1. Introduction
Stimuli-responsive luminescent materials are materials with intelligent response characteristics that can change their fluorescent colour by external stimuli such as force[
1,
2], temperature[
3,
4,
5], pH[
6,
7], light[
8,
9,
10,
11], magnetic field[
12], etc[
13,
14,
15]. Among them acid stimulus-responsive fluorescent materials play a crucial role in anti-counterfeiting and security. Adding acid-stimuli-responsive fluorescent materials to a label changes the colour or pattern of the label when exposed to acid. This can be used to protect products from counterfeiting and tampering[
16,
17]. Developing acid-stimulation responsive materials that reveal hidden information when exposed to acidic environments[
18,
19]. This can be used to create anti-counterfeiting documents and security markings.
The controlled erasure of fluorescent molecules is crucial for securely transmitting encrypted information. However, most current research on erasable materials still relies on the attenuation of fluorescent signals. The development of molecular materials that can be removed with common solvents presents a more convenient method for tamper-resistant removal. Nevertheless, this method of conveniently erasing information still requires further improvement[
20,
21,
22,
23].
Most systems discussed so far suffer from the aggregation-caused quenching (ACQ) effect, which is very limited in practical applications involving solid substrates. On the other hand, aggregation-induced emission (AIE) molecules, which Tang and coworkers pioneered[
24,
25], emit bright fluorescence in the solid or aggregated state but usually show weak or even no fluorescence in solution, which endows solid materials with fascinating fluorescent properties. Therefore, introducing AIE properties into smart response anti-counterfeiting materials is an ideal strategy to solve the limitations of ACQ.
Therefore, we have designed a tetraphenylethylene (TPE)-based molecule decorated with diaminotriazine (DAT) groups, which shows strong AIE properties and can hardly dissolve in any common solvent. However, the molecule can easily react with various organic and inorganic acids by protonating the N atoms in the triazine rings. Over 90% or more fluorescence diminution occurs upon reaction, while TPEPhDAT, after protonation, can be quickly removed with common MeOH solvents. We demonstrate that this material can be processed into paper with encrypted information for anti-counterfeiting applications by solution methods.
2. Results and Discussion
2.1. Synthesis and Structural Elucidation of TPEPhDAT
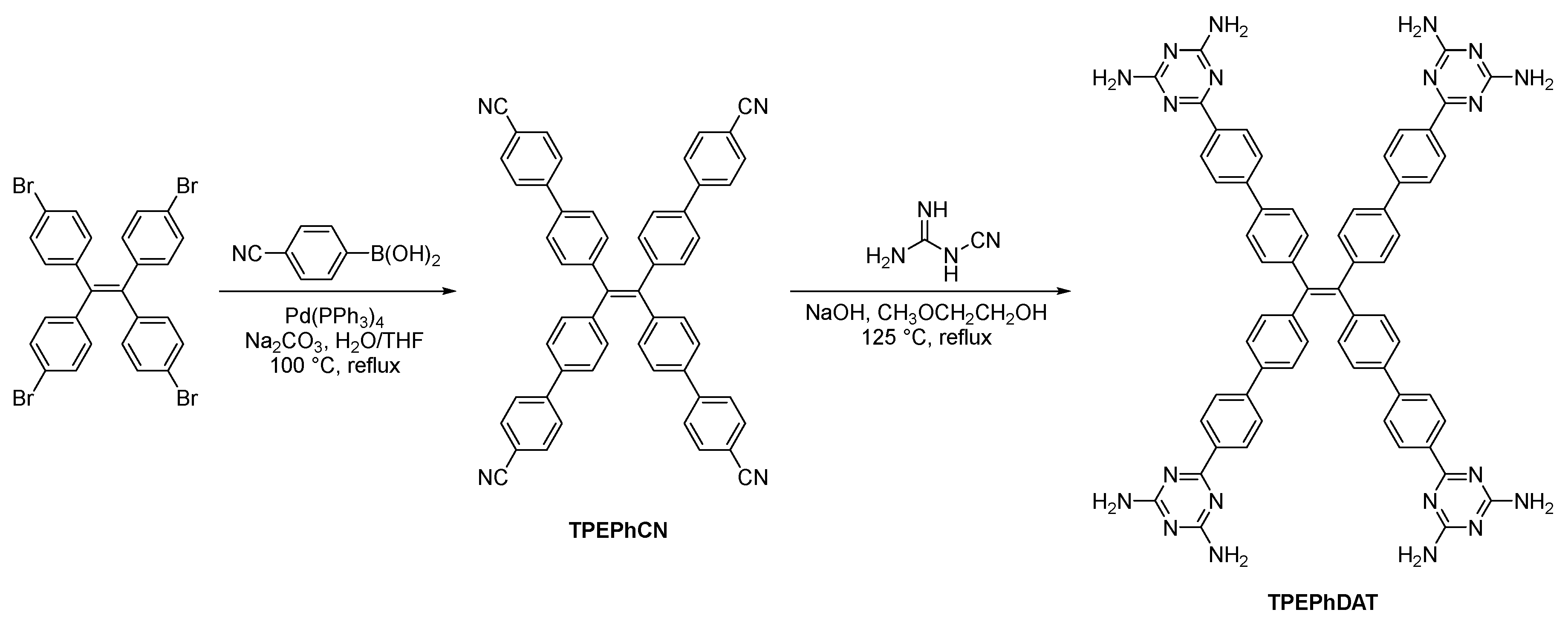
TPEPhDAT was synthesized according to a previous report and was obtained by a two-step reaction[
26,
27], the synthetic route of which is shown in
Scheme 1. 1,1,2,2-tetrakis(4-bromophenyl)ethene and 4-cyanophenylboronic acid underwent Suzuki-Miyaura coupling to obtain extended
TPEPhCN. The yellowish
TPEPhDAT could be easily obtained in 80% yield by reacting the corresponding nitrile with dicyandiamide. The structure of
TPEPhDAT was characterized and confirmed by
1H NMR and
13C NMR (
Figures S1–S4).
2.2. AIE Characteristic of TPEPhDAT
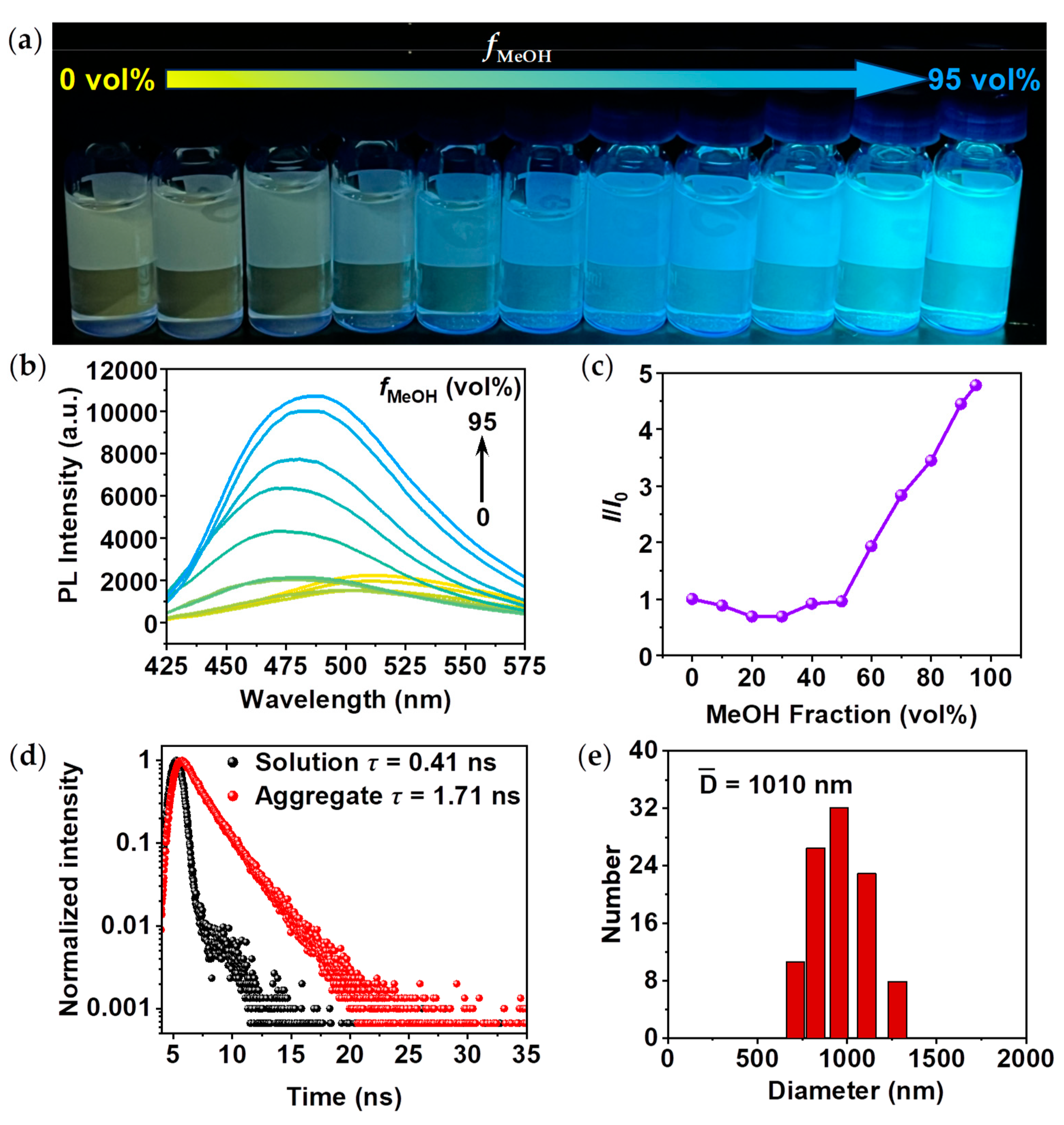
The AIE properties of
TPEPhDAT were explored in the DMSO/MeOH system (
Figure 1a). In this system, DMSO is a good solvent, and MeOH is a poor solvent. The luminescence of
TPEPhDAT is extremely weak in pure DMSO, when the volume fraction of MeOH (
fMeOH) was increased from 0 vol% to 30 vol%, the fluorescence emission spectra were continuously blue-shifted and the intensity of the emission peaks decreased slightly. This particular fluorescence change has been identified in existing reports[
28,
29,
30], and this phenomenon can be attributed to suppression of proximity effect (SOPE)[
31,
32]. When
fMeOH was greater than 30 vol%, the intensity of the emission peaks was gradually enhanced, reaching a maximum at
fMeOH of 95 vol% (
Figure 1b). Compared with the pure DMSO system, the fluorescence intensity increased about 5-fold (
Figure 1c). Besides, the fluorescence spectra of the aggregates were blue-shifted compared with that of the solution (
Figure 1b). The above phenomenon results from the joint action of the solvation effect and AIE effect[
33,
34,
35].
