1. Introduction
Mammarenavirus wenzhouense (WENV) is a single-stranded negative-sense RNA virus, belonging to the family of
Arenaviridae [
1], which was firstly identified in Asian house shrews in China, and named after the place of discovery [
2]. Subsequently, it was found that the virus was widely distributed in Asia, and identified from a wide range of host animals, such as
R. norvegicus, R. rattus,
R. losea,
R. exulans,
R. tanezumi,
R. nitidus,
Niviventer niviventer,
Suncus murinus,
R. flavipectus,
Mus musculus,
Apodemus agrarius and
T. belangeri, and the viral RNA were detected in multiple organs and tissues from field rodents including heart, liver, spleen, lung, spleen, intestine, and the respiratory tract samples[
3,
4,
5,
6,
7,
8]. WENV specific IgG antibody was detected by ELISA with a positive rate of 17.4% (89 / 510) in the sera collected from patients with dengue/influenza-like illness[
5,
6], and a seroprevalence rate of 4.6% was reported in a serological survey in the general population[
4]. Mammarenaviruses are generally associated with a specific rodent species as reservoir, and which can be asymptomatically shed into feces, urine and saliva of the infected rodent reservoir for weeks or months [
9]. Humans and host animals usually become infected through contact with infected rodents or inhalation of infectious rodent excreta or secretions [
10,
11]. The pathogenicity of WENV to human remains, however, the evolution of WENV under changing natural selection pressures may make it a potential threat to human health. It was accepted that pathogens within their hosts are not isolated but interact and evolve within a large community of microorganisms that can be commensal or pathogenic [
12,
13]. The co-circulation and co-infection of WENV with Hantaan or Seoul virus in small mammals and humans had been reported recently [
8], which could affect the virus-rodent interactions, the eco-evolutionary and virological perspectives of the two viruses, and pose a risk of emergence of recombinant viral variants with increased fitness for circulation and a zoonotic potential.
So far, there is few report about the dynamics characteristics of WENV infection in the rodent host. In this study, we evaluated the process of WENV replication and shedding in experimental infections in SD rats by controlling time since inoculation, which may enhance the understanding of WENV transmission within populations of their reservoirs, and provide helpful information for the early warning and prediction of emerging infectious diseases originating from rodents.
2. Materials and Methods
2.1. Animal Handling and Viral Inoculations
Animals were handled using approved protocols according to the guidelines from National Institute for Viral Disease Control and Prevention, China CDC. At 6 to 8 weeks of age, 52 male SD rats (
Rattus norvegicus) were i.m. inoculated with WENV (strain DG4) suspended in 0.2 ml of Eagle minimum essential medium (EMEM). WENV was isolated from the lung of a field rodent
Rattus flavipectus and passaged one times in SD rats [
8]. Homogenates intended for inoculation were diluted with a 10-fold excess of EMEM, sham-inoculated control rats were inoculated i.m. with tissue homogenates from an uninfected rat. Two rats were raised in each individual ventilated cage (IVC). 54 animals were divided into four groups; group 1 was set to includes 5 IVC, both rats in each IVC were inoculated; group 2 includes 20 IVC, one was inoculated with the virus and the other was sham-inoculated in each IVC, which was set to evaluate the possibility of WENV horizontal transmission to cage mate in IVC system; group 3 includes one IVC, both animals were sham-inoculated as control; group 4 includes 2 rats in one IVC as no injection control. The vital signs of the rats, including weight, fur, trauma, emotions, mucosal elasticity, and behavioral activity, were observed and recorded daily till the terminal experiments. Blood samples were collected from each rat from group 1 prior to infection, and then every other day post-inoculation from the tail using a capillary tube. Fecal samples were obtained from rats in each IVC of group 1 everyday post-inoculation (pi). On day 7, 14, 21, 28 pi, 5 cages of rats from group 2 were anesthetized respectively with tribromoethanol, and blood samples were collected from the arterial vessels in the axilla at the side of the thorax, and organs of heart, liver, kidneys, lungs, spleen, thymus, testes, brain, and intestine were collected from the animals. After samples were collected on day 28 pi, all animals were killed as that described above.
