Submitted:
31 July 2024
Posted:
01 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Extraction of GLP
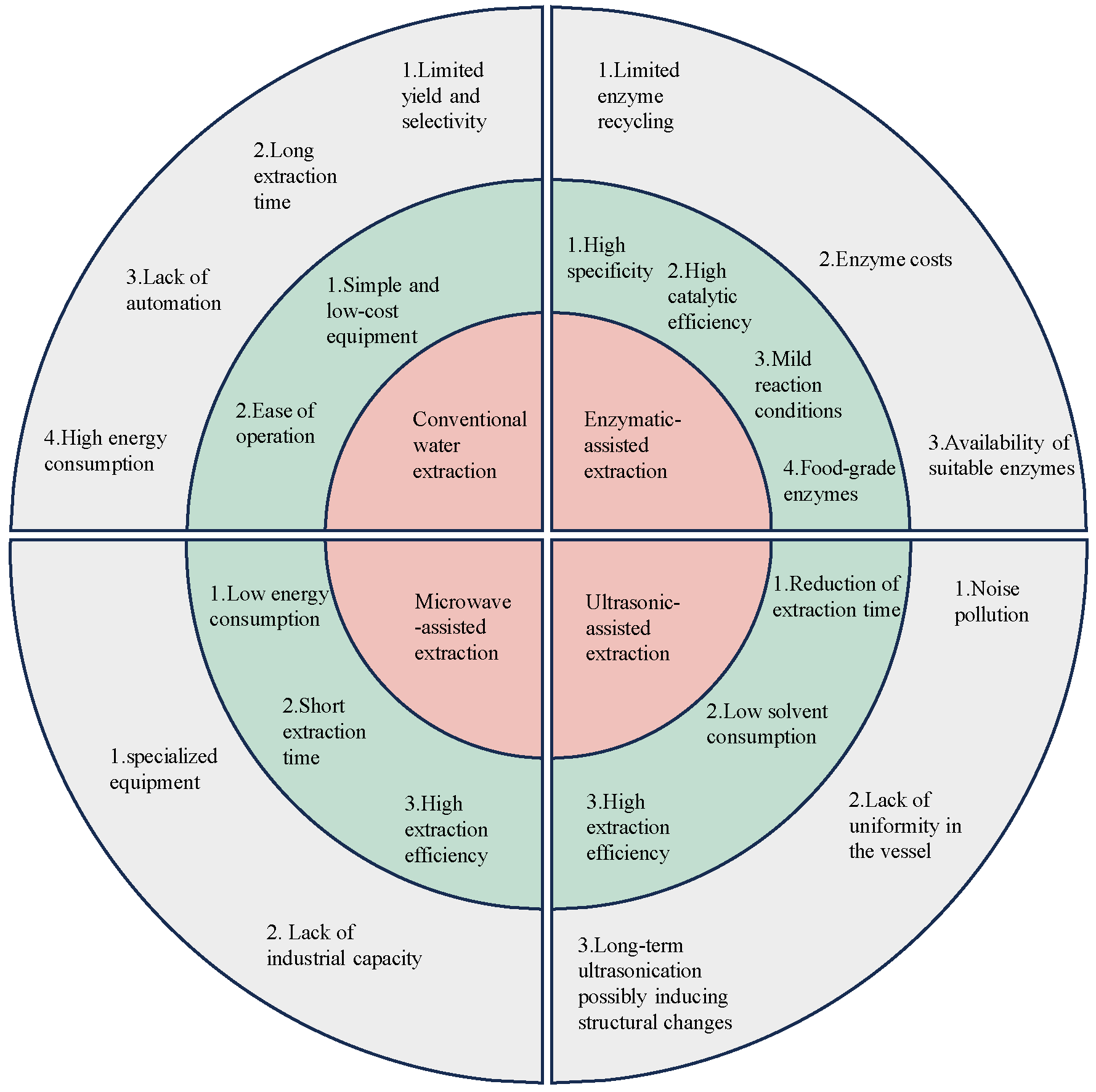
2.1. Traditional Extraction Method
2.2. Microwave-Assisted Extraction Method
2.3. Ultrasonic-Assisted Extraction Method
2.4. Enzymatic-Assisted Extraction Method
2.5. Other Extraction Techniques
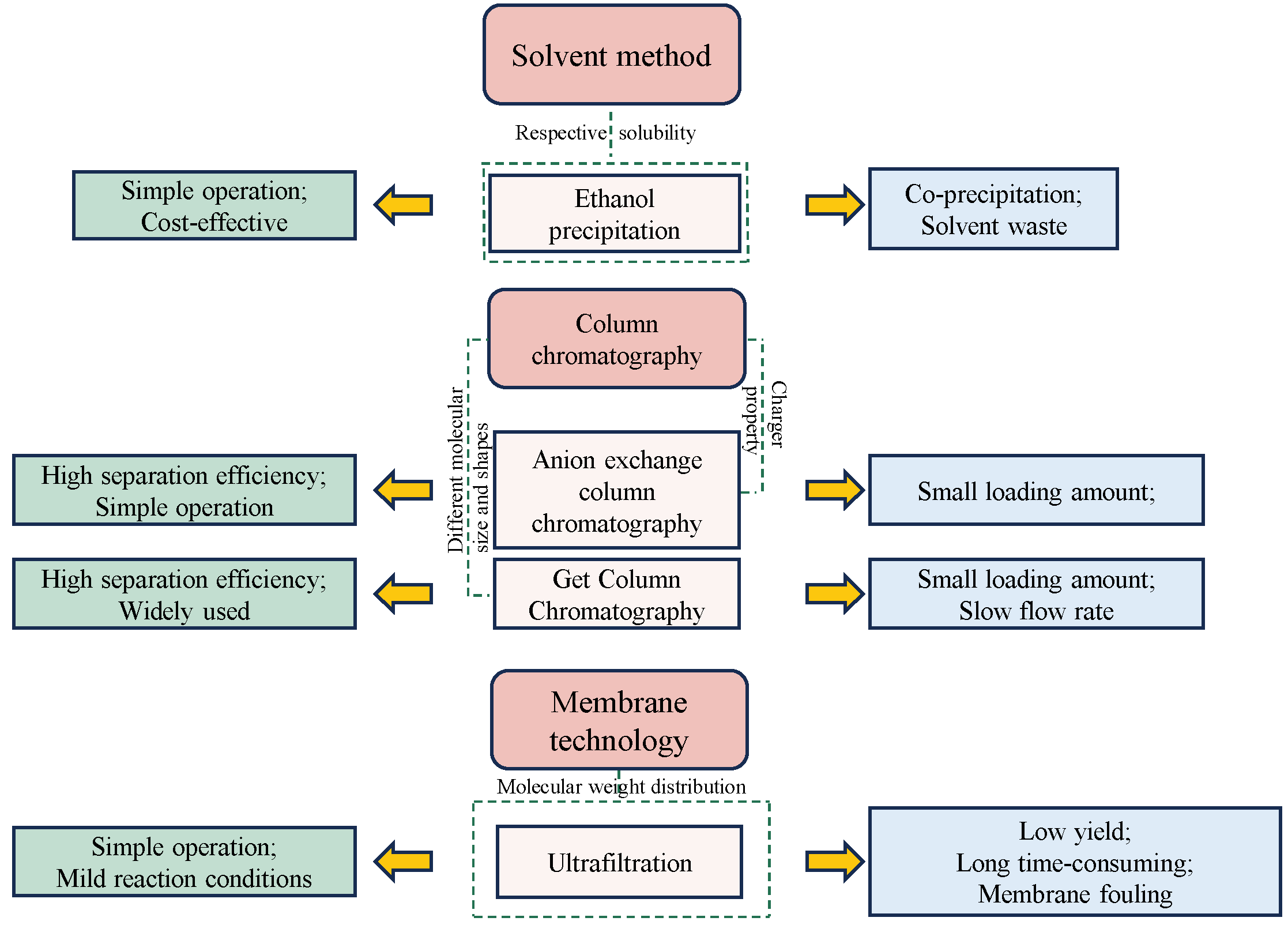
3. Purification of GLP
4. Structural Characterization of GLP
4.1. Monosaccharide Composition
4.2. Molecular Weight
4.3. Glycosidic Linkages
5. Biological Activities of GLP
5.1. Antioxidant Activities
5.2. Immuno-Modulatory Activity
5.3. Anti-Tumor Activity
5.4. Intestinal Health and Gut Microbiota
6. Conclusion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. , Marine Bioactive Compounds and Their Health Benefits: A Review. Comprehensive Reviews in Food Science and Food Safety 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, Y.; Zhang, G. , Marine peptides as potential anti-aging agents: Preparation, characterization, mechanisms of action, and future perspectives. Food Chemistry 2024, 460, 140413. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, L.; Ma, Y.; Huan, L.; Wang, Y.; Xia, B.; Wang, G. , Economically important red algae resources along the Chinese coast: History, status, and prospects for their utilization. Algal Research 2020, 46, 101817. [Google Scholar] [CrossRef]
- Wei, Z.; You, J.; Wu, H.; Yang, F.; Long, L.; Liu, Q.; Huo, Y.; He, P. , Bioremediation using Gracilaria lemaneiformis to manage the nitrogen and phosphorous balance in an integrated multi-trophic aquaculture system in Yantian Bay, China. Marine Pollution Bulletin 2017, 121, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, D.Y.; Israel, A.; Abelson, A. , A novel two-stage seaweed integrated multi-trophic aquaculture. Reviews in Aquaculture 2019, 11, 246–262. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; dos Santos, D.Y.A.C. , A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Research 2019, 37, 288–306. [Google Scholar] [CrossRef]
- Pang, Y.; Peng, Z.; Ding, K. , An in-depth review: Unraveling the extraction, structure, bio-functionalities, target molecules, and applications of pectic polysaccharides. Carbohydrate Polymers 2024, 343, 122457. [Google Scholar] [CrossRef]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviðsson, G.O.; Nordberg Karlsson, E. , Extraction and Modification of Macroalgal Polysaccharides for Current and Next-Generation Applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Shi, M.; Yan, Y.; Liu, Y. , An Insight into the Research Concerning Panax ginseng C. A. Meyer Polysaccharides: A Review. Food Reviews International 2022, 38, 1149–1165. [Google Scholar] [CrossRef]
- Zhang, X.; Aweya, J.J.; Huang, Z.-X.; Kang, Z.-Y.; Bai, Z.-H.; Li, K.-H.; He, X.-T.; Liu, Y.; Chen, X.-Q.; Cheong, K.-L. , In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydrate Polymers 2020, 234, 115894. [Google Scholar] [CrossRef]
- Liu, Q.-M.; Yang, Y.; Maleki, S.J.; Alcocer, M.; Xu, S.-S.; Shi, C.-L.; Cao, M.-J.; Liu, G.-M. , Anti-Food Allergic Activity of Sulfated Polysaccharide from Gracilaria lemaneiformis is Dependent on Immunosuppression and Inhibition of p38 MAPK. Journal of Agricultural and Food Chemistry 2016, 64, 4536–4544. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, W.; Song, W.; Chen, H.; Teng, A.; Liu, A. , Partial characterization and anti-tumor activity of an acidic polysaccharide from Gracilaria lemaneiformis. Carbohydrate Polymers 2012, 88, 1313–1318. [Google Scholar] [CrossRef]
- Sun, Y.; He, H.; Wang, Q.; Yang, X.; Jiang, S.; Wang, D. , A Review of Development and Utilization for Edible Fungal Polysaccharides: Extraction, Chemical Characteristics, and Bioactivities. Polymers 2022, 14, 4454. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Yang, F.-C.; Chang, J.-S. , Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydrate Polymers 2021, 251, 117006. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Franke, K.; Kießling, M.; Fischer, S.; Töpfl, S.; Heinz, V.; Becker, T. , Influence of hydrothermal treatment on the structural modification of spent grain specific carbohydrates and the formation of degradation products using model compounds. Carbohydrate Polymers 2018, 184, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Hu, X.; Xiang, H.; Chen, S.; Li, L.; Qi, B.; Li, C.; Liu, S.; Yang, X. , Structural characterization and hypolipidemic activity of Gracilaria lemaneiformis polysaccharide and its degradation products. Food Chemistry: X 2022, 14, 100314. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Pang, D.; Wen, L.; You, L.; Huang, R.; Kulikouskaya, V. , In vitro digestibility and prebiotic activities of a sulfated polysaccharide from Gracilaria Lemaneiformis. Journal of Functional Foods 2020, 64, 103652. [Google Scholar] [CrossRef]
