Submitted:
01 August 2024
Posted:
01 August 2024
You are already at the latest version
Abstract
Keywords:
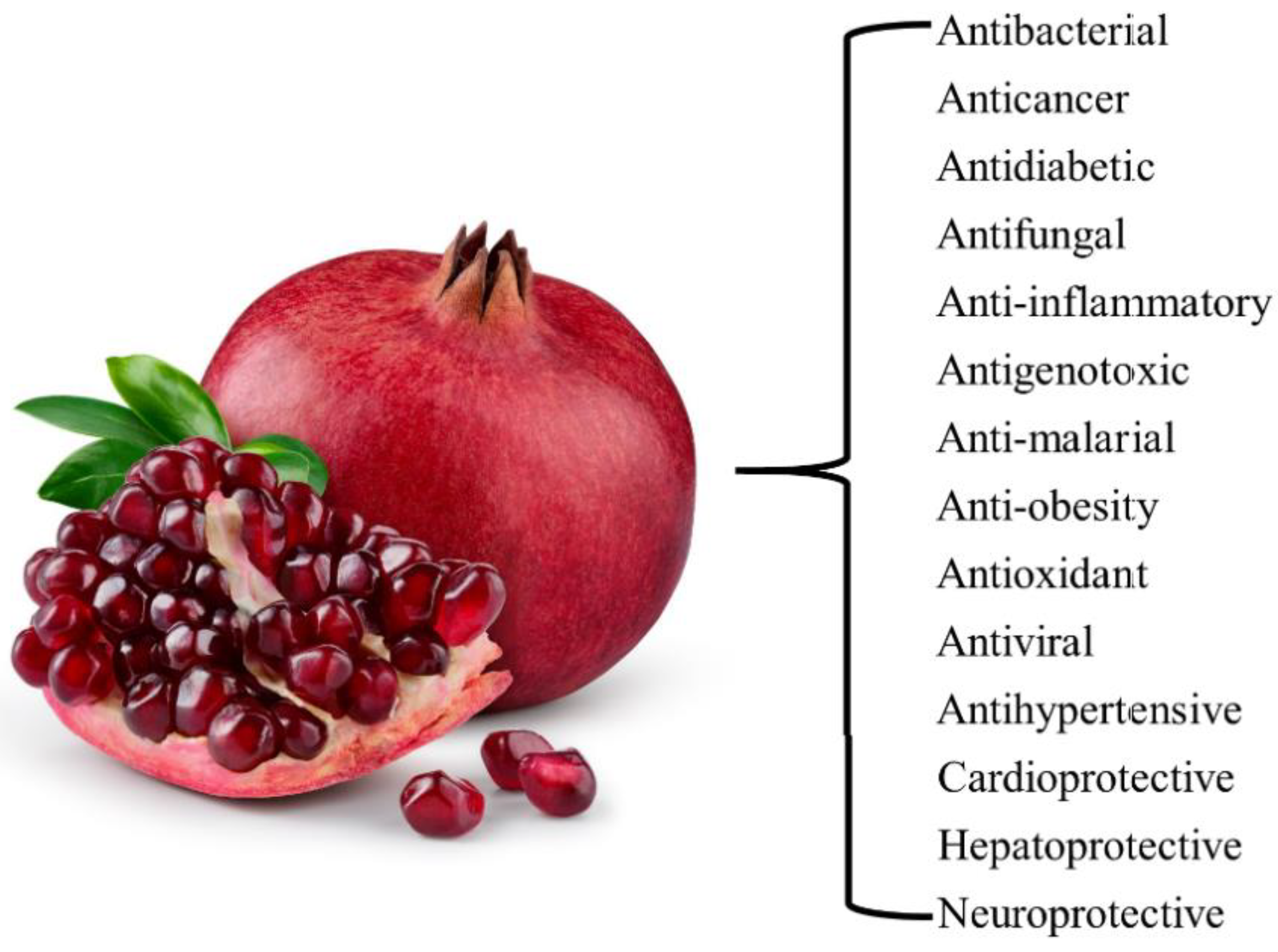
1. Introduction
2. Pomegranate Extracts
3. Phytochemicals Present in the Pomegranate Extract
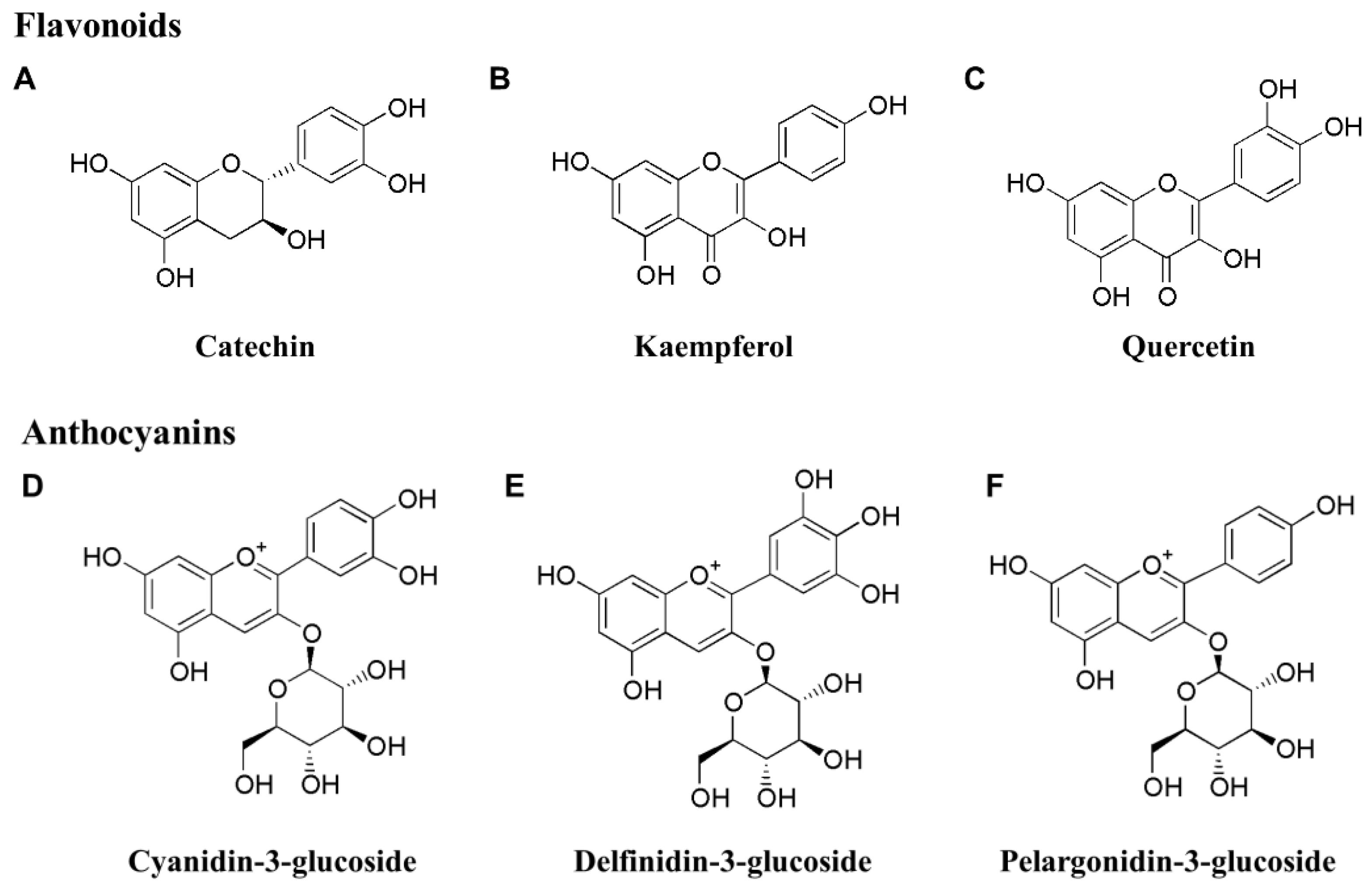
3.1. Flavonoids
3.2. Anthocyanins
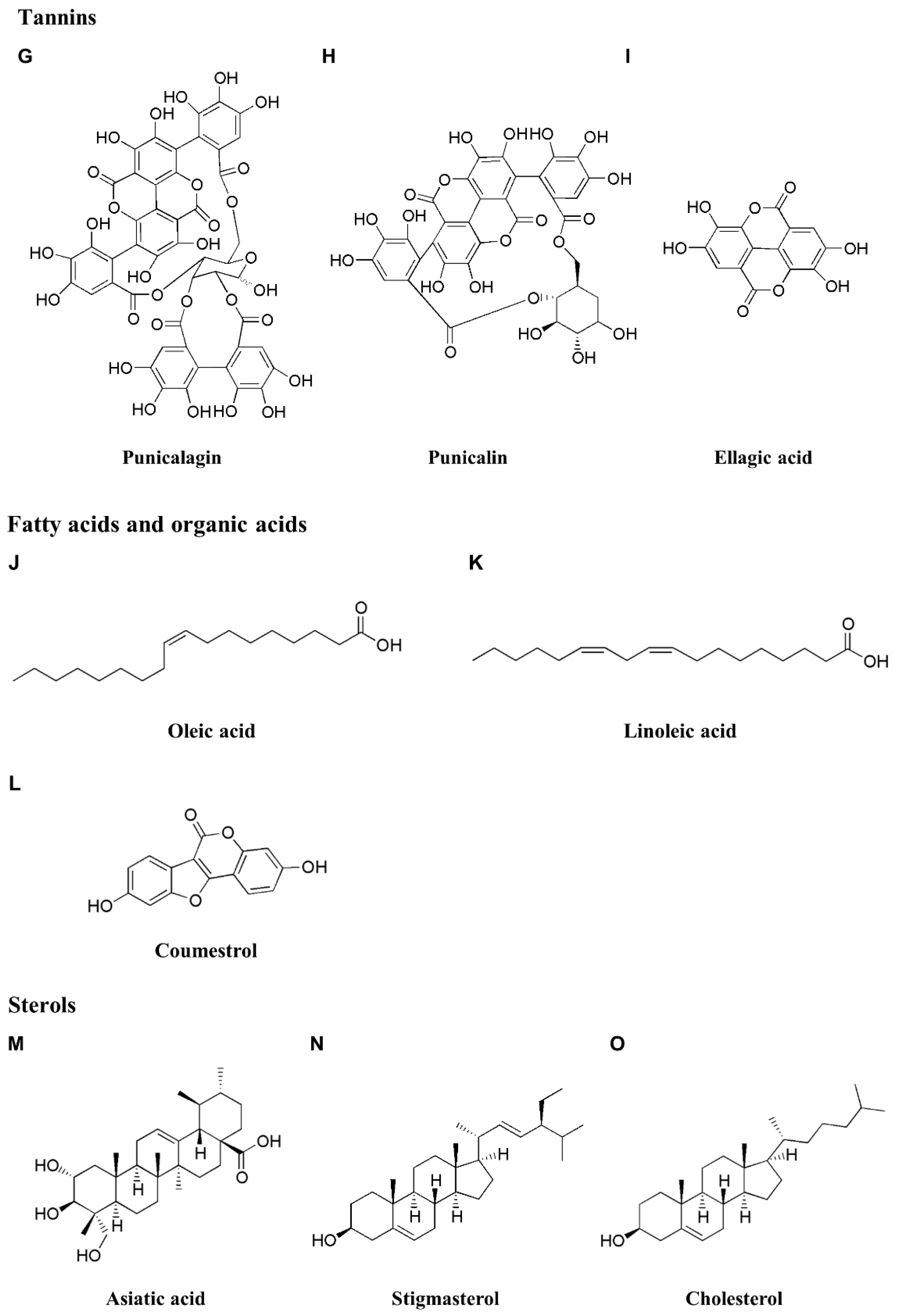
3.3. Tannins
3.4. Fatty and Organic Acids
3.5. Sterols
4. Role of Pomegranate Extracts in Alleviating Peri- and Post-menopausal Symptoms
4.1. Peri- and Post-Menopausal Symptoms
4.2. Effect of Pomegranate Extract on Peri- and Post-Menopausal Symptoms
5. Role of Pomegranate Extracts in PCOS
5.1. PCOS
5.2. Effect of Pomegranate Extract on PCOS
6. Role of Pomegranate Extracts in Breast Cancer
6.1. Breast Cancer
6.2. Effect of Pomegranate Extract on Breast Cancer
7. Clinical Effects of Pomegranate Extract on Women’s Health
7.1. Clinical Effects of Pomegranate Extract Against Peri- and Post-Menopausal Symptoms
7.2. Clinical Effects of Pomegranate Extract on PCOS
7.3. Clinical Effects of Pomegranate Extract on Breast Cancer
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Montefusco, A.; Durante, M.; Migoni, D.; De Caroli, M.; Ilahy, R.; Pék, Z.; Helyes, L.; Fanizzi, F.P.; Mita, G.; Piro, G.; et al. Analysis of the phytochemical composition of pomegranate fruit juices, peels and kernels: A comparative study on four cultivars grown in southern Italy. Plants (Basel) 2021, 10, 2521. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ya’akov, I.; Tian, L.; Amir, R.; Holland, D. Primary metabolites, anthocyanins, and hydrolyzable tannins in the pomegranate fruit. Front. Plant Sci. 2019, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Kosseva, M.R.; Joshi, V.K.; Panesar, P.S. Science and Technology of Fruit Wine Production; Elsevier Science: Amsterdam, The Netherlands, 2016; p. 24. [Google Scholar]
- Eghbali, S.; Askari, S.F.; Avan, R.; Sahebkar, A. Therapeutic effects of Punica granatum (pomegranate): An updated review of clinical trials. J. Nutr. Metab. 2021, 2021, 5297162. [Google Scholar] [CrossRef] [PubMed]
- Paris, H.S. Origin and emergence of the sweet dessert watermelon, Citrullus lanatus. Ann. Bot. 2015, 116, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Langley, P. Why a pomegranate? BMJ 2000, 321, 1153–1154. [Google Scholar] [CrossRef]
- Wetzstein, H.Y.; Zhang, Z.; Ravid, N.; Wetzstein, M.E. Characterization of attributes related to fruit size in pomegranate. HortScience 2011, 46, 908–912. [Google Scholar] [CrossRef]
- Kumar, N.V.; Godara, A.; Mirza, A.J.I.J.C.M.A.S. Characteristics of flowering and fruiting description of pomegranate (Punica granatum L.). J. Am. Soc. Hortic. Sci. 2020, 9, 401–412. [Google Scholar] [CrossRef]
- Kahramanoglu, I.; Usanmaz, S. Pomegranate Production and Marketing; CRC Press: 2016; pp. 16.