2.2. Enzyme-Linked Immunosorbent Assay
Plasma was used to detect anti-WENV immunoglobulin G (IgG) using an enzyme-linked immunosorbent assay, in which microtiter plates were coated overnight at 4°C with purified recombinant produced nucleocapsid (N) protein (0.4 μg/well) of WENV or recombinant N protein of Seoul virus diluted in carbonate buffer as described previously (8). Thawed plasma samples, as well as positive and negative control samples were diluted 1:100 in phosphate-buffered saline (PBS)–Tween (PBS-T) with 5% skim milk powder and added in duplicate to antigen-coated wells. The plates were incubated at 37°C for 1 h, and washed with PBS-T, and secondary antibody (horseradish peroxidase (HRP)-conjugated anti-rat IgG diluted 1:1000 or HRP-conjugated N protein in PBS with 5% skim milk powder) was added. The plates were incubated for 1 h at 37°C, and washed with PBS-T, and TMB peroxidase substrate buffer (3,3’,5,5’tetramethylbenzidine and hydrogen peroxide) was added to each well, which was terminated after 10 to 15 min by adding 2 N H2SO4 to each well. The optical density (OD) was measured at 450 nm with a reference wavelength of 620 nm, and the average OD for N protein of Seoul virus duplicates was set as negative control. Samples were considered positive if the OD value was ≥ 2.1 times of the values of the OD from negative control wells.
2.3. Quantitative TaqMan Reverse Transcription PCR (qRT-PCR)
Aliquots of 100-200 mg of tissue, feces or 140 µL blood from SD rats were used to prepare RNA. The tissue and feces were placed in a 2ml tube containing 0.5 mL dulbecco’s modified eagle medium (DMEM) and mechanically homogenized using a TOMY Micro Smash MS-100. The homogenates were centrifuged at 12,000 g for 5 min and the supernatant was preserved at -80 °C for RNA extraction and virus isolation. RNeasy Mini Kit (Qiagen, Hilden, Germany) was used to extract total RNA from 140 µL of the homogenates supernatant and QiaAmp viral RNA Mini Kit is used for RNA extraction from 140 µL blood samples following the manufacturer’s instructions. TaqMan quantitative real-time RT- PCR (qRT-PCR) assay was performed as described previously [
8]. One step fluorescent quantitative RT-PCR kit (AgPath-ID Onestep RT-PCR Kit, ABI) was used for viral RNA detection. The reaction conditions were 50 ℃ for 30 min; 95 ℃ 10 min, 95 ℃ 15 s, 60 ℃ for 45 s, 40 cycles. A standard curve containing dilutions ranging from 1 × 10
1 copies to 1 × 10
7 copies of template was used on each 96-well plate, and viral RNA copy numbers were determined according to the standard curve, and the cut-off cycle threshold (Ct) value for a positive reaction was set at 35 cycles.
2.4. RT-PCR and Sequencing
To examine whether the WENV strain had undergone mutations during passage from the field captured specimen and later passages in SD rats, we compared the complete sequence of L segment and S segment obtained from the lungs from field captured rattus norvegicus and those of passages specimen, using direct sequencing of viral amplification products. The genomic RNA of L and S segments were amplified by RT-PCR with a Taq PCR Master Mix Kit (Sangon, China) using degenerate primers. PCRs were set up in a final volume of 50 µL. The thermal cycling conditions involved initial denaturation for 2 min at 95 °C, followed by 30 cycles of 20 s at 95 °C, 30 s at 55 °C and 30 s at 72 °C, the final extension lasted 10 min at 72 °C. Each PCR product was sequenced by Sanger sequencing. The obtained viral genomic sequences and amino acid sequence of open reading frame (ORF) were aligned by Clustal W embedded in Mega11 software[
14].
2.5. Virus Isolation
To evaluate WENV replication and shedding in SD rats, the homogenates supernatant of qRT-PCR positive tissue samples of lung and feces were diluted in DMEM containing ampicillin (400 µg/ml), streptomycin (100 µg/ml) with a ratio of 1:10, and an additional gentamicin (50 µg/ml) was added in the dilutions of the homogenates of feces. 500 µL of each diluted solution was loaded onto freshly prepared Vero, DH82 monolayer cells in T25 cell culture flasks respectively following common virus isolation technology using mammalian cells, and 200 µL of each dilutes was inoculated in SD rats with intraperitoneal injection (i.p.). The cells for virus rescue were incubated in 5 mL maintenance DMEM medium containing 2% FBS at 37℃ supplemented with CO2 5% for 10 days after absorbance of the loaded homogenates dilutes with intermittently shaking for about 2 hours. Then, the cells were passaged twice, and the results of virus rescue were determined with immunofluorescence assay using polyclonal rabbit serum against the recombinant N protein of WENV, and qRT-PCR method.
2.6. Statistical Analyses
The copies of viral RNA detected were calculated using a standard curve containing dilutions of in vitro transcribed reference viral RNA, and the statistical significance was analyzed using either the Pearson χ2 or continuity correction χ2 test. Analyses were performed using the SPSS software (ver. 16.0). Values of P < 0.05 were considered statistically significant.
