1. Introduction
Pain after cardiac surgery (CS), ranging from moderate to severe, is reported by up to 60% of patients in the first 2 days [
1], with persistent postoperative pain affecting 37% patients in the first 6 months after CS, and in 17% of the patient beyond 2 years after CS [
2]. As a result, poor control of postoperative pain can worse patient’s quality of life, since even with improvements in cardiovascular symptoms after CS may be minimized by the chronic nature of postoperative pain [
3].
Despite advances in cardiovascular surgery, with minimally invasive CS and transcatheter valve therapies, acute pain after traditional open surgery still peaks during the first 48 hours. This pain after CS is due to post-cardiac surgery trauma, including sternotomy, sternal retraction, radial artery/saphenous vein dissection and harvesting, and chest tube insertion [
4].
Traditionally, postoperative pain management relies on opioid administration. In CS, opioids were the cornerstone of anesthesia, with reports of intraoperative dosages as high as 3mg.kg
-1 of morphine [
5] or 100 mcg.kg
-1 of fentanyl [
6]. Although opioids are effective, relatively inexpensive, and available in various pharmaceutical forms, the adverse effects typically associated with opioids, such as sedation, respiratory depression, or paralytic ileus, can delay and impair postoperative recovery [
4].
Furthermore, opioid abuse is identified as a significant cause of morbidity and mortality in the United States, with postoperative opioid use being a contributing factor [
7]. A recent study showed that almost 10% of patients after CS continued to use opioids over 90 days after surgery and the higher the prescribed opioid dose at discharge the greater the risk of persistent postoperative opioid use [
8].
Therefore, measures such as multimodal analgesia strategies have been implemented to reduce opioid use, especially in opioid-naive patients [
7]. However, reducing the use of opioids poses a challenge for healthcare providers, as inadequately controlled postoperative pain can be as high as 80% and lead to chronic pain syndrome, continuous opioid use, increased morbidity, impaired functionality and quality of life, delayed recovery time, and higher costs to healthcare systems [
9].
Multimodal analgesia combines different analgesics and techniques that block or modulate pain stimulus in every point of its pathway from periphery to cerebral cortex relying on the “aggregation of marginal gains” theory, in what small gains in each stage can result in a greater improvement that may produce superior analgesia while decreasing opioid use and opioid-related side effects [
7,
10]. The administration of acetaminophen, gabapentinoids, NMDA antagonists, alpha-2 agonists, lidocaine, nonsteroidal and steroidal anti-inflammatory drugs, or regional nerve block techniques are the most common used approaches [
11]. Based on these documented benefits, The Society for Enhanced Recovery After Cardiac Surgery (ERAS
® Cardiac) strongly recommends the inclusion of an opioid-sparing multimodal strategy [
12] and have recently published updated recommendations specifically addressing postoperative analgesia in CS [
13]. Implementation of cumulative anesthesia-related strategies, mainly focusing on multimodal analgesia, indeed improved rates of early extubation and affected the duration of stay after CS in ERACS scenario [
14].
Considering the significance of postoperative pain control in cardiovascular surgery, particularly through reducing opioid use and potentially incorporating regional anesthesia techniques, this article aims to present the main multimodal analgesia strategies for postoperative CS through a literature review.
2. Materials and Methods
This is a literature review in which primary articles published preferably within the last 10 years were considered eligible for evidence-based medicine assessment, except for the articles used as references for background and theoretical concepts that were selected regardless of publication date. Only articles published in Portuguese, English, or Spanish were selected, and the following reference databases were used: Biblioteca Virtual de Saúde (BVS), Web of Science; CAPES; SciELO; PubMed, and LILACS. Search strategy used the following keywords or MeSH (Medical Subject Headings): “Pain, Postoperative”; “Cardiac Surgical Procedures”; “Analgesics, Opioid”; “Enhanced Recovery After Surgery”; “Perioperative Care”; “Regional Anesthesia”; “Multimodal analgesia”; “Adjuvants, Pharmaceutic”; “Adrenergic alpha-2 Receptor Agonists”; “Methadone”; “Ketamine”; “Lidocaine”; “Anti-Inflammatory Agents, Non-Steroidal”; “Acetamminophen”; “Gabapentin”; “Pregabalin”. After completing search in each database, duplicate references were excluded. In this manner, 906 articles were selected for review, and 82 were included in the writing of this review.
Within the context of Evidence-Based Medicine, critical appraisal of articles is an essential skill for evidence-based practice, focusing on mitigating biases and integrating the best external evidence into clinical care. Several meta-analyses and clinical trials have served as models for the development of various health guidelines, particularly in the context of postoperative pain control management in conventional cardiovascular surgery, generating various speculations in the scientific community regarding therapeutic measures involving the use of opioids, multimodal analgesia, and adjunct therapy [
15].
The Critical Appraisal Tools (CATs) in the selection of the 82 articles in this study were utilized. CATs are structured checklists that allow for assessing the methodological quality of a study based on a set of 20 criteria. CATs are based on algorithms to understand the study design type, three separate tools (for analytical studies, descriptive studies, and literature reviews), additional tools to support the evaluation process, and guidance for synthesizing evidence and drawing conclusions on a specific topic’s evidence scope. Although the toolset was developed to assist in creating national guidelines associated with infection prevention and control, physicians, reviewers, and academics can use it to assess any quantitative health-related research [
15]. To be included in the review, the study should fulfil at least 80% of the set of 20 criteria and have at least 80% consensus between the authors.
Following the evaluation of individual items in each study type, each CAT also provides requirements for inferring an overall conclusion about the study’s evidence quality based on item evaluation. Quality is categorized as high, medium, or low. While a randomized clinical trial is a strong study design, it is possible to have a low-quality trial or a high-quality study. Therefore, a study’s evidence quality distinguishes itself from the study design strength when evaluating the overall evidence quality.
3. Results
3.1. The Basic Pathophysiology of Pain
Pain after CS can arise from various sources such as visceral, musculoskeletal, or neurogenic origins (
Table 1). Visceral pain from the heart reaches the central nervous system through pathways involving the vagus nerve, cervical sympathetic chain, and upper-thoracic sympathetic ganglia. Most pain related to heart issues post-surgery is often due to inadequate blood supply (ischemia) caused by conditions like coronary artery vasospasm, atherosclerosis, or acute insufficiency [
16].
The most common persistent pain following CS typically arises from myofascial structures such as muscles, bones, tendons, and ligaments. Besides the impact of surgical trauma, which activates peripheral neurons and releases chemical mediators like histamine and serotonin, pain can also be influenced by patient positioning during surgery and the use of surgical instruments. For instance, the sternal retractor can lead to complications such as fractured ribs, dislocation of the costochondral junction, costochondritis, and rib-spine articulation issues [
16].