- He, L.; Yan, X.; Liang, J.; Li, S.; He, H.; Xiong, Q.; Lai, X.; Hou, S.; Huang, S. , Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem. Carbohydrate Polymers 2018, 198, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liu, D.; Yin, J.-Y.; Nie, S.-P. , Consecutive and progressive purification of food-derived natural polysaccharide: Based on material, extraction process and crude polysaccharide. Trends in Food Science & Technology 2020, 99, 76–87. [Google Scholar]
- Hu, Q.; He, Y.; Wang, F.; Wu, J.; Ci, Z.; Chen, L.; Xu, R.; Yang, M.; Lin, J.; Han, L.; Zhang, D. , Microwave technology: A novel approach to the transformation of natural metabolites. Chinese Medicine 2021, 16, 87. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Marti-Quijal, F.J.; Barba, F.J.; Altintas, Z. , Current emerging trends in antitumor activities of polysaccharides extracted by microwave- and ultrasound-assisted methods. International Journal of Biological Macromolecules 2022, 202, 494–507. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, Y.; Chen, H.; Sun, P. , Extraction, Structural Characterization, and Potential Antioxidant Activity of the Polysaccharides from Four Seaweeds. International Journal of Molecular Sciences 2016, 17, 1988. [Google Scholar] [CrossRef] [PubMed]
- Hashemifesharaki, R.; Xanthakis, E.; Altintas, Z.; Guo, Y.; Gharibzahedi, S.M.T. , Microwave-assisted extraction of polysaccharides from the marshmallow roots: Optimization, purification, structure, and bioactivity. Carbohydrate Polymers 2020, 240, 116301. [Google Scholar] [CrossRef] [PubMed]
- Guzik, P.; Kulawik, P.; Zając, M.; Migdał, W. , Microwave applications in the food industry: An overview of recent developments. Critical Reviews in Food Science and Nutrition 2022, 62, 7989–8008. [Google Scholar] [CrossRef] [PubMed]
- Arrutia, F.; Adam, M.; Calvo-Carrascal, M.Á.; Mao, Y.; Binner, E. , Development of a continuous-flow system for microwave-assisted extraction of pectin-derived oligosaccharides from food waste. Chemical Engineering Journal 2020, 395, 125056. [Google Scholar] [CrossRef]
- Wu, J.; Chen, R.; Tan, L.; Bai, H.; Tian, L.; Lu, J.; Gao, M.; Bai, C.; Sun, H.; Chi, Y. , Ultrasonic disruption effects on the extraction efficiency, characterization, and bioactivities of polysaccharides from Panax notoginseng flower. Carbohydrate Polymers 2022, 291, 119535. [Google Scholar] [CrossRef] [PubMed]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. , Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. International Journal of Environmental Research and Public Health 2021, 18. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Luo, X.; Wang, M.; Wang, Z.; Guo, J.; Kong, F.; Bi, Y. , Synthesis, characterization, in vitro antioxidant and hypoglycemic activities of selenium nanoparticles decorated with polysaccharides of Gracilaria lemaneiformis. International Journal of Biological Macromolecules 2021, 193, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Yan, X.; Cheong, K.-L.; Liu, Y. , Extraction, purification, and characterization of polysaccharides from marine algae Gracilaria lemaneiformis with anti-tumor activity. Process Biochemistry 2018, 73, 197–203. [Google Scholar] [CrossRef]
- Giovannoni, M.; Gramegna, G.; Benedetti, M.; Mattei, B. , Industrial Use of Cell Wall Degrading Enzymes: The Fine Line Between Production Strategy and Economic Feasibility. Frontiers in Bioengineering and Biotechnology 2020, 8, 356. [Google Scholar] [CrossRef]
- Fournière, M.; Latire, T.; Lang, M.; Terme, N.; Bourgougnon, N.; Bedoux, G. , Production of Active Poly- and Oligosaccharidic Fractions from Ulva sp. by Combining Enzyme-Assisted Extraction (EAE) and Depolymerization. Metabolites 2019, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Łubek-Nguyen, A.; Ziemichód, W.; Olech, M. , Application of Enzyme-Assisted Extraction for the Recovery of Natural Bioactive Compounds for Nutraceutical and Pharmaceutical Applications. Applied Sciences 2022, 12, 3232. [Google Scholar] [CrossRef]
- Wu, S.; Lu, M.; Wang, S. , Amylase-assisted extraction and antioxidant activity of polysaccharides from Gracilaria lemaneiformis. 3 Biotech 2017, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiao, Q.; Weng, H.; Zhang, Y.; Yang, Q.; Xiao, A. , Extraction of sulfated agar from Gracilaria lemaneiformis using hydrogen peroxide-assisted enzymatic method. Carbohydrate Polymers 2020, 232, 115790. [Google Scholar] [CrossRef] [PubMed]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Bursać Kovačević, D.; Elez Garofulić, I.; Dragović-Uzelac, V. , Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Marine Drugs 2020, 18, 168. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Nadar, S.S.; Rathod, V.K. , Integrated strategies for enzyme assisted extraction of bioactive molecules: A review. International Journal of Biological Macromolecules 2021, 191, 899–917. [Google Scholar] [CrossRef]
