Submitted:
30 July 2024
Posted:
31 July 2024
You are already at the latest version
Abstract
Keywords:
1. Importance of Recognizing Subtypes of Periodontitis for Scientific Discovery
2. Consensus (the Process) Pros and Cons
3. World Workshop Case Definition of Periodontitis
4. Conclusions for the Consensus Section
5. Localized Juvenile Periodontitis: History
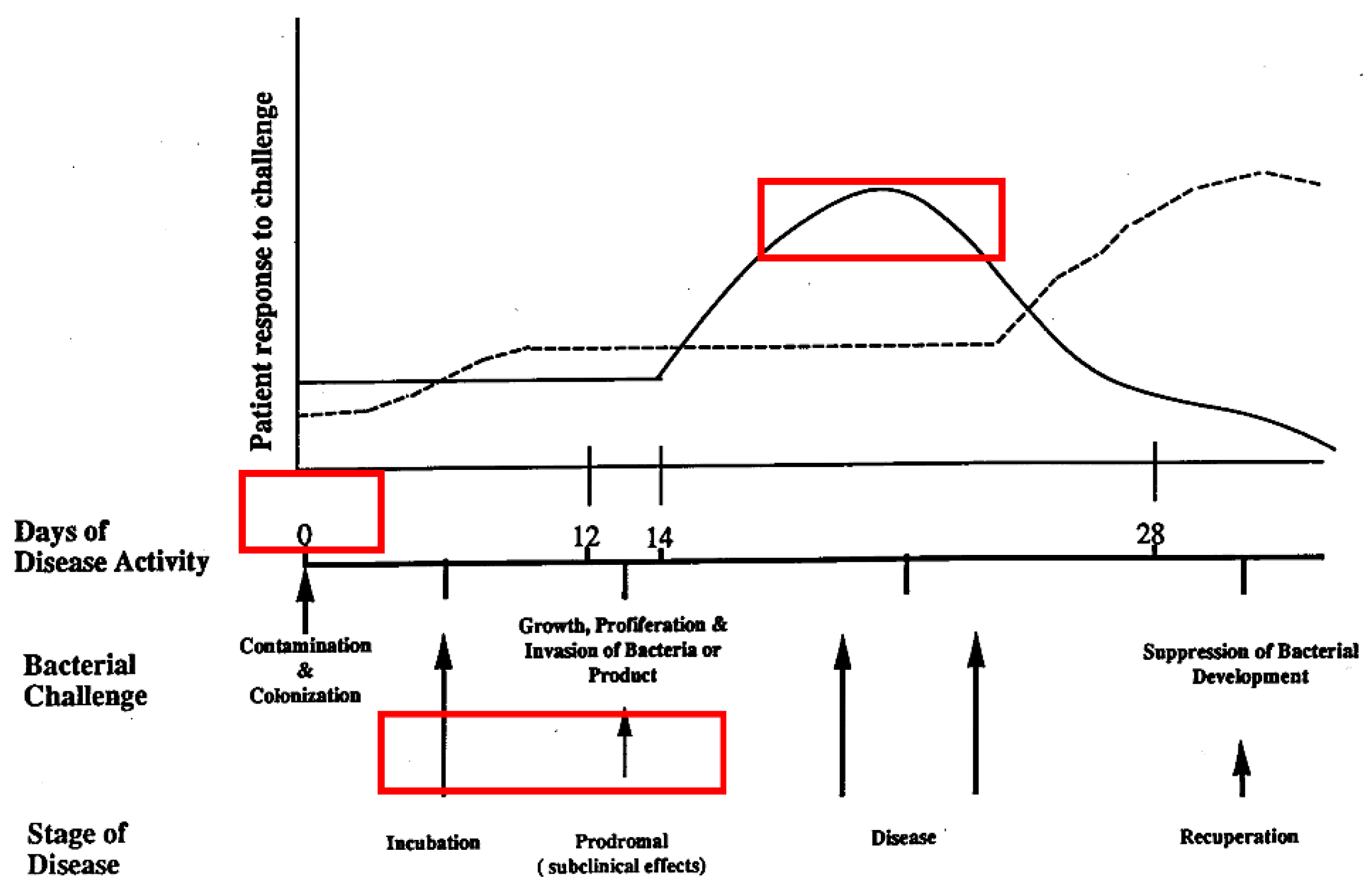
6. Bradford Hill Guidelines for Distinguishing Causation from Correlation
7. The Changing landscape of Infectious Diseases: The Oral Cavity as a New Focus
8. Oral Microbiology and Immunology and Infectious Diseases
9. Immunology and Studies of Host Responsiveness to Infectious Agents
10. Measurements as Determinants of Treatment Failure or Success
11. Future Challenges and Suggestions to the Clinical and Research Community
| Hill criteria | Example | Feasibility Yes/No? |
Impact of study and reference [ ] |
| 1.Temporal relationship | Exposure to agent precedes outcome | Yes | Longitudinal; healthy controls; age approp Aa; [41,158] Longitudinal; health controls age approp Aa + consort;[47,159] |
| 2.Strength of Association | Size of association determined statistically | Yes | Show stats Aa ; [41] Show stats Aa + consort; [47,159] |
| 3.Dose-Response | ^exposure> ^response | Yes | Measure consort vs Aa alone; [47,159] |
| 4.Consistency | Experiments reproduced |
Yes | Show consort X-sect; [160,161] Show consort longitude;[47,159] |
| 5.Plausibility | Assoc agree with pathobiological explanations |
Yes | Cdt has impact; [162] Ltx has impact; [99] Consortia passed from mother with disease to Child: local debridement improves inflammation, but consort remains; [163]. Consort metabolomics; [98] |
| 6.Experimental evidence |
Disease altered By intervention |
Yes | Tetracycline admin reduces disease; [164] Tetracycline eliminates Aa and reduces disease; [165] Amox/Metra reduces disease, no antibiotic no improve; [166] |
| 7.Alternative explanation |
Rule out other explanations | ? Open ? Open |
[41,47,159] |
| 8. Specificity | Cause produces effect | Yes | Flp and no disease; [167] Ltx and more bone loss; [168] Pga B is modified and disease is reduced; [169] |
| 9.Coherence | Theory consistent with Existing knowledge |
Yes | Ltx and infections; [170] Cdt and infections; [171] Metabolomics and consortia; [98] |
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. Journal of Periodontology 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Periodontal diseases: diagnosis. Ann Periodontol 1996, 1, 37–215. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Clin Periodontol 2018, 45 Suppl 20, S1–S8. [Google Scholar] [CrossRef]
- Greene, F.L. Cancer staging in outcomes assessment. J Surg Oncol 2014, 110, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Schneider, J.; Suh, Y.J. Survival analysis for patients with metachronous contralateral breast cancer: Insights from a retrospective study. Oncol Lett 2024, 28, 390. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.N. The case for periodontosis as a clinical entity. Journal of Periodontology 1971, 42, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Marazita, M.L.; Burmeister, J.A.; Gunsolley, J.C.; Koertge, T.E.; Lake, K.; Schenkein, H.A. Evidence for autosomal dominant inheritance and race-specific heterogeneity in early-onset periodontitis. Journal of Periodontology 1994, 65, 623–630. [Google Scholar] [CrossRef]
- Loe, H.; Brown, L.J. Early onset periodontitis in the United States of America. Journal of Periodontology 1991, 62, 608–616. [Google Scholar] [CrossRef]
- Fine, D.H.; Kaplan, J.B.; Kachlany, S.C.; Schreiner, H.C. How we got attached to Actinobacillus actinomycetemcomitans: A model for infectious diseases. Periodontol 2000 2006, 42, 114–157. [Google Scholar] [CrossRef]
- Fine, D.H.; Cohen, D.W.; Bimstein, E.; Bruckmann, C. A ninety-year history of periodontosis: the legacy of Professor Bernhard Gottlieb. Journal of Periodontology 2015, 86, 1–6. [Google Scholar] [CrossRef]
- Miguel, M.V.M.; Shaddox, L.M. Grade C molar-incisor pattern periodontitis in young adults: What have we learned so far? Pathogens 2024, accepted in press, 1–21. [Google Scholar] [CrossRef]
- Zambon, J.J. Authors’ response. J Am Dent Assoc 2020, 151, 160–161. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, F.M.; Tinoco, E.M.; Govil, M.; Marazita, M.L.; Vieira, A.R. Aggressive periodontitis is likely influenced by a few small effect genes. J Clin Periodontol 2009, 36, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Armitage, G.C.; Genco, R.J.; Griffen, A.L.; Diehl, S.R. Unique etiologic, demographic, and pathologic characteristics of localized aggressive periodontitis support classification as a distinct subcategory of periodontitis. J Am Dent Assoc 2019, 150, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Pallos, D.; Acevedo, A.C.; Mestrinho, H.D.; Cordeiro, I.; Hart, T.C. Novel cathepsin C mutation in a Brazilian family with Papillon-Lefevre syndrome: case report and mutation update. J Dent Child (Chic) 2010, 77, 36–41. [Google Scholar] [PubMed]