3. Results
3.1. Virus Preparation
To prepare the WENV (strain DG4) stock, five 6 - 8 weeks old SD male rats were inoculated with the homogenate of a WENV RNA positive lung tissue from wild Rattus norvegicus captured in Jiangxi Province, China. The 5 rats received 200μl of 1:10 dilution of the homogenate in EMEM by intraperitoneal injection (I.P.). Blood samples had been collected from tail every other day since day 1 prior to inoculation, to examine them for viral RNA by qRT-PCR and seroreactivity to N antigen via ELISA. They were killed at 7 d, and a viral stock was prepared from a pooled homogenate of lung and spleen, which was used for all subsequent infections. The viral RNA copies of this homogenate was about 5 × 107 copies/mL determined using qRT-PCR.
3.2. General Conditions of the Inoculated SD Rats
No significant symptoms were observed from the SD rats during the 4-week post inoculation with WENV stocks compared to the sham-inoculated ones. Animals were killed on days 7, 14, 21 and 28 d pi respectively, heart, liver, kidney, lungs, spleen, thymus, testicles, brain, and intestine were collected, bleeding points were only observed in the lungs and thymus of the inoculated rats collected at 14 d pi, 21 d pi. No lesions were observed in the other organs.
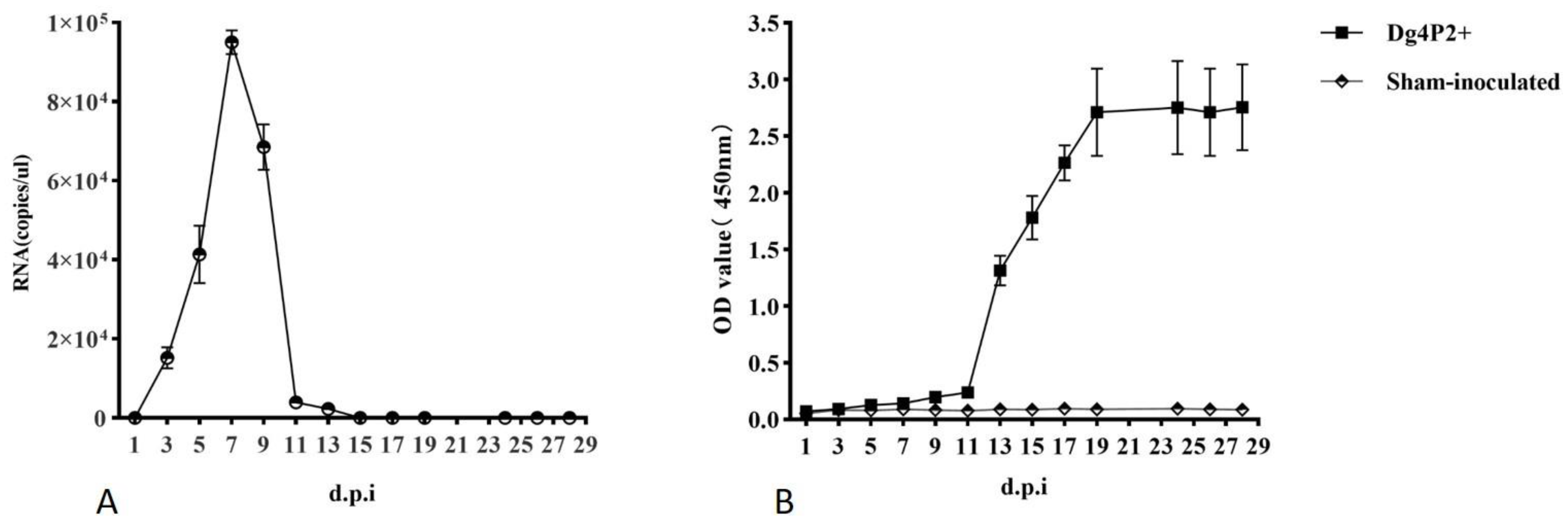
3.3. Viral RNA and Plasma Antibody Responses in Tail Blood
Blood samples were collected from each rat from group 1 prior to infection, and then every other day post-inoculation from the tail using a capillary tube. Testing of viral RNA and viral specific antibodies using qRT-PCR and ELISAs. Viral RNA was detected at 3 d pi, and remained detectable for about 12 days till 15 d pi, peaked at 6~8 d pi, with a number of viral RNA copies at a range of 10
5 copies/μL (
Figure 1A). Anti-N antibodies were evident in tail blood by 5 d in three of five samples, but seroconversion was complete in all of the remaining animals at later time points, and the OD value reached their maximum at 19 d pi, and remained at this level in the later stage (
Figure 1B).