In dilute DMSO solution, the benzene ring rotor of TPE undergoes dynamic intramolecular rotation, and the excited state energy is dissipated in the form of non-radiative leaps, resulting in weak luminescence. As the
fMeOH increases, the molecule appears aggregated, and the rotation of the benzene ring rotor is spatially limited. The restricted intramolecular rotation (RIR) suppresses the abovementioned non-radiative decay[
24,
36], and the excited state molecules can only return to the ground state by radiative decay, significantly enhancing the fluorescence. When
fMeOH = 95 vol%, the absorption intensity of the ultraviolet-visible (UV-vis) spectra underwent a significant decrease, and at the same time, the tail elevation phenomenon appeared at the long wavelength band of the absorption spectrum (
Figure S5), indicating that the addition of MeOH caused the aggregation of
TPEPhDAT in the system[
37,
38].
Figure 1d shows the fluorescence lifetime decay curves of
TPEPhDAT in DMSO solution and DMSO/MeOH mixture (
fMeOH = 95 vol%). The τ value of
TPEPhDAT increases from 0.41 ns to 1.71 ns as the MeOH fraction in the mix is increased from 0 vol% to 95 vol%. The longer fluorescence lifetime reflects that
TPEPhDAT is more immobilized and aggregated[
39,
40]. In the meantime, when
fMeOH = 95 vol%, aggregates with a hydrodynamic diameter of about 1 μm appeared, as confirmed by dynamic light scattering (DLS,
Figure 1e) and scanning electron microscopy (SEM,
Figure S6).
2.3. Acidochromism
The nitrogen atoms in the DAT moiety are considered effective proton acceptors[
7,
41,
42]. Given this, we investigated the possible acid-induced fluorescence discolouration properties of
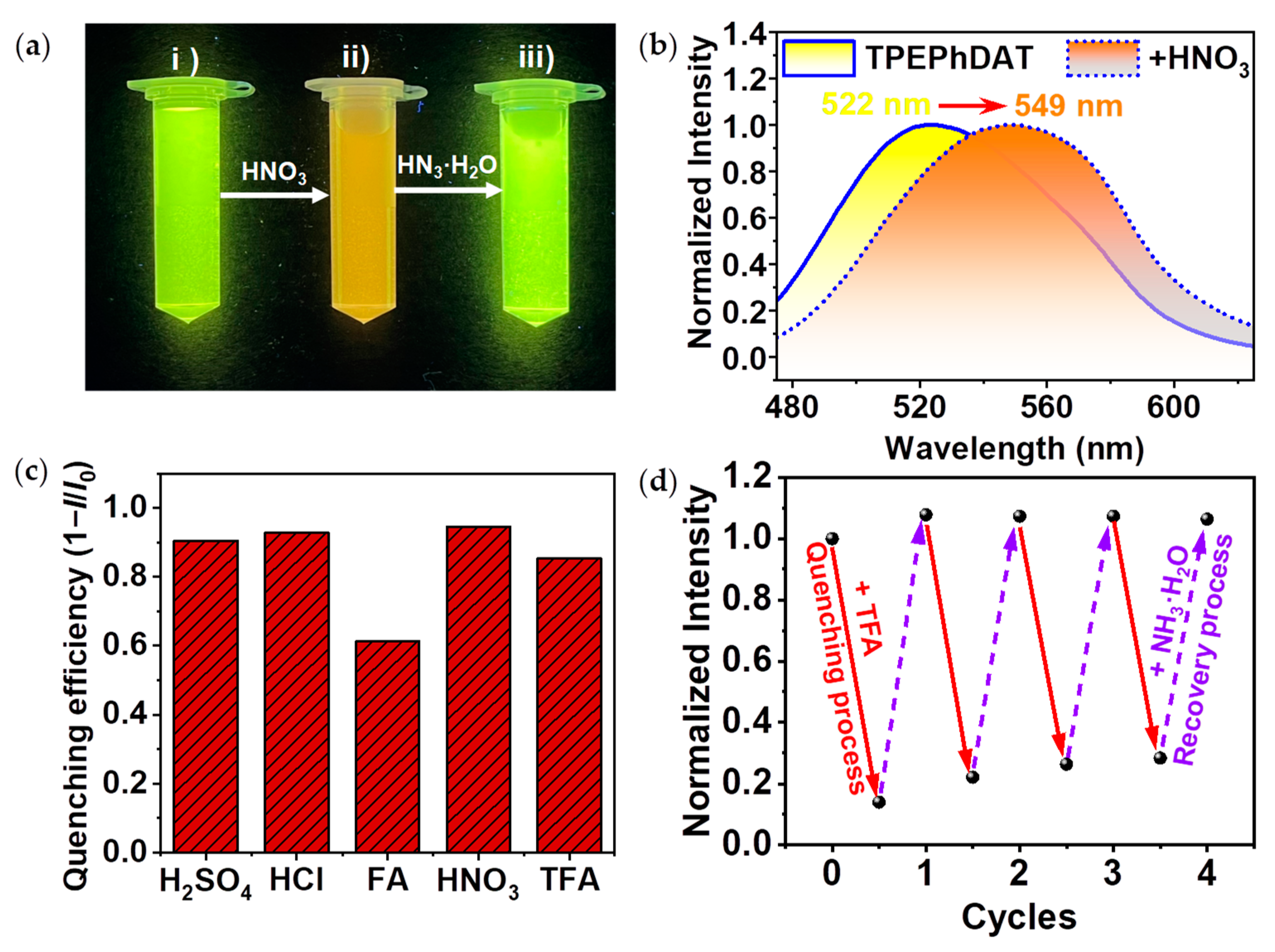
TPEPhDAT. In an aqueous dispersion of
TPEPhDAT, an excess of HNO
3 was added dropwise, and an immediate change in fluorescence was observed (
Figure 2a). As shown in
Figure 2b, the aqueous dispersion of
TPEPhDAT showed bright yellow fluorescence with the maximum emission peak at 522 nm (
λex = 350 nm). In contrast, the dropwise addition of HNO
3 completely disappeared the initial emission band at 522 nm, and a new peak at 549 nm corresponded to the orange emission. The fluorescence of
TPEPhDAT+HNO3 was extremely weak, and the quenching rate reached 95%. In addition, we also discussed the acid-induced fluorescence discolouration properties of four acids, including HCl, trifluoroacetic acid (TFA), H
2SO
4, and formic acid (FA), in addition to HNO
3 (
Figure S7). The results showed that similar results were presented with HNO
3, and the quenching efficiency of HCl, H
2SO
4, and TFA could reach more than 85%, while FA was less acidic, and the quenching efficiency could only reach 61% (
Figure 2c). The results of the absolute quantum yield (
Φ) tests are in close agreement with those of the fluorescence quenching (
Figure S8). In an aqueous solution, the
Φ of
TPEPhDAT can reach 57.36%, which decreases to different degrees after adding different acids, especially after adding HNO
3. The
Φ can be reduced to 6.85%. It is proved that the fluorescence change of
TPEPhDAT is caused by hydrogen ions (H
+) but not by anions (NO
3−), and the stronger the acidity, the more pronounced the effect of acid discolouration.
