Preprint
Review
Antibody Drug Conjugates in the Treatment of Urothelial Cancer: The Dawn of a New Era
This version is not peer-reviewed.
Submitted:
31 July 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
This review critically evaluates the role of Antibody-drug conjugates (ADCs) in the treatment of urothelial cancer, exploring their therapeutic potential and addressing the scientific and clinical challenges encountered in the development of these agents. It also discusses the recent approvals and clinical trials that are shaping the future of ADC therapy in this field. Through a detailed examination of the mechanism of action of ADCs, including the bystander effect, this paper aims to highlight both the successes and the limitations of these drugs in clinical settings. The main objective of this work is to provide a thorough overview of the current state of ADCs in urothelial cancer treatment, elucidating how these drugs can offer improved outcomes for patients. By discussing the broader implications of ADC technology and its integration into existing treatment paradigms, this review outlines the next steps in research and application necessary to overcome the existing challenges and harness the full potential of ADCs in cancer therapy.
Keywords:
antibody-drug conjugates
; urothelial cancer
; enfortumab vedotin
; bystander effect
1. Introduction
Urothelial cancer is the ninth most common cancer worldwide [1]. It is characterized by high recurrence and progression rates; thus, effective treatments are urgently needed. Its prognosis depends on the initial stage presentation. Most cases are diagnosed with non-muscle invasive disease (NMIBC), where local treatments are the most common form of therapy, usually a combination of intravesical instillations and maximum transurethral excision. For the rest of the patients with muscle-invasive disease, some form of systemic treatment will be administered in the course of their disease. Chemotherapy is the most widely used form of systemic treatment, and it can be administered as neoadjuvant or adjuvant treatment in the early stages, as a part of the bladder preservation approach, and in the advanced-metastatic setting. The agent associated with the most significant clinical benefit is cisplatin (usually as part of multidrug regimens). Agents such as carboplatin, taxanes, and vinflunine represent clinically meaningful alternatives for patients not fit or already exposed to cisplatin, especially in the later lines of therapy[2,3,4].
Immunotherapy -in the form of anti-PD-1/L1 monoclonal antibodies- has also been part of the therapeutic algorithm for urothelial cancer. Initially, it was used in the platinum refractory / cisplatin-ineligible setting with mixed results [5,6,7]. In the first-line setting, three of the four trials that tested the concurrent combination of immunotherapy with chemotherapy were negative. Until very recently, the current standard was the administration of 1st line chemotherapy with a platinum agent (cisplatin either or carboplatin), and for those patients who had not progressed, immunotherapy (agent: avelumab) is then administered as maintenance treatment (switched maintenance) following the scheme of Javelin Bladder 100 trial. Alternatively, for the cisplatin-eligible patients, there is also the choice of combining immunotherapy (agent: nivolumab) upfront with chemotherapy, following the scheme of the Checkmate 901 trial, the only positive trial of this combination so far [8,9]. Immunotherapy has also successfully increased the overall survival of patients with high-risk early disease when administered as an adjuvant treatment after cystectomy [10,11]. For the minority of patients harboring certain DNA alterations in their tumors, such as fibroblast growth factor receptor (FGFR) gene alterations, treatment with erdafitinib, a tyrosine kinase inhibitor, is also a valid option [12].
Despite these promising advances that significantly increased the therapeutic choices and offered a meaningful benefit to some patients, there are still unmet needs in the treatment of this highly lethal disease since its treatment in advanced stages has seen limited progress over the last few decades. We can expect a new class of drugs that can benefit patients irrespective of cisplatin eligibility, platinum resistance, or absence of PD-L1 positivity in the future, and this is precisely the case we will examine for antibody-drug conjugates.
2. Mechanisms of Action, Toxicity and Resistance
2.1. Mechanisms of Action
ADCs, or antibody-drug conjugates, are carriers that mimic the Trojan horse method in their complex mode of operation. Utilizing monoclonal antibodies, they can deliver high doses of cytotoxic payloads directly to tumor cells or the structural elements of the tumor microenvironment (TME). This allows them to exert their anti-tumor activity and cause cell death while having little to no effect on normal cells and selectively driving cancer cells to apoptosis. Three primary components define the ADCs architecture: the monoclonal antibody (mAb) that is rapidly released into the targeted tumor cells and ultra-selectively targets tumor antigens thus enchasing specificity; the cytotoxic payload engineered to break DNA or tubulin mechanism within the targeted cell, resulting in high efficacy, and finally the molecular binder that bridges the two, forming the antibody-drug conjugate. Immunoglobulin G is the hardware used the most for the antibody production of ADCs, taking advantage of their potential cross-reactivity, binding affinity, and immunogenicity. After exposure to ADCs, activation of immune responses is often observed, resulting in the generation of antibodies that ultimately attach to the ADC, undermining its role and effectiveness. Following this consideration, it is critical to point out the drug-to-antibody proportion as a factor that needs to be constantly assessed. Namely, a high ratio leads to the formation of aggregates and rapid destruction of the antibody-drug conjugate (ADC), while a low ratio leads to reduced effectiveness. Ultimately, the drug-antibody linkage should be adjusted to be stable enough while circulating in the bloodstream. A premature release of the cytotoxic payload in the serum can result in excessive toxicity and inevitably reduced effectiveness at the tumor site. Additionally, the linker must be capable of being cleaved after the ADC has been internalized into the target cell [13]. Upon target cells are at a proximal distance, the ADC (antibody-drug conjugate) identifies the antigens, attaches to the cell surface, and, through endocytosis, follows the internalization of the ADC-antigen complex into the cancer cell. The transitional path of the newly formed complex starts as an early endosome, followed by maturation into late endosomes, and ultimately fusing with lysosomes. As the procedure continues, either an enzymatic digestion in the lysosomes or a chemical process disengages the cytotoxic active payload from the monoclonal antibody (mAb), exposing targeted cells to detrimental effects by triggering cellular apoptosis or premature death [14]. In addition, the Fc region of the monoclonal antibody enhances immune-related cytotoxicity, including antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent phagocytosis (ADP) and complement-dependent cytotoxicity (CDC) [15]. Significant progress in Genetic engineering techniques, leading to improved antibody effectiveness in the Fc region [16]. The use of ADCs offers the opportunity to expose malignant cells to high doses of toxic anti-neoplastic agents. Even higher compared to what would be possible with a standard, non-antibody-directed infusion. At the same time, minimal toxicity is present to healthy cells surrounding the tumor microenvironment, given the lack of respective key antigens. An additional characteristic of ADC activity is the payload cell membrane permeability. An ability that could facilitate the apoptosis of bystander cells following the payload spillage from a targeted tumor cell. This is described as the Bystander effect. The capability, as mentioned above, may be affected by the drug (payload) to antibody ratio (DAR), which determines drug potency and serum clearance. The increased toxicity of bystander-killing payloads allows them to overcome tumor heterogeneity, enhancing the clinical potential of antibody-drug conjugates (ADC). Modern ADCs have been engineered to have a bystander-killing effect and a higher anti-neoplastic efficacy through linker optimization and conjugation [17]. This new drug category in comparison with standard chemotherapy, offers several advantages. This includes increased drug tolerability, significant cytotoxicity even at low drug concentrations, stability in the bloodstream and lysosomes, and reduced systemic toxicity—important characteristics that lead to broader therapeutic implementation [18]. Figure 1 summarizes the mode of ADC action
2.2. Toxicity
A fundamental part of the ADCs design is the ability to minimize the exposure of the cytotoxic compound to healthy tissues, resulting in a narrow band of rather manageable toxicities. Among the most frequent are vomiting, diarrhea, nausea, and fatigue. Apart from these, severe toxicities of G3 or G4 have been reported, such as Steven Johnson, peripheral neuropathy, and myelosuppression. There is an association with peripheral neuropathy for the ADCs that use cleavable linkers bound to tubulin inhibitors. Clinical manifestations may include tingling, numbness, or pain in the extremities. All cleavable linkers block tubulin polymerization and have a strong association with early payload release, resulting in a rather expected toxicity profile. Given that microtubules significantly participate in the axonal transport as an important partner to the maturation and growth of neurons. As a result of myelosuppression, anemia, neutropenia, and thrombocytopenia of any grade may occur. Neutropenia is predominant, mostly due to the off-target Fc receptor-mediated uptake of ADCs into immune cells. Other reported toxicities that could lead to dose adjustments mainly include hepatotoxicity (for payload MMAF, DM1, and calicheamicin), skin toxicity (for payload MMAE and PBD), and ocular toxicity (for payload MMAF and DM4). Upon administration, Sacituzumab govitecan and Trastuzumab deruxtecan may present gastrointestinal side effects. In addition, Trastuzumab deruxtecan has an established association with an increased risk of interstitial lung disease (ILD), in most cases, requires dose adjustment in order to even fatal outcomes. As these novel therapeutic options extend their implementations, it is of the utmost importance for clinicians to be able to identify potential life-threatening toxicities and deep pharmacogenetic analysis to identify SNPs or potential mutations in key genes. Aiming to improve the tolerability and efficacy of ADCs [18].
2.3. Resistance
Inevitably, drug resistance occurs under treatment pressure. Malignant cells have a well-known ability to develop resistance mechanisms to avoid drug control. The exact mechanisms contributing to acquired ADC resistance are mostly unknown, but it is hypothesized that they may be related to individual parts of ADCs. The mechanisms related to this ability could be categorized into antigen down-regulation end or high-regulation, the drug efflux pumps, lysosomal function abnormality, and irregularity of signaling pathways controlling the cell cycle. Antigen-related resistance has a profound impact on the efficacy of ADCs. In this context, an association was spotted between treatment with brentuximab vedotin and antigen levels affecting the ADCs efficacy, whose multiple treatment cycles correlated with CD30 downregulation and, consequently, increased tumor cell resistance to MMAE. This example demonstrates how the presence of appropriate antigen ligands could potentially challenge the hypothesis of modulating the sensitivity of ADCs. Current research emphasizes that ligands, like neuregulin, which facilitates the heterodimerization of HER2 with HER3 and HER4, may reduce the efficacy of TDM-1. According to the following resistance-induced theory, ABC transporters are a family of transmembrane proteins that facilitate drug efflux across cell membranes. This is significant for ADCs, too, because these ABC transporters are substrates for many of the cytotoxic agents used in ADCs. Their role is to pump out various functional components from the tumor cellular cytoplasm, including therapeutic pharmaceutical payloads. That evacuation procedure increases resistance to potentially curative treatment. Given this mechanism, it is observed that cyclic dosing of TDM-1 induces an increase in ABCC1 transporter levels, resulting in acquired drug resistance. In addition to the above, impaired lysosomal function notably reduces the ADCs' therapeutic capability. This escape mechanism is involved in the acidification of lysosomal PH, especially those with non-cleavable and lysosomal protease-sensitive cleavable linkers. When linkers are cleaved by lysosomes, an acid hydrolase begins to take place, releasing a cytotoxic payload into the cytoplasm of targeted tumor cells. Malignant cells often acquire the ability to emit this process upon constant therapeutic exposure, altering the pH of the lysosomal compartment. This clever technique aims to slow down the proteases' catabolic procedure. Nevertheless, perturbations in signaling pathways involved in cell cycle regulation and alterations in apoptotic regulation may also modulate tumor cell sensitivity to ADC. In addition to the mechanisms mentioned above for ADCs, endocytic uptake is crucial; it can occur through various pathways such as clathrin-caveolin-independent endocytosis, clathrin-mediated (CME), and caveolin-mediated. Caveolae-mediated endocytosis responds to trastuzumab etamine (TDM-1) as a recently described resistance mechanism. It is of great importance to understand drug resistance mechanisms and implement effective strategies to overcome them. Regarding antigen down-regulation or deletion, novel ultra-specific combinations with other targeted cytotoxic molecules could be an appropriate response. Bi-payloads and immune-stimulating payloads may contribute to increased ADC effectiveness. New bispecific antibodies bearing characteristics of increasing tumor cell affinity, enhanced internalization efficiency, and notable resistance management could be future options. For cases exhibiting endocytosis deficiency, developing ADCs that do not internalize but instead directly release their payload into the tumor cell for absorption could also be a promising approach [18].
