Submitted:
30 July 2024
Posted:
01 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Patient Demographics and Clinical Characteristics
2.2. DNA Quality and Sequencing Metrics
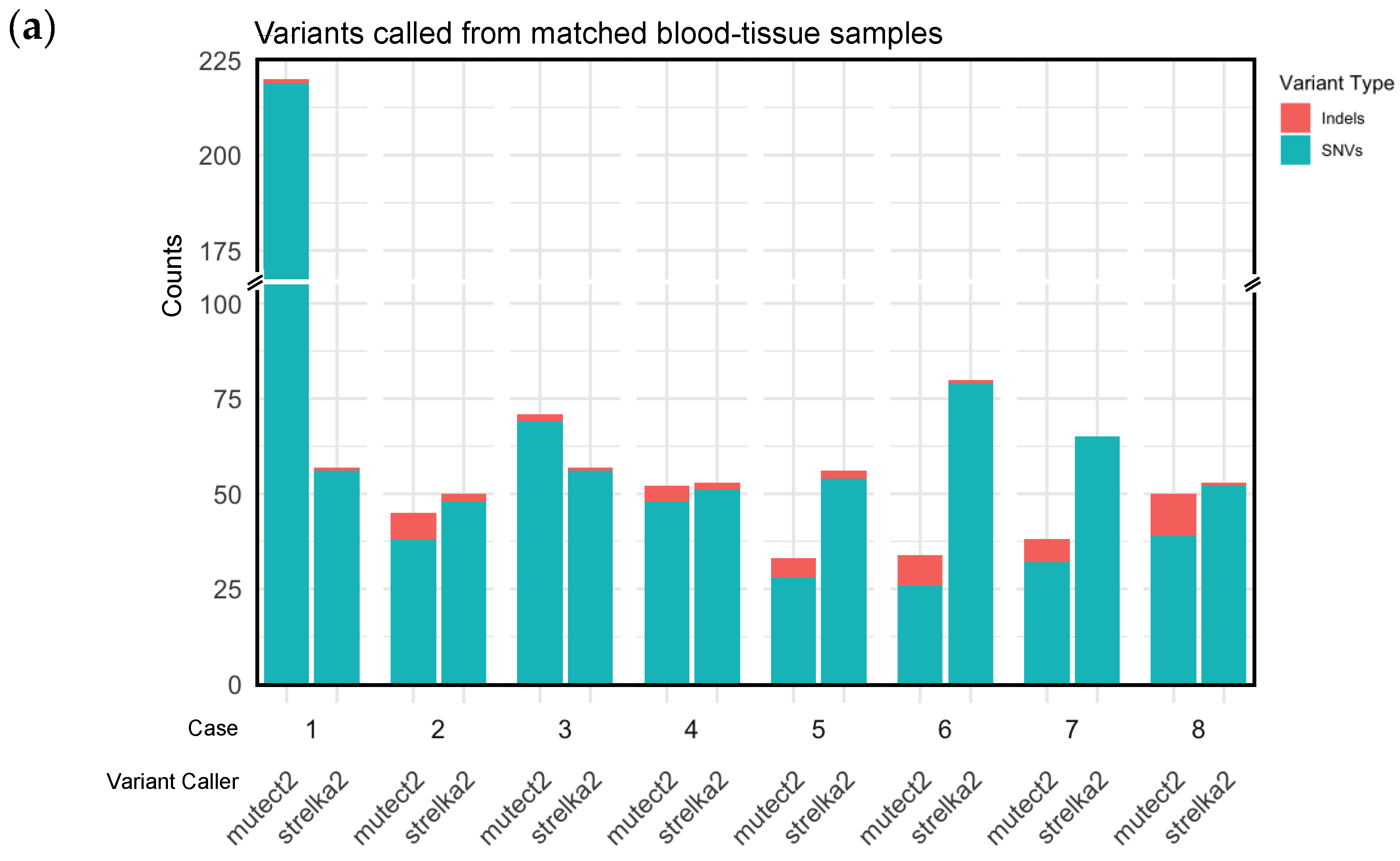
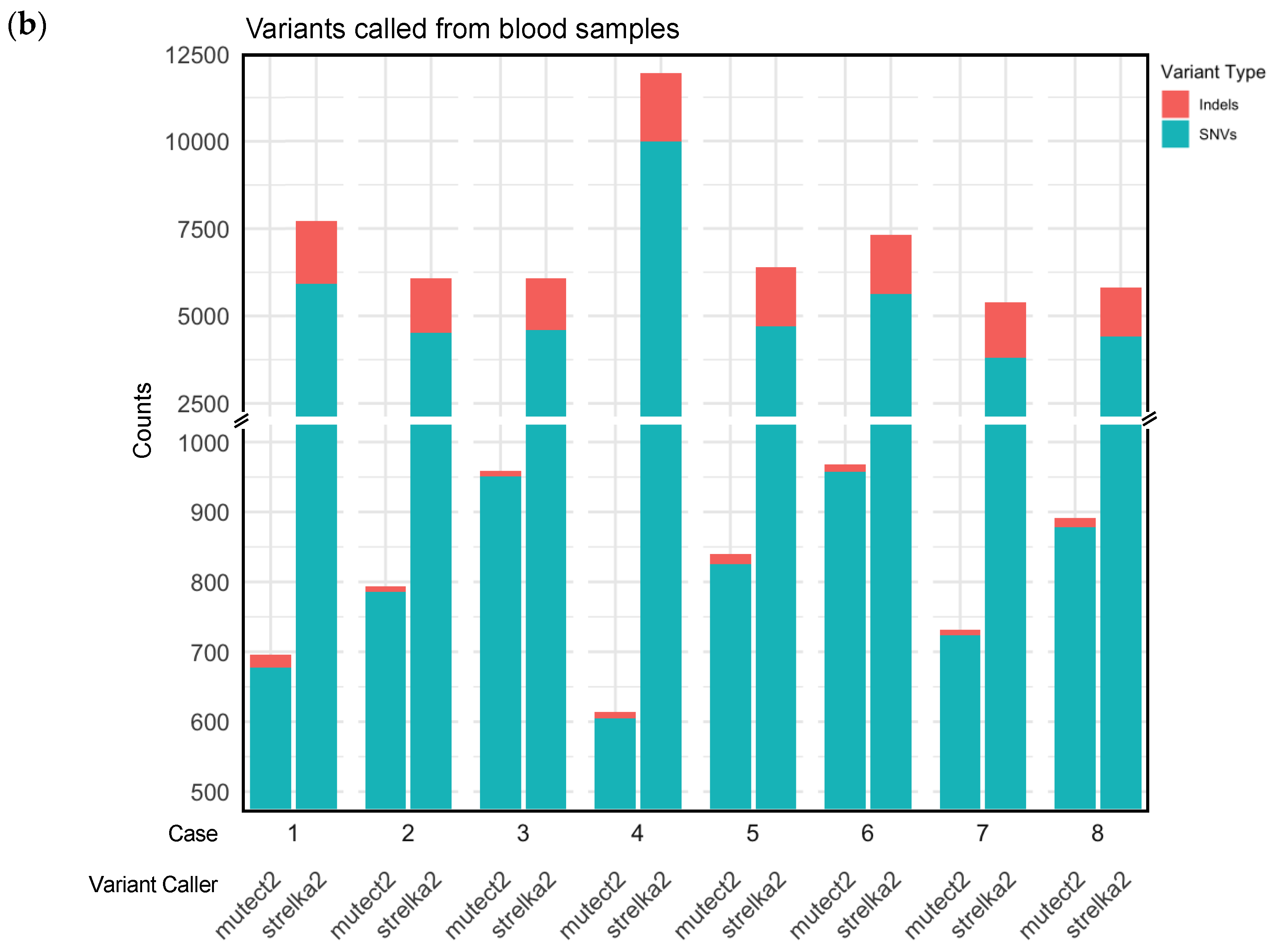
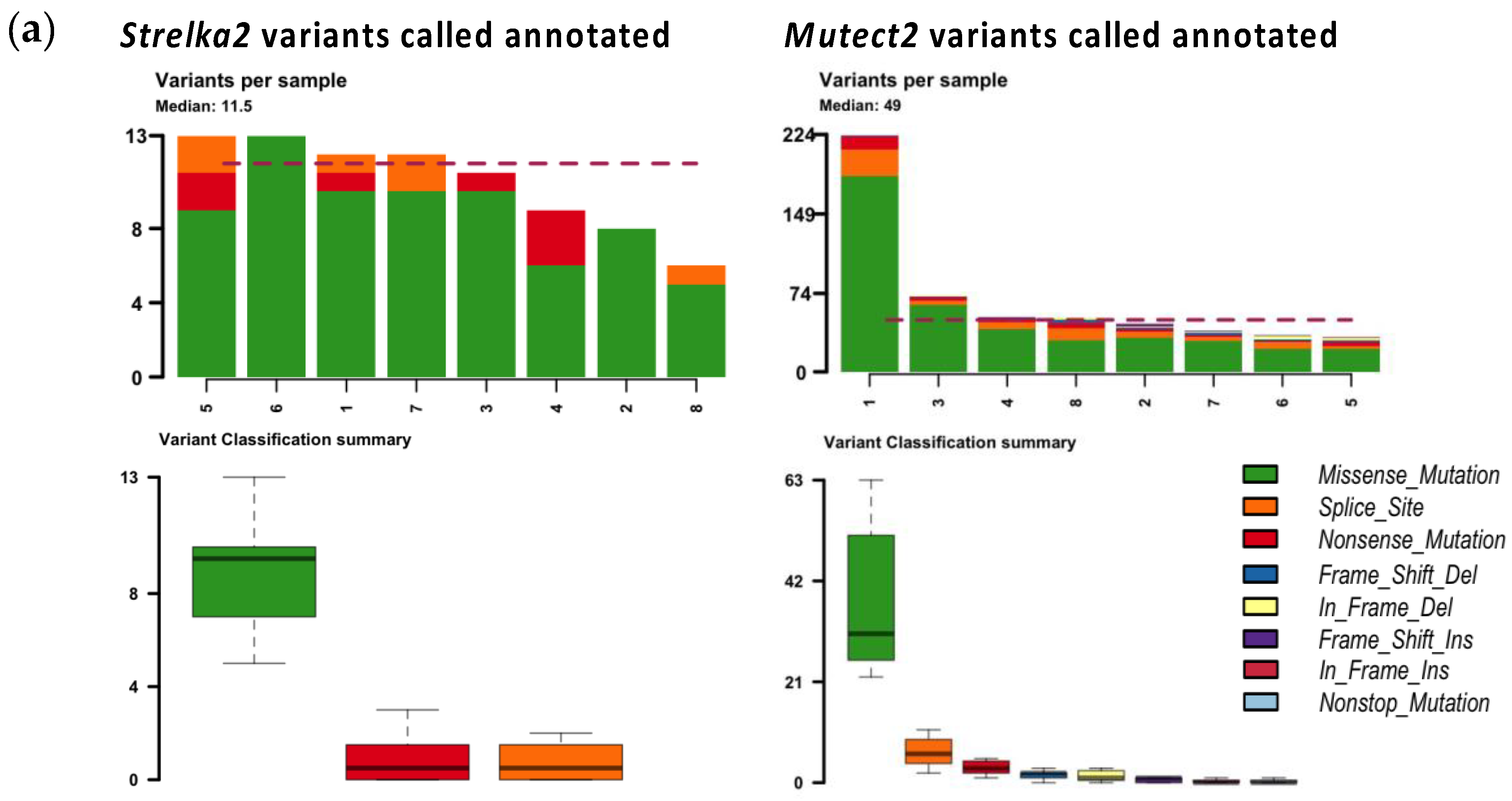
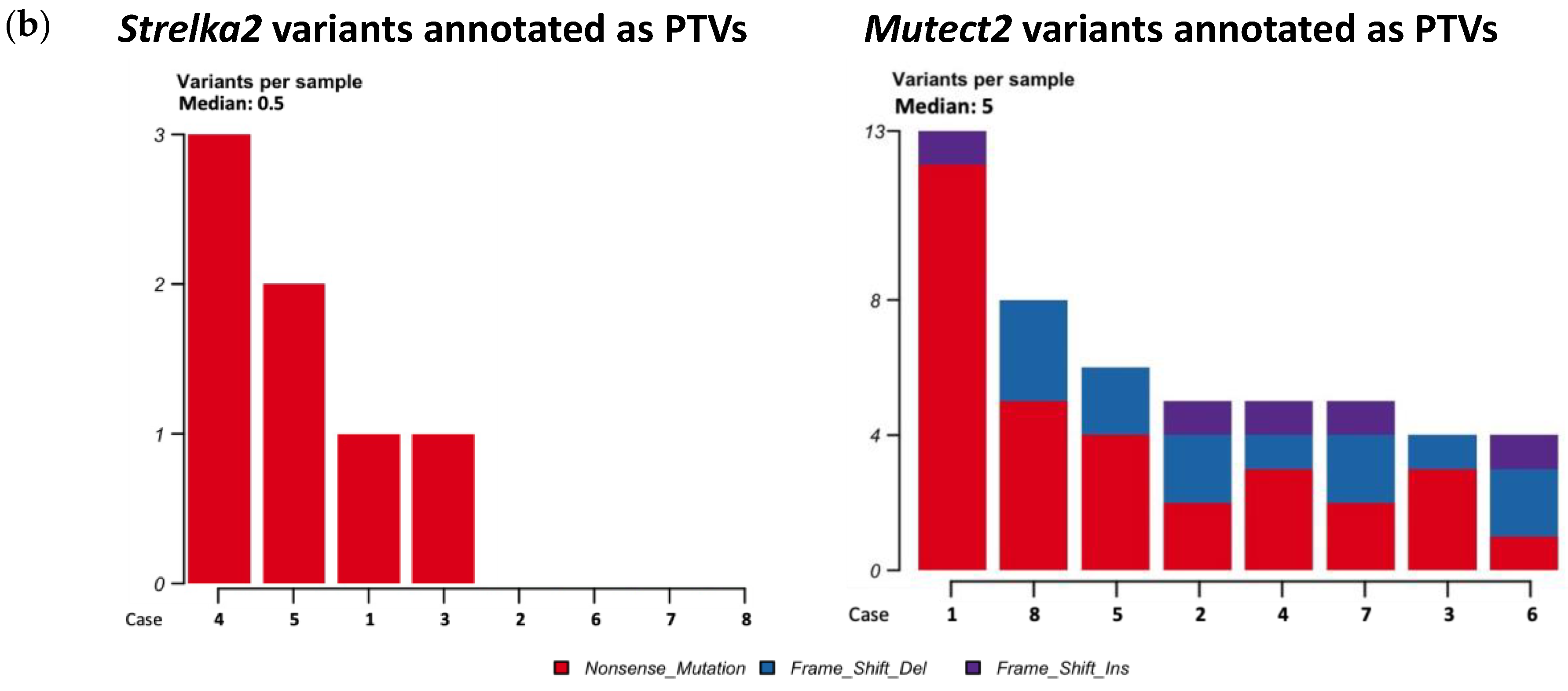
2.3. Somatic variants identified from WES
- Variants called from blood samples: Variants identified in blood samples (Supplementary Table S4), and
- Variants called from paired blood/tissue samples: Variants identified in the tissue sample that were not present in the corresponding blood sample (Table 2).
| Case | Somatic variants | SNVs1 | Indels2 | PTVs3 | Pathogenic4 | Pathogenic / Likely Pathogenic4 | Likely Pathogenic4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strelka2 | Mutect2 | Strelka2 | Mutect2 | Strelka2 | Mutect2 | Strelka2 | Mutect2 | Strelka2 | Mutect2 | Strelka2 | Mutect2 | Strelka2 | Mutect2 | |
| 1 | 57 | 224 | 56 | 219 | 1 | 1 | 1 | 13 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 50 | 46 | 48 | 38 | 2 | 7 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 57 | 72 | 56 | 69 | 1 | 2 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 53 | 52 | 51 | 48 | 2 | 4 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 56 | 33 | 54 | 28 | 2 | 5 | 2 | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 80 | 35 | 79 | 26 | 1 | 8 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 65 | 39 | 65 | 32 | 0 | 6 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 53 | 52 | 52 | 39 | 1 | 11 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Median (range) | 56.5 (50-80) |
49 (23-224) |
55 (48-79) |
38.5 (26-219) |
1 (0-2) |
5.5 (1-11) |
0.5 (0-3) |
5 (4-13) |
0 (0-0) |
0 (0-0) |
0 (0-0) |
0 (0-0) |
0 (0-0) |
0 (0-0) |
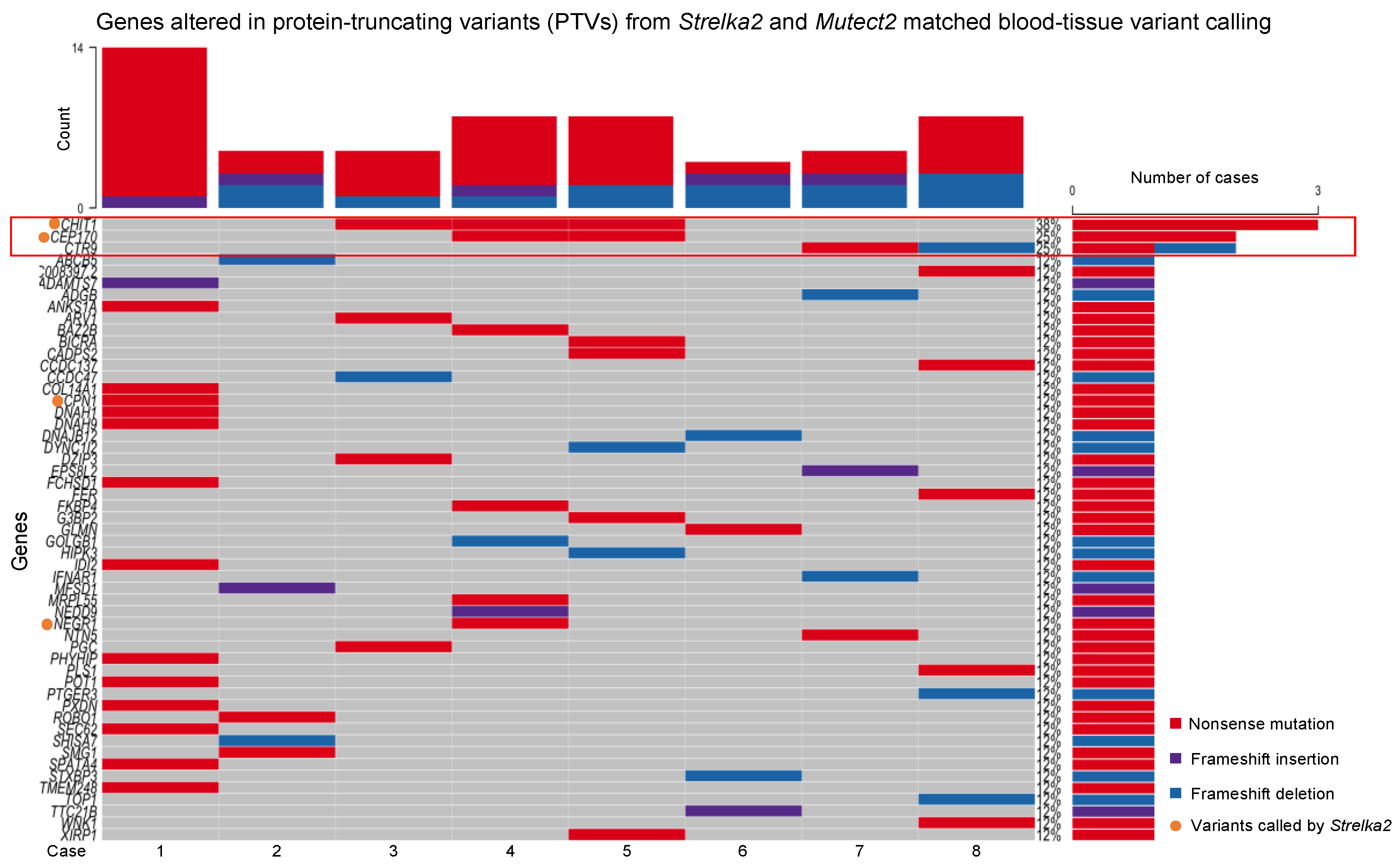
- CHIT1, altered in Cases 3, 4 and 5, nonsense mutations
- CEP170, altered in Cases 4 and 5, nonsense mutations
- CTR9, altered in Cases 7 and 8, nonsense mutation and frameshift deletion, respectively
2.4. Validation of Somatic Variants with Sanger Sequencing
3. Discussion
4. Materials and Methods
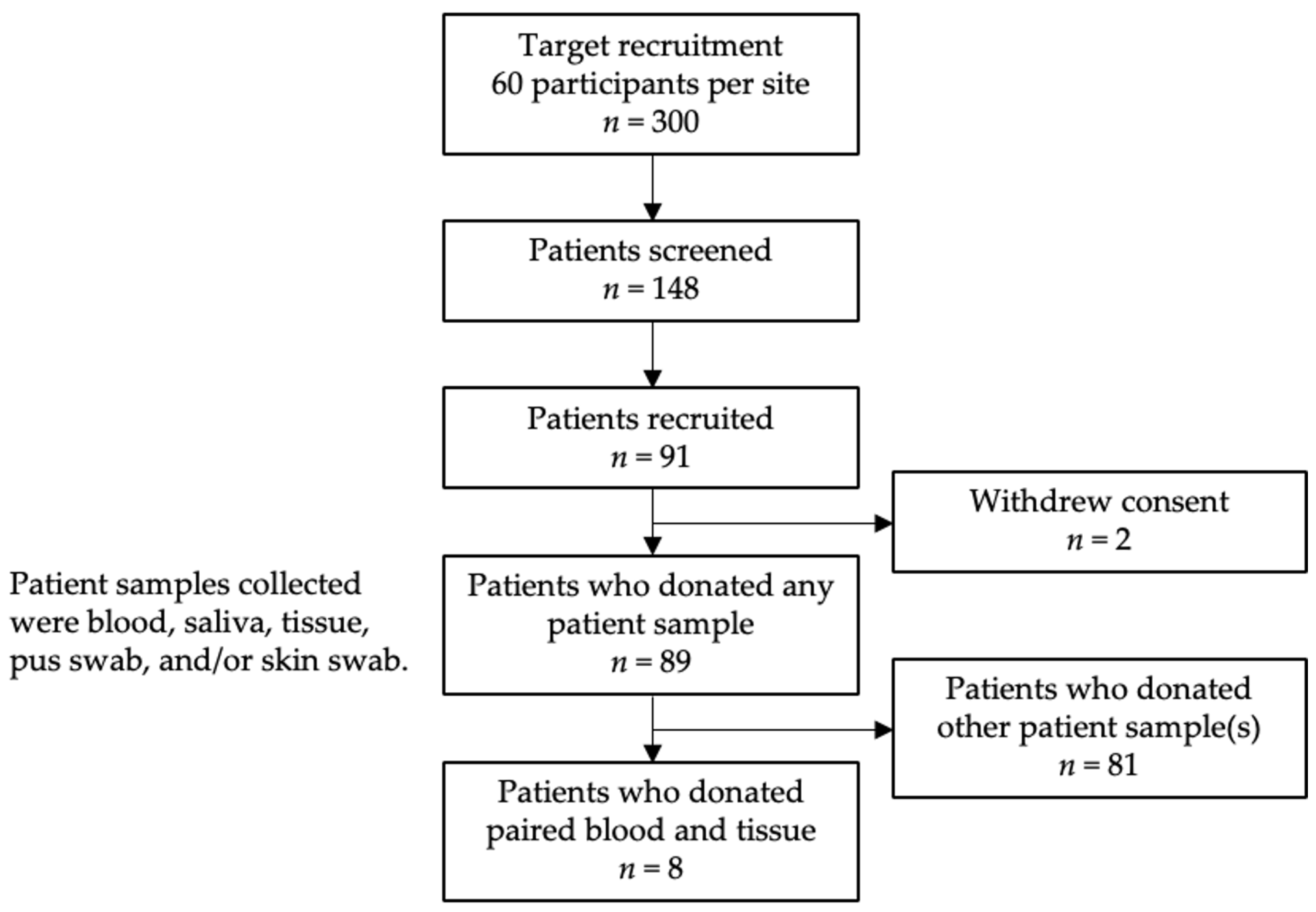
4.1. Patient Recruitment
4.2. Sample Collection
4.3. DNA Extraction and Sequencing
4.4. Quality Control and Somatic Variant Calling
- Catalogue of Somatic Mutations in Cancer (COSMIC) Cancer Gene Census (CGC) (data source dated 15 March 2012) [61]
- NCBI dbSNP (data source dated 18 April 2018) [64]
- Human DNA repair genes (data source dated 24 May 2018) [65]
- GENCODE (v34) [68]
- Genome Aggregation Database (gnomAD) (v3.1.2) [69]
