Submitted:
30 July 2024
Posted:
01 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. HIV Epidemic and Health Disparities
1.2. Preexposure Prophylaxis (PrEP) Medications
1.3. PrEP Uptake and Challenges
1.4. Pharmacist’s Role Expansion to Increase PrEP Uptake in High-Risk Population
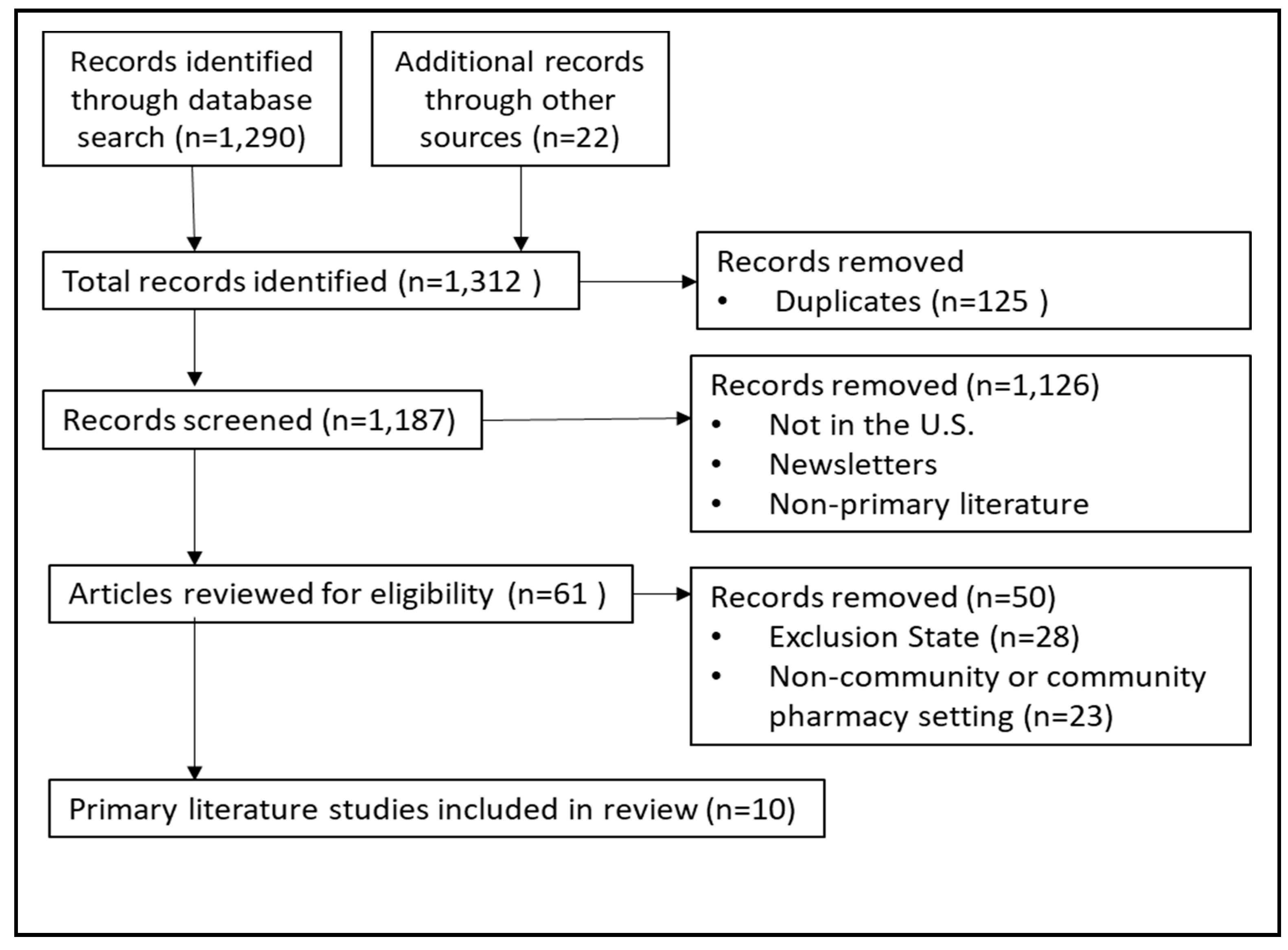
2. Methods
3. Results
3.1. Surveys and Interviews
3.2. Intervention Reports
3.3. Clinical Trial in Progress
4. Discussion
4.1. How Does a Pharmacist Provide HIV and PrEP Services in the Community Setting in Prescriptive Authority Restricted States?
