Preprint
Communication
Pd EnCat™ 30 Recycling in Suzuki Cross-Coupling Reactions
Altmetrics
Downloads
74
Views
22
Comments
0
A peer-reviewed article of this preprint also exists.
supplementary.pdf (232.16KB )
This version is not peer-reviewed
Submitted:
30 July 2024
Posted:
02 August 2024
You are already at the latest version
Alerts
Abstract
Pd EnCat™ 30 is a palladium catalyst broadly used in several hydrogenation and cross-coupling reactions. It is known for its numerous beneficial features, which include high-yielding performance, easy recovery, and reusability. However, the available data regarding its recyclability in Suzuki coupling reactions are limited to a few reaction cycles and, therefore, fail to explore its full potential. Our work focuses on investigating the extent of Pd EnCat™ 30 reusability in Suzuki cross-coupling reactions by measuring its performance according to isolated yields of product. Our findings demonstrate that Pd EnCat™ 30 can be reused over a minimum of 30 reaction cycles, which is advantageous in terms of cost reduction and more sustainable chemical production.
Keywords:
Subject: Chemistry and Materials Science - Organic Chemistry
1. Introduction
Cross-coupling reactions are among the most studied processes within synthetic organic chemistry. Their importance stems from the fact that they allow the formation of new carbon-carbon bonds between two fragments, by use of a metal catalyst and a base [1].
The broad range of products synthesizable via cross-coupling reactions make the latter fundamental in several academic and industrial fields, such as pharmaceuticals, natural products, and agrochemicals production [2].
Suzuki-Miyaura cross-coupling reactions, also called Suzuki reactions, were invented by Akira Suzuki in 1979, when the first stereospecific coupling of alkenylboranes and alkynylhalides by use of a homogeneous catalyst, namely tetrakis(triphenylphosphine)palladium, was reported [3].
Palladium was and still is the most used metal catalyst of choice in Suzuki coupling processes, due to its higher performance in comparison to more abundant earth metals, such as copper, nickel, and iron, for example [2,4].
However, homogeneous palladium catalysts typically display high sensitivity towards moisture, oxygen, and high temperature, which strictly limits their use to reactions in inert atmosphere, deoxygenated solvents, and ultimately impedes their reusability. Moreover, their use implies high catalytic loading and requires multi-step purification techniques, which cause a significant drop in product yield [5,6,7,8].
Consequently, numerous efforts were made to develop novel heterogeneous palladium catalysts that could overcome these limitations and could make Suzuki reactions more efficient and environmentally friendly [2,6,7].
A common downside of the most used heterogeneous palladium catalysts resides in metal leaching [8]. This phenomenon involves the release of metal ions into the reaction mixture, which leads to both prompt decrease in catalytic activity, and metal contamination of the crude products. Therefore, recyclability of most heterogeneous palladium catalysts is limited, and removal of the metal particles requires additional purification steps, which has a major impact on chemical production costs [6,8].
In the search of recoverable and reusable palladium catalysts, one of the most successful achievements involved the stabilization of the metal nanoparticles by microencapsulation in polymer beads, which gave rise to the EnCat™ catalysts family [9].
Pd EnCat™ 30 is one of its members, where palladium acetate is microencapsulated in a polyurea matrix. Easy catalyst recovery, high thermal/bench-stability, and reusability are only a few of the several beneficial features that Pd EnCat™ 30 displays, which make this catalyst highly more efficient and sustainable than its predecessors [9,10].
Nevertheless, only a few reported studies focus on its recyclability properties in Suzuki reactions, and often encompass a limited number of reaction cycles, an increase in reaction time, and single measurements exclusively [11,12,13]. To bridge these gaps in the literature, we investigated Pd EnCat™ 30 reusability by use of a conventional high-yielding Suzuki reaction to produce a substituted biaryl adduct in constant reaction conditions.
2. Results
Reagents and reaction conditions were selected after evaluating similar procedures reported in the literature [14,15,16]. The chosen synthetic method stood out for the considerable reduction in reaction time, due to the use of microwave irradiation, and its high yielding outcome [17]. This procedure was employed to prepare 18 different adducts, which consisted of mono-/polysubstituted biphenyls, phenylnaphthalene, and a phenylthiophene representative all shown in the Supporting Information. While this procedure was effective in delivering all the desired products, the obtained isolated yields fluctuated between 64% and 95%.