We attempted to treat acidified
TPEPhDAT with ammonia. We found that the addition of excess ammonia fully restored the fluorescence, which was still fully restored after four cycles of repeated treatment with TFA and ammonia (
Figure 2d). When the solution was treated with TFA and ammonia alternately, the fluorescence emission could be switched several times between "off and on" states. This implies that the reaction is entirely reversible.
2.4. Mechanism of TPEPhDAT Binding to Acid
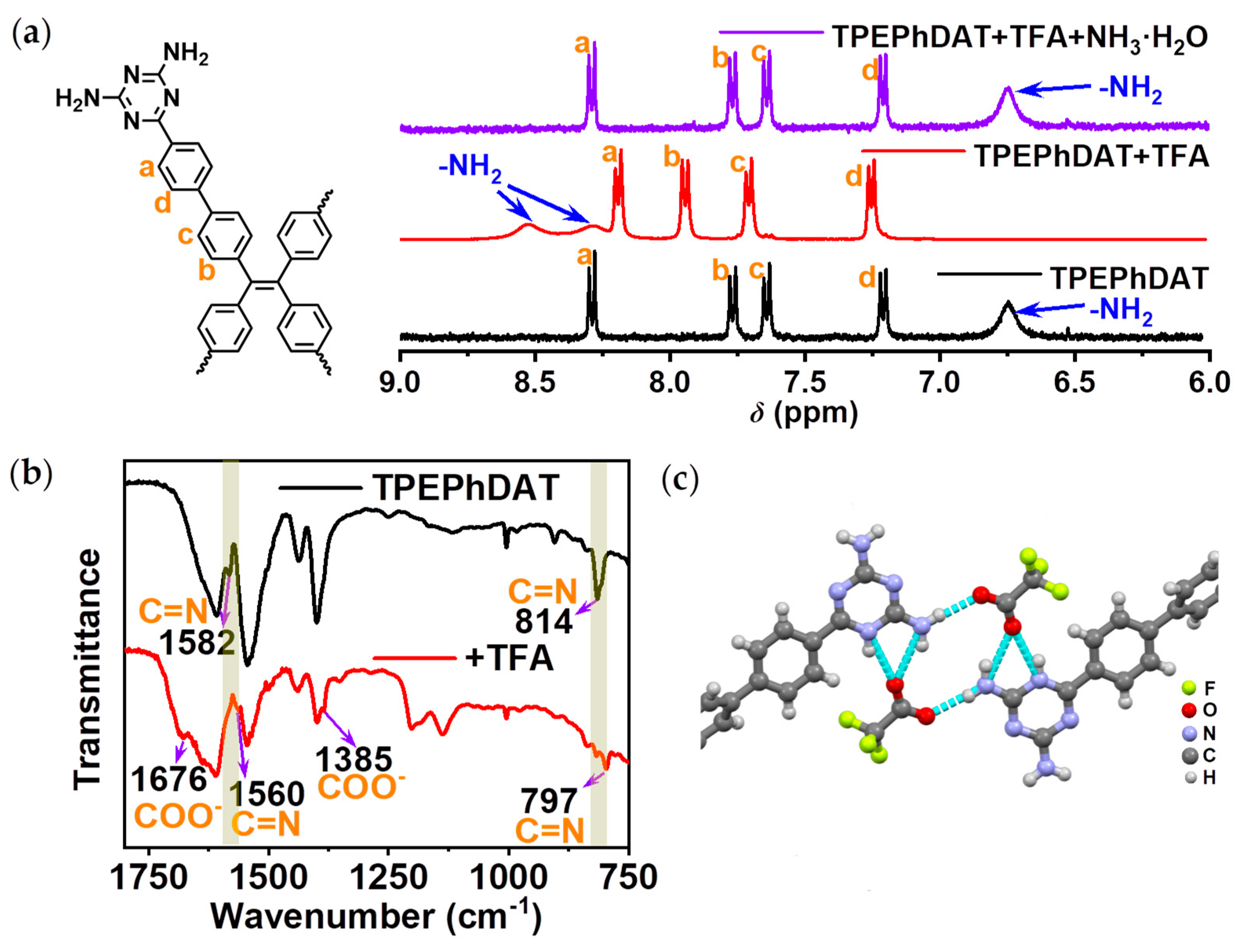
To further understand the binding behaviour at the molecular level, we performed
1H NMR measurements using TFA as an example. The two protons of −NH
2 in
TPEPhDAT show a single broad peak. In contrast, after being protonated, these two protons show two separate single peaks that shifted to a lower field than before protonation (
Figure 3a). The non-equivalence of the two amino protons is because the bond between C-4 and −NH
2 has a partially double-bonded nature after being protonated, resulting in a blockage of its rotation (
Figure S9)[
43,
44].
This suggests that the protonation of
TPEPhDAT occurs on the triazine ring and not on −NH
2. Fourier transform infrared (FT-IR) spectroscopy further confirms this. As shown in
Figure 3b, the peaks at 1582 and 814 cm
−1 significantly shift to 1560 and 797 cm
−1 upon the addition of TFA. These changes are attributed to the deformation of the triazine ring from an ideal hexagonal shape upon protonation[
45,
46,
47]. The peaks of deprotonated −COOH were also observed (1676 and 1385 cm
−1)[
48]. This indicates that upon the reaction of TFA with
TPEPhDAT, the −COOH of TFA loses its proton and forms −COO
−. The changes in FT-IR spectra provided evidence for the protonation of the triazine ring. In aqueous solutions, triazine cyclonitrogen is generally more alkaline than nitrogen in −NH
2[
49,
50,
51,
52], because its lone pair electrons do not participate in conjugation and are more vulnerable to proton attack. In addition, the strong electron absorption of the triazine ring causes the electronegativity of amino nitrogen to easily transfer to the triazine ring (
Figure S10)[
53], preventing amino nitrogen from binding with protons.
Given this, we prepared co-crystals of
TPEPhDAT with TFA by solvothermal methods (
Figure S11). As can be seen from the crystal structure (
Figure 3c;
Figure S12,
Table S1), the protonation of
TPEPhDAT occurs on the triazine ring rather than on −NH
2. This crystal structure provides a possible site for the binding of
TPEPhDAT to acids.
2.5. Possible Mechanism of Acid-induced Fluorescence Quenching in TPEPhDAT
As can be seen in
Figure 4a, the
TPEPhDAT molecule has weak intramolecular charge transfer (ICT) properties. When
TPEPhDAT reacts with TFA, the triazine ring exhibits a strong nucleophilic ability and can effectively attack the −COOH of TFA to generate the corresponding salt. Upon completion of protonation, the ICT effect of
TPEPhDAT was enhanced, achieving a significant change in fluorescence from the initial yellow to orange[
54,
55]. Therefore, simple deprotonation methods can help restore the probe's fluorescence properties. For example,
TPEPhDAT can be simply deprotonated by adding ammonia, thus restoring the initial properties of the fluorescent probe.
To elucidate the chemical sensing system's quenching mechanism, time-resolved fluorescence spectroscopy was utilized to investigate the quenching dynamics (
Figure 4b). The results revealed that the fluorescence lifetimes of
TPEPhDAT were 2.29 ns and 0.62 ns before and after adding 0.25 M H
2SO
4, respectively, indicating a dynamic quenching process[
56]. Furthermore, simple deprotonation treatment can restore the fluorescence of
TPEPhDAT; for instance, dropping ammonia can restore the fluorescence lifetime to its initial state (2.10 ns).