3.4. Dynamic of Experimental WENV Infection in SD Rats
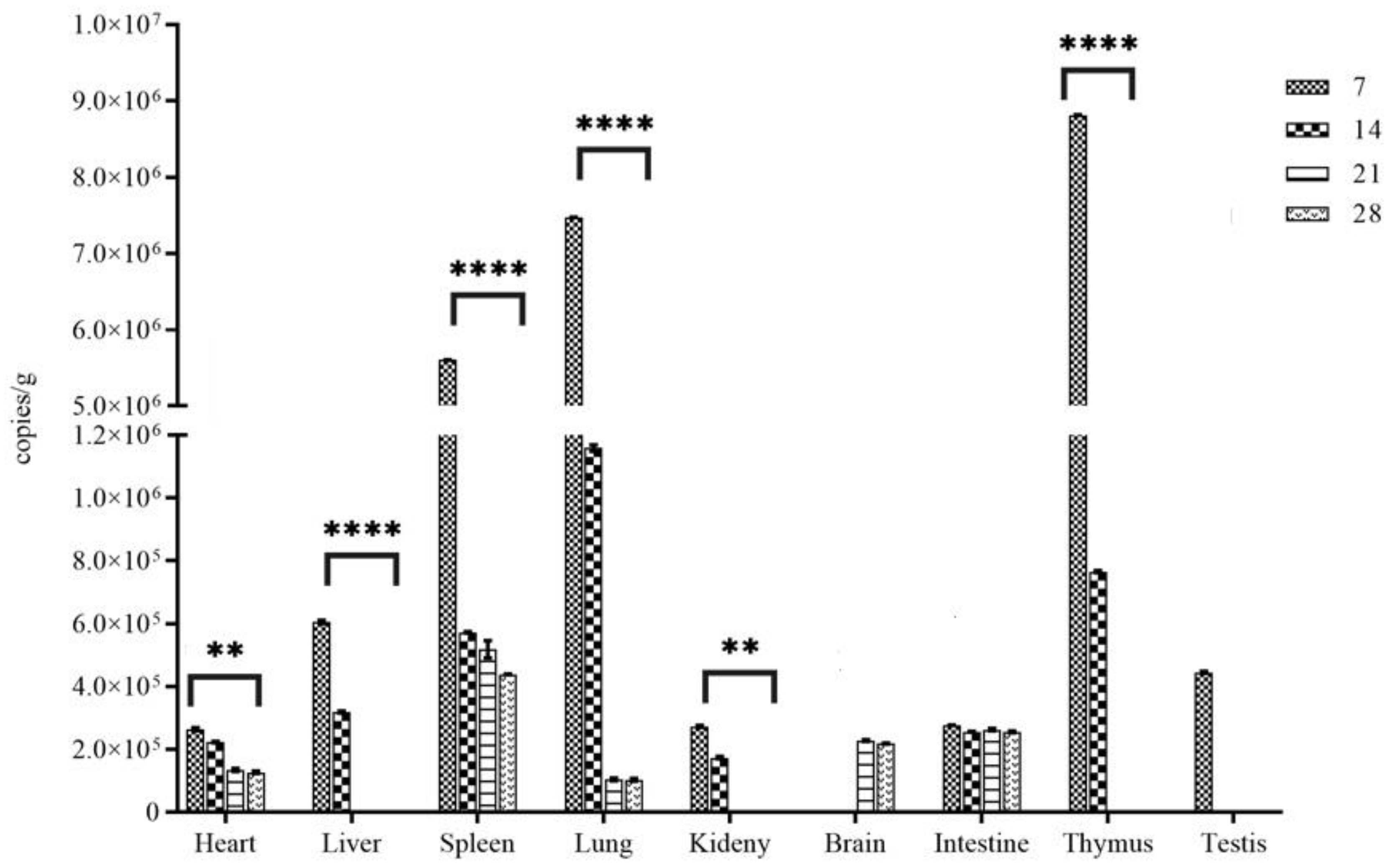
Animals from group 2 were killed on days 7, 14, 21 and 28 d pi respectively, to examine five 5 cages of SD rats in IVC system at each interval by qRT-PCR and ELISA, therefore a minimum of five infected rats and five sham inoculated rats were tested.