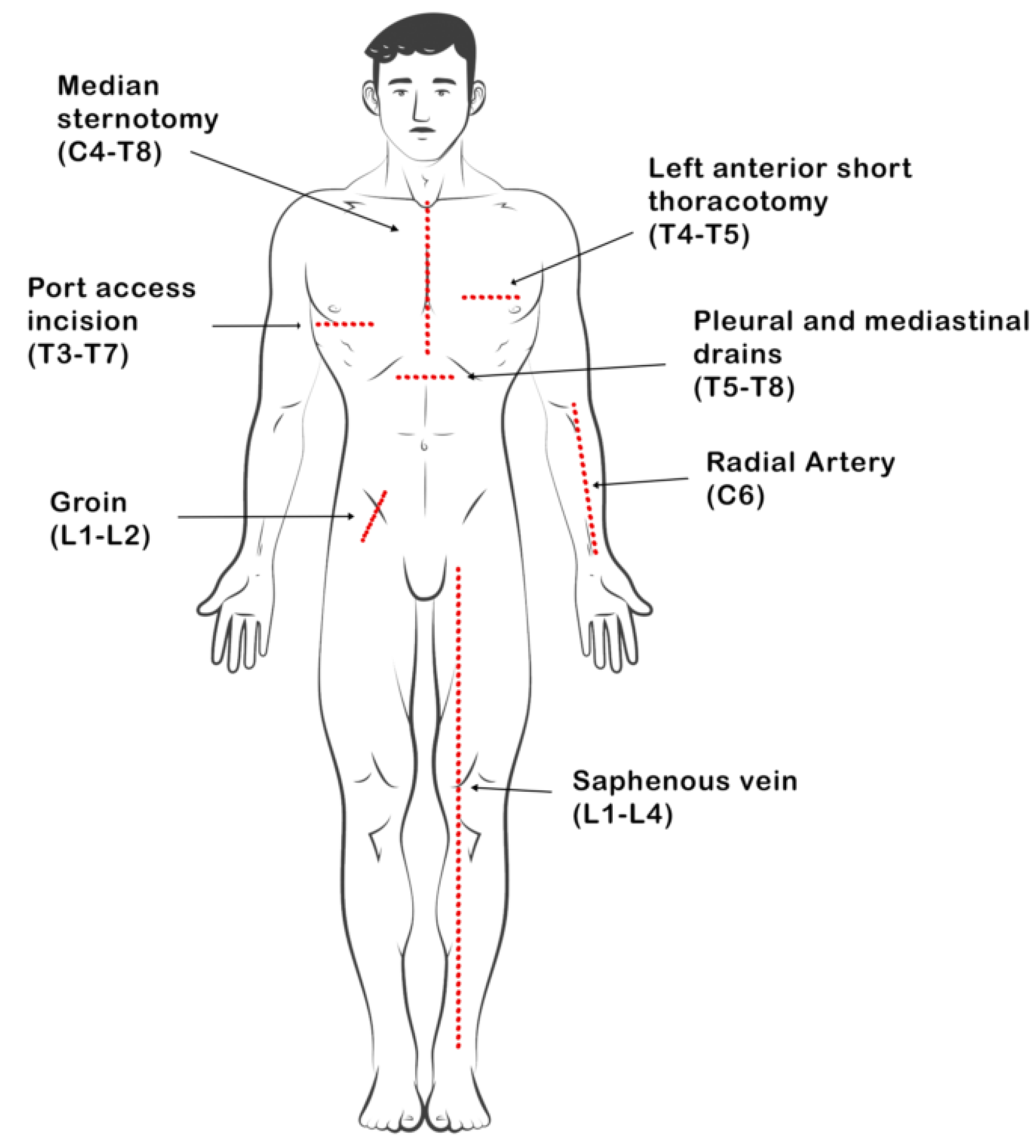
Furthermore, harvesting the internal mammary artery during procedures like coronary artery bypass, has been linked to a specific neuropathic pain syndrome post-surgery. This pain is believed to arise from nerve injuries resulting from the procedure, leading to an irritated state referred to as neuritis. Symptoms of this pain may include burning or lancinating sensations, worsening at night, and exacerbation upon stretching the affected nerve. Patients may also experience muscle twitching, hypersensitivity, abnormal sensations (paresthesia), or altered sensation to touch (dysesthesia) [
16]. Commonly used incisions include cervical, thoracic, and lumbar dermatomes (
Figure 1).
3.2. Opioids
Opioids, as already discussed, are effective for acute pain treatment and, in this respect, are widely used after surgery, including CS. The side effects of the use of this class of drugs are repeatedly observed in both acute and chronic use. These include constipation, postoperative nausea and vomiting (PONV), respiratory depression and hyperalgesia. In addition, tolerance develops with chronic use of opioids, in which higher doses are required to acquire the same analgesic effects. It is also found that opioids can even decrease the rate of wound healing [
17].
Recent recommendations in ERACS are based on a “opioid stewardship” approach, which means the careful and appropriate utilization of opioids to manage surgical pain effectively and enhance postoperative recovery outcomes [
13]. Despite concerns regarding opioid-related adverse events and persistent opioid use, opioids continue to play a vital role in managing acute pain and providing intraoperative anesthesia. Opioid stewardship emphasizes the importance of using opioids judiciously to ensure patient comfort, promote functional recovery, and avoid compromising optimal pain control.
Although there is a trend towards reducing opioid use in cardiac surgeries and ERAS settings, methadone, despite being classified as an opioid, emerges as an attractive alternative with a unique profile within the multimodal strategy. The prerogatives of its use for analgesia after CS are diverse. First, due to its prolonged action (24-36 hours of efficacy with a single dose), it can promote a more stable basal control of acute pain during this period of intense painful stimuli [
18]. Its activity on the NMDA receptor is also considered a potential mechanism for improved quality and more consistent control of pain in the postoperative period following cardiac and non-cardiac procedures in adults, and some evidence supports the hypothesis that NMDA receptor antagonism may reduce the development of chronic pain syndromes [
18,
19]. Additionally, methadone inhibits the reuptake of neurotransmitters serotonin and norepinephrine in the CNS and may potentially provide a mood-elevating effect postoperatively, as well as act on inhibitory descending pain pathways [
20].
A systematic review showed that intraoperative administration of methadone decreased postoperative acute pain, reduces opioid consumption compared to morphine and fentanyl [
21]. This confirms that methadone could be an opioid to be utilized during cardiothoracic procedures to alleviate acute post-surgical pain. In line with this study, other authors assessed the effect of intraoperative intravenous methadone in patients undergoing CS on postoperative opioid requirements and surgical recovery. It was observed that methadone was safe and significantly reduced the intraoperative and postoperative opioid needs in the first 24 hours after surgery [
22].
The largest clinical trial assessing methadone in CS compared patients randomized to receive either 0.3 mg.kg
-1 of methadone or 12 μg.kg
-1 of fentanyl intraoperatively and methadone group reduced postoperative morphine requirements, improved pain scores, and enhanced patient-perceived quality of pain management without increasing adverse events [
23]. In a follow-up of this study, a pain questionnaire assessing weekly frequency (primary outcome) and pain intensity at 1-, 3-, 6-, and 12-months post-surgery was administered. The results showed that patients randomized to receive methadone for CS reported lower postoperative pain frequency even at 1 month compared to patients randomized to receive fentanyl [
24].
In pediatric patients, an observational prospective study showed that Intraoperative methadone use, in doses up to 0.4 mg.kg
-1, was associated with a decrease in perioperative opioid exposure in patients undergoing congenital heart surgery during the first 36 hours and was not associated with neither adverse events nor prolonged durations of mechanical ventilation or intensive care unit stay [
25].
Considering methadone as a component of a multimodal analgesia, a recent retrospective study in adult patients undergoing cardiac surgeries were assigned to one of three analgesic regimens: opioids only or two multimodal regimens. The opioid regimen comprised intraoperative fentanyl and patient-controlled analgesia pumps. Multimodal regimen 1 involved preoperative extended-release oxycodone, intraoperative ketamine infusion, and postoperative morphine suppository. Multimodal regimen 2 included intraoperative methadone infusion and dexmedetomidine. It was observed that multimodal analgesic regimens, particularly those incorporating methadone and dexmedetomidine, significantly reduced total opioid usage and pre-discharge opioid consumption in cardiac surgical patients [
26].