3. ADCs and Urothelial Cancer
3.1. Nectin 4
Nectins are calcium-independent immunoglobulin-like transmembrane molecules belonging to a family of four members that mediate cell-cell adhesion functions. Nectin 4 is highly expressed in embryonal and placentas tissues. Nectin 4 is also overexpressed in various human cancer types such as gastric, breast, ovarian, lung, pancreatic, and bladder. Nectin 4 is a significant contributor to cell growth and highly malignant tumor phenotype since its expression is implicated in cell migration and invasion. Various studies in cases of urothelial cancer indicate a level of expression around 80% for bladder cancer and 65,7% for upper urinary tract cancer, with strong expression recognized as an adverse prognostic factor [20,21,22].
3.1.1. Enforumab Vedotin
Enfortumab Vedotin (EV) is an ADC targeting Nectin 4; it is the most widely used ADC in urothelial cancer and the only one- so far- with regulatory approval in both the EU and the USA. EV contains a fully human IgG1 monoclonal antibody (AGS-22M6), which binds with high affinity against Nectin 4 and is conjugated with a cleavable linker to its payload monomethyl auristatin E (MMAE), a microtubule-disrupting toxin. The resulting DAR is approximately 4. Targeted delivery of MMAE results in cell-cycle arrest and apoptosis [22,23,24].
The clinical development of EV began in 2011 with its phase I trial (NCT01409135). This trial, which enrolled 42 patients with metastatic urothelial cancer (mUC), provided evidence about EV's safety and antitumor activity, paving the way for its further clinical use. Subsequent phase I dose escalation and expansion study EV101 (NCT02091999), established the dosing scheme of EV. EV has a half-life of approximately two days and is administered on days 1,8 and 15 of every 28-day cycle at a dose of 1.25mg/m2 [24,25].
EV-201 was a two-cohort, single-arm phase II trial and is considered the pivotal trial of EV in mUC after platinum and anti-PD1/PDL1 therapy. Cohort I enrolled 128 patients previously treated with platinum and anti-PD-1/L1 therapy. Patients were heavily pretreated, with a median of three systemic therapies (range: 1-6). The objective response rate was 44% (95% CI,35.1% to 53.2%), the median time to response was 1.84 months, the median duration of response was 7.6 months. 12% of the patients achieved complete response, and for them, the duration of response ranged from 3.6 to 11.3 months. At the time of analysis, 44% of all responders had ongoing responses. Responses were observed irrespective the site of metastasis, the presence of poor prognostic characteristics and regardless the of patient's response to prior anti-PD-1/L1 therapy. Cohort II enrolled 91 cisplatin-ineligible patients who were previously treated with only anti-PD-1/L1 therapy. The objective response rate was 52% (95% CI 41–62), median duration of response (DOR) was 10.9 months. The median progression-free survival (PFS) was 5.8 months for both cohorts, and the median overall survival (OS) was 11.7 months for cohort I and 14.7 months for cohort II [26,27].
As it concerned safety, EV-201 trial revealed a distinct pattern of toxicity for EV, with peripheral neuropathy, rash, hyperglycemia, and infusion-related reactions prespecified as composite terms. Peripheral neuropathy occurred in 50% of patients, almost all (94%) of which were grade 2 or less, with peripheral sensory more common than motor neuropathy. Treatment-related rash occurred in 48% of patients, most of which were low grade, with onset in the 1st cycle. Only 2 patients discontinued treatment because of rash (one case was reported as Stevens-Johnson syndrome). Hyperglycemia occurred in 11% of patients, with only one grade IV case discontinuing treatment [26,27].
After the promising results of those earlier phase trials, the randomized phase 3 EV-301 trial evaluated EV as compared with chemotherapy, in mUC patients who had previously received treatment with a platinum compound and an anti-PD-1/L1 agent. The trial enrolled 608 patients in a 1:1 ratio to receive EV or chemotherapy (docetaxel, paclitaxel or vinflunine). The primary endpoint was OS, and after 11.1 months of median follow-up, treatment with EV resulted in 30% lower risk of death (hazard ratio, 0.70; 95% confidence interval [CI], 0.56 to 0.89; p= 0.001), indicating significantly longer OS with EV. Treatment with EV resulted in significantly longer PFS with 38% lower risk of progression or death (95% CI, 0.51 to 0.75; p<0.001). Median OS and PFS were 12.88 and 5.55 months respectively for EV vs 8.97 and 3.71 for the chemotherapy group. Among patients who responded to treatment, the EV group confirmed more than double the responses from the chemotherapy group (40.6% vs 17.9%). There were no new or unexpected toxicity events, and the already described toxicity of EV was regarded as mild to moderate and overall manageable [28]. Based on the OS data of this confirmatory trial, the U.S. FDA granted EV regular approval on July 9, 2021. In May 2022 EV was authorized by the EMA for use across E.U. [29,30].
There is accumulated evidence that the major drugs with clinical activity in mUC ADCs and anti-PD-1/L1 can act synergistically. The biological rationale behind this hypothesis is quite strong. ADCs have the potential to induce immunogenic cell death, and thus the release of damage-associated molecular patterns (DAMPs). DAMPs are easily recognized by elements of innate and adaptive immunity and via cross-presentation by cytotoxic T cells. These T cells are also a prime target for immune-modulating agents such as anti-PD-1/L1, increasing their antigen (tumor)-specific cytotoxicity. Thus, by combining EV with an anti-PD-1 like pembrolizumab the antitumor activity is enhanced versus either agent alone, since their respective immune properties act complementary. Based on these theories many clinical trials explored the possibility of combining these agents.
EV-103 (NCT03288545) was the first trial to assess the combination of EV and pembrolizumab in previously untreated advanced urothelial cancer (aUC) patients. EV-103 is a three-cohort (dose escalation, cohorts A and K), phase Ib/II study evaluating the safety, tolerability, and antitumor activity of first-line EV in combination with pembrolizumab. Cohort A enrolled 45 cisplatin-ineligible aUC/mUC patients. Confirmed ORR after a median of 9 cycles was 73.3% with a CR rate of 15.6%. The median DOR and OS were 25.6 and 26.1, respectively. No new safety signals were reported, and the safety profile was manageable. Since this combination appears to offer higher antitumor activity than that of carboplatin-based chemotherapy it received Breakthrough Therapy designation by the U.S FDA in February 2020. Cohort K randomized 149 patients to receive EV plus pembrolizumab or EV monotherapy in a 1:1 ratio. The combination arm exhibited a confirmed ORR of 64.5%, with rapid (median time to response was two months) and durable responses, with 65.4% of responders maintaining a response at 12 months [31,32].
EV-302 is a phase III, randomized trial comparing the efficacy of EV plus pembrolizumab combination with that of platinum-based chemotherapy in the first-line setting. The trial enrolled 886 patients in a 1:1 ratio to receive EV and pembrolizumab or platinum-based chemotherapy, cisplatin, or carboplatin -depending on their status as cisplatin eligible- with gemcitabine. Treatment was to be continued until occurrence of disease progression, unacceptable toxicity or completion of the maximum cycles, (six for chemotherapy, 35 for pembrolizumab); maintenance therapy was permitted according to the local standard. The primary endpoints were PFS and OS [33].
EV-302 is a positive trial in both its co-primary endpoints. Patients treated with EV and pembrolizumab had 55% lower risk of disease progression or death (HR, 0.45; 95% CI, 0.38 to 0.54; p<0.001) and 53% lower risk of death (HR, 0.47; 95% CI, 0.38 to 0.58; p<0.001) compared with the patients treated with chemotherapy. The median duration of PFS was 12.5 months for the EV-pembrolizumab group vs 6.3 months for the chemotherapy group, and the median was OS 31.5 months and 16.1 months, respectively. PFS and OS was consistent in all prespecified patient groups regardless of cisplatin eligibility status or PDL-1 positivity. At the 12-month time point, 78.2% of patients in the EV-pembrolizumab group were alive vs 61.4% in the chemotherapy group. ORR was higher in the EV-pembrolizumab group with 67.7% vs 44.4% in the chemotherapy group; 29.1% of patients achieved complete response vs 12.5% in the chemotherapy group. Among responders, 67.3% and 59.6% were still in remission in the EV-pembrolizumab group at 12 and 18 months, respectively, vs 35.2% and 19.3% for the chemotherapy group. The median duration of response in the EV-pembrolizumab group was not reached at the time of the data cutoff point (August 8, 2023). At the time of data cutoff, almost one-third of patients in the EV-pembrolizumab arm were still on treatment, vs none in the chemotherapy group. The most common subsequent therapy was platinum-based chemotherapy for the former and anti-PD-1/L1 for the latter, including 143 patients who received maintenance therapy (135 of them avelumab). Regarding safety, the toxicity profile was consistent with what was already known from previous trials for each agent. Fewer grade 3 or higher adverse events were reported for the patients in the EV-pembrolizumab group (55.9%) than those in the chemotherapy group (69.5%). Despite that fact, more patients discontinued treatment in the EV-pembrolizumab group (29.5%) than in the chemotherapy group (21.4%) as a result of treatment-related adverse events with the most common peripheral sensory neuropathy and anemia, respectively. Treatment-related deaths occurred in 4 patients in each group (<1%) [33,34].
3.2. TROP-2
TROP-2 is a transmembrane glycoprotein that is overexpressed among different types of epithelial cancers, such as urothelial carcinoma, while maintaining low expression in normal tissues [35]. Its presence correlates with poor prognosis and aggressive tumor behavior [19,36,37].
3.2.1. Sagituzumab Govitecan
Sacituzumab Govitecan(SG) utilizes α variation of humanized mAb against TROP-2, namely hRS7 IgG1κ conjugated with the active metabolite of irinotecan SN-38. Its role is to inhibit nuclear topoisomerase I (Topo-I), a milestone for DNA stability [38]. Sacituzumab Govitecan's engineered design is notable for its fast breakdown once it reaches the TROP-2-expressing cells and enchased linker stability in the bloodstream. The result is improved specificity and effectiveness [37,39]. The stable topoisomerase inhibitor uses a breakable linker to connect itself to a monoclonal antibody for therapy to be employed directly into the malignant cells without an off-target effect [40]. This unique design has increased importance because the cleavable nature of the linker almost guarantees that the cytotoxic payload is delivered within the tumor microenvironment [40]. Consequently, the desired outcome of minimal systemic exposure and toxicity is attained. Following the rationale mentioned above, the clinical effectiveness of SG was studied in locally advanced or mUC disease settings, including patients refractory to platinum-based chemotherapy and a checkpoint inhibitor (CPI) treatment. IMMU-132-01, a Phase I/II basket study, resulted in a median PFS of 7.3m and OS 18.9m; the median DOR was 12.6 months. Complete response (CR) for 2 subjects and 12 partial responses (PR), ORR 31%. Promising outcomes for SG, resulting in clinical efficacy for patients with mUC, including local relapsed or visceral disease, after receiving a standard chemotherapy regimen and anti-PD-1 or PD-L1 inhibitor therapies [41].
