Submitted:
30 July 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Samples
2.2. Animals and Experimental Design
2.3. Pig Performance Assays
2.4. Standard Diet and Muscle Chemical Composition Analysis
2.5. Functional Diet: Preparation and Analysis
2.6. HPLC-UV Analysis
2.6.1. Chemicals
2.6.2. Preparation of Extracts with Different Solvents
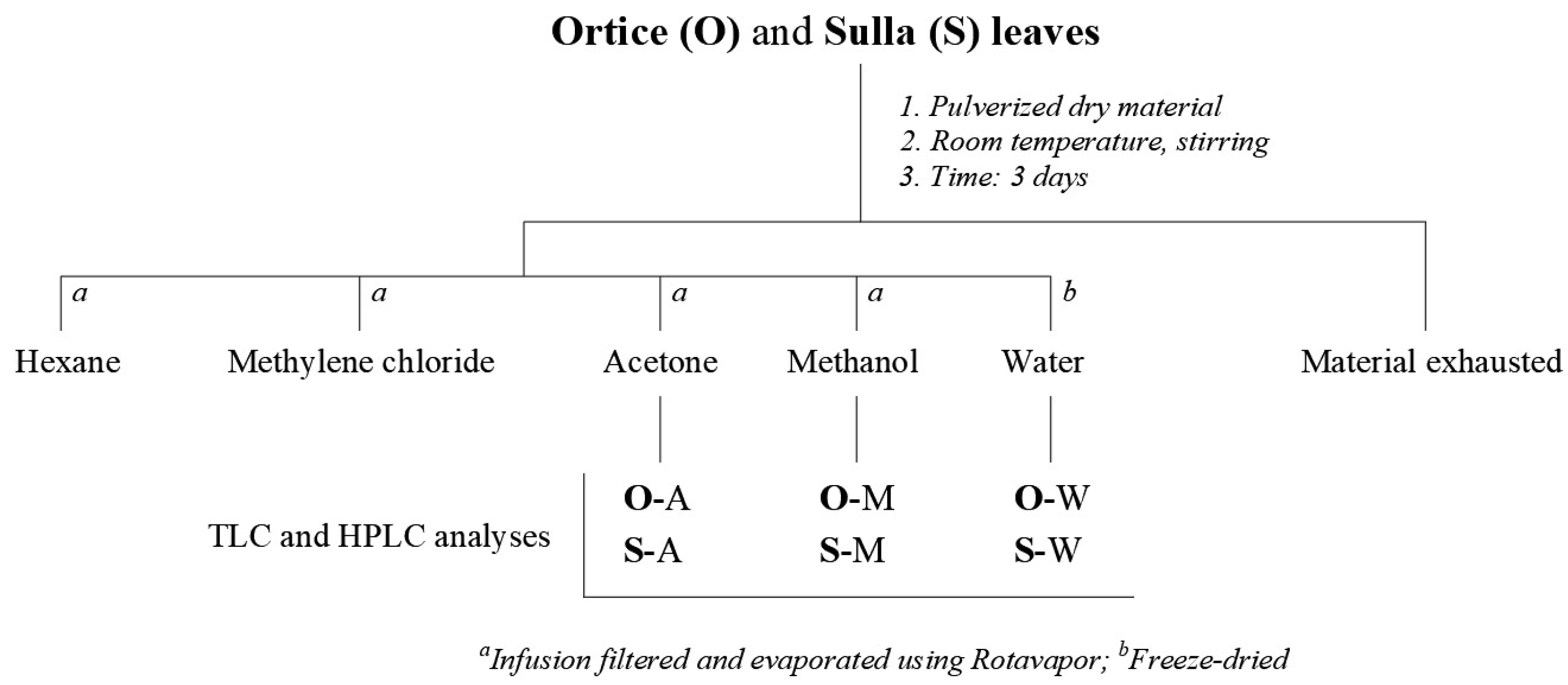
2.6.3. General Experimental Procedures
2.7. Fatty Acids Analysis of Meat and Diet
2.8. Statistical Analysis
3. Results
3.1. Pig Performances and Chemical Composition of Muscle
3.2. Functional Diet Analysis
3.2.1. Total Polyphenols Content, Total Flavonoid Content, and Antioxidant Activity
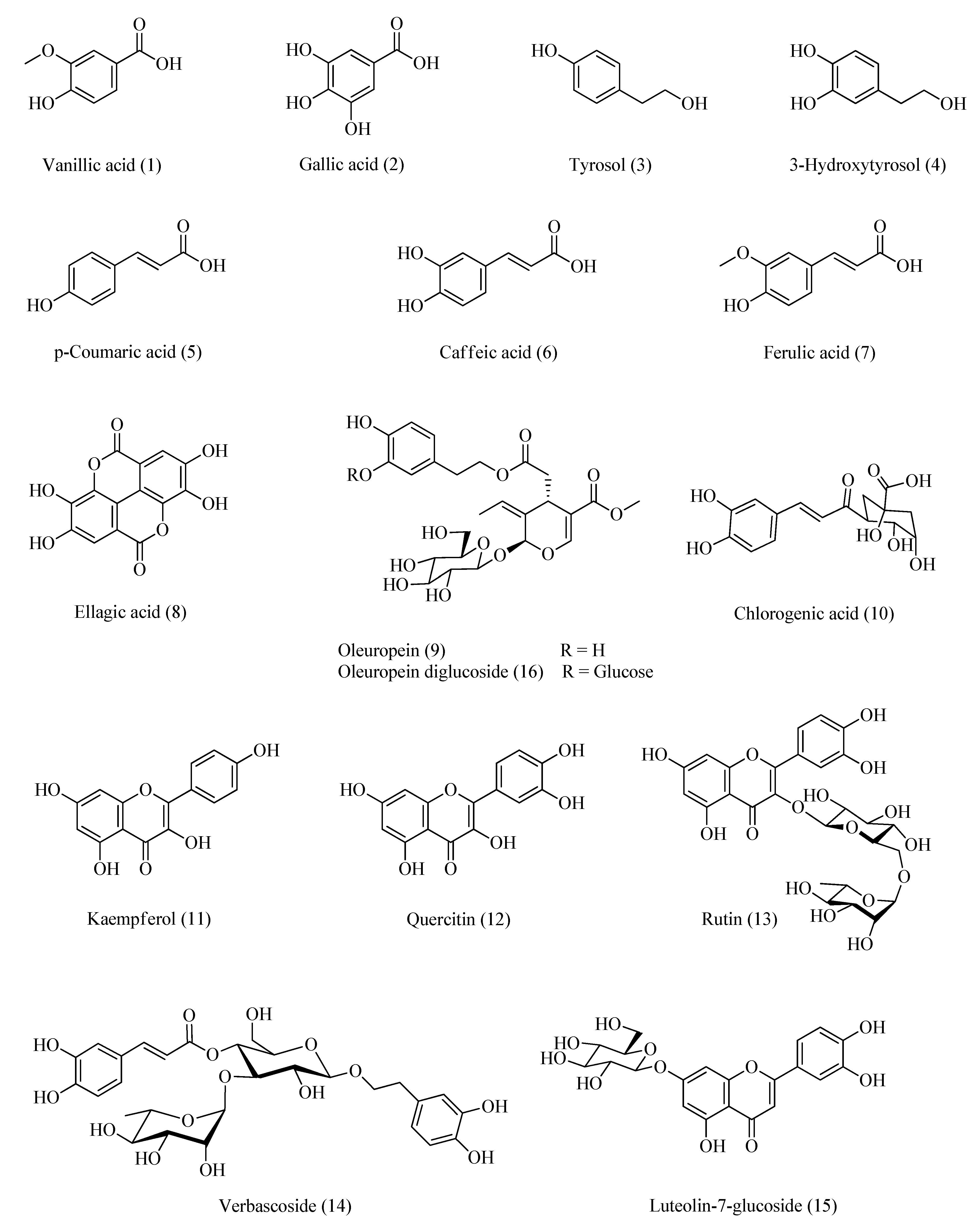
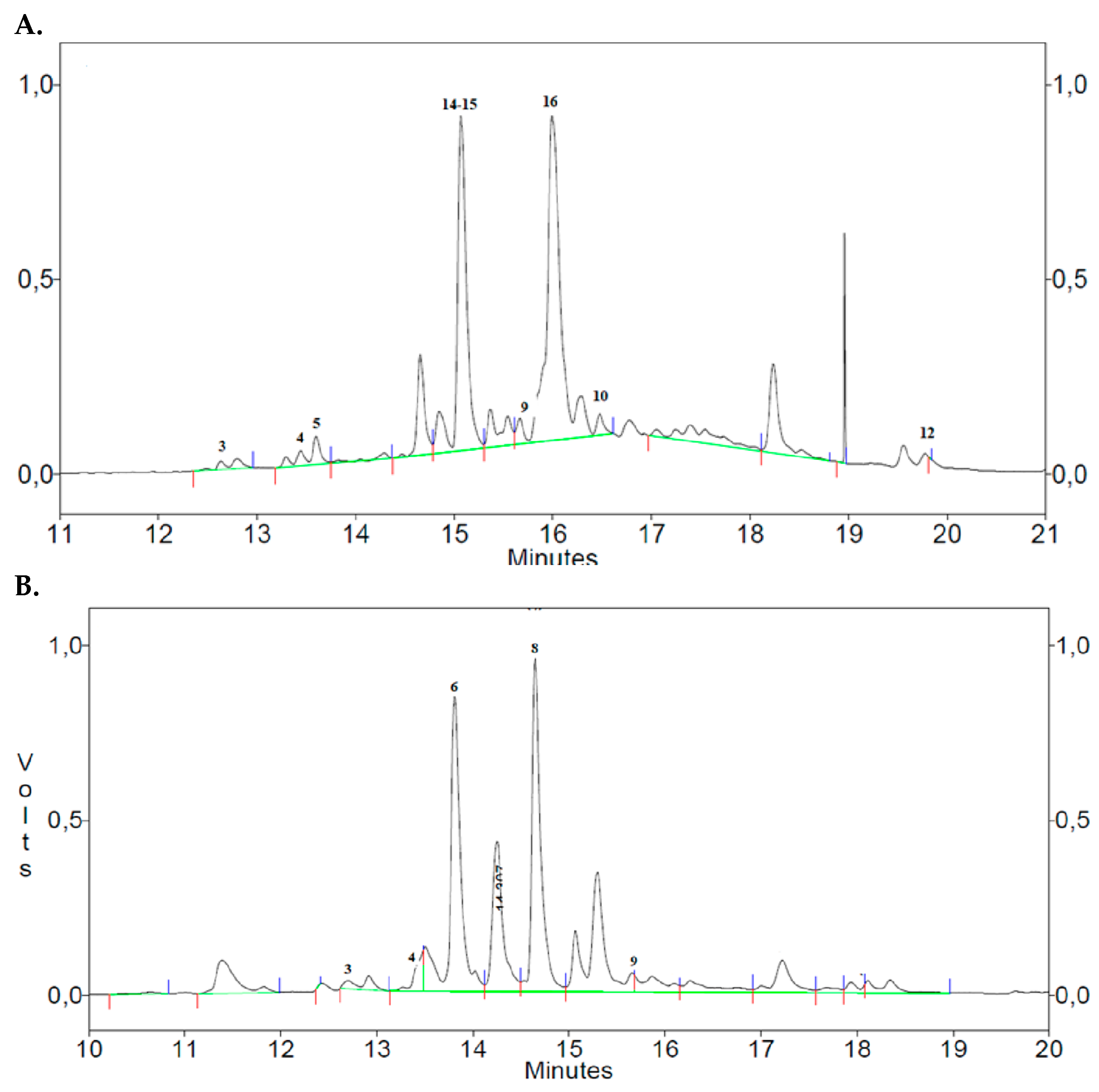
3.2.2. HPLC-UV Analysis
3.3. Fatty Acid Profile
3.3.1. Dietary Fatty Acid Profile
3.3.2. Muscle Fatty Acid Profile
4. Discussion
5. Conclusions and Future Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malenica, D.; Kass, M.; Bhat, R. Sustainable Management and Valorization of Agri-Food Industrial Wastes and By-Products as Animal Feed: For Ruminants, Non-Ruminants and as Poultry Feed. Sustainability 2023, 15, 117. [Google Scholar] [CrossRef]
- Hukerdi, Y. J.; Nasri, M. F.; Rashidi, L.; Ganjkhanlou, M.; Emami, A. Effects of dietary olive leaves on performance, carcass traits, meat stability and antioxidant status of fattening Mahabadi male kids. Meat Sci. 2019, 153, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Ferlisi, F.; Tang, J.; Cappelli, K.; Trabalza-Marinucci, M. Dietary supplementation with olive oil co-products rich in polyphenols: a novel nutraceutical approach in monogastric animal nutrition. Front. Vet. Sci. 2023, 10, 1272274. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, R.; Budriesi, R.; Clodoveo, M.L.; Biotti, G.; Micucci, M.; Ragusa, A.; Curci, F.; Muraglia, M.; Corbo, F.; Franchini, C. Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation. Molecules 2021, 26, 1072. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P. E. S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J. M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Berbel, J.; Posadillo, A. Review and Analysis of Alternatives for the Valorisation of Agro-Industrial Olive Oil By-Products. Sustainability 2018, 10, 237. [Google Scholar] [CrossRef]
- Mahfuz S, Shang Q, Piao X. Phenolic compounds as natural feed additives in poultry and swine diets: a review. J Anim Sci Biotechnol. 2021, 12, 48. [Google Scholar] [CrossRef]
- Di Domenico, M.; Feola, A.; Ambrosio, P.; Pinto, F.; Galasso, G.; Zarrelli, A.; Di Fabio, G.; Porcelli, M.; Scacco, S.; Inghingolo, F.; Quagliuolo, L.; Ballini, A.; Boccellino, M. Antioxidant Effect of Beer Polyphenols and Their Bioavailability in Dental-Derived Stem Cells (D-dSCs) and Human Intestinal Epithelial Lines (Caco-2) Cells. Stem Cells Int. 2020, 1, 8835813. [Google Scholar] [CrossRef] [PubMed]
- Vastolo, A.; Calabró, S.; Liotta, L.; Musco, N.; Di Rosa, A.R.; Cutrignelli, M.I.; Chiofalo, B. In Vitro Fermentation and Chemical Characteristics of Mediterranean By-Products for SwineNutrition. Animals 2019, 9, 556. [Google Scholar] [CrossRef]