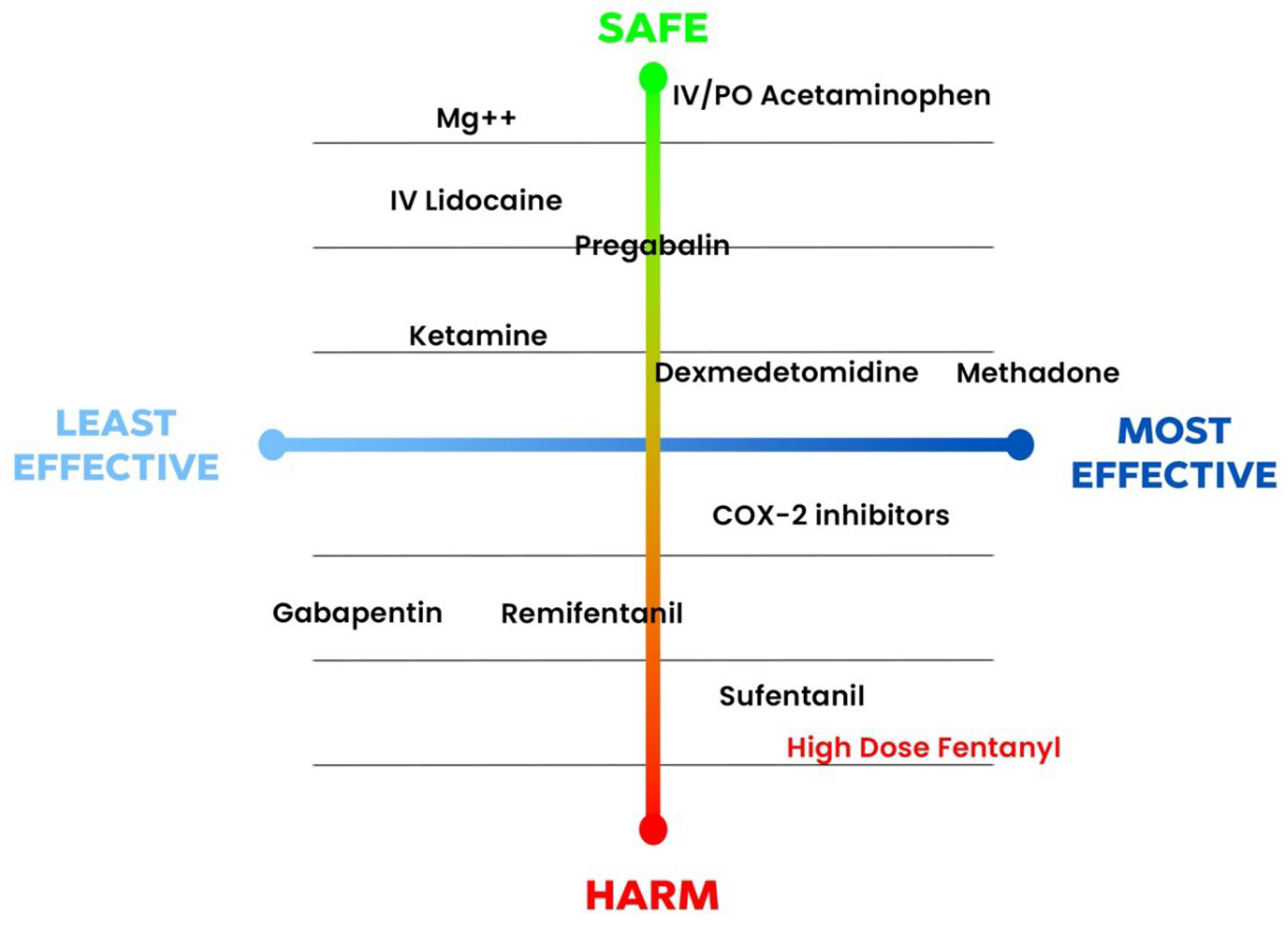
Due to the above-mentioned evidence, methadone has been considered one of the most effective strategies in controlling postoperative pain after CS (
Figure 2) [
13]. Methadone may be the opioid that helps greatly limit the use of other opioids and with promising role in enhanced recovery protocols [
27]. All these recent findings have led to an increased utilization of methadone in CS over time. This trend aligns with the implementation of enhanced recovery pathways, affirming improved pain management post-cardiac surgery with minimal side effects [
28].
Tramadol is another opioid medication that worth mentioning in multimodal analgesia for CS, as it is recommended in first ERACS guidelines [
12]. Tramadol is a centrally acting analgesic with a double-action mechanism: one based on the mu-receptor and the other on serotonin and noradrenalin reuptake inhibition at the central synaptic level. Unlike traditional opioids, it does not interact with neither hemodynamic or respiratory function, nor does not cause tolerance [
29]. Its recommendation for postoperative analgesia in CS results from an old clinical trial that showed a 25% reduction in postoperative morphine consumption after a single dose prior extubation [
30]. A more recent study showed that, in combination with oral acetaminophen, tramadol improved analgesia and reduced morphine requirement up to 50% after coronary artery bypass surgery [
29].
3.3. Paracetamol/Acetaminophen
Paracetamol is generally used as an adjuvant analgesic with central properties and has been shown to reduce inflammation in perioperative setting, however, is not commonly studied in CS, except for the intravenous formulation. The DEXACET trial showed that paracetamol reduces opioid consumption, produce similar pain scores, and potentially reduce delirium - a possible consequence of poor pain control or opioid side effects [
31]. Another trial in CS comparing 1 g of intravenous acetaminophen every 6 hours for 24 hours with placebo, showed reduced pain after CS, but not opioid consumption in acetaminophen group [
32].
In oral formulation, acetaminophen (375 mg) in combination with tramadol (37.5 mg), reduced cumulative morphine consumption after CS in 50% [
29] when given preoperatively and every 6 hours until 48 hours postoperatively [
29]. The relative cost-effectiveness of oral administration compared to intravenous administration is controversial, therefore, despite the higher bioavailability of the intravenous form, oral paracetamol is encouraged in major surgery (maximum dose, 3-4 g/24 hours), unless contraindicated due to an inability to tolerate oral medication or in the presence of significant liver dysfunction [
33]. Due to these presented evidence and safety profile, acetaminophen is recommended in ERACS guidelines in 1g dose every 8 hours as an opioid-sparing strategy [
12].
3.4. Gabapentinoids
Gabapentin is an amino acid, an analogue of gamma-aminobutyric acid (GABA) found to be effective as an anticonvulsant drug. Pregabalin is another GABA analogue closely related to gabapentin. Both medications are also used as adjunctive therapy in pain management. Despite their close structural resemblance to GABA, gabapentin and pregabalin do not directly act on GABA receptors. Both drugs bind to the α2δ subunit of voltage-gated N-type calcium (Ca2+) channels. This appears to form the basis of their primary mechanism of action, which involves reducing Ca2+ ion influx, predominantly affecting presynaptic channels in the dorsal horn of the spinal cord [
34].
One of the most emblematic trials assessing gabapentin, a preoperative single dose (600 mg) reduced opioid consumption and improved pain scores compared to placebo, but it also increased sedation and was associated with a modest increase in postoperative mechanical ventilation [
35]. Conversely, another study comparing a higher preoperative dose (1,200 mg) followed by scheduled gabapentin (600 mg twice daily for the next two postoperative days) resulted in similar opioid consumption and pain scores, with no difference in side effects [
36].
Pregabalin is considered a successor of gabapentin. The administration of pregabalin 150 mg preoperatively and followed by 75 mg twice a day, for 5 days, reduced postoperative opioid consumption, the incidence of confusion on the first postoperative day and increased time to extubation when compared with placebo after CS. Three months after operation, patients in the pregabalin group experienced less pain during movement [
37]. Another study showed that the same pregabalin schedule, however until 48 hours postoperavely, reduced pain scores at rest and deep breath and reduced consumption of tramadol in the post-operative period without delaying extubation and causing excessive sedation [
38].
In combination with ketamine infusion, adults without chronic pain undergoing any elective CS with sternotomy, who were randomized to receive either pregabalin alone (150 mg preoperatively and twice daily for 14 postoperative days) or pregabalin in combination with a 48-hour postoperative intravenous infusion of ketamine at 0.1 mg.kg
-1.hr
-1. The primary outcomes were the prevalence of clinically significant pain at 3 and 6 months after surgery. In total, 150 patients were randomized. It was observed that the prevalence of pain was lower at 3 months postoperatively with pregabalin alone (6% [3 out of 50]) and in combination with ketamine (2% [1 out of 50]) compared to the control group (34% [17 out of 50]; odds ratio = 0.126 [0.022 to 0.5], P = 0.0008; and 0.041 [0.0009 to 0.28], P < 0.0001, respectively) and at 6 months for pregabalin alone (6% [3 out of 50]) and in combination with ketamine 0% (0 out of 50) compared to the control group (28% [14 out of 50]; odds ratio = 0.167 [0.029 to 0.7], P = 0.006; and 0.000 [0 to 0.24], P < 0.0001) [
39].
A metanalysis in CS, found that pregabalin decreased postoperative pain scores; reduced opioid consumption when it was continued in the postoperative period; and did not increase duration of mechanical ventilation, sedation or other side effects. While gabapentin did not reduce postoperative opioid consumption after CS; but may reduce pain scores at the expense of increased duration of mechanical ventilation [
40]. In a nutshell, it seems that regarding gabapentinoids, pregabalin is preferable and is recommended as an opioid stewardship strategy [
13].
3.5. N-Methyl-D-Aspartate (NMDA) Receptor Antagonists
Ketamine is a derivative of phencyclidine that is partially water-soluble and highly lipophilic. Of the two stereoisomers of ketamine, the S(+) form is more potent than the R(−) isomer. The main observed effect is likely produced by the inhibition of the N-methyl-D-aspartate (NMDA) receptor complex. The use of ketamine has always been limited due to its unpleasant psychotomimetic side effects. However, this drug represents a relevant alternative in certain circumstances, mainly due to its potent analgesic effects with minimal respiratory depression [
41].