- Human Genome Organisation (HUGO) Gene Nomenclature Committee (HGNC) Database (data source dated 30 November 2017) [70]
4.5. Validating Called Variants with Sanger Sequencing
4.6. Gene-Set Analysis
4.7. Data and Sample Availability Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Jarrah, A.; Taranikanti, V.; Lakhtakia, R.; Al-Jabri, A.; Sawhney, S. Idiopathic Granulomatous Mastitis: Diagnostic strategy and therapeutic implications in Omani patients. Sultan Qaboos Univ Med J 2013, 13, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.S.; Ho, P.J.; Liow, J.J.K.; Tan, Q.T.; Goh, S.S.N.; Li, J.; Hartman, M. A meta-analysis of idiopathic granulomatous mastitis treatments for remission and recurrence prevention. Front Med (Lausanne) 2024, 11, 1346790. [Google Scholar] [CrossRef]
- Hugon-Rodin, J.; Plu-Bureau, G.; Hugol, D.; Gompel, A. Management of granulomatous mastitis: a series of 14 patients. Gynecol Endocrinol 2012, 28, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Baslaim, M.M.; Khayat, H.A.; Al-Amoudi, S.A. Idiopathic granulomatous mastitis: a heterogeneous disease with variable clinical presentation. World J Surg 2007, 31, 1677–1681. [Google Scholar] [CrossRef]
- Yukawa, M.; Watatani, M.; Isono, S.; Fujiwara, Y.; Tsujie, M.; Kitani, K.; Hara, J.; Kato, H.; Takeyama, H.; Kanaizumi, H.; et al. Management of granulomatous mastitis: a series of 13 patients who were evaluated for treatment without corticosteroids. Int Surg 2015, 100, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Goulabchand, R.; Hafidi, A.; Van de Perre, P.; Millet, I.; Maria, A.T.J.; Morel, J.; Quellec, A.L.; Perrochia, H.; Guilpain, P. Mastitis in Autoimmune Diseases: Review of the Literature, Diagnostic Pathway, and Pathophysiological Key Players. J Clin Med 2020, 9. [Google Scholar] [CrossRef]
- Pevzner, M.; Dahan, A. Mastitis While Breastfeeding: Prevention, the Importance of Proper Treatment, and Potential Complications. J Clin Med 2020, 9. [Google Scholar] [CrossRef]
- Ong, S.S.; Xu, J.; Sim, C.K.; Khng, A.J.; Ho, P.J.; Kwan, P.K.W.; Ravikrishnan, A.; Tan, K.B.; Tan, Q.T.; Tan, E.Y.; et al. Profiling Microbial Communities in Idiopathic Granulomatous Mastitis. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Dilaveri, C.; Degnim, A.; Lee, C.; DeSimone, D.; Moldoveanu, D.; Ghosh, K. Idiopathic Granulomatous Mastitis. Breast J 2024, 2024, 6693720. [Google Scholar] [CrossRef]
- Ong, S.S.; Sim, J.X.Y.; Chan, C.W.; Ho, P.J.; Lim, Z.L.; Li, J.; Hartman, M. Current Approaches to Diagnosing and Treating Idiopathic Granulomatous Mastitis in Asia: A Summary from In-Depth Clinician Interviews. SSRN Preprints with The Lancet https://ssrn.com/abstract=4908728. 2024. [Google Scholar]