- Di Meo, M.C; De Cristofaro, G.A.; Imperatore, R.; Rocco, M.; Giaquinto, D.; Palladino, A.; Zotti, T.; Vito, P.; Paolucci, M.; Varricchio, E. Microwave-Assisted Extraction of Olive Leaf from Five Italian Cultivars: Effects of Harvest-Time and Extraction Conditions on Phenolic Compounds and In Vitro Antioxidant Properties. ACS Food Sci. Technol. 2021, 2, 31–40. [Google Scholar] [CrossRef]
- Saija, A.; Trombetta, D.; Tomaino, A.; Lo Cascio, R.; Princi, P.; Uccella, N; Bonina, F.; Castelli, F. `In vitro’ evaluation of the antioxidant activity and biomembrane interaction of the plant phenols oleuropein and hydroxytyrosol. Int J Pharm. [CrossRef]
- Paiva-Martins, F.; Barbosa, S.; Pinheiro, V.; Mourão, J. L.; Outor-Monteiro, D. The effect of olive leaves supplementation on the feed digestibility, growth performances of pigs and quality of pork meat. Meat Sci. 2009, 82(4), 438–443. [Google Scholar] [CrossRef] [PubMed]
- Qwele, K.; Hugo, A.; Oyedemi, S. O.; Moyo, B.; Masika, P. J.; Muchenje, V. Chemical composition, fatty acid content and antioxidant potential of meat from goats supplemented with Moringa (Moringa oleifera) leaves, sunflower cake and grass hay. Meat Sci. 2013, 93(3), 455–4. [Google Scholar] [CrossRef] [PubMed]
- Poutaraud, A.; Michelot-Antalik, A.; Plantureux, S. Grasslands: a source of secondary metabolites for livestock health. J. Agric. Food Chem 2017, 65(31), 6535–6553. [Google Scholar] [CrossRef] [PubMed]
- Tava, A.; Biazzi, E.; Ronga, D.; Mella, M.; Doria, F.; D’Addabbo, T.; Candido, V.; Avato, P. Chemical identification of specialized metabolites from sulla (Hedysarumcoronarium L.) collected in southern Italy. Molecules 2021, 26, 4606. [Google Scholar] [CrossRef] [PubMed]
- Rivero, M. J.; Rodríguez-Estévez, V.; Pietrosemoli, S.; Carballo, C.; Cooke, A. S.; Kongsted, A. G. Forage consumption and its effects on the performance of growing swine—Discussed in relation to European wild boar (Sus Scrofa L.) in semi-extensive systems: A review. Animals 2019, 9, 457. [Google Scholar] [CrossRef]
- Priolo, A.; Bella, M.; Lanza, M.; Galofaro, V.; Biondi, L.; Barbagallo, D.; Ben Salem, H.; Pennisi, P. Carcass and meat quality of lambs fed fresh sulla (Hedysarum coronarium L.) with or without polyethylene glycol or concentrate. Small Rumin. Res. 2005, 59, 281–288. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, P.; Reyes-Palomo, C.; Sanz-Fernández, S.; Rufino-Moya, P.J.; Zafra, R.; Martínez-Moreno, F.J.; Rodríguez-Estévez, V.; Díaz-Gaona, C. Antiparasitic Tannin-Rich Plants from the South of Europe for Grazing Livestock: A Review. Animals 2023, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Ponte, M.; Maniaci, G.; Di Grigoli, A.; Gannuscio, R.; Ashkezary, M.R.; Addis, M.; Pipi, M.; Alabiso, M.; Todaro, M.; Bonanno, A. Feeding Dairy Ewes with Fresh or Dehydrated Sulla (Sulla coronarium L.) Forage. 2. Effects on Cheese Enrichment in Bioactive Molecules. Animals 2022, 12, 2462. [Google Scholar] [CrossRef]
- Gladine, C.; Rock, E.; Morand, C.; Cauchart, D.; Durand, D. Bioavailability and antioxidant capacity of plant extracts rich in polyphenols, given as a single acute dose, in sheep made highly susceptible to lipoperoxidation. Br. J. Nutr. 2007, 98, 691–701. [Google Scholar] [CrossRef]