- Amos Fawole, O.; Linus Opara, U. Composition of trace and major minerals in different parts of pomegranate (Punica granatum) fruit cultivars. BFJ 2012, 114, 1518–1532. [Google Scholar] [CrossRef]
- Mayuoni-Kirshinbaum, L.; Porat, R. Agriculture. The flavor of pomegranate fruit: A review. J. Sci. Food Agric. 2014, 94, 21–27. [Google Scholar] [CrossRef]
- Buyuran, F. Pomegranates and Saffron: A Culinary Journey to Azerbaijan, 2nd ed.; AZ Cookbook: 2015. pp. 1–86.
- Saparbekova, A.A.; Kantureyeva, G.O.; Kudasova, D.E.; Konarbayeva, Z.K.; Latif, A.S. Potential of phenolic compounds from pomegranate (Punica granatum L.) by-product with significant antioxidant and therapeutic effects: A narrative review. Saudi J. Bio. Sci. 2023, 30, 103553. [Google Scholar] [CrossRef]
- de Oliveira, F.L.; Arruda, T.Y.P.; da Silva Lima, R.; Casarotti, S.N.; Morzelle, M.C. Pomegranate as a natural source of phenolic antioxidants: A review. J. Food Bioact. 2020, 9, 10–22. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Lampakis, D.; Skenderidis, P.; Leontopoulos, S. Technologies and extraction methods of polyphenolic compounds derived from pomegranate (Punica granatum) peels. A mini review. Processes 2021, 9, 236. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Bălan, A.; Dima, L.; Toma, S.I.; Bîgiu, N.F.; Blidaru, A. Pharmacological and therapeutic properties of Punica granatum phytochemicals: possible roles in breast cancer. Molecules 2021, 26, 1054. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing factors affecting the phytochemical and nutritional properties of pomegranate (Punica granatum L.) peel waste: A review. Molecules 2020, 25, 4690. [Google Scholar] [CrossRef] [PubMed]
- Milošević, M.; Vulić, J.; Kukrić, Z.; Lazić, B.; Četojević-Simin, D.; Čanadanović-Brunet, J. Polyphenolic composition, antioxidant and antiproliferative activity of edible and inedible parts of cultivated and wild pomegranate (Punica granatum L.). Food Technol. Biotechnol. 2023, 61, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Solano, J.A.; Bautista, M.; Espinosa-Juárez, J.V.; Moreno-Rocha, L.A.; Betanzos-Cabrera, G.; Salanță, L.C.; De la, O.A.M.; Olvera-Hernández, E.G.; Jaramillo-Morales, O.A. Differential antinociceptive efficacy of peel extracts and lyophilized juices of three varieties of Mexican pomegranate (Punica granatum L.) in the formalin test. Plants (Basel) 2022, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.; Tzulker, R.; Glazer, I.; Bar-Ya’akov, I.; Wiesman, Z.; Tripler, E.; Bar-Ilan, I.; Fromm, H.; Borochov-Neori, H.; Holland, D.; et al. Environmental conditions affect the color, taste, and antioxidant capacity of 11 pomegranate accessions’ fruits. J. Agric. Food Chem. 2009, 57, 9197–9209. [Google Scholar] [CrossRef]
- Mohammad, S.M.; Kashani, H.H. Chemical composition of the plant Punica granatum L.(Pomegranate) and its effect on heart and cancer. J. Med. Plants Res. 2012, 6, 5306–5310. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; Ramadan, W.S.; Harakeh, S.; Al Jaouni, S.K.; Bharali, D.J.; Mousa, S.A.; Almuhayawi, S.M. The potential role of pomegranate and its nano-formulations on cerebral neurons in aluminum chloride induced Alzheimer rat model. Saudi J. Biol. Sci. 2020, 27, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Faddladdeen, K.A.; Ojaimi, A.A. Protective effect of pomegranate (Punica granatum) extract against diabetic changes in adult male rat liver: Histological study. J. Microsc. Ultrastruct. 2019, 7, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Schell, J.; Scofield, R.H. Dietary fruits and arthritis. Food Funct. 2018, 9, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Michicotl-Meneses, M.M.; Thompson-Bonilla, M.d.R.; Reyes-López, C.A.; García-Pérez, B.E.; López-Tenorio, I.I.; Ordaz-Pichardo, C.; Jaramillo-Flores, M.E. Inflammation markers in adipose tissue and cardiovascular risk reduction by pomegranate juice in obesity induced by a hypercaloric diet in Wistar rats. Nutrients 2021, 13, 2577. [Google Scholar] [CrossRef] [PubMed]
- Alshinnawy, A.; Elsayed, W.; Taha, A.; Sayed, A.; Salem, A. Astragalus membranaceus and Punica granatum alleviate infertility and kidney dysfunction induced by aging in male rats. Turk. J. Biol. 2020, 44, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Özen, C.; Abu-Reidah, I.M.; Chigurupati, S.; Patra, J.K.; Horbanczuk, J.O.; Jóźwik, A.; Tzvetkov, N.T.; Uhrin, P.; Atanasov, A.G. Vasculoprotective effects of pomegranate (Punica granatum L.). Front. Pharmacol. 2018, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Kupnik, K.; Primožič, M.; Vasić, K.; Knez, Ž.; Leitgeb, M. A Comprehensive study of the antibacterial activity of bioactive juice and extracts from pomegranate (Punica granatum L.) peels and seeds. Plants (Basel) 2021, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Usha, T.; Middha, S.K.; Sidhalinghamurthy, K.R. Pomegranate peel and its anticancer activity: a mechanism-based review. 2020, 223-250. [CrossRef]