4.2. How Do Important Stakeholders Feel about Pharmacists Providing HIV and PrEP Services in the Community Setting in Prescriptive Authority Restricted States?
4.3. What Gaps Are Yet to Be Filled to Implement HIV and PrEP Services in the Community Pharmacy Setting in Prescriptive Authority Restricted States?
5. Conclusion
Acknowledgments
References
- UNAIDS 2023 Report. UNAIDS - Global Report 2023. https://thepath.unaids.org/ (accessed 2024-05-27).
- U.S. Centers for disease control and prevention AtlasPlus - Map. Explore CDC’s Atlas Plus HIV+Hepatitis+STD+TB+Social Determinants of Health. https://gis.cdc.gov/grasp/nchhstpatlas/maps.html (accessed 2024-05-26).
- HIV Surveillance Report: Diagnoses, Deaths, and Prevalence of HIV in the United States and 6 Territories and Freely Associated States, 2022. https://stacks.cdc.gov/view/cdc/156509 (accessed 2024-05-27).
- US Public Health Service: PREEXPOSURE PROPHYLAXIS FOR THE PREVENTION OF HIV INFECTION IN THE UNITED STATES – 2021 UPDATE, A CLINICAL PRACTICE GUIDELINE. 2021.
- Commissioner, O. of the. FDA Approves First Injectable Treatment for HIV Pre-Exposure Prevention. FDA. https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention (accessed 2024-05-26).
- CDC. Ending the HIV Epidemic in the US (EHE). Ending the HIV Epidemic in the US (EHE). https://www.cdc.gov/ehe/index.html (accessed 2024-05-27).
- CDC. HIV in the U.S. by the Numbers – 2021. NCHHSTP Newsroom. https://www.cdc.gov/nchhstp-newsroom/factsheets/hiv-in-us-by-the-numbers-2021.html (accessed 2024-05-27).
- Toward PrEP Access For All: An analysis of policies, approaches, and strategies in the Southern United States. PrEP4All. https://prep4all.org/publication/sac-report/ (accessed 2024-05-27).
- Sophus, A.I.; Mitchell, J.W. A Review of Approaches Used to Increase Awareness of Pre-Exposure Prophylaxis (PrEP) in the United States. AIDS Behav 2019, 23(7), 1749–1770. [Google Scholar] [CrossRef] [PubMed]
- Berenbrok, L.A.; Tang, S.; Gabriel, N.; Guo, J.; Sharareh, N.; Patel, N.; Dickson, S.; Hernandez, I. Access to Community Pharmacies: A Nationwide Geographic Information Systems Cross-Sectional Analysis. J Am Pharm Assoc (2003) 2022, 62 (6), 1816-1822.e2. [CrossRef]
- Hogue, M. CEO Blog. Pharmacists expand access to PrEP in 17 states. American Pharmacists Association. https://www.pharmacist.com/CEO-Blog/ (accessed 2024-05-27).
- Home | ClinicalTrials.gov. https://clinicaltrials.gov/ (accessed 2023-11-18).