Of the five inoculated rats examined at 7 d, all five animals were viral RNA positive in heart, liver, kidney, lung, spleen, intestine, testes, and thymus gland, the number of viral RNA copies detected in the lung, thymus and spleen, at a range of approximately 6.0~9.0 * 10
7 copies/g, was significantly higher than that in other tissue samples (P< 0.01) (
Figure 2). By 14 d, except for brain and testes, the other tissues of the 5 animals were positive for viral RNA, although the RNA load decreased (
Figure 2), coincident with the appearance of high titers of antibodies in blood. By 21 d and onward, the five animals examined showed viral RNA in the tissue samples with significantly decreased number of copies, except liver, kidney, thymus gland and testis wherein it was undetectable in all specimens (
Figure 2). In brain tissue, viral RNA was only detected in samples collected on 21 and 28 d pi (
Figure 2).
For the five sham inoculated rats examined at each interval at the same time as that for the inoculated rats in the same cages, WENV RNA was detected in the lung tissue samples from 2 of 5 rats at 14 dpi, 1 of 5 rats at 21 dpi using qRT-PCR, which suggested that horizontal contact infection occurred among cage-mates.
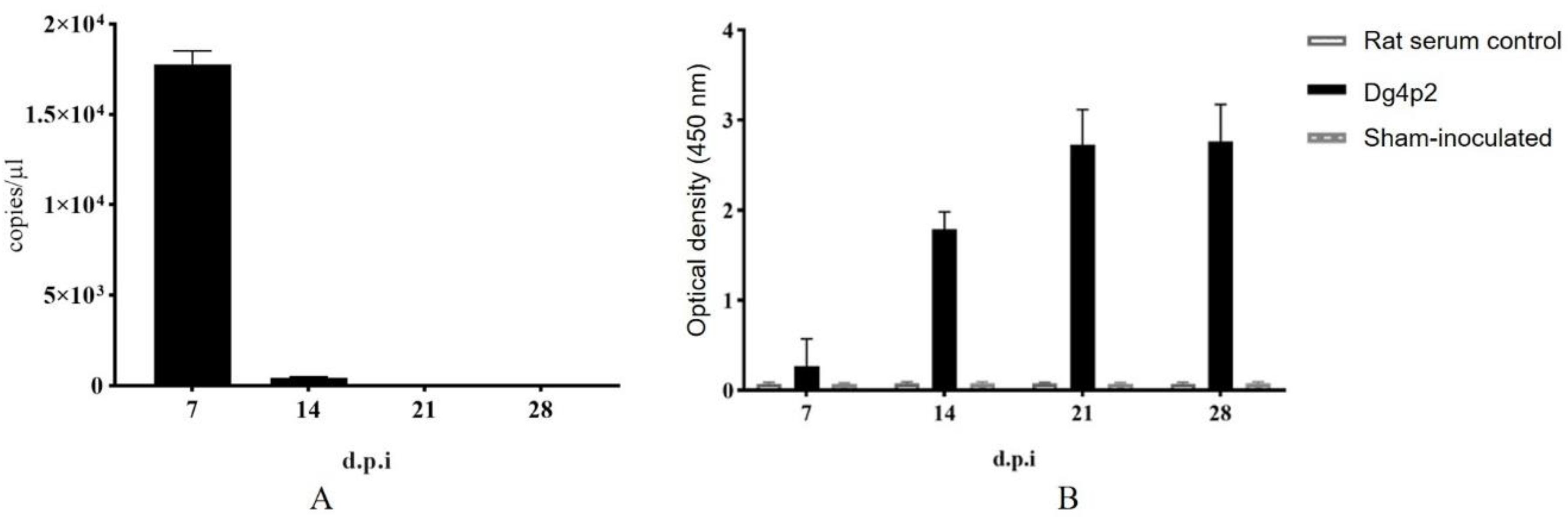
Arterial blood samples were also collected on days 7, 14, 21 and 28 d pi, viral RNA was detected in the arterial blood using qRT-PCR from all the animals at 7 d and 14 d, with a maximum level of 10
7 copies/mL at day 7, but at the other time points, no animal was positive (
Figure 3A). Anti-N antibodies were detectable in all five inoculated rats collected at 7 d pi, and reached their maximum at 21 d pi (
Figure 3B).
3.5. Virus Shedding and Rescue
Fecal samples were collected from cages with inoculated SD rats of group 1 everyday post-inoculation. WENV RNA in feces was detected on 6 d pi, and remained detectable for 5 days until 11 d pi using qRT-PCR, while it could not be detected at other time points (
Figure 4A). The PCR amplification products obtained from feces were sequenced and verified as WENV RNA. To evaluate the infectivity of WENV shedding in feces of inoculated SD rats, the freshly prepared homogenates of feces were inoculated onto monolayer Vero and DH82 cells, as well as in SD rats with intraperitoneal injection (i.p.). The development of viral RNA was detected in the tail blood from the inoculated SD rats with freshly prepared homogenates of feces, with a level of 10
5 copies/μL WENV RNA at 7 dpi (
Figure 4B). And antibodies against N protein were detected after 9 d pi in the blood collected from the tail (
Figure 4C). However, WENV was failed to be recovered from the fecal samples through mammalian cells culture in this study as previous reports (7, 8). To determine whether experimental passage of WENV through rats may have selected for a genetic variant that is more readily transmitted under experimental conditions than the original wild virus, the complete sequence of L and S genomes from the wild-caught rat, lung samples from rats of passage 2 and 3, collected at 7 d pi were sequenced and compared. However, no variants were detected.