- Amri, Z.; Ben Khedher, M.R.; Zaibi, M.S.; Kharroubi, W.; Turki, M.; Ayadi, F.; Hammami, M. Anti-diabetic effects of pomegranate extracts in long-term high fructose-fat fed rats. Clin. Phytoscience 2020, 6, 55. [Google Scholar] [CrossRef]
- Jahani, M.; Pira, M.; Aminifard, M.H. Antifungal effects of essential oils against Aspergillus niger in vitro and in vivo on pomegranate (Punica granatum) fruits. Sci. Hortic. 2020, 264, 109188. [Google Scholar] [CrossRef]
- Stefanou, V.; Papatheodorou, S.; Tsakni, A.; Lougovois, V.; Talelli, A.; Panourgias, G.; Dariatos, A.; Tsaknis, I. Immunology. Anti-inflammatory properties of pomegranate. Int. J. Adv. Res. MicroBiol. Immunol. 2020, 2, 1–13. [Google Scholar]
- Uluman, E.; Kilicle, P.A. The investigation of the possible antigenotoxic in vivo effects of pomegranate (Punica granatum L.) peel extract on mitomycin-C genotoxicity. Turk. J. Vet. Anim. Sci. 2020, 44, 382–390. [Google Scholar] [CrossRef]
- Raj, A.A.S.; Rekhaa, V.P.S.; Suvedha, P.; Priya, N.R. Pomegranate: constituents, biological properties, therapeutic applications and its safety–A Review. Int. J. Adv. Life Sci. 2014, 7, 534–548. [Google Scholar]
- Mayasankaravalli, C.; Deepika, K.; Esther Lydia, D.; Agada, R.; Thagriki, D.; Govindasamy, C.; Chinnadurai, V.; Othman Gatar, O.M.; Khusro, A.; Kim, Y.O.; et al. Profiling the phyto-constituents of Punica granatum fruits peel extract and accessing its in-vitro antioxidant, anti-diabetic, anti-obesity, and angiotensin-converting enzyme inhibitory properties. Saudi J. Biol. Sci. 2020, 27, 3228–3234. [Google Scholar] [CrossRef] [PubMed]
- Jalili, S.; Tabatabee Naini, A.; Ashrafi, M.; Aminlari, M. Antioxidant activity of pericarp extract from different varieties of pomegranate fruit. J. Agr. Sci. Tech. 2020, 22, 95–107. [Google Scholar]
- Salles, T.S.; Meneses, M.D.F.; Caldas, L.A.; Sá-Guimarães, T.E.; de Oliveira, D.M.; Ventura, J.A.; Azevedo, R.C.; Kuster, R.M.; Soares, M.R.; Ferreira, D.F. Virucidal and antiviral activities of pomegranate (Punica granatum) extract against the mosquito-borne Mayaro virus. Parasit. Vectors 2021, 14, 1–8. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Plaza, M.; Marina, M.L.; García, M.C. Sustainable extraction of proteins and bioactive substances from pomegranate peel (Punica granatum L.) using pressurized liquids and deep eutectic solvents. Innov. Food Sci. Emerg. Technol. 2020, 60, 102314. [Google Scholar] [CrossRef]
- Niewiadomska, J.; Kasztura, M.; Janus, I.; Chełmecka, E.; Stygar, D.M.; Frydrychowski, P.; Wojdyło, A.; Noszczyk-Nowak, A. Punica granatum L. extract shows cardioprotective effects measured by oxidative stress markers and biomarkers of heart failure in an animal model of metabolic syndrome. Antioxidants 2023, 12, 1152. [Google Scholar] [CrossRef]
- Murtaza, S.; Khan, J.A.; Aslam, B.; Faisal, M.N. Pomegranate peel extract and quercetin possess antioxidant and hepatoprotective activity against concanavalin a-induced liver injury in mice. Pak. Vet. J. 2021, 41, 197–202. [Google Scholar] [CrossRef]
- Emami Kazemabad, M.J.; Asgari Toni, S.; Tizro, N.; Dadkhah, P.A.; Amani, H.; Akhavan Rezayat, S.; Sheikh, Z.; Mohammadi, M.; Alijanzadeh, D.; Alimohammadi, F. Pharmacotherapeutic potential of pomegranate in age-related neurological disorders. Front. Aging Neurosci. 2022, 14, 955735. [Google Scholar] [CrossRef]
- Conidi, C.; Drioli, E.; Cassano, A. Perspective of membrane technology in pomegranate juice processing: a review. Foods 2020, 9, 889. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Waheed, S.; Siddique, N.; Rahman, A.; Zaidi, J.; Ahmad, S. INAA for dietary assessment of essential and other trace elements in fourteen fruits harvested and consumed in Pakistan. J. Radioanal. Nucl. Chem. 2004, 260, 523–531. [Google Scholar] [CrossRef]
- Aloqbi, A.; Omar, U.; Yousr, M.; Grace, M.; Lila, M.A.; Howell, N. Antioxidant activity of pomegranate juice and punicalagin. Nat. Sci. 2016, 8, 235–246. [Google Scholar] [CrossRef]
- Paul, A.; Radhakrishnan, M. Pomegranate seed oil in food industry: Extraction, characterization, and applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- Afaq, F.; Saleem, M.; Krueger, C.G.; Reed, J.D.; Mukhtar, H. Anthocyanin-and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int. J. Cancer 2005, 113, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Walid, E.; Hedia, H.; Nizar, T.; Yassine, Y.; Nizar, N.; Ali, F. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plant Res. 2012, 6, 4724–4730. [Google Scholar] [CrossRef]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef]
- Kohno, H.; Suzuki, R.; Yasui, Y.; Hosokawa, M.; Miyashita, K.; Tanaka, T.J.C.s. Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci. 2004, 95, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Maphetu, N.; Unuofin, J.O.; Masuku, N.P.; Olisah, C.; Lebelo, S.L.J.B. Pharmacotherapy. Medicinal uses, pharmacological activities, phytochemistry, and the molecular mechanisms of Punica granatum L.(pomegranate) plant extracts: A review. Biomed. Pharmacother. 2022, 153, 113256. [Google Scholar] [CrossRef] [PubMed]
