Submitted:
01 August 2024
Posted:
01 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
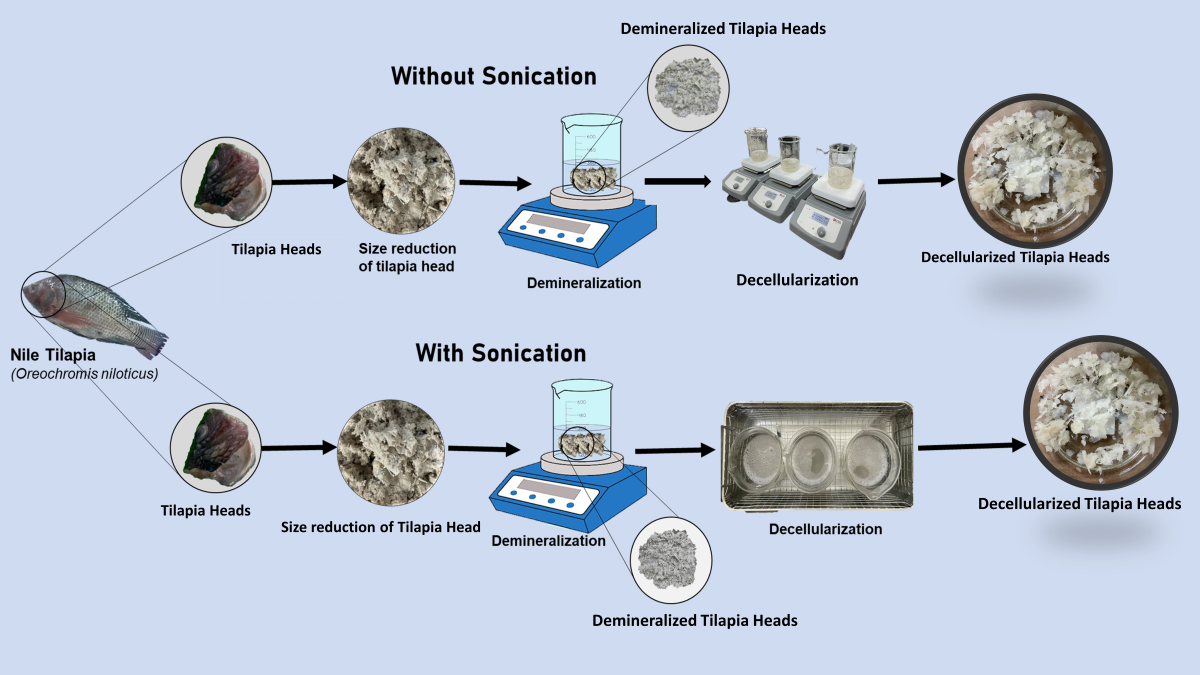
2.1. Sample Preparation and Demineralization