4. Discussion
Rodents represent the largest order of living mammals with wide distribution, diverse species, strong reproductive ability, and strong adaptability in the world [
15], which are known to be reservoir hosts for at least 60 zoonotic diseases [
16]. WENV was first identified in Wenzhou China, and subsequently found in broad areas of China, Cambodia, Thailand, and Laos, demonstrating a wide distribution of WENV infection in rodents and humans in Asia[
3,
4,
5,
6,
7,
8]. The rodent hosts of WENV, such as
Rattus norvegicus and
Mus musculus, are commonly found in human dwellings and in peridomestic habitats, which could facilitate frequent spillover of the virus to humans [
17].
In this study, we intended to reveal the dynamics of WENV infection in its rodent reservoirs at acute phase. All animals remained asymptomatic within 28 days after inoculation of 6 - 8 weeks old SD rats. Pathogenesis of arenavirus diseases is believed to involve initial replication at the site of infection in nonreservoir hosts, usually following aerosol deposition in the lung [
18]. WENV replicated to high titers in lung, thymus and spleen of the inoculated rats (>10
6 RNA copies per g) at 7 d pi, and a low titer of viral RNA was detected from heart, liver, kidney, brain, intestine and testis (~10
5 RNA copies per g). Lungs were found with the highest level of viral loads, which could be the primary site of replication for WENV. It was suggested that the viral replication seems to occur systematically all along the acute phase of infection [
19]. In brain tissue, viral RNA could be detected in samples at 21 and 28 d pi, while in other organs, viral RNA could not be detected, except for the liver, spleen, and small intestine. The results demonstrated that WENV could naturally infect brain tissue, with a delay of about 2 weeks compared to other organs, indicating that the virus could cross the blood-brain barrier and pose a potential risk of developing central nervous system diseases in humans after getting infected. It was reported that the mechanism of arenavirus clearance from organs of acutely infected rats was associated with T-cell mediated immune response to viral infection, which had been found in the clearance of lymphocytic choriomeningitis virus (LCMV) in infected animals[
20]. Generally, it is accepted that the kinetics of virus clearance in different organs varied significantly; virus is usually cleared from the liver within 30 days, but neurons can contain viral antigen for 90 days or more, and clearance of virus and antigen from the kidneys requires more than 200 days[
18]. A relative stable level of viral RNA was detected in small intestine, and brain tissues at 21 and 28 d pi in this study, whether that is related to persistent infection of WENV remains unclear. Several studies carried out on hantaviruses highlighted that the transition from acute to chronic phases could occur at different times from the first 2 - 3 weeks to 2 - 3 months of infection[
21,
22,
23].
In this study, a transient viremia of WENV occurred between 5 and 15 days in SD rats, which was similar to a previous report, where viral RNA was detected in sera at 3 d pi, and remained detectable till 15 d pi [
7]. The determinants of the period of viremia in the mature host are unknown, both host genetic component and viral genes play important role in maintenance of the persistent viremic response [
18]. It is well known that arenavirus readily infect mammals, however, strains of animals vary in susceptibility to virus infection, such as the MHA strain of hamsters is highly susceptible, while those of the LVG strain are resistant to Pichinde virus infection [
18].
Only fecal samples were collected in this study to examine the shedding of WENV, viral RNA was detected at 6 d pi, and transmission of WENV among cage-mates were detected at 14 (2/5) and 21 d pi. (1/5) under IVC system. During the feeding period of experimental animals, no significant mutual biting was found between rats, and no obvious bite marks were found from the animals, which could indicate that the transmission should not have been caused by mutual biting between animals. Virus shedding could be affected by the mode of transmission, the quantity of virus inoculated or some individual factors[
19]. Arenavirus had been found in a various array of excreta/secreta of their infected rodent reservoirs, such as urina, saliva and feces [
18]. WENV genomic RNA was detected from the respiratory tract samples of rodents collected from Cambodia, Thailand, and Laos[
6], in addition, a periodic shedding could occur during the persistent phase of arenavirus infection.