- Boussetta, N.; Turk, M.; De Taeye, C.; Larondelle, Y.; Lanoisellé, J.L.; Vorobiev, E. , Effect of high voltage electrical discharges, heating and ethanol concentration on the extraction of total polyphenols and lignans from flaxseed cake. Industrial Crops and Products 2013, 49, 690–696. [Google Scholar] [CrossRef]
- Ju, T.; Deng, Y.; Xi, J. , Optimization of Circulating Extraction of Polysaccharides from Gracilaria Lemaneiformis Using Pulsed Electrical Discharge. ACS Sustainable Chemistry & Engineering 2019, 7, 3593–3601. [Google Scholar]
- Ju, T.; Xi, J. , Continuous extraction optimization, molecular structures and antioxidant activities of polysaccharide from Gracilariopsis lemaneiformis using liquid-phase pulsed discharge. Separation and Purification Technology 2020, 236, 116241. [Google Scholar] [CrossRef]
- Niu, G.; You, G.; Zhou, X.; Fan, H.; Liu, X. , Physicochemical properties and in vitro hypoglycemic activities of hsian-tsao polysaccharide fractions by gradient ethanol precipitation method. International Journal of Biological Macromolecules 2023, 231, 123274. [Google Scholar] [CrossRef]
- Zou, P.; Yang, X.; Huang, W.-W.; Zhao, H.-T.; Wang, J.; Xu, R.-B.; Hu, X.-L.; Shen, S.-Y.; Qin, D. , Characterization and Bioactivity of Polysaccharides Obtained from Pine Cones of Pinus koraiensis by Graded Ethanol Precipitation. Molecules 2013, 18, 9933–9948. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gong, Y.; Yao, W.; Chen, X.; Xian, J.; You, L.; Fardim, P. , Structural characterization and protective effects of polysaccharide from Gracilaria lemaneiformis on LPS-induced injury in IEC-6 cells. Food Chemistry: X 2021, 12, 100157. [Google Scholar] [CrossRef] [PubMed]
- Shi, L. , Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. International Journal of Biological Macromolecules 2016, 92, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Bai, Y.; Zhang, Z.; Cai, W.; Del Rio Flores, A. , The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-B.; Li, B.; Guo, L.-W.; Pan, L.-M.; Zhu, H.-X.; Tang, Z.-S.; Xing, W.-H.; Cai, Y.-Y.; Duan, J.-A.; Wang, M.; Xu, S.-N.; Tao, X.-B. , Current and Future Use of Membrane Technology in the Traditional Chinese Medicine Industry. Separation & Purification Reviews 2022, 51, 484–502. [Google Scholar]
- Shao, W.; Zhang, H.; Duan, R.; Xie, Q.; Hong, Z.; Xiao, Z. , A rapid and scalable integrated membrane separation process for purification of polysaccharides from Enteromorpha prolifera. Natural Product Research 2019, 33, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, S.; Chen, X.; Xu, Q.; Ma, Y.; You, L.; Kulikouskaya, V.; Xiao, J.; Piao, J. , Structural characteristic of a sulfated polysaccharide from Gracilaria Lemaneiformis and its lipid metabolism regulation effect. Food & Function 2020, 11, 10876–10885. [Google Scholar]
- Eder, S.; Zueblin, P.; Diener, M.; Peydayesh, M.; Boulos, S.; Mezzenga, R.; Nyström, L. , Effect of Polysaccharide Conformation on Ultrafiltration Separation Performance. Carbohydrate Polymers 2021, 260, 117830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Teng, J.; Zou, H.; Zhang, W.; Cheng, S.; Zhang, M.; Lin, H. , The molecular weight-fouling matrix: A novel dissection of polysaccharide interactions in ultrafiltration processes. Separation and Purification Technology 2024, 345, 127340. [Google Scholar] [CrossRef]
- Sun, H.; Qi, D.; Xu, J.; Juan, S.; Zhe, C. , Fractionation of polysaccharides from rapeseed by ultrafiltration: Effect of molecular pore size and operation conditions on the membrane performance. Separation and Purification Technology 2011, 80, 670–676. [Google Scholar] [CrossRef]
- Xie, J.-H.; Shen, M.-Y.; Nie, S.-P.; Zhao, Q.; Li, C.; Xie, M.-Y. , Separation of water-soluble polysaccharides from Cyclocarya paliurus by ultrafiltration process. Carbohydrate Polymers 2014, 101, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Jonsson, G.; Meyer, A.S. , Membrane technology for purification of enzymatically produced oligosaccharides: Molecular and operational features affecting performance. Separation and Purification Technology 2009, 70, 1–11. [Google Scholar] [CrossRef]
- Kravtchenko, T.P.; Voragen, A.G.J.; Pilnik, W. , Studies on the intermolecular distribution of industrial pectins by means of preparative ion-exchange chromatography. Carbohydrate Polymers 1992, 19, 115–124. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, F.; Ming, K.; Wang, D.; Hu, Y.; Liu, J. , Polysaccharides from Traditional Chinese Medicines: Extraction, Purification, Modification, and Biological Activity. Molecules 2016, 21, 1705. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ai, C.; Yao, H.; Zhao, C.; Xiang, C.; Hong, T.; Xiao, J. , Guideline for the extraction, isolation, purification, and structural characterization of polysaccharides from natural resources. eFood 2022, 3, e37. [Google Scholar] [CrossRef]