- Usta, C.; Ozdemir, S.; Schiariti, M.; Puddu, P.E. The pharmacological use of ellagic acid-rich pomegranate fruit. Int. J. Food Sci. Nutr. 2013, 64, 907–913. [Google Scholar] [CrossRef]
- Venusova, E.; Kolesarova, A.; Horky, P.; Slama, P. Physiological and immune functions of punicalagin. Nutrients 2021, 13, 2150. [Google Scholar] [CrossRef]
- Yilmaz, E.; Arikanoğlu, Z.; Turkoğlu, A.; Kiliç, E.; Yüksel, H.; Gümüş, M.J.E.R.f.M.; Sciences, P. The protective effects of pomegranate on liver and remote organs caused by experimental obstructive jaundice model. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 767–772. [Google Scholar]
- Ketnawa, S.; Reginio Jr, F.C.; Thuengtung, S.; Ogawa, Y. Changes in bioactive compounds and antioxidant activity of plant-based foods by gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4684–4705. [Google Scholar] [CrossRef] [PubMed]
- Yamini, S.; Paswan, V.K.; Shehata, A.M.; Choubey, M.; Bunkar, D.S.; Venkatesh, V. Pomegranate (Punica granatum L.) seed: A review on nutritional profile, functional food properties, health benefits, and safety aspects. Ann. phytomedicine 2023, 12, 93–104. [Google Scholar] [CrossRef]
- Suman, M.; Bhatnagar, P. A review on proactive pomegranate one of the healthiest foods. Int. J. Chem. Stud. 2019, 7, 189–194. [Google Scholar]
- Akbari, A. An overview of the characteristics and function of vitamin C in various tissues: relying on its antioxidant function. Zahedan J. Res. Med. Sci. 2016, 18, e4037. [Google Scholar] [CrossRef]
- Mirjalili, S.A. Pomegranate worth in women’s health-a review. Amazon. J. Plant Res. 2020, 4, 679–691. [Google Scholar] [CrossRef]
- Tashkandi, H.M. Therapeutic potential of pomegranate (Punica granatum Linn.) against breast cancer. Indian J. Pharm. Sci. 2023, 85, 156–166. [Google Scholar] [CrossRef]
- Karak, P. Biological activities of flavonoids: An overview. IJPSR 2019, 10, 1567–1574. [Google Scholar] [CrossRef]
- Prakash, D.; Gupta, C. Phytopharmaceutical applications of nutraceutical and functional foods. In Complementary and Alternative Medicine: Breakthroughs in Research and Practice; IGI Global: 2019; pp. 182–204.
- Brodowska, K.M. Natural flavonoids: classification, potential role, and application of flavonoid analogues. 2017, 7, 108–123. [Google Scholar] [CrossRef]
- Yisimayili, Z.; Chao, Z. A review on phytochemicals, metabolic profiles and pharmacokinetics studies of the different parts (juice, seeds, peel, flowers, leaves and bark) of pomegranate (Punica granatum L.). Food Chem. 2022, 395, 133600. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Özen, C.; Abu-Reidah, I.M.; Chigurupati, S.; Patra, J.K.; Horbanczuk, J.O.; Jóźwik, A.; Tzvetkov, N.T.; Uhrin, P.; Atanasov, A.G. Vasculoprotective effects of pomegranate (Punica granatum L.). Front. Pharmacol. 2018, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Vučić, V.; Grabež, M.; Trchounian, A.; Arsić, A. Composition and potential health benefits of pomegranate: a review. Curr. Pharm. Des. 2019, 25, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Jafari, T.; Fallah, A.A.; Bahrami, M.; Lorigooini, Z. Effects of pomegranate peel extract and vitamin E on oxidative stress and antioxidative capacity of hemodialysis patients: A randomized controlled clinical trial. J. Funct. Foods 2020, 72, 104069. [Google Scholar] [CrossRef]
- Suručić, R.; Travar, M.; Petković, M.; Tubić, B.; Stojiljković, M.P.; Grabež, M.; Šavikin, K.; Zdunić, G.; Škrbić, R. Pomegranate peel extract polyphenols attenuate the SARS-CoV-2 S-glycoprotein binding ability to ACE2 Receptor: In silico and in vitro studies. Bioorg. Chem. 2021, 114, 105145. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and bioactive components of pomegranate waste used in food and cosmetic applications: A review. Foods 2021, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Bozkurt, T.; Ergun, Z. Fatty acid composition and antioxidant capacity of pomegranate seed oil. GSCBPS 2021, 15, 103–110. [Google Scholar] [CrossRef]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The Bioavailability and biological activities of phytosterols as modulators of cholesterol metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef]
- Marsoul, A.; Ijjaali, M.; Elhajjaji, F.; Taleb, M.; Salim, R.; Boukir, A. Phytochemical screening, total phenolic and flavonoid methanolic extract of pomegranate bark (Punica granatum L): Evaluation of the inhibitory effect in acidic medium 1 M HCl. Mater. Today: Proc. 2020, 27, 3193–3198. [Google Scholar] [CrossRef]
- Puneeth, H.; Chandra, S. A review on potential therapeutic properties of pomegranate (Punica granatum L.). Plant Sci. Today 2020, 7, 9–16. [Google Scholar] [CrossRef]
- Wong, T.L.; Strandberg, K.R.; Croley, C.R.; Fraser, S.E.; Nagulapalli Venkata, K.C.; Fimognari, C.; Sethi, G.; Bishayee, A. Pomegranate bioactive constituents target multiple oncogenic and oncosuppressive signaling for cancer prevention and intervention. Semin. Cancer Biol. 2021, 73, 265–293. [Google Scholar] [CrossRef] [PubMed]