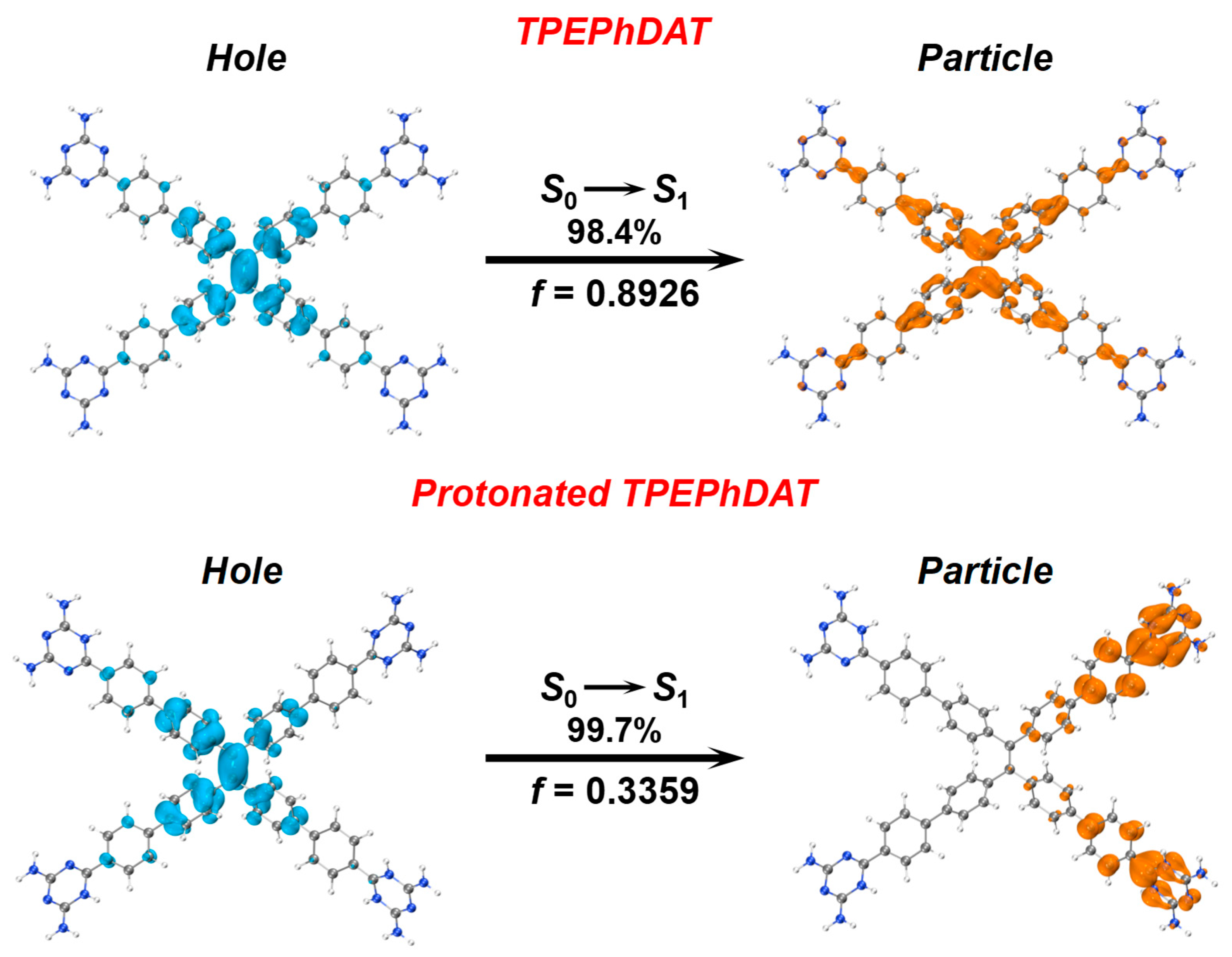
In addition, we have investigated the mechanism by which TFA quenches the fluorescence of
TPEPhDAT by time-dependent density functional theory (TDDFT) calculations. As shown in
Figure 5, on the natural transition orbitals (NTOs) of the bare
TPEPhDAT probe, most of the "hole" of
TPEPhDAT is localized on the TPE units of the backbone. At the same time, the "particle" of
TPEPhDAT is distributed throughout the molecular backbone. This result fits well with the weak ICT state of the
S0 → S1 transition in the
TPEPhDAT molecule. After protonation, it can be seen that the "hole" is mainly located on the TPE unit, which is similar to the
TPEPhDAT probe before protonation. In contrast, in the
S0 → S1 transition, the "particle" is mainly located on the triazine ring unit of the backbone. As a result, a large orbital separation between the hole and the particle is observed, and the oscillator strength (
f) correspondingly decreases from 0.8926 for the bare
TPEPhDAT probe to 0.3359 for the protonated
TPEPhDAT probe. The molecular energy is also reduced simultaneously after protonation (
Figure S13). These results indicate that the protonated
TPEPhDAT probe has stronger ICT properties[
17,
57]. This shows that the theoretical calculations agree with the sensing mechanism."
Generally, the fluorescence lifetime of the molecule increases with the enhancement of ICT[
58]. However, the fluorescence lifetime decreases after protonation, and ICT enhancement increases the molecule's dipole moment, which leads to a decrease in the energy difference between the excited and ground states. As a result, excited state electrons are more likely to return to the ground state via nonradiative pathways, thereby shortening the fluorescence lifetime. As shown in
Figure S14, after the
TPEPhDAT proton, the dihedral angle between the DAT group and the neighbouring phenyl group increased from −0.11° to 25.35°. The molecule became more distorted, and a similar twisted intramolecular charge transfer (TICT) effect may have occurred[
59,
60,
61,
62], which increased the fluorescence nonradiative leap pathway and led to a shorter fluorescence lifetime.
2.6. Erasable Anti-counterfeiting Applications
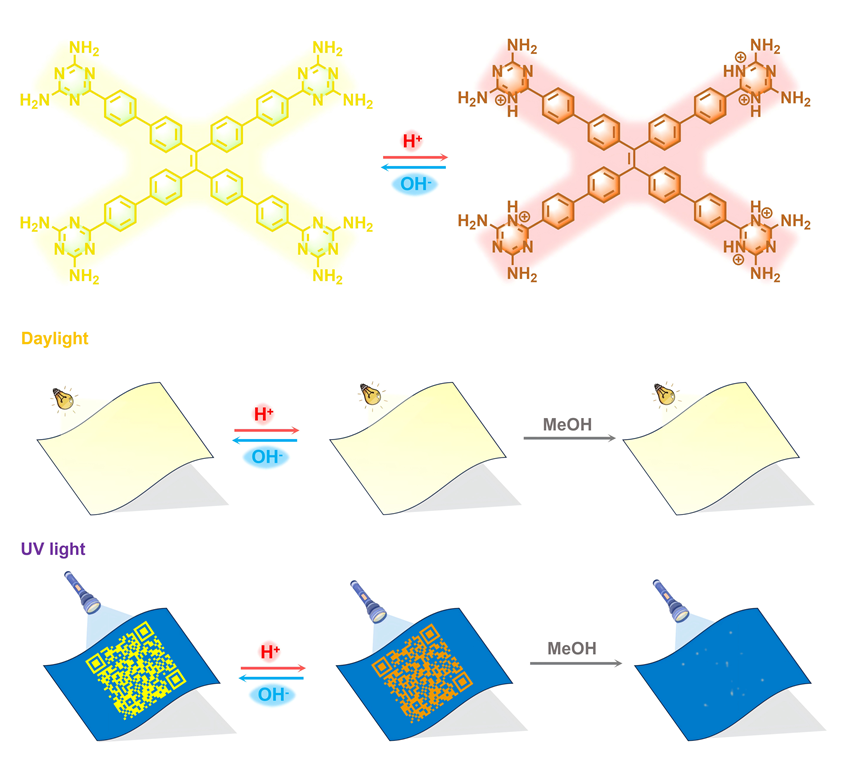
TPEPhDAT, as an AIE-type fluorescent molecule, skillfully avoids the limitations of ACQ molecules on solid substrates. Therefore, we designed an anti-counterfeiting paper using solution processing. In addition, we have taken advantage of the reversible conversion of fluorescence of TPEPhDAT in acidic and alkaline environments and the excellent solubility of acidified TPEPhDAT in MeOH for processing and erasure of encrypted information.