- Diehl, S.R.; Wu, T.; Michalowicz, B.S.; Brooks, C.N.; Califano, J.V.; Burmeister, J.A.; Schenkein, H.A. Quantitative measures of aggressive periodontitis show substantial heritability and consistency with traditional diagnoses. Journal of Periodontology 2005, 76, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.W. Development of orphan drugs for rare diseases. Clin Exp Pediatr 2024, 67, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.N.; Kerns, D.G. Acute necrotizing ulcerative gingivitis-periodontitis: a literature review. Mil Med 1998, 163, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M. Disparities and social determinants of periodontal diseases. Periodontol 2000 2024. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Proenca, L.; Mendes, J.J. The 2018 periodontitis case definition improves accuracy performance of full-mouth partial diagnostic protocols. Sci Rep 2020, 10, 7093. [Google Scholar] [CrossRef]
- Fredman, G.; Oh, S.F.; Ayilavarapu, S.; Hasturk, H.; Serhan, C.N.; Van Dyke, T.E. Impaired phagocytosis in localized aggressive periodontitis: rescue by Resolvin E1. PLoS One 2011, 6, e24422. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Patil, A.G.; Loos, B.G. Classification and diagnosis of aggressive periodontitis. Journal of Periodontology 2018, 89 Suppl 1, S103–S119. [Google Scholar] [CrossRef]
- Endersby, J. Lumpers and splitters: Darwin, Hooker, and the search for order. Science 2009, 326, 1496–1499. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S. The Emperor of All Maladies: A Biography of Cancer; Scribner: New York, USA, 2010. [Google Scholar]
- Doll, R. The Pierre Denoix Memorial Lecture: nature and nurture in the control of cancer. Eur J Cancer 1999, 35, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Snyderman, R.; Phillips, J.K.; Mergenhagen, S.E. Biological activity of complement in vivo. Role of C5 in the accumulation of polymorphonuclear leukocytes in inflammatory exudates. J Exp Med 1971, 134, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Snyderman, R. Personalized health care: from theory to practice. Biotechnol J 2012, 7, 973–979. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatric Dentistry Council on Clinical, A. Policy on use of a caries-risk assessment tool (CAT) for infants, children, and adolescent. Pediatr Dent 2005, 27, 25–27. [Google Scholar]
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol Rev 1986, 50, 353–380. [Google Scholar] [CrossRef]
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology (Reading) 2003, 149, 279–294. [Google Scholar] [CrossRef]
- Folayan, M.N.O.; Amalia, R.; Kemoli, A.; Sun, I.G.; Duangthip, D.; Abodunrin, O.; Virtanen, J.I.; Masumo, R.M.; Vukovic, A.; Al-Batayneh, O.B.; et al. Can the sustainable development goal 9 support an untreated early childhood caries elimination agenda? BMC Oral Health 2024, 24, 776. [Google Scholar] [CrossRef]
- Sheykholeslam, Z.; Buonocore, M.G. Bonding of resins to phosphoric acid-etched enamel surfaces of permanent and deciduous teeth. J Dent Res 1972, 51, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, A.Y.; Geissler, K.H.; Dick, A.W.; Kranz, A.M. Clinician characteristics associated with fluoride varnish applications during well-child visits. Am J Manag Care 2024, 30, e203–e209. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, C.L.; Edelstein, B.L.; Basch, C.E.; Wolf, R.L.; Koch, P.A.; McKeague, I.; Leu, C.S.; Andrews, H. Protocol for a family-centered behavioral intervention to reduce early childhood caries: the MySmileBuddy program efficacy trial. BMC Oral Health 2021, 21, 246. [Google Scholar] [CrossRef] [PubMed]
- Lienhart, G.; Elsa, M.; Farge, P.; Schott, A.M.; Thivichon-Prince, B.; Chaneliere, M. Factors perceived by health professionals to be barriers or facilitators to caries prevention in children: a systematic review. BMC Oral Health 2023, 23, 767. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. A brief history of periodontics in the United States of America: Pioneers and thought-leaders of the past, and current challenges. Periodontol 2000 2020, 82, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Diamond, I.R.; Grant, R.C.; Feldman, B.M.; Pencharz, P.B.; Ling, S.C.; Moore, A.M.; Wales, P.W. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014, 67, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, P.J.; Kolias, A.G.; Tajsic, T.; Adeleye, A.; Aklilu, A.T.; Apriawan, T.; Bajamal, A.H.; Barthelemy, E.J.; Devi, B.I.; Bhat, D.; et al. Consensus statement from the International Consensus Meeting on the Role of Decompressive Craniectomy in the Management of Traumatic Brain Injury : Consensus statement. Acta Neurochir (Wien) 2019, 161, 1261–1274. [Google Scholar] [CrossRef]