2.2. Decellularization Process
2.3. Evaluation of the dECM
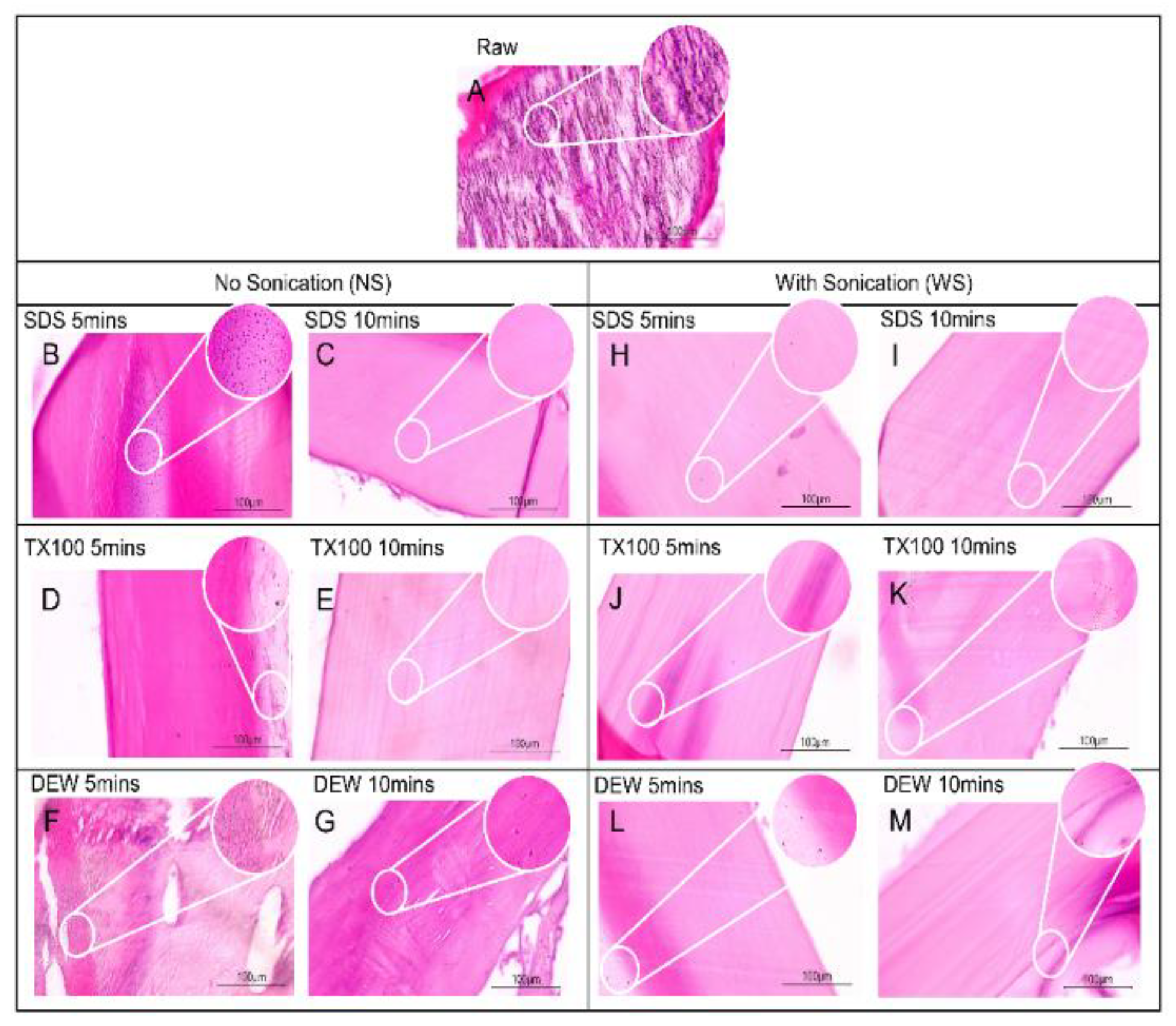
2.3.1. Hematoxylin and Eosin (H&E) Staining
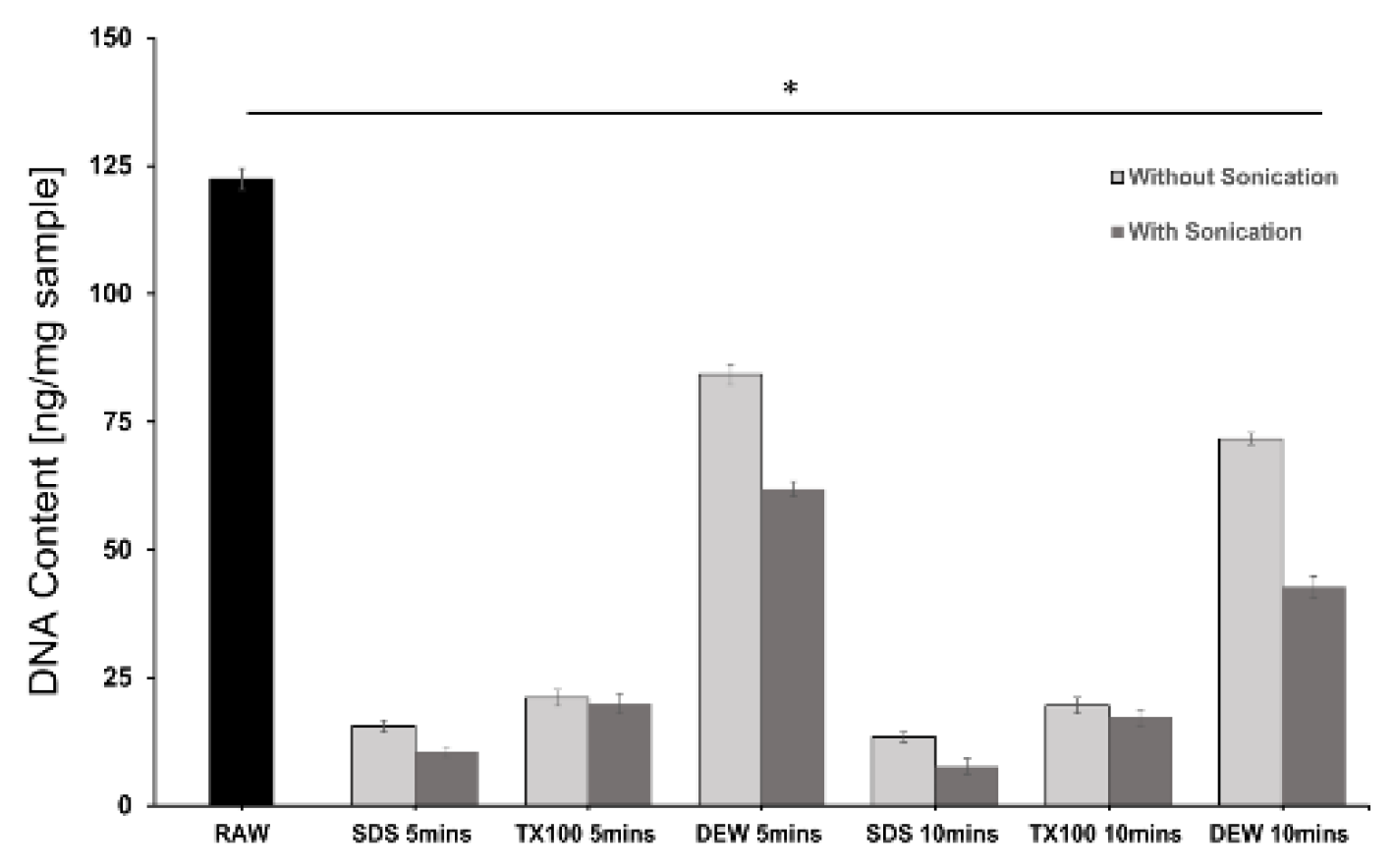
2.3.2. DNA Quantification