- Altintoprak, F.; Kivilcim, T.; Ozkan, O.V. Aetiology of idiopathic granulomatous mastitis. World J Clin Cases 2014, 2, 852–858. [Google Scholar] [CrossRef]
- Wolfrum, A.; Kummel, S.; Theuerkauf, I.; Pelz, E.; Reinisch, M. Granulomatous Mastitis: A Therapeutic and Diagnostic Challenge. Breast Care (Basel) 2018, 13, 413–418. [Google Scholar] [CrossRef]
- Sathyanarayanan, A.; Manda, S.; Poojary, M.; Nagaraj, S.H. Exome Sequencing Data Analysis. In Encyclopedia of Bioinformatics and Computational Biology, Ranganathan, S., Gribskov, M., Nakai, K., Schönbach, C., Eds.; Academic Press: Oxford, 2019; pp. 164–175. [Google Scholar]
- Sprissler, R.; Perkins, B.; Johnstone, L.; Babiker, H.M.; Chalasani, P.; Lau, B.; Hammer, M.; Mahadevan, D. Rare Tumor-Normal Matched Whole Exome Sequencing Identifies Novel Genomic Pathogenic Germline and Somatic Aberrations. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, Y.; Chen, X.; Chen, J.; Lin, S.; Li, X.; Du, H. Systematic comparison of somatic variant calling performance among different sequencing depth and mutation frequency. Sci Rep 2020, 10, 3501. [Google Scholar] [CrossRef]
- Jin, J.; Chen, Z.; Liu, J.; Du, H.; Zhang, G. Towards an accurate and robust analysis pipeline for somatic mutation calling. Front Genet 2022, 13, 979928. [Google Scholar] [CrossRef]
- Cai, L.; Yuan, W.; Zhang, Z.; He, L.; Chou, K.C. In-depth comparison of somatic point mutation callers based on different tumor next-generation sequencing depth data. Sci Rep 2016, 6, 36540. [Google Scholar] [CrossRef]
- Alioto, T.S.; Buchhalter, I.; Derdak, S.; Hutter, B.; Eldridge, M.D.; Hovig, E.; Heisler, L.E.; Beck, T.A.; Simpson, J.T.; Tonon, L.; et al. A comprehensive assessment of somatic mutation detection in cancer using whole-genome sequencing. Nat Commun 2015, 6, 10001. [Google Scholar] [CrossRef]
- Kroigard, A.B.; Thomassen, M.; Laenkholm, A.V.; Kruse, T.A.; Larsen, M.J. Evaluation of Nine Somatic Variant Callers for Detection of Somatic Mutations in Exome and Targeted Deep Sequencing Data. PLoS One 2016, 11, e0151664. [Google Scholar] [CrossRef]
- Kim, S.; Scheffler, K.; Halpern, A.L.; Bekritsky, M.A.; Noh, E.; Kallberg, M.; Chen, X.; Kim, Y.; Beyter, D.; Krusche, P.; et al. Strelka2: fast and accurate calling of germline and somatic variants. Nat Methods 2018, 15, 591–594. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Carson, A.R.; Smith, E.N.; Matsui, H.; Braekkan, S.K.; Jepsen, K.; Hansen, J.B.; Frazer, K.A. Effective filtering strategies to improve data quality from population-based whole exome sequencing studies. BMC Bioinformatics 2014, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Boulygina, E.A.; Borisov, O.V.; Valeeva, E.V.; Semenova, E.A.; Kostryukova, E.S.; Kulemin, N.A.; Larin, A.K.; Nabiullina, R.M.; Mavliev, F.A.; Akhatov, A.M.; et al. Whole genome sequencing of elite athletes. Biol Sport 2020, 37, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Ng, C.K.Y.; Lim, R.S.; Jiang, T.; Kumar, S.; Li, X.; Wali, V.B.; Piscuoglio, S.; Gerstein, M.B.; Chagpar, A.B.; et al. Reliability of Whole-Exome Sequencing for Assessing Intratumor Genetic Heterogeneity. Cell Rep 2018, 25, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Lyu, N.; Guan, L.L.; Ma, H.; Wang, X.J.; Wu, B.M.; Shang, F.H.; Wang, D.; Wen, H.; Yu, X. Failure to Identify Somatic Mutations in Monozygotic Twins Discordant for Schizophrenia by Whole Exome Sequencing. Chin Med J (Engl) 2016, 129, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Ren, L.; Chen, Z.; Fang, L.T.; Zhao, Y.; Lack, J.; Guan, M.; Zhu, B.; Jaeger, E.; Kerrigan, L.; et al. Toward best practice in cancer mutation detection with whole-genome and whole-exome sequencing. Nat Biotechnol 2021, 39, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Barbitoff, Y.A.; Abasov, R.; Tvorogova, V.E.; Glotov, A.S.; Predeus, A.V. Systematic benchmark of state-of-the-art variant calling pipelines identifies major factors affecting accuracy of coding sequence variant discovery. BMC Genomics 2022, 23, 155. [Google Scholar] [CrossRef] [PubMed]
- Belien, J.; Swinnen, S.; D’Hondt, R.; de Juan, L.V.; Dedoncker, N.; Matthys, P.; Bauer, J.; Vens, C.; Moylett, S.; Dubois, B. CHIT1 at diagnosis predicts faster disability progression and reflects early microglial activation in multiple sclerosis. Nat Commun 2024, 15, 5013. [Google Scholar] [CrossRef] [PubMed]
- Dymek, B.; Sklepkiewicz, P.; Mlacki, M.; Guner, N.C.; Nejman-Gryz, P.; Drzewicka, K.; Przysucha, N.; Rymaszewska, A.; Paplinska-Goryca, M.; Zagozdzon, A.; et al. Pharmacological Inhibition of Chitotriosidase (CHIT1) as a Novel Therapeutic Approach for Sarcoidosis. J Inflamm Res 2022, 15, 5621–5634. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.M.; Verrecchia, E.; Sicignano, L.L.; Massaro, M.G.; Antuzzi, D.; Covino, M.; Pasciuto, G.; Richeldi, L.; Manna, R. The Use of Chitotriosidase as a Marker of Active Sarcoidosis and in the Diagnosis of Fever of Unknown Origin (FUO). J Clin Med 2021, 10. [Google Scholar] [CrossRef]