- Veeraperumal, S.; Qiu, H.-M.; Zeng, S.-S.; Yao, W.-Z.; Wang, B.-P.; Liu, Y.; Cheong, K.-L. , Polysaccharides from Gracilaria lemaneiformis promote the HaCaT keratinocytes wound healing by polarised and directional cell migration. Carbohydrate Polymers 2020, 241, 116310. [Google Scholar] [CrossRef]
- Ren, Y.; Zheng, G.; You, L.; Wen, L.; Li, C.; Fu, X.; Zhou, L. , Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. Journal of Functional Foods 2017, 33, 286–296. [Google Scholar] [CrossRef]
- Lecacheux, D.; Brigand, G. , Preparative fractionation of natural polysaccharides by size exclusion chromatography. Carbohydrate Polymers 1988, 8, 119–130. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liu, Z.; Zhao, M.; Liu, P. , Purification, Characterization, and Antioxidant Activity of Polysaccharides Isolated from Cortex Periplocae. Molecules 2017, 22, 1866. [Google Scholar] [CrossRef]
- Yang, X.Q.; Liu, M.Q.; Qi, B.; Li, L.H.; Deng, J.C.; Hu, X.; Wu, Y.Y.; Hao, S.X. , Extraction, purification and partial characterizations of polysaccharides from Gracilaria lemaneiformis. Advanced Materials Research 2014, 881, 776–780. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, Z.-J.; Xie, D.; Sun, X.; Yang, W.; Zhao, X.; Xu, N. , Characterization and Potential Antitumor Activity of Polysaccharide from Gracilariopsis lemaneiformis. Marine Drugs 2017, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. , Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Research International 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.-L.; Liu, K.; Chen, W.; Zhong, S.; Tan, K. , Recent progress in Porphyra haitanensis polysaccharides: Extraction, purification, structural insights, and their impact on gastrointestinal health and oxidative stress management. Food Chemistry: X 2024, 22, 101414. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Gong, Y.; Li, L.; Hu, X.; You, L. , The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chemistry: X 2022, 13, 100252. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Shi, S.; Wang, H.; Liu, R.; Li, N.; Chen, Y.; Wang, S. , Neutral monosaccharide composition analysis of plant-derived oligo- and polysaccharides by high performance liquid chromatography. Carbohydrate Polymers 2016, 136, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Suárez, M.J.; Redondo-Cuenca, A.; Rodríguez-Sevilla, M.D.; de las Heras Martínez, M. , Characterization of Nonstarch Polysaccharides Content from Different Edible Organs of Some Vegetables, Determined by GC and HPLC: Comparative Study. Journal of Agricultural and Food Chemistry 2003, 51, 5950–5955. [Google Scholar] [CrossRef] [PubMed]
- Bakky, M.A.H.; Tran, N.T.; Zhang, M.; Zhang, Y.; Liang, H.; Wang, Y.; Zhang, Y.; Ma, H.; Zheng, H.; Li, S. , In vitro fermentation of Gracilaria lemaneiformis and its sulfated polysaccharides by rabbitfish gut microbes. International Journal of Biological Macromolecules 2023, 246, 125561. [Google Scholar] [CrossRef] [PubMed]
- Jol, C.N.; Neiss, T.G.; Penninkhof, B.; Rudolph, B.; De Ruiter, G.A. , A Novel High-Performance Anion-Exchange Chromatographic Method for the Analysis of Carrageenans and Agars Containing 3,6-Anhydrogalactose. Analytical Biochemistry 1999, 268, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Navarro, D.A.; Stortz, C.A. , Determination of the configuration of 3,6-anhydrogalactose and cyclizable α-galactose 6-sulfate units in red seaweed galactans. Carbohydrate Research 2003, 338, 2111–2118. [Google Scholar] [CrossRef]
- Xie, X.-T.; Zhang, X.; Liu, Y.; Chen, X.-Q.; Cheong, K.-L. , Quantification of 3,6-anhydro-galactose in red seaweed polysaccharides and their potential skin-whitening activity. 3 Biotech 2020, 10, 189. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Mao, G.; Guo, Y.; Wang, G.; Sun, X.; Xu, N.; Zhang, Z. , Structural Characterization of Gracilariopsis lemaneiformis Polysaccharide and Its Property in Delaying Cellular Senescence. Frontiers in Nutrition 2022, 9, 876992. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Yang, L.; Chen, M.; Yu, J.; Zhang, S.; Ju, Y. , The hypoglycemic effect of a polysaccharide (GLP) from Gracilaria lemaneiformis and its degradation products in diabetic mice. Food & Function 2015, 6, 2542–2549. [Google Scholar]
- Bajwa, B.; Xing, X.; Terry, S.A.; Gruninger, R.J.; Abbott, D.W. , Methylation-GC-MS/FID-Based Glycosidic Linkage Analysis of Unfractionated Polysaccharides in Red Seaweeds. Marine Drugs 2024, 22, 192. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Deng, L.; Chen, X.; Ouyang, P.; Li, Y.; Tang, X.; Fan, L.; Tan, H.; Mei, S.; Ye, H.; Wu, K.; Luo, H. , Structural characterization and anti-pigmentation of a novel heteropolysaccharide from Gracilaria lemaneiformis via α-MSH/MC1R pathway. Journal of Functional Foods 2023, 107, 105650. [Google Scholar] [CrossRef]