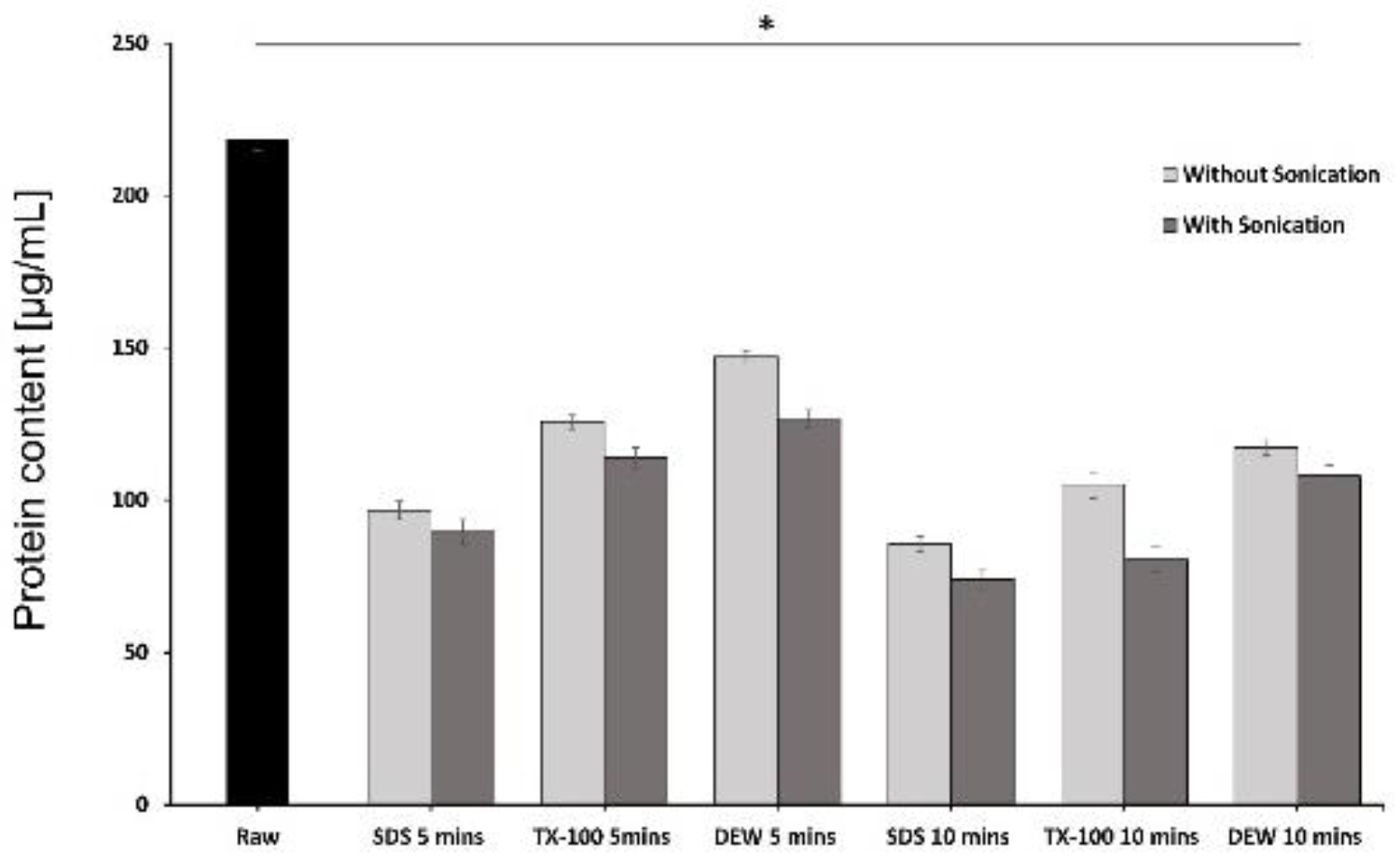
2.3.3. Protein Quantification
2.3.4. Attenuated Total Reflectance- Fourier Transform Infrared Spectroscopy (ATR-FTIR) Analysis