An important issue needs to be addressed experimentally is the kinetic of humoral antibodies appearance in WENV infection, which will be helpful to understand the immune mechanisms under reservoir/WENV interactions. Antibody seroconversion was detected at 5 dpi, high level of antibodies was found at 15 dpi, and antibody response next increased and persisted until the end of experiment. During the process of LCMV infection in mice, a vigorous antibody response to LCMV antigens was insufficient to clear virus, but antibodies, complexed with circulating viral antigen, lodges in the renal glomeruli and arterial walls, could lead to chronic glomerulonephritis and arteritis [
18].
In summary, in this experimental infection of WENV in SD rats, by controlling time since inoculation, several organs, blood, and fecal samples were analyzed simultaneously, that have enabled to discriminate the kinetic of viremic response, virus shedding in feces and horizontal transmission, as well as the sites for viral replication at the acute phase of infection and the potential sites for viral maintenance. Although the mechanism underlying needs further investigation, the results provide important insights into the infection, transmission, and prevalence of WENV.
Author Contributions
Methodology, S. Du. X. Deng, T. Liu, T. Tian, Z. Zheng, Q. Lin, Z. Li; validation, X. Huang, A. Li, and Q. Wang; formal analysis, S. Du. X. Deng, X. Huang, J. Li; resources, S. Wang; data curation, S. Du. X. Deng, X. Huang, S. Wang; writing—original draft preparation, S. Du. X. Deng; conceptualization, writing—review and editing, J.Li; project administration, C. Li, S. Wang; funding acquisition, J.Li. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Project of Capital Clinical Diagnosis and Treatment Technology Research and Transformation, grant number Z221100007422076 and ZDGWNLJS24-28. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
All procedures involving animal experiments were reviewed, approved, and conducted in strict accordance with the Animal Experimental Ethical Inspection of the Laboratory Animal Centre, National Institute for Viral Disease Control and Prevention, China CDC (Ethics Approval Number:20210917072; 9 September 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data underlying this article are available in this manuscript and in its online Supporting Information.
Acknowledgments
We thank the colleagues from Laboratory Animal Center, China CDC, for their enormous assistance in animal feeding and monitoring; We express our gratitude to Mr. Quanfu Zhang for his kind discussions in WENV rescue using animals and mammalian cells.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Walker, P. J.; Siddell, S. G.; Lefkowitz, E. J.; Mushegian, A. R.; Adriaenssens, E. M.; Alfenas-Zerbini, P.; Davison, A. J.; Dempsey, D. M.; Dutilh, B. E.; Garcia, M. L.; Harrach, B.; Harrison, R. L.; Hendrickson, R. C.; Junglen, S.; Knowles, N. J.; Krupovic, M.; Kuhn, J. H.; Lambert, A. J.; Lobocka, M.; Nibert, M. L.; Oksanen, H. M.; Orton, R. J.; Robertson, D. L.; Rubino, L.; Sabanadzovic, S.; Simmonds, P.; Smith, D. B.; Suzuki, N.; Van Dooerslaer, K.; Vandamme, A. M.; Varsani, A.; Zerbini, F. M. , Changes to virus taxonomy and to the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2021). Arch Virol 2021, 166, (9), 2633–2648. [Google Scholar] [CrossRef]
- Li, K.; Lin, X. D.; Wang, W.; Shi, M.; Guo, W. P.; Zhang, X. H.; Xing, J. G.; He, J. R.; Wang, K.; Li, M. H.; Cao, J. H.; Jiang, M. L.; Holmes, E. C.; Zhang, Y. Z. , Isolation and characterization of a novel arenavirus harbored by Rodents and Shrews in Zhejiang province, China. Virology 2015, 476, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Y.; Guo, C.; Xia, Y.; Bao, H. M.; Zhu, Y. S.; Guo, Z. M.; Wei, Y. H.; Lu, J. H. , Genomic characterization of Wenzhou mammarenavirus detected in wild rodents in Guangzhou City, China. One Health 2021, 13, 100273. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liu, S.; Song, J.; Han, L.; Zhang, H.; Wu, C.; Wang, C.; Zhou, H.; Wang, J. , Seroprevalence of Wenzhou virus in China. Biosaf Health 2020, 2, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, X.; Liu, H.; Wang, L.; Zhou, J.; Han, X.; Zhu, Y.; Yang, W.; Pan, H.; Zhang, Y.; Shi, Z. , Prevalence of Wenzhou virus in small mammals in Yunnan Province, China. PLoS Negl Trop Dis 2019, 13, e0007049. [Google Scholar] [CrossRef] [PubMed]