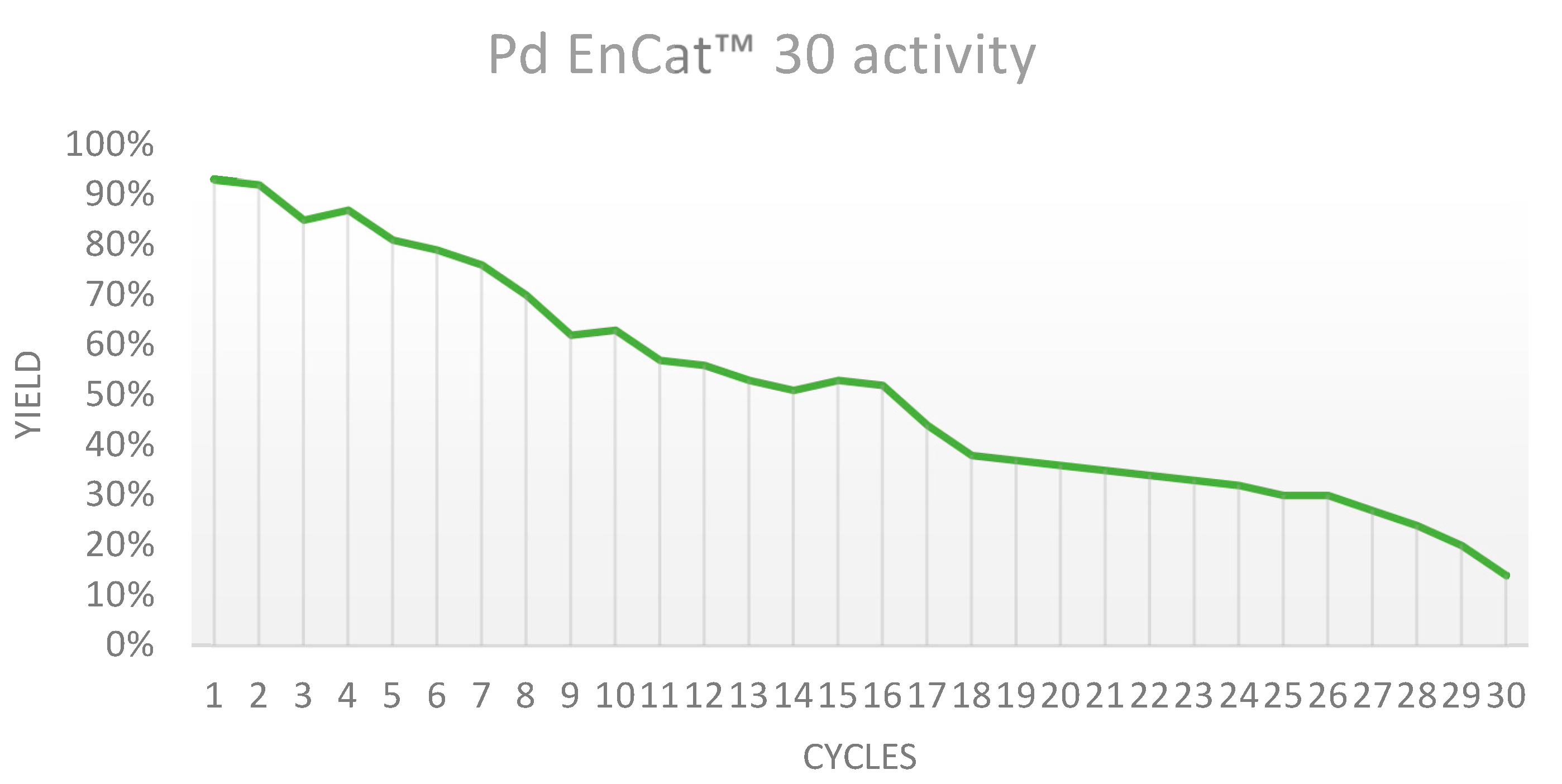
As a result, the synthesis of 2-methoxy-4'-nitrobiphenyl from 4-bromonitrobenzene and 2-methoxyphenyl boronic acid was chosen due to the high yield achieved (93%) in only 20 minutes, and the prompt isolation of the product by flash column chromatography. (Scheme 1)
After each reaction cycle, the catalyst was recovered by simple filtration of the crude upon reaching 50 °C, acetone washing, and air-drying for approximately 10 minutes.