2.3.5. Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.3.6. Differential Scanning Calorimetry (DSC)
2.3.7. Residual Detergent Determination
2.3.8. Statistical Analysis
3. Results
3.1. Hematoxylin and Eosin (H&E) Staining
3.2. DNA Quantification
3.3. Protein Quantification
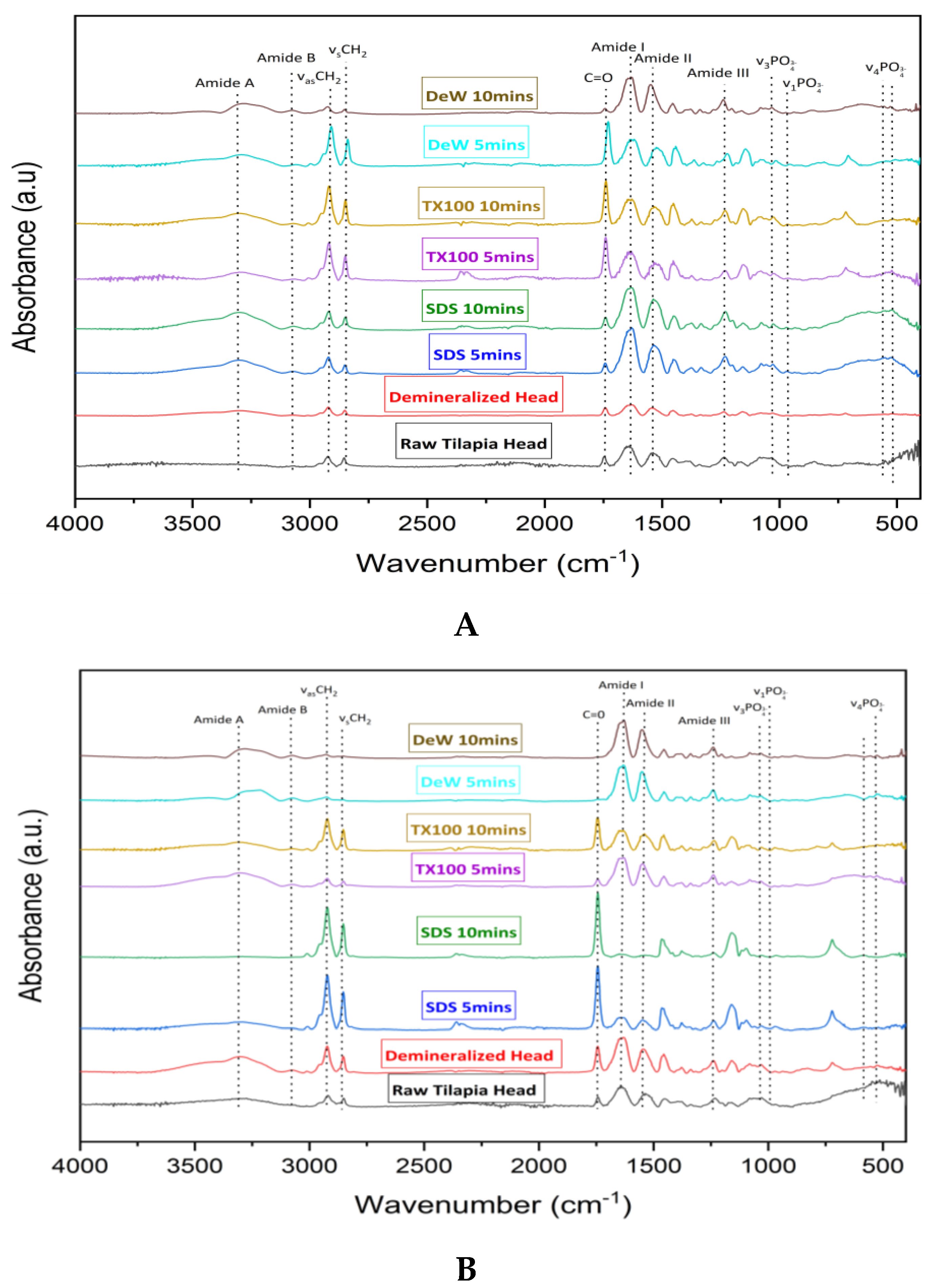
3.4. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR) Analysis