- Blasdell, K. R.; Duong, V.; Eloit, M.; Chretien, F.; Ly, S.; Hul, V.; Deubel, V.; Morand, S.; Buchy, P. , Evidence of human infection by a new mammarenavirus endemic to Southeastern Asia. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yu, H.; Xu, L.; Zhao, Z.; Zhang, P.; Qu, Y.; He, B.; Tu, C. , Virome profiling of rodents in Xinjiang Uygur Autonomous Region, China: Isolation and characterization of a new strain of Wenzhou virus. Virology 2019, 529, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Xie, Y.; Deng, X.; Xia, Z.; Wu, W.; Huang, X.; Chen, Z.; Li, A.; Li, C.; Wang, Q.; Sun, L.; Guo, M.; Wang, S.; Liang, M.; Li, D.; Liu, X.; Li, J. , Co-circulation and co-infection of hantaviruses and Wenzhou mammarenavirus in small mammals and humans in Jiangxi, China. Front Microbiol 2023, 14, 1225255. [Google Scholar] [CrossRef] [PubMed]
- Charrel, R. N.; de Lamballerie, X. , Zoonotic aspects of arenavirus infections. Vet Microbiol 2010, 140, 213–20. [Google Scholar] [CrossRef] [PubMed]
- Sarute, N.; Ross, S. R. , New World Arenavirus Biology. Annu Rev Virol 2017, 4, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Buchmeier, M. J.; Peters, C. J.; Torre, J. C. D. L. , Arenaviridae., 6th ed.; Fields virology: USA, 2010; Vol. 2, p 1284-1298. [Google Scholar]
- Moutailler, S.; Valiente Moro, C.; Vaumourin, E.; Michelet, L.; Tran, F. H.; Devillers, E.; Cosson, J. F.; Gasqui, P.; Van, V. T.; Mavingui, P.; Vourc’h, G.; Vayssier-Taussat, M. , Co-infection of Ticks: The Rule Rather Than the Exception. PLoS Negl Trop Dis 2016, 10, e0004539. [Google Scholar] [CrossRef] [PubMed]
- Vayssier-Taussat, M.; Albina, E.; Citti, C.; Cosson, J. F.; Jacques, M. A.; Lebrun, M. H.; Le Loir, Y.; Ogliastro, M.; Petit, M. A.; Roumagnac, P.; Candresse, T. , Shifting the paradigm from pathogens to pathobiome: new concepts in the light of meta-omics. Front Cell Infect Microbiol 2014, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. , MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Capizzi, D.; Bertolino, S.; Mortelliti, A. , Rating the rat: Global patterns and research priorities in impacts and management of rodent pests. Mammal Review 2014, 44, 148–162. [Google Scholar] [CrossRef]
- Taylor, P. J.; Arntzen, L.; Hayter, M.; Iles, M.; Frean, J.; Belmain, S. , Understanding and managing sanitary risks due to rodent zoonoses in an African city: beyond the Boston Model. Integr Zool 2008, 3, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Kock, R. , Drivers of disease emergence and spread: is wildlife to blame? Onderstepoort J Vet Res 2014, 81, E1–4. [Google Scholar] [CrossRef] [PubMed]
- Buchmeier, M. J.; Peters, C. J.; Torre, J. C. D. L. , Arenaviridae: the virus and their replication., 6th ed.; Fields virology: USA, 2007; Vol. 2, p 1284-1298. [Google Scholar]
- Madrieres, S.; Castel, G.; Murri, S.; Vulin, J.; Marianneau, P.; Charbonnel, N. , The Needs for Developing Experiments on Reservoirs in Hantavirus Research: Accomplishments, Challenges and Promises for the Future. Viruses 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J. A.; Oldstone, M. B. , Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus. VI. Migration and activity in vivo in acute and persistent infection. J Immunol 1986, 136, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Easterbrook, J. D.; Klein, S. L. , Seoul virus enhances regulatory and reduces proinflammatory responses in male Norway rats. J Med Virol 2008, 80, 1308–18. [Google Scholar] [CrossRef]
- Hutchinson, K. L.; Rollin, P. E.; Peters, C. J. , Pathogenesis of a North American hantavirus, Black Creek Canal virus, in experimentally infected Sigmodon hispidus. Am J Trop Med Hyg 1998, 59, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Botten, J.; Mirowsky, K.; Kusewitt, D.; Ye, C.; Gottlieb, K.; Prescott, J.; Hjelle, B. , Persistent Sin Nombre virus infection in the deer mouse (Peromyscus maniculatus) model: sites of replication and strand-specific expression. J Virol 2003, 77, 1540–50. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).