- Di Trana, A.; Bonanno, A.; Cecchini, S.; Giorgio, D.; Di Grigoli, A.; Claps, S. Effects of Sulla forage (Sulla coronarium L.) on the oxidative status and milk polyphenol content in goats. J. Dairy Sci. 2015, 98, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Botsoglou, E.; Govaris, A.; Ambrosiadis, I.; Fletouris, D. Lipid and protein oxidation of α-linolenic acid-enriched pork during refrigerated storage as influenced by diet supplementation with olive leaves (Olea europea L.) or α-tocopheryl acetate. Meat Sci. 2012, 92, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional compounds from olive pomace to obtain high-added value foods–a review. J. Sci. Food Agr. 2021, 101(1), 15–26. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, M.C.; Salzano, A.; Zotti, T.; Palladino, A.; Giaquinto, D.; Maruccio, L.; Romanucci, R.; Rocco, M.; Zarrelli, A.; D’Occhio, M.J. , Campanile, G.; Varricchio, E. Plasma fatty acid profile in Italian Holstein-Friesian dairy cows supplemented with natural polyphenols from the olive plant Olea Europaea L. Vet. Anim. Sci. 2023, 21, 100298. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, M. C.; Giacco, A.; Zarrelli, A.; Mandrone, V. M.; D’Angelo, L.; Silvestri, E.; De Girolamo, P.; Varricchio, E. Effects of Olea europaea L. Polyphenols on the Animal Welfare and Milk Quality in Dairy Cows. Animals 2023, 13(20), 3225. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Martins, F.; Barbosa, S.; Silva, M.; Monteiro, D.; Pinheiro, V.; Mourão, J.L.; Fernandes, J.; Rocha, S.; Belo, L.; Santos-Silva, A. The effect of olive leaf supplementation on the constituents of blood and oxidative stability of red blood cells. J. Funct. Foods 2014, 9, 271–279. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Ribeirinha, T.; Silva, A.; Gonçalves, R.; Pinheiro, V.; Mourão, J. L.; Outor-Monteiro, D. Effects of the dietary incorporation of olive leaves on growth performance, digestibility, blood parameters and meat quality of growing pigs. J. Sci. Food Agric. 2014, 94(14), 3023–3029. [Google Scholar] [CrossRef]
- Ponnampalam, E. N.; Sinclair, A. J.; Holman, B. W. The sources, synthesis and biological actions of omega-3 and omega-6 fatty acids in red meat: An overview. Foods 2021, 10(6), 1358. [Google Scholar] [CrossRef] [PubMed]
- Dugan, M. E.; Vahmani, P.; Turner, T. D.; Mapiye, C.; Juárez, M.; Prieto, N.; Beaulieu, A. D.; Zijlstra, R. T.; Patience, J. F.; Aalhus, J. L. Pork as a source of omega-3 (n-3) fatty acids. J. Clin. Med. 2015, 4(12), 1999–2011. [Google Scholar] [CrossRef]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis, 16th ed.; AOAC: Washington, DC, USA, 1995. [Google Scholar]
- DellaGreca, M.; Previtera, L.; Purcaro, R.; Zarrelli, A. Cinnamic ester derivatives from Oxalis pes-caprae (Bermuda buttercup). J. Nat. Prod. 2007, 70, 1664–1667. [Google Scholar] [CrossRef]
- Crescenzo, R.; Bianco, F.; Mazzoli, A.; Giacco, A.; Cancelliere, R.; Di Fabio, G.; Zarrelli, A.; Liverini, G.; Iossa, S. Fat quality influences the obesogenic effect of high-fat diets. Nutrients 2015, 7, 9475–9491. [Google Scholar] [CrossRef] [PubMed]
- Pollio, A.; Zarrelli, A.; Romanucci, V.; Di Mauro, A.; Barra, F.; Pinto, G.; Crescenzi, E.; Roscetto, E.; Palumbo, G. Polyphenolic profile and targeted bioactivity of methanolic extracts from Mediterranean ethnomedicinal plants on human cancer cell lines. Molecules 2016, 21, 395. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Martins, F.; Gordon, M. H. Isolation and characterization of the antioxidant component 3,4-dihydroxyphenylethyl 4-formyl-3-formylmethyl-4-hexanoate from olive (Olea europaea). J. Agric. Food Chem. 2001, 49, 4214–4219. [Google Scholar] [CrossRef] [PubMed]