- Ahmed, K.K.M.; Al Dhubaib, B.E. Zotero: A Bibliographic Assistant to Researcher. J Pharmacol Pharmacother 2011, 2(4), 303–305. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.M.; Endres, K.; Derrick, C.; Cooper, A.; Fabel, P.; Okeke, N.L.; Ahuja, D.; Corneli, A.; McKellar, M.S. A Survey of South Carolina Pharmacists’ Readiness to Prescribe Human Immunodeficiency Virus Pre-exposure Prophylaxis. JAACP : Journal of the American College of Clinical Pharmacy 2023, 6 (4), 329–338. [CrossRef]
- Crawford, N.D.; Josma, D.; Morris, J.; Hopkins, R.; Young, H.N. Pharmacy-Based Pre-Exposure Prophylaxis Support among Pharmacists and Men Who Have Sex with Men. Journal of the American Pharmacists Association 2020, 60 (4), 602–608. [CrossRef]
- Hopkins, R.; Josma, D.; Morris, J.; Klepser, D.G.; Young, H.N.; Crawford, N.D. Support and Perceived Barriers to Implementing Pre-Exposure Prophylaxis Screening and Dispensing in Pharmacies: Examining Concordance between Pharmacy Technicians and Pharmacists. Journal of the American Pharmacists Association 2021, 61(1), 115–120. [Google Scholar] [CrossRef] [PubMed]
- Shaeer, K.M.; Sherman, E.M.; Shafiq, S.; Hardigan, P. Exploratory Survey of Florida Pharmacists’ Experience, Knowledge, and Perception of HIV Pre-Exposure Prophylaxis. J Am Pharm Assoc (2003) 2014, 54(6), 610–617. [Google Scholar] [CrossRef] [PubMed]
- Angela, B. Hoth; Shafer, C.; Dillon, D.B.; Mayer, R.; Walton, G.; Ohl, M.E. Iowa TelePrEP: A Public-Health-Partnered Telehealth Model for Human Immunodeficiency Virus Preexposure Prophylaxis Delivery in a Rural State. Sexually transmitted diseases 2019, 46(8), 507–512. [Google Scholar] [CrossRef]
- Chasco, E.E.; Shafer, C.; Dillon, D.M.; Owens, S.; Ohl, M.E.; Hoth, A.B. Bringing Iowa TelePrEP to Scale: A Qualitative Evaluation. American journal of preventive medicine 2021, 61(5), S108–S117. [Google Scholar] [CrossRef] [PubMed]
- Khosropour, C.M.; Backus, K.V.; Means, A.R.; Beauchamps, L.; Johnson, K.; Golden, M.R.; Mena, L. A Pharmacist-Led, Same-Day, HIV Pre-Exposure Prophylaxis Initiation Program to Increase PrEP Uptake and Decrease Time to PrEP Initiation. AIDS Patient Care and STDs 2020, 34(1), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Taliaferro, T.; Layson-Wolf, C.; Seung, H.; Banjo, O.; Tran, D. Impact of Pharmacist-Led Program on Knowledge of College Students about Pre-Exposure Prophylaxis. Journal of the American Pharmacists Association 2021, 61(4), S30–S38. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.J.K. FINISHING HIV: An Ending the HIV Epidemic (EHE) Model for Latinx Integrating One-Stop-Shop Pre-Exposure Prophylaxis (PrEP) Services, a Social Network Support Program and a National Pharmacy Chain; Clinical trial registration NCT06406049; clinicaltrials.gov, 2024. https://clinicaltrials.gov/study/NCT06406049 (accessed 2023-12-31).
- Crawford, N. Advancing Pre-Exposure Prophylaxis (PrEP) Access in Pharmacies to Improve PrEP Uptake in Disadvantaged Areas; Clinical trial registration NCT04393935; clinicaltrials.gov, 2023. https://clinicaltrials.gov/study/NCT04393935 (accessed 2023-12-31).
- Pharmacists’ Authority to Initiate PrEP and PEP and Engage in Collaborative Practice Agreements | NASTAD. https://nastad.org/resources/pharmacists-authority-engage-collaborative-practice-agreements-and-initiate-prep-pep-and (accessed 2024-07-04).
- Cocohoba, J.; Tweedie, L.; Frank, M.; McElya, B.; Witt, E. Legislation Expanding Pharmacist Scope of Practice to Furnish Human Immunodeficiency Virus Pre-Exposure Prophylaxis: A Content Analysis. JACCP: JOURNAL OF THE AMERICAN COLLEGE OF CLINICAL PHARMACY 2024, 7 (1), 25–30. [CrossRef]
- Pellegrino, A.N.; Martin, M.T.; Tilton, J.J.; Touchette, D.R. Medication Therapy Management Services: Definitions and Outcomes. Drugs 2009, 69(4), 393–406. [Google Scholar] [CrossRef] [PubMed]
- Census profile: Houston-The Woodlands-Sugar Land, TX Metro Area. Census Reporter. http://censusreporter.org/profiles/31000US26420-houston-the-woodlands-sugar-land-tx-metro-area/ (accessed 2024-07-05).