The role of ketamine as an adjuvant for pain management in CS dates back to the early 2000s when a small-dose S(+)-ketamine infusion, as an adjunct to PCA oxycodone, exerted an opioid-sparing effect without hemodynamic side-effects after sternotomy and improved patient satisfaction in CABG patients [
42]. However, recent trials have shown that either single induction bolus of 0.5-1.0 mg.kg
-1 [
43] or a bolus of 0.5 mg.kg
-1 followed by a continuous infusion of 0.5 mg.kg
-1.h
-1[
44], were not able to reduce pain scores, opioid consumption or delirium after CS. In one of these studies, patients who received ketamine had more negative experiences [
43].
In the context of CS, the results diverge from non-cardiac settings, wherein perioperative intravenous ketamine reduces postoperative analgesic requirements and pain severity across various surgical procedures and administration timings, irrespective of study size or pain intensity levels. Central nervous system (CNS) adverse events exhibited minimal disparity between ketamine and control groups. Perioperative intravenous ketamine likely marginally reduces PONV [
45]. In addition to this, a previous mentioned study showed that, ketamine infusion associated with pregabalin resulted in less chronic pain after 3 and 6 months, when compared to pregabalin alone [
39]. Although included as an opioid-sparing strategy in pain management after CS [
13] and has been shown to be one of the process measures that effectively reduced intubation time and length of stay (through an intraoperative infusion of 0.25 mg.kg
-1.h
-1) [
14], more studies are needed to further prove its effectiveness.
3.6. Alpha-2 Agonists
Dexmedetomidine is a highly selective α2-adrenergic agonist. This drug is the active S-enantiomer of medetomidine, a highly selective α2-adrenergic agonist imidazoline derivative. Dexmedetomidine produces its selective α2-agonist effects by activating α2 receptors in the CNS. Hypnosis presumably results from the stimulation of α2 receptors in the locus ceruleus, while the analgesic effect originates at the level of the spinal cord. Its sedative effects are associated with the activation of endogenous sleep pathways [
46].
Intravenous dexmedetomidine has been the focus of various randomized clinical trials and meta-analyses in CS, which have reported a range of benefits, including earlier extubation and reduced incidences of arrhythmias, delirium, and length of hospital stay [
47,
48]. It has shown promise as an analgesic, providing a significant reduction in the need for opioids and improved pain for up to 24 hours after CS [
49,
50]. It has been recommended as a component of stewardship opioid strategy after CS in ERACS context in 0.5-1.5 mg.kg
-1.h
-1 [
12,
13]. Dexmedetomidine in 0.2-0.7 mg.kg
-1.h
-1 infusion, administered at the time of cardiopulmonary bypass and throughout transport to the ICU, was one of the process measures that effectively reduced time to extubation and length of hospital stay in one hospital during ERACS implementation [
14].
A recent large trial with almost 800 patients assessing the effectiveness of dexmedetomidine in reducing atrial fibrillation and postoperative delirium brought disappointing results. Dexmedetomidine infusion, initiated at anesthetic induction and continued for 24 hours, did not decrease postoperative atrial arrhythmias and, on the other hand, increased delirium in patients recovering from CS. In addition, patients who received dexmedetomidine had more clinically important hypotension episodes, which might be the cause of increased delirium [
51]. Although well-designed, this study has points of concern as both anesthesia techniques and medications were not standardized, as intraoperative opioids and benzodiazepines were given at the discretion of the anesthesiologist. Moreover, anesthesia was usually induced with midazolam, thiopental, etomidate, propofol, and sufentanil or fentanyl, or both. The absence of standard anesthetic techniques can make delirium assessment and quantification of postoperative opioid consumption difficult.
3.7. Dexamethasone and Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
NSAIDs are a unique group of medications that target cyclooxygenase (COX), an enzyme essential for prostaglandin (PG) biosynthesis from arachidonic acid. COX has two isoforms: COX-1, found in most tissues, plays a role in various bodily functions, while COX-2, present in inflammatory cells, produces pro-inflammatory mediators. NSAIDs can either inhibit both COX-1 and COX-2 or selectively inhibit COX-2. Arachidonic acid, released in response to stimuli, is converted by COX to produce various PGs, facilitating the inflammatory response mechanism [
52].
Prostaglandins are molecules that are released from cells and act locally through G protein-coupled receptors. The most common type in the body is PGE2, which is continuously produced by COX-1. Inflammation can cause an increase in PGE2 production by COX-2. These enzymes have preferences for various prostaglandins. PGE2 and PGI2 play a role in increasing the sensitivity of pain receptors and neurons, contributing to pain perception both in the periphery, reducing excitability threshold, and in the spinal dorsal horn neurons, contributing to central sensitization [
53].
When administered as part of a multimodal regimen, NSAIDs or COX-2 inhibitors are associated with a reduction in pain and opioid usage, which may result in a decrease in some opioid-related side effects [
54,
55]. In CS, there is enough evidence to support that NSAIDs reduces pain scores and postoperative opioid consumption [
56]. However, even with evidence showing similar adverse events compared to placebo [
56], concerns about potential renal injuries and gastrointestinal complications limit their widespread use in CS [
57]. Similarly, COX-2 inhibitors significantly reduced postoperative morphine consumption and improved pain perception after coronary artery bypass graft, however at the expense of increase in composite adverse events, mainly of thrombotic nature [
58]. Another study also found that COX-2 inhibitors were associated with increased incidence of cardiovascular events after CABG [
59]. These results were sufficient to The Food and Drug Administration (FDA) issue a formal alert regarding NSAIDs in patients undergoing coronary revascularization.
Nonetheless, perioperative care physicians are still using NSAIDs selectively in CS setting, despite 2005 FDA’s blackbox. An observational study showed that one-third of cardiac patients, with lower preoperative risk, received ketorolac postoperatively for pain control and no increase in any adverse events was found. The authors concluded that ketorolac appears to be safe for use as a postoperative analgesic when administered selectively after cardiac operations and raise the question of the need for a black box warning recommending against the use of ketorolac for all CS patients [
60].
A recent trial in pediatric patients undergoing CS has shown that ibuprofen 10mg.kg
-1 as a component of a multimodal analgesia improved postoperative analgesia in terms of reduction of opioid consumption and pain scores, without increasing renal dysfunction [
61]. In adults, a combination of ketorolac intraoperatively and ibuprofen postoperatively for 4 days as components of multimodal analgesic therapy offered significantly better analgesia with significantly less PONV than a traditional opiate regimen [
62].
Dexamethasone, as a glucocorticoid, have analgesic benefits possibly related to anti-inflammatory properties and should be considered as part of a multimodal perioperative pain regimen. Several meta-analyses examined perioperative dexamethasone and indicated that patients who received dexamethasone (4–10 mg or >0.1 mg.kg
-1) had lower pain scores, used less opioids, and required less rescue analgesia [
63,
64]. In CS, there is concern regarding glucocorticoids due to reference studies evaluating high doses, as large as 1 mg.kg
-1, of dexamethasone, which have been associated with increased myocardial injury [
65] and elevated blood glucose levels [
66]. No benefits in reducing mortality were registered and analgesia was not assessed. There are some evidences that, as a component of multimodal analgesia, a single 8 mg dexamethasone dose can significantly reduce pain and PONV [
62], besides improving quality of recovery after CS [
67].
3.8. Intravenous Lidocaine
Intravenous (IV) lidocaine infusions may be effective to reduce systemic inflammation and are indicated as part of a multimodal analgesic approach for visceral surgery when other local anesthetic approaches such as regional analgesia are not possible [
27]. In this scenario, recent metanalysis shows that in non-cardiac surgery, IV lidocaine was associated with a decrease in postoperative pain and opioid consumption [
68,
69] and possibly faster return of bowel function and decreased length of hospital stay [
69]. However, in CS studies intraoperative IV lidocaine has not shown a difference in postoperative pain or in opioid consumption [
70,
71]. One possible mechanism for this difference is that the key mechanism responsible for the analgesic effects of IV lidocaine infusions, its metabolism to a glycine receptor inhibitor causing an anti-nociceptive effect, may be disrupted by the abrupt changes in glycine concentrations that occur in response to cardiopulmonary bypass [
71].