- Pelosi, L.; Aranyi, Z.; Beekman, R.; Bland, J.; Coraci, D.; Hobson-Webb, L.D.; Padua, L.; Podnar, S.; Simon, N.; van Alfen, N.; et al. Expert consensus on the combined investigation of ulnar neuropathy at the elbow using electrodiagnostic tests and nerve ultrasound. Clin Neurophysiol 2021, 132, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Markowitz, K.; Furgang, D.; Fairlie, K.; Ferrandiz, J.; Nasri, C.; McKiernan, M.; Gunsolley, J. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. J Clin Microbiol 2007, 45, 3859–3869. [Google Scholar] [CrossRef]
- Haubek, D.; Ennibi, O.K.; Poulsen, K.; Vaeth, M.; Poulsen, S.; Kilian, M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet 2008, 371, 237–242. [Google Scholar] [CrossRef]
- Fine, D.H.; Armitage, G.C.; Griffen, A.L.; Diehl, S.R. Authors’ response. J Am Dent Assoc 2020, 151, 160. [Google Scholar] [CrossRef] [PubMed]
- Clyne, B.; Sharp, M.K.; M, O.N.; Pollock, D.; Lynch, R.; Amog, K.; Ryan, M.; Smith, S.M.; Mahtani, K.; Booth, A.; et al. An international modified Delphi process supported updating the web-based “right review” tool. J Clin Epidemiol 2024, 170, 111333. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M. Aggressive periodontitis: case definition and diagnostic criteria. Periodontol 2000 2014, 65, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000 2014, 64, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Billings, M.; Holtfreter, B.; Papapanou, P.N.; Mitnik, G.L.; Kocher, T.; Dye, B.A. Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. Journal of Periodontology 2018, 89 (Suppl. 1), S140–S158. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Markowitz, K.; Fairlie, K.; Tischio-Bereski, D.; Ferrendiz, J.; Furgang, D.; Paster, B.J.; Dewhirst, F.E. A consortium of Aggregatibacter actinomycetemcomitans, Streptococcus parasanguinis, and Filifactor alocis is present in sites prior to bone loss in a longitudinal study of localized aggressive periodontitis. J Clin Microbiol 2013, 51, 2850–2861. [Google Scholar] [CrossRef] [PubMed]
- Needleman, I.; Garcia, R.; Gkranias, N.; Kirkwood, K.L.; Kocher, T.; Iorio, A.D.; Moreno, F.; Petrie, A. Mean annual attachment, bone level, and tooth loss: A systematic review. Journal of Periodontology 2018, 89 (Suppl. 1), S120–S139. [Google Scholar] [CrossRef] [PubMed]
- Leow, N.M.; Moreno, F.; Marletta, D.; Hussain, S.B.; Buti, J.; Almond, N.; Needleman, I. Recurrence and progression of periodontitis and methods of management in long-term care: A systematic review and meta-analysis. J Clin Periodontol 2022, 49 (Suppl. 24), 291–313. [Google Scholar] [CrossRef] [PubMed]
- Bik, E.M.; Long, C.D.; Armitage, G.C.; Loomer, P.; Emerson, J.; Mongodin, E.F.; Nelson, K.E.; Gill, S.R.; Fraser-Liggett, C.M.; Relman, D.A. Bacterial diversity in the oral cavity of 10 healthy individuals. The ISME journal 2010, 4, 962–974. [Google Scholar] [CrossRef]
- Proctor, D.M.; Shelef, K.M.; Gonzalez, A.; Davis, C.L.; Dethlefsen, L.; Burns, A.R.; Loomer, P.M.; Armitage, G.C.; Ryder, M.I.; Millman, M.E.; et al. Microbial biogeography and ecology of the mouth and implications for periodontal diseases. Periodontol 2000 2019, 82, 26–41. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Baik, J.E.; Lagana, S.M.; Han, R.P.; Raab, W.J.; Sahoo, D.; Dalerba, P.; Wang, T.C.; Han, Y.W. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/beta-catenin modulator Annexin A1. EMBO Rep 2019, 20. [Google Scholar] [CrossRef]
- Han, Y.W.; Redline, R.W.; Li, M.; Yin, L.; Hill, G.B.; McCormick, T.S. Fusobacterium nucleatum Induces Premature and Term Stillbirths in Pregnant Mice: Implication of Oral Bacteria in Preterm Birth. Infect Immun 2004, 72, 2272–2279. [Google Scholar] [CrossRef]
- Miller, W.D. The Micro-organisms of the Human Mouth: The Local and General Diseases which are Caused by Them; Classics of Dentistry Library: 1890.
- Kritchevsky, B.; Seguin, P. The Pathogenesis and Treatment of Pyorrhea Alveolaris. The Dental Cosmos: a monthly record of dental science 1918, 60, 781–784. [Google Scholar]
- Beckwith, T.D.; Williams, A.; Rose, E.T. The role of bacteria in pyorrhea. Medical journal and record 1929, 129, 333–336. [Google Scholar]
