran, W.; Raz, R. Risk factors for the development of extended-spectrum beta-lactamase- producing bacteria in nonhospitalized patients. Eur J Clin Microbiol Infect Dis. 2004, 23, 23,163–167. [Google Scholar] [CrossRef] [PubMed]
- Urbánek, K.; Kolář, M.; Lovečková, Y.; Strojil, J.; Šantavá, L. Influence of third-generation cephalosporin utilization on the occurrence of ESBL-positive Klebsiella pneumoniae strains. J Clin Pharm Ther, 2007, 32, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Dhole, S.; Mahakalkar, C.; Kshirsagar, S.; Bhargava, A. Antibiotic Prophylaxis in Surgery: Current Insights and Future Directions for Surgical Site Infection Prevention,” Cureus, 2023, 15,1-6. [CrossRef]
- Arumugham, V. B. ; Gujarathi,R.; Cascella,M. Third Generation Cephalosporins,.2019. [Online]. Available: https://www.researchgate.net/publication/358040422.
- D’Arcy, N.; Ashiru-Oredope, D.; Olaoye, O.; Afriyie, D.; Akello, Z.; Ankrah, D.; Asima, D.; Banda, D.; Barrett, S. ; Brandish, C et al. Antibiotic prescribing patterns in Ghana, Uganda, Zambia and Tanzania hospitals: Results from the global point prevalence survey (G-PPS) on antimicrobial use and stewardship interventions implemented,” Antibiotics, 2021, 10, 1122. [Google Scholar] [CrossRef]
- World Health Organization, Global guidelines for the prevention of surgical site infection., Second edition. Geneva, 2018.
- WHO, “The WHO AWaRe (Access, Watch, Reserve) antibiotic book,” Dec. 2022.
- Jamaluddin, N.; Periyasamy, P.; Lau, C.; Ponnampalavanar, S.; Lai, P.; Ramli, R.; Tan, T.; Kori, N.; Yin, M. ; Azman, et al., Point prevalence survey of antimicrobial use in a malaysian tertiary care university hospital,” Antibiotics, 2021,10,531. [CrossRef]
- Anand, P.; Tiroyakgosi, C.; Mpinda-Joseph, P.; Morokotso, M.; Matome, M.; Sinkala, F.; Gaolebe, M.; Malone, B.; Molosiwa, E.; Shanmugam, M.; et al. , Point prevalence study of antimicrobial use among hospitals across Botswana; findings and implications. Expert Rev Anti Infect Ther, 2019, 17, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Umeokonkwo, C.; Madubueze, U.; Onah, C.; Okedo-Alex, I.; Adeke, A.; Versporten, A.; Goossens, H.; Igwe-Okomiso, D.; Okeke, K.; Azuogu, B.; et al. Point prevalence survey of antimicrobial prescription in a tertiary hospital in South East Nigeria: A call for improved antibiotic stewardship. J Glob Antimicrob Resist, 2019, 17, 291–295. [Google Scholar] [CrossRef]
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health, 2018, 6, 619–629. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).