Pd EnCat™ 30 recycling was initially explored by 10-fold repetition of the reaction described in Scheme 1 in triplicate, which led to a 30 % drop in yields from 93 % to 62 %, as shown in Figure 1.
The obtained yields were consistent between the 3 repetitions for each of the 10 cycles with low standard deviations of the yield ranging from 0.22 (first cycle) to 2.78 (sixth cycle). Mean values of isolated yields and standard deviations for all cycles can be found in the Supporting Information.
Furthermore, Pd EnCat™ 30 was recycled 30 times to address its full reusability potential, and these results are shown in Figure 2.
The catalytic activity of Pd EnCat™ 30 dropped consistently over the first 18 runs, with exception of small increases at the 4th, 14th, and 15th experiments. Surprisingly, between the 19th and the 26th runs, the drop in yields slowed down to a steady -1 % measured at each repetition.
Eventually, the catalytic depletion accelerated over the last 4 runs by dropping quickly from 30% to 14%, which indicated that further recycling of Pd EnCat™ 30 was no longer beneficial if compared to the amounts of reagents and solvents used and was subsequently stopped.
3. Discussion
The reaction was also performed for 30 minutes but no increase in yield was observed. Moreover, different concentrations of boronic acid were employed, which demonstrated that 1.1 equivalents led to the highest product yields. With equimolar amounts, for example, the yield dropped to 43 %. Considering the high rate of conversion with the parameters shown in Scheme 1, no further optimization seemed necessary.
Alternatively, the product can also be isolated by use of crystallization and recrystallization techniques, which allow the recovery of the residual starting organohalide and boronic acid. After concentration of the crude product and solubilization in a minimum volume of acetone, an excess of diethyl ether is slowly added to the vessel under vigorous stirring, which causes tetrabutylammonium acetate/bromide and boron-containing species to precipitate out of solution. Upon filtration, the filtrate is concentrated. By use of an excess of n-pentane, unreacted 2-methoxyboronic acid is crystallized upon heating the vessel, letting it slowly cool down to room temperature, and by isolating the acid crystals via filtration. Unreacted 4-bromonitrobenzene can be recovered by 4-fold recrystallization in a minimum amount of EtOH:H2O (9:1) solvent system.
Finally, the pure product is obtained by collecting and concentrating the ethanolic filtrates under reduced pressure.
4. Materials and Methods
Experiments were performed by use of CEM Discover® 2.0 microwave synthesizer (30 PSI, 250 W), after 1 min. prestirring. Flash column chromatography was performed with a Grace Reveleris® Prep Flash Chromatography System and 55 mL/min. solvent flow rate. Product separation was achieved by use of Claricep™ CS-series screw-on irregular 80 g columns. Pd EnCat™ 30 was removed by use of a glass fritted filter with 40-60 μm pore size.
NMR analyses of the product were conducted in CDCl3 using a Bruker AVIII-600 MHz NMR spectrometer equipped with a CPP-TCI cryogenically cooled probe. 1H and 13C spectra were calibrated using the residual TMS signal (0.0 ppm) and CDCl3 signal (77.16 ppm), respectively, and processed with TopSpin 4.1.4. TLC analyses were performed on ALUGRAM SIL G/UV254 TLC plates with 0.2 mm silica gel and pentane:ethyl acetate (4:1) solvent system. The plates were visualized by use of a UV-lamp at 254 nm wavelength. Pd EnCat™ 30 (0.4 mmol/g Pd loading), Celite® 545, 4-bromonitrobenzene (99%) and 2-methoxyphenyl boronic acid (95%) were purchased from Merck and used without further purification. Tetrabutylammonium acetate (98%) was purchased from BLD Pharmatech Ltd. Solvents were HPLC grade and purchased from VWR Chemicals. Purification of the product was achieved by use of pentane:ethyl acetate as eluent (gradient: 0 B% for 7 min., 0 – 2 B% over 10 min., 2 B% 5 min.)
Synthesis of 2-methoxy-4'-nitrobiphenyl
In a 35 mL microwave vial, 4-bromobenzene (2 mmol, 1 eq., 408.1 mg), 2-methoxyphenyl boronic acid (2.2 mmol, 1.1 eq., 352 mg), tetrabutylammonium acetate (6 mmol, 3 eq., 1.846 g), and Pd EnCat™ 30 (0.2 mmol, 0.1 eq., 500 mg) were added to 15 mL of EtOH 96% aqueous solution. The mixture was irradiated in a microwave apparatus at 120 °C for 20 minutes. Upon cooling to 50 °C, the crude product was filtered, diluted with acetone, adsorbed on 9 g of celite 545, and concentrated under reduced pressure. 2-Methoxy-4'-nitrobiphenyl was purified by flash column chromatography as bright yellow crystals (424.9 mg, 1.85 mmol, 93%).