- Woodward, C.; Fisher, M.A. Drug treatment of common STDs: Part II. Vaginal infections, pelvic inflammatory disease and genital warts. Am Fam Physician 1999, 60, 1716–1722. [Google Scholar] [PubMed]
- Malhotra, M.; Sood, S.; Mukherjee, A.; Muralidhar, S.; Bala, M. Genital Chlamydia trachomatis: an update. Indian J Med Res 2013, 138, 303–316. [Google Scholar]
- Radu, O.; Pantanowitz, L. Kaposi sarcoma. Arch Pathol Lab Med 2013, 137, 289–294. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun 1999, 67, 3703–3713. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. Microbiology: Ditch the term pathogen. Nature 2014, 516, 165–166. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. What is a pathogen? Ann Med 2002, 34, 2–4. [Google Scholar] [CrossRef]
- Pirofski, L.; Casadevall, A. The Damage-Response Framework as a Tool for the Physician-Scientist to Understand the Pathogenesis of Infectious Diseases. J Infect Dis 2018, 218, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Pirofski, L.A.; Casadevall, A. The meaning of microbial exposure, infection, colonisation, and disease in clinical practice. Lancet Infect Dis 2002, 2, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Learned and unlearned concepts in periodontal diagnostics: a 50-year perspective. Periodontol 2000 2013, 62, 20–36. [Google Scholar] [CrossRef]
- Newman, M.G.; Socransky, S.S.; Savitt, E.D.; Propas, D.A.; Crawford, A. Studies of the microbiology of periodontosis. Journal of Periodontology 1976, 47, 373–379. [Google Scholar] [CrossRef]
- Slots, J. The predominant cultivable organisms in juvenile periodontitis. Scand J Dent Res 1976, 84, 1–10. [Google Scholar] [CrossRef]
- Loesche, W.J. Chemotherapy of dental plaque infections. Oral Sci Rev 1976, 9, 65–107. [Google Scholar]
- Loesche, W.J. Clinical and microbiological aspects of chemotherapeutic agents used according to the specific plaque hypothesis. J Dent Res 1979, 58, 2404–2412. [Google Scholar] [CrossRef]
- Klinger, R. Untersuchungen uber menschliche aktinomycose. Zentralbl. Bakteriol. (Orig) 1912, 62, 191–200. [Google Scholar]
- Marsh, P.D.; Zaura, E. Dental biofilm: ecological interactions in health and disease. J Clin Periodontol 2017, 44 Suppl 18, S12–S22. [Google Scholar] [CrossRef]
- Moore, W.E.; Moore, L.H.; Ranney, R.R.; Smibert, R.M.; Burmeister, J.A.; Schenkein, H.A. The microflora of periodontal sites showing active destructive progression. J Clin Periodontol 1991, 18, 729–739. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J Clin Periodontol 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontol 2000 2005, 38, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. In Sickness and in Health-What Does the Oral Microbiome Mean to Us? An Ecological Perspective. Adv Dent Res 2018, 29, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.J.; Macdonald, J.B. Hemin and vitamin K compounds as required factors for the cultivation of certain strains of Bacteroides melaninogenicus. J Bacteriol 1960, 80, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, K.; Khandelwal, R.L.; Heinrich, S.E. Glycogen synthetic and degradative activities by Actinomyces viscosus and Actinomyces naeslundii of root surface caries and noncaries sites. Caries Res 1988, 22, 217–225. [Google Scholar] [CrossRef]
- Moore, W.E.C.; Moore, L.V.H. The bacteria of periodontal disease. Periodontol 2000 1994, 5, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Schachtele, C.F.; Loken, A.E.; Schmitt, M.K. Use of specifically labeled sucrose for comparison of extracellular glucan and fructan metabolism by oral streptococci. Infect Immun 1972, 5, 263–266. [Google Scholar] [CrossRef]
- Kachlany, S.C.; Fine, D.H.; Figurski, D.H. Secretion of RTX leukotoxin by Actinobacillus actinomycetemcomitans. Infect Immun 2000, 68, 6094–6100. [Google Scholar] [CrossRef] [PubMed]
- Pavloff, N.; Potempa, J.; Pike, R.N.; Prochazka, V.; Kiefer, M.C.; Travis, J.; Barr, P.J. Molecular cloning and structural characterization of the Arg-gingipain proteinase of Porphyromonas gingivalis. Biosynthesis as a proteinase-adhesin polyprotein. J Biol Chem 1995, 270, 1007–1010. [Google Scholar] [CrossRef]
- Chu, L.; Bramanti, T.E.; Ebersole, J.L.; Holt, S.C. Hemolytic activity in the periodontopathogen Porphyromonas gingivalis: kinetics of enzyme release and localization. Infect Immun 1991, 59, 1932–1940. [Google Scholar] [CrossRef]
- Gibbons, R.J.; Macdonald, J.B. Degradation of collagenous substrates by Bacteroides melaninogenicus. J Bacteriol 1961, 81, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 1994, 8, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Ivanyi, L.; Challacombe, S.J.; Lehner, T. The specificity of serum factors in lymphocyte transformation in periodontal disease. Clin Exp Immunol 1973, 14, 491–500. [Google Scholar] [PubMed]
