Submitted:
31 July 2024
Posted:
31 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Localization and Samples Analysis
2.1.1. Butter Production
2.2. Samples Analysis
2.2.1. Fatty Acid Profile in Milk and Butter
2.2.2 Fatty Acid Profile in Pasture and Supplement
2.2.3. Butter Hardness
2.2.4. Differentials Scanning Calorimeter (DSC)
2.2.5. Color
2.3. Statistical Analysis
3. Results
3.2. Butter production from two farms (GRZ and C)
3.2.1. Fatty acid profile in Butter
3.2.2. Techno-Functional Characteristics of Butter from Two Farms (GRZ and C)
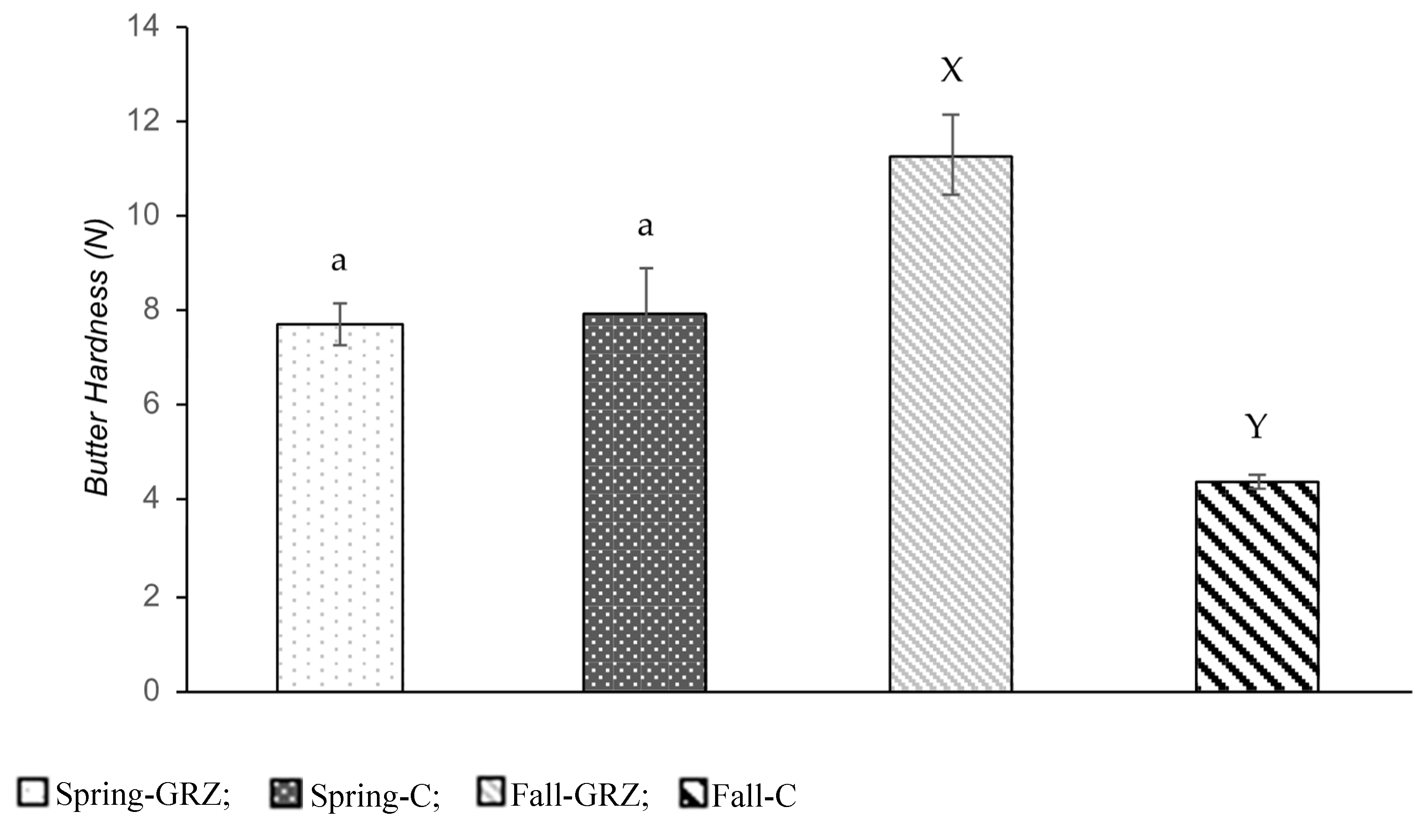
- Butter Hardness
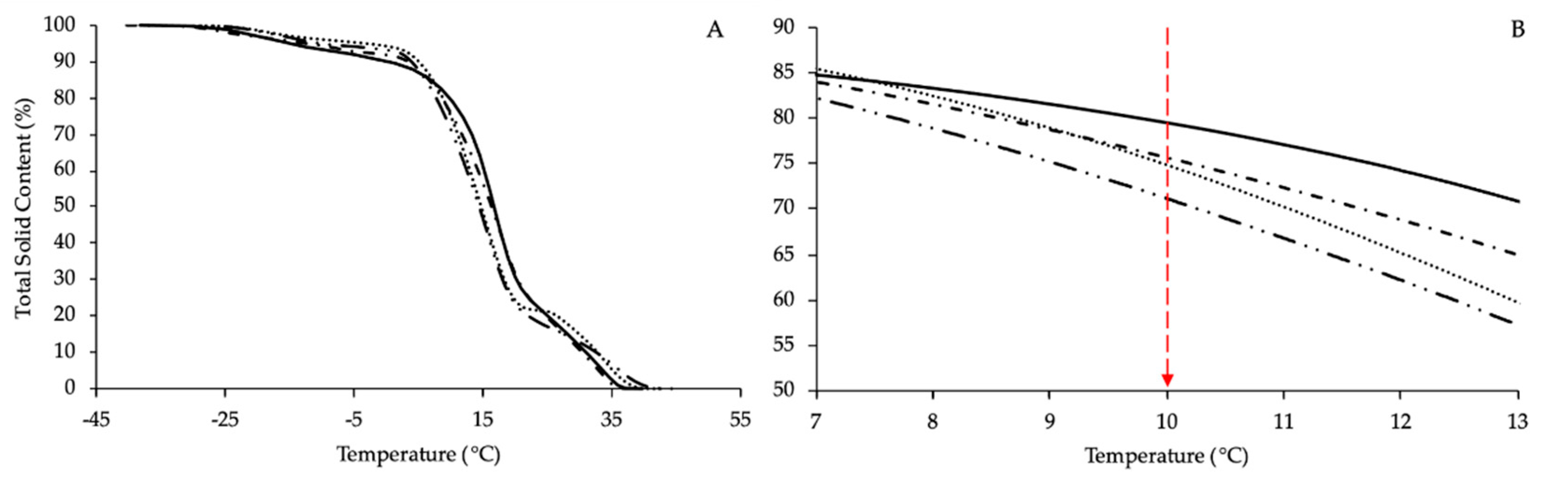
- Differential Scanning Calorimetry (DSC)
- Color
4. Discussion
4.1. Fatty Acid Profile in Milk
4.2. Fatty Acid Profile in Butter
4.3. Physical Properties of Butter
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harvatine, K. J.; Boisclair, Y. R.; Bauman, D. E. Recent Advances in the Regulation of Milk Fat Synthesis. Animal, 2009, 3(1), 40–54. [Google Scholar] [CrossRef] [PubMed]
- Delmotte, C.; Rondia, P.; Dehareng, F.; Laloux, J.; Famerée, J. An Oleaginous Supplement to Improve the Nutritional Quality of Goat’s Milk and Cheese (Whole or Extruded Linseed, Rapeseed Cake). In Nutritional and foraging ecology of sheep and goats. ; Papachristou T.G, Parissi Z.M., Ben Salem H., Morand-Fehr P., Eds.; CIHEAM / FAO / NAGREF: Zaragosa, 2009; pp 445–451.
- Lock, A. Update on Dietary and Management Effects on Milk Fat; Michigan, 2010; pp 15–26.
- Li, X. Z.; Yan, C. G.; Lee, H. G.; Choi, C. W.; Song, M. K. Influence of Dietary Plant Oils on Mammary Lipogenic Enzymes and the Conjugated Linoleic Acid Content of Plasma and Milk Fat of Lactating Goats. Anim Feed Sci Technol, 2012, 174 (1–2), 26–35. [CrossRef]
- Zullo, A.; De Francesco, V.; Scaccianoce, G.; Hassan, C.; Panarese, A.; Piglionica, D.; Panella, C.; Morini, S.; Ierardi, E. Quadruple Therapy with Lactoferrin for Helicobacter Pylori Eradication: A Randomised, Multicentre Study. Digestive and Liver Disease, 2005, 37(7), 496–500. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, M.; Park, J. H.; Ki, K. S.; Lim, D. H.; Kim, S. B.; Park, S. M.; Jeong, H. Y.; Park, B. Y.; Kim, T. Il. The Effect of Lactation Number, Stage, Length, and Milking Frequency on Milk Yield in Korean Holstein Dairy Cows Using Automatic Milking System. Asian-Australas J Anim Sci, 2017, 30(8), 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, G. F.; Gagliostro, G. A.; Bargo, F.; Delahoy, J. E.; Muller, L. D. Effects of Fat Supplementation on Milk Production and Composition by Dairy Cows on Pasture: A Review. Livest Prod Sci, 2004, 86(1-3), 1–18. [Google Scholar] [CrossRef]
- Markiewicz-Kęszycka, M.; Czyżak-Runowska, G.; Lipińska, P.; Wójtowski, J. Fatty Acid Profile of Milk - A Review. Bulletin of the Veterinary Institute in Pulawy, 2013, 57(2), 135–139. [Google Scholar] [CrossRef]
- Sanhueza C, J.; Nieto K, S.; Valenzuela B., A. Ácido Linoléico Conjugado: Un Ácido Graso Con Isomería TRANS Potencialmente Beneficioso. Revista chilena de nutrición, 2002, 29(2). [Google Scholar] [CrossRef]
- Tudisco, R.; Chiofalo, B.; Addi, L.; Lo Presti, V.; Rao, R.; Calabro’, S.; Musco, N.; Grossi, M.; Cutrignelli, M. I.; Mastellone, V.; et al. Effect of Hydrogenated Palm Oil Dietary Supplementation on Milk Yield and Composition, Fatty Acids Profile and Stearoyl-CoA Desaturase Expression in Goat Milk. Small Ruminant Research, 2015, 132, 72–78. [Google Scholar] [CrossRef]
- Siurana, A.; Calsamiglia, S. A Metaanalysis of Feeding Strategies to Increase the Content of Conjugated Linoleic Acid (CLA) in Dairy Cattle Milk and the Impact on Daily Human Consumption. Anim Feed Sci Technol, 2016, 217, 13–26. [Google Scholar] [CrossRef]
- Field, C. J.; Blewett, H. H.; Proctor, S.; Vine, D. Human Health Benefits of Vaccenic Acid. Applied Physiology, Nutrition, and Metabolism, 2009, 34(5), 979–991. [Google Scholar] [CrossRef] [PubMed]
- Benito, P.; Caballero, J.; Moreno, J.; Gutiérrez-Alcántara, C.; Muñoz, C.; Rojo, G.; Garcia, S.; Soriguer, F. C. Effects of Milk Enriched with ω-3 Fatty Acid, Oleic Acid and Folic Acid in Patients with Metabolic Syndrome. Clinical Nutrition, 2006, 25(4), 581–587. [Google Scholar] [CrossRef] [PubMed]
- Turpeinen, A. M.; Mutanen, M.; Aro, A.; Salminen, I.; Basu, S.; Palmquist, D. L.; Griinari, J. M. Bioconversion of Vaccenic Acid to Conjugated Linoleic Acid in Humans. Am J Clin Nutr, 2002, 76(3), 504–510. [Google Scholar] [CrossRef] [PubMed]
- Fievez, V.; Colman, E.; Castro-Montoya, J. M.; Stefanov, I.; Vlaeminck, B. Milk Odd- and Branched-Chain Fatty Acids as Biomarkers of Rumen Function-An Update. Anim Feed Sci Technol, 2012, 172 (1–2), 51–65. [CrossRef]
- Cai, Q.; Huang, H.; Qian, D.; Chen, K.; Luo, J.; Tian, Y.; Lin, T.; Lin, T. 13-Methyltetradecanoic Acid Exhibits Anti-Tumor Activity on T-Cell Lymphomas In Vitro and In Vivo by Down-Regulating p-AKT and Activating Caspase-3. PLoS One, 2013, 8(6), e65308. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.; West, J.; Koulman, A. A Review of Odd-Chain Fatty Acid Metabolism and the Role of Pentadecanoic Acid (C15:0) and Heptadecanoic Acid (C17:0) in Health and Disease. Molecules, 2015, 20(2), 2425–2444. [Google Scholar] [CrossRef] [PubMed]
- Pfeuffer, M.; Jaudszus, A. Pentadecanoic and Heptadecanoic Acids: Multifaceted Odd-Chain Fatty Acids. Advances in Nutrition. American Society for Nutrition July 1, 2016, pp 730–734. [CrossRef]
- Abdoul-Aziz, S. K. A.; Zhang, Y.; Wang, J. Milk Odd and Branched Chain Fatty Acids in Dairy Cows: A Review on Dietary Factors and Its Consequences on Human Health. Animals. MDPI November 1, 2021. [CrossRef]
- Vlaeminck, B.; Fievez, V.; Cabrita, A. R. J.; Fonseca, A. J. M.; Dewhurst, R. J. Factors Affecting Odd- and Branched-Chain Fatty Acids in Milk: A Review. Animal Feed Science and Technology. December 15, 2006, pp 389–417. [CrossRef]
- Ulbricht, T. L. V.; Southgate, D. A. T. Coronary Heart Disease: Seven Dietary Factors. The Lancet, 1991, 338(8773), 985–992. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Hadzhinikolova, L. Agricultural Academy. Bulgarian Journal of Agricultural Science, 2015, 21, 180–185. [Google Scholar]
- Simopoulos, A. P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Experimental Biology and Medicine. June 2008, pp 674–688. [CrossRef]
- Tóth, T.; Mwau, P. J.; Bázár, G.; Andrássy-Baka, G.; Hingyi, H.; Csavajda, É.; Varga, L. Effect of Feed Supplementation Based on Extruded Linseed Meal and Fish Oil on Composition and Sensory Properties of Raw Milk and Ultra-High Temperature Treated Milk. Int Dairy J, 2019, 99, 104552. [Google Scholar] [CrossRef]
- O’Callaghan, T. F.; Hennessy, D.; McAuliffe, S.; Kilcawley, K. N.; O’Donovan, M.; Dillon, P.; Ross, R. P.; Stanton, C. Effect of Pasture versus Indoor Feeding Systems on Raw Milk Composition and Quality over an Entire Lactation. J Dairy Sci, 2016, 99(12), 9424–9440. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.; Schnepps, A.; Pichler, A.; Meixner, O. Cow Milk versus Plant-Based Milk Substitutes: A Comparison of Product Image and Motivational Structure of Consumption. Sustainability, 2019, 11(18), 5046. [Google Scholar] [CrossRef]
- Stampa, E.; Schipmann-Schwarze, C.; Hamm, U. Consumer Perceptions, Preferences, and Behavior Regarding Pasture-Raised Livestock Products: A Review. Food Qual Prefer, 2020, 82, 103872. [Google Scholar] [CrossRef]
- Verkerk, G. Pasture-Based Dairying: Challenges and Rewards for New Zealand Producers. Theriogenology, 2003, 59(2), 553–561. [Google Scholar] [CrossRef] [PubMed]
- Fariña, S. R.; Chilibroste, P. Opportunities and Challenges for the Growth of Milk Production from Pasture: The Case of Farm Systems in Uruguay. Agric Syst, 2019, 176. [Google Scholar] [CrossRef]
- Bargo, F.; Delahoy, J. E.; Schroeder, G. F.; Baumgard, L. H.; Muller, L. D. Supplementing Total Mixed Rations with Pasture Increase the Content of Conjugated Linoleic Acid in Milk. Anim Feed Sci Technol, 2006, 131(3-4), 226–240. [Google Scholar] [CrossRef]
- Weiss, W. P.; Hogan, J. S.; Smith, K. L. Changes in Vitamin C Concentrations in Plasma and Milk from Dairy Cows After an Intramammary Infusion of Escherichia Coli. J Dairy Sci, 2004, 87(1), 32–37. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.; Buccioni, A.; Petacchi, F.; Serra, A.; Banni, S.; Antongiovanni, M.; Secchiari, P. Effect of Forage/Concentrate Ratio and Soybean Oil Supplementation on Milk Yield, and Composition from Sarda Ewes. Animal Research, 2006, 55(4), 273–285. [Google Scholar] [CrossRef]
- Collomb, M.; Bisig, W.; Bütikofer, U.; Sieber, R.; Bregy, M.; Etter, L. Fatty Acid Composition of Mountain Milk from Switzerland: Comparison of Organic and Integrated Farming Systems. Int Dairy J, 2008, 18(10-11), 976–982. [Google Scholar] [CrossRef]
- Gagliostro. GA. Nutritional Control of Conjugated Linoleic Acid (CLA) Content in Milk and Natural Functional Foods. 1. Effects on Human Health. Rev. Arg. Prod. Anim., 2004, 24, 3–4. [Google Scholar]
- Esposito, G.; Masucci, F.; Napolitano, F.; Braghieri, A.; Romano, R.; Manzo, N.; Di Francia, A. Fatty Acid and Sensory Profiles of Caciocavallo Cheese as Affected by Management System. J Dairy Sci, 2014, 97(4), 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Alothman, M.; Hogan, S. A.; Hennessy, D.; Dillon, P.; Kilcawley, K. N.; O’Donovan, M.; Tobin, J.; Fenelon, M. A.; O’Callaghan, T. F. The “Grass-Fed” Milk Story: Understanding the Impact of Pasture Feeding on the Composition and Quality of Bovine Milk. Foods. MDPI Multidisciplinary Digital Publishing Institute 2019. [CrossRef] [PubMed]
- Grille, L.; Adrien, M. L.; Méndez, M. N.; Chilibroste, P.; Olazabal, L.; Damián, J. P. Milk Fatty Acid Profile of Holstein Cows When Changed from a Mixed System to a Confinement System or Mixed System with Overnight Grazing. Int J Food Sci, 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Dauber, C.; Carreras, T.; Britos, A.; Carro, S.; Cajarville, C.; Gámbaro, A.; Jorcin, S.; López, T.; Vieitez, I. Elaboration of Goat Cheese with Increased Content of Conjugated Linoleic Acid and Transvaccenic Acid: Fat, Sensory and Textural Profile. Small Ruminant Research, 2021, 199(6), 106379. [Google Scholar] [CrossRef]
- Coakley, M.; Barrett, E.; Murphy, J. J.; Ross, R. P.; Devery, R.; Stanton, C. Cheese Manufacture with Milk with Elevated Conjugated Linoleic Acid Levels Caused by Dietary Manipulation. J Dairy Sci, 2007, 90, 2919–2927. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Devi, P.; Sharma, N.; Yallappa, I.; Somagond, M.; Somagond, Y. M. Impact of Thermal Stress on Milk Production, Composition and Fatty Acid Profile in Dairy Cows: A Review. ~ 1278 ~ Journal of Entomology and Zoology Studies, 2020, 8(5), 1278–1283. [Google Scholar]
- Vibart, R. E.; Fellner, V.; Burns, J. C.; Huntington, G. B.; Green, J. T. Performance of Lactating Dairy Cows Fed Varying Levels of Total Mixed Ration and Pasture. Journal of Dairy Research, 2008, 75(4), 471–480. [Google Scholar] [CrossRef] [PubMed]
- Maniaci, G.; Di Grigoli, A.; Bonanno, A.; Giosuè, C.; Ilardi, V.; Alabiso, M. Fatty Acids as Biomarkers of the Production Season of Caciocavallo Palermitano Cheese. Animals, 2021, 11(9). [Google Scholar] [CrossRef] [PubMed]
- Tzamaloukas, O.; Neofytou, M. C.; Simitzis, P. E.; Miltiadou, D. Effect of Farming System (Organic vs. Conventional) and Season on Composition and Fatty Acid Profile of Bovine, Caprine and Ovine Milk and Retail Halloumi Cheese Produced in Cyprus. Foods, 2021, 10(5), 1016. [Google Scholar] [CrossRef] [PubMed]
- Paszczyk, B.; Polak-śliwińska, M.; Zielak-Steciwko, A. E. Chemical Composition, Fatty Acid Profile, and Lipid Quality Indices in Commercial Ripening of Cow Cheeses from Different Seasons. Animals, 2022, 12(2). [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B. M.; Vessby, B.; Uusitupa, M.; Berglund, L.; Pedersen, E.; Riccardi, G.; Rivellese, A. A.; Tapsell, L.; Hermansen, K. Effects of Dietary Saturated, Monounsaturated, and N−3 Fatty Acids on Blood Pressure in Healthy Subjects. Am J Clin Nutr, 2006, 83(2), 221–226. [Google Scholar] [CrossRef] [PubMed]
- Armas, L. A. G.; Frye, C. P.; Heaney, R. P. Effect of Cow’s Milk on Human Health. In Beverage Impacts on Health and Nutrition; Springer International Publishing: Cham, 2016; pp. 131–150. [Google Scholar] [CrossRef]
- Lamarche, B.; Givens, D. I.; Soedamah-Muthu, S.; Krauss, R. M.; Jakobsen, M. U.; Bischoff-Ferrari, H. A.; Pan, A.; Després, J.-P. Does Milk Consumption Contribute to Cardiometabolic Health and Overall Diet Quality? Canadian Journal of Cardiology, 2016, 32(8), 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cortés, P.; Juárez, M.; de la Fuente, M. A. Milk Fatty Acids and Potential Health Benefits: An Updated Vision. Trends Food Sci Technol, 2018, 81, (August). 1–9. [Google Scholar] [CrossRef]
- Girard, M.; Dohme-Meier, F.; Wechsler, D.; Goy, D.; Kreuzer, M.; Bee, G. Ability of 3 Tanniferous Forage Legumes to Modify Quality of Milk and Gruyère-Type Cheese. J Dairy Sci, 2016, 99(1), 205–220. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, T. F.; Mannion, D. T.; Hennessy, D.; McAuliffe, S.; O’Sullivan, M. G.; Leeuwendaal, N.; Beresford, T. P.; Dillon, P.; Kilcawley, K. N.; Sheehan, J. J.; et al. Effect of Pasture versus Indoor Feeding Systems on Quality Characteristics, Nutritional Composition, and Sensory and Volatile Properties of Full-Fat Cheddar Cheese. J Dairy Sci, 2017, 100(8), 6053–6073. [Google Scholar] [CrossRef]
- Glover, K. E.; Budge, S.; Rose, M.; Rupasinghe, H. P. V.; MacLaren, L.; Green-Johnson, J.; Fredeen, A. H. Effect of Feeding Fresh Forage and Marine Algae on the Fatty Acid Composition and Oxidation of Milk and Butter. J Dairy Sci, 2012, 95(6), 2797–2809. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Dairy Cattle, Natl. Acad Sci, Ed.; Washington, DC. 2001.
- Méndez, M. N.; Grille, L.; Mendina, G. R.; Robinson, P. H.; Adrien, M. de L.; Meikle, A.; Chilibroste, P. Performance of Autumn and Spring Calving Holstein Dairy Cows with Different Levels of Environmental Exposure and Feeding Strategies. Animals, 2023, 13(7), 1211. [Google Scholar] [CrossRef] [PubMed]
- BSI. Methods for Chemical Analysis of Butter (BS769). In British Standards Method 769 ; London, UK, 1961.
- AOAC. 2001. Fat in Milk Rose-Gottlieb Method, Method No. 905.02. In Official methods of analysis of AOAC International; W. Horowitz, Ed.; AOAC International.: Gaithersburg, 2001. [Google Scholar]
- IUPAC. Preparation of Fatty Acid Methyl Ester. Standard Method 2.301. In Standard methods for analysis of oils, fats and derivatives; Standard methods for analysis of oils, fats and derivatives, 1987.
- AOCS. 1990. Official Methods and Recommended Practices of the American Oil Chemists’s Society. Champaign: American Oil Chemists’ Society.; 1990.
- Hara, A.; Radin, N. S. Lipid Extraction of Tissues with a Low-Toxicity Solvent. Anal Biochem, 1978, 90(1), 420–426. [Google Scholar] [CrossRef]
- IDF International Dairy Federation. Butter – Determination of Firmness-IDF Standard 187:2005; ISO16305. In International Dairy Federation ; Brusselas, 2005.
- Grompone, M.A; Irigaray, B.; Rodríguez, D.; Maidana, S.; Sammán, N. Composition and Thermal Behavior of Oils from Native Seeds and Fruits of Argentina and Uruguay. J Food Sci Eng, 2015, 5(2). [Google Scholar] [CrossRef]
- Jensen, R. G. The Composition of Bovine Milk Lipids: January 1995 to December 2000. J Dairy Sci, 2002, 85(2), 295–350. [Google Scholar] [CrossRef] [PubMed]
- Moscovici Joubran, A.; Pierce, K. M.; Garvey, N.; Shalloo, L.; O’Callaghan, T. F. Invited Review: A 2020 Perspective on Pasture-Based Dairy Systems and Products. Journal of Dairy Science. Elsevier Inc. July 1, 2021, pp 7364–7382. [CrossRef]
- Barca, J.; Carriquiry, M.; Olazabal, L.; Fajardo, M.; Chilibroste, P.; Meikle, A. Milk Fatty Acid Profile from Cows Fed with Mixed Rations and Different Access Time to Pastureland during Early Lactation. J Anim Physiol Anim Nutr (Berl), 2018, 102(3), 620–629. [Google Scholar] [CrossRef] [PubMed]
- Grille, L.; Escobar, D.; Méndez, M. N.; Adrien, M. de L.; Olazabal, L.; Rodríguez, V.; Pelaggio, R.; Chilibroste, P.; Meikle, A.; Damián, J. P. Different Conditions during Confinement in Pasture-Based Systems and Feeding Systems Affect the Fatty Acid Profile in the Milk and Cheese of Holstein Dairy Cows. Animals, 2023, 13(8). [Google Scholar] [CrossRef]
- Ferlay, A.; Bernard, L.; Meynadier, A.; Malpuech-Brugère, C. Production of Trans and Conjugated Fatty Acids in Dairy Ruminants and Their Putative Effects on Human Health: A Review. Biochimie, 2017, 141, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Glasser, F.; Ferlay, A.; Bernard, L.; Rouel, J.; Doreau, M. Diet, Rumen Biohydrogenation and Nutritional Quality of Cow and Goat Milk Fat. European Journal of Lipid Science and Technology, 2007, 109(8), 828–855. [Google Scholar] [CrossRef]
- Ran-Ressler, R. R.; Bae, S.; Lawrence, P.; Wang, D. H.; Thomas Brenna, J. Branched-Chain Fatty Acid Content of Foods and Estimated Intake in the USA. British Journal of Nutrition, 2014, 112(4), 565–572. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Khan, N. A.; Liu, X.; Jiang, X.; Sun, F.; Zhang, S.; Sun, Y.; Zhang, Y.; Li, X. Profiles of Odd- and Branched-Chain Fatty Acids and Their Correlations With Rumen Fermentation Parameters, Microbial Protein Synthesis, and Bacterial Populations Based on Pure Carbohydrate Incubation in Vitro. Front Nutr, 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Elgersma, A. Grazing Increases the Unsaturated Fatty Acid Concentration of Milk from Grass-Fed Cows: A Review of the Contributing Factors, Challenges and Future Perspectives. European Journal of Lipid Science and Technology. Wiley-VCH Verlag September 1, 2015, pp 1345–1369. [CrossRef]
- Chilliard, Y.; Ferlay, A.; Loor, J.; Rouel, J.; Martin, B. Trans and Conjugated Fatty Acids in Milk from Cows and Goats Consuming Pasture or Receiving Vegetable Oils or Seeds. Ital J Anim Sci, 2002, 1(4), 243–254. [Google Scholar] [CrossRef]
- Nudda, A.; Correddu, F.; Cesarani, A.; Pulina, G.; Battacone, G. Functional Odd- and Branched-Chain Fatty Acid in Sheep and Goat Milk and Cheeses. Dairy, 2021, 2(1), 79–89. [Google Scholar] [CrossRef]
- León JM; Pabón ML; Carulla JE. Relación Entre Las CaracterÌsticas de La Pastura y El Contenido de Ácido Linoleico Conjugado (ALC) En La Leche. . Rev Colomb Cienc Pecu , 2011, 24, 63–73.
- Pariza, M. W.; Park, Y.; Cook, M. E. The Biologically Active Isomers of Conjugated Linoleic Acid. Prog Lipid Res, 2001, 40(4), 283–298. [Google Scholar] [CrossRef] [PubMed]
- Bauman, D. E.; Mather, I. H.; Wall, R. J.; Lock, A. L. Major Advances Associated with the Biosynthesis of Milk. J Dairy Sci, 2006, 89(4), 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, J.; Bialek, M.; Adamska, A.; Zbikowska, A. Differentiation of Geographical Origin of Cream Products in Poland According to Their Fatty Acid Profile. Food Chem, 2015, 178, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Lobos-Ortega, I.; Pizarro-Aránguiz, N.; Urrutia, N. L.; Silva-Lemus, M.; Pavez-Andrades, P.; Subiabre-Riveros, I.; Torres-Püschel, D. Determination of Nutritional Health Indexes of Fresh Bovine Milk Using near Infrared Spectroscopy. Grasas y Aceites, 2022, 73 (2), e458. [CrossRef]
- Pastorini, M.; Pomiés, N.; Repetto, J. L.; Mendoza, A.; Cajarville, C. Productive Performance and Digestive Response of Dairy Cows Fed Different Diets Combining a Total Mixed Ration and Fresh Forage. J Dairy Sci, 2019, 102(5), 4118–4130. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cortés, P.; Domenech, F. R.; Rueda, M. C.; de la Fuente, M. Á.; Schiavone, A.; Marín, A. L. M. Odd-and Branched-Chain Fatty Acids in Lamb Meat as Potential Indicators of Fattening Diet Characteristics. Foods, 2021, 10(1). [Google Scholar] [CrossRef] [PubMed]
- Nutrient Requirements of Dairy Cattle. National Academies of Science, Engineering and Medicine , 8th ed.; The National Academies Press, Ed.; 2021.
- Roy, A.; Ferlay, A.; Shingfield, K. J.; Chilliard, Y. Examination of the Persistency of Milk Fatty Acid Composition Responses to Plant Oils in Cows given Different Basal Diets, with Particular Emphasis on Trans -C 18:1 Fatty Acids and Isomers of Conjugated Linoleic Acid. Animal Science, 2006, 82(4), 479–492. [Google Scholar] [CrossRef]
- Lawless, F.; Murphy, J.; Harrington, D.; Devery, R.; Stanton, C. Elevation of Conjugated Cis-9, Trans-11-Octadecadienoic Acid in Bovine Milk Because of Dietary Supplementation. J Dairy Sci, 1998, 81, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J. J.; Connolly, J. F.; McNeill, G. P. Effects on Cow Performance and Milk Fat Composition of Feeding Full Fat Soyabeans and Rapeseeds to Dairy Cows at Pasture. Livest Prod Sci, 1995, 44(1), 13–25. [Google Scholar] [CrossRef]
- Khiaosa-ard, R.; Klevenhusen, F.; Soliva, C. R.; Kreuzer, M.; Leiber, F. Transfer of Linoleic and Linolenic Acid from Feed to Milk in Cows Fed Isoenergetic Diets Differing in Proportion and Origin of Concentrates and Roughages. Journal of Dairy Research, 2010, 77(3), 331–336. [Google Scholar] [CrossRef] [PubMed]
- Techeira, N.; Keel, K.; Garay, A.; Harte, F.; Mendoza, A.; Cartaya, A.; Fariña, S.; López-Pedemonte, T. Milk Fatty Acid Profile from Grass Feeding Strategies on 2 Holstein Genotypes: Implications for Health and Technological Properties. JDS Communications, 2023, 4(3), 169–174. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, A. G.; Ghazani, S. M. Perspective: A Commentary on Elevated Palmitic Acid Levels in Canadian Butter and Their Relationship to Butter Hardness. J Dairy Sci, 2021, 104(9), 9380–9382. [Google Scholar] [CrossRef] [PubMed]
- Walstra P; Geurts T.J.; Noomen A.; Jellema A.; Van Boekel M.A.J.S. Ciencia de La Leche y Tecnología de Los Productos Lácteos; Acribia, E. S. A., Ed.; 2001.
- Chamberlain, M. B.; Veltri, B. C.; Taylor, S. J.; Pareas, J. W.; Jimenez-Flores, R.; Juchem, S. O.; Getachew, G.; DePeters, E. J. Feeding Lactating Holstein Cows a Lipid Source High in Palmitic Acid Changes the Fatty Acid Composition and Thermal Properties of Lipids in Milk and Butter. Prof Anim Sci, 2016, 32(5), 672–680. [Google Scholar] [CrossRef]
- Tomaszewska-Gras, J. Melting and Crystallization DSC Profiles of Milk Fat Depending on Selected Factors. J Therm Anal Calorim, 2013, 113(1), 199–208. [Google Scholar] [CrossRef]
- El-Hadad, S. S.; Tikhomirova, N.; Zahran, H.; Abd El-Aziz, M.; Sayed, R. The Effect of Minor Components of Wheat Germ Oil on Thermal Behavior, Crystallization Characteristics, and Oxidative Stability of Butter Oil. Egypt J Chem, 2024, 0 (0), 0–0. [CrossRef]
- Nozière, P.; Graulet, B.; Lucas, A.; Martin, B.; Grolier, P.; Doreau, M. Carotenoids for Ruminants: From Forages to Dairy Products. Anim Feed Sci Technol, 2006, 131 (3–4), 418–450. [CrossRef]

| Spring | Fall | ||||||||||||||
| HP | LP | HP | LP | ||||||||||||
| Past | Suppl | Total diet** | Past* | Suppl | Total diet** | Past | Suppl | Total diet** | Past* | Suppl | Total diet** | ||||
| NDF | 47.3± 4.8 |
30.3± 7.3 |
43.1± 3.5 |
46.3± 1.7 |
36.2± 1.8 |
37.2± 2.6 |
36.7± 6.9 |
43.9± 7.6 |
40.1± 5.7 |
36.9± 2.4 |
44.7± 5.8 |
44.5± 5.9 |
|||
| ADF | 26.2± 2.3 |
14.3± 6.1 |
23.4± 1.5 |
26.3± 1.5 |
19.8± 2.5 |
20.6± 2.2 |
20.1± 5.0 |
24.1± 6.5 |
22.0± 3.6 |
20.9± 1.7 |
26.5± 7.2 |
26.5± 7.2 |
|||
| Lipids | 3.7± 0.9 |
4.8± 1.6 |
4.1± 1.1 |
2.6± 0.06 |
3.9± 0.3 |
4.2± 0.5 |
6.6± 4.1 |
3.9± 1.2 |
5.4± 2.5 |
6.9± 0.1 |
5.8± 1.5 |
5.8± 1.5 |
|||
| Spring | Fall | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acids (g/100 g fat) | HP | LP | HP | LP | ||||||||||||
| Past | Suppl | Past | Suppl | Past | Suppl | Past | Suppl | |||||||||
| 10:0 | 0.36 ± 0.20 | 0.11 ± 0.03 | 0.82 ± 0.44 | 0.41 ± 0.22 | 0.06 ± 0.05 | 0.03 ± 0.05 | 0.20 ± 0 | 0.29 ± 0.18 | ||||||||
| 12:0 | 0.74 ± 0.21 | 0.14 ± 0.05 | 1.3 ± 0.55 | 0.61 ± 0.27 | 0.36 ± 0.08 | 0.34 ± 0.27 | 0.30 ± 0 | 0.45 ± 0.42 | ||||||||
| 14:0 | 2.09 ± 0.79 | 0.50 ± 0.15 | 4.62 ± 1.53 | 1.69 ± 0.70 | 0.95 ± 0.31 | 0.56 ± 0.23 | 0.63 ± 0.05 | 1.29 ± 1.08 | ||||||||
| 16:0 | 18.25 ± 2.91 | 15.23 ± 2.19 | 24.82 ± 2.36 | 16.49 ± 1.73 | 12.6 ± 1.5 | 15.3 ± 1.4 | 12.4 ± 0.1 | 14 ± 3.1 | ||||||||
| 16:1 cis | 0.37 ± 0.19 | 0.17 ± 0.06 | 0.60 ± 0.27 | 0.33 ± 0.08 | 0.03 ± 0.08 | 0.29 ± 0.21 | 0.18 ± 0.05 | 0.29 ± 0.37 | ||||||||
| 17:0 | 0.36 ± 0.27 | 0.11 ± 0.03 | 0.44 ± 0.05 | 0.13 ± 0.12 | 0 ± 0 | 0 ± 0 | 0.05 ± 0.10 | 0.03 ± 0.05 | ||||||||
| 18:0 | 3.1 ± 0.97 | 2.06 ± 0.46 | 5.24 ± 0.33 | 3.49 ± 0.42 | 2.21 ± 0.32 | 2.49 ± 0.48 | 2.08 ± 0.05 | 4.35 ± 1.60 | ||||||||
| 18:1 trans-vaccenic | 0.25 ± 0.13 | 0.05 ± 0.08 | 0.52 ± 0.16 | 0.12 ± 0.13 | 0.11 ± 0.07 | 0.13 ± 0.13 | 0.35 ± 0.10 | 0.08 ± 0.09 | ||||||||
| 18:1 cis | 7.21 ± 4.19 | 28.81 ± 6.01 | 11.44 ± 1.15 | 22.8 ± 2.76 | 5.00 ± 1.57 | 27.68 ± 5.71 | 3.90 ± 0.60 | 22.66 ± 2.50 | ||||||||
| 18:2 trans | 1.03 ± 1.00 | 0 ± 0 | 0.58 ± 0.11 | 0.07 ± 0.10 | 0.20 ± 0.19 | 0.13 ± 0.10 | 0.15 ± 0.10 | 0.22 ± 0.20 | ||||||||
| 18:2 cis | 13.41 ± 5.05 | 46.43 ± 4.86 | 10.18 ± 0.38 | 41.37 ± 4.35 | 13.77 ±3.04 | 41.81 ± 5.82 | 14.18 ± 1.35 | 37.90 ± 10.02 | ||||||||
| 20:0 | 0.79 ± 0.39 | 0.43 ± 0.05 | 0.58 ± 0.11 | 0.49 ± 0.14 | 0.38 ± 0.08 | 0.62 ± 0.14 | 0.40 ± 0 | 0.53 ± 0.21 | ||||||||
| 20:1 | 0.04 ± 0.08 | 0.39 ± 0.14 | 0 ± 0 | 0.11 ± 0.09 | 0.10 ± 0.10 | 0.49 ± 0.09 | 0 ± 0 | 0.19 ± 0.18 | ||||||||
| 18:3 cis | 42.42 ± 12.93 | 3.41 ± 1.66 | 31.72 ± 11.61 | 8.36 ± 4.11 | 58.66 ± 7.61 | 6.84 ± 5.47 | 57.60 ± 2.40 | 13.36 ± 9.32 | ||||||||
| 22:0 | 0.51 ± 0.12 | 0.24 ± 0.06 | 0.54 ± 0.05 | 0.33 ± 0.14 | 0.66 ± 0.24 | 0.53 ± 0.13 | 0.55 ± 0.10 | 0.48 ± 0.16 | ||||||||
| 24:0 | 0.47 ± 0.12 | 0.3 ± 0.08 | 0.32 ± 0.16 | 0.27 ± 0.12 | 0.40 ± 0.07 | 0.55 ± 0.25 | 0.53 ± 0.05 | 0.38 ± 0.11 | ||||||||
| SFA | 27.21 ± 5.52 | 19.17 ± 2.64 | 40.22 ± 7.28 | 24.73 ± 3.65 | 18.77 ± 3.60 | 18.36 ± 1.57 | 19.25 ± 2.10 | 18.76 ± 2.01 | ||||||||
| MUFAs cis | 7.63 ± 4.31 | 29.37 ± 5.89 | 12.4 ± 1.37 | 23.25 ± 2.76 | 19.56 ± 8.65 | 22.14 ± 15.97 | 6.68 ± 7.15 | 8.47 ± 6.18 | ||||||||
| PUFAs cis | 55.83 ± 9.16 | 49.84 ± 5.56 | 41.9 ± 11.23 | 49.73 ± 3.95 | 56.80 ± 8.91 | 56.47 ± 16.42 | 66.80 ± 6.80 | 67.38 ± 6.93 | ||||||||
| Trans | 1.28 ± 1.08 | 0.05 ± 0.08 | 1.1 ± 0.27 | 0.19 ± 0.19 | 0.26 ± 0.30 | 0.15 ± 0.18 | 0.38 ± 0.25 | 0.38 ± 0.22 | ||||||||
| Total fat | 3.73 ± 1.00 | 4.86 ± 1.63 | 2.62 ± 0.06 | 3.95 ± 0.30 | 5.96 ± 1.17 | 9.03 ± 5.52 | 5.78 ± 2.00 | 4.41 ± 0.68 | ||||||||
| Spring | Fall | ||||||
|---|---|---|---|---|---|---|---|
| Fatty acids (g/100 g fat) |
GRZ | C | GRZ | C | |||
| 16:0 | 18.5 ± 2.5 | 17.0 ± 2.1 | 15.4 ± 1.4 | 12.6 ± 1.6 | |||
| 18:0 | 3.7 ± 0.7 | 3.5 ± 0.3 | 2.6 ± 0.4 | 4.0 ± 0.6 | |||
| 18:1 n9 cis | 11.3 ± 5.1 | 18.2 ± 1.9 | 14.0 ± 3.3 | 26.2 ± 1.5 | |||
| 18:2 n6 cis | 21.7 ± 4.9 | 34.6 ± 2.3 | 25.5 ± 4.4 | 47 ± 5.2 | |||
| 18:3 n3 cis | 29.2 ± 7.2 | 15.1 ± 2.8 | 32.2 ± 6.5 | 4.2 ± 5.8 | |||
| Fatty Acid (g/100g fat) | Spring | Fall | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | P | SEM | p-value | T | P | SEM | p-value | |||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | T | P | T*P | 1 | 2 | 3 | 4 | 5 | T | P | T*P | |||||||||||
| 4:0 | HP | 3.03 | 2.92 | 3.54 | 3.43 | 2.51 | 2.76 | 0.09 | ns | 0.022 | ns | HP | 3.16 | 3.20 | 3.31 | 2.94 | 3.49 | 2.87 | 0.13 | ns | ns | ns | ||||
| LP | 2.86 | 2.58 | 3.18 | 2.61 | 2.55 | 3.37 | LP | 3.30 | 3.81 | 3.13 | 3.26 | 3.28 | 3.04 | |||||||||||||
| 6:0 | HP | 1.82 | 1.93a | 2.12a | 1.91a | 1.49a | 1.63b | 0.05 | ns | 0.050 | 0.045 | HP | 2.00 | 1.86 | 2.15 | 1.92 | 2.25 | 1.82 | 0.10 | ns | ns | ns | ||||
| LP | 1.86 | 1.73a | 2.04a | 1.64a | 1.70a | 2.21a | LP | 2.13 | 2.48 | 2.04 | 2.13 | 2.09 | 1.90 | |||||||||||||
| 8:0 | HP | 1.06 | 1.18 | 1.23 | 1.01 | 0.87 | 1.00 | 0.05 | ns | ns | ns | HP | 1.23 | 1.07 | 1.35 | 1.24 | 1.40 | 1.09 | 0.08 | ns | ns | ns | ||||
| LP | 1.13 | 1.10 | 1.19 | 1.00 | 1.04 | 1.34 | LP | 1.30 | 1.47 | 1.30 | 1.28 | 1.31 | 1.12 | |||||||||||||
| 10:0 | HP | 2.37 | 2.77 | 2.78 | 2.15 | 2.04 | 2.12 | 0.16 | ns | ns | ns | HP | 2.78 | 2.75 | 2.93 | 2.73 | 3.10 | 2.39 | 0.29 | ns | 0.015 | ns | ||||
| LP | 2.64 | 2.64 | 2.71 | 2.41 | 2.52 | 2.89 | LP | 2.62 | 2.65 | 2.54 | 2.69 | 2.83 | 2.39 | |||||||||||||
| 11:0 | HP | 0.30 | 0.35 | 0.34 | 0.26 | 0.26 | 0.28 | 0.02 | ns | ns | ns | HP | 0.36 | 0.31 | 0.42 | 0.36 | 0.41 | 0.29 | 0.03 | ns | ns | ns | ||||
| LP | 0.32 | 0.30 | 0.33 | 0.29 | 0.32 | 0.39 | LP | 0.31 | 0.27 | 0.35 | 0.33 | 0.32 | 0.27 | |||||||||||||
| 12:0 | HP | 2.78 | 3.26 | 3.20 | 2.47 | 2.50 | 2.49 | 0.10 | ns | ns | ns | HP | 3.22 | 2.93 | 3.49 | 3.23 | 3.63 | 2.81 | 0.42 | ns | ns | ns | ||||
| LP | 3.08 | 3.07 | 3.19 | 2.91 | 2.89 | 3.32 | LP | 2.95 | 2.91 | 2.82 | 3.03 | 3.23 | 2.75 | |||||||||||||
| 14:0 | HP | 10.61 | 11.56 | 11.50 | 10.08 | 10.20 | 9.72 | 0.42 | ns | ns | ns | HP | 10.98 | 11.01 | 11.16 | 11.03 | 11.50 | 10.21 | 0.85 | ns | ns | ns | ||||
| LP | 10.86 | 10.84 | 10.88 | 10.75 | 10.66 | 11.17 | LP | 10.15 | 10.61 | 9.89 | 10.21 | 10.54 | 9.51 | |||||||||||||
| 15:0 | HP | 1.37 | 1.42 | 1.36 | 1.35 | 1.34 | 1.38 | 0.14 | ns | ns | ns | HP | 1.20 | 1.17 | 1.26 | 1.21 | 1.23 | 1.11 | 0.06 | ns | ns | ns | ||||
| LP | 1.21 | 1.23 | 1.19 | 1.19 | 1.13 | 1.29 | LP | 1.04 | 1.03 | 1.04 | 1.08 | 1.01 | 1.02 | |||||||||||||
| 16:0 | HP | 29.46 | 29.75 | 29.69 | 29.14 | 30.00 | 28.71 | 1.00 | ns | ns | ns | HP | 30.75 | 31.40 | 30.59 | 30.43 | 31.07 | 30.26 | 1.16 | ns | ns | ns | ||||
| LP | 29.94 | 29.30 | 29.65 | 30.29 | 29.73 | 30.71 | LP | 28.79 | 30.00 | 28.41 | 28.06 | 29.19 | 28.28 | |||||||||||||
| 17:0 | HP | 0.82 | 0.79 | 0.81 | 0.82 | 0.83 | 0.87 | 0.06 | ns | ns | ns | HP | 0.71 | 0.64 | 0.68 | 0.76 | 0.73 | 0.76 | 0.04 | ns | ns | ns | ||||
| LP | 0.69 | 0.71 | 0.69 | 0.69 | 0.65 | 0.71 | LP | 0.67 | 0.62 | 0.70 | 0.68 | 0.64 | 0.72 | |||||||||||||
| 18:0 | HP | 10.08 | 9.27 | 9.73 | 10.72 | 10.24 | 10.42 | 0.45 | ns | ns | ns | HP | 9.15 | 9.12 | 8.52 | 9.59 | 8.56 | 9.95 | 0.85 | ns | ns | ns | ||||
| LP | 10.10 | 11.03 | 10.49 | 9.92 | 10.12 | 8.92 | LP | 10.93 | 11.19 | 11.44 | 10.57 | 10.37 | 11.09 | |||||||||||||
| Total SFA | HP | 63.88 | 65.36 | 66.46 | 63.55 | 62.45 | 61.59 | 1.08 | ns | ns | ns | HP | 65.55 | 64.94 | 66.02 | 65.59 | 67.51 | 63.71 | 2.13 | ns | ns | ns | ||||
| LP | 64.91 | 64.71 | 66.01 | 63.87 | 63.49 | 66.46 | LP | 64.34 | 67.19 | 63.84 | 63.46 | 64.95 | 62.24 | |||||||||||||
| Fatty Acid (g/100g fat) | Spring | Fall | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | P | SEM | p-value | T | P | SEM | p-value | |||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | T | P | T*P | 1 | 2 | 3 | 4 | 5 | T | P | T*P | |||||||||||||
| 14:0 iso | HP | 0.17 | 0.16 | 0.15 | 0.18 | 0.17 | 0.19 | 0.01 | ns | ns | ns | HP | 0.13 | 0.13 | 0.12 | 0.14 | 0.12 | 0.12 | 0.01 | ns | ns | ns | ||||||
| LP | 0.13 | 0.14 | 0.12 | 0.13 | 0.11 | 0.13 | LP | 0.11 | 0.10 | 0.12 | 0.11 | 0.11 | 0.10 | |||||||||||||||
| 15:0 iso | HP | 0.36a | 0.36 | 0.35 | 0.36 | 0.33 | 0.37 | 0.02 | 0.029 | ns | ns | HP | 0.30a | 0.27 | 0.31 | 0.32 | 0.29 | 0.30 | 0.01 | 0.003 | ns | ns | ||||||
| LP | 0.28b | 0.30 | 0.32 | 0.25 | 0.24 | 0.27 | LP | 0.24b | 0.25 | 0.24 | 0.23 | 0.22 | 0.24 | |||||||||||||||
| 15:0 anteiso | HP | 0.69a | 0.74 | 0.71 | 0.70 | 0.64 | 0.68 | 0.03 | 0.008 | ns | ns | HP | 0.56a | 0.52 | 0.57 | 0.58 | 0.56 | 0.56 | 0.01 | 0.0004 | ns | ns | ||||||
| LP | 0.48b | 0.49 | 0.48 | 0.48 | 0.46 | 0.51 | LP | 0.43b | 0.45 | 0.43 | 0.44 | 0.43 | 0.42 | |||||||||||||||
| 16:0 iso | HP | 0.34a | 0.35 | 0.34 | 0.34 | 0.33 | 0.35 | 0.01 | 0.003 | ns | ns | HP | 0.29 | 0.26 | 0.27 | 0.31 | 0.30 | 0.30 | 0.02 | ns | ns | ns | ||||||
| LP | 0.30b | 0.33 | 0.29 | 0.30 | 0.29 | 0.31 | LP | 0.28 | 0.25 | 0.30 | 0.30 | 0.28 | 0.28 | |||||||||||||||
| 17:0 iso | HP | 0.43a | 0.37 | 0.46 | 0.45 | 0.44 | 0.44 | 0.02 | 0.005 | ns | ns | HP | 0.38 | 0.34 | 0.38 | 0.40 | 0.37 | 0.44 | 0.02 | ns | 0.003 | ns | ||||||
| LP | 0.34b | 0.36 | 0.35 | 0.34 | 0.33 | 0.33 | LP | 0.33 | 0.29 | 0.35 | 0.34 | 0.30 | 0.37 | |||||||||||||||
| 17:0 anteiso | HP | 0.53 | 0.55 | 0.53 | 0.52 | 0.50 | 0.55 | 0.03 | ns | ns | ns | HP | 0.48 | 0.43 | 0.48 | 0.50 | 0.50 | 0.48 | 0.02 | ns | ns | ns | ||||||
| LP | 0.44 | 0.46 | 0.43 | 0.44 | 0.43 | 0.47 | LP | 0.43 | 0.38 | 0.46 | 0.45 | 0.41 | 0.46 | |||||||||||||||
| 18:0 iso | HP | 0.054a | 0.057 | 0.053 | 0.052 | 0.052 | 0.057 | 0.001 | 0.025 | 0.040 | ns | HP | 0.063 | 0.059 | 0.053 | 0.060 | 0.067 | 0.077 | 0.01 | ns | ns | ns | ||||||
| LP | 0.048b | 0.051 | 0.048 | 0.046 | 0.046 | 0.050 | LP | 0.045 | 0.045 | 0.048 | 0.043 | 0.039 | 0.050 | |||||||||||||||
| Total BCFA | HP | 2.57a | 2.58 | 2.59 | 2.59 | 2.46 | 2.64 | 0.09 | 0.006 | ns | ns | HP | 2.19ª | 2.01 | 2.18 | 2.30 | 2.21 | 2.27 | 0.06 | 0.007 | 0.030 | ns | ||||||
| LP | 2.02b | 2.13 | 2.03 | 1.98 | 1.91 | 2.06 | LP | 1.86b | 1.77 | 1.95 | 1.92 | 1.78 | 1.91 | |||||||||||||||
| Fatty Acid (g/100g fat) | Spring | Fall | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | P | SEM | p-value | T | P | SEM | p-value | |||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | T | P | T*P | 1 | 2 | 3 | 4 | 5 | T | P | T*P | |||||||||
| 14:1 cis | HP | 0.85 | 0.99 | 0.92 | 0.79 | 0.81 | 0.76 | 0.05 | ns | ns | ns | HP | 1.01 | 1.00 | 1.17 | 0.99 | 1.04 | 0.88 | 0.11 | ns | ns | ns | ||
| LP | 0.92 | 0.89 | 0.88 | 0.92 | 0.84 | 1.05 | LP | 0.84 | 0.76 | 0.91 | 0.86 | 0.87 | 0.79 | |||||||||||
| 16:1 cis | HP | 1.29 | 1.34 | 1.27 | 1.28 | 1.35 | 1.23 | 0.12 | ns | ns | ns | HP | 1.59a | 1.58 | 1.71 | 1.53 | 1.59 | 1.54 | 0.05 | 0.022 | ns | ns | ||
| LP | 1.36 | 1.39 | 1.31 | 1.41 | 1.23 | 1.44 | LP | 1.31b | 1.17 | 1.42 | 1.33 | 1.26 | 1.39 | |||||||||||
| 18:1 elaidic | HP | 0.23 | 0.26 | 0.17 | 0.22 | 0.26 | 0.26 | 0.04 | ns | ns | ns | HP | 0.22b | 0.31 | 0.22 | 0.24 | 0.20 | 0.11 | 0.04 | 0.015 | ns | ns | ||
| LP | 0.34 | 0.32 | 0.34 | 0.39 | 0.41 | 0.25 | LP | 0.38a | 0.33 | 0.34 | 0.41 | 0.36 | 0.46 | |||||||||||
| 18:1 trans-vaccenic | HP | 4.11a | 4.14 | 3.84 | 4.22 | 4.24 | 4.10 | 0.28 | 0.012 | ns | ns | HP | 3.55 | 3.80 | 3.45 | 3.44 | 3.39 | 3.70 | 0.41 | ns | ns | ns | ||
| LP | 2.36b | 2.28 | 2.35 | 2.58 | 2.38 | 2.20 | LP | 2.40 | 1.91 | 2.41 | 2.58 | 2.34 | 2.73 | |||||||||||
| 18:1 cis | HP | 19.68 | 18.00 | 18.04 | 19.97 | 21.69 | 20.68 | 0.73 | ns | ns | ns | HP | 19.68 | 20.68 | 18.65 | 19.43 | 18.01 | 21.62 | 1.83 | ns | ns | ns | ||
| LP | 21.04 | 21.32 | 20.54 | 21.81 | 22.23 | 19.29 | LP | 21.75 | 20.77 | 22.78 | 21.27 | 21.01 | 22.94 | |||||||||||
| Total MUFA cis | HP | 21.82 | 20.34 | 20.23 | 22.04 | 23.84 | 22.67 | 0.68 | ns | ns | ns | HP | 22.28 | 23.26 | 21.52 | 21.94 | 20.65 | 24.04 | 0.73 | ns | ns | ns | ||
| LP | 23.32 | 23.61 | 22.73 | 24.14 | 24.30 | 21.79 | LP | 23.90 | 22.70 | 25.11 | 23.45 | 23.13 | 25.12 | |||||||||||
| Fatty Acid (g/100g fat) | Spring | Fall | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | P | SEM | p-value | T | P | SEM | p-value | |||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | T | P | T*P | 1 | 2 | 3 | 4 | 5 | T | P | T*P | |||||||||
| 18:2 trans | HP | 1.12a | 1.07 | 1.05 | 1.09 | 1.19 | 1.19 | 0.04 | 0.003 | ns | ns | HP | 1.27a | 1.22 | 1.21 | 1.22 | 1.24 | 1.45 | 0.04 | 0.031 | ns | ns | ||
| LP | 0.89b | 0.79 | 0.79 | 1.17 | 0.86 | 0.82 | LP | 1.12b | 1.00 | 1.13 | 1.09 | 1.04 | 1.33 | |||||||||||
| 18:2 cis | HP | 1.72 | 1.44 | 1.54 | 1.91 | 1.80 | 1.91 | 0.32 | ns | ns | ns | HP | 1.48 | 1.33 | 1.51 | 1.55 | 1.33 | 1.69 | 0.08 | <.0001 | ns | ns | ||
| LP | 2.54 | 2.42 | 2.53 | 2.55 | 2.64 | 2.58 | LP | 2.86 | 2.45 | 2.62 | 2.99 | 3.07 | 3.19 | |||||||||||
| 18:2 CLA | HP | 1.65a | 1.86 | 1.51 | 1.58 | 1.70 | 1.62 | 0.11 | 0.014 | ns | ns | HP | 1.43a | 1.53 | 1.53 | 1.47 | 1.37 | 1.29 | 0.15 | 0.033 | ns | ns | ||
| LP | 0.89b | 0.79 | 0.83 | 0.96 | 0.93 | 0.94 | LP | 0.86b | 0.66 | 0.97 | 0.90 | 0.85 | 0.90 | |||||||||||
| 18:3 n3 cis | HP | 0.89a | 0.82 | 0.89 | 0.97 | 0.87 | 0.90 | 0.08 | 0.033 | ns | ns | HP | 0.53 | 0.47 | 0.52 | 0.56 | 0.55 | 0.57 | 0.03 | ns | 0.03 | ns | ||
| LP | 0.54b | 0.36 | 0.50 | 0.63 | 0.60 | 0.63 | LP | 0.50 | 0.37 | 0.54 | 0.54 | 0.54 | 0.53 | |||||||||||
| Total PUFA cis | HP | 2.61 | 2.27 | 2.43 | 2.88 | 2.67 | 2.82 | 0.24 | ns | ns | ns | HP | 2.02b | 1.80 | 2.03 | 2.11 | 1.87 | 2.26 | 0.08 | <.0001 | ns | ns | ||
| LP | 3.09 | 2.78 | 3.03 | 3.17 | 3.24 | 3.21 | LP | 3.37a | 2.82 | 3.16 | 3.53 | 3.61 | 3.72 | |||||||||||
| Spring | Fall | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid (g/100g fat) | Butter | MilkB | Butter | MilkB | ||||||||||||||||
| GRZ | C | SEM | p-value | GRZ | C | SEM | p-value | GRZ | C | SEM | p-value | GRZ | C | SEM | p-value | |||||
| 4:0 | 3.84 | 2.61 | 0.58 | ns | 2.36 | 2.97 | 0.44 | ns | 2.75a | 2.62b | 0.02 | 0.043 | 2.74 | 2.78 | 0.22 | ns | ||||
| 6:0 | 2.33 | 1.70 | 0.25 | ns | 1.59 | 1.82 | 0.27 | ns | 1.86a | 1.59 b | 0.01 | 0.002 | 2.00 | 1.56 | 0.13 | ns | ||||
| 8:0 | 1.28 | 1.87 | 0.57 | ns | 1.04 | 1.03 | 0.13 | ns | 1.12a | 0.92 b | 0.01 | 0.003 | 1.30 | 0.91 | 0.12 | ns | ||||
| 10:0 | 2.8 | 2.78 | 0.25 | ns | 2.52 | 2.33 | 0.11 | ns | 2.71a | 1.99 b | 0.04 | 0.005 | 3.13 | 1.86 | 0.32 | ns | ||||
| 11:0 | 0.34a | 0.32b | 0.002 | 0.040 | 0.33 | 0.34 | 0.01 | ns | 0.29a | 0.23 b | 0.01 | 0.040 | 0.38 | 0.22 | 0.05 | ns | ||||
| 12:0 | 3.20a | 3.00b | 0.01 | 0.006 | 3.13 | 2.65 | 0.25 | ns | 3.27a | 2.33 b | 0.02 | 0.001 | 3.73 | 2.16 | 0.41 | ns | ||||
| 14:0 iso | 0.18a | 0.16b | 0.002 | 0.046 | 0.14 | 0.15 | 0.02 | ns | 0.11a | 0.09 b | 0.002 | 0.020 | 0.12 | 0.10 | 0.01 | ns | ||||
| 14:0 | 11.37 | 11.06 | 0.17 | ns | 11.56 | 10.14 | 0.43 | ns | 11.36a | 8.86 b | 0.05 | 0.001 | 11.83 | 7.92 | 0.73 | ns | ||||
| 15:0 iso | 0.36 | 0.40 | 0.01 | ns | 0.32 | 0.31 | 0.03 | ns | 0.25a | 0.19b | 0.004 | 0.009 | 0.28 | 0.23 | 0.01 | ns | ||||
| 15:0 anteiso | 0.76a | 0.67b | 0.01 | 0.012 | 0.66 | 0.59 | 0.04 | ns | 0.48a | 0.39b | 0.01 | 0.010 | 0.57a | 0.43b | 0.01 | 0.004 | ||||
| 14:1 | 0.93b | 1.03a | 0.01 | 0.027 | 0.96 | 0.95 | 0.14 | ns | 0.83 | 0.72 | 0.03 | ns | 0.95 | 0.66 | 0.06 | ns | ||||
| 15:0 | 1.55b | 1.62a | 0.01 | 0.025 | 1.50 | 1.60 | 0.09 | ns | 1.16a | 0.99b | 0.004 | 0.001 | 1.34 | 1.07 | 0.05 | ns | ||||
| 16:0 iso | 0.38 | 0.40 | 0.01 | ns | 0.32 | 0.32 | 0.03 | ns | 0.27 | 0.27 | 0.003 | ns | 0.30 | 0.27 | 0.01 | ns | ||||
| 16:0 | 29.79 | 31.14 | 0.45 | ns | 33.25 | 30.99 | 1.94 | ns | 34.23a | 28.29b | 0.43 | 0.010 | 32.61a | 25.81b | 0.58 | 0.014 | ||||
| 17:0 iso | 0.48 | 0.5 | 0.02 | ns | 0.43 | 0.36 | 0.03 | ns | 0.45a | 0.33b | 0.01 | 0.008 | 0.38 | 0.42 | 0.04 | ns | ||||
| 17:0 anteiso | 0.63 | 0.69 | 0.01 | ns | 0.53 | 0.56 | 0.05 | ns | 0.42a | 0.19b | 0.01 | 0.006 | 0.48 | 0.51 | 0.03 | ns | ||||
| 16:1 | 1.34b | 1.71a | 0.03 | 0.016 | 1.49 | 1.57 | 0.17 | ns | 1.47 | 1.35 | 0.02 | ns | 1.50 | 1.36 | 0.10 | ns | ||||
| 17:0 | 0.94b | 1.00a | 0.01 | 0.030 | 0.88 | 0.86 | 0.04 | ns | 0.75a | 0.69b | 0.01 | 0.022 | 0.80 | 0.82 | 0.05 | ns | ||||
| 18:0 iso | 0.1 | 0.08 | 0.03 | ns | 0.06 | 0.05 | 0.003 | ns | 0.06 | 0.06 | 0.01 | ns | 0.06 | 0.06 | 0.01 | ns | ||||
| 18:0 | 9.06 | 8.97 | 0.26 | ns | 8.30 | 9.06 | 1.27 | ns | 9.36b | 11.16a | 0.10 | 0.006 | 8.65b | 11.81a | 0.51 | 0.048 | ||||
| 18:1 elaidic | 0.22 | 0.19 | 0.03 | ns | 0.20 | 0.29 | 0.04 | ns | 0.22b | 0.41a | 0.03 | 0.035 | 0.17b | 0.50a | 0.04 | 0.035 | ||||
| 18:1 trans-vaccenic | 3.22a | 1.89b | 0.15 | 0.024 | 3.65 | 2.33 | 0.11 | 0.015 | 2.89b | 2.97a | 0.01 | 0.009 | 3.14 | 2.57 | 0.26 | ns | ||||
| 18:1 cis | 17.65b | 19.95a | 0.35 | 0.043 | 17.33 | 20.80 | 1.85 | ns | 18.10b | 25.73a | 0.22 | 0.002 | 17.72 | 27.28 | 1.83 | ns | ||||
| 18:2 trans | 0.81 | 0.90 | 0.08 | ns | 1.10 | 0.88 | 0.16 | ns | 1.01b | 1.86a | 0.05 | 0.008 | 1.19 | 1.34 | 0.08 | ns | ||||
| 18:2 cis | 2.00a | 1.77b | 0.02 | 0.010 | 1.90 | 1.53 | 0.15 | ns | 1.70b | 2.84a | 0.06 | 0.005 | 1.74b | 3.23a | 0.24 | 0.048 | ||||
| 18:2 CLA | 1.44a | 0.98b | 0.07 | 0.042 | 1.57 | 1.21 | 0.12 | ns | 0.92 | 0.91 | 0.005 | ns | 1.06 | 0.97 | 0.08 | ns | ||||
| 18:3 cis | 1.09 | 1.02 | 0.07 | ns | 1.07a | 0.96b | 0.02 | 0.050 | 0.56 | 0.55 | 0.02 | ns | 0.60 | 0.58 | 0.02 | ns | ||||
| 20:0 | 0.18 | 0.18 | 0.003 | ns | 0.18 | 0.17 | 0.03 | ns | 0.15a | 0.14b | 0.001 | 0.011 | 0.15 | 0.17 | 0.01 | ns | ||||
| Total SFA | 66.67 | 66.25 | 1.40 | ns | 66.65 | 63.95 | 2.01 | ns | 69.02a | 59.79b | 0.45 | 0.005 | 68.66a | 57.09b | 1.68 | 0.040 | ||||
| Total MUFAcis | 19.92b | 22.68a | 0.39 | 0.037 | 19.78 | 23.31 | 1.54 | ns | 20.76b | 28.17a | 0.22 | 0.002 | 20.17 | 29.30 | 1.86 | ns | ||||
| Total PUFAcis | 3.1 | 2.78 | 0.08 | ns | 2.97 | 2.50 | 0.15 | ns | 2.27b | 3.38a | 0.07 | 0.008 | 2.34 | 3.81 | 0.26 | ns | ||||
| Total BCFA | 2.87 | 2.90 | 0.03 | ns | 2.46 | 2.34 | 0.18 | ns | 2.04a | 1.51b | 0.02 | 0.002 | 2.19 | 2.01 | 0.08 | ns | ||||
| Total Trans | 4.26a | 2.98b | 0.20 | 0.046 | 4.95 | 3.51 | 0.29 | ns | 4.12b | 5.24a | 0.08 | 0.009 | 4.26 | 5.20 | 0.21 | ns | ||||
| Spreadability index (C16:0/C18:1) | 1.69 | 1.56 | 0.05 | ns | 1.93 | 1.52 | 0.25 | ns | 1.89b | 1.10a | 0.04 | 0.005 | 1.85 | 0.95 | 0.13 | 0.037 | ||||
| Tpeak (°C) | ΔH (J/g) | Tendset (°C) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | |||||
| Spring-GRZ | 15.7 ± 0.4 | 34.1 ± 0.9 | 96.9 ± 2.9 | 39.9 ± 0.3 | ||
| Spring-C | 18.2 ± 0.5 | 33.1 ± 0.8 | 97.9 ± 3.0 | 36.4 ± 0.4 | ||
| Fall-GRZ | 17.2 ± 0.8 | 33.7 ± 0.9 | 112.9 ± 3.3 | 37.1 ± 0.3 | ||
| Fall-C | 16.4 ± 0.7 | 35.9 ± 0.9 | 89.4 ± 2.6 | 42.4 ± 0.5 | ||
| Spring | Fall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GRZ | C | SEM | p-value | GRZ | C | SEM | p-value | |||
| L* | 55.08 | 57.16 | 0.58 | ns | 59.24 | 58.95 | 0.14 | ns | ||
| a* | -5.05 | -5.09 | 0.04 | ns | -4.57 | -4.57 | 0.13 | ns | ||
| b* | 22.88 | 23.26 | 1.00 | ns | 28.39a | 15.71b | 0.46 | 0.003 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