Nonetheless, IV lidocaine reduced the incidence of postoperative cognitive dysfunction after CS when administered as a bolus of 1.5 mg.kg
-1 followed by a 4-mg.min
-1 infusion in CS, which raised interest of intraoperative infusion of this medication [
72]. However, a larger randomized controlled trial has recently shown that IV lidocaine did not alter any quality-of-life outcomes after 6 weeks of CS. Furthermore, even at the 1-year follow-up there continued to be no difference in cognitive score change, cognitive deficit, or quality of life in patients who received IV lidocaine [
73]. In summary, the available evidence does not support the use of perioperative lidocaine infusion for CS patients.
3.9. Regional Anesthesia
Regional anesthesia, encompassing both neuroaxial and peripheral nerve blocks, plays a crucial role in multimodal analgesia as it can effectively block pain sensation originating from the site of surgical incision or manipulation. This occurs blocking the transmission of action potentials from the periphery to the central nervous system at various points [
13]. When performing regional nerve blocks in surgical settings, it is essential to consider the distribution of nerves in both the incision and drainage sites. Specifically, for procedures such as median sternotomy or thoracotomy, the focus should be on targeting the perforating branches of the intercostal nerves originating from the thoracic spine nerves (T1-T11) [
74].
In the past, thoracic epidural analgesia (TEA), spinal anesthesia (SA) and paravertebral blocks (PVBs) were commonly used regional techniques for postoperative pain control. However, concerns such as increased risk of epidural hematomas from systemic heparinization, hemodynamic instability due to sympathetic blockade, technical complexities, and difficulties in postoperative pain management with neuraxial blocks have limited the utilization of TEA and PVBs in cardiac surgical patients [
75]. Due to both these concerns and the widespread use of ultrasound in regional anesthesia, several regional analgesic techniques were developed in recent years using different approaches in thoracic region (anterior, lateral, and posterior), most of them relying on ultrasound-guided fascial plane blocks, in the perioperative pain management of patients undergoing CS [
75].
The most popular novel ultrasound-guided blocks for CS are: erector spinae block (ESPB), serratus anterior muscle plane block (SAPB), pectoral muscles blocks (PECS I and PECS II), transversus thoracic muscle plane block (TTMB) and pecto-intercostal fascial plane block (PIFB) [
76]. In view of the various nomenclatures given to the same regional block in the literature, there was recently a Delphi consensus of regional anesthesia experts that resulted in the standardization of block names [
77]. Thus, PIFB and TTMB are now referred to as superficial and deep parasternal intercostal plane (PIP) block, respectively. In addition to this, pectoral muscles plane blocks are still matter of debate, since PECS I had its name changed to interpectoral plane block and PECS II had its name changed to pectoserratus plane block, however with low agreement rate between the authors [
77].
The ESP block and PVB are posterior approaches of fascial plane blocks, and normally covers the posterolateral region of the chest, with variable anterior coverage past the midclavicular line [
74]. PECS I, PECS II and SAPB (superficial or deep) are anterolateral approaches which normally covers the lateral region of the chest, being suitable for drains, thoracotomies associated with minimal invasive CS [
74,
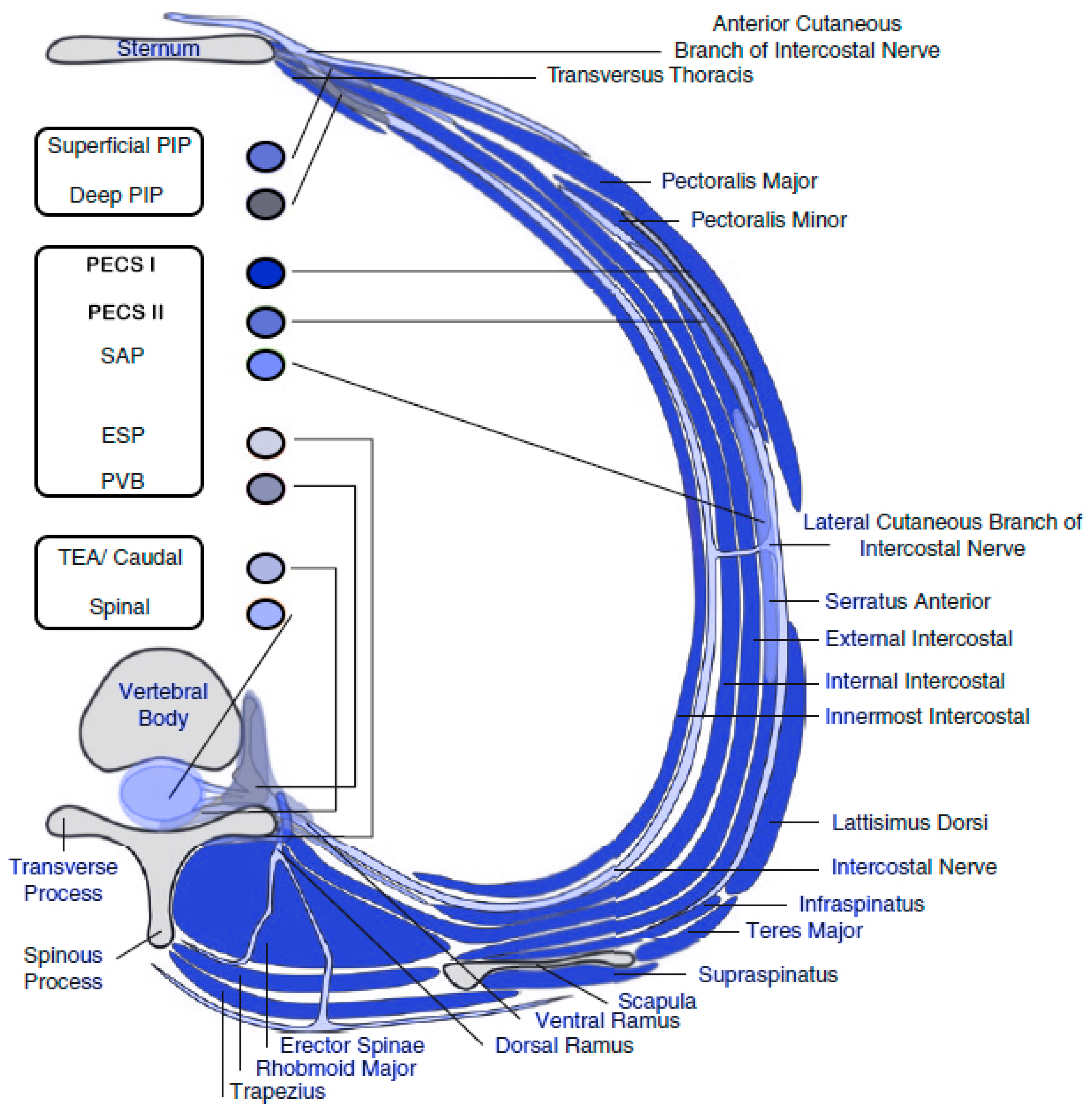
76]. Lastly, the parasternal intercostal plane blocks, either superficial or deep, are anterior approaches and well cover anterior cutaneous intercostal branches, being suitable for sternotomy (
Figure 3) [
74,
76]. Although the deep parasternal intercostal plane block covers more parasternal interspaces compared to the superficial block with a single injection, the proximity to the internal mammary artery and the severe consequences of an arterial injury, raises concerns about the deep technique [
78].
Compared either to placebo or absence of regional anesthesia, the fascial plane blocks of the chest reduce both pain and opioid consumption after CS [
80,
81]. Among all fascial plane blocks, one network metanalysis showed that, to date, ESPB was the most effective treatment with a greater reduction in postoperative opioid consumption compared to others [
80]. As a component of multimodal anesthesia, regional blocks were associated to reduced time to extubation and length of stay [
14] and should be performed for postoperative pain control in ERACS context [
13]. A summary of all fascial plane blocks is depicted in
Table 2.