When used alone, pembrolizumab and SG have shown effectiveness in controlling solid tumors and have acceptable safety characteristics that do not overlap significantly. These treatments have been tested in patients with platinum-relapsed/refractory mUC. The hypothesis that a combination of these two agents would be secure and efficacious in this context was tested in cohorts of the TROPHY-U-01 trial. This is a phase II trial (NCT03547973) aimed to determine if the combination of sacituzumab govitecan (SG) with pembrolizumab could increase the (ORR and DOR, leading to improved clinical benefit rates (CBRs). The trial enrolled patients who had not previously been treated with checkpoint inhibitors and had progressive disease mUC after receiving platinum-based chemotherapy in or within 12 months after the neoadjuvant stage. The initial report with a median follow-up of 9.1 months resulted in an ORR of 27% (31 of 113; 95% CI, 19.5 to 36.6); 77% decrease in measurable disease, mDOR of 7.2 months (95% CI, 4.7 to 8.6 months), mPFS of 5.4 months (95% CI, 3.5 to 7.2 months) and OS 10.9 months (95% CI, 9.0 to 13.8 months). With longer follow-up, it was pointed out that ORR remained high at 28%. Subject to confirmation of its therapeutic benefits from Cohort 1 of the TROPHY-U-01 trial, the fast-tracked designation led to accelerated approval by the FDA for the use of SG in treating mUC that has already been treated with platinum-based chemotherapy and a checkpoint inhibitor (CPI) treatment [42].
Cohort 3 of TROPHY-U-01 study included 41 patients, 78% with visceral metastases. A median follow-up of 14.8 months resulted in ORR of 41% (95% confidence interval 26.3 to 57.9) and CR of 20%, CBR was 46% (95% CI 30.7 to 62.6), Median DOR was 11.1 months (95% CI 4.8 to not estimable). Median PFS was 5.3 months (95% CI 3.4 to 10.2), and median OS reached 12.7 months (range, 10.7-NE). To fully explore SG's potential, cohort 3 additionally explored SG in combination with a range of other treatments, including cisplatin, avelumab maintenance in first-line cisplatin-ineligible mUC, and zimberelimab an anti PD-1 monoclonal antibody [43]. TROPiCS-04, a phase III confirmatory study of SG monotherapy in mUC in refractory disease after platinum-based chemotherapy and ICI. At the 2024 ASCO annual meeting, the published results did not meet the initial hypothesis, concluding a negative study. Although the investigators observed trends in minimal numerical improvement of OS and secondary endpoints of PFS and ORR were also shown [44]. SURE-01 (NCT05226117) designed to evaluate sacituzumab govitecan (SG) as neoadjuvant therapy before radical cystectomy. Given higher toxicity rate than expected the authors applied a protocol amendment consisting of a dose reduction and G-CSF prophylaxis. As a result, the preliminary data is encouraging. Demonstrated hypothesis-generating complete responses with monotherapy sacituzumab govitecan-hziy (Trodelvy) as neoadjuvant treatment following surgery in patients with muscle-invasive bladder cancer (MIBC)[15]. Future research should focus on investigating the pharmacokinetics and dynamics of the linker technology. This could lead to the creation of improved versions that are more effective and less toxic [37].
3.2.2. Datopotamab Deruxtecan
Datopotamab Deruxtecan (Dato-DXd, DS-1062a) is a novel humanized anti-TROP2 IgG1 monoclonal antibody ADC, representing a significant advancement in the therapeutic landscape of urothelial carcinoma through its targeted approach. It is designed to selectively deliver a Topo I inhibitor intracellularly, namely Dato-DXd via a tetra peptide-based cleavable linker. Applying the DXd-ADC technology cluster with the optimized DAR of 4 is anticipated to expand the potential therapeutic capability [45]. This theory was tested in the ongoing phase 1 TROPION-PanTumor01 study. Preliminary results as published in 2024 ASCO Genitourinary Cancers Symposium suggested an encouraging antitumor activity as salvage therapy for unrespectable advanced or metastatic UC after first line. Confirmed ORR of 27.8% (95% CI 9.7–53.5), DCR of 77.8% (95% CI 52.4–93.6) [46].
3.3. HER2
HER2, renowned for its involvement in the development of breast cancer, is also associated with urothelial carcinoma, where its expression can range widely, from 23.42% HER2 1+, 54.95% HER2 2+ to 4.50% HER2 expression 3+. This disparity in expression levels has a significant impact on both prognosis and response to treatment [47]. According to the recently released study at the 2024 ASCO GU conference, there is an inverse correlation between the expression of HER2 IHC and the PD-L1 CPS score [48].
3.3.1. Trastuzumab Deruxtecan
Fam-trastuzumab deruxtecan-nxki (T-DXd) builds upon the concept of targeted HER2 therapy but with enhanced efficacy and a broader application spectrum. Concists of an anti-HER2 ADC that combines an antibody, a tetrapeptide-based cleavable linker and a payload of topoisomerase I inhibitor (DXd). T-DXd harbors a twin antitumor effect. In the first place by inhibiting the double-stranded DNA breakage and through topoisomerase cancer cell apoptosis. Second, it acts on proximal cancer cells with low HER2 expression through the "bystander effect" [49]. Amplification of HER2 In UC range from 6% to 17%, while incidence ranges from 6% to 80% [50]. In the past various trials tested anti-HER2 therapies for treatment of UC. Nevertheless, they have failed. Targeting HER2 with ADCs may represent a more promising strategy [51]. Given the ability of ADCs to employ cytotoxic therapy to tumor cells with low HER2 levels (IHC 1+, 2+ with FISH test negative), HER2 positive report on UC will be subject to greater scrutiny [52]. DESTINY-PanTumor01 a phase II trial tested patients expressing HER2 activating mutations in a context of metastatic disease from different primary sites. Patients with urothelial malignancy achieved objective response (ORR) of 29% [53]. Following DESTINY-PanTumor02 an open-label phase II basket trial (NCT04482309) with 267 patients expressing receptors pocitivity HER2 3+ or 2+, originated from different primary sites, who received monotherapy of Fam-trastuzumab deruxtecan-nxki [54]. Patients with bladder malignancy marked an overall response rate (ORR) of 39% (95% CI 24.2–55.5%). Of note is the fact that patients with IHC HER2 3+ disease reported ORR of 56% vs 35% for IHC HER2 2+ disease. Based on these results On April 5, 2024, T-DXd is the first HER2-targeted ADC to be granted the Food and Drug Administration accelerated approval for adult patients with unresectable or metastatic HER2-positive (IHC 3+) solid tumors including UC who have received prior systemic treatment and have no satisfactory alternative treatment options [55]. Currently T-DXd is a treatment option for patients with mUC in the US. This option highlights the need to define HER2 protein expression in this malignancy and ensure routine testing.
3.3.2. Disitamab Vedotin
Disitamab vedotin (DV) also known as RC48, contains a monoclonal antibody that targets HER2 with higher affinity than trastuzumab, its valine- citrulline linker is cleavable, ensuring on-target cytotoxicity upon endocytosis by the tumor cell, and its payload is MMAE. The drug has intracellular cell killing as well as bystander effect on adjacent cells. Its DAR is 4 to 1 [56,57].
Phase I trial of DV enrolled 4 patients with HER2+ mUC; in this trial, two patients reported with partial response and two with stable disease, proving the drug's antitumor activity. The clinical development of DV continued with two open-label phase II trials, both conducted in China, RC48-C005 (ClinicalTrials.gov identifier: NCT03507166) and RC48-C009 (ClinicalTrials.gov identifier: NCT03809013) studies. In these very similar studies, 107 patients with HER2-positive (IHC 2+/3+) laUC/mUC were enrolled and had previously progressed on at least one line of systemic chemotherapy. DV was administered intravenously every 2 weeks at a dose of 2mg/kg. The primary end-point was ORR. 65% of patients had received at least two lines of previous systematic treatment, and 25% had received an anti-PD-1/L1 treatment. ORR was 50%, with 32% of patients achieving stable disease as their best response, and 18% experienced disease progression. The ORR was numerically higher for patients with higher HER2 expression. The median DoR was 7.3 months, median PFS was 5.9 months and median OS was 14.2 months. In terms of safety, the most reported TRAE resulting in treatment discontinuation was peripheral sensory neuropathy (11.2%), no grade 4 or 5 TRAEs were observed. Peripheral sensory neuropathy and leukopenia were the most common grade 3 TRAEs reported. Based on these results, DV was approved in China in January 2022 for platinum-refractory patients with mUC [57,58,59,60]. DV has also gained Breakthrough therapy designation by the US FDA since September 2020.
DV has also been examined in other -smaller- phase II trials. In the NCT04073602 trial, DV was administered in 19 patients with tumors lacking HER2 expression. ORR was 26%, indicating a certain degree of response even in the absence of HER2 expression. In addition to monotherapy, DV-based combinations also have been explored. The combination with toripalimab, an anti-PD-1 antibody, resulted in a response rate of 73%, in the NCT04264936 phase II trial, by the cutoff date of 18 November 2022. This trial had enrolled 41patiens, 61% of them without prior exposure to any treatment. Median PFS was 9.2 months, and 2-year OS rate is 63.2%. A phase III study is ongoing to compare this combination with the standard of care [57,61,62]. Currently, there are also two larger trials testing the combination of DV with pembrolizumab in non-Chinese patients. RC48G001 (NCT04879329) is a phase II trial, with three cohorts. Patients in cohorts A and B with tumors expressing HER2+/ HER2 low, respectively, are previously treated with platinum-containing therapy and will receive DV monotherapy, while patients in cohort C are treatment-naïve and will receive either monotherapy or the combination of DV with pembrolizumab. DV-001 (NCT05911295), is a phase III randomized trial, where previously untreated patients with HER2-expressing laUC/mUC will receive in a 1:1 ratio DV and pembrolizumab or platinum-containing chemotherapy (maintenance with avelumab is permitted). The primary endpoints are PFS and OS [63,64]. Outside the context of prospective clinical trials, DV combinations with anti-PD-1/L1 has also been tested in patients with muscle-invasive and la UC, with promising results [65].
3.4. EPCAM
3.4.1. Oportuzumab Monatox (Vicineum)
EpCAM (epithelial cell adhesion molecule) is a transmembrane glycoprotein that is overexpressed in various human tumors, including urothelial carcinomas. Targeting the extracellular domain of EpCAM is clinically relevant, and clinical trials in urothelial cancer have been underway since 2010. The drug used is oportuzumab monatox (OM), known as vicineum, a fusion protein of a humanized single-chain antibody against EpCAM linked to Pseudomonas exotoxin. OM binds to the surface of EpCAM-positive cancer cells and exotoxin releases apoptosis. OM is used in BCG-refractory non-muscle invasive bladder cancer (NMIBC) patients as intravesical instillation, thus reducing systemic toxicity [56,66,67].
Phase I study of OM enrolled 64 patients with BCG-refractory or intolerant patients with grade 2 or 3, stage Ta/T1 transitional cell carcinomas or in-situ carcinomas. Patients were administered 6 weekly instillations, achieving CR at a rate of 39% at the 12-week endpoint. No patients were removed from the study due to toxicity. The subsequent open-label phase II study enrolled 46 patients and reported similar outcomes, with 44% of the patients achieving CR. The phase III registrational trial of OM, the VISTA study, enrolled 133 patients divided in three cohorts, two depending on CIS recurrence from BCG treatment and the third recruited patients with papillary recurrence < 6 months from BCD treatment. Intrvesical instillation of 30mg OM was administered two times per week for 6 weeks followed by weekly for 6 weeks and then every 2 weeks for up to 2 years. The primary end-point was CR rate and duration of response in Cohort 1 (CIS recurrence < 6 months from BCG treatment). At 3 months,the primary end point of CR was 39% in cohort 1. Treatment was well tolerated with only 4% experiencing treatment-related grade ≥ 3 toxicity, most commonly urinary tract infections. In July 2022, the US FDA rejected the approval of Vicineum, leading to the pausing of its further development by the manufacturer Sesen Bio [66,68,69,70].