- Aslan, M.; Dogan, S.; Kucuksayan, E. , Oxidative stress and potential applications of free radical scavengers in glaucoma. Redox Report 2013, 18, 76–87. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. , Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends in Pharmacological Sciences 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. , ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Islam, M.T. , Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurological Research 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Wu, Y.; Sun, X.; Xu, N. , Synthesized sulfated and acetylated derivatives of polysaccharide extracted from Gracilariopsis lemaneiformis and their potential antioxidant and immunological activity. International Journal of Biological Macromolecules 2019, 124, 568–572. [Google Scholar] [CrossRef]
- Long, X.; Hu, X.; Pan, C.; Xiang, H.; Chen, S.; Qi, B.; Liu, S.; Yang, X. , Antioxidant Activity of Gracilaria lemaneiformis Polysaccharide Degradation Based on Nrf-2/Keap-1 Signaling Pathway in HepG2 Cells with Oxidative Stress Induced by H2O2. Marine Drugs 2022, 20, 545. [Google Scholar] [CrossRef]
- Fang, T.; Zhang, X.; Hu, S.; Yu, Y.; Sun, X.; Xu, N. , Enzymatic Degradation of Gracilariopsis lemaneiformis Polysaccharide and the Antioxidant Activity of Its Degradation Products. Marine Drugs 2021, 19, 270. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; Hussain, A.; Haque, S.; Reshi, M.S. , Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; dos Anjos, P.M.F.; Nogueira-Machado, J.A. , Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death & Disease 2018, 9, 119. [Google Scholar]
- Okoduwa, S.I.R.; Umar, I.A.; Ibrahim, S.; Bello, F.; Habila, N. , Age-dependent alteration of antioxidant defense system in hypertensive and type-2 diabetes patients. Journal of Diabetes & Metabolic Disorders 2015, 14, 32. [Google Scholar]
- Zhang, Z.; Li, X.; Xu, X.; Mao, G.; Sun, X.; Xu, N.; Wang, X. , Gracilariopsis lemaneiformis Polysaccharide Attenuates D-Galactose–Induced Aging of Mice by Regulating Oxidative Stress and Gut Microbiota. Frontiers in Marine Sciences 2022, 9. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Y.; Sun-Waterhouse, D.; You, L.; Fu, X. , Advantages of the polysaccharides from Gracilaria lemaneiformis over metformin in antidiabetic effects on streptozotocin-induced diabetic mice. RSC Advances 2017, 7, 9141–9151. [Google Scholar] [CrossRef]
- Lu, Y.; Mei, S.; Wang, P.; Ouyang, P.; Liao, X.; Ye, H.; Wu, K.; Ma, X. , Protective effects of Gracilaria lemaneiformis extract against ultraviolet B-induced damage in HaCaT cells. Pharmacognosy Magazine 2020, 16. [Google Scholar]
- Miller, A.H.; Haroon, E.; Felger, J.C. , The Immunology of Behavior—Exploring the Role of the Immune System in Brain Health and Illness. Neuropsychopharmacology 2017, 42, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Antushevich, H. , Interplays between inflammasomes and viruses, bacteria (pathogenic and probiotic), yeasts and parasites. Immunology Letters 2020, 228, 1–14. [Google Scholar] [CrossRef]
- Lemke, G. , How macrophages deal with death. Nature Reviews Immunology 2019, 19, 539–549. [Google Scholar] [CrossRef]
- Mosser, D.M.; Hamidzadeh, K.; Goncalves, R. , Macrophages and the maintenance of homeostasis. Cellular & Molecular Immunology 2021, 18, 579–587. [Google Scholar]
- Becher, B.; Spath, S.; Goverman, J. , Cytokine networks in neuroinflammation. Nature Reviews Immunology 2017, 17, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. , Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. , Inflammation and cardiovascular disease: From mechanisms to therapeutics. American Journal of Preventive Cardiology 2020, 4, 100130. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.-M.; Veeraperumal, S.; Tan, K.; Zhong, S.; Cheong, K.-L. , The in vitro anti-inflammatory mechanism of Porphyra haitanensis oligosaccharides on lipopolysaccharide-induced injury in IEC-6 cells. Journal of Functional Foods 2024, 112, 106005. [Google Scholar] [CrossRef]
- Cronkite, D.A.; Strutt, T.M. , The Regulation of Inflammation by Innate and Adaptive Lymphocytes. Journal of Immunology Research 2018, 2018, 1467538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Che, H.; Li, C.; Jin, T. , Food Allergens of Plant Origin. Foods 2023, 12, 2232. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Zhou, Y.-C.; Liu, Y.; Huang, J.-Y.; Liu, H.; Liu, C.-F.; Liu, W.-H.; Liu, G.-M.; Liu, Q.-M. , Fermented Gracilaria lemaneiformis polysaccharides alleviate food allergy by regulating Treg cells and gut microbiota. International Journal of Biological Macromolecules 2024, 269, 132215. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Shu, Z.; Liu, M.; Zeng, R.; Wang, Y.; Liu, H.; Cao, M.; Su, W.; Liu, G. , Sulfated oligosaccharide of Gracilaria lemaneiformis protect against food allergic response in mice by up-regulating immunosuppression. Carbohydrate Polymers 2020, 230, 115567. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Ma, L.; Gu, F.; Liao, K.; Liu, Y.; Zhang, Y.; Liu, H.; Hong, Y.; Cao, M.; Liu, W.-H.; Liu, C.; Liu, G. , Sulfate oligosaccharide of Gracilaria lemaneiformis modulates type 1 immunity by restraining T cell activation. Carbohydrate Polymers 2022, 288, 119377. [Google Scholar] [CrossRef]