- Ebersole, J.L.; Dawson, D., 3rd; Emecen-Huja, P.; Nagarajan, R.; Howard, K.; Grady, M.E.; Thompson, K.; Peyyala, R.; Al-Attar, A.; Lethbridge, K.; et al. The periodontal war: microbes and immunity. Periodontol 2000 2017, 75, 52–115. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H. Incorporating new technologies in periodontal diagnosis into training programs and patient care: a critical assessment and a plan for the future. Journal of Periodontology 1992, 63, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Listgarten, M.A. Periodontal probing: what does it mean? J Clin Periodontol 1980, 7, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Cugini, C.; Ramasubbu, N.; Tsiagbe, V.K.; Fine, D.H. Dysbiosis From a Microbial and Host Perspective Relative to Oral Health and Disease. Front Microbiol 2021, 12, 617485. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. What is a host? Incorporating the microbiota into the damage-response framework. Infect Immun 2015, 83, 2–7. [Google Scholar] [CrossRef]
- Loe, H.; Silness, J. Periodontal disease in pregancy. Prevalence and severity. Acta Odontol Scand 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Page, R.C.; Schroeder, H.E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest 1976, 34, 235–249. [Google Scholar]
- Taubman, M.A.; Valverde, P.; Han, X.; Kawai, T. Immune response: the key to bone resorption in periodontal disease. Journal of Periodontology 2005, 76, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Sanz, M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontol 2000 2020, 83, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Taichman, N.S.; Dean, R.T.; Sanderson, C.J. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infect Immun 1980, 28, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Taichman, N.S.; Wilton, J.M. Leukotoxicity of an extract from Actinobacillus actinomycetemcomitans for human gingival polymorphonuclear leukocytes. Inflammation 1981, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bamashmous, S.; Kotsakis, G.A.; Kerns, K.A.; Leroux, B.G.; Zenobia, C.; Chen, D.; Trivedi, H.M.; McLean, J.S.; Darveau, R.P. Human variation in gingival inflammation. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wright, C.J.; Dingming, H.; Uriarte, S.M.; Lamont, R.J. Oral community interactions of Filifactor alocis in vitro. PLoS One 2013, 8, e76271. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Claesson, R.; Aberg, C.H.; Haubek, D.; Lindholm, M.; Jasim, S.; Oscarsson, J. Genetic Profiling of Aggragatibacter actinomycetemcomitans Serotype B Isolated from Periodontitis Patients Living in Sweden. Pathogens 2019, 8, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Patel, M.; Socransky, S.S. Microbiological changes associated with four different periodontal therapies for the treatment of chronic periodontitis. Oral Microbiol Immunol 2008, 23, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Microbial mechanisms in the pathogenesis of destructive periodontal diseases: a critical assessment. J Periodontal Res 1991, 26, 195–212. [Google Scholar] [CrossRef]
- Usman, M.; Hameed, Y.; Ahmad, M. Does human papillomavirus cause human colorectal cancer? Applying Bradford Hill criteria postulates. Ecancermedicalscience 2020, 14, 1107. [Google Scholar] [CrossRef]
- Blaser, M.J. Missing microbes : how the overuse of antibiotics is fueling our modern plagues, First edition. ed.; Henry Holt and Company: New York, 2014. [Google Scholar]
- Del Romero, J.; Moreno Guillen, S.; Rodriguez-Artalejo, F.J.; Ruiz-Galiana, J.; Canton, R.; De Lucas Ramos, P.; Garcia-Botella, A.; Garcia-Lledo, A.; Hernandez-Sampelayo, T.; Gomez-Pavon, J.; et al. Sexually transmitted infections in Spain: Current status. Rev Esp Quimioter 2023, 36, 444–465. [Google Scholar] [CrossRef] [PubMed]
- Okell, C.C.; Elliott, S.D. Bacteriaaemia and oral sepsis with special reference to the aetiology of subacute endocarditis. The Lancet 1935, 869–872. [Google Scholar] [CrossRef]