Another phase I trial combines OM with durvalumab (an anti-PD-L1 checkpoint inhibitor) in high-grade NMIBC after BCG failure (NCT03258593). The trial enrolled 15 patients, and its final results were recently presented at the 2024 American Urological Association meeting. The combination was well tolerated and resulted in a 47% CR rate at the 12-week time point, with three patients showing no evidence of disease for more than 12 months [71].
3.5. Tissue Factor
3.5.1. Tisotumab Vedotin
Tissue factor (TF) is highly expressed on the surface of many solid tumor cells, including ovarian, prostate, bladder, esophageal, endometrial, and lung tumors. Tisotuman vedotin (TV) comprises a human monoclonal antibody specific for TF conjugated to the microtubule-disrupting agent MMAE via a protease-cleavable linker.
TV has been examined in the phase I-II InnovaTV 201 trial, a multicenter dose-escalation and dose-expansion trial to assess its safety administration in patients. The trial enrolled in both phases a total of 174 patients with metastatic solid tumors, of whom 17 had bladder cancer. Of the 15 bladder cancer patients eligible for response analysis, 4 (26,7%) demonstrated objective response. Overall, the trial has shown that TV is safe to administer and has an encouraging antitumor activity in heavily pretreated patients with tumors known to express TF. Its safety profile is consistent with other MMAE-containing ADCs, with epistaxis and conjunctivitis appearing more frequently [72]. Over the next years, the development of TV focused more on patients with cervical cancer. In April 2024, the FDA granted full approval to TV for the treatment of patients with recurrent or metastatic cervical cancer who have disease progression on or after treatment with chemotherapy.
A list of approved indications of ADCs in urothelial cancer can be found in Table 1.
4. Discussion and Future Perspectives
The impressive results of the EV-302 trial establish a new standard of care for the 1st line treatment of urothelial carcinomas. The combination has already gained approval from the US FDA, leading to fundamental changes in the treatment landscape of this disease as we have known it for the last four decades. The combination of EV plus pembrolizumab produced unprecedented benefits in both OS and PFS, irrespective of cisplatin eligibility and PDL-1 positivity [73]. These two prerequisites have correlated with benefits in previous trials of chemotherapy and immunotherapy with ICIs. The question is, how did that happen? Cisplatin has been the mainstay of treatment in the front line for decades, and most trials were built upon the hypothesis that cisplatin was unreplaceable. With the introduction of immunotherapy, again, the only positive trial of concurrent administration enrolled patients who were exclusively cisplatin eligible, while in the switch maintenance Javelin Bladder 100 trial, there was a patient selection strategy depending on their response to platinum (cisplatin or carboplatin-based) chemotherapy [74]. In the EV-302 trial, the immunotherapy arm is an ICI as in other trials, so the difference in the beneficial impact has to be attributed to the MMAE, the payload component of the ADC. Indeed, there is evidence that MMAE – a potent cytotoxic on its own- has properties that justify its excellent combination with ICIs. Human dendritic cells exposed to MMAE upregulate costimulatory molecules and display enhanced T cell-stimulatory capacity. Preclinical studies have shown that EV induces early hallmarks of immunogenic cell death in vitro, immunomodulatory changes in mouse xenografts, and gene expression patterns associated with immunogenic cell death. We must understand this exciting interplay between EV and tumor microenvironment to gain insight toward restoring resistance mechanisms and further augmenting its therapeutic effect [75,76]. It is worth mentioning that similar results of increased response have also been with the combination of DV and toripalimab, another example of MMAE containing ADC and an ICI [77].
These positive results already mentioned create new questions about how we can fully exploit the potential of these new drugs. One aspect is how fast we can move those drugs into the earlier phases of the disease, following the example of nivolumab in the adjuvant setting. The other aspect is how we can better control the significant toxicity of these combinations in order to maximize the benefit for our patients. The third consequence is that from now on, we are expecting a more considerable patient population eligible for second and later lines of therapy, and this underlies the importance of adjusting the treatment algorithm accordingly.
Moving forward ADCs in the earliest stages of the disease is challenging given the strongly established role of cisplatin-based chemotherapy in both the neoadjuvant and adjuvant setting. Even in the ChekcMate 274 trial, it is difficult to disregard the HR difference between the patients exposed to neoadjuvant cisplatin versus those who were not [78]. At least three major phase III clinical trials are underway, combining EV with ICIs in adjuvant and neoadjuvant settings. EV-304/Keynote B-15 trial enrolls cisplatin-eligible patients using the combination of EV plus pembrolizumab before and after cystectomy versus cisplatin plus gemcitabine combination. The primary endpoint is EFS, and the trial has an accrual target of 784 patients. The other two trials enroll cisplatin-ineligible patients, and this is the patient population with fewer treatment options. VOLGA trial has three arms: one without any intervention except cystectomy, one with EV plus Durvalumab and Tremelimumab as neoadjuvant treatment followed by cystectomy and Durvalumab plus tremelimumab in the adjuvant setting, and one with EV plus durvalumab, with durvalumab only after cystectomy. The EV-303/ Keynote 905 trial follows a similar randomization scheme with EV plus pembrolizumab in both the neoadjuvant and adjuvant treatment in one arm, pembrolizumab monotherapy in the second arm, and cystectomy alone in the third arm. When both trials are complete, they will have enrolled more than 1600 patients, defining the place of EV/ICIs combination in this patient population [79,80,81,82]. Similar trials are underway for the other ADCs (SG, DV) but in earlier phases of clinical development. The most important clinical trials of ADCs in urothelial cancer are summarised in Table 2.
The toxicity issue is a significant concern, especially in the context of the increasing ADCs plus ICIs combinations to come. The point to address is not excessive toxicity, since patients in chemotherapy arms experienced numerically more serious adverse events, but the management of a unique toxicity profile, its early detection, and the proper education of patients and non-oncology specialists. The most common grade 3 AEs for the EV-P combination of any grade are peripheral sensory neuropathy (50.0%), pruritus (39.8%), alopecia (33.2%), maculopapular rash (32.7%), fatigue (29.3%), and diarrhea (27.5%). Since both agents share common toxicities in some regard, distinguishing might be a challenge. Peripheral neuropathy can be treatment-limiting, and skin reactions can sometimes be life-threatening. Neurologic toxicity can cause sensory, motor, and autonomic dysfunction. It is progressive with rapid onset, and the current recommendation is to hold treatment if grade 3 or more permanently. It has a median resolution time of 7 months and can severely affect a patient’s quality of life. The key to this toxicity is patient education to facilitate early detection. Cutaneous toxicity can be grave; 7.7% of patients experienced grade ≥ 3 maculopapular rash. Initiation of systemic steroid is recommended when there is grade 3 and referral to a dermatologist for recurrent or more serious events. What makes EV dermatological toxicity unique is the way it combines the specificity of an antibody-drug conjugate targeting nectin-4 with the delivery of the MMAE payload to the skin. This causes the observed apoptosis of keratinocytes and the strong cytotoxicity of a microtubule-disrupting agent [83,84]. Hyperglycemia is less frequent (5% grade 3 or more), affecting patients with higher BMI and higher values of A1C at baseline, indicating that these are the patients who need more vigorous laboratory controls. Pneumonitis is severely underreported and overlaps with immune-induced pneumonitis. Its onset time necessitated longer follow-up and specific patient education about its often vague initial symptoms. Yoon et al. reported that the median time from the initiation of EV therapy to the onset of pneumonia was 13 weeks [85]. Therefore, clinicians should consider not only early TRAEs, as discussed, but also delayed TRAEs such as pneumonia, peripheral neuropathy, and hyperglycemia. The adverse events that emerge with EV-P treatment and patient performance status should be considered for the subsequent therapy decision, especially cumulative neurotoxicity in future cisplatin or taxane administration.
TRAEs of any grade occurred in more than 90% of patients in phase 2 or 3 trials, with high discontinuation rates. Despite toxicity, patient-reported outcomes indicate that patients treated with the combination demonstrated significant improvement in worse pain and general health status/ quality of life. Data from phase 2 and 3 trials of the EV also showed no impact on quality of life and an improvement in some aspects [24,25,26,27]. Dose modifications, including dose reductions, are a common tool in addressing toxicity management. Recommended dose modifications can assist in managing adverse events, allowing patients to have a more extended treatment exposure and more pronounced clinical benefit while maintaining a functional status. In the future, more specific interventions have to be answered as to what extent EV administration can be reduced to six months while maintaining immunotherapy exposure, if we can reduce the dose to patients with established responses, and, of course, to design future trials to assess the efficacy of those dose de-escalation strategies.
Patients eventually will relapse after 1st line treatment. Often, due to unacceptable toxicity or disease progression, up to approximately two-thirds of patients with mUC do not receive second-line treatment [86]. The emerging new standards of care for the 1st line raise questions and challenges for the oncology community. However, the RR and the OS with EV-P are considerably high; ultimately, resistance build-up induced by mechanisms, such as resistance to the monomethyl auristatin payload, decreased expression in nectin-4, immune evasion, insufficiency of drug internalization and increase in efflux pumps [87]. Currently, an unmet need for a 2nd line regimen is evolving about the accepted and established standard of care for metastatic urothelial carcinoma (mUC) after progression on EV-P. Approved therapies for this setting include platinum-based regimens, sacituzumab govitecan, erdafitinib, and clinical trials. There is insufficient data to consider switch maintenance strategies in the 2nd line.
For the patients not already exposed to EV, presented data support the use of EV in patients with la/ mUC who have previously received platinum-containing chemotherapy plus PD-1 or PD-L1 inhibitor. At the 2024 ASCO Genitourinary Cancers Symposium, Nizam et al. stated that a retrospective analysis showed that patients with advanced urothelial carcinoma who received the EV after maintenance avelumab had outcomes similar to data that led to its FDA-approved indication in chemotherapy and ICI refractory mUC [88].
The randomized Phase 3 THOR trial with Erdafitinib, an oral pan-FGFR1–4 inhibitor, markedly increased effectiveness versus ChT and is recommended in patients with selected FGFR DNA fusions and mutations who have previously been treated with ChT and an ICI [12]. In the retrospective study UNITE, the ORR of erdafitinib following EV was 31%, with mPFS of 5 months and mOS of 10 months [89].
Sacituzumab govitecan can be recommended in patients previously treated with chemotherapy and an ICI [87]. Real-world studies have also evaluated SG following EV and immunotherapy [90,91]. Toxicity includes diarrhea and cytopenias, which are more prominent in patients with UGT1A1 polymorphisms.
There is insufficient trial data to demonstrate an OS benefit beyond 2nd line therapy [92]. However, after EV-P, treatments not previously used may be considered for third- and fourth-line therapy. In the current treatment algorithm for mUC, is there any optimal sequence of ADCs? Reading the results from a cohort of real-world data for patients with advanced mUC and prior exposure to EV conclude that Sacituzumab govitecan (SG) resulted in limited clinical efficacy ORR of 10% (95% CI 4.3%, 18.3%) compared to previous reports. A correlation was observed between the administration of SG immediately following EV and enhanced clinical results: 2.1 months (95% CI 1.9, 2.5) and 6.0 months (95% CI 4.5, 7.0) were the median PFS and OS, respectively. After SG, no correlation (p>0.8) existed between the response to EV and ORR, PFS, or OS. Improved PFS (HR=0.43, 95% CI 0.21, 0.87, p = 0.02) and ORR (p = 0.028) were linked to sequencing SG immediately after EV, but not OS [93].