- Baral, S.; Kaphle, H.P. Health-related quality of life among menopausal women: A cross-sectional study from Pokhara, Nepal. PloS One 2023, 18, e0280632. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N. Perimenopause: from research to practice. J. Womens Health (Larchmt) 2016, 25, 332–339. [Google Scholar] [CrossRef]
- Johnson, A.; Roberts, L.; Elkins, G. Complementary and alternative medicine for menopause. J. Evid. Based Integr. Med. 2019, 24, 2515690x19829380. [Google Scholar] [CrossRef] [PubMed]
- Sussman, M.; Trocio, J.; Best, C.; Mirkin, S.; Bushmakin, A.G.; Yood, R.; Friedman, M.; Menzin, J.; Louie, M. Prevalence of menopausal symptoms among mid-life women: findings from electronic medical records. BMC Womens Health 2015, 15, 58. [Google Scholar] [CrossRef]
- Yu, Q.; Chae, H.D.; Hsiao, S.M.; Xie, J.; Blogg, M.; Sumarsono, B.; Kim, S. Prevalence, severity, and associated factors in women in East Asia with moderate-to-severe vasomotor symptoms associated with menopause. Menopause 2022, 29, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Theis, S.; Baumgartner, S.J.; Janka, H.; Kolokythas, A.; Skala, C.; Stute, P. Quality of life in menopausal women in the workplace - a systematic review. Climacteric 2023, 26, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Minkin, M.J. Menopause: Hormones, lifestyle, and optimizing aging. Obstet. Gynecol. Clin. North Am. 2019, 46, 501–514. [Google Scholar] [CrossRef]
- Moeini, R.; Shirafkan, H.; Gorji, N. Pomegranate effects on the health aspects of women during peri-and postmenopause: A systematic review and meta-analysis. Phytother. Res. 2023, 38, 368–383. [Google Scholar] [CrossRef]
- Newson, L. Menopause and cardiovascular disease. Post Reprod. Health 2018, 24, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Assaf, A.R.; Bushmakin, A.G.; Joyce, N.; Louie, M.J.; Flores, M.; Moffatt, M. The relative burden of menopausal and post-menopausal symptoms versus other major conditions: A retrospective analysis of the medical expenditure panel survey data. Am. Health Drug Benefits 2017, 10, 311–321. [Google Scholar] [PubMed]
- Wee, J.-H.; Jung, H.J.; Jung, K.O.; Sung, H.M.; Shin, Y.-R.; Park, J.-H.; Seo, H.-Y.; Lim, J.-M.; Chae, H.-J.; Lee, K.Y. Pomegranate extract improves menopausal syndrome in ovariectomized rats. J. Korean Soc. Food Sci. Nutr. 2015, 44, 506–515. [Google Scholar] [CrossRef]
- Mori-Okamoto, J.; Otawara-Hamamoto, Y.; Yamato, H.; Yoshimura, H. Pomegranate extract improves a depressive state and bone properties in menopausal syndrome model ovariectomized mice. J. Ethnopharmacol. 2004, 92, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Shaban, N.Z.; Talaat, I.; Elrashidy, F.; Hegazy, A.; Sultan, A. Therapeutic role of Punica granatum (pomegranate) seed oil extract on bone turnover and resorption induced in ovariectomized rats. J. Nutr. Health Aging 2017, 21, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Kum, E.J.; Kwon, D.H.; Lee, H. The effect of pomegranate extracts on the menopausal syndromes. J. Exp. Biomed. Sci. 2009, 15, 217–227. [Google Scholar]
- Kaban, I.; Kaban, A.; Tunca, A.F.; Aka, N.; Kavak, H.; Akar, F. Effect of pomegranate extract on vagina, skeleton, metabolic and endocrine profiles in an ovariectomized rat model. J. Obstet. Gynaecol. Res. 2018, 44, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Spilmont, M.; Léotoing, L.; Davicco, M.-J.; Lebecque, P.; Mercier, S.; Miot-Noirault, E.; Pilet, P.; Rios, L.; Wittrant, Y.; Coxam, V. Pomegranate and its derivatives can improve bone health through decreased inflammation and oxidative stress in an animal model of post-menopausal osteoporosis. Eur. J. Nutr. 2014, 53, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Lentscher, J.A.; Slocum, B.; Torrealday, S. Polycystic ovarian syndrome and fertility. Clin. Obstet. Gynecol. 2021, 64, 65–75. [Google Scholar] [CrossRef]
- Minocha, N. Polycystic ovarian disease or polycystic ovarian syndrome: How to identify and manage-a review. Arch. Pharm. Pract. 2020, 11, 102–106. [Google Scholar]
- Bahmani, M.; Shokri, S.; Akhtar, Z.N.; Abbaszadeh, S.; Manouchehri, A. The effect of pomegranate seed oil on human health, especially epidemiology of polycystic ovary syndrome; a systematic review. JBRA Assist. Reprod. 2022, 26, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Abasian, Z.; Rostamzadeh, A.; Mohammadi, M.; Hosseini, M.; Rafieian-Kopaei, M. A review on role of medicinal plants in polycystic ovarian syndrome: pathophysiology, neuroendocrine signaling, therapeutic status and future prospects. Middle East Fertil. Soc. J. 2018, 23, 255–262. [Google Scholar] [CrossRef]
- Purwar, A.; Nagpure, S. Insulin resistance in polycystic ovarian syndrome. Cureus 2022, 14, e30351. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Haseeb, A.; Rehman, A. Relationship of polycystic ovarian syndrome with cardiovascular risk factors. Diabetes Metab. Syndr. 2018, 12, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Franks, S. Chapter 27 - Genetic and environmental factors in the etiology of polycystic ovary syndrome. In The Ovary (Third Edition), Leung, P.C.K., Adashi, E.Y., Eds.; Academic Press: 2019; pp. 437–459.