- Savournin, C.; Baghdikian, B.; Elias, R.; Dargouth-Kesraoui, F.; Boukef, K.; Balansard, G. Rapid high-performance liquid chromatography analysis for the quantitative determination of oleuropein in Olea europaea leaves. J Agric Food Chem. 2001, 49, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Benavente-Garcıa, O.; Castillo, J.; Lorente, J.; Ortuño, A. D. R. J.; Del Rio, J. A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68(4), 457–462. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic Compounds and Antimicrobial Activity of Olive (Olea europaea L. Cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Pino, F.; Millan-Linares, MC.; Villanueva-Lazo, A.; Fernandez-Prior, Á.; Montserrat-de-la-Paz, S. In vivo evidences of the health-promoting properties of bioactive compounds obtained from olive by-products and their use as food ingredient. Crit Rev Food Sci Nutr. 2023, 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Molinu, M.G.; Sulas, L.; Campesi, G.; Re, G.A.; Sanna, F.; Piluzza, G. Subterranean Clover and Sulla as Valuable and Complementary Sources of Bioactive Compounds for Rainfed Mediterranean Farming Systems. Plants 2023, 12, 417. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Deng, C.; Chen, Q.; Zhao, S.; Li, P.; Wu, T.; Hou, Y.; Yi, D. Protective effects and mechanisms of ellagic acid on intestinal injury in piglets infected with porcine epidemic diarrhea virus. Front. Immunol. 2024, 15, 1323866. [Google Scholar] [CrossRef]
- Wang, F.; Chen, J.; Yin, Y.; Yang, M.; Xiao, Y.; Cheng, Y.; Yin, L.; Fu, C. The effects of dietary ellagic acid supplementation on growth performance, immune response, antioxidant activity, digestive enzyme activities, and intestinal functions in yellow-feathered broilers. J. Anim. Sci. 2022, 100(12), skac301. [Google Scholar] [CrossRef]
- Wen, X.; Zhou, M.; Lu, Q.; Liu, B.; Shi, X.; Zhao, J. Addition of ellagic acid improved the immune ability and delayed the apoptosis of ovarian granulosa cells of Guizhou black goat. Anim. Prod. Sci. 2023, 64(1), AN23310. [Google Scholar] [CrossRef]
- Echegaray, N.; Munekata, P.E.S.; Centeno, J.A.; Domínguez, R.; Pateiro, M.; Carballo, J.; Lorenzo, J.M. Total Phenol Content and Antioxidant Activity of Different Celta Pig Carcass Locations as Affected by the Finishing Diet (Chestnuts or Commercial Feed). Antioxidants 2021, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary Polyphenol Supplementation in Food Producing Animals: Effects on the Quality of Derived Products. Animals 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- DeJong, S. & Lanari, M. C. Extracts of olive polyphenols improve lipid stability in cooked beef and pork: Contribution of individual phenolics to the antioxidant activity of the extract. Food Chem. 2009, 116, 892–897. [Google Scholar] [CrossRef]
- Botsoglou, E.; Govaris, A.; Christaki, E.; Botsoglou, N. Effect of dietary olive leaves and/or α-tocopheryl acetate supplementation on microbial growth and lipid oxidation of turkey breast fillets during refrigerated storage. Food Chem. 2010, 121(1), 17–22. [Google Scholar] [CrossRef]