4. Conclusions
Multimodal treatment of pain after CS is feasible, rational and has been associated with improved outcomes compared to traditional care. As both the surgical techniques and technology of the devices improves in CS scenario, the perioperative analgesia strategy must follow these advancements providing effective pain control without the frequent adverse side effects caused by the traditional opioid-based analgesia, as well as providing faster patient recovery. To date, methadone seems to be the most effective opioid-sparing drug in controlling pain after CS, however it still has opioid related side effects, and other strategies should be combined such as gabapentinoids, acetaminophen, low dexamethasone doses, NSAIDs (in selected cases), dexmedetomidine and even, with lower evidence, ketamine infusion. Although safety concerns with traditional regional anesthesia techniques (spinal anesthesia, epidural and paravertebral blocks), ultrasound-guided fascial plane blocks have shown to be effective, safe and, regardless of the chosen technique, should now be a component of the multimodal analgesia strategy following CS by ERACS recommendations.
References
- Raksamani, K.; Wongkornrat, W.; Siriboon, P.; Pantisawat, N. Pain management after cardiac surgery: are we underestimating post sternotomy pain? Journal of the Medical Association of Thailand = Chotmaihet thangphaet 2013, 96, 824–828. [Google Scholar]
- Guimarães-Pereira, L.; Reis, P.; Abelha, F.; Azevedo, L.F.; Castro-Lopes, J.M. Persistent postoperative pain after cardiac surgery: a systematic review with meta-analysis regarding incidence and pain intensity. Pain 2017, 158, 1869–1885. [Google Scholar] [CrossRef]
- Viana, L.; Oliveira, E.; Oliveira, C.M.B.; Moura, E.C.R.; Viana, L.H.L.; Nina, V.; Farkas, E.; Leal, P.D.C. Assessment of pain and quality of life in patients undergoing cardiac surgery: a cohort study. Revista da Associacao Medica Brasileira (1992) 2023, 69, 473–478. [Google Scholar] [CrossRef]
- Barr, L.F.; Boss, M.J.; Mazzeffi, M.A.; Taylor, B.S.; Salenger, R. Postoperative Multimodal Analgesia in Cardiac Surgery. Critical care clinics 2020, 36, 631–651. [Google Scholar] [CrossRef]
- Lowenstein, E.; Hallowell, P.; Levine, F.H.; Daggett, W.M.; Austen, W.G.; Laver, M.B. Cardiovascular response to large doses of intravenous morphine in man. The New England journal of medicine 1969, 281, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Quintin, L.; Whalley, D.G.; Wynands, J.E.; Morin, J.E.; Burke, J. High dose fentanyl anaesthesia with oxygen for aorto-coronary bypass surgery. Canadian Anaesthetists’ Society Journal 1981, 28, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Wick, E.C.; Grant, M.C.; Wu, C.L. Postoperative Multimodal Analgesia Pain Management With Nonopioid Analgesics and Techniques: A Review. JAMA surgery 2017, 152, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.R.; Chen, Z.; Khurshan, F.; Groeneveld, P.W.; Desai, N.D. Development of Persistent Opioid Use After Cardiac Surgery. JAMA cardiology 2020, 5, 889–896. [Google Scholar] [CrossRef]
- Gan, T.J. Poorly controlled postoperative pain: prevalence, consequences, and prevention. Journal of pain research 2017, 10, 2287–2298. [Google Scholar] [CrossRef]
- Fleming, I.O.; Garratt, C.; Guha, R.; Desai, J.; Chaubey, S.; Wang, Y.; Leonard, S.; Kunst, G. Aggregation of Marginal Gains in Cardiac Surgery: Feasibility of a Perioperative Care Bundle for Enhanced Recovery in Cardiac Surgical Patients. Journal of cardiothoracic and vascular anesthesia 2016, 30, 665–670. [Google Scholar] [CrossRef]
- Noss, C.; Prusinkiewicz, C.; Nelson, G.; Patel, P.A.; Augoustides, J.G.; Gregory, A.J. Enhanced Recovery for Cardiac Surgery. Journal of cardiothoracic and vascular anesthesia 2018, 32, 2760–2770. [Google Scholar] [CrossRef]
- Engelman, D.T.; Ben Ali, W.; Williams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khoynezhad, A.; Gerdisch, M.; Levy, J.H.; et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA surgery 2019, 154, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.C.; Chappell, D.; Gan, T.J.; Manning, M.W.; Miller, T.E.; Brodt, J.L. Pain management and opioid stewardship in adult cardiac surgery: Joint consensus report of the PeriOperative Quality Initiative and the Enhanced Recovery After Surgery Cardiac Society. The Journal of thoracic and cardiovascular surgery 2023, 166, 1695–1706.e1692. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.C.; Isada, T.; Ruzankin, P.; Whitman, G.; Lawton, J.S.; Dodd, O.J.; Barodka, V. Results from an enhanced recovery program for cardiac surgery. The Journal of thoracic and cardiovascular surgery 2020, 159, 1393–1402.e1397. [Google Scholar] [CrossRef] [PubMed]
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ open 2016, 6, e011458. [Google Scholar] [CrossRef] [PubMed]
- Cogan, J. Pain management after cardiac surgery. Seminars in cardiothoracic and vascular anesthesia 2010, 14, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Ingason, A.B.; Geirsson, A.; Gudbjartsson, T.; Muehlschlegel, J.D.; Sigurdsson, M.I. The Incidence of New Persistent Opioid Use Following Cardiac Surgery via Sternotomy. The Annals of thoracic surgery 2022, 113, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.S.; Szokol, J.W. Intraoperative Methadone in Surgical Patients: A Review of Clinical Investigations. Anesthesiology 2019, 131, 678–692. [Google Scholar] [CrossRef]
- Kharasch, E.D. Intraoperative methadone: rediscovery, reappraisal, and reinvigoration? Anesthesia and analgesia 2011, 112, 13–16. [Google Scholar] [CrossRef]
- Pontes, J.P.J.; Braz, F.R.; Módolo, N.S.P.; Mattar, L.A.; Sousa, J.A.G.; Navarro, E.L.L.H. Intra-operative methadone effect on quality of recovery compared with morphine following laparoscopic gastroplasty: a randomised controlled trial. Anaesthesia 2021, 76, 199–208. [Google Scholar] [CrossRef]
- Lobova, V.A.; Roll, J.M.; Roll, M.L.C. Intraoperative Methadone Use in Cardiac Surgery: A Systematic Review. Pain medicine (Malden, Mass.) 2021, 22, 2827–2834. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.J.; Song, P.; Nault, K.M. Impact of intraoperative methadone use on postoperative opioid requirements after cardiac surgery. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists 2022, 79, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.S.; Szokol, J.W.; Avram, M.J.; Greenberg, S.B.; Marymont, J.H.; Shear, T.; Parikh, K.N.; Patel, S.S.; Gupta, D.K. Intraoperative Methadone for the Prevention of Postoperative Pain: A Randomized, Double-blinded Clinical Trial in Cardiac Surgical Patients. Anesthesiology 2015, 122, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.S.; Avram, M.J.; Greenberg, S.B.; Shear, T.D.; Deshur, M.A.; Dickerson, D.; Bilimoria, S.; Benson, J.; Maher, C.E.; Trenk, G.J.; et al. Postoperative Pain and Analgesic Requirements in the First Year after Intraoperative Methadone for Complex Spine and Cardiac Surgery. Anesthesiology 2020, 132, 330–342. [Google Scholar] [CrossRef]
- Robinson, J.D.; Caruso, T.J.; Wu, M.; Kleiman, Z.I.; Kwiatkowski, D.M. Intraoperative Methadone Is Associated with Decreased Perioperative Opioid Use Without Adverse Events: A Case-Matched Cohort Study. Journal of cardiothoracic and vascular anesthesia 2020, 34, 335–341. [Google Scholar] [CrossRef]
- Eisenbraun, A.; Schroeder, D.; Schaff, H.V.; Martin, E.; Wittwer, E.D. Single-Center Retrospective Comparison of Opioid-Based and Multimodal Analgesic Regimens in Adult Cardiac Surgery. Journal of cardiothoracic and vascular anesthesia 2023, 37, 1179–1187. [Google Scholar] [CrossRef]