To confirm the utility of acid vapour fumigation for fluorescence discolouration under solid substrates. We obtained protonated
TPEPhDAT samples by direct fumigation with acid vapour in a closed environment (
Figure S15a)[
63]. The phenomenon was very similar to the change in water, with nearly 95.7% fluorescence quench and 38 nm redshift (
Figure S15b). UV-vis spectra confirmed the redshift in absorption (
Figure S15c).
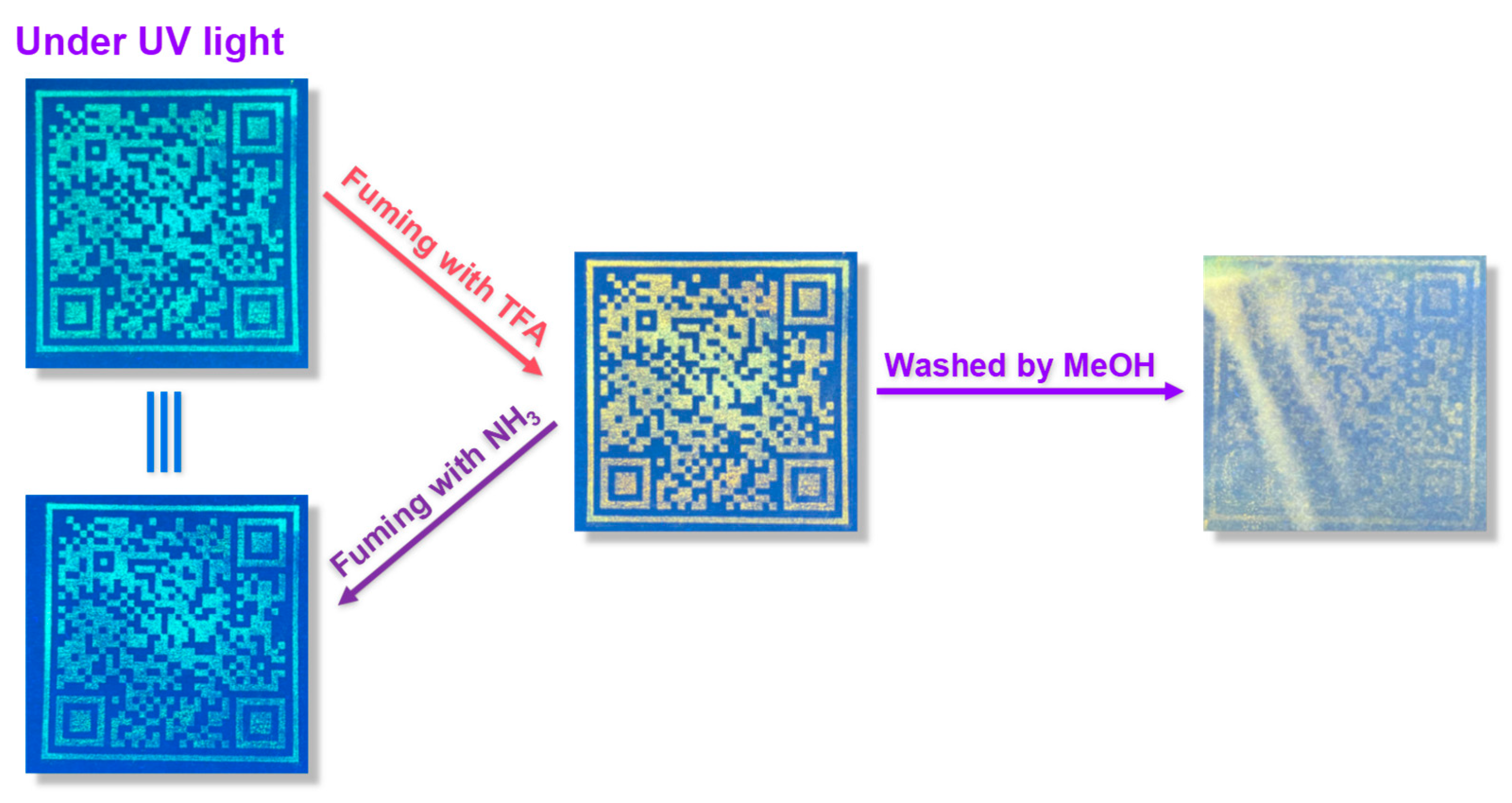
As shown in
Figure 6, a QR code written in security ink is stamped on the yellow printing paper. The yellow paper is similar to the colour of the
TPEPhDAT solid, which can hide the information well and realize that it is unrecognizable under daylight (
Figure S16), and only the information can be read under UV light. After fuming with TFA, the fluorescence is quenched under UV light, and the colour changes from green to orange. At the same time, after treatment with NH
3, the fluorescence can be restored entirely, realizing the reversible processing of encrypted information. Finally, it is again treated with an acidic solution. Currently, most of the acidified
TPEPhDAT can be removed by rinsing with MeOH, thus realizing the erasure of encrypted information under UV light.
3. Materials and Methods
3.1. Materials
1,1,2,2-tetrakis(4-bromophenyl)ethene, tetrakis(triphenylphosphine)palladium [Pd(PPh3)4], 4-cyanophenylboronic acid, and 2-methoxyethanol were supplied by Adamas (Shanghai, China). Sodium carbonate (Na2CO3), potassium carbonate (K2CO3), sodium sulfate (Na2SO4), sodium hydroxide (NaOH), dicyandiamide, sulfuric acid (H2SO4), dichloromethane (DCM), hydrochloric acid (HCl), methanol (MeOH), ethanol (EtOH), and silica gel were supplied by Greagent (Shanghai, China). Trifluoroacetic acid (TFA), ammonium hydroxide (NH3·H2O), nitric acid (HNO3), formic acid (FA), and tetrahydrofuran (THF) were supplied by Shanghai Hushi Chemical Co., Ltd. (Shanghai, China). Petroleum ether (PE) was supplied by Shanghai Titan Technology Co., Ltd. (Shanghai, China).
3.1.1. Synthesis of tetrakis[4-(4'-cyanophenyl)phenyl]ethene (TPEPhCN)
In a 500 mL round-bottomed flask, 1,1,2,2-tetrakis(4-bromophenyl)ethene (2.5 g, 3.86 mmol), Na2CO3 (1 g, 9.43 mmol), 4-cyanophenylboronic acid (3.13 g, 21.28 mmol), and Pd(PPh3)4 (127 mg, 0.11 mmol) were added to a mixed solvent system consisting of THF (100 mL) and distilled water (50 mL) in the mixture. The mixture was stirred at 100°C for 24 h under a nitrogen atmosphere. The mixture was cooled to room temperature. Quench the mixture with 10% K2CO3 solution (30 mL). Extract the mixture with DCM (50 mL × 3). The organic layer was collected, washed twice with saturated saline, and dried with anhydrous Na2SO4. The filtrate was filtered and concentrated under reduced pressure. The crude material was purified by silica gel column chromatography using PE and DCM as elution solvents (PE/DCM = 4:1). Removal of the solvent gave a pale yellow solid in 59% yield. 1H NMR (400 MHz, CDCl3) δ 7.67 (dd, J = 8.7 Hz, 16H), 7.42 (d, J = 8.4 Hz, 8H), 7.21 (d, J = 8.4 Hz, 8H).
3.1.2. Synthesis of 6,6',6'',6'''-(ethene-1,1,2,2-tetrayltetrakis([1,1'-biphenyl]-4',4-diyl))tetrakis(1,3,5-triazine-2,4-diamine) (TPEPhDAT)
In a 500 mL round-bottomed flask, tetrakis[4-(4'-cyanophenyl)phenyl]ethene (1 g, 1.36 mmol), NaOH (1 g, 25 mmol), and dicyandiamide (0.5 g, 5.95 mmol) were added to 2-methoxyethanol (40 mL) and stirred at 125°C for 36 h under a nitrogen atmosphere. The mixture was then cooled to room temperature and poured into MeOH. The precipitated solid was filtered, washed with DCM and boiling water, respectively, and dried under vacuum at 90 °C to give a yellowish solid in 80% yield. 1H NMR (400 MHz, DMSO-d6) δ 8.28 (d, J = 7.7 Hz, 8H), 7.75 (d, J = 7.9 Hz, 8H), 7.63 (d, J = 7.7 Hz, 8H), 7.20 (d, J = 7.6 Hz, 8H), 6.74 (s, 16H).