- Botsoglou, E.; Govaris, A.; Ambrosiadis, I.; Fletouris, D.; Papageorgiou, G. Effect of olive leaf (Olea europea L.) extracts on protein and lipid oxidation in cooked pork meat patties enriched with n-3 fatty acids. J Sci Food Agric. 2014, 94, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Mylonaki, S.; Kiassos, E.; Makris, D. P.; Kefalas, P. Optimisation of the extraction of olive (Olea europaea) leaf phenolics using water/ethanol-based solvent systems and response surface methodology. Anal Bioanal Chem. 2008, 392, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Leskovec, J.; Rezar, V.; Nemec Svete, A.; Salobir, J.; Levart, A. Antioxidative Effects of Olive Polyphenols Compared to Vitamin E in Piglets Fed a Diet Rich in N-3 PUFA. Animals 2019, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Wood, J. D.; Enser, M.; Fisher, A. V.; Nute, G. R.; Sheard, P. R.; Richardson, R. I. ; Hughes,S.I.; Whittington, F. M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Joven, M.; Pintos, E.; Latorre, M. A.; Suárez-Belloch, J.; Guada, J. A.; Fondevila, M. Effect of replacing barley by increasing levels of olive cake in the diet of finishing pigs: Growth performances, digestibility, carcass, meat and fat quality. Anim Feed Sci Technol. 2014, 197, 185–193. [Google Scholar] [CrossRef]
- Tsala, A.; Mpekelis, V.; Karvelis, G.; Tsikakis, P.; Goliomytis, M.; Simitzis, P. Effects of Dried Olive Pulp Dietary Supplementation on Quality Characteristics and Antioxidant Capacity of Pig Meat. Foods 2020, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, A.; Mugnai, C.; Ruggeri, S.; Mattioli, S.; Castellini, C. Fatty acid composition of meat and estimated indices of lipid metabolism in different poultry genotypes reared under organic system. Poultry Sci. 2012, 91(8), 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Losacco, C.; Laudadio, V.; Schiavitto, M.; Tufarelli, V. Perspectives and advantages of using olive (Olea europaea) by-products as a dietary supplement for rabbit production and health. S. Afr. J. Anim. Sci. 2023, 53(5), 737–754. [Google Scholar] [CrossRef]
- Ferrer, P.; Calvet, S.; García-Rebollar, P.; de Blas, C.; Jiménez-Belenguer, A. I.; Hernández, P.; Piquer, O.; Cerisuelo, A. Partially defatted olive cake in finishing pig diets: Implications on performance, faecal microbiota, carcass quality, slurry composition and gas emission. Animal 2020, 14(2), 426–434. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-García, A.; Vieira-Aller, C. Improving Fatty Acid Profile in Native Breed Pigs Using Dietary Strategies: A Review. Animals 2023, 13, 1696. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F. Quality of Meat and Meat Products Produced from Southern European Pig Breeds. Meat Sci. 2012, 90, 511–518. [Google Scholar] [CrossRef]
| Family name | Cultivar name | Scientific name | English name |
|---|---|---|---|
| Oleaceae | Ortice | Olea europaea | Olive tree |
| Brassicaceae | - | Hedysarium coronarium | Wild Radish |
| Components | % DM |
|---|---|
| Standard Diet | |
| Maize | 45.00 |
| Barley | 27.00 |
| Wheat | 15.00 |
| Soy Protein 46% | 8.00 |
| Bran | 5.00 |
| Pasture grasses | |
| Sulla a | 18.24 |
| a. Standard Diet | ||
| Parameters | DM [%] | |
| Moisture | 11.15 | |
| Dry matter | 88.85 | |
| Crude protein | 13.00 | |
| Ash | 1.94 | |
| Crude Fats | 2.78 | |