- Ajmal, N.; Khan, S.Z.; Shaikh, R. Polycystic ovary syndrome (PCOS) and genetic predisposition: A review article. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 3, 100060. [Google Scholar] [CrossRef]
- Wawrzkiewicz-Jałowiecka, A.; Kowalczyk, K.; Trybek, P.; Jarosz, T.; Radosz, P.; Setlak, M.; Madej, P. In search of new therapeutics-molecular aspects of the PCOS pathophysiology: Genetics, hormones, metabolism and beyond. Int. J. Mol. Sci. 2020, 21, 7054. [Google Scholar] [CrossRef] [PubMed]
- Hossein, K.J.; Leila, K.J.; koukhdan Ebrahim, T.; Nazanin, S.J.; Farzad, P.; Elham, R.J.B.; Journal, P. The effect of pomegranate juice extract on hormonal changes of female Wistar rats caused by polycystic ovarian syndrome. Biomed. Pharmacol. J. 2015, 8, 971–977. [Google Scholar] [CrossRef]
- Ibrahim, M.; Sadek, M.; Eldin, H.S.J.M. Role of pomegranate extract in restoring endometrial androgen receptor expression, proliferation, and pinopodes in a rat model of polycystic ovary syndrome. Morphologie 2022, 106, 145–154. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Sharma, P.; McClees, S.F.; Afaq, F.J.M. Pomegranate for prevention and treatment of cancer: an update. Molecules 2017, 22, 177. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: a review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Britt, K.L.; Cuzick, J.; Phillips, K.-A. Key steps for effective breast cancer prevention. Nat. Rev. Cancer 2020, 20, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Ronnenberg, A.; Puleo, E.; Chatterton, R.T., Jr.; Dorgan, J.F.; Seeram, N.P.; Sturgeon, S.R. Effects of pomegranate juice on hormonal biomarkers of breast cancer risk. Nutr. Cancer 2015, 67, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Khwairakpam, A.D.; Bordoloi, D.; Thakur, K.K.; Monisha, J.; Arfuso, F.; Sethi, G.; Mishra, S.; Kumar, A.P.; Kunnumakkara, A.B. Possible use of Punica granatum (pomegranate) in cancer therapy. Pharmacol. Res. 2018, 133, 53–64. [Google Scholar] [CrossRef]
- Shirode, A.B.; Kovvuru, P.; Chittur, S.V.; Henning, S.M.; Heber, D.; Reliene, R. Antiproliferative effects of pomegranate extract in MCF-7 breast cancer cells are associated with reduced DNA repair gene expression and induction of double strand breaks. Mol. Carcinog. 2014, 53, 458–470. [Google Scholar] [CrossRef]
- Chen, H.S.; Bai, M.H.; Zhang, T.; Li, G.D.; Liu, M. Ellagic acid induces cell cycle arrest and apoptosis through TGF-β/Smad3 signaling pathway in human breast cancer MCF-7 cells. Int. J. Oncol. 2015, 46, 1730–1738. [Google Scholar] [CrossRef]
- Kim, N.D.; Mehta, R.; Yu, W.; Neeman, I.; Livney, T.; Amichay, A.; Poirier, D.; Nicholls, P.; Kirby, A.; Jiang, W.; et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res. Treat. 2002, 71, 203–217. [Google Scholar] [CrossRef]
- Costantini, S.; Rusolo, F.; De Vito, V.; Moccia, S.; Picariello, G.; Capone, F.; Guerriero, E.; Castello, G.; Volpe, M.G. Potential anti-inflammatory effects of the hydrophilic fraction of pomegranate (Punica granatum L.) seed oil on breast cancer cell lines. Molecules 2014, 19, 8644–8660. [Google Scholar] [CrossRef]
- Toi, M.; Bando, H.; Ramachandran, C.; Melnick, S.J.; Imai, A.; Fife, R.S.; Carr, R.E.; Oikawa, T.; Lansky, E.P. Preliminary studies on the anti-angiogenic potential of pomegranate fractions in vitro and in vivo. Angiogenesis 2003, 6, 121–128. [Google Scholar] [CrossRef]
- Xue, P.; Zhang, G.; Zhang, J.; Ren, L. Synergism of ellagic acid in combination with radiotherapy and chemotherapy for cancer treatment. Phytomedicine 2022, 99, 153998. [Google Scholar] [CrossRef]
- Kim, H.J.; Kwon, D.H.; Kum, E.J. Effects of Punica granatum (pomegranate) extracts on the menopause women. Biomed. Sci. Lett. 2010, 16, 53–64. [Google Scholar]
- Huber, R.; Gminski, R.; Tang, T.; Weinert, T.; Schulz, S.; Linke-Cordes, M.; Martin, I.; Fischer, H.; Tang, T. Pomegranate (Punica granatum) seed oil for treating menopausal symptoms: An individually controlled cohort study. Altern. Ther. Health Med. 2017, 23, 28–34. [Google Scholar] [PubMed]
- Auerbach, L.; Rakus, J.; Bauer, C.; Gerner, C.; Ullmann, R.; Wimmer, H.; Huber, J. Pomegranate seed oil in women with menopausal symptoms: a prospective randomized, placebo-controlled, double-blinded trial. Menopause 2012, 19, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Adel-Mehraban, M.S.; Tansaz, M.; Mohammadi, M.; Yavari, M. Effects of pomegranate supplement on menopausal symptoms and quality of life in menopausal women: A double-blind randomized placebo-controlled trial. Complement. Ther. Clin. Pract. 2022, 46, 101544. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, M.; Mahjoub, S. Effects of aerobic exercise and pomegranate extract on antioxidant markers in women post-menopausal with type 2 diabetes. HMJ 2017, 21, 128–136. [Google Scholar]
- Pomegranate extract biomarker study in osteopenic women. 2010. Available online: https://classic.clinicaltrials.gov/show/NCT01219140 (accessed on 5 February 2024).
- Sakarya, U. Effect of pomegranate supplementation on symptom severity in women with premenstrual syndrome. 2024. Available online: https://classic.clinicaltrials.gov/show/NCT06201702 (accessed on 5 February 2024).
- Esmaeilinezhad, Z.; Babajafari, S.; Sohrabi, Z.; Eskandari, M.H.; Amooee, S.; Barati-Boldaji, R. Effect of synbiotic pomegranate juice on glycemic, sex hormone profile and anthropometric indices in PCOS: A randomized, triple blind, controlled trial. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 201–208. [Google Scholar] [CrossRef]
- Esmaeilinezhad, Z.; Barati-Boldaji, R.; Brett, N.R.; de Zepetnek, J.O.T.; Bellissimo, N.; Babajafari, S.; Sohrabi, Z. The effect of synbiotics pomegranate juice on cardiovascular risk factors in PCOS patients: a randomized, triple-blinded, controlled trial. J. Endocrinol. Invest. 2020, 43, 539–548. [Google Scholar] [CrossRef]
- Abedini, M.; Ghasemi-Tehrani, H.; Tarrahi, M.J.; Amani, R. The effect of concentrated pomegranate juice consumption on risk factors of cardiovascular diseases in women with polycystic ovary syndrome: A randomized controlled trial. Phytother. Res. 2021, 35, 442–451. [Google Scholar] [CrossRef]