3.1.3. Synthesis of TPEPhDAT-TFA single crystalline
6 mg of TPEPhDAT was added to a 2 mL vial containing 200 µL of TFA, followed by 80 µL of EtOH, capped and placed in an oven at 70°C. After 12 h, the orange single crystals were collected for single-crystal X-ray diffraction analysis.
Details of the crystal data, data collection, structure solution, and refinement are shown in
Table S1. CCDC 2345516 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via
http://www.ccdc.cam.ac.uk/conts/retrieving.html
3.1.4. The Fabrication of Anti-Counterfeiting Inks
20 mg of TPEPhDAT was taken and ground thoroughly for 10 min. Then, 20 mL of EtOH was added and sonicated for 15 min to ensure the solids were well dispersed.
3.1.5. Density Functional Theory (DFT) Calculations
All the DFT calculations were carried out using the Gaussian 16 (version C.01) package. The ground-state geometries were optimized under the B3LYP/6-31G(d,p) level. The DFT-D3 dispersion correction with BJ-damping was applied to correct the weak interaction to improve the calculation accuracy. The IEFPCM implicit solvation model was used to account for the solvation effect. The absorption properties were obtained by time-dependent density functional theory (TDDFT) with the B3LYP function at the same basis set level. Orbital energy level analysis and Electron excitation analysis were performed using Multiwfn software.
3.2. Methods
Solution 1H and 13C NMR spectra were collected by Bruker AVANCE III 400 MHz spectrometers (Bruker Corporation, German). The 13C NMR spectra of TPEPhDAT was tested in DMSO-d6 with the addition of 10 equivalent TFA. X-ray single crystal diffraction was collected by BL17B1 High throughput Protein Crystallography Beamline in Shanghai Synchrotron Radiation Facility and Bruker D8 Venture MetalJet X-ray diffractometer equipped with a Photon II detector (Bruker Corporation, German). Fourier-Transform Infrared (FT-IR) spectra were collected by Thermo Fisher Nicolet iS10 (Thermo Fisher Corporation, America). PL spectra were recorded on a Hitachi F-7000 fluorescence spectrometer (Hitachi Corporation, Japan). Photoluminescence lifetime was obtained on FLS1000 (Edinburg Instruments, Livingstone, UK). Solid ultraviolet-visible (UV-vis) spectra were tested with a Lambda 650S UV-vis spectrophotometer (PERKIN ELMER, America). Liquid UV-vis spectra were tested by a UV-9000S UV-vis spectrophotometer (METASH, China). Dynamic light scattering (DLS) was examined on a Nano-ZS90 (Malvern Instruments, England). Scanning electron microscope (SEM) images were obtained on a LaB6 VEGA 3 XMU scanning electron microscope (TESCAN, Czech Republic) at an acceleration voltage of 200 V − 30 kV.
4. Conclusions
This paper reports a DAT group-modified TPE molecule, TPEPhDAT, prepared by Suzuki-Miyaura coupling and cyclization reactions. The DMSO/MeOH system exhibits excellent AIE properties, and the luminescence intensity of its solid-state aggregates is about five times higher than that of dilute solutions. Since the triazine ring is easily protonated, TPEPhDAT exhibits an apparent acid-induced fluorescence discolouration behaviour. The protonation occurred on the triazine ring rather than −NH2, as confirmed by various means, including 1H NMR, FT-IR, single crystals, etc. Reversible switching between yellow and orange-red fluorescence is achieved by using alternating treatment of TFA with ammonia. TDDFT calculations show that the electron-withdrawing capacity of the triazine unit is enhanced after TFA protonation, leading to a decrease in the band gap and a change in the distribution of the electron cloud, which is the fundamental cause of the wavelength redshift and fluorescence quenching. Based on their acid-induced discolouration behaviour and the property that MeOH can quickly shift them out after protonation, they are processed into paper with encrypted information for anti-counterfeiting by solution method. It can be seen that this property of reversible switching of fluorescence under alternating acid-base stimulation makes the molecule potentially applicable in the field of information processing and anti-counterfeiting.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Figure S1:
1H NMR spectra of
TPEPhCN; Figure S2:
1H NMR spectra of
TPEPhDAT; Figure S3:
13C NMR spectra of
TPEPhDAT; Figure S4: Localized enlarged
13C NMR spectra of
TPEPhDAT; Figure S5: UV-vis absorption spectra of
TPEPhDAT in solution (
fMeOH = 0 vol%) and aggregate (
fMeOH = 95 vol%); Figure S6: SEM pictures of aggregates at
fMeOH = 95 vol%; Figure S7: Emission spectra of
TPEPhDAT dispersed in water (2 mL) before and after the addition of aqueous solution of (
a) H
2SO
4 (200 µL, 0.25 M), (
b) HCl (200 µL, 0.5 M), (
c) FA (200 µL, 0.5 M), (
d) TFA (200 µL, 0.5 M) and (
e) HNO
3 (200 µL, 0.5 M); Figure S8: Absolute quantum yield of
TPEPhDAT before and after addition of various acids; Figure S9: The possible structure of the protonated triazine ring; Figure S10: Electrostatic potentials mapped on isodensity surfaces of
TPEPhDAT; Figure S11: Microscopic optical image for
TPEPhDAT-TFA; Figure S12: Schematic representation of
TPEPhDAT after protonation by TFA; Figure S13: Calculated absorption energy of bared and protonated
TPEPhDAT monomer; Figure S14: Dihedral angle between triazine ring and neighboring benzene ring before and after
TPEPhDAT protonation; Figure S15: (
a) Schematic diagram of
TPEPhDAT fumigated TFA. Fluorescence spectra (
b) and UV-vis absorption spectra (
c) of
TPEPhDAT before and after fumigation of TFA; Figure S16: Pictures of reversible switching of fluorescence of
TPEPhDAT in acidic and alkaline environments and its erasure by MeOH (under daylight); Table S1: Crystallographic data of
TPEPhDAT-TFA.
Author Contributions
Conceptualization, Z.L. and P.L.; methodology, J.L. and X.G.; software, J.L. and Q.N.; validation, J.L., X.G. and Q.N.; formal analysis, J.L., X.G., Q.N., M.J. and Y.W.; investigation, J.L. and X.G.; resources, Z.L., P.L., H.S. and B.C.; data curation, J.L., M.J. and Y.W.; writing—original draft preparation, J.L.; writing—review and editing, J.L., Z.L. and P.L.; visualization, Z.L. and P.L.; supervision, P.L. and Z.L.; project administration, P.L. and Z.L.; funding acquisition, T.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (HCPNU2024R1), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the staff at SSRF BL17B1 of the National Facility for Protein Science in Shanghai (NFPS), Shanghai Advanced Research Institute, CAS, for technical support in X-ray diffraction data collection and analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tong, S.; Dai, J.; Sun, J.; Liu, Y.; Ma, X.; Liu, Z.; Ma, T.; Tan, J.; Yao, Z.; Wang, S.; et al. Fluorescence-based monitoring of the pressure-induced aggregation microenvironment evolution for an AIEgen under multiple excitation channels. Nat. Commun. 2022, 13, 5234. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.X.; Yin, S.Y.; Wei, Z.W.; Qiu, Q.F.; Zhu, N.X.; Fan, Y.N.; Pan, M.; Su, C.Y. Pressure-Induced Multiphoton Excited Fluorochromic Metal-Organic Frameworks for Improving MPEF Properties. Angew. Chem. Int. Ed. 2019, 58, 14379–14385. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, H.; Kagawa, T.; Noborio, T.; Kanezashi, M.; Ogata, A.; Tsuru, T. Ultrafast Synthesis of Silica-Based Molecular Sieve Membranes in Dielectric Barrier Discharge at Low Temperature and Atmospheric Pressure. J. Am. Chem. Soc. 2020, 143, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Xu, W.; Wang, G.; Liu, K.; Wang, Z.; Shi, Q.; Lin, S.; Liu, Z.; Fang, Y. A Mild-Stimuli-Responsive Fluorescent Molecular System Enables Multilevel Anti-Counterfeiting and Highly Adaptable Temperature Monitoring. Adv. Funct. Mater. 2022, 32, 2207895. [Google Scholar] [CrossRef]