It is not recommended to rechallenge with a single-agent ICI without more proof. There is ongoing discussion about the place of PD-L1 testing in the context of UC treatment. A consensus could not be reached on giving EV-P after completing adjuvant immune therapy with nivolumab. Therefore, it may be considered [94].
Due to high treatment costs, access is often limited to recently approved treatments such as SG and erdafitinib. Additionally, unfit for platinum-based chemotherapy patients may consider other chemotherapies, such as taxane-based regimens. The benefit of this option is considered limited, with time on treatment up to 12 weeks before death, progression, or intolerance [86]. Palliative RT as external beam irradiation remains a treatment option for patients in this setting, especially for symptomatic patients with urinary symptoms such as hematuria. In those patients, hypofractionated palliative RT effectiveness is similar to a multifractionated treatment regarding symptom improvement with rapid palliation of symptoms [95].
After disease progression, the question is whether Nectin-4 is still a viable target. Many new trials start with new Nectin-4-directed ADCs with new antibodies, linkers, and payloads. The goal is to make a less toxic but more effective drug for patients who haven't responded to treatment yet or who haven't been treated at all. Future research in this area should primarily focus on finding biomarkers that would make it easier to choose a more successful treatment plan for each patient from the range of options available to them. Furthermore, research into new ADCs targeting novel tumor antigens is a promising area, given the established effectiveness of ADCs for patients with urothelial carcinoma. Research on improved immunotherapy strategies remains relevant as the cancer is amenable to immune modulation. Platinum-based chemotherapy continues to stand strong as an element in the systemic treatment of metastatic urothelial carcinoma.
5. Conclusions
The advent of ADCs has opened new avenues for the treatment of this aggressive cancer type, providing hope for more personalized and effective therapies. ADCs represent a promising approach in the arsenal against cancer, particularly urothelial carcinoma. Still, their successful integration into clinical practice requires a nuanced understanding of their mechanism, including the critical bystander effect, and a strategic approach to overcoming the inherent challenges associated with this innovative therapy.
Supplementary Materials
Figure 1 was created by adobe illustrator 2024.
Author Contributions
Both authors N.P., D.Z., had equal contribution to the work reported.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
N.P. has received speaker fees and honoraria from Pfizer, GSK, MSD, Ipsen, Roche, Astra Zeneca, Janssen and Astellas, D.Z., sponsored presentation, 2024 by Bayer AG.
Abbreviations
AEadverse event rate or incidence; ADC, antibody-drug conjugates; AVLM, avelumab; BCG, bacillus calmette guerin; CTX, standard chemotherapy, often physician’s choice; DV, Disitamab vedotin; DoR, Duration of response; EV, enfortumab vedotin; Gem, gemcitabine; ICI/CPI, immune checkpoint inhibitor; LA, laboratory abnormalities; LA/mUC, locally advanced or metastatic urothelial cancer; LFR, Local-Recurrence Free; MIBC, muscle-invasive bladder cancer; MMAE, monomethyl auristatin E; MTD, max tolerated dose; OM, oportuzumab monatox; ORR, objective response rate; OS, overall survival; P, pembrolizumab; pCR, pathologic complete response; PFS, progression-free survival; Plat (platinum), cisplatin or carboplatin; RP2D, recommended phase II dose; SG, sacituzumab govitecan; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan; Taxane, paclitaxel or docetaxel; TRAE, treatment-related AE; TV, tisotumab vedotin.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R. L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M. D.; Chen, G. J.; Oh, W. K.; Bellmunt, J.; Roth, B. J.; Petrioli, R.; Dogliotti, L.; Dreicer, R.; Sonpavde, G. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol 2012, 23, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Sonpavde, G.; Pond, G. R.; Choueiri, T. K.; Mullane, S.; Niegisch, G.; Albers, P.; Necchi, A.; Di Lorenzo, G.; Buonerba, C.; Rozzi, A.; et al. Single-agent Taxane Versus Taxane-containing Combination Chemotherapy as Salvage Therapy for Advanced Urothelial Carcinoma. Eur Urol 2016, 69, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Pistamaltzian, N.; Tzannis, K.; Pissanidou, V.; Peroukidis, S.; Milaki, G.; Karavasilis, V.; Mitsogiannis, I.; Varkarakis, I.; Papatsoris, A.; Dellis, A.; et al. Treatment of relapsed urothelial bladder cancer with vinflunine: real-world evidence by the Hellenic Genitourinary Cancer Group. Anticancer Drugs 2016, 27, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Balar, A. V.; Castellano, D. E.; Grivas, P.; Vaughn, D. J.; Powles, T.; Vuky, J.; Fradet, Y.; Lee, J. L.; Fong, L.; Vogelzang, N. J.; et al. Efficacy and safety of pembrolizumab in metastatic urothelial carcinoma: results from KEYNOTE-045 and KEYNOTE-052 after up to 5 years of follow-up. Ann Oncol 2023, 34, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Durán, I.; van der Heijden, M. S.; Loriot, Y.; Vogelzang, N. J.; De Giorgi, U.; Oudard, S.; Retz, M. M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E. R.; Vaena, D.; Grimm, M. O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017, 18, 312–322. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, MS.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, JP.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. CheckMate 901 Trial Investigators. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef]
- Powles, T.; Park, SH.; Caserta, C.; Valderrama, BP.; Gurney, H.; Ullén, A.; Loriot, Y.; Sridhar, SS.; Sternberg, CN.; Bellmunt, J.; et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After ≥2 Years of Follow-Up. J Clin Oncol. 2023, 41, 3486–3492. [Google Scholar] [CrossRef]
- Bajorin, DF.; Witjes, JA.; Gschwend, JE.; Schenker, M.; Valderrama, BP.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, SF.; Park, SH.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2021, 384(22), 2102–2114. [Google Scholar] [CrossRef]
- Galsky M., :; et al. Extended Follow-up from CheckMate 274 Including the First Report of Overall Survival Outcomes. Presented during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th 2024. [Google Scholar]
- Loriot, Y.; Matsubara, N.; Park, S. H.; Huddart, R. A.; Burgess, E. F.; Houede, N.; Banek, S.; Guadalupi, V.; Ku, J. H.; Valderrama, B. P.; et al. Erdafitinib or Chemotherapy in Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2023, 389, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S. E.; VanderWeele, D. J. Antibody-drug conjugates and predictive biomarkers in advanced urothelial carcinoma. Front Oncol 2022, 12, 1069356. [Google Scholar] [CrossRef] [PubMed]
- Birrer, M. J.; Moore, K. N.; Betella, I.; Bates, R. C. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J Natl Cancer Inst 2019, 111, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Natsume, A.; Niwa, R.; Satoh, M. Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC. Drug Des Devel Ther 2009, 3, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xie, G.; Deng, X.; Zhang, Y.; Jia, Z.; Huang, Z. Antibody-drug conjugates in urinary tumors: clinical application, challenge, and perspectives. Front Oncol 2023, 13, 1259784. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shen, Z.; Zhao, W.; Lu, J.; Song, Y.; Shen, L.; Lu, Y.; Wu, M.; Shi, Q.; Zhuang, W.; et al. Rational Identification of Novel Antibody-Drug Conjugate with High Bystander Killing Effect against Heterogeneous Tumors. Adv Sci (Weinh) 2024, 11, e2306309. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, F.; Dal Bo, M.; Macor, P.; Toffoli, G. A comprehensive overview on antibody-drug conjugates: from the conceptualization to cancer therapy. Front Pharmacol 2023, 14, 1274088. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, E.; Fujita, K.; Nakano, K.; Kuwahara, K.; Minami, T.; Kato, T.; Hatano, K.; Kawashima, A.; Uemura, M.; Takao, T.; et al. Trop-2 in Upper Tract Urothelial Carcinoma. Curr Oncol 2022, 29, 3911–3921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Shen, Q.; Yin, W.; Huang, H.; Liu, Y.; Ni, Q. High expression of Nectin-4 is associated with unfavorable prognosis in gastric cancer. Oncol Lett 2018, 15, 8789–8795. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Wang, L.; Wu, Y.; Hao, J.; Wang, Z.; Lu, W.; Wang, X. A.; Zhang, F.; Cao, Y.; et al. A novel PI3K/AKT signaling axis mediates Nectin-4-induced gallbladder cancer cell proliferation, metastasis and tumor growth. Cancer Lett 2016, 375, 179–189. [Google Scholar] [CrossRef]
- Shafique, M. A.; Haseeb, A.; Siddiq, M. A.; Mussarat, A.; Rangwala, H. S.; Mustafa, M. S. Current and Emerging Treatments for Urothelial Carcinoma: A Focus on Enfortumab Vedotin. Cancer Manag Res 2023, 15, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Challita-Eid, P. M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D. R.; et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res 2016, 76, 3003–3013. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, B. A.; Catalano, M.; Maiello, E.; Roviello, G. Enfortumab vedotin in metastatic urothelial carcinoma: the solution EVentually? Front Oncol 2023, 13, 1254906. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J. E.; Heath, E.; Perez, R.; Merchan, J.; Lang, J.; Ruether, D.; Petrylak, D.; Sangha, R.; Smith, D. C.; Sridhar, S.; et al. Interim analysis of a phase I dose escalation trial of ASG-22CE (ASG-22ME; enfortumab vedotin), an antibody drug conjugate (ADC), in patients (Pts) with metastatic urothelial cancer (mUC). Annals of oncology 2016, 27, vi273–vi273. [Google Scholar] [CrossRef]
- Rosenberg, J. E.; O'Donnell, P. H.; Balar, A. V.; McGregor, B. A.; Heath, E. I.; Yu, E. Y.; Galsky, M. D.; Hahn, N. M.; Gartner, E. M.; Pinelli, J. M.; et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2019, 37, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Yu, E. Y.; Petrylak, D. P.; O'Donnell, P. H.; Lee, J. L.; van der Heijden, M. S.; Loriot, Y.; Stein, M. N.; Necchi, A.; Kojima, T.; Harrison, M. R.; et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2021, 22, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J. E.; Powles, T.; Sonpavde, G. P.; Loriot, Y.; Duran, I.; Lee, J. L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Mamtani, R.; et al. EV-301 long-term outcomes: 24-month findings from the phase III trial of enfortumab vedotin versus chemotherapy in patients with previously treated advanced urothelial carcinoma. Ann Oncol 2023, 34, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Astellas Pharma US, I. Padcev (enfortumab vedotin-ejfv) with Keytruda (pembrolizumab) Approved by FDA as the First and Only ADC Plus PD-1 to Treat Advanced Bladder Cancer. 2023. https://www.drugs.com/newdrugs/padcev-enfortumab-vedotin-ejfv-keytruda-pembrolizumab-approved-fda-first-only-adc-plus-pd-1-6161.html (accessed 2023).
- AGENCY, E. M.; Padcev : EPAR - Product information. 29/11/2023. https://www.ema.europa.eu/en/medicines/human/EPAR/padcev.