- Silver, J.G.; Martin, A.W.; McBride, B.C. Experimental transient bacteraemias in human subjects with varying degrees of plaque accumulation and gingival inflammation. J Clin Periodontol 1977, 4, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Proctor, D.M.; Shelef, K.M.; Gonzalez, A.; Davis, C.L.; Dethlefsen, L.; Burns, A.R.; Loomer, P.M.; Armitage, G.C.; Ryder, M.I.; Millman, M.E.; et al. Microbial biogeography and ecology of the mouth and implications for periodontal diseases. Periodontol 2000 2020, 82, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.M.; Sprockett, D.D.; Bowyer, R.C.E.; Kurushima, Y.; Relman, D.A.; Williams, F.M.K.; Steves, C.J. Influential factors of saliva microbiota composition. Sci Rep 2022, 12, 18894. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Schreiner, H. Oral microbial interactions from an ecological perspective: a narrative review. Front Oral Health 2023, 4, 1229118. [Google Scholar] [CrossRef] [PubMed]
- Taichman, N.S.; Tsai, C.C.; Baehni, P.C.; Stoller, N.; McArthur, W.P. Interaction of inflammatory cells and oral microorganisms. IV. In vitro release of lysosomal constituents from polymorphonuclear leukocytes exposed to supragingival and subgingival bacterial plaque. Infect Immun 1977, 16, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E.; Horoszewicz, H.U.; Genco, R.J. The polymorphonuclear leukocyte (PMNL) locomotor defect in juvenile periodontitis. Study of random migration, chemokinesis and chemotaxis. Journal of Periodontology 1982, 53, 682–687. [Google Scholar] [CrossRef]
- Fives-Taylor, P.M.; Meyer, D.H.; Mintz, K.P.; Brissette, C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontol 2000 1999, 20, 136–167. [Google Scholar] [CrossRef]
- Zambon, J.J. Periodontal diseases: microbial factors. Ann Periodontol 1996, 1, 879–925. [Google Scholar] [CrossRef]
- Aruni, W.; Chioma, O.; Fletcher, H.M. Filifactor alocis: The Newly Discovered Kid on the Block with Special Talents. J Dent Res 2014, 93, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Patil, A.G.; Velusamy, S.K. Aggregatibacter actinomycetemcomitans (Aa) Under the Radar: Myths and Misunderstandings of Aa and Its Role in Aggressive Periodontitis. Front Immunol 2019, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Offenbacher, S.; Katz, V.; Fertik, G.; Collins, J.; Boyd, D.; Maynor, G.; McKaig, R.; Beck, J. Periodontal infection as a possible risk factor for preterm low birth weight. Journal of Periodontology 1996, 67, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Furgang, D.; McKiernan, M.; Tereski-Bischio, D.; Ricci-Nittel, D.; Zhang, P.; Araujo, M.W. An investigation of the effect of an essential oil mouthrinse on induced bacteraemia: a pilot study. J Clin Periodontol 2010, 37, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Riggio, M.P.; Walker, K.F.; MacKenzie, D.; Shearer, B. Bacteraemia following periodontal procedures. J Clin Periodontol 2005, 32, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Shanson, D. New British and American guidelines for the antibiotic prophylaxis of infective endocarditis: do the changes make sense? A critical review. Curr Opin Infect Dis 2008, 21, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.K.; Stagaman, K.; Dethlefsen, L.; Bohannan, B.J.; Relman, D.A. The application of ecological theory toward an understanding of the human microbiome. Science 2012, 336, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Tabak, L.A.; Levine, M.J.; Mandel, I.D.; Ellison, S.A. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol 1982, 11, 1–17. [Google Scholar] [CrossRef]
- Mandel, I.D. The role of saliva in maintaining oral homeostasis. J Am Dent Assoc 1989, 119, 298–304. [Google Scholar] [CrossRef]
- Rosebury, T.; Linton, R.W.; Buchbinder, L. A Comparative Study of Dental Aciduric Organisms and Lactobacillus Acidophilus. J Bacteriol 1929, 18, 395–412. [Google Scholar] [CrossRef]
- Hemmens, E.S.; Harrison, R.W. Studies on the Anaerobic Bacterial Flora of Suppurative Periodontitis. The Journal of Infectious Diseases 1942, 70, 131–146. [Google Scholar] [CrossRef]
- Rosebury, T.; Clark, A.R.; Macdonald, J.B.; O’Connell, D.C. Studies of fusospirochetal infection. III. Further studies of a guinea pig passage strain of fusospirochetal infection, including the infectivity of sterile exudate filtrates, of mixed cultures through ten transfers, and of recombined pure cultures. J Infect Dis 1950, 87, 234–248. [Google Scholar] [CrossRef]
- Keyes, P.H.; Fitzgerald, R.J. Dental caries in the Syrian hamster. IX. Arch Oral Biol 1962, 7, 267–277. [Google Scholar] [CrossRef]
- Loe, H.; Theilade, E.; Jensen, S.B. Experimental Gingivitis in Man. Journal of Periodontology 1965, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol 2000 2020, 83, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Kiel, R.A.; Anderhalden, K. Clinical and microbiological effects of subgingival restorations with overhanging or clinically perfect margins. J Clin Periodontol 1983, 10, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Theilade, E.; Wright, W.H.; Jensen, S.B.; Loe, H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res 1966, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; London, J. Adhere today, here tomorrow: oral bacterial adherence. J Bacteriol 1993, 175, 3247–3252. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Socransky, S.S.; Manganiello, S.D. The oral microbiota of man from birth to senility. Journal of Periodontology 1971, 42, 485–496. [Google Scholar] [CrossRef]