- Shen, F.; Yang, W.; Cui, J.; Hou, Y.; Bai, G. Small-Molecule Fluorogenic Probe for the Detection of Mitochondrial Temperature In Vivo. Anal. Chem. 2021, 93, 13417–13420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, L.; Han, Y.; Wang, Z.; Li, H.; Sun, S.; Xu, Y. A pH-sensitive ESIPT molecule with aggregation-induced emission and tunable solid-state fluorescence multicolor for anti-counterfeiting and food freshness detection. Chem. Eng. J. 2022, 428, 130986. [Google Scholar] [CrossRef]
- Hisaki, I.; Suzuki, Y.; Gomez, E.; Ji, Q.; Tohnai, N.; Nakamura, T.; Douhal, A. Acid Responsive Hydrogen-Bonded Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, X.; Wang, G.; Li, B.; Chen, H.; Li, H.; Zhao, Y. Photoresponsive supramolecular coordination polyelectrolyte as smart anticounterfeiting inks. Nat. Commun. 2021, 12, 1363. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Feng, Y.; Ye, X.; Li, Z.; Tao, S.; Jiang, D. Light-Gating Crystalline Porous Covalent Organic Frameworks. J. Am. Chem. Soc. 2024, 146, 10953–10962. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Wang, H.-P.; Zhang, L.-Y.; Wei, S.-C.; Wei, Z.-W.; Pan, M.; Su, C.-Y. Coordinative-to-covalent transformation, isomerization dynamics, and logic gate application of dithienylethene based photochromic cages. Chem. Sci. 2020, 11, 8885–8894. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, J.; He, X.; Tao, W.; Shi, Y.; Xu, J.; Xu, L.; Tang, M.; Shen, D.; Bi, H.; et al. Tuned Intra- and Intermolecular Photoreactions of Tridentate Cyanostilbenes with Distinct Aggregated-State Photomechanical and Dispersed-State Photochromic Behaviors. Adv. Funct. Mater. 2023, 33, 2212886. [Google Scholar] [CrossRef]
- Hu, Z.B.; Li, L.H.; Han, Y.; Zhang, J.; Li, J.; Chen, Z.; Wu, S.; Zhang, Y.; Ye, H.Y.; Song, Y. A new insight into the unique magneto-optical effect of layered perovskite (C6H5C2H3FNH3)2MnCl4. Aggregate 2022, 4, e294. [Google Scholar] [CrossRef]
- Wei, W.; He, L.; Han, G.; Lu, Y.; Shi, S.; Yuan, Z.; Wang, X.; Li, Y.; Chen, B.; Zhang, Z.; et al. Stimulus-responsive hydrogen-bonded organic frameworks: Construction strategies, research progress and applications. Coord. Chem. Rev. 2024, 507, 215760. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, S.; Tao, W.; Guo, J.; Xie, S.; Ding, Y.; Xu, G.; Chen, C.; Sun, X.; Zhang, Z.; et al. Multiple yet switchable hydrogen-bonded organic frameworks with white-light emission. Nat. Commun. 2022, 13, 1882. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; He, X.; Zheng, Z.; He, D.; Li, Q.; Gong, J.; Zhang, J.; Sung, H.H.Y.; Williams, I.D.; Lam, J.W.Y.; et al. Robust Supramolecular Nano-Tunnels Built from Molecular Bricks**. Angew. Chem. Int. Ed. 2021, 60, 7148–7154. [Google Scholar] [CrossRef]
- Zhang, T.L.; Zhao, C.; Liu, Z.J.; Xu, T.Y.; Lin, H.Y.; Zhou, S.W.; Li, Y.H.; Tong, F. Reversible Acid-Base Responsive Fluorescence Changes of Solutions and Crystals Based on Anthracenyl Pyridyl Derivatives. ChemPhotoChem 2023, 7, e202300194. [Google Scholar] [CrossRef]
- Zeng, C.Y.; Deng, W.J.; Zhao, K.Q.; Redshaw, C.; Donnio, B. Phenanthrothiophene-Triazine Star-Shaped Discotic Liquid Crystals: Synthesis, Self-Assembly, and Stimuli-Responsive Fluorescence Properties. Chem. - Eur. J. 2024, 30, e202400296. [Google Scholar] [CrossRef]
- Yang, J.; Chen, M.; Li, P.; Cheng, F.; Xu, Y.; Li, Z.; Wang, Y.; Li, H. Self-healing hydrogel containing Eu-polyoxometalate as acid-base vapor modulated luminescent switch. Sens. Actuators, B 2018, 273, 153–158. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.; Guo, R.; Yao, D.; Guo, W.; Liu, S.; Li, Z.; Wang, Y.; Li, H. Self-Healing Material with Reversible Luminescence Switch Behavior. ACS Appl. Mater. Interfaces 2020, 12, 54026–54034. [Google Scholar] [CrossRef] [PubMed]
- Kalita, A.; Malik, A.H.; Sarma, N.S. Stimuli-Responsive Naphthalene Diimide as Invisible Ink: A Rewritable Fluorescent Platform for Anti-Counterfeiting. Chem. - Asian J. 2020, 15, 1074–1080. [Google Scholar] [CrossRef]
- Wu, J.; Jiao, X.; Chen, D.; Li, C. Dual-stimuli responsive color-changing nanofibrous membranes as effective media for anti-counterfeiting and erasable writing. Colloids Surf., A 2021, 621, 126626. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Huang, L.; Song, R.; Yang, J.; Zhang, H. A novel photochromic complex based on a viologen ligand with rapid inkless and erasable printing, anti-counterfeiting and amine-selective responses. Inorg. Chem. Commun. 2023, 153, 110839. [Google Scholar] [CrossRef]
- Yu, C.-M.; Wang, P.-H.; Liu, Q.; Cai, L.-Z.; Guo, G.-C. Modulating Fading Time of Photochromic Compounds by Molecular Design for Erasable Inkless Printing and Anti-counterfeiting. Cryst. Growth Des. 2021, 21, 1323–1328. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Leung, N.L.C.; Tang, B.Z. AIE macromolecules: syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; He, Y.; Arman, H.D.; Krishna, R.; Wang, H.; Weng, L.; Chen, B. A microporous six-fold interpenetrated hydrogen-bonded organic framework for highly selective separation of C2H4/C2H6. Chem. Commun. 2014, 50, 13081–13084. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, B.; Wu, H.; Hu, T.-L.; Yao, Z.; Zhou, W.; Xiang, S.; Chen, B. A Flexible Microporous Hydrogen-Bonded Organic Framework for Gas Sorption and Separation. J. Am. Chem. Soc. 2015, 137, 9963–9970. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-L.; Li, Z.; Wu, J.-R.; Lu, T.; Yang, Y.-W. Intramolecular Through-Space Interactions Induced Emission of Pillar[4]arene[1]dicyanobenzene. Chem. Mater. 2022, 34, 10181–10189. [Google Scholar] [CrossRef]
- Yu, Y.; Xing, H.; Zhou, Z.; Liu, J.; Sung, H.H.Y.; Williams, I.D.; Halpert, J.E.; Zhao, Z.; Tang, B.Z. How do molecular interactions affect fluorescence behavior of AIEgens in solution and aggregate states? Sci. China Chem. 2021, 65, 135–144. [Google Scholar] [CrossRef]
- Lee, M.M.S.; Yu, E.Y.; Yan, D.; Chau, J.H.C.; Wu, Q.; Lam, J.W.Y.; Ding, D.; Kwok, R.T.K.; Wang, D.; Tang, B.Z. The Role of Structural Hydrophobicity on Cationic Amphiphilic Aggregation-Induced Emission Photosensitizer-Bacterial Interaction and Photodynamic Efficiency. ACS Nano 2023, 17, 17004–17020. [Google Scholar] [CrossRef]