- O'Donnell, P. H.; Milowsky, M. I.; Petrylak, D. P.; Hoimes, C. J.; Flaig, T. W.; Mar, N.; Moon, H. H.; Friedlander, T. W.; McKay, R. R.; Bilen, M. A.; et al. Enfortumab Vedotin With or Without Pembrolizumab in Cisplatin-Ineligible Patients With Previously Untreated Locally Advanced or Metastatic Urothelial Cancer. J Clin Oncol 2023, 41, 4107–4117. [Google Scholar] [CrossRef] [PubMed]
- Hoimes, C. J.; Flaig, T. W.; Milowsky, M. I.; Friedlander, T. W.; Bilen, M. A.; Gupta, S.; Srinivas, S.; Merchan, J. R.; McKay, R. R.; Petrylak, D. P.; et al. Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer. J Clin Oncol 2023, 41, 22–31. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B. P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S. H.; Shin, S. J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med 2024, 390, 875–888. [Google Scholar] [CrossRef]
- Niegisch, G. Enfortumab Vedotin and Pembrolizumab - A New Perspective on Urothelial Cancer. N Engl J Med 2024, 390, 944–946. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D. M.; Sharkey, R. M. Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: A case study of anti-TROP-2 sacituzumab govitecan. MAbs 2019, 11, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Shvartsur, A.; Bonavida, B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer 2015, 6, 84–105. [Google Scholar] [CrossRef] [PubMed]
- Sasso, J. M.; Tenchov, R.; Bird, R.; Iyer, K. A.; Ralhan, K.; Rodriguez, Y.; Zhou, Q. A. The Evolving Landscape of Antibody–Drug Conjugates: In Depth Analysis of Recent Research Progress. Bioconjugate Chemistry 2023, 34, 1951–2000. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D. M.; Sharkey, R. M. Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert Opin Biol Ther 2020, 20, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Song, C. H.; Jeong, M.; In, H.; Kim, J. H.; Lin, C.-W.; Han, K. H. Trends in the Development of Antibody-Drug Conjugates for Cancer Therapy. Antibodies 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lim, K. S.; Blackburn, B. J.; Yun, J.; Putnam, C. W.; Bull, D. A.; Won, Y. W. The Potential of Topoisomerase Inhibitor-Based Antibody-Drug Conjugates. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Messersmith, W. A.; Kio, E. A.; Berlin, J. D.; Vahdat, L.; Masters, G. A.; Moroose, R.; Santin, A. D.; Kalinsky, K.; Picozzi, V.; et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol 2021, 32, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Petrylak, D. P.; Rezazadeh Kalebasty, A.; Fléchon, A.; Jain, R. K.; Gupta, S.; Bupathi, M.; Beuzeboc, P.; Palmbos, P.; Balar, A. V.; et al. TROPHY-U-01, a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors: updated safety and efficacy outcomes. Ann Oncol 2024, 35, 392–401. [Google Scholar] [CrossRef]
- Grivas, P.; Pouessel, D.; Park, C. H.; Barthelemy, P.; Bupathi, M.; Petrylak, D. P.; Agarwal, N.; Gupta, S.; Fléchon, A.; Ramamurthy, C.; et al. Sacituzumab Govitecan in Combination With Pembrolizumab for Patients With Metastatic Urothelial Cancer That Progressed After Platinum-Based Chemotherapy: TROPHY-U-01 Cohort 3. J Clin Oncol 2024, 42, 1415–1425. [Google Scholar] [CrossRef]
- Paz-Ares, L. G.; Juan-Vidal, O.; Mountzios, G. S.; Felip, E.; Reinmuth, N.; Marinis, F. d.; Girard, N.; Patel, V. M.; Takahama, T.; Owen, S. P.; et al. Sacituzumab Govitecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non–Small Cell Lung Cancer: The Randomized, Open-Label Phase III EVOKE-01 Study. Journal of Clinical Oncology 0 (0), JCO.24.00733. [CrossRef]
- Okajima, D.; Yasuda, S.; Maejima, T.; Karibe, T.; Sakurai, K.; Aida, T.; Toki, T.; Yamaguchi, J.; Kitamura, M.; Kamei, R.; et al. Datopotamab Deruxtecan, a Novel TROP2-directed Antibody-drug Conjugate, Demonstrates Potent Antitumor Activity by Efficient Drug Delivery to Tumor Cells. Mol Cancer Ther 2021, 20, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Lisberg, A.; Drakaki, A.; Meric-Bernstam, F.; Alhalabi, O.; Kojima, T.; Kato, M.; Spira, A. I.; Salkeni, M. A.; Heist, R.; Gao, X.; et al. Datopotamab deruxtecan in locally advanced/metastatic urothelial cancer: Preliminary results from the phase 1 TROPION-PanTumor01 study. Journal of Clinical Oncology 2024, 42, 603–603. [Google Scholar] [CrossRef]
- Zhu, K.; Chang, Y.; Zhao, D.; Guo, A.; Cao, J.; Wu, C.; Guan, Y.; Ding, S. Expression of HER2 in high-grade urothelial carcinoma based on Chinese expert consensus and the clinical effects of disitamab vedotin-tislelizumab combination therapy in the treatment of advanced patients. Front Pharmacol 2024, 15, 1355081. [Google Scholar] [CrossRef] [PubMed]
- Aggen, D. H.; Shah, N. J.; Zheng, J.; Alam, S. M.; Balar, O.; Niederhausern, A.; Regazzi, A. M.; Ratna, N.; Funt, S. A.; Teo, M. Y.; et al. HER2 and PD-L1 immunohistochemistry (IHC) expression, and HER2 genomic alterations: Associations and clinical outcomes for advanced bladder cancer. Journal of Clinical Oncology 2024, 42, 538–538. [Google Scholar] [CrossRef]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 2016, 107, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Werner, L.; Bamias, A.; Fay, A. P.; Park, R. S.; Riester, M.; Selvarajah, S.; Barletta, J. A.; Berman, D. M.; de Muga, S.; et al. HER2 as a target in invasive urothelial carcinoma. Cancer Med 2015, 4, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Oh, D. Y.; Bang, Y. J. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol 2020, 17, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Nally, E.; Young, M.; Wells, C.; Fairhead, R.; Baines, K.; Cheney-Lowe, H.; Jackson-Spence, F.; Powles, T. Is HER2 the New NECTIN4 in Advanced Urothelial Cancer? Eur Urol Focus 2024, 10, 219–221. [Google Scholar] [CrossRef]
- Li, B. T.; Meric-Bernstam, F.; Bardia, A.; Naito, Y.; Siena, S.; Aftimos, P.; Anderson, I.; Curigliano, G.; de Miguel, M.; Kalra, M.; et al. Trastuzumab deruxtecan in patients with solid tumours harbouring specific activating HER2 mutations (DESTINY-PanTumor01): an international, phase 2 study. Lancet Oncol 2024, 25, 707–719. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D. Y.; Banerjee, S.; González-Martín, A.; Jung, K. H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J Clin Oncol 2024, 42, 47–58. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive solid tumors. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2 (accessed 2024).
- Kim, J. H.; Chang, I. H. A novel strategy for treatment of bladder cancer: Antibody-drug conjugates. Investig Clin Urol 2022, 63, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Liu, Y.; Zhou, X.; Shen, P.; Xue, R.; Zhang, M. Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Deliv 2022, 29, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Zhu, C.; Yu, P.; Wang, X.; Wang, Y.; Wang, J.; Yu, J.; Wang, K. Emerging strategy for the treatment of urothelial carcinoma: Advances in antibody-drug conjugates combination therapy. Biomed Pharmacother 2024, 171, 116152. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Sun, M.; Getz, T.; Ho, B.; Nauseef, J. T.; Tagawa, S. T. Antibody-drug conjugates for urothelial carcinoma. Urol Oncol 2023, 41, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Wang, L.; He, Z.; Shi, Y.; Luo, H.; Han, W.; Yao, X.; Shi, B.; Liu, J.; Hu, C.; et al. Efficacy and Safety of Disitamab Vedotin in Patients With Human Epidermal Growth Factor Receptor 2-Positive Locally Advanced or Metastatic Urothelial Carcinoma: A Combined Analysis of Two Phase II Clinical Trials. J Clin Oncol 2024, 42, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Zhou, L.; Yang, K.; Zhang, S.; Xu, H.; Yan, X.; Li, S.; Li, J.; Cui, C.; Chi, Z.; et al. Disitamab vedotin, a novel humanized anti-HER2 antibody-drug conjugate (ADC), combined with toripalimab in patients with locally advanced or metastatic urothelial carcinoma: An open-label phase 1b/2 study. Journal of Clinical Oncology 2023, 41, 4566–4566. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, R.; Yu, C.; Hong, Z.; Lin, L.; Li, T.; Chen, J. Disitamab vedotin in combination with immune checkpoint inhibitors for locally and locally advanced bladder urothelial carcinoma: a two-center's real-world study. Front Pharmacol 2023, 14, 1230395. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Yu, E. Y.; Iyer, G.; Campbell, M. T.; Loriot, Y.; Santis, M. D.; O'Donnell, P. H.; Burgess, E. F.; Necchi, A.; Krieger, L. E. M.; et al. Phase 2 clinical study evaluating the efficacy and safety of disitamab vedotin with or without pembrolizumab in patients with HER2-expressing urothelial carcinoma (RC48G001). Journal of Clinical Oncology 2023, 41, TPS594–TPS594. [Google Scholar] [CrossRef]
- Galsky, M.; Grande, E.; Necchi, A.; Koontz, M.; Iyer, G.; Campbell, M.; Drakaki, A.; Loriot, Y.; Sokolowski, K.; Zhang, W.; et al. Phase 3 open-label, randomized, controlled study of disitamab vedotin with pembrolizumab versus chemotherapy in patients with previously untreated locally advanced or metastatic urothelial carcinoma that expresses HER2 (DV-001). Journal of Clinical Oncology 2024, 42, TPS717–TPS717. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Y.; Chen, Z.; Zeng, X.; Hu, Z.; Yang, C. Neoadjuvant therapy with Disitamab vedotin in treating muscle-invasive bladder cancer: A case report. Heliyon 2023, 9, e15157. [Google Scholar] [CrossRef] [PubMed]
- Alameddine, R.; Mallea, P.; Shahab, F.; Zakharia, Y. Antibody Drug Conjugates in Bladder Cancer: Current Milestones and Future Perspectives. Curr Treat Options Oncol 2023, 24, 1167–1182. [Google Scholar] [CrossRef] [PubMed]
- Sarfaty, M.; Rosenberg, J. E. Antibody-Drug Conjugates in Urothelial Carcinomas. Curr Oncol Rep 2020, 22. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, A.; Tucci, M.; Audisio, A.; Di Prima, L.; Pisano, C.; Turco, F.; Delcuratolo, M. D.; Di Maio, M.; Scagliotti, G. V.; Buttigliero, C. Antibody-Drug Conjugates in Urothelial Carcinoma: A New Therapeutic Opportunity Moves from Bench to Bedside. Cells 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.; Entwistle, J.; Cizeau, J.; Niforos, D.; Loewen, S.; Chapman, W.; MacDonald, G. C. A Phase I study of an intravesically administered immunotoxin targeting EpCAM for the treatment of nonmuscle-invasive bladder cancer in BCGrefractory and BCG-intolerant patients. Drug Des Devel Ther 2010, 4, 313–320. [Google Scholar] [CrossRef] [PubMed]
- R Dickstein, N. W., B Cowan, C Dunshee, M Franks, F Wolk, L Belkoff, S Castellucci, J Holzbeierlein, G Kulkarni, A Weizer, D Lamm, S Ali, J; Epstein, G. A., H Youssoufian, W Kassouf. VISTA, PHASE 3 TRIAL OF VICINIUM, AN EPCAM-TARGETED PSEUDOMONAS EXOTOXIN, IN BCG-UNRESPONSIVE NON-MUSCLE INVASIVE BLADDER CANCER. SESEN Bio. 2018. https://sesenbio.com/wp-content/uploads/2019/01/BLADDR_Congress_2018_Posterl.pdf (accessed 2018).