- McEvoy, M.D.; Raymond, B.L.; Krige, A. Opioid-Sparing Perioperative Analgesia Within Enhanced Recovery Programs. Anesthesiology clinics 2022, 40, 35–58. [Google Scholar] [CrossRef]
- Edwards, J.N.; Whitney, M.A.; Smith, B.B.; Fah, M.K.; Buckner Petty, S.A.; Durra, O.; Sell-Dottin, K.A.; Portner, E.; Wittwer, E.D.; Milam, A.J. The role of methadone in cardiac surgery for management of postoperative pain. BJA open 2024, 10, 100270. [Google Scholar] [CrossRef]
- Altun, D.; Çınar, Ö.; Özker, E.; Türköz, A. The effect of tramadol plus paracetamol on consumption of morphine after coronary artery bypass grafting. Journal of clinical anesthesia 2017, 36, 189–193. [Google Scholar] [CrossRef]
- But, A.K.; Erdil, F.; Yucel, A.; Gedik, E.; Durmus, M.; Ersoy, M.O. The effects of single-dose tramadol on post-operative pain and morphine requirements after coronary artery bypass surgery. Acta anaesthesiologica Scandinavica 2007, 51, 601–606. [Google Scholar] [CrossRef]
- Subramaniam, B.; Shankar, P.; Shaefi, S.; Mueller, A.; O’Gara, B.; Banner-Goodspeed, V.; Gallagher, J.; Gasangwa, D.; Patxot, M.; Packiasabapathy, S.; et al. Effect of Intravenous Acetaminophen vs Placebo Combined With Propofol or Dexmedetomidine on Postoperative Delirium Among Older Patients Following Cardiac Surgery: The DEXACET Randomized Clinical Trial. Jama 2019, 321, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Mamoun, N.F.; Lin, P.; Zimmerman, N.M.; Mascha, E.J.; Mick, S.L.; Insler, S.R.; Sessler, D.I.; Duncan, A.E. Intravenous acetaminophen analgesia after cardiac surgery: A randomized, blinded, controlled superiority trial. The Journal of thoracic and cardiovascular surgery 2016, 152, 881–889.e881. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, I.; Poeran, J.; Zubizarreta, N.; Babby, J.; Serban, S.; Goldberg, A.T.; Greenstein, A.J.; Memtsoudis, S.G.; Mazumdar, M.; Leibowitz, A.B. Impact of Intravenous Acetaminophen on Perioperative Opioid Utilization and Outcomes in Open Colectomies: A Claims Database Analysis. Anesthesiology 2018, 129, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Fabritius, M.L.; Geisler, A.; Petersen, P.L.; Wetterslev, J.; Mathiesen, O.; Dahl, J.B. Gabapentin in procedure-specific postoperative pain management - preplanned subgroup analyses from a systematic review with meta-analyses and trial sequential analyses. BMC anesthesiology 2017, 17, 85. [Google Scholar] [CrossRef]
- Menda, F.; Köner, O.; Sayın, M.; Ergenoğlu, M.; Küçükaksu, S.; Aykaç, B. Effects of single-dose gabapentin on postoperative pain and morphine consumption after cardiac surgery. Journal of cardiothoracic and vascular anesthesia 2010, 24, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Rapchuk, I.L.; O’Connell, L.; Liessmann, C.D.; Cornelissen, H.R.; Fraser, J.F. Effect of gabapentin on pain after cardiac surgery: a randomised, double-blind, placebo-controlled trial. Anaesthesia and intensive care 2010, 38, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, A.; Suojaranta-Ylinen, R.; Hammarén, E.; Kontinen, V.K.; Raivio, P.; Tarkkila, P.; Rosenberg, P.H. Pregabalin has an opioid-sparing effect in elderly patients after cardiac surgery: a randomized placebo-controlled trial. British journal of anaesthesia 2011, 106, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Jagadeesh, A.M. Efficacy of perioperative pregabalin in acute and chronic post-operative pain after off-pump coronary artery bypass surgery: a randomized, double-blind placebo controlled trial. Annals of cardiac anaesthesia 2013, 16, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Cooper, J.; Rahman, J.; Sharma, C.; Langford, R. Prolonged Perioperative Use of Pregabalin and Ketamine to Prevent Persistent Pain after Cardiac Surgery. Anesthesiology 2019, 131, 119–131. [Google Scholar] [CrossRef]
- Maitra, S.; Baidya, D.K.; Bhattacharjee, S.; Som, A. [Perioperative gabapentin and pregabalin in cardiac surgery: a systematic review and meta-analysis]. Revista brasileira de anestesiologia 2017, 67, 294–304. [Google Scholar] [CrossRef]
- Mion, G.; Villevieille, T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS neuroscience & therapeutics 2013, 19, 370–380. [Google Scholar] [CrossRef]
- Lahtinen, P.; Kokki, H.; Hakala, T.; Hynynen, M. S(+)-ketamine as an analgesic adjunct reduces opioid consumption after cardiac surgery. Anesthesia and analgesia 2004, 99, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Avidan, M.S.; Maybrier, H.R.; Abdallah, A.B.; Jacobsohn, E.; Vlisides, P.E.; Pryor, K.O.; Veselis, R.A.; Grocott, H.P.; Emmert, D.A.; Rogers, E.M.; et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet (London, England) 2017, 390, 267–275. [Google Scholar] [CrossRef]
- Cameron, M.; Tam, K.; Al Wahaibi, K.; Charghi, R.; Béïque, F. Intraoperative Ketamine for Analgesia Post-Coronary Artery Bypass Surgery: A Randomized, Controlled, Double-Blind Clinical Trial. Journal of cardiothoracic and vascular anesthesia 2020, 34, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Brinck, E.C.; Tiippana, E.; Heesen, M.; Bell, R.F.; Straube, S.; Moore, R.A.; Kontinen, V. Perioperative intravenous ketamine for acute postoperative pain in adults. The Cochrane database of systematic reviews 2018, 12, Cd012033. [Google Scholar] [CrossRef]
- Afonso, J.; Reis, F. Dexmedetomidine: current role in anesthesia and intensive care. Revista brasileira de anestesiologia 2012, 62, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Elgebaly, A.S.; Sabry, M. Sedation effects by dexmedetomidine versus propofol in decreasing duration of mechanical ventilation after open heart surgery. Annals of cardiac anaesthesia 2018, 21, 235–242. [Google Scholar] [CrossRef]
- Wu, M.; Liang, Y.; Dai, Z.; Wang, S. Perioperative dexmedetomidine reduces delirium after cardiac surgery: A meta-analysis of randomized controlled trials. Journal of clinical anesthesia 2018, 50, 33–42. [Google Scholar] [CrossRef]
- Habibi, V.; Kiabi, F.H.; Sharifi, H. The Effect of Dexmedetomidine on the Acute Pain After Cardiothoracic Surgeries: A Systematic Review. Brazilian journal of cardiovascular surgery 2018, 33, 404–417. [Google Scholar] [CrossRef]
- Priye, S.; Jagannath, S.; Singh, D.; Shivaprakash, S.; Reddy, D.P. Dexmedetomidine as an adjunct in postoperative analgesia following cardiac surgery: A randomized, double-blind study. Saudi journal of anaesthesia 2015, 9, 353–358. [Google Scholar] [CrossRef]
- Turan, A.; Duncan, A.; Leung, S.; Karimi, N.; Fang, J.; Mao, G.; Hargrave, J.; Gillinov, M.; Trombetta, C.; Ayad, S.; et al. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): a randomised placebo-controlled trial. Lancet (London, England) 2020, 396, 177–185. [Google Scholar] [CrossRef]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iranian journal of pharmaceutical research : IJPR 2011, 10, 655–683. [Google Scholar] [PubMed]
- Grosser, T.; Smyth, E.; FitzGerald, G.A.J.G.; therapeutics, G.s.t.p.b.o. Anti-inflammatory, antipyretic, and analgesic agents. pharmacotherapy of gout 2011, 12, 959–1004. [Google Scholar]
- De Oliveira, G.S., Jr.; Agarwal, D.; Benzon, H.T. Perioperative single dose ketorolac to prevent postoperative pain: a meta-analysis of randomized trials. Anesthesia and analgesia 2012, 114, 424–433. [Google Scholar] [CrossRef]