- Tu, Y.; Yu, Y.; Xiao, D.; Liu, J.; Zhao, Z.; Liu, Z.; Lam, J.W.Y.; Tang, B.Z. An Intelligent AIEgen with Nonmonotonic Multiresponses to Multistimuli. Adv. Sci. 2020, 7, 2001845. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, Y.; Shen, H.; Lam, J.W.Y.; Sun, J.; Zhang, H.; Tang, B.Z. Regulating the proximity effect of heterocycle-containing AIEgens. Nat. Commun. 2023, 14, 3772. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Bian, G.; Chen, Y.; Ouyang, M.; Hu, B.; Zhang, C. Cyanostilben-based derivatives: mechanical stimuli-responsive luminophors with aggregation-induced emission enhancement. Photochem. Photobiol. Sci. 2012, 11, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, S.; Zheng, M.; Deng, Q.; Zheng, C.; Li, J.; Huang, F. A novel fluorometric turn-on assay for lipase activity based on an aggregation-induced emission (AIE) luminogen. Sens. Actuators, B 2017, 238, 765–771. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Turley, A.T.; Wang, L.; McGonigal, P.R.; Tu, Y.; Li, Y.; Wang, Z.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Aggregate Science: From Structures to Properties. Adv. Mater. 2020, 32, 2001457. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Lu, L.; Feng, Y.; Zheng, J.-F.; Deng, L.; Chen, E.-Q.; Ren, X.-K. Synthesis, Aggregation-Induced Emission, and Liquid Crystalline Structure of Tetraphenylethylene–Surfactant Complex via Ionic Self-Assembly. J. Phys. Chem. C 2016, 120, 27577–27586. [Google Scholar] [CrossRef]
- Tang, B.Z.; Geng, Y.; Lam, J.W.Y.; Li, B.; Jing, X.; Wang, X.; Wang, F.; Pakhomov, A.B.; Zhang, X.X. Processible Nanostructured Materials with Electrical Conductivity and Magnetic Susceptibility: Preparation and Properties of Maghemite/Polyaniline Nanocomposite Films. Chem. Mater. 1999, 11, 1581–1589. [Google Scholar] [CrossRef]
- Gao, M.; Hong, Y.; Chen, B.; Wang, Y.; Zhou, W.; Wong, W.W.H.; Zhou, J.; Smith, T.A.; Zhao, Z. AIE conjugated polyelectrolytes based on tetraphenylethene for efficient fluorescence imaging and lifetime imaging of living cells. Polym. Chem. 2017, 8, 3862–3866. [Google Scholar] [CrossRef]
- Kuimova, M.K.; Yahioglu, G.; Levitt, J.A.; Suhling, K. Molecular Rotor Measures Viscosity of Live Cells via Fluorescence Lifetime Imaging. J. Am. Chem. Soc. 2008, 130, 6672–6673. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, T.; Chen, X.; Chen, Q.; Hao, J.; Song, W.; Zeng, Y.; Xue, P. Guest-Regulated Luminescence and Force-Stimuli Response of a Hydrogen-Bonded Organic Framework. ACS Appl. Mater. Interfaces 2021, 13, 32270–32277. [Google Scholar] [CrossRef]
- Zadehnazari, A.; Khosropour, A.; Altaf, A.A.; Rosen, A.S.; Abbaspourrad, A. Tetrazine-Linked Covalent Organic Frameworks With Acid Sensing and Photocatalytic Activity. Adv. Mater. 2024, 36, 2311042. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.D.; Miles, H.T.; Bradley, R.B. Nuclear Magnetic Resonance Studies of Methyl Derivatives of Cytosine. J. Am. Chem. Soc. 2002, 87, 5575–5582. [Google Scholar] [CrossRef] [PubMed]
- Miles, H.T.; Bradley, R.B.; Becker, E.D. Tautomerism and Site of Protonation of 1-Methylcytosine: Proof by Nuclear Magnetic Resonance Spin-Spin Coupling. Science 1963, 142, 1569–1571. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, G.; Wang, Z.; Zhang, X. Generation of 2D organic microsheets from protonated melamine derivatives: suppression of the self assembly of a particular dimension by introduction of alkyl chains. Chem. Sci. 2012, 3, 3227–3230. [Google Scholar] [CrossRef]
- Perpétuo, G.J.; Janczak, J. Solid-state supramolecular architectures formed by co-crystallization of melamine and 2-, 3- and 4-fluorophenylacetic acids. J. Mol. Struct. 2018, 1152, 237–247. [Google Scholar] [CrossRef]
- Marchewka, M.K.; Pietraszko, A.; Feki, H.; Abid, Y. Crystal structure and vibrational spectra of melaminium 2,5-dinitrophenolate monohydrate: FT-IR, FT-Raman and quantum chemical calculations. Vib. Spectrosc. 2011, 56, 255–264. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Wang, C.; Gao, X.; Zhou, Y.; Chen, B.; Li, P. Self-Healing Hydrogen-Bonded Organic Frameworks for Low-Concentration Ammonia Capture. J. Am. Chem. Soc. 2023, 146, 627–634. [Google Scholar] [CrossRef]
- Gündüz, T.; Gündüz, N.; Kılıç, E.; Kenar, A. Titrations in non-aqueous media. Part III. Basicity order of aniline, N-Alkyl- and N-aryl-substituted anilines and pyridine in nitrobenzene solvent. The Analyst 1986, 111, 1103–1105. [Google Scholar] [CrossRef]
- Dzidic, I. Relative gas-phase basicities of some amines, anilines, and pyridines. Application of some Broensted acids as reactants in chemical ionization mass spectrometry. J. Am. Chem. Soc. 2002, 94, 8333–8335. [Google Scholar] [CrossRef]
- Mucci, A.; Domain, R.; Benoit, R.L. Solvent effect on the protonation of some alkylamines. Can. J. Chem. 1980, 58, 953–958. [Google Scholar] [CrossRef]
- Benoit, R.L.; Mackinnon, M.J.; Bergeron, L. Basicity of N-substituted anilines and pyridine in dimethylsulfoxide. Can. J. Chem. 1981, 59, 1501–1504. [Google Scholar] [CrossRef]
- Lv, Y.; Liang, J.; Xiong, Z.; Yang, X.; Li, Y.; Zhang, H.; Xiang, S.; Chen, B.; Zhang, Z. Smart-Responsive HOF Heterostructures with Multiple Spatial-Resolved Emission Modes toward Photonic Security Platform. Adv. Mater. 2023, 36, 2309130. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Abdurahman, A.; Liu, G.; Liu, H.; Zhang, Y.; Zhang, M. An instantaneously-responded, ultrasensitive, reutilizable fluorescent probe to sarin substitute both in solution and in gas phase. Sens. Actuators B 2020, 322, 128611. [Google Scholar] [CrossRef]
- Zheng, P.; Abdurahman, A.; Zhang, Z.; Feng, Y.; Zhang, Y.; Ai, X.; Li, F.; Zhang, M. A simple organic multi-analyte fluorescent prober: One molecule realizes the detection to DNT, TATP and Sarin substitute gas. J. Hazard. Mater. 2021, 409, 124500. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, B. Configuration-regulated highly luminescent hydrogen-organic frameworks for detection of phenelzine and propofol. Inorg. Chem. Front. 2024, 11, 1099–1107. [Google Scholar] [CrossRef]
- Gu, K.; Meng, Z.; Liu, X.; Wu, Y.; Qi, X.; Ren, Y.; Yu, Z.-Q.; Tang, B.Z. A gated strategy stabilizes room-temperature phosphorescence. Aggregate 2023, 4, e337. [Google Scholar] [CrossRef]
- Shen, H.; Li, Y.; Li, Y. Self-assembly and tunable optical properties of intramolecular charge transfer molecules. Aggregate 2020, 1, 57–68. [Google Scholar] [CrossRef]
- Hanaoka, K.; Iwaki, S.; Yagi, K.; Myochin, T.; Ikeno, T.; Ohno, H.; Sasaki, E.; Komatsu, T.; Ueno, T.; Uchigashima, M.; et al. General Design Strategy to Precisely Control the Emission of Fluorophores via a Twisted Intramolecular Charge Transfer (TICT) Process. J. Am. Chem. Soc. 2022, 144, 19778–19790. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.m.; Yuk, S.b.; Namgoong, J.w.; Kim, J.p. Mechanofluorochromism of Triphenylamine-BODIPY: Effect of twisted intramolecular charge transfer and restriction in rotation on fluorescence. Dyes Pigm. 2021, 185, 108864. [Google Scholar] [CrossRef]
- Sasaki, S.; Drummen, G.P.C.; Konishi, G.-i. Recent advances in twisted intramolecular charge transfer (TICT) fluorescence and related phenomena in materials chemistry. J. Mater. Chem. C 2016, 4, 2731–2743. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, J.; Miao, R.; Song, H.; Ma, Y.; Zhou, M.; Fang, Y. Integrating Aggregation Induced Emission and Twisted Intramolecular Charge Transfer via Molecular Engineering. Adv. Funct. Mater. 2023, 34, 2311404. [Google Scholar] [CrossRef]
- Xia, G.; Jiang, Z.; Shen, S.; Liang, K.; Shao, Q.; Cong, Z.; Wang, H. Reversible Specific Vapoluminescence Behavior in Pure Organic Crystals through Hydrogen-Bonding Docking Strategy. Adv. Opt. Mater. 2019, 7, 1801549. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).