- Hou, C.; Yin, M.; Lan, P.; Wang, H.; Nie, H.; Ji, X. , Recent progress in the research of Angelica sinensis (Oliv.) Diels polysaccharides: Extraction, purification, structure and bioactivities. Chemical and Biological Technologies in Agriculture 2021, 8, 13. [Google Scholar] [CrossRef]
- Yao, W.; Qiu, H.-M.; Cheong, K.-L.; Zhong, S. , Advances in anti-cancer effects and underlying mechanisms of marine algae polysaccharides. International Journal of Biological Macromolecules 2022, 221, 472–485. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Chen, Y.; Liang, C.-L.; Liu, H.; Qiu, F.; Dai, Z. , Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomedicine & Pharmacotherapy 2020, 121, 109570. [Google Scholar]
- Ji, H.-y.; Yu, J.; Dong, X.-d.; Liu, A.-j. , Preparation of soluble dietary fibers from Gracilaria lemaneiformis and its antitumor activity in vivo. Journal of Food Measurement and Characterization 2019, 13, 1574–1582. [Google Scholar] [CrossRef]
- Kondoh, N.; Mizuno-Kamiya, M. , The Role of Immune Modulatory Cytokines in the Tumor Microenvironments of Head and Neck Squamous Cell Carcinomas. Cancers 2022, 14, 2884. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Li, H.; Wu, J.; Xu, X.; Sun, X.; Zhao, X.; Xu, N. , Transcriptome Profiling Reveals the Antitumor Mechanism of Polysaccharide from Marine Algae Gracilariopsis lemaneiformis. PLoS ONE 2016, 11, e0158279. [Google Scholar] [CrossRef] [PubMed]
- Seyrek, K.; Ivanisenko, N.V.; Wohlfromm, F.; Espe, J.; Lavrik, I.N. , Impact of human CD95 mutations on cell death and autoimmunity: A model. Trends in Immunology 2022, 43, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Luo, L.; Chen, X.; Zhao, X.; He, J.; Chen, H.; Wan, P.; Chen, D.; Pan, J. , Structural characterization of a sulfated polysaccharide from Gracilariopsis lemaneiformis and its potentiation of cisplatin antitumor activity in Colon-26 carcinoma tumor-bearing mice by inducing ferroptosis. Food & Function 2023, 14, 3712–3721. [Google Scholar]
- Li, J.; Cao, F.; Yin, H.-l.; Huang, Z.-j.; Lin, Z.-t.; Mao, N.; Sun, B.; Wang, G. , Ferroptosis: Past, present and future. Cell Death & Disease 2020, 11, 88. [Google Scholar]
- Fan, Y.; Pedersen, O. , Gut microbiota in human metabolic health and disease. Nature Reviews Microbiology 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Zhang, P. , Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. International Journal of Molecular Sciences 2022, 23, 9588. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. , Microbiota revolution: How gut microbes regulate our lives. World journal of gastroenterology 2023, 29, 4368–4383. [Google Scholar] [CrossRef] [PubMed]
- Kudelka, M.R.; Stowell, S.R.; Cummings, R.D.; Neish, A.S. , Intestinal epithelial glycosylation in homeostasis and gut microbiota interactions in IBD. Nature Reviews Gastroenterology & Hepatology 2020, 17, 597–617. [Google Scholar]
- Haneishi, Y.; Furuya, Y.; Hasegawa, M.; Picarelli, A.; Rossi, M.; Miyamoto, J. , Inflammatory Bowel Diseases and Gut Microbiota. International Journal of Molecular Sciences 2023, 24, 3817. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-Y.; Tan, K.; Zhong, S.; Cheong, K.-L. , Marine algal polysaccharides as future potential constituents against non-alcoholic steatohepatitis. International Journal of Biological Macromolecules 2023, 250, 126247. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wei, J.; Liu, P.; Zhang, Q.; Tian, Y.; Hou, G.; Meng, L.; Xin, Y.; Jiang, X. , Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism 2021, 117, 154712. [Google Scholar] [CrossRef] [PubMed]
- Chakaroun, R.M.; Massier, L.; Kovacs, P. , Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients 2020, 12, 1082. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. , The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Veeraperumal, S.; Zhong, S.; Cheong, K.-L. , Fucoidan-Derived Functional Oligosaccharides: Recent Developments, Preparation, and Potential Applications. Foods 2023, 12, 878. [Google Scholar] [CrossRef]
- Divyashri, G.; Karthik, P.; Murthy, T.P.K.; Priyadarshini, D.; Reddy, K.R.; Raghu, A.V.; Vaidyanathan, V.K. , Non-digestible oligosaccharides-based prebiotics to ameliorate obesity: Overview of experimental evidence and future perspectives. Food Science and Biotechnology 2023, 32, 1993–2011. [Google Scholar] [CrossRef]