- Mazur, M.; Zielinska, A.; Grzybowski, M.M.; Olczak, J.; Fichna, J. Chitinases and Chitinase-Like Proteins as Therapeutic Targets in Inflammatory Diseases, with a Special Focus on Inflammatory Bowel Diseases. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Cho, S.J.; Weiden, M.D.; Lee, C.G. Chitotriosidase in the Pathogenesis of Inflammation, Interstitial Lung Diseases and COPD. Allergy Asthma Immunol Res 2015, 7, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Q.; Shao, Z.M. Identification of immune-related prognostic biomarkers in triple-negative breast cancer. Transl Cancer Res 2024, 13, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Wiechmann, S.; Ruprecht, B.; Siekmann, T.; Zheng, R.; Frejno, M.; Kunold, E.; Bajaj, T.; Zolg, D.P.; Sieber, S.A.; Gassen, N.C.; et al. Chemical Phosphoproteomics Sheds New Light on the Targets and Modes of Action of AKT Inhibitors. ACS Chem Biol 2021, 16, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Hernandez, C.; van Beelen Granlund, A.; Bruland, T.; Sandvik, A.K.; Koch, S.; Ostvik, A.E.; Munch, A. Transcriptomic Profiling of Collagenous Colitis Identifies Hallmarks of Nondestructive Inflammatory Bowel Disease. Cell Mol Gastroenterol Hepatol 2021, 12, 665–687. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, B.; Liu, L.; Sun, S.; Sun, S. Centrosome dysfunction: a link between senescence and tumor immunity. Signal Transduct Target Ther 2020, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, J.W.; Yoo, K.D.; Yoo, J.Y.; Lee, J.P.; Kim, D.K.; Chin, H.J.; Kim, Y.S.; Yang, S.H. Cln 3-requiring 9 is a negative regulator of Th17 pathway-driven inflammation in anti-glomerular basement membrane glomerulonephritis. Am J Physiol Renal Physiol 2016, 311, F505–519. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Bae, E.; Kwon, S.H.; Yu, M.Y.; Cha, R.H.; Lee, H.; Kim, D.K.; Lee, J.P.; Ye, S.K.; Yoo, J.Y.; et al. Transcriptional modulation of the T helper 17/interleukin 17 axis ameliorates renal ischemia-reperfusion injury. Nephrol Dial Transplant 2019, 34, 1481–1498. [Google Scholar] [CrossRef]
- Vinayagam, R.; Cox, J.; Webb, L. Granulomatous Mastitis: A Spectrum of Disease. Breast Care (Basel) 2009, 4, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Corominas, J.; Smeekens, S.P.; Nelen, M.R.; Yntema, H.G.; Kamsteeg, E.J.; Pfundt, R.; Gilissen, C. Clinical exome sequencing-Mistakes and caveats. Hum Mutat 2022, 43, 1041–1055. [Google Scholar] [CrossRef]
- Duan, J.; Liu, H.; Zhao, L.; Yuan, X.; Wang, Y.P.; Wan, M. Detection of False-Positive Deletions from the Database of Genomic Variants. Biomed Res Int 2019, 2019, 8420547. [Google Scholar] [CrossRef]