- Elia, N.; Lysakowski, C.; Tramèr, M.R. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology 2005, 103, 1296–1304. [Google Scholar] [CrossRef]
- Bainbridge, D.; Cheng, D.C.; Martin, J.E.; Novick, R. NSAID-analgesia, pain control and morbidity in cardiothoracic surgery. Canadian journal of anaesthesia = Journal canadien d’anesthesie 2006, 53, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Abou-Arab, O.; Yakoub-Agha, M.; Moussa, M.D.; Mauriat, P.; Provenchère, S.; Fellahi, J.L.; Besnier, E. Nonsteroidal Antiinflammatory Drugs Used in Cardiac Surgery: A Survey of Practices and New Insights for Future Studies. Journal of cardiothoracic and vascular anesthesia 2024, 38, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Ott, E.; Nussmeier, N.A.; Duke, P.C.; Feneck, R.O.; Alston, R.P.; Snabes, M.C.; Hubbard, R.C.; Hsu, P.H.; Saidman, L.J.; Mangano, D.T. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. The Journal of thoracic and cardiovascular surgery 2003, 125, 1481–1492. [Google Scholar] [CrossRef]
- Nussmeier, N.A.; Whelton, A.A.; Brown, M.T.; Langford, R.M.; Hoeft, A.; Parlow, J.L.; Boyce, S.W.; Verburg, K.M. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. The New England journal of medicine 2005, 352, 1081–1091. [Google Scholar] [CrossRef]
- Oliveri, L.; Jerzewski, K.; Kulik, A. Black box warning: is ketorolac safe for use after cardiac surgery? Journal of cardiothoracic and vascular anesthesia 2014, 28, 274–279. [Google Scholar] [CrossRef]
- Abdelbaser, I.; Abo-Zeid, M.; Hayes, S.; Taman, H.I. The Analgesic Effects of the Addition of Intravenous Ibuprofen to a Multimodal Analgesia Regimen for Pain Management After Pediatric Cardiac Surgery: A Randomized Controlled Study. Journal of cardiothoracic and vascular anesthesia 2023, 37, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Steinbrüchel, D.A.; Wanscher, M.J.; Andersen, L.W.; Navne, A.; Lilleoer, N.B.; Olsen, P.S. Multimodal analgesia versus traditional opiate based analgesia after cardiac surgery, a randomized controlled trial. Journal of cardiothoracic surgery 2014, 9, 52. [Google Scholar] [CrossRef]
- De Oliveira, G.S., Jr.; Almeida, M.D.; Benzon, H.T.; McCarthy, R.J. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 2011, 115, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Waldron, N.H.; Jones, C.A.; Gan, T.J.; Allen, T.K.; Habib, A.S. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. British journal of anaesthesia 2013, 110, 191–200. [Google Scholar] [CrossRef]
- Dvirnik, N.; Belley-Cote, E.P.; Hanif, H.; Devereaux, P.J.; Lamy, A.; Dieleman, J.M.; Vincent, J.; Whitlock, R.P. Steroids in cardiac surgery: a systematic review and meta-analysis. British journal of anaesthesia 2018, 120, 657–667. [Google Scholar] [CrossRef]
- Dieleman, J.M.; Nierich, A.P.; Rosseel, P.M.; van der Maaten, J.M.; Hofland, J.; Diephuis, J.C.; Schepp, R.M.; Boer, C.; Moons, K.G.; van Herwerden, L.A.; et al. Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. Jama 2012, 308, 1761–1767. [Google Scholar] [CrossRef]
- Murphy, G.S.; Sherwani, S.S.; Szokol, J.W.; Avram, M.J.; Greenberg, S.B.; Patel, K.M.; Wade, L.D.; Vaughn, J.; Gray, J. Small-dose dexamethasone improves quality of recovery scores after elective cardiac surgery: a randomized, double-blind, placebo-controlled study. Journal of cardiothoracic and vascular anesthesia 2011, 25, 950–960. [Google Scholar] [CrossRef]
- Bi, Y.; Ye, Y.; Ma, J.; Tian, Z.; Zhang, X.; Liu, B. Effect of perioperative intravenous lidocaine for patients undergoing spine surgery: A meta-analysis and systematic review. Medicine 2020, 99, e23332. [Google Scholar] [CrossRef] [PubMed]
- Cooke, C.; Kennedy, E.D.; Foo, I.; Nimmo, S.; Speake, D.; Paterson, H.M.; Ventham, N.T. Meta-analysis of the effect of perioperative intravenous lidocaine on return of gastrointestinal function after colorectal surgery. Techniques in coloproctology 2019, 23, 15–24. [Google Scholar] [CrossRef]
- Insler, S.R.; O’Connor, M.; Samonte, A.F.; Bazaral, M.G. Lidocaine and the inhibition of postoperative pain in coronary artery bypass patients. Journal of cardiothoracic and vascular anesthesia 1995, 9, 541–546. [Google Scholar] [CrossRef]
- Boswell, M.R.; Moman, R.N.; Burtoft, M.; Gerdes, H.; Martinez, J.; Gerberi, D.J.; Wittwer, E.; Murad, M.H.; Hooten, W.M. Lidocaine for postoperative pain after cardiac surgery: a systematic review. Journal of cardiothoracic surgery 2021, 16, 157. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, X.; Li, J.; Xiao, F.; Liu, X.; Meng, M. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesthesia and analgesia 2002, 95, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Klinger, R.Y.; Cooter, M.; Bisanar, T.; Terrando, N.; Berger, M.; Podgoreanu, M.V.; Stafford-Smith, M.; Newman, M.F.; Mathew, J.P. Intravenous Lidocaine Does Not Improve Neurologic Outcomes after Cardiac Surgery: A Randomized Controlled Trial. Anesthesiology 2019, 130, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Mittnacht, A.J.C.; Shariat, A.; Weiner, M.M.; Malhotra, A.; Miller, M.A.; Mahajan, A.; Bhatt, H.V. Regional Techniques for Cardiac and Cardiac-Related Procedures. Journal of cardiothoracic and vascular anesthesia 2019, 33, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Nooli, N.P.; Goldhammer, J.E.; Linganna, R.E.; Herman, M.; Kalagara, H. Fascial Plane Blocks as Regional Analgesia Techniques for Cardiac Surgeries: a Technical Description and Evidence Update. Current Anesthesiology Reports 2024, 14, 63–74. [Google Scholar] [CrossRef]
- Smith, L.M.; Barrington, M.J. Ultrasound-guided blocks for cardiovascular surgery: which block for which patient? Current opinion in anaesthesiology 2020, 33, 64–70. [Google Scholar] [CrossRef] [PubMed]
- El-Boghdadly, K.; Wolmarans, M.; Stengel, A.D.; Albrecht, E.; Chin, K.J.; Elsharkawy, H.; Kopp, S.; Mariano, E.R.; Xu, J.L.; Adhikary, S.; et al. Standardizing nomenclature in regional anesthesia: an ASRA-ESRA Delphi consensus study of abdominal wall, paraspinal, and chest wall blocks. Regional anesthesia and pain medicine 2021, 46, 571–580. [Google Scholar] [CrossRef]
- Douglas, R.N.; Kattil, P.; Lachman, N.; Johnson, R.L.; Niesen, A.D.; Martin, D.P.; Ritter, M.J. Superficial versus deep parasternal intercostal plane blocks: cadaveric evaluation of injectate spread. British journal of anaesthesia 2024, 132, 1153–1159. [Google Scholar] [CrossRef]
- Hamilton, C.; Sabouri, A.S. Regional Anesthesia and Perioperative Acute Pain Management in Pediatric and Adult Congenital Heart Surgical Patients. In Congenital Heart Disease in Pediatric and Adult Patients: Anesthetic and Perioperative Management; Dabbagh, A., Hernandez Conte, A., Lubin, L.N., Eds.; Springer International Publishing: Cham, 2023; pp. 853–888. [Google Scholar]
- Dost, B.; De Cassai, A.; Balzani, E.; Tulgar, S.; Ahiskalioglu, A. Effects of ultrasound-guided regional anesthesia in cardiac surgery: a systematic review and network meta-analysis. BMC anesthesiology 2022, 22, 409. [Google Scholar] [CrossRef]
- Schmedt, J.; Oostvogels, L.; Meyer-Frießem, C.H.; Weibel, S.; Schnabel, A. Peripheral Regional Anesthetic Techniques in Cardiac Surgery: A Systematic Review and Meta-Analysis. Journal of cardiothoracic and vascular anesthesia 2024, 38, 403–416. [Google Scholar] [CrossRef]
- Balan, C.; Bubenek-Turconi, S.I.; Tomescu, D.R.; Valeanu, L. Ultrasound-Guided Regional Anesthesia-Current Strategies for Enhanced Recovery after Cardiac Surgery. Medicina (Kaunas, Lithuania) 2021, 57. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).