- Rivera-Marquez, G.; Walter, B.; Dolan, R.; Belfield, S.; Merino, M.; Gurram, S.; … Valera, V.A. MP16-20 FINAL RESULTS OF A PHASE I, SINGLE-ARM CLINICAL TRIAL OF THE COMBINATION OF DURVALUMAB AND VICINEUM IN BCG UNRESPONSIVE, HIGH-RISK NON-MUSCLE-INVASIVE BLADDER CANCER PATIENTS (NCT03258593). Journal of Urology 2024, 211. [Google Scholar] [CrossRef]
- de Bono, J. S.; Concin, N.; Hong, D. S.; Thistlethwaite, F. C.; Machiels, J. P.; Arkenau, H. T.; Plummer, R.; Jones, R. H.; Nielsen, D.; Windfeld, K.; et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1-2 trial. Lancet Oncol 2019, 20, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Hoimes, C.J.; Flaig, T.W.; Milowsky, M.I.; Friedlander, T.W.; Bilen, M.A.; Gupta, S.; Srinivas, S.; Merchan, J.R.; McKay, R.R.; Petrylak, D.P.; Sasse, C.; Moreno, B.H.; Yu, Y.; Carret, A.S.; Rosenberg, J.E. Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2023, 41, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S. H.; Caserta, C.; Valderrama, B. P.; Gurney, H.; Ullén, A.; Loriot, Y.; Sridhar, S. S.; Sternberg, C. N.; Bellmunt, J.; et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After ≥2 Years of Follow-Up. J Clin Oncol 2023, 41, 3486–3492. [Google Scholar] [CrossRef]
- Gray, E.; Ulrich, M.; Epp, A.; Younan, P.; Sahetya, D.; Hensley, K.; Allred, S.; Huang, L. Y.; Hahn, J.; Gahnberg, K.; et al. SGN-B7H4V, an investigational vedotin ADC directed to the immune checkpoint ligand B7-H4, shows promising activity in preclinical models. J Immunother Cancer 2023, 11. [Google Scholar] [CrossRef]
- Oncology, A. o. annals of Oncology; 2022; pp. S808–S869. [Google Scholar]
- Li, C.; Zhu, J.; Yu, W. Efficacy and safety of disitamab vedotin combined with toripalimab as neoadjuvant treatment in patients with HER2 positive locally advanced muscle-invasive urothelial bladder cancer. Journal of Clinical Oncology 2024, 42, 631–631. [Google Scholar] [CrossRef]
- Bajorin, D. F.; Witjes, J. A.; Gschwend, J. E.; Schenker, M.; Valderrama, B. P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S. F.; Park, S. H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med 2021, 384, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Hoimes, C.; Bedke, J.; Loriot, Y.; Nishiyama, H.; Fang, X.; Kataria, R.; Homet, B.; Galsky, M. KEYNOTE-B15/EV-304: Randomized phase 3 study of perioperative enfortumab vedotin plus pembrolizumab versus chemotherapy in cisplatin-eligible patients with muscle-invasive bladder cancer (MIBC). Journal of Clinical Oncology 2021, 39, TPS4587–TPS4587. [Google Scholar] [CrossRef]
- Powles, T.; Meeks, J.; Galsky, M.; Van der Heijden, M.; Nishiyama, H.; Al-Ahmadie, H.; Gupta, A.; Ye, J.; Donegan, S.; Ghiorghiu, D.; et al. A phase III, randomized, open label, multicenter, global study of efficacy and safety of durvalumab in combination with gemcitabine+cisplatin (G+C) for neoadjuvant treatment followed by durvalumab alone for adjuvant treatment in muscle-invasive bladder cancer (MIBC) (NIAGARA). Journal of Clinical Oncology 2019, 37, TPS4592–TPS4592. [Google Scholar] [CrossRef]
- Powles, T.; Drakaki, A.; Teoh, J.; Grande, E.; Fontes-Sousa, M.; Porta, C.; Wu, E.; Goluboff, E.; Ho, S.; Hois, S.; et al. A phase 3, randomized, open-label, multicenter, global study of the efficacy and safety of durvalumab (D) + tremelimumab (T) + enfortumab vedotin (EV) or D + EV for neoadjuvant treatment in cisplatin-ineligible muscle-invasive bladder cancer (MIBC) (VOLGA). Journal of Clinical Oncology 2022, 40, TPS579–TPS579. [Google Scholar] [CrossRef]
- Galsky, M. D.; Hoimes, C. J.; Necchi, A.; Shore, N.; Witjes, J. A.; Steinberg, G.; Bedke, J.; Nishiyama, H.; Fang, X.; Kataria, R.; et al. Perioperative pembrolizumab therapy in muscle-invasive bladder cancer: Phase III KEYNOTE-866 and KEYNOTE-905/EV-303. Future Oncol 2021, 17, 3137–3150. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J. E.; Sonpavde, G. P.; Loriot, Y.; Durán, I.; Lee, J. L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021, 384, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S. T.; Balar, A. V.; Petrylak, D. P.; Kalebasty, A. R.; Loriot, Y.; Fléchon, A.; Jain, R. K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J Clin Oncol 2021, 39, 2474–2485. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Shin, S.J.; Kim, H.C.; Kim, Y.S.; Lee, H.J.; Keam, B.; Choi, Y.J.; Kim, Y.J.; Park, I.; Park, S.H.; Lee, J.L. Enfortumab vedotin-related pneumonitis is more common than expected and could lead to acute respiratory failure. Eur J Cancer. 2022, 174, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Mathew Thomas, V.; Jo, Y.; Tripathi, N.; Roy, S.; Chigarira, B.; Narang, A.; Gebrael, G.; Hage Chehade, C.; Sayegh, N.; Galarza Fortuna, G.; et al. Treatment Patterns and Attrition With Lines of Therapy for Advanced Urothelial Carcinoma in the US. JAMA Netw Open 2024, 7, e249417. [Google Scholar] [CrossRef]
- Brown, J. R.; Koshkin, V. S. Therapies After Progression on Enfortumab Vedotin and Pembrolizumab: Navigating Second-line Options for Metastatic Urothelial Carcinoma in the New Treatment Landscape. Eur Urol Focus 2024, 10, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Nizam, A.; Jindal, T.; Jiang, C.; Alhalabi, O.; Bakaloudi, D.; Talukder, R.; Davidsohn, M.; Nguyen, C.; Oh, E.; Taylor, A.; et al. Outcomes in patients (pts) with advanced urothelial carcinoma (aUC) treated with enfortumab vedotin (EV) after switch maintenance avelumab (MAv) in the UNITE study. Journal of Clinical Oncology 2024, 42, 537–537. [Google Scholar] [CrossRef]
- Jiang, C.; Hwang, H.; Jindal, T.; Qiao, W.; Epstein, I.; Nguyen, C.; Gupta, S.; Shah, S.; Bilen, M.; Milowsky, M.; et al. Sequencing of erdafitinib (erda) and enfortumab vedotin (EV) in patients (pts) with fibroblast growth factor receptor (FGFR2/3) altered (alt) advanced urothelial cancer (aUC): Analysis of UNITE database. Journal of Clinical Oncology 2024, 42, 616–616. [Google Scholar] [CrossRef]
- Jindal, T.; Jiang, C. Y.; Alhalabi, O.; Bakaloudi, D. R.; Talukder, R.; Davidsohn, M. P.; Nizam, A.; Taylor, A. K.; Nguyen, C. B.; Kilari, D.; et al. Biomarkers of response to sacituzumab govitecan (SG) and efficacy after treatment with enfortumab vedotin (EV) in advanced urothelial carcinoma (aUC): Analysis of the UNITE study. Journal of Clinical Oncology 2023. [Google Scholar] [CrossRef]
- Vlachou, E.; Hahn, N.; Dabb, A.; Johnson, B.; Lefande, M.; Hoffman-Censits, J. Evaluation of clinical benefit of sacituzumab govitecan (SG) following enfortumab vedotin (EV) in advanced urothelial cancer (UC): Real-world experience. Journal of Clinical Oncology 2023, 41, 523–523. [Google Scholar] [CrossRef]
- Bellmunt, J.; Théodore, C.; Demkov, T.; Komyakov, B.; Sengelov, L.; Daugaard, G.; Caty, A.; Carles, J.; Jagiello-Gruszfeld, A.; Karyakin, O.; et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009, 27, 4454–4461. [Google Scholar] [CrossRef] [PubMed]
- Sternschuss, M.; Toumbacaris, N.; Das, J. P.; Powles, T.; Bajorin, D. F.; Kotecha, R. R.; Laccetti, A. L.; Xiao, H.; Feld, E.; McHugh, D. J.; et al. Clinical outcomes of sacituzumab govitecan (SG) after prior exposure to enfortumab vedotin (EV) in patients with metastatic urothelial carcinoma (mUC). Journal of Clinical Oncology 2024, 42, 4581–4581. [Google Scholar] [CrossRef]
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B. P.; Ravaud, A.; Shariat, S. F.; et al. ESMO Clinical Practice Guideline interim update on first-line therapy in advanced urothelial carcinoma. Ann Oncol 2024, 35, 485–490. [Google Scholar] [CrossRef]
- Vassiliou, V.; Katsila, T.; Sodergren, S. C.; Kardamakis, D. Radiotherapy in Metastatic Urothelial Carcinoma: Rationale and Clinical Applications. Anticancer Res 2022, 42, 3767–3778. [Google Scholar] [CrossRef]
Figure 1.
Mechanisms of ADCs action, including the Bystander effect.
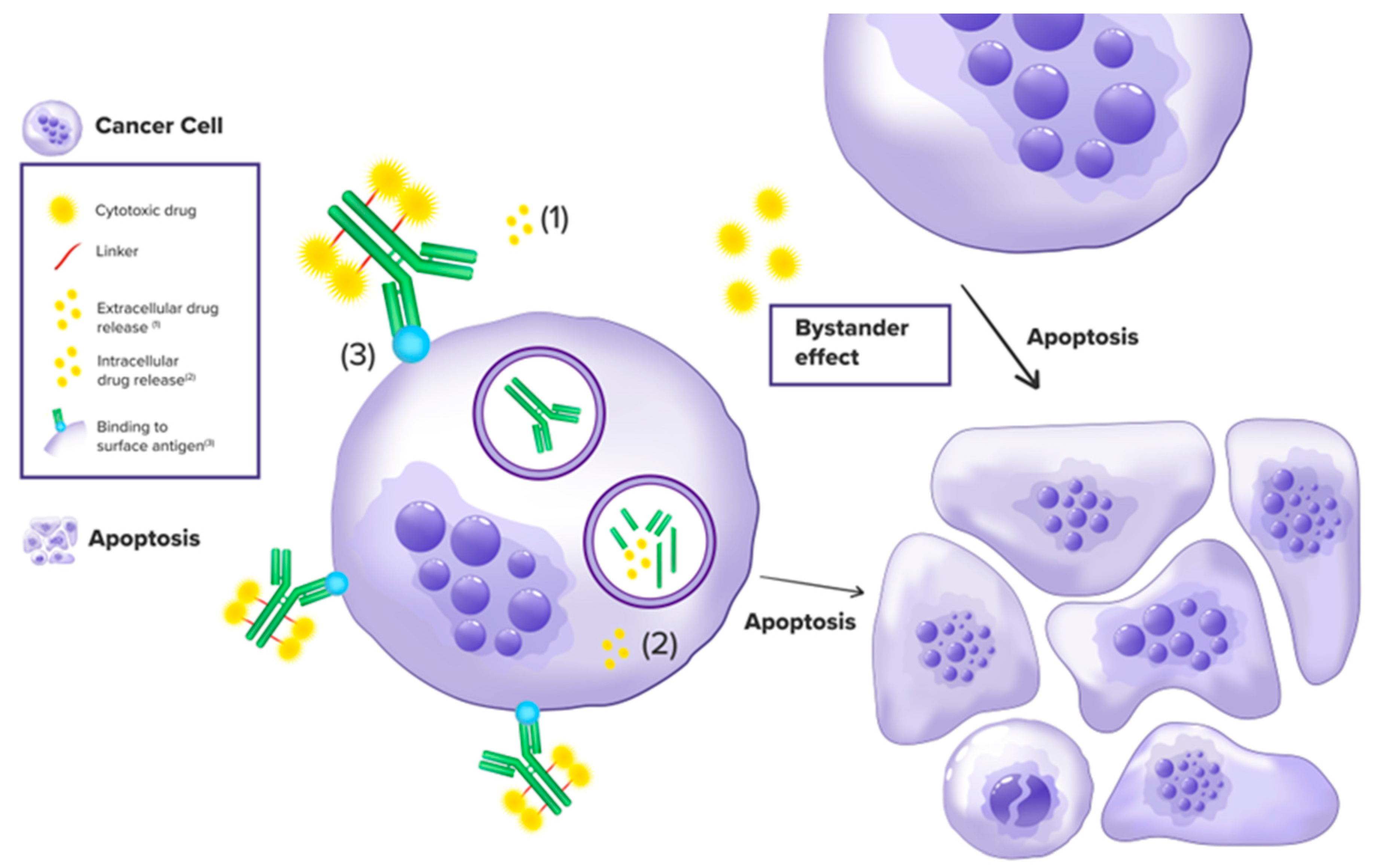
Table 1.