| Raw fibre | 4.05 | |
| Starch | 52.17 | |
| b. Sulla (H. coronarium L.) | ||
| Parameters | F.M. [%] | D.M. [%] |
| Moisture | 81.76 | |
| Dry matter | 18.24 | |
| Crude protein | 4.56 | 25.00 |
| Ash | 2.07 | 11.36 |
| NDF 1 | 6.57 | 36.00 |
| ADF 2 | 3.79 | 20.80 |
| Dietary treatment1 | SEM6 | p-value | ||
|---|---|---|---|---|
| Control | OL | |||
| Final BW2, kg | 204 | 200 | 2.016 | 0.261 |
| Carcass weight, kg | 200 | 185 | 5.560 | 0.563 |
| Total DMI3, g/d | 3.54 | 3.26 | 0.156 | 0.674 |
| ADG4, g/d | 750 | 650 | 9.261 | 0.633 |
| FCR 5, g DMI/g ADG | 4.72 | 5.02 | 0.192 | 0.105 |
| Chemical composition | ||||
| Moisture | 71.65 | 72.20 | 0.245 | 0.321 |
| Crude protein | 23.78 | 23.54 | 0.112 | 0.055 |
| Ether extract | 3.06 | 3.01 | 0.091 | 0.186 |
| Ash | 1.51 | 1.50 | 0.067 | 0.156 |
| Phenolic Extracts | TPC (mg GAE/g) | TFC (mg QE/g) | Inhibition % |
|---|---|---|---|
| Standard Diet | 8.14 ± 0.30 | 1.71 ± 0.20 | 28.21 ± 1.20 |
| Olive leaves extract | 19.13 ± 0.75 | 3.50 ± 0.43 | 51.10 ± 2.98 |
| Enriched Diet | 33.59 ± 0.88 | 2.98 ± 0.32 | 70.20 ± 2.36 |
| Sulla (H. coronarium) | 17.47 ± 0.52 | 3.02 ± 0.26 | 63.20 ± 2.00 |
| A.Ortice olive leaves | ||||||
| No. Peak | Compound | λ (nm) | RT (min) | r2 | Phenolic compounds concentration (mg/g) | |
| 14 | Verbascoside | 280 | 14 | 0.98 | 0.098 ± 0.05 | |
| 15 | Luteolin-7-glucoside | 280 | 15 | 0.99 | 0.098 ± 0.04 | |
| 16 | Oleuropein diglucoside | 280 | 16 | 0.99 | 0.100 ± 0.09 | |
| B. Sulla plant | ||||||
| No. Peak | Compound | λ (nm) | RT (min) | r2 | Phenolic compounds concentration (mg/g) | |
| 6 | Caffeic acid | 280 | 14 | 0.99 | 0.068 ± 0.04 | |
| 8 | Ellagic acid | 280 | 14.7 | 0.99 | 0.096 ± 0.01 | |
| Fatty acid | Items | Standard diet (g/100 g) |
Ortice olive leaf extract (g/100 g) |
Enriched diet (g/100g) |
Sulla extract |
SEM | p-value |
|---|---|---|---|---|---|---|---|
| Myristic | C14:0 | 0.88 | 1.14 | 0.70 | 0.51 | 0.13 | ns |
| Palmitic | C16:0 | 39.44 | 38.00 | 37.01 | 26.11 | 2.64 | <0.001 |
| Trans-Palmitoleic | C16:1n7t | 0.09 | 0.06 | 0.05 | 0.00 | 0.02 | ns |
| Palmitoleic | C16:1n7 | 0.15 | 0.25 | 0.18 | 0.70 | 0.13 | ns |
| Stearic | C18:0 | 9.73 | 10.55 | 11.01 | 13.37 | 0.78 | <0.001 |
| Oleic | C18:1n9 | 16.35 | 36.18 | 19.30 | 2.56 | 6.90 | <0.001 |
| Trans-Linoleic | C18:2n6t | 0.30 | 0.13 | 0.18 | 0.00 | 0.06 | ns |
| Linoleic | C18:2n6 | 30.96 | 11.28 | 29.11 | 9.27 | 5.73 | <0.001 |
| Gamma-Linolenic | C18:3n6 | 0.16 | 0.02 | 0.12 | 0.00 | 0.04 | ns |
| Eicosenoic | C20:1n9 | 0.10 | 0.18 | 0.08 | 0.23 | 0.03 | ns |
| Alpha-Linolenic | C18:3n3 | 0.08 | 0.06 | 1.01 | 46.59 | 11.55 | <0.001 |
| Eicosadienoic | C20:2n6 | 0.19 | 0.24 | 0.08 | 0.00 | 0.05 | ns |
| Dihomo-γ-linolenic | C20:3n6 | 0.12 | 0.05 | 0.10 | 0.00 | 0.03 | ns |
| Arachidonic | C20:4n6 | 0.77 | 1.01 | 0.68 | 0.38 | 0.13 | ns |
| Lignoceric | C24:0 | 0.15 | 0.36 | 0.14 | 0.05 | 0.07 | ns |
| Eicosapentaenoic (EPA) | C20:5n3 | 0.23 | 0.09 | 0.05 | 0.11 | 0.04 | ns |
| Nervonic | C24:1n9 | 0.06 | 0.04 | 0.06 | 0.00 | 0.01 | ns |
| Docosahexaenoic (DHA) | C22:6n3 | 0.01 | 0.04 | 0.01 | 0.12 | 0.03 | ns |
| Total | |||||||
| ∑SFA | 50.20 | 50.05 | 48.86 | 40.04 | 2.11 | <0.001 | |
| ∑MUFA | 16.84 | 36.80 | 19.67 | 3.49 | 5.93 | <0.001 | |
| ∑PUFA | 32.96 | 13.15 | 31.47 | 56.47 | 7.69 | <0.001 | |