1H NMR (600 MHz, CDCl3) δ 8.23 (d, J = 8.8 Hz, 2H), 7.68 (d, J = 8.8 Hz, 2H), 7.39 (m, J = 4.4 Hz, 1H), 7.31 (q, J = 3.0 Hz, 1H), 7.06 (t, J = 7.5 Hz, 1H), 7.01 (d, J = 8.3 Hz, 1H), 3.82 (s, 3H).
13C NMR (151 MHz, CDCl3) δ 156.47, 146.68, 145.54, 130.72, 130.40, 130.28, 128.28, 123.26, 121.16, 111.51, 55.62.
5. Conclusions
Our investigation assessed the recyclability potential of Pd EnCat™ 30. To the best of our knowledge, our work is the first study reporting recyclability data over numerous experiments and demonstrates that Pd EnCat™ 30 can be successfully reused over 30 reaction cycles. Starting from 93 %, the isolated product yield drops to 70 % at the 8th cycle and 51 % at the 14th, to slowly reach 30 % at the 26th and quickly drops to 14 % at the 30th. Catalyst recycling plays a crucial role in advancing towards greener chemical production and can have a major impact on costs reduction. At the same time, the reuse of precious palladium minimizes the necessity for catalyst disposal and diminishes the demand for rare earth metals mining [2,18].
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, L.D’A. and C.S.; methodology, L.D’A.; software, L.D’A.; validation, L.D’A.; formal analysis, L.D’A. and C.S.; investigation, L.D’A.; resources, L.D’A.; writing—original draft preparation, L.D’A.; writing—review and editing, L.D’A. and C.S.; visualization, L.D’A.; project administration, L.D’A. and C.S.; funding acquisition, C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by VILLUM FONDEN, grant number 50405. The APC was funded by VILLUM FONDEN, grant number 50405.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors have no acknowledgements to report.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ghosh, I.; Shlapakov, N.; Karl, T.A.; Düker, J.; Nikitin, M.; Burykina, J.V.; Ananikov, V.P.; König, B. General cross-coupling reactions with adaptive dynamic homogeneous catalysis. Nature 2023, 619, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Horbaczewskyj, C.S.; Fairlamb, I.J. Pd-catalyzed cross-couplings: On the importance of the catalyst quantity descriptors, mol% and ppm. Organic Process Research & Development 2022, 26, 2240–2269. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Letters 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Lipshutz, B.; Gallou, F.; Luescher, M. The impact of earth-abundant metals as a replacement for Pd in cross coupling reactions. ChemRxiv 2024. [Google Scholar] [CrossRef]
- McAfee, S.M.; McCahill, J.S.; Macaulay, C.M.; Hendsbee, A.D.; Welch, G.C. Utility of a heterogeneous palladium catalyst for the synthesis of a molecular semiconductor via Stille, Suzuki, and direct heteroarylation cross-coupling reactions. RSC Advances 2015, 5, 26097–26106. [Google Scholar] [CrossRef]
- Mpungose, P.P.; Vundla, Z.P.; Maguire, G.E.; Friedrich, H.B. The current status of heterogeneous palladium catalysed Heck and Suzuki cross-coupling reactions. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- King, A.K.; Brar, A.; Li, G.; Findlater, M. Homogeneous and recyclable palladium catalysts: Application in Suzuki–Miyaura cross-coupling reactions. Organometallics 2023, 42, 2353–2358. [Google Scholar] [CrossRef]