- Li, Y.-F.; Wu, B.; Chen, J.-p.; Veeraperumal, S.; Wei, J.-C.; Tan, K.-s.; Zhong, S.; Cheong, K.-L. , Prebiotic characteristics of added-value polysaccharides from jackfruit peel waste during in vitro digestion and fecal fermentation. LWT 2023, 187, 115330. [Google Scholar] [CrossRef]
- Xie, X.-T.; Cheong, K.-L. , Recent advances in marine algae oligosaccharides: Structure, analysis, and potential prebiotic activities. Critical Reviews in Food Science and Nutrition 2022, 62, 7703–7717. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Chen, X.-Q.; Liu, Y.; Cheong, K.-L. , Ultrasonic/microwave-assisted extraction, simulated digestion, and fermentation in vitro by human intestinal flora of polysaccharides from Porphyra haitanensis. International Journal of Biological Macromolecules 2020, 152, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Moseiko, V.; Romanenko, M.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Vaiserman, A. , Sex differences in the phylum-level human gut microbiota composition. BMC Microbiology 2021, 21, 131. [Google Scholar] [CrossRef] [PubMed]
- Wardman, J.F.; Bains, R.K.; Rahfeld, P.; Withers, S.G. , Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nature Reviews Microbiology 2022, 20, 542–556. [Google Scholar] [CrossRef]
- McKee, L.S.; La Rosa, S.L.; Westereng, B.; Eijsink, V.G.; Pope, P.B.; Larsbrink, J. , Polysaccharide degradation by the Bacteroidetes: Mechanisms and nomenclature. Environmental Microbiology Reports 2021, 13, 559–581. [Google Scholar] [CrossRef] [PubMed]
- Hehemann, J.-H.; Kelly, A.G.; Pudlo, N.A.; Martens, E.C.; Boraston, A.B. , Bacteria of the human gut microbiome catabolize red seaweed glycans with carbohydrate-active enzyme updates from extrinsic microbes. Proceedings of the National Academy of Sciences 2012, 109, 19786–19791. [Google Scholar] [CrossRef]
- Yu, B.; Lu, Z.; Zhong, S.; Cheong, K.-L. , Exploring potential polysaccharide utilization loci involved in the degradation of typical marine seaweed polysaccharides by Bacteroides thetaiotaomicron. Frontiers in Microbiology 2024, 15, 1332105. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Liu, G.; Shen, J.; Chen, G.; Mei, X.; Chang, Y.; Xue, C. , The α-linkage in funoran and agarose could be hydrolyzed by a GH96 family enzyme: Discovery of the α-funoranase. Carbohydrate Polymers 2024, 338, 122201. [Google Scholar] [CrossRef]
- Ho Do, M.; Seo, Y.S.; Park, H.-Y. , Polysaccharides: Bowel health and gut microbiota. Critical Reviews in Food Science and Nutrition 2021, 61, 1212–1224. [Google Scholar] [CrossRef]
- Sun, X.; Duan, M.; Liu, Y.; Luo, T.; Ma, N.; Song, S.; Ai, C. , The beneficial effects of Gracilaria lemaneiformis polysaccharides on obesity and the gut microbiota in high fat diet-fed mice. Journal of Functional Foods 2018, 46, 48–56. [Google Scholar] [CrossRef]
- Kim, C.H. , Microbiota or short-chain fatty acids: Which regulates diabetes? Cellular & Molecular Immunology 2018, 15, 88–91. [Google Scholar]
- van der Hee, B.; Wells, J.M. , Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends in Microbiology 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Mandaliya, D.K.; Patel, S.; Seshadri, S. , The Combinatorial Effect of Acetate and Propionate on High-Fat Diet Induced Diabetic Inflammation or Metaflammation and T Cell Polarization. Inflammation 2021, 44, 68–79. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. , Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Critical Reviews in Food Science and Nutrition 2019, 59, S130–S152. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.-L.; Xie, X.-T.; Zhou, T.; Malairaj, S.; Veeraperumal, S.; Zhong, S.; Tan, K. , Exploring the therapeutic potential of porphyran extracted from Porphyra haitanensis in the attenuation of DSS-induced intestinal inflammation. International Journal of Biological Macromolecules 2024, 271, 132578. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Sun, R.; Cong, Y.; Liu, Z. , Critical roles of G protein-coupled receptors in regulating intestinal homeostasis and inflammatory bowel disease. Mucosal Immunology 2022, 15, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Felizardo, R.J.F.; de Almeida, D.C.; Pereira, R.L.; Watanabe, I.K.M.; Doimo, N.T.S.; Ribeiro, W.R.; Cenedeze, M.A.; Hiyane, M.I.; Amano, M.T.; Braga, T.T.; Ferreira, C.M.; Parmigiani, R.B.; Andrade-Oliveira, V.; Volpini, R.A.; Vinolo, M.A.R.; Mariño, E.; Robert, R.; Mackay, C.R.; Camara, N.O.S. , Gut microbial metabolite butyrate protects against proteinuric kidney disease through epigenetic- and GPR109a-mediated mechanisms. The FASEB Journal 2019, 33, 11894–11908. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ma, Y.; Xiao, J.; You, L.; Pedisić, S.; Liao, L. , The possible mechanism of the protective effect of a sulfated polysaccharide from Gracilaria Lemaneiformis against colitis induced by dextran sulfate sodium in mice. Food and Chemical Toxicology 2021, 149, 112001. [Google Scholar] [CrossRef]