Approved ADCs for UC. Approvals are updated until July 2024.
| ADC UC | Year Approved | Target Receptor | Cleavable Linker | Payload | Payload Action |
|---|---|---|---|---|---|
| Enfortumab vedotin | 2019 FDA, 2022 EMA |
Nectin-4 | Enzyme | MMAE | Microtubule inhibitor |
| Sacituzumab govitecan | 2021 FDA, 2021 EMA |
Trop2 | Acid | SN-38 | Topoisomerase-DNA complex Inhibitor |
| Disitamab vedotin | 2020 FDA | HER2 | Protease | MMAE | Microtubule inhibitor |
| Trastuzumab deruxtecan | 2024 FDA | HER2 | Enzyme | Deruxtecan | Topoisomerase-DNA complex Inhibitor |
| Tisotumab vedotin | 2024 FDA | Tissue Factor (TF) | Protease | MMAE | Microtubule inhibitor |
Table 2.
Selected trials of ADCs in urothelial cancer.
| Trial, NCT number | Phase | Regimen | Study Population | Primary and/or Co-Primary End Points |
|---|---|---|---|---|
| EV-301, NCT03474107 |
III | EV | Evaluate Enfortumab Vedotin vs Chemotherapy in Subjects With Previously Treated Locally Advanced or Metastatic Urothelial Cancer | OS 12.88 months (m) (10.58 to 15.21) PSF1 5.55 m (5.32 to 5.82) ORR 40.6% (34.90 to 46.54) DCR 71.9% (66.30 to 76.99) DoR 7.39 m (5.59 to 9.46) |
| EV-201, Cohort 2, NCT03219333 | II | EV | Locally Advanced or Metastatic Urothelial Cancer Who Previously Received Immune Checkpoint Inhibitor (CPI) Therapy | ORR 51.7% (39.8 to 61.3) DoR 10.9 m (5.78 to NA) PFS 5.8 m (5.03 to 8.28) |
| EV-103, cohort H, NCT03288545 | I/II | EV | Neoadjuvant in cisplatin-ineligible MIBC naive to systemic therapy | pCR 36.4% (95% CI, 17.2, 59.3) |
| EV-103, cohort L, NCT03288545 | I/II | EV | Perioperative, previously untreated, cis-ineligible MIBC | pCR 34% (95%CI, 21.2, 48.8) |
| EV-103, Cohort A, NCT03288545 | I/II | EV + P | First line in platinum ineligible LA/mUC refractory to prior therapies. | ORR 73.3% (95% CI, 58.1-85.4) DCR 84.4% (95% CI: 70.5, 93.5) PFS 12.7 m (95% CI: 6.11, -) OS 26.1 m (95% CI: 15.51, -) |
| EV-103, Cohort B, NCT03288545 |
I/II | EV + P | Second line in LA/mUC refractory to platinum therapies. | ORR |
| EV-103, Cohort D, NCT03288545 |
I/II | EV + Cis | First line in platinum-eligible LA/mUC. | ORR |
| EV-103, Cohort E, NCT03288545 |
I/II | EV + Carbo | First line in cisplatin-ineligible, carboplatin-eligible LA/mUC. | ORR |
| EV-103, Cohort F, NCT03288545 |
I/II | EV + Gem | First and second line in platinum ineligible LA/mUC refractory to prior therapies | ORR |
| EV-103,CohortG, NCT03288545 | I/II | EV + Plt + P | First line in platinum-eligible LA/mUC | ORR |
| EV-103, Cohort J, NCT03288545 |
I/II | EV + P | Neoadjuvant in cisplatin-ineligible MIBC naive to systemic therapy | ORR |
| EV-302, NCT04223856 |
III | EV + P vs. Gem + Plat |
La/mUC and no prior systemic therapy for advanced disease who are ineligible for cisplatin-containing chemotherapy |
mOS 31.5m (95% CI, 25.4-not reached [NR]) |
| 516–003, Cohort 9, NCT03606174 | I/II | EV + P + Si | LA/mUC refractory to platinum and ICI therapies | ORR |
| KEYNOTE-905/EV-303, NCT03924895 |
III | EV + P vs. P vs. Cystectomy |
Neoadjuvant therapy or only surgery in cisplatin-ineligible MIBC; Arm A, neoadjuvant P followed by RC + PLND and adjuvant P, Arm B, RC + PLND followed by observation, Arm C, neoadjuvant EV + P followed by RC + PLND and adjuvant EV + P and adjuvant P. |
pCR |
| Keynote-B15/ EV-304, NCT04700124 | III | EV + P vs. CTX |
Neoadjuvant in cisplatin-eligible MIBC; Arm A, neoadjuvant EV + P followed by adjuvant EV + adjuvant P after RC + PLND Arm B, neoadjuvant Gem + Cis followed by observation after RC + PLND |
pCR, PFS, OS |
| VOLGA, NCT04960709 | III | EV + Du + Tr vs. EV + Du |
Neoadjuvant in cisplatin-ineligible systemic therapy-naive MIBC; Arm A, Du + T + EV, Arm B, Du + EV, Arm C, no neoadjuvant treatment (SoC) |
pCR, EFS |
| NCT04878029 | Ib | EV + Caboz | Cabozantinib in Combination With Enfortumab Vedotin (EV) in the Treatment of Locally Advanced or Metastatic Urothelial Cancer | recommended phase II dose (RP2D), ORR,PFS, OS |
| NCT05775471 | II | EV + P | Pembrolizumab and Enfortumab Vedotin with Pembrolizumab Prior to and After Radical Nephroureterectomy for High-Risk Upper Tract Urothelial Cancer. |
ORR, recurrence-free survival |
| NCT04963153 | I | EV + Erdb | Phase Ib Trial of Erdafitinib Combined With Enfortumab Vedotin Following Platinum and PD1/L1 Inhibitors for Metastatic Urothelial Carcinoma With FGFR2/3 Genetic Alterations | ORR, DoR, PFS, OS |
| SKB264, NCT04152499 | II | SKB264 ADC targeting TROP2 | La/Unresectable/Metastatic Solid Tumors who are refractory to Available Standard Therapies including Urothelial carcinoma. | ORR |
| SURE-01, NCT05226117 | II | SG | Neoadjuvant in cisplatin-ineligible MIBC naive to systemic therapy | pCR 36.4% (95% CI, 14.9%-64.8%) |
| NCT05581589 | II | Adj SG | Sacituzumab Govitecan as Neoadjuvant Therapy in Pts With Non-Urothelial Muscle Invasive Bladder Cancer |
pCR, RFS, OS |
| TROPiCS-4 / Immu132–13, NCT04527991 | III | SG vs CTX | LA/mUC refractory to platinum and anti-PD-1/PD-L1 Therapies | OS not met |
| Trophy-U-01, Cohort 4, NCT03547973 | II | SG + Cis + AVLM | Platinum naive and cisplatin-eligible LA/mUC with responders receiving Avelumab maintenance | ORR, PFS |
| Trophy-U-01, Cohort 4, NCT03547973 | II | SG + Cis + Zi | Platinum naive and cisplatin-eligible LA/mUC with responders receiving Avelumab maintenance | ORR, PFS |
| Trophy-U-01, Cohort 5, NCT03547973 | II | SG Zi or AVLM or Zi | LA/mUC maintenance therapy following Gem-Cis | ORR, PFS |
| Trophy-U-01, Cohort 6, NCT03547973 | II | SG or SG + Zi or SG + Zi + Do or GC | Cisplatin-ineligible, treatment-naive LA/mUC | ORR, PFS |
| Trophy-U-01, Cohort 3, NCT03547973 | II | SG + P | Sacituzumab Govitecan in Unresectable Locally Advanced/Metastatic Urothelial Cancer | ORR 41% (95% CI, 26.3 to 57.9; 20% CR rate), CBR 46% (95% CI, 30.7 to 62.6), mDOR 11.1 m (95% CI, 4.8 to not NE), mPFS 5.3 m (95% CI, 3.4 to 10.2) mOS 12.7 m (10.7-NE) |
| MORPHEUS-UC, NCT03869190 |
IB/II | SG + At | PD1-expressing LA/mUC refractory to platinum therapy | ORR, pCRR criteria not met |
| JAVELIN Bladder Medley, NCT05327530 |
II | AVLM + SoC vs SG + AVLM |
Maintenance Treatment in Participants with Locally Advanced or Metastatic Urothelial Carcinoma Whose Disease Did Not Progress with First Line Platinum-Containing Chemotherapy (JAVELIN Bladder Medley) Group B: Avelumab + Sacituzumab Govitecan |
PFS, OS, OR, DoR |
| DestinyPanTumor02, Cohort 2 NCT04482309 | II | T-DXd | HER2-positive solid tumor including bladder cancer | ORR 39% (24.2 to 55.5) mDoR 8.7m (4.3 to 11.8) PFS 7.0m (4.2 to 9.7) mOS 12.8m (11.2 to 15.1) |
| DestinyPanTumor01, NCT04639219 | II | T-DXd | Trastuzumab deruxtecan in patients with solid tumours harbouring specific activating HER2 mutations | ORR 29.4% (95% CI 20·8–39·3; 30 of 102 patients) |
| Keynote D78/RC48G001, NCT04879329, Cohort A, Cohort B. |
II b | DV | HER2-positive, platinum-refractory LA/mUC. HER2 low expressing, platinum refractory LA/mUC |
ORR |
| KeynoteD78/RC48G001 NCT04879329, Cohort C. |
IIb | DV vs. DV + P, |
HER2-positive, platinum eligible, treatment-naive LA/mUC | ORR |
| RC48-C016, NCT05302284 |
III | DV vs. Gem + Plat |
HER2-positive platinum-eligible treatment-naive LA/mUC; Arm A, DV, until PD, unacceptable toxicity, or voluntary withdrawal, Arm B, Gem + Cis or Carbo until loss of clinical benefit, unacceptable toxicity, investigator or participant decision to withdraw from therapy, or death. |
PFS, OS |
| NCT06210490 | II | DV + Rt vs SoC |
Efficacy and Safety of Disitamab Vedotin in Combination With Radiotherapy for the Adjuvant Treatment of HER2 Overexpressing UTUC Patients With High Risk Factors for Recurrence After Radical Surgery | DFS, OS, MFS, Local-Recurrence Free (LRF) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Downloads
223
Views
83
Comments
0
Subscription
Notify me about updates to this article or when a peer-reviewed version is published.
MDPI Initiatives
Important Links
© 2025 MDPI (Basel, Switzerland) unless otherwise stated