- Abedini, M.; Ramezani-Jolfaie, N.; Ghasemi-Tehrani, H.; Tarrahi, M.J.; Amani, R. The effect of concentrated pomegranate juice on biomarkers of inflammation, oxidative stress, and sex hormones in overweight and obese women with polycystic ovary syndrome: A randomized controlled trial. Phytother. Res. 2023, 37, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Duan, Y.; Ma, F.; Lou, L. Punicalagin inhibits the viability, migration, invasion, and EMT by regulating GOLPH3 in breast cancer cells. J. Recept. Signal Transduct. Res. 2020, 40, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Nallanthighal, S.; Elmaliki, K.M.; Reliene, R. Pomegranate extract alters breast cancer stem cell properties in association with inhibition of epithelial-to-mesenchymal transition. Nutr. Cancer 2017, 69, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Bhatia, D.; Bishayee, A. Anti-inflammatory mechanism involved in pomegranate-mediated prevention of breast cancer: the role of NF-κB and Nrf2 signaling pathways. Nutrients 2017, 9, 436. [Google Scholar] [CrossRef]
- Bishayee, A.; Mandal, A.; Bhattacharyya, P.; Bhatia, D. Pomegranate exerts chemoprevention of experimentally induced mammary tumorigenesis by suppression of cell proliferation and induction of apoptosis. Nutr. Cancer 2016, 68, 120–130. [Google Scholar] [CrossRef]
| Chemical class | Phytochemicals |
|---|---|
| Amino acids | Aspartic acid, Glutamic acid, Methionine, Proline, Valine |
| Anthocyanins | Cyanidin-3-O-glucoside, Cyanidin-3,5-di-O-glucosdie, Delphinidin-3-O-glucoside, Delphinidin-3,5-di-O-glucoside, Pelargonidin-3-O-glucoside, Pelargonidin-3,5-di-O-glucoside |
| Ellagitannins | Casuarinin, Corilagin, Gallagyldilacton, Punicalagin, Punicalin |
| Flavan-3-ols | Catechin, Epicatechin, Epigallocatechin-3-gallate |
| Flavonols | Quercetin, Isoquercetin, Rutin |
| Hydroxybenzoic acid/Hydroxycinnamic acid | Caffeic acid, Chlorogenic acid, Ellagic acid, Gallic acid, Quinic acid |
| Indoleamines | Melatonin, Serotonin, Tryptamine |
| Organic acids | Ascorbic acid, Citric acid, Fumaric acid, Malic acid, Succinic acid, Tartaric acid |
| Sugars | Glucose, Fructose, Sucrose |
| Chemical class | Phytochemicals |
|---|---|
| Conjugated fatty acids | Punicic acid |
| Non-conjugated fatty acids | Linoleic acid, Oleic acid, Palmitic acid, Stearic acid |
| Hydroxybenzoic acid | 3,3’-Di-O-methylellagic acid, Ellagic acid, 3,3’-4’-Tri-O-methylellagic acid |
| Isoflavones | Daidzein, Genistein |
| Lignins | Coniferyl-9-O-[β-dapiofuranosyl(1→6)-O-β-D-glucopyranoide, Icariside D1, Phenylethyl rutinoside, Sinapyl-9-O-[βD-apiofuranosyl(1→6)-O-β-D-glucopyranoide |
| Sterols and steroids | Camesterol, Cholesterol, 17-α-Estradiol, Estriol, Estrone, β-Sitosterol, Stigmasterol, Testosterone |
| Tocopherols | γ-Tocopherol |
| Triterpenes | Oleanolic acid, Ursolic acid |
| Conditions | Interventions | Dosage/Duration | Brief Summary | Outcomes | Refs. |
|---|---|---|---|---|---|
| Menopausal Symptoms | |||||
| MSs women (Experience of menstrual irregularities lasting for more than 3 months or amenorrhea persisting for over 1 year postmenopause) |
PE, Placebo |
125 mg of PE per 500 mg tablet, 12 tablets per day (= 1.5 g of PE per day) / 8 weeks | Effects of PE on the MSs women | Decrease in HFs score, HFs VAS, sweating VAS, HFs duration, MRS, KI, MSs frequency, and MENQOL | [119] |
| MSs women (Mean duration of MSs of 46 months) |
PSO, Placebo |
1000 mg of PSO per day in 2 capsules / 8 weeks | Investigating the safety and effectiveness of PSO for MSs | Reduction in MRS symptoms (HFs); Improvement in urogenital tract symptoms (dry vagina) | [120] |
| Post-MSs women (12 months of amenorrhea) |
PSO, Placebo |
Two doses of 30 mg PSO per day / 12 weeks | Investigating the potential effects of PSO on MSs | Decrease in the score of the menopause rating scale II, the number of HFs, and the sum score of vegetative somatic symptoms; Improvement in sleeping disorders | [121] |
| MSs women | Pomegranate supplement, Placebo |
3 mL three times per day / 4 weeks | Effects of pomegranate supplement on MSs and quality of life in menopausal women | Decrease in modified-KI and MENQOL | [122] |
| Post-MSs women with type 2 diabetes | CW, PE, TW, TPE | 150 mL of PE per day / 6 weeks | Effects of aerobic exercise and PE on antioxidant markers in post-MSs women with type 2 diabetes | Increase in GPX, SOD, GSH, and TAC; The highest levels of the antioxidants in TPE | [123] |
| Osteopenic women | 2 PE capsules, 2 placebo capsules |
2 PE capsules per day / 6 month | Changes in biomarkers related to bone absorption and formation in post-MSs women | Urinary NTX and serum P1NP in post-MSs women (Completed, No study results posted) |
[124] |
| Pre-MSs women | PE, Placebo |
2 mL of PE three times per day / 10 days during the 3 menstrual cycles (from 7 days before to 3 days after the estimated onset of menstruation) | Effect of pomegranate supplementation on symptom severity in pre-MSs women | Pre-MSs scale (Recruiting) | [125] |
| PCOS | |||||
| PCOS patients | SPJ, PJ, SB, PB | 2 L of PJ, SB, SPJ, PB per week / 8 weeks | Effect of SPJ on glycemic, sex hormone profile, and anthropometric indices in PCOS | Decrease in insulin resistance, FBS, testosterone, BMI, waist, and hip circumference; Increase in insulin sensitivity | [126] |
| PCOS patients | SPJ, PJ, SB, PB | 300 mL of PJ, SB, SPJ, PB per day / 8 weeks | The effect of SPJ on cardiovascular risk factors in PCOS patients | Decrease in TC, LDL-C, MDA, hs-CRP, and blood pressure; Increase in HDL-C and TAC | [127] |
| Women with PCOS (18–40 years and BMI of ≥ 25 kg/m2 ) |
CPJ, Placebo |
45 mL of CPJ in combination with 180 mL water per day / 8 weeks | The effect of CPJ consumption on risk factors of cardiovascular diseases in women with PCOS | Decrease in systolic and diastolic blood pressure and TG levels, TG/HDL-C ratio; Increase in LDL-C and HDL-C | [128] |
| Women with PCOS (18–40 years and BMI of ≥ 25 kg/m2) |
CPJ, Placebo |
45 mL of CPJ in combination with 180 mL water per day / 8 weeks | The effect of CPJ on biomarkers of inflammation, oxidative stress, and sex hormones in overweight and obese women with PCOS | Decrease in serum testosterone levels | [129] |
| Breast Cancer | |||||
| Healthy post-MSs women | 100% commercial PJ, 100% apple juice (control) |
8 ounces (4 ounces in the morning and 4 ounces in the early evening) per day / 3 weeks | effects of PJ on hormonal biomarkers of breast cancer risk | Decrease in estrone and testosterone levels (normal weight women) |
[111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