- Mukai, S.; Yamada, Y. Catalyst recycling in the Suzuki coupling reaction: Toward a greener synthesis in the pharmaceutical industry. Knowledge 2022, 3, 1–17. [Google Scholar] [CrossRef]
- Pears, D.A.; Treacher, K.E.; Nisar, M. , REAXA Ltd. Microencapsulated catalyst-ligand system 2016. U.S. Patent 9,399,211, 4 August 2015. [Google Scholar]
- Ley, S.V.; Ramarao, C.; Gordon, R.S.; Holmes, A.B.; Morrison, A.J.; McConvey, I.F.; Shirley, I.M.; Smith, S.C.; Smith, M.D. Polyurea-encapsulated palladium (II) acetate: A robust and recyclable catalyst for use in conventional and supercritical media. Chemical communications 2002, 10, pp–1134. [Google Scholar] [CrossRef]
- da Silva, J.F.M.; Perez, A.F.Y.; de Almeida, N.P. An efficient and new protocol for phosphine-free Suzuki coupling reaction using palladium-encapsulated and air-stable MIDA boronates in an aqueous medium. RSC advances 2014, 4, 28148–28155. [Google Scholar] [CrossRef]
- Baxendale, I.R.; Pitts, M.R. Microwave flow chemistry: The next evolutionary step in synthetic chemistry? Chimica Oggi 2006, 24. [Google Scholar] [CrossRef]
- Lee, C.K.; Holmes, A.B.; Ley, S.V. , McConvey, I.F.; Al-Duri, B.; Leeke, G.A.; Santos, R.C.; Seville, J.P. Efficient batch and continuous flow Suzuki cross-coupling reactions under mild conditions, catalysed by polyurea-encapsulated palladium (II) acetate and tetra-n-butylammonium salts. Chemical communications 2005, 16, pp–2175. [Google Scholar] [CrossRef]
- Zhu, L.; Duquette, J.; Zhang, M. An improved preparation of arylboronates: Application in one-pot Suzuki biaryl synthesis. The Journal of organic chemistry 2003, 68, 3729–3732. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.; Xin, B. Synthesis of biaryls and polyaryls by ligand-free Suzuki reaction in aqueous phase. The Journal of organic chemistry 2006, 71, 3994–3997. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Cross-coupling reactions of organoboranes: An easy way to construct C-C bonds (Nobel Lecture). Angewandte Chemie International Edition 2011, 30, 6722–6737. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Gowdahalli, K.; Krzeminski, J.; Amin, S. Microwave-assisted Suzuki cross-coupling reaction, a key step in the synthesis of polycyclic aromatic hydrocarbons and their metabolites. The Journal of organic chemistry 2007, 72, 8987–8989. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, S.; Braddock, D.C.; Wilton-Ely, J.D. Strategies for sustainable palladium catalysis. Coordination Chemistry Reviews 2021, 442, p–213925. [Google Scholar] [CrossRef]
Scheme 1.
Reaction scheme of the Suzuki cross-coupling reaction used for investigating Pd EnCat™ 30 recyclability. TBAA: tetrabutylammonium acetate.
Scheme 1.
Reaction scheme of the Suzuki cross-coupling reaction used for investigating Pd EnCat™ 30 recyclability. TBAA: tetrabutylammonium acetate.
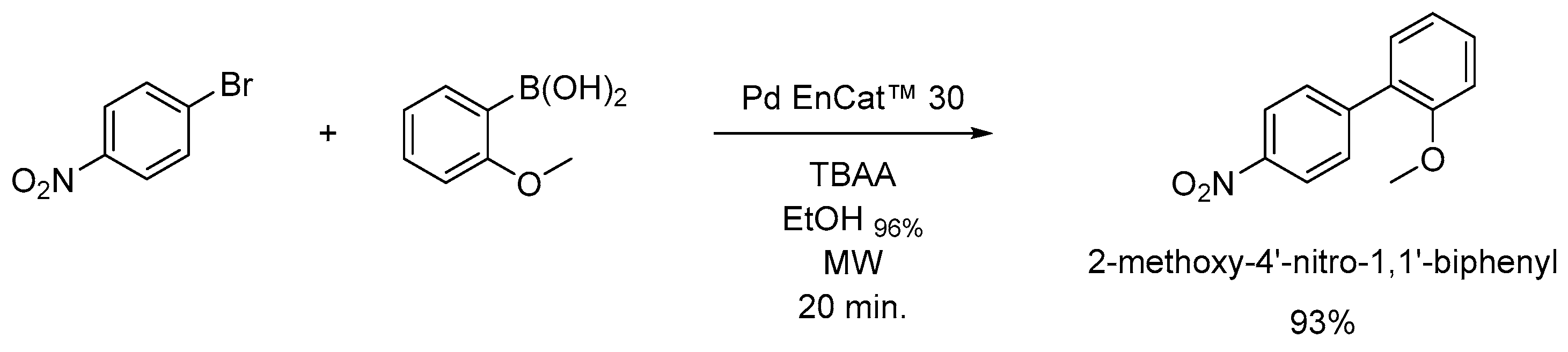
Figure 1.