| ∑n-6 PUFA | 32.50 | 12.73 | 30.27 | 9.65 | 5.09 | <0.001 | |
| ∑n-3 PUFA | 0.32 | 0.19 | 1.07 | 46.82 | 10.89 | <0.001 | |
| n-6/n-3 PUFA |
101.56 | 67.00 | 28.29 | 0.21 | 19.20 | <0.001 | |
| ∑SFA/ ∑MUFA |
2.98 | 1.36 | 2.48 | 11.47 | 2.01 | <0.001 | |
| ∑SFA/ ∑PUFA |
1.52 | 3.81 | 1.55 | 0.71 | 0.58 | <0.001 |
| Musculus Longissimus dorsi | |||||
|---|---|---|---|---|---|
| Fatty acid | Items | Control diet | OL diet | SEM | p-value |
| Myristic | C14:0 | 2.17 | 1.67 | 0.18 | ns |
| Palmitic | C16:0 | 29.16 | 28.98 | 0.06 | ns |
| Trans-Palmitoleic | C16:1n7t | 0.02 | 0.02 | 0.00 | ns |
| Palmitoleic | C16:1n7 | 0.15 | 0.12 | 0.01 | ns |
| Stearic | C18:0 | 7.82 | 5.35 | 0.87 | <0.001 |
| Trans-Oleic | C18:1n9t | 0.01 | 0.05 | 0.01 | ns |
| Oleic | C18:1n9 | 51.16 | 55.63 | 1.59 | <0.001 |
| Trans-Linoleic | C18:2n6t | 0.03 | 0.03 | 0.00 | ns |
| Linoleic | C18:2n6 | 8.12 | 6.26 | 0.66 | <0.05 |
| Gamma-Linolenic | C18:3n6 | 0.27 | 0.30 | 0.01 | ns |
| Eicosenoic | C20:1n9 | 0.27 | 0.21 | 0.02 | ns |
| Alpha-Linolenic | C18:3n3 | 0.03 | 0.04 | 0.00 | ns |
| Eicosadienoic | C20:2n6 | 0.18 | 0.21 | 0.01 | ns |
| Dihomo-γ-linolenic | C20:3n6 | 0.06 | 0.05 | 0.00 | ns |
| Arachidonic | C20:4n6 | 0.46 | 0.40 | 0.02 | ns |
| Lignoceric | C24:0 | 0.00 | 0.49 | 0.17 | ns |
| Eicosapentaenoic (EPA) | C20:5n3 | 0.01 | 0.01 | 0.00 | ns |
| Nervonic | C24:1n9 | 0.01 | 0.01 | 0.00 | ns |
| Docosatetraenoic | C22:4n6 | 0.07 | 0.06 | 0.00 | ns |
| Docosatetraenoic-N6 | C22:5n6 | 0.01 | 0.01 | 0.00 | ns |
| Docosatetraenoic-N3 | C22:5n3 | 0.04 | 0.04 | 0.00 | ns |
| Docosahexaenoic (DHA) | C22:6n3 | 0.01 | 0.11 | 0.04 | ns |
| Total | |||||
| SFA | 39.14 | 33.97 | 1.84 | <0.001 | |
| MUFA | 51.61 | 60.29 | 3.08 | <0.001 | |
| PUFA | 9.25 | 5.75 | 1.24 | <0.05 | |
| Omega6 | 8.99 | 5.19 | 1.35 | <0.01 | |
| Omega3 | 0.08 | 0.33 | 0.09 | ns | |
| n6/n3 | 119.80 | 15.95 | 36.82 | <0.001 | |
| Musculus Diaphragm | |||||
| Fatty acid | Items | Control diet | OL diet | SEM | p-value |
| Myristic | C14:0 | 1.14 | 1.33 | 0.07 | ns |
| Palmitic | C16:0 | 25.35 | 24.61 | 0.26 | ns |
| Trans-Palmitoleic | C16:1n7t | 0.01 | 0.02 | 0.00 | ns |
| Palmitoleic | C16:1n7 | 1.83 | 1.99 | 0.06 | ns |
| Stearic | C18:0 | 14.47 | 13.30 | 0.41 | <0.05 |
| Trans-Oleic | C18:1n9t | 0.90 | 1.06 | 0.06 | ns |
| Oleic | C18:1n9 | 35.02 | 37.07 | 0.73 | <0.001 |
| Trans-Linoleic | C18:2n6t | 0.02 | 0.04 | 0.01 | ns |
| Linoleic | C18:2n6 | 14.30 | 13.33 | 0.34 | ns |
| Gamma-Linolenic | C18:3n6 | 0.01 | 0.01 | 0.00 | ns |
| Eicosenoic | C20:1n9 | 0.68 | 0.98 | 0.11 | ns |
| Alpha-Linolenic | C18:3n3 | 5.31 | 5.32 | 0.00 | ns |
| Eicosadienoic | C20:2n6 | 0.09 | 0.06 | 0.01 | ns |
| Dihomo-γ-linolenic | C20:3n6 | 0.08 | 0.09 | 0.00 | ns |
| Arachidonic | C20:4n6 | 0.32 | 0.27 | 0.02 | ns |
| Lignoceric | C24:0 | 0.32 | 0.38 | 0.02 | ns |
| Eicosapentaenoic (EPA) | C20:5n3 | 0.03 | 0.04 | 0.01 | ns |
| Nervonic | C24:1n9 | 0.03 | 0.02 | 0.00 | ns |
| Docosatetraenoic | C22:4n6 | 0.01 | 0.01 | 0.00 | ns |
| Docosatetraenoic-N6 | C22:5n6 | 0.02 | 0.02 | 0.00 | ns |
| Docosatetraenoic-N3 | C22:5n3 | 0.01 | 0.01 | 0.00 | ns |
| Docosahexaenoic (DHA) | C22:6n3 | 0.10 | 0.10 | 0.00 | ns |
| Total | |||||
| SFA | 38.20 | 39.71 | 0.54 | ns | |
| MUFA | 44.17 | 41.16 | 1.07 | <0.05 | |