- Lowe, A.M.; Yansouni, C.P.; Behr, M.A. Causality and gastrointestinal infections: Koch, Hill, and Crohn’s. Lancet Infect Dis 2008, 8, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Ritz, H.L. Microbial population shifts in developing human dental plaque. Arch Oral Biol 1967, 12, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, P.P.; White, B.A.; Garcia, R.I.; Listgarten, M.A. The dentogingival epithelial surface area revisited. J Periodontal Res 2001, 36, 48–55. [Google Scholar] [CrossRef]
- Bergenholtz, G. Effect of bacterial products on inflammatory reactions in the dental pulp. Scand J Dent Res 1977, 85, 122–129. [Google Scholar] [PubMed]
- Bik, E.M.; Eckburg, P.B.; Gill, S.R.; Nelson, K.E.; Purdom, E.A.; Francois, F.; Perez-Perez, G.; Blaser, M.J.; Relman, D.A. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A 2006, 103, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Phillips, D.; Hickey, J.W.; Kennedy-Darling, J.; Venkataraaman, V.G.; Samusik, N.; Goltsev, Y.; Schurch, C.M.; Nolan, G.P. CODEX multiplexed tissue imaging with DNA-conjugated antibodies. Nat Protoc 2021, 16, 3802–3835. [Google Scholar] [CrossRef] [PubMed]
- Mark Welch, J.L.; Dewhirst, F.E.; Borisy, G.G. Biogeographty of the Oral Microbiome: The Site-Specific Hyothesis. Ann Rev Microbiol. 2019, 73, 335–358. [Google Scholar] [CrossRef]
- Lakschevitz, F.S.; Hassanpour, S.; Rubin, A.; Fine, N.; Sun, C.; Glogauer, M. Identification of neutrophil surface marker changes in health and inflammation using high-throughput screening flow cytometry. Exp Cell Res 2016, 342, 200–209. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Oshrain, H.I.; Salind, A.; Mandel, I.D. A method for collection of subgingival plaque and calculus. Journal of Periodontology 1968, 39, 322–325. [Google Scholar] [CrossRef]
- Fine, D.H.; Greene, L.S. Microscopic evaluation of root surface associations in vivo. J Periodontal Res 1984, 19, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Wecke, J.; Wolf, V.; Fath, S.; Bernimoulin, J.P. The occurrence of treponemes and their spherical bodies on polytetrafluoroethylene membranes. Oral Microbiol Immunol 1995, 10, 278–283. [Google Scholar] [CrossRef]
- Fine, D.H.; Markowitz, K.; Furgang, D.; Velliyagounder, K. Aggregatibacter actinomycetemcomitans as an early colonizer of oral tissues: epithelium as a reservoir? J Clin Microbiol 2010, 48, 4464–4473. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J.; Koo, H. Oral polymicrobial communities: Assembly, function, and impact on diseases. Cell Host Microbe 2023, 31, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontol 2000 2022, 89, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Holtfreter, B.; Kuhr, K.; Borof, K.; Tonetti, M.S.; Sanz, M.; Kornman, K.; Jepsen, S.; Aarabi, G.; Volzke, H.; Kocher, T.; et al. ACES: A new framework for the application of the 2018 periodontal status classification scheme to epidemiological survey data. J Clin Periodontol 2024, 51, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, M.C. Platelet-streptococcal interactions in endocarditis. Crit Rev Oral Biol Med 1996, 7, 222–236. [Google Scholar] [CrossRef]
- Peters, J.; Robinson, F.; Dasco, C.; Gentry, L.O. Subacute bacterial endocarditis due to Actinobacillus actinomycetemcomitans. Am J Med Sci 1983, 286, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Haubek, D.; Poulsen, K.; Kilian, M. Microevolution and patterns of dissemination of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans. Infect Immun 2007, 75, 3080–3088. [Google Scholar] [CrossRef]
- Fine, D.H.; Toruner, G.A.; Velliyagounder, K.; Sampathkumar, V.; Godboley, D.; Furgang, D. A lactotransferrin single nucleotide polymorphism demonstrates biological activity that can reduce susceptibility to caries. Infect Immun 2013, 81, 1596–1605. [Google Scholar] [CrossRef]
- Esmaeilyfard, R.; Bonyadifard, H.; Paknahad, M. Dental Caries Detection and Classification in CBCT Images Using Deep Learning. Int Dent J 2024, 74, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Twetman, S.; Fisher, J.; Marsh, P.D. Understanding dental caries as a non-communicable disease. Br Dent J 2021, 231, 749–753. [Google Scholar] [CrossRef]