- HIV Testing Sites & Care Services Locator. https://locator.hiv.gov/map (accessed 2024-07-05).
- PrEP Locator: A national database for US PrEP providers. US PrEP Provider Directory. https://preplocator.org/ (accessed 2024-07-05).
- Gregory, P.A.; Austin, Z. How Do Patients Develop Trust in Community Pharmacists? Res Social Adm Pharm 2021, 17(5), 911–920. [Google Scholar] [CrossRef] [PubMed]
- Kolanczyk, D.M.; Merlo, J.R.; Bradley, B.; Flannery, A.H.; Gibson, C.M.; McBane, S.; Murphy, J.A.; Noble, J.M.; Noble, M.B.; Patton, H.M.; Rosselli, J.L.; Stone, R.H.; Thornby, K. 2023 Update to the American College of Clinical Pharmacy Pharmacotherapy Didactic Curriculum Toolkit. J Am Coll Clin Pharm 2024, 7(3), 255–269. [Google Scholar] [CrossRef]
- Home Page - National HIV PrEP Curriculum. https://www.hivprep.uw.edu/ (accessed 2024-07-18).
- Saberi, P.; Su, H.; Mendiola, J.; Gruta, C.; Lutes, E.R.; Dong, B.; Bositis, C.; Chu, C. HIV Pre-Exposure Prophylaxis Champion Preceptorship Training for Pharmacists and Nurses in the United States. JACCP: JOURNAL OF THE AMERICAN COLLEGE OF CLINICAL PHARMACY 2024, 7 (5), 458–470. [CrossRef]
- Broekhuis, J.M.; Scarsi, K.K.; Sayles, H.R.; Klepser, D.G.; Havens, J.P.; Swindells, S.; Bares, S.H. Midwest Pharmacists’ Familiarity, Experience, and Willingness to Provide Pre-Exposure Prophylaxis (PrEP) for HIV. PLoS ONE 2018, 13(11), e0207372–e0207372. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | State/Setting | Methods | Results |
|---|---|---|---|
| Burns CM et al (2023) [14] | South Carolina Retail, hospital, independent, community, specialty, academia |
43-question online survey through a pharmacist listserv | More than half of pharmacists (n=129) responded ready and willing to prescribe PrEP. |
| Crawford ND et al (2020) [15] | Georgia Community pharmacies in zip codes with high HIV incidence |
Semi-structured interviews of pharmacists and MSM clients | Participants (n=14) reported in supportive of HIV, STI, and PrEP screenings. |
| Hopkins R et al (2021) [16] | Georgia Community pharmacies in zip codes with high HIV incidence |
Semi-structured interviews of pharmacists and pharmacy technicians | Community pharmacists (n=7) and pharmacy technicians (n=6) were supportive of implementation of HIV and PrEP screening. |
| Shaeer KM et al (2014) [17] | Florida Community pharmacies |
In-person and online survey | Pharmacists (n=225) reported limited understanding of PrEP and more education was needed. |
| Author (Year) | State/ Setting | Methods | Results |
|---|---|---|---|
| Holt AB (2019) [18] Chasco EE et al. (2021) [19] |
Iowa Statewide public health departments and University of Iowa collaboration |
The pharmacists provided counseling and prescribed PrEP through a collaborative practice model. | 12 public health partners referred 708 individuals over 18 month period. 258 individuals received TelePrEP service and 167 individuals initiated PrEP. |
| Khosropour CM et al. (2020) [20] | Mississippi An academic affiliated HIV/STD testing center located next to the state health department STD clinic |
The clinical pharmacist received patient referrals from a STD clinic, collected medical history, prescribed PrEP through collaborative practice agreements, and provided a follow up appointment. | 69 patients were referred to clinical pharmacy service over a 7-month period. 80% received a PrEP prescription on the same day, 77% filled the PrEP prescription and 43% of those who filled the prescription attended a follow up appointment. |
| Taliaferro T et al.(2021) [21] | Washington, DC College campus |
The pharmacist provided a 30-minute education to college students about HIV prevention and PrEP. | Participants (n=102) reported an increase in perception of HIV and PrEP knowledge after the education compared to baseline. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