- Kim, C.H. , Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cellular & Molecular Immunology 2023, 20, 341–350. [Google Scholar]
- Gonçalves, P.; Araújo, J.R.; Di Santo, J.P. , A Cross-Talk Between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease. Inflammatory Bowel Diseases 2018, 24, 558–572. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Liu, Y.; Tang, S.; Zhang, W.; Yu, Q.; Shi, C.; Cheong, K.-L. , Gracilaria lemaneiformis polysaccharides alleviate colitis by modulating the gut microbiota and intestinal barrier in mice. Food Chemistry: X 2022, 13, 100197. [Google Scholar] [CrossRef]
- Xie, X.-T.; Zheng, L.-X.; Duan, H.-M.; Liu, Y.; Chen, X.-Q.; Cheong, K.-L. , Structural characteristics of Gracilaria lemaneiformis oligosaccharides and their alleviation of dextran sulphate sodium-induced colitis by modulating the gut microbiota and intestinal metabolites in mice. Food & Function 2021, 12, 8635–8646. [Google Scholar]
- Zhang, S.; Zhang, M.; Li, W.; Ma, L.; Liu, X.; Ding, Q.; Yu, W.; Yu, T.; Ding, C.; Liu, W. , Research progress of natural plant polysaccharides inhibiting inflammatory signaling pathways and regulating intestinal flora and metabolism to protect inflammatory bowel disease. International Journal of Biological Macromolecules 2023, 253, 126799. [Google Scholar] [CrossRef]
- Chand Dakal, T.; Dhabhai, B.; Agarwal, D.; Gupta, R.; Nagda, G.; Meena, A.R.; Dhakar, R.; Menon, A.; Mathur, R.; Mona; Yadav, V. ; Sharma, A., Mechanistic basis of co-stimulatory CD40-CD40L ligation mediated regulation of immune responses in cancer and autoimmune disorders. Immunobiology 2020, 225, 151899. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-H.; Zheng, Y.-Y.; Ouyang, J.-M. , Antioxidant Activities and Cytotoxicity of the Regulated Calcium Oxalate Crystals on HK-2 Cells of Polysaccharides from Gracilaria lemaneiformis with Different Molecular Weights. Foods 2023, 12, 1031. [Google Scholar] [CrossRef]
- Long, X.; Hu, X.; Zhou, S.; Xiang, H.; Chen, S.; Li, L.; Liu, S.; Yang, X. , Optimized Degradation and Inhibition of α-glucosidase Activity by Gracilaria lemaneiformis Polysaccharide and Its Production In Vitro. Marine Drugs 2022, 20, 13. [Google Scholar] [CrossRef]
- Chen, M.-z.; Xie, H.-g.; Yang, L.-w.; Liao, Z.-h.; Yu, J. , In vitro anti-influenza virus activities of sulfated polysaccharide fractions from Gracilaria lemaneiformis. Virologica Sinica 2010, 25, 341–351. [Google Scholar] [CrossRef]
| Bioactivities | Mechanism | Structural information of GLP | Ref |
|---|---|---|---|
| Antioxidant | Radicals (hydroxyl, DPPH, and ABTS) scavenging capacity, regulation of antioxidant enzyme levels (MDA, SOD) in HK-2 cells, and decreased ROS levels. | Mw: 106, 49.6, 10.5, 6.14, 5.06, 3.71 and 2.42 kDa | [146] |
| Antioxidant | Radicals (ABTS, hydroxyl, and nitrite) scavenging capacity. | Glc: Gal: Xyl: Fuc= 4.48: 18.76: 1.811: 5.968;刘Mw: 591 kDa | [22] |
| Antioxidant | Decreased senescence-associated β-galactosidase activity and suppression of p21 and p53 gene expression. | Mw: 2.7 kDa刘alternating α-(1→3)- and β-(1→4)-Gal. | [71] |
| Antitumor | Inhibition of tumor cell proliferation in vitro through the apoptosis-related Fas/FasL signaling pathway. | Mw: 123.06, 14.29, 64.78, and 57.02 kDa刘Gal: Glc= 11.68: 1 | [61] |
| Antitumor | Inhibition of tumor cell proliferation in vitro, enhancement of NK cell activity, and increased levels of serum cytokines in vivo. | Mw: 20958 Da刘Ara: Xyl: Man: Glc: Gal= 0.22: 0.23: 0.11: 0.17: 1.00 | [104] |
| Hypoglycemic | Regulation of blood sugar levels. Increases in SOD, GSH-Px, and total antioxidant capacity. | Mw: 121.89, 57.02, and 14.29 kDa | [72] |
| Hypoglycemic | Inhibition of α-glucosidase activity. | Mw: 1478 kDa刘34.35% Glc and 57.37% Gal | [147] |
| Intestinal health | Modulation of gut microbiota and an increase in short-chain fatty acids (SCFAs). | alternating 4-linked 3,6-anhydro-α-L-Gal and 3-linked β-D-Gal units with sulfate residues | [142] |
| Intestinal health | Modulate gut microbiota, increase short-chain fatty acids (SCFAs), and enhance the expression of tight junction proteins and MUC-2. | Mw: 22.38 kDa刘Ara: Fuc: Xyl: Man: Glc: Gal= 1.14: 1.45: 7.92: 4.23: 13.48: 71.78 | [17,139] |
| Anti-influenza virus | Inhibit viral replication and decrease viral adsorption ability. | Mw: 4.9, 52, and 67 kDa刘AnGal and Gal | [148] |
| Wound healing | Promotes cell proliferation and migration through activation of the PI3K/aPKC signaling pathway. Enhances epithelial layer thickness and collagen deposition in vivo. | Mw: 157 kDa刘alternating 4-linked 3,6-anhydro-α-L-Gal and 3-linked β-D-Gal units with sulfate residues | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