- Arteche-Lopez, A.; Avila-Fernandez, A.; Romero, R.; Riveiro-Alvarez, R.; Lopez-Martinez, M.A.; Gimenez-Pardo, A.; Velez-Monsalve, C.; Gallego-Merlo, J.; Garcia-Vara, I.; Almoguera, B.; et al. Sanger sequencing is no longer always necessary based on a single-center validation of 1109 NGS variants in 825 clinical exomes. Sci Rep 2021, 11, 5697. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Huang, H.D.; Yeh, K.T.; Chang, J.G. Evaluation of whole exome sequencing by targeted gene sequencing and Sanger sequencing. Clin Chim Acta 2017, 471, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.N. Whole-exome sequencing for finding de novo mutations in sporadic mental retardation. Genome Biol 2010, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Lian, T.; Lin, J.; Li, S.; Zheng, H.; Cheng, C.; Ye, J.; Jing, Z.; Wang, X.; Huang, W. Whole-exome sequencing improves genetic testing accuracy in pulmonary artery hypertension. Pulm Circ 2018, 8, 2045894018763682. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.H.; Chen, S.X.; Cheng, L.Y.; Rodriguez, A.Y.; Tang, R.; Cabrera, K.; Zhang, D.Y. Confirming putative variants at </= 5% allele frequency using allele enrichment and Sanger sequencing. Sci Rep 2021, 11, 11640. [Google Scholar] [CrossRef]
- Hamilton, A.; Tetreault, M.; Dyment, D.A.; Zou, R.; Kernohan, K.; Geraghty, M.T.; Consortium, F.C.; Care4Rare Canada, C.; Hartley, T.; Boycott, K.M. Concordance between whole-exome sequencing and clinical Sanger sequencing: implications for patient care. Mol Genet Genomic Med 2016, 4, 504–512. [Google Scholar] [CrossRef]
- Tattini, L.; D’Aurizio, R.; Magi, A. Detection of Genomic Structural Variants from Next-Generation Sequencing Data. Front Bioeng Biotechnol 2015, 3, 92. [Google Scholar] [CrossRef]
- Mahmodlou, R.; Dadkhah, N.; Abbasi, F.; Nasiri, J.; Valizadeh, R. Idiopathic granulomatous mastitis: dilemmas in diagnosis and treatment. Electron Physician 2017, 9, 5375–5379. [Google Scholar] [CrossRef]
- Pourhoseingholi, M.A.; Vahedi, M.; Rahimzadeh, M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench 2013, 6, 14–17. [Google Scholar]
- Maione, C.; Palumbo, V.D.; Maffongelli, A.; Damiano, G.; Buscemi, S.; Spinelli, G.; Fazzotta, S.; Gulotta, E.; Buscemi, G.; Lo Monte, A.I. Diagnostic techniques and multidisciplinary approach in idiopathic granulomatous mastitis: a revision of the literature. Acta Biomed 2019, 90, 11–15. [Google Scholar] [CrossRef]
- Hanssen, F.; Garcia, M.U.; Folkersen, L.; Pedersen, A.S.; Lescai, F.; Jodoin, S.; Miller, E.; Seybold, M.; Wacker, O.; Smith, N.; et al. Scalable and efficient DNA sequencing analysis on different compute infrastructures aiding variant discovery. NAR Genom Bioinform 2024, 6, lqae031. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Juhos, S.; Larsson, M.; Olason, P.I.; Martin, M.; Eisfeldt, J.; DiLorenzo, S.; Sandgren, J.; Diaz De Stahl, T.; Ewels, P.; et al. Sarek: A portable workflow for whole-genome sequencing analysis of germline and somatic variants. F1000Res 2020, 9, 63. [Google Scholar] [CrossRef]
- Ewels, P.A.; Peltzer, A.; Fillinger, S.; Patel, H.; Alneberg, J.; Wilm, A.; Garcia, M.U.; Di Tommaso, P.; Nahnsen, S. The nf-core framework for community-curated bioinformatics pipelines. Nat Biotechnol 2020, 38, 276–278. [Google Scholar] [CrossRef]
- Di Tommaso, P.; Chatzou, M.; Floden, E.W.; Barja, P.P.; Palumbo, E.; Notredame, C. Nextflow enables reproducible computational workflows. Nat Biotechnol 2017, 35, 316–319. [Google Scholar] [CrossRef]
- Kurtzer, G.M.; Sochat, V.; Bauer, M.W. Singularity: Scientific containers for mobility of compute. PLoS One 2017, 12, e0177459. [Google Scholar] [CrossRef] [PubMed]
- Maxime U Garcia, F.H., Anders Sune Pedersen, Gisela Gabernet, WackerO, SusiJo, et al. nf-core/sarek: Sarek 3.3.0 - Rapaselet. Zenodo 2023. [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv: Genomics (q-bio.GN). [CrossRef]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 2011, 43, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics 2013, 43, 11 10 11–11 10 33. [Google Scholar] [CrossRef]
- Sondka, Z.; Bamford, S.; Cole, C.G.; Ward, S.A.; Dunham, I.; Forbes, S.A. The COSMIC Cancer Gene Census: describing genetic dysfunction across all human cancers. Nat Rev Cancer 2018, 18, 696–705. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Riley, G.R.; Jang, W.; Rubinstein, W.S.; Church, D.M.; Maglott, D.R. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res 2014, 42, D980–985. [Google Scholar] [CrossRef]
- Harrison, S.M.; Riggs, E.R.; Maglott, D.R.; Lee, J.M.; Azzariti, D.R.; Niehaus, A.; Ramos, E.M.; Martin, C.L.; Landrum, M.J.; Rehm, H.L. Using ClinVar as a Resource to Support Variant Interpretation. Curr Protoc Hum Genet 2016, 89, 8 16 11–8 16 23. [Google Scholar] [CrossRef]
- Sherry, S.T.; Ward, M.; Sirotkin, K. dbSNP-database for single nucleotide polymorphisms and other classes of minor genetic variation. Genome Res 1999, 9, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.D.; Mitchell, M.; Lindahl, T. Human DNA repair genes, 2005. Mutat Res 2005, 577, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Sijmons, R.H.; Burger, G.T. Familial cancer database: a clinical aide-memoire. Fam Cancer 2001, 1, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Sijmons, R.H.; Burger, G.T. The Use of a Diagnostic Database in Clinical Oncogenetics. Hered Cancer Clin Pract. 2003, 1, 31–33. [Google Scholar] [CrossRef]
- Harrow, J.; Frankish, A.; Gonzalez, J.M.; Tapanari, E.; Diekhans, M.; Kokocinski, F.; Aken, B.L.; Barrell, D.; Zadissa, A.; Searle, S.; et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res 2012, 22, 1760–1774. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, S.; Singer-Berk, M.; Watts, N.A.; Phu, W.; Goodrich, J.K.; Solomonson, M.; Genome Aggregation Database, C.; Rehm, H.L.; MacArthur, D.G.; O’Donnell-Luria, A. Variant interpretation using population databases: Lessons from gnomAD. Hum Mutat 2022, 43, 1012–1030. [Google Scholar] [CrossRef] [PubMed]
- Seal, R.L.; Braschi, B.; Gray, K.; Jones, T.E.M.; Tweedie, S.; Haim-Vilmovsky, L.; Bruford, E.A. Genenames.org: the HGNC resources in 2023. Nucleic Acids Res 2023, 51, D1003–D1009. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsdottir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform 2013, 14, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3--new capabilities and interfaces. Nucleic Acids Res 2012, 40, e115. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef]
- Koressaar, T.; Lepamets, M.; Kaplinski, L.; Raime, K.; Andreson, R.; Remm, M. Primer3_masker: integrating masking of template sequence with primer design software. Bioinformatics 2018, 34, 1937–1938. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 2016, 44, W90–97. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr Protoc 2021, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag New York: 2016.
| Demographic and clinical parameters | IGM n = 8 |
|---|---|
| Demographics | |
| Median age at diagnosis (years, IQR) | 33.0 (27.3-34.5) |
| Ethnicity (n, %) | |
| Chinese | 4 (50) |
| Malay | 3 (38) |
| Others | 1 (12) |
| Body mass index (kg/m2, IQR) | 27.770 (23.460-33.454) |
| Education level (n, %) | |
| Up to secondary school | 3 (38) |
| Secondary school to pre-university | 3 (38) |
| Tertiary education | 2 (25) |
| Patient characteristics | |
| Parity (n, %) | |
| Yes | 6 (75) |
| No | 2 (25) |
| Number of children (n, %) | |
| No children | 2 (25) |
| 1-2 children | 5 (62) |
| More than 2 children | 1 (12) |
| Smoking (n, %) | |
| Yes | 2 (25) |
| No | 6 (75) |
| Chronic illness1 diagnosis (n, %) | |
| Yes | 2 (25) |
| No | 6 (75) |
| Family history2 of breast cancer (n, %) | |
| Yes | 1 (12) |
| No | 7 (88) |
| Case | Somatic variants | SNVs1 | Indels2 | PTVs3 | Pathogenic4 | Pathogenic / Likely Pathogenic4 | Likely Pathogenic4 |
|---|---|---|---|---|---|---|---|
| 1 | 3 | 3 | 0 | 0 | 0 | 0 | 0 |
| 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| 3 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| 4 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| 5 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| 6 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| 7 | 3 | 3 | 0 | 0 | 0 | 0 | 0 |
| 8 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| Median (range) | 2 (1-3) | 2 (1-3) | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