| PUFA | 20.28 | 17.98 | 0.81 | <0.05 | |
| Omega6 | 14.73 | 12.95 | 0.63 | ns | |
| Omega3 | 5.45 | 5.00 | 0.16 | ns | |
| n6/n3 | 2.70 | 2.59 | 0.04 | ns | |
| Musculus Semimembranosus | |||||
| Fatty acid | Items | Control diet | OL diet | SEM | p-value |
| Myristic | C14:0 | 2.10 | 1.67 | 0.15 | ns |
| Palmitic | C16:0 | 23.41 | 22.23 | 0.42 | <0.001 |
| Trans-palmitoleic | C16:1n7t | 0.22 | 0.20 | 0.01 | ns |
| Palmitoleic | C16:1n7 | 2.37 | 2.88 | 0.18 | ns |
| Stearic | C18:0 | 12.14 | 11.18 | 0.34 | <0.05 |
| Trans-Oleic | C18:1n9t | 0.02 | 0.32 | 0.11 | ns |
| Oleic | C18:1n9 | 37.05 | 39.19 | 0.76 | <0.001 |
| Trans-Linoleic | C18:2n6t | 0.22 | 0.28 | 0.02 | ns |
| Linoleic | C18:2n6 | 13.77 | 11.00 | 0.98 | <0.05 |
| Gamma-Linolenic | C18:3n6 | 0.00 | 0.06 | 0.02 | ns |
| Eicosenoic | C20:1n9 | 0.00 | 0.00 | 0.00 | ns |
| Alpha-Linolenic | C18:n3 | 3.04 | 4.24 | 0.42 | <0.05 |
| Eicosadienoic | C20:2n6 | 0.65 | 1.05 | 0.14 | ns |
| Dihomo-γ-linolenic | C20:3n6 | 0.01 | 0.01 | 0.00 | ns |
| Arachidonic | C20:4n6 | 0.51 | 1.00 | 0.17 | ns |
| Lignoceric | C24:0 | 3.46 | 3.57 | 0.04 | ns |
| Eicosapentaenoic (EPA) | C20:5n3 | 0.12 | 0.12 | 0.00 | ns |
| Nervonic | C24:1n9 | 0.10 | 0.14 | 0.02 | ns |
| Docosatetraenoic | C22:4n6 | 0.01 | 0.01 | 0.00 | ns |
| Docosatetraenoic-N6 | C22:5n6 | 0.45 | 0.52 | 0.02 | ns |
| Docosatetraenoic-N3 | C22:5n3 | 0.26 | 0.27 | 0.01 | ns |
| Docosahexaenoic (DHA) | C22:6n3 | 0.13 | 0.10 | 0.01 | ns |
| Total | |||||
| SFA | 41.11 | 36.39 | 1.67 | <0.001 | |
| MUFA | 39.75 | 45.34 | 1.98 | <0.001 | |
| PUFA | 19.15 | 18.28 | 0.31 | ns | |
| Omega6 | 14.52 | 11.80 | 0.96 | <0.01 | |
| Omega3 | 3.54 | 4.92 | 0.49 | ns | |
| n6/n3 | 4.11 | 2.40 | 0.61 | ns | |
| Musculus Psoas Major | |||||
| Fatty acid | Items | Control diet | OL diet | SEM | p-value |
| Myristic | C14:0 | 1.70 | 2.00 | 0.11 | ns |
| Palmitic | C16:0 | 24.57 | 24.54 | 0.01 | ns |
| Trans-Palmitoleic | C16:1n 7t | 0.02 | 0.05 | 0.01 | ns |
| Palmitoleic | C16:1 7 | 3.42 | 2.63 | 0.28 | ns |
| Stearic | C18:0 | 9.94 | 7.97 | 0.70 | <0.001 |
| Trans-Oleic | C18:1n9t | 0.01 | 0.02 | 0.00 | ns |
| Oleic | C18:1n9 | 50.47 | 55.37 | 1.74 | <0.001 |
| Trans-Linoleic | C18:2n6t | 0.01 | 0.05 | 0.01 | ns |
| Linoleic | C18:2n6 | 7.80 | 5.77 | 0.72 | <0.05 |
| Gamma-Linolenic | C18:3n6 | 0.08 | 0.04 | 0.01 | ns |
| Eicosenoic | C20:1n9 | 0.01 | 0.05 | 0.01 | ns |
| Alpha-Linolenic | C18:3n3 | 0.09 | 0.07 | 0.01 | ns |
| Eicosadienoic | C20:2n6 | 0.82 | 0.26 | 0.20 | ns |
| Dihomo-γ-linolenic | C20:3n6 | 0.13 | 0.10 | 0.01 | ns |
| Arachidonic | C20:4n6 | 0.52 | 0.42 | 0.03 | ns |
| Lignoceric | C24:0 | 0.02 | 0.42 | 0.14 | ns |
| Eicosapentaenoic (EPA) | C20:5n3 | 0.08 | 0.05 | 0.01 | ns |
| Nervonic | C24:1n9 | 0.11 | 0.06 | 0.02 | ns |
| Docosatetraenoic | C22:4n6 | 0.12 | 0.05 | 0.02 | ns |
| Docosatetraenoic-N6 | C22:5n6 | 0.01 | 0.00 | 0.00 | ns |
| Docosatetraenoic-N3 | C22:5n3 | 0.00 | 0.01 | 0.00 | ns |
| Docosahexaenoic (DHA) | C22:6n3 | 0.01 | 0.01 | 0.00 | ns |
| Total | |||||
| SFA | 36.24 | 34.15 | 0.74 | ns | |
| MUFA | 54.06 | 61.25 | 2.55 | <0.001 | |
| PUFA | 9.70 | 4.61 | 2.25 | <0.001 | |
| Omega 6 | 8.67 | 3.81 | 1.73 | <0.001 | |
| Omega 3 | 0.19 | 0.60 | 0.15 | ns | |
| n6/n3 | 45.66 | 6.34 | 11.88 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