Isolated yields of 2-methoxy-4'-nitrobiphenyl measured across 10 experiments conducted in triplicate.
Figure 1.
Isolated yields of 2-methoxy-4'-nitrobiphenyl measured across 10 experiments conducted in triplicate.
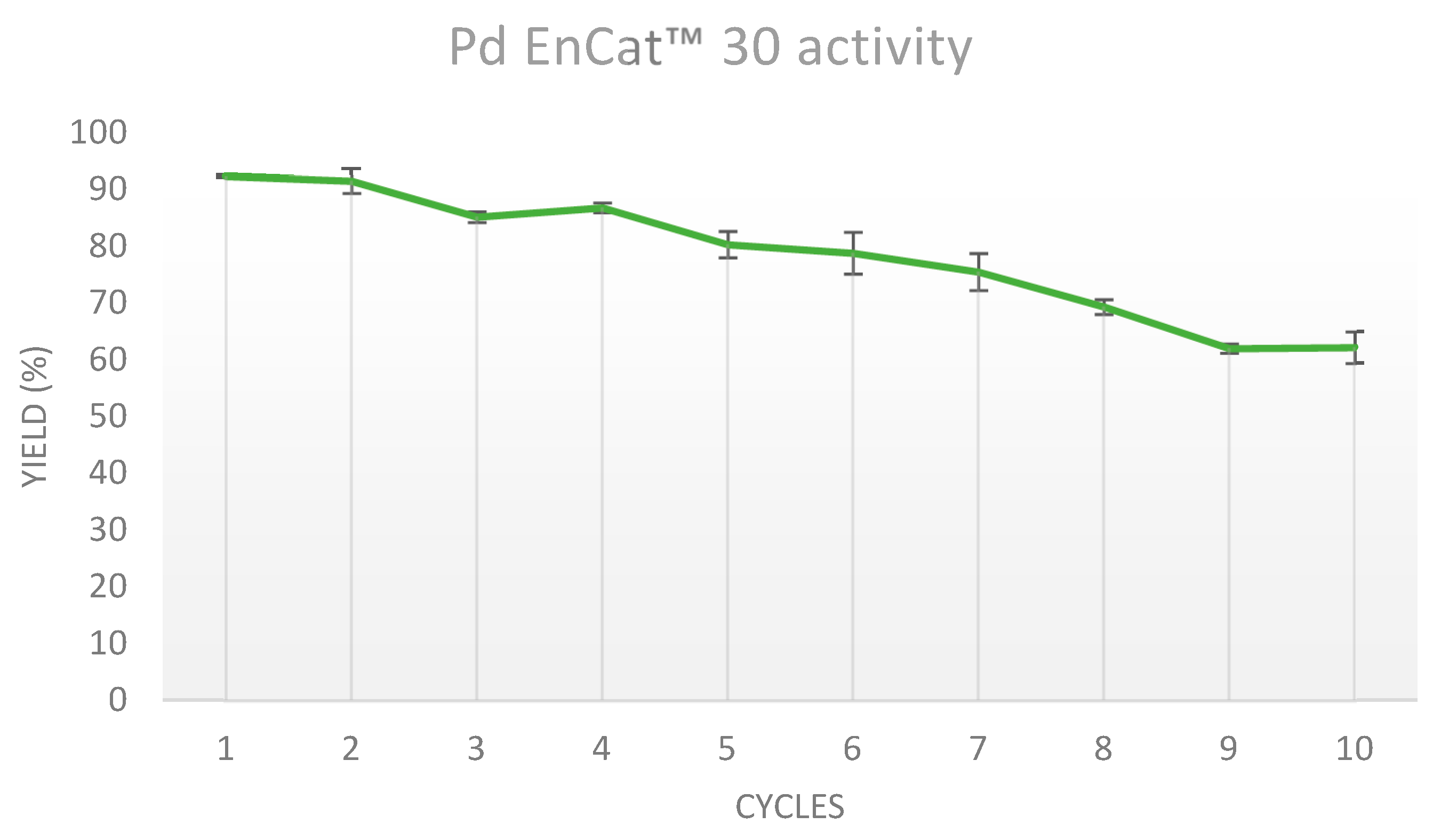
Figure 2.
Isolated yields of 2-methoxy-4'-nitrobiphenyl measured across 30 experiments.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated





