The
Cinnamomum camphora belongs to the Laurales order, Lauraceae family, and
Cinnamomum genus. It is one of the important ornamental and greening tree species in southern China and is extensively planted in the South China region [
1]. Due to its rich content of chemical defense substances such as
camphora, the
C. camphora is generally resistant to various phytophagous insects. However, the practice of cultivating large areas of monoculture
C. camphora plantations has led to a uniformity in forest structure, which in turn has weakened the trees' resistance to pests. Increasing pest species damaging
C. camphora forests have been reported, including the
C. camphora nest moth
Orthaga achatina Butler,
Pagiophloeus tsushimanus Morimoto, and
Orthaga olivacea Warre [
1,
2,
3,
4]. Among these,
P. tsushimanus was originally described by Morimoto and belongs to the Coleoptera order, Curculionidae family, Molytinae subfamily, Hylobiini tribe, and
Pagiophloeus genus. This species, a new record for China first reported in 2014, bores into and damages camphor trees and is currently only found in Shanghai [
2,
5,
6]. The life cycle of
P. tsushimanus includes adults, eggs, larvae, and pupae [
2]. Both adults and larvae can damage
C. camphora. After emerging, adults climb towards the canopy to feed on the tender outer bark of twigs to replenish nutrients, while larvae feed on the phloem and cambium, producing large amounts of frass [
2]. The collective damage from both adults and larvae significantly reduces the
C. camphora's economic and aesthetic value; high pest densities can weaken trees and even cause them to die off completely [
2,
7].
C. camphora forests in various administrative districts of Shanghai have been severely affected by this pest, presenting a trend of spread that poses a serious threat to
C. camphora resources in surrounding areas [
2,
8].
To date, research on the
P. tsushimanus has been very limited, focusing primarily on pest identification, characteristics of damage, life cycle, adult mating and oviposition behaviors, development, and the stability of reference genes [
2,
5,
7,
8,
9,
10]. However, there have been no studies reported on the pest's preference for different plants within the Lauraceae family, leaving an assessment of the risk of host shifting unexplored. Nutritional components such as proteins, sugars, and amino acids differ among plants, and these variations can affect the growth, survival, and reproduction of insects, thereby influencing their adaptability to different plants [
10,
11]. Phytophagous insects and their host plants undergo continuous natural selection and mutual adaptation through long-term coevolution, leading to differences in insect preferences for various plants [
12]. The choice of suitable host plants directly impacts the development and survival of the insects and the growth performance of their offspring, and even the dynamics of the entire population [
13,
14,
15]. In scenarios where the preferred host is absent, female insects may be compelled to lay eggs on non-host plants. If the larvae successfully adapt to some of these non-hosts, the emerging females may continue to choose these new hosts for oviposition, leading to host shifts [
16]. Field surveys have found that
P. tsushimanus feeds exclusively on the camphor tree
C. camphora, but indoor rearing experiments have shown that it can also complete its life cycle on other Lauraceae plants such as
Cinnamomum chekiangensis and
Phoebe chekiangensis. This indicates that these species could serve as potential alternative hosts and pose a risk of host shifting [
7].
C. camphora,
C. chekiangensis, and
P. chekiangensis, all Lauraceae species, share the same distribution areas in East China and are important urban greening trees in Shanghai and its surrounding areas [
7,
17,
18]. Therefore, this study examines the feeding, oviposition, and olfactory behavioral responses of adult
P. tsushimanus toward these three Lauraceae plants, aiming to assess the potential risk of host shifting and to provide a reference for monitoring the pest damage.
1. Materials and Methods
1.1. Main Instruments and Equipment
Instruments used include an atmospheric sampler (QC-1B, Beijing Labor Protection Science Research Institute), Linalool,β-Caryophyllene, α-Phellandrene, trans-Nerolidol, and a "Y"-shaped olfactometer (custom-made by Nanjing Zebra Experimental Equipment Co., Ltd.): constructed of glass, with an inner diameter of 4 cm, a main arm length of 40 cm, and two side arms each 20 cm long at a 60-degree angle to each other. The ends of the side arms are connected to the odor source bottles through Teflon tubing. Air is first drawn through an activated carbon filter to purify it, followed by a distillation water filter to increase humidity, using the atmospheric sampler. An equal airflow rate (300 mL/min) passes through either the odor source or control and then converges in the main arm tube from the side arms, entering the atmospheric sampler through the main arm's outlet.
1.2. Test Insects
Larvae and pupae of the P. tsushimanus were collected from a 15-year-old camphor water conservation forest in Mao Gang Town, Songjiang District, Shanghai (30°56'6.15"N, 121°12'32.76"E). They were brought back to the Entomology Laboratory at the school and reared individually in 6-well plates (2.5 cm diameter per well) using a homemade semi-artificial diet for camphor until pupation and adult emergence. Newly emerged adults were provided with fresh 2-year-old tender camphor branches to feed on and replenish nutrients, kept in reserve.
1.3. Selectivity Tests of P. tsushimanus Adults on Three Lauraceae Plants
1.3.1. Choice Test
A total of 36 unmated, healthy adult insects, both males and females, were subjected to starvation for 24 hours. The plants chosen for the test—C. camphora, C. chekiangensis (both Cinnamomum species), and P. chekiangensis (a species of Phoebe)—were used to determine adult feeding and oviposition preferences. Each rearing box (18 cm long × 12 cm wide × 6 cm high), containing 10 males and 10 females, was simultaneously furnished with 10 cm long branches of the three plant species for the insects to choose from, with branches replaced weekly. There were three replicates, each containing two rearing boxes. Daily observations and recordings were made of the feeding area and egg-laying quantity on the three types of plants over a period of three months. The area fed upon was calculated using a method involving grid paper coated with sulfuric acid, tallying the marked squares.
1.3.2. No-Choice Test
The status of the test insects and the pre-experiment handling methods were the same as in
Section 1.3.1. The adult insects' feeding areas and oviposition quantities were measured on single species of Lauraceae plants. Each rearing box (same dimensions as 1.3.1, containing 10 male and 10 female adults) was separately furnished with branches of
C. camphora,
C. chekiangensis, or
P. chekiangensis (same dimensions as
Section 1.3.1) for the insects to feed on, with branches replaced weekly. Three replicates were set for each plant type, with each replicate containing two rearing boxes. Daily observations and recordings were made of the feeding area and egg-laying quantity on the three types of plants over a period of three months.
1.4. Behavioral Response Measurements of P. tsushimanus female and male Adults to Three Lauraceae Plants
1.4.1. Behavioral Responses of female and male Adults to Three Lauraceae Plants and Air Control
The status of the test insects and the pre-experiment handling methods were the same as in 1.3.1. Five segments of approximately 10 cm long 2-year-old branches of
C. camphora,
C. chekiangensis, or
P. chekiangensis were placed in one of the odor source bottles of the "Y"-shaped olfactometer, while the other bottle was left empty as an air control. After each test, new odor source bottles were used, and the used ones were sequentially cleaned with acetone and distilled water, then dried for reuse. During the experiment, a single adult was introduced into the main arm of the olfactometer, and the port was sealed with ground glass stoppers. The atmospheric sampler was activated for 30 seconds before timing began. If the test insect moved beyond the halfway mark of a selected arm and remained active for ≥ 3 minutes near the odor source area, it was recorded as having made a choice for the odor source connected to that arm. If no choice was made within 5 minutes, the insect was recorded as non-responsive, and the test concluded. Each type of odor source was tested in three sets of 12 adults each, for both male and female insects. The environmental conditions were maintained at 2,000 lx illumination, a temperature of 26 ± 1°C, and relative humidity of 55 ± 5%, ensuring even lighting in both choice arms.
SRR: selection response rates; ST: adults number in the odor source tube; CT: adults number in the control tube.
SC: selection coefficient: ST: adults number in the odor source tube; CT: adults number in the control tube.
Positive values in the selection coefficient calculations indicate positive chemotaxis, while negative values indicate negative chemotaxis.
1.4.2. Behavioral Response of female and male Adults Among Three Lauraceae Plants
The condition of the test insects and the pre-experiment handling methods were the same as in
Section 1.3.1. For each test, five segments approximately 10 cm long from either 2-year-old
C. camphora,
C. chekiangensis, or
P. chekiangensis branches were placed in one of the odor source bottles of the "Y"-shaped olfactometer, while another type of branch was placed in the other bottle. Three comparative groups were established:
C. camphora vs.
C. chekiangensis,
C. camphora vs.
P. chekiangensis, and
C. chekiangensis vs.
P. chekiangensis. Each comparison was tested in three sets, with each set consisting of 12 adults. The testing and calculation methods followed those described in
Section 1.4.1.
1.5. Electroantennography (EAG) Measurements of P. tsushimanus female and male Adults to Four Components of Camphor Trees
In the laboratory, EAG was used to measure the electrophysiological responses of single or mixed components of camphor tree volatiles in the toothed beaked elephant, in order to screen for components with antennal electrophysiological activity. Linalool, trans-Nerolidol,α-Phellandrene, and β-Caryophyllene are the main components of camphor volatiles [
24]. Therefore, these four volatile compounds of camphor trees were selected as the objects for EAG determination.
Before the experiment, six standard compounds were prepared into solutions with concentrations of 0.001, 0.01, 0.1, 1, and 10 μg/μL using liquid paraffin. In addition, a mixed component solution was prepared in a 1:1 ratio of 1 μg/μL of standard compound solutions of camphor, basilene, and linalool for later use. EAG measurement method: Refer to the method in section 3.1.3 for the antenna connection method. First, mix the prepared volatile standard solution with a vortex mixer, and use a pipette to draw 10 μL and drop it onto a qualitative filter paper (5 cm × 0.5 cm) strip. After natural evaporation for 10 seconds, insert the filter paper into a Pasteur tube (10 mm × 20 mm × 20 mm). The base of the Pasteur tube is connected to the gas purification device, and the end is inserted into the small hole of the odor mixing tube. The flow rate of each stimulation airflow is 60 mL/s, the stimulation time is 0.5 s, and the time interval between two stimulations is 60 s. The testing time is during the day, and the test environment temperature is 27 ± 1 ℃. The testing sequence follows the following cycle: liquid paraffin → compound → liquid paraffin → compound → liquid paraffin. The concentration of each single component (6 in total) or mixed component (3 in total) (selecting compounds with high antennal potential response for mixing) was tested from low to high, with liquid paraffin as the blank control group. 10 male and 10 female adult insects were tested for each treatment and control group, with 2 antennae tested for each.
1.6. Data Analysis
EAG reaction value analysis is based on the methods reported in previous studies [20-23]. The detailed operations are as follows: the maximum amplitude of negative polarity deflection (−mV) induced by a stimulus was used to measure the EAG response. The absolute EAG response (mV) to each test stimulus was subtracted by the mean response to the two nearest mineral oil controls in order to compensate for solvent and mechanosensory artifacts. The resultant EAG value was corrected based on the reduction of the EAG amplitude to the standard stimulus to compensate for the decline of the antennal responsiveness during the experiment.
One-Way ANOVA and Tukey's HSD test were used for statistical analysis of feeding area, oviposition quantity, and selection coefficient. Chi-square tests were used to compare the selection response rates of male and female adults to different odor sources; independent sample t-tests were used to compare the selection coefficients between male and female adults to different odor sources. All statistical analyses were conducted in SPSS 20.0 software.
2. Results and Analysis
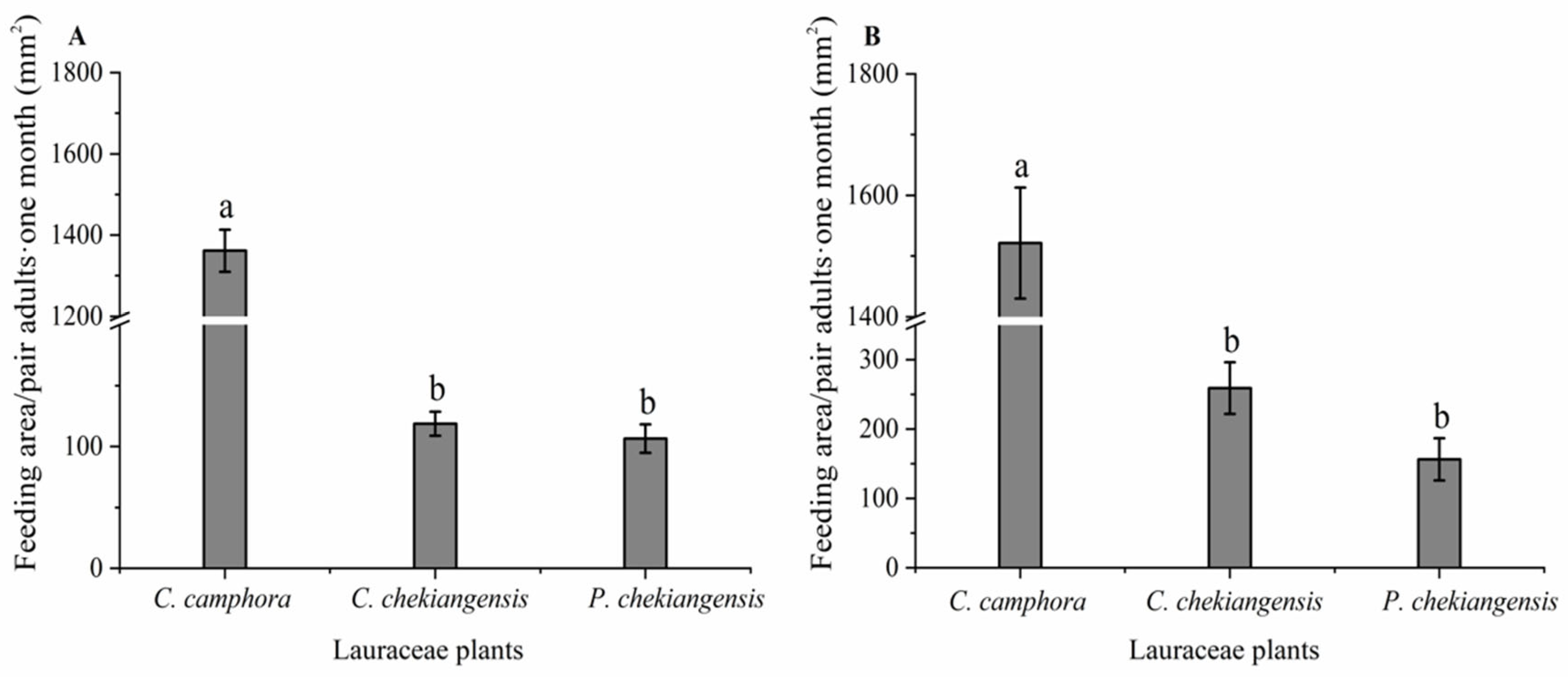
2.1. Feeding Preferences of Adult P. tsushimanus Toward C. camphora, C. chekiangensis, and P. chekiangensis
As indicated in
Figure 1, under both choice (
F = 534.791;
df = 2, 15;
P < 0.01) and no-choice (
F = 162.260;
df = 2, 15;
P < 0.01) conditions, significant differences were observed in the feeding areas on branches of
C. camphora,
C. chekiangensis, and
P. chekiangensis by adult. Under choice conditions, adults significantly preferred feeding on
C. camphora compared to
C. chekiangensis and
P. chekiangensis, with no significant difference in feeding area between
C. chekiangensis and
P. chekiangensis. Similarly, under no-choice conditions, the feeding area on
C. camphora was significantly larger compared to both
C. chekiangensis and
P. chekiangensis, with no significant differences between
C. chekiangensis and
P. chekiangensis.
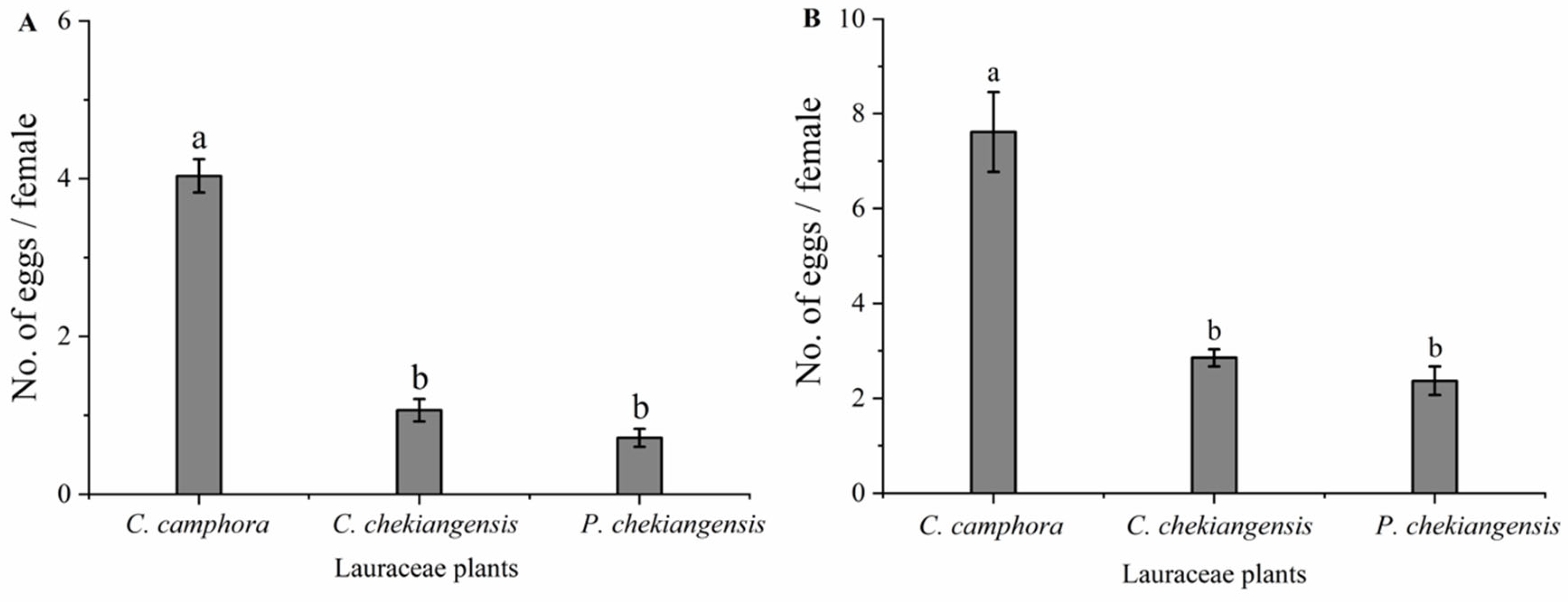
2.2. Oviposition Preferences of Female Adults towards C. camphora, C. chekiangensis, and P. chekiangensis
Under both choice (
F = 129.096;
df = 2, 15;
P < 0.01) and no-choice (
F = 35.835;
df = 2, 15;
P < 0.01) conditions, significant differences were observed in the number of eggs laid per female per month on branches of
C. camphora,
C. chekiangensis, and
P. chekiangensis (
Figure 2). Under both conditions, the egg-laying rate on
C. camphora branches was significantly higher than on the other two plants, while no significant differences were found between
C. chekiangensis and
P. chekiangensis (
Figure 2). Under non-selective conditions, the oviposition rate on
C. chekiangensis was significantly higher than under selective conditions; however, the oviposition rates on
C. chekiangensis and
P. chekiangensis did not differ significantly between selective and non-selective conditions.
2.3. Behavioral Responses of Adult P. tsushimanus to C. camphora, C. chekiangensis, and P. chekiangensis
2.3.1. Behavioral Responses of Adults to Three Lauraceae Plants Versus Air Control
According to
Figure 3, both female (
χ2 = 5.974,
P < 0.01) and male (
χ2 = 4.010,
P < 0.05) adults showed a significantly higher response rate towards
C. camphora compared to the control. Females did not show a significant preference for either
C. chekiangensis (
χ2 = 0.724,
P > 0.05) or
P. chekiangensis (
χ2 = 1.290,
P > 0.05) compared to the control, while males demonstrated a significantly lower preference rate towards
C. chekiangensis (
χ2 = 4.010,
P < 0.05) and
P. chekiangensis (
χ2 = 4.010,
P < 0.05) compared to the control. There were no significant differences in the response rates towards
C. camphora (
χ2 = 0.106,
P > 0.05),
C. chekiangensis (
χ2 = 0.403,
P > 0.05), or
P. chekiangensis (
χ2 = 0.655,
P > 0.05) between males and females.
Table 1 shows that both female (selection coefficient = 0.363) and male (selection coefficient = 0.306) adults exhibited a positive chemotactic response towards
C. camphora, indicating an attraction. Both females (selection coefficient = -0.131) and males (selection coefficient = -0.306) displayed a negative chemotactic response towards
C. chekiangensis, suggesting repellence, and similarly, both showed negative responses towards
P. chekiangensis (female selection coefficient = -0.178, male selection coefficient = -0.310). There were no significant differences between females and males in the selection coefficients towards
C. camphora (
t = 0.535,
P > 0.05),
C. chekiangensis (
t = 2.251,
P > 0.05), or
P. chekiangensis (
t = 0.717,
P > 0.05).
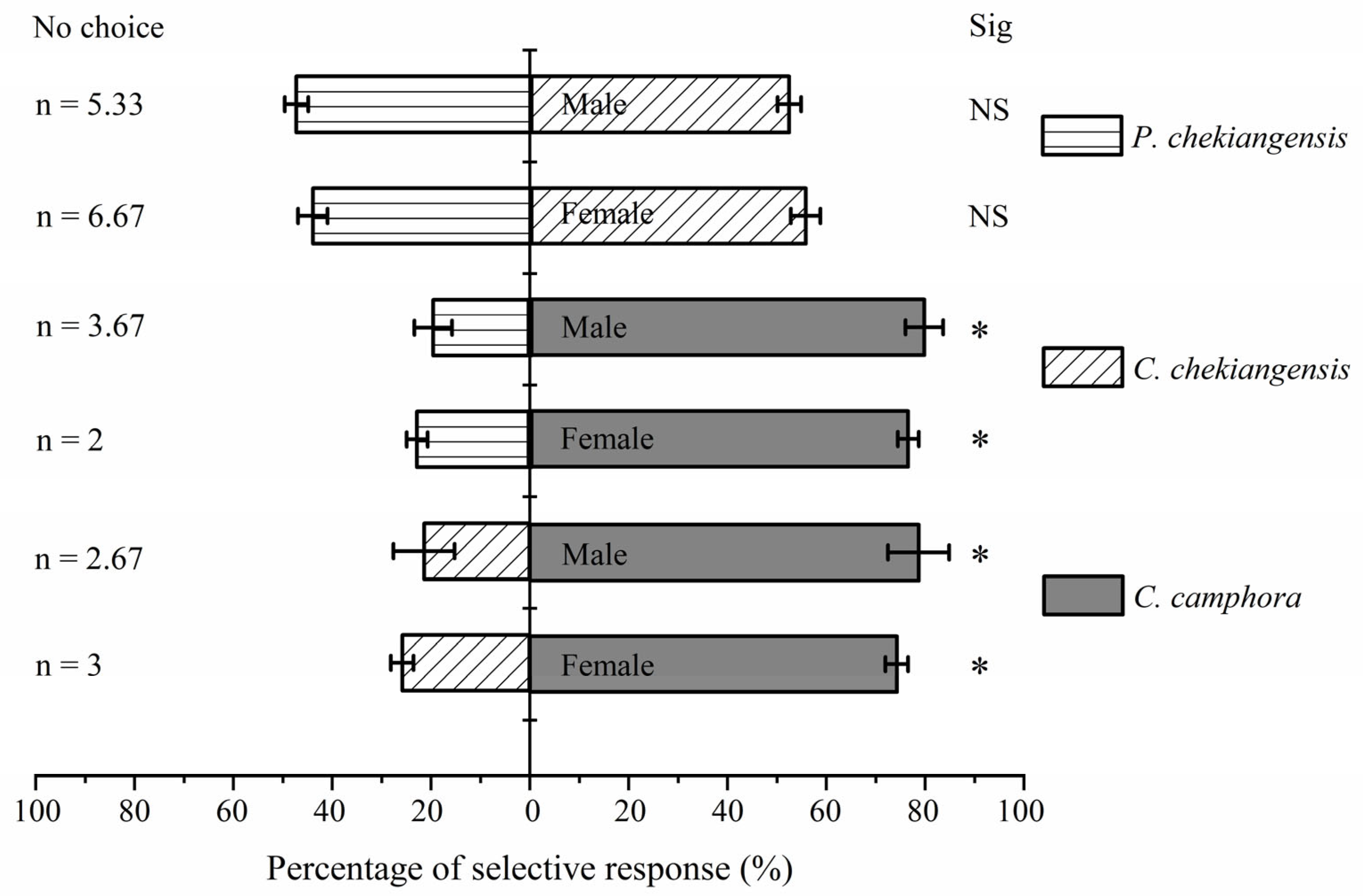
2.3.2. Behavioral Responses of Adults Among Three Lauraceae Plants
Figure 4 shows that both female (
χ2 = 11.227,
P < 0.05) and male (
χ2 = 17.120,
P < 0.05) adults significantly preferred
C. camphora over
C. chekiangensis. Similarly, the preference rates for
C. camphora over
P. chekiangensis were also significantly higher for both female (
χ2 = 14.583,
P < 0.05) and male (
χ2 = 18.484,
P < 0.05) adults. However, there was no significant difference in the preference rates between
C. chekiangensis and
P. chekiangensis for both female (
χ2 = 0.502,
P > 0.05) and male (
χ2 = 0.020,
P > 0.05) adults.
Table 2 indicates that the positive chemotactic responses of both female (selection coefficient = 0.485) and male (selection coefficient = 0.573) adults were stronger towards
C. camphora compared to
C. chekiangensis; and for
C. camphora over
P. chekiangensis (female selection coefficient = 0.537, male selection coefficient = 0.602). The positive chemotactic responses were also stronger towards
C. chekiangensis compared to
P. chekiangensis (female selection coefficient = 0.114, male selection coefficient = 0.048). There were no significant differences between females and males in their selection coefficients for the three plants (
C. camphora vs.
C. chekiangensis:
t = -0.665,
P > 0.05;
C. camphora vs.
P. chekiangensis:
t = -0.748,
P > 0.05;
C. chekiangensis vs.
P. chekiangensis:
t = 0.875,
P > 0.05).
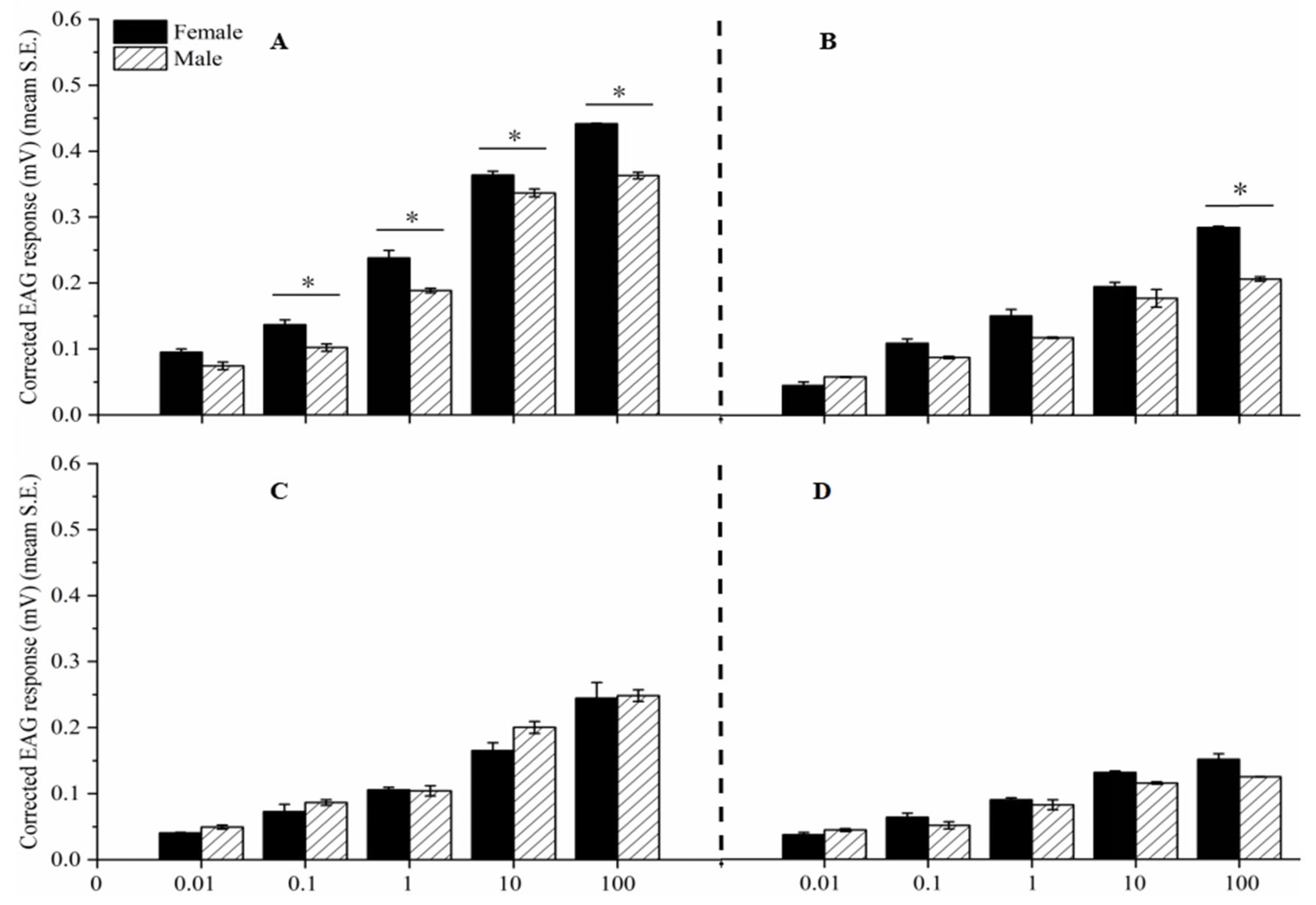
2.4. EAG responses of female and male Adults to Four Components
The EAG response values of female and male adult antennae to four different concentrations of a single compound component increased with increasing concentration, and there were significant differences in EAG response values between different concentrations (
P < 0.05) (
Figure 5).
There is a significant difference in the EAG response values between female and male adults to the same concentration of Linalool (0.01, 0.1, 1, and 10 μg/μL) (
t = 3.759,
P < 0.05;
t = 4.055,
P < 0.05;
t = 3.313,
P < 0.05;
t = 15.48,
P < 0.05) and β-Caryophyllene (10 μg/μL) (
t = 20.291,
P < 0.05) of the same compound. There was no significant difference in EAG response values between female and male adult insects to other compounds (
Figure 5).
At a concentration of 10 μg/μL, the EAG response values of female adults to four single component compounds were as follows: Linalool (0.4415 mV) > β-Caryophyllene (0.2850 mV) > α-Phellandrene (0.2447 mV) > trans-Nerolidol (0.1515 mV); The EAG response values of male adult insects to four single components of compounds, from high to low, are as follows: Linalool (0.3631 mV) > α-Phellandrene (0.2483 mV) > β-Caryophyllene (0.2068 mV) > trans-Nerolidol (0.1249 mV) (
Figure 5).
3. Discussion
Since the
P. tsushimanus can complete its life cycle on camphor tree,
Cinnamomum chekiangensis, and
Phoebe chekiangensis—all belonging to the Lauraceae family with
Cinnamomum chekiangensis and the camphor tree within the
Cinnamomum genus, and
Phoebe chekiangensis in the
Phoebe genus [
8,
25,
26]—these plants are closely related genetically. Additionally, these three species overlap in their distribution in the East China region and are significant for urban greening in Shanghai and its surrounding areas [
18,
24]. Consequently, this study selects
C. chekiangensis and
P. chekiangensis as candidate plants to investigate the selection behavior of adults towards volatiles emitted by these Lauraceae plants, providing crucial insights for assessing the risk of host shifting in this pest.
C. camphora is generally resistant to various phytophagous insects, yet both female and male adults showed a preference for feeding and, particularly in females, for ovipositing on
C. camphora branches under both choice and no-choice conditions. This preference could be attributed to the differences in the main secondary metabolites among these Lauraceae species [
1,
27,
28], which influence adult feeding and oviposition behaviors [
8]. Volatile chemical compounds play a pivotal role in guiding these behaviors [
29], and non-volatile chemicals also significantly affect feeding and oviposition preferences, as evidenced by previous studies where larvae fed on semi-artificial diets made from branches of these three Lauraceae species. Larvae consuming diets containing camphor components had the shortest developmental periods, lowest mortality rates, and highest pupation rates and weight gain [
8]. Female adults are likely to choose host plants that maximize the survival and growth potential of their offspring, a behavior driven by selective pressures aimed at maximizing offspring survival and population growth [
30,
31,
32]. However, the choice of plants for feeding and oviposition by insects is also influenced by environmental factors such as altitude, slope orientation, temperature, humidity, and the physical characteristics of the plants, such as branch thickness. For example, the longhorn beetle
Aphrodisium sauteri Matsushita shows a preference for feeding on
Cyclobalanopsis myrsinaefolia (Blume) Oersted and tends to oviposit on branches with bark thicknesses of 1-2 mm and diameters of 15-25 mm [
33]. Therefore, future studies on the oviposition habits of the
P. tsushimanus should consider a broader range of influencing factors.
This study also observed that adults feed and oviposit on the branches of
P. chekiangensis and
C. chekiangensis. In line with earlier research findings, larvae of the
P. tsushimanus can complete their lifecycle when fed semi-artificial diets made from branches of these two plants. This adaptability under suboptimal food conditions through physiological and biochemical adjustments suggests that
C. chekiangensis and
P. chekiangensis could serve as alternative hosts for adults, indicating a potential risk of host shifting [
8,
34]. Additionally, under pressures such as a scarcity of preferred host plants, the
P. tsushimanus might adapt to other species like
C. chekiangensis and
P. chekiangensis by modulating its growth, development, and detoxification metabolism [
8]. Therefore, in field surveys monitoring the damage caused by this pest, there should also be an intensified effort to monitor other Lauraceae plants to detect any damage and to prevent potential host shifts.
The selection of host plants by phytophagous insects is influenced by genetic factors, volatile compounds from host plants, and the growth and development performance of offspring [
35,
36]. Insects can genetically acquire the ability to recognize volatiles from host plants. For instance, Corbet (1985) proposed the early detection hypothesis of chemical residues, emphasizing that chemical residues from host plants are stored in the hemolymph of larvae or on the surface of pupae and are ultimately detected by adults after emergence [
37]. Recent studies also suggest that the sensitivity to chemical cues exists in the central nervous system (CNS) of larvae and persists into adulthood [
38]. However, there are opposing views suggesting that substantial reconstruction of the CNS during metamorphosis may prevent the transfer of chemical sensory memory [
39,
40]. It is generally believed that there is a close relationship between adult phytophagous insects' choice of host plants and the growth and development of their offspring. However, some argue that the feeding and oviposition preferences of female adults may not necessarily correlate with the performance of their offspring [
27,
30]. Because the nutritional quality of host plants can undergo unpredictable changes over time, it becomes challenging for female adults to quickly determine the most suitable plants for the growth and development of their offspring, thus increasing the difficulty in recognizing changes in plant nutritional quality [
1,
41]. Volatile compounds from host plants play a crucial role in host recognition and oviposition site selection by adult insects, with the composition and concentration of these volatiles directly influencing adult insects' choice behaviors [
42,
43]. Weevils exhibit selective preferences for volatiles from different plants; for example, the maize weevil
Sitophilus zeamais Motschulsky shows a stronger preference for wheat Triticum aestivum L. and rough rice over other crops like peanuts
Arachis hypogaea Leguminosae, buckwheat
Fagopyrum esculentum Mench, and sorghum
Sorghum bicolor (L.) Moench [
44]. Significant electrophysiological response of female and male adults to four volatile compounds (Linalool,β-Caryophyllene, α-Phellandrene, and trans-Nerolidol) of camphor tree was discovered in this study. Among them, female and male adult insects had a stronger electroantennogram response to Linalool. These results preliminarily indicate that male and female adults exhibit a positive chemotactic response to camphor volatiles, but a negative chemotactic response to volatiles from
C. chekiangensis and
P. chekiangensis. Future research will determine the differences in volatile composition of
C. camphora,
C. chekiangensis, and
P. chekiangensis, and use chemical ecology theory to analyze the host selection preferences of male and female adults. At the same time, by employing techniques from entomology, insect physiology, electroantennography, and analytical chemistry, it would be possible to isolate and identify key volatile components from host plants and test their biological activity. This would provide a basis for understanding why
P. tsushimanus prefer camphor and aid in developing attractants for managing this pest.
Combining the results of adult feeding choices, oviposition preferences, and positive chemotactic responses to camphor volatiles, it is evident that
P. tsushimanus adults exhibit an olfactory preference for camphor volatiles. This study is crucial for assessing the risk of host shifts in this pest and can be applied to its management practices. The preference of insects for certain plants is associated with differences in the plant's volatile compounds [
28,
29]. The positive chemotactic response of
P. tsushimanus adults to camphor volatiles suggests that key factors causing this phenomenon likely include significant differences in the volatile components or concentrations released by camphor compared to
C. chekiangensis and
P. chekiangensis. Identifying whether single or mixed components of these differences play a major role in adult olfactory choice requires further research. This studies could help elucidate the mechanisms behind the
P. tsushimanus's preference for different plants and aid in the development of plant-based attractants for this pest.
Author Contributions
Conceptualization, Q.J. and D.H.; Methodology, C.C., Q.J. and D.H.; Investigation, C.C., S.L., and Z.L.; Data curation, C.C., S.L., and Z.L.; Writing—original draft, C.C., S.L. and Z.L.; Writing—review and editing, C.C., Q.J. and D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Science and Technology Commission of Shanghai Municipality (18391903200), the innovation platform construction project of Zhaoqing University (202413004), the scientific research fund funding project of Zhaoqing University (QN202331), and the doctoral Initiation Fund Project of Zhaoqing University (611/230009).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, H.; Wang, J.; Song, L.; Cao, X.S.; Yao, X.; Tang, F.; Yue, Y.D. GC×GC-TOFMS analysis of essential oils composition from leaves, twigs and seeds of Cinnamomum camphora L. Presl and their insecticidal and repellent activities. Molecules 2016, 21, 423. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, C.C.; Li, S.Y.; Zhu, H.; Fan, B.Q.; Wang, Y.; Su, P.; Han, Y.Y.; Hao, D.J. Biological traits and life history of Pagiophloeus tsushimanus(Coleoptera:Curculionidae), a weevil pest on camphor trees in China. J. Forestry Res. 2021, 32, 1979–1988. [Google Scholar] [CrossRef]
- Yan, Q.; Li, H.D.; Chen, Y.; Ye, Z.F.; You, X.F.; Zhou, J.X.; Mu, L.F.; Liu, S.J.; Kong, X.B.; Khuhro, S.A.; Dong, S. Identification and field evaluation of the sex pheromone of Orthaga achatina (Lepidoptera: Pyralidae). J. Chem. Ecol. 2018, 44, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.J.; Xu, F.L.; Hua, F.L.; Lu, J.D.; Zhang, C.G.; Chen, X.X. A camphor insect pest-bionomics of Orthaga olivacea. Chin. Bull. Entomol. 2008, 45, 562–565. (In Chinese) [Google Scholar]
- Zhang, C.C.; Gu, T.Z.; Su, P.; Fan, B.Q.; Wang, Y.; Hao, D.J. Identification and phylogenetic position of Pagiophloeus tsushimanus based on COI and rDNA sequences. For. Res. 2018, 31, 78–87. (In Chinese) [Google Scholar]
- Morimoto, K. The family Curculionidae of Japan. I. subfamily Hylobxinae. Esakia 1982, 19, 51–121. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Chen, C.; Jia, Z.Y.; Li, Q.; Tang, Z.Z.; Zhong, M.F.; Zhu, H.; Hao, D.J. Offspring performance and female preference of Pagiophloeus tsushimanus (Coleoptera: Curculionidae) on three Lauraceae tree species: a potential risk of host shift caused by larval experience. J. Appl. Entomol. 2021, 145, 530–542. [Google Scholar] [CrossRef]
- Li, S.Y.; Chen, C.; Li, H.; Fan, B.Q.; Wang, Y.; Hao, D.J. Effects of feeding on diets containing components of different plants on the development and detoxifying enzyme activities in Pagiophloeus tsushimanus (Coleptera: Curculionidae) larvae. Acta Entomologica Sinica 2019, 62, 1286–1296. (In Chinese) [Google Scholar]
- Jia, Z.Y.; Chen, C.; Ma, Y.X.; Li, S.Y.; Fan, B.Q.; Wang, Y.; Hao, D.J. Effects of temperature on growth and development of Pagiophloeus tsushimanus Morimoto. J. of Nanjing For. University (Natural Sciences Edition) 2020, 44, 131–136. [Google Scholar]
- Chen, C.; LI, S.Y.; Zhu, H.; Fan, B.Q.; Wang, Y.; Hao, D. Identification and evaluation of reference genes for gene expression analysis in the weevil pest Pagiophloeus tsushimanus using RT-qPCR. J. Asia-Pac. Entomol. 2020, 23, 336–344. [Google Scholar] [CrossRef]
- Song, W. Effects of temperature and host on the individual growth and development of Phenacoccus solenopsis Tinsley; (In Chinese). Anhui Agricultural University: Hefei, 2016. [Google Scholar]
- Gullan, P.J.; Cranston, P.S. The insects: an outline of entomology (third edition); Blackwell Publishing Ltd: OxFord, 2009; pp. 211–239. [Google Scholar]
- Franziska, B.; Georg, P. Sequestration of plant defense compounds by insects: from mechanisms to insect-plant coevolution. Annu. Rev. Entomol. 2022, 67, 163–180. [Google Scholar]
- Jaenike, J. On optimal oviposition behavior in phytophagous insects. Academic Press 1978, 14, 350–356. [Google Scholar] [CrossRef]
- Carrasco, D.; Larsson, M.C.; Anderson, P. Insect host plant selection in complex environments. Curr. Opin. in Insect Sci. 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Griese, E.; Pineda, A.; Pashalidou, F.G.; Iradi, E.P.; Hilker, M.; Dicke, M.; Fatouros, N.E. Plant responses to butterfly oviposition partly explain preference-performance relationships on different brassicaceous species. Oecologia 2020, 192, 463–475. [Google Scholar] [CrossRef]
- Ballabeni, P.; Rahier, M. Performance of leaf beetle larvae on sympatric host and non-host plants. Entomol. Exp. Appl. 2000, 97, 175–181. [Google Scholar] [CrossRef]
- Wu, M.T.; Ni, L.; Lu, H.X.; Xu, H.Y.; Zou, S.Q.; Zou, X.X. Terpenoids and their biological activities from Cinnamomum: a review. J. Chem. 2020. [Google Scholar] [CrossRef]
- Hu, D.Y.; Xu, D.M.; Chu, M.L.; Zhang, C.C.; Gan, W.X. Brief introduction on main chemical constituents of Cinnamomum Camphora essential oil. China Forest Products Industry 2019, 56, 61–64. (In Chinese) [Google Scholar]
- Germinara, G.S.; Pistillo, M.; Griffo, R.; Garonna, A.P.; Palma, A.D. Electroantennographic responses of Aromia bungii (Faldermann, 1835) (Coleoptera, Cerambycidae) to a range of volatile compounds. Insects 2019, 10, 274. [Google Scholar] [CrossRef]
- Light, D.M.; Kamm, J.A.; Buttery, R.G. Electroantennogram response of alfalfa seed chalcid, Bruchophagus roddi (Hymenoptera: Eurytomidae) to host- and non host-plant volatiles. J. Chem. Ecol. 1992, 18, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Raguso, R.A.; Light, D.M. Electroantennogram responses of male Sphinx perelegans hawkmoths to floral and ‘green leaf volatiles’. Entomol. Exp. Appl. 1998, 86, 287–293. [Google Scholar] [CrossRef]
- Otter, C.J.; Tchicaya, T.; Schutte, A.M. Effects of age, sex and hunger on the antennal olfactory sensitivity of tsetse flies. Physiol. Entomol. 1991, 16, 173–182. [Google Scholar] [CrossRef]
- Moudrý, V.; Devillers, R. Quality and usability challenges of global marine biodiversity databases: an example for marine mammal data. Ecological Informatics 2020, 56. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B. Ethnobotany, pharmacology and phytochemistry of the genus phoebe (Lauraceae). Mini-Rev. Org. Chem. 2013, 10, 12–26. [Google Scholar] [CrossRef]
- Wu, M.T.; Ni, L.; Lu, H.X.; Xu, H.Y.; Zou, S.Q.; Zou, X.X. Terpenoids and their biological activities from Cinnamomum: A review. J. Chem. [CrossRef]
- Yang, Q.; Chen, S.P.; Zou, L.Q.; Lian, P.H. GC-MS analysis of chemical components of essential oil from leaves of Cinnamomum chekiangensis. Biomass Chemical Engineering 2009, 43, 25–27. [Google Scholar]
- Chen, Y.X.; Shi, H.F.; Xue, X.M.; Pan, B. Analysis and comparison of volatile components of four species of phoebe based on GC-MS. Journal of Central South University of Forestry & Technology 2019, 39, 92–96. [Google Scholar]
- Jiang, M. Effects of volatiles from host plant on the tropism, growth and reproduction of Agasicles hygrophila; (In Chinese). Fujian Agriculture and Forestry University: Fuzhou, 2019. [Google Scholar]
- Thompson, J.N. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol. Exp. Appl. 1988, 47, 3–14. [Google Scholar] [CrossRef]
- Mayhew, P.J. Adaptive patterns of host plant selection by phytophagous insects. Oikos 1997, 79, 417–428. [Google Scholar] [CrossRef]
- Kuang, X.J.; Ge, F.; Xue, F.S. Influence of environment factors and individual differences to female fecundity in insect. Journal of Environmental Entomology 2016, 38, 1275–1281. (In Chinese) [Google Scholar]
- Zhang, Q.D.; Ji, B.Z.; Xu, T.; Liu, S.W.; Wu, G.X.; Wang, T.X.; Chen, Z.M. Feeding and oviposition preferences of Aphrodisium sauteri. Chin. J. Appl. Entomol. 2011, 48, 626–633. (In Chinese) [Google Scholar]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Dai, J.Q.; Han, S.C.; Du, J.W. Progress Studies on behavioral effects of semiochemicals of host plant to insects. Journal of Environmental Entomology 2010, 32, 407–414. (In Chinese) [Google Scholar]
- Corbet, S.A. Insect chemosensory resPonses: A chemical legacy hypothesis. Ecological Entomology 1985, 10, 143–153. [Google Scholar] [CrossRef]
- Petit, C.; Dupas, S.; Thiéry, D.; Capdevielle-Dulac, C.; Ru, B.; Harry, M.; Calatayud, P. Do the mechanisms modulating host preference in holometabolous phytophagous insects depend on their host plant specialization? a quantitative literature analysis. J. Pest Sci. 2017, 90, 797–805. [Google Scholar] [CrossRef]
- Tissot, M.; Stocker, R.F. Metamorphosis in Drosophila and other insects: the fate of neurons throughout the stages. Prog. Neurobiol. 2000, 62, 89–111. [Google Scholar] [CrossRef]
- Blackiston, D.J.; Casey, E.S.; Weiss, M.R. Retention of memory through metamorphosis: can a moth remember what it learned as a caterpillar? PLoS One 2008, 3, e1736. [Google Scholar] [CrossRef]
- Craig, T.P.; Itami, J.K. Evolution of preference and performance relationships. In specialization, speciation, and radiation. The evolutionary biology of herbivorous insects (ed. K.J. tilmon); University of CaliFornia Press, 2008; pp. 20–28. [Google Scholar]
- Wang, C.Z.; Qin, J.D. Insect-plant co-evolution: multitrophic interactions concerning Helicoverpa species. Chin. Bull. Entomol. 2007, 44, 311–319. (In Chinese) [Google Scholar]
- Qin, J.D. The relationship between insects and plants: on the interaction and evolution of insects and plants; Science Press: Beijing, 1987; 227. (In Chinese) [Google Scholar]
- Collins, J.K.; Mulder, P.G.; Grantham, R.A.; Reid, W.; Smith, M.W.; Eikenbary, R. Assessing feeding preferences of pecan weevil (Coleoptera: Curculionidae) adults using a hardee olfactometer. J. Kans. Entomol. Soc. 1997, 70, 181–188. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).