- Sioson, P.B.; Furgang, D.; Steinberg, L.M.; Fine, D.H. Proximal caries in juvenile periodontitis patients. Journal of Periodontology 2000, 71, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Zambon, J.J. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol 1985, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hoglund Aberg, C.; Kwamin, F.; Claesson, R.; Dahlen, G.; Johansson, A.; Haubek, D. Progression of attachment loss is strongly associated with presence of the JP2 genotype of Aggregatibacter actinomycetemcomitans: a prospective cohort study of a young adolescent population. J Clin Periodontol 2014, 41, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Razooqi, Z.; Tjellstrom, I.; Hoglund Aberg, C.; Kwamin, F.; Claesson, R.; Haubek, D.; Johansson, A.; Oscarsson, J. Association of Filifactor alocis and its RTX toxin gene ftxA with periodontal attachment loss, and in synergy with Aggregatibacter actinomycetemcomitans. Front Cell Infect Microbiol 2024, 14, 1376358. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L.M.; Huang, H.; Lin, T.; Hou, W.; Harrison, P.L.; Aukhil, I.; Walker, C.B.; Klepac-Ceraj, V.; Paster, B.J. Microbiological characterization in children with aggressive periodontitis. J Dent Res 2012, 91, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.S.; Fernandes, J.G.; Li, L.; Huang, H.; Aukhil, I.; Harrison, P.; Diaz, P.I.; Shaddox, L.M. Evaluation of microbiome in primary and permanent dentition in grade C periodontitis in young individuals. Journal of Periodontology 2024. [Google Scholar] [CrossRef]
- Shenker, B.J.; Walker, L.P.; Zekavat, A.; Korostoff, J.; Boesze-Battaglia, K. Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin-Induces Cell Cycle Arrest in a Glycogen Synthase Kinase (GSK)-3-Dependent Manner in Oral Keratinocytes. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Monteiro, M.F.; Altabtbaei, K.; Kumar, P.S.; Casati, M.Z.; Ruiz, K.G.S.; Sallum, E.A.; Nociti-Junior, F.H.; Casarin, R.C.V. Parents with periodontitis impact the subgingival colonization of their offspring. Sci Rep 2021, 11, 1357. [Google Scholar] [CrossRef]
- Novak, M.J.; Stamatelakys, C.; Adair, S.M. Resolution of early lesions of juvenile periodontitis with tetracycline therapy alone: long-term observations of 4 cases. Journal of Periodontology 1991, 62, 628–633. [Google Scholar] [CrossRef]
- Mandell, R.L.; Tripodi, L.S.; Savitt, E.; Goodson, J.M.; Socransky, S.S. The effect of treatment on Actinobacillus actinomycetemcomitans in localized juvenile periodontitis. Periodontology 1986, 57, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.A.; Branco-de-Almeida, L.S.; Wolf, S.; Hovencamp, N.; Treloar, T.; Harrison, P.; Aukhil, I.; Gong, Y.; Shaddox, L.M. Long-term clinical response to treatment and maintenance of localized aggressive periodontitis: a cohort study. J Clin Periodontol 2017, 44, 158–168. [Google Scholar] [CrossRef]
- Schreiner, H.C.; Sinatra, K.; Kaplan, J.B.; Furgang, D.; Kachlany, S.C.; Planet, P.J.; Perez, B.A.; Figurski, D.H.; Fine, D.H. Tight-adherence genes of Actinobacillus actinomycetemcomitans are required for virulence in a rat model. Proc Natl Acad Sci U S A 2003, 100, 7295–7300. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, H.; Li, Y.; Cline, J.; Tsiagbe, V.K.; Fine, D.H. A comparison of Aggregatibacter actinomycetemcomitans (Aa) virulence traits in a rat model for periodontal disease. PLoS One 2013, 8, e69382. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, C.; Varudharasu, D.; Shanmugam, M.; Gopal, P.; Ragunath, C.; Thomas, L.; Nitz, M.; Ramasubbu, N. Structural and functional analysis of de-N-acetylase PgaB from periodontopathogen Aggregatibacter actinomycetemcomitans. Mol Oral Microbiol 2017, 32, 324–340. [Google Scholar] [CrossRef] [PubMed]
- Ristow, L.C.; Welch, R.A. RTX Toxins Ambush Immunity’s First Cellular Responders. Toxins (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Azzi-Martin, L.; Touffait-Calvez, V.; Everaert, M.; Jia, R.; Sifre, E.; Seeneevassen, L.; Varon, C.; Dubus, P.; Menard, A. Cytolethal Distending Toxin Modulates Cell Differentiation and Elicits Epithelial to Mesenchymal Transition. J Infect Dis 2024, 229, 1688–1701. [Google Scholar] [CrossRef]
| Disease Category |
Pub Med Years |
Web of Science Years |
Pub Med Years |
|||
| 2012-17 | 2018-23 | 2012-17 | 2018-23 | 2012-17 | 2018-23 | |
| Periodontitis | 6,492 | 6,287 | 10,181 | 16,420 | 13,000 | 18,926 |
| Aggressive Periodontitis |
884 | 534 | 1.053 | 817 | 1,030 | 679 |
| Localized Aggressive Periodontitis |
179 | 114 | 134 | 102 | 118 | 80 |
| *Stage III Grade C Periodontitis |
*148 | *138 | *135 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