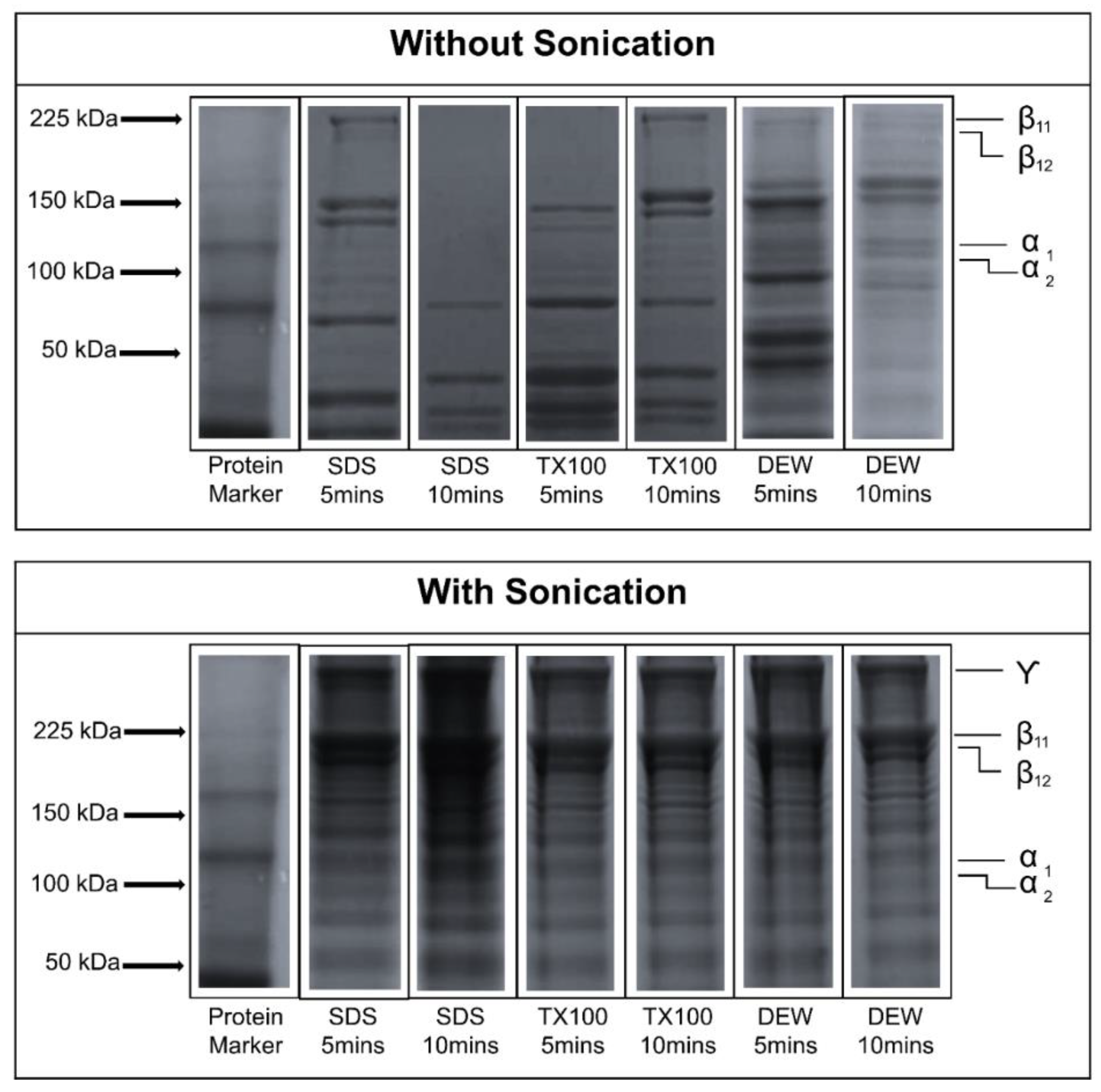
3.5. Sodium Dodecyl Sulfate-Polycrylamide Gel Electrophoresis (SDS-PAGE)
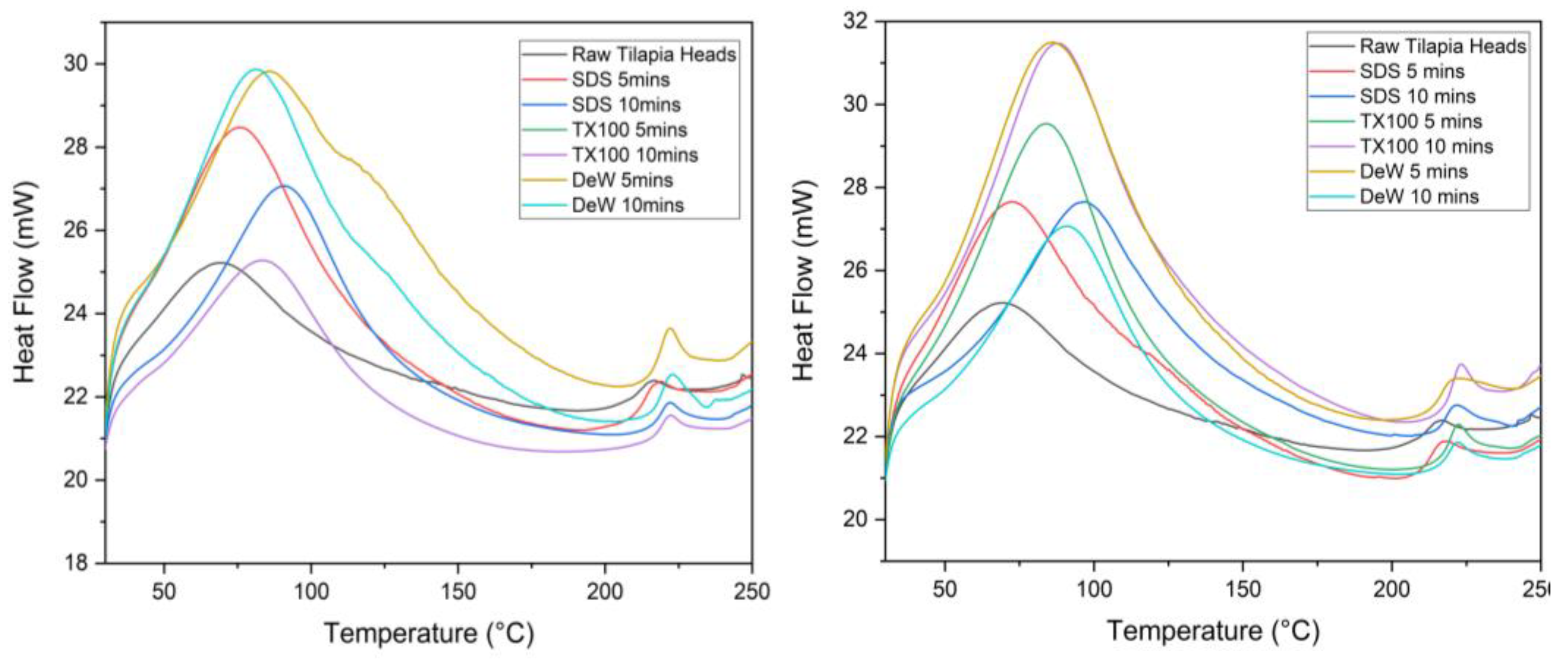
3.5. Differential Scanning Calorimetry (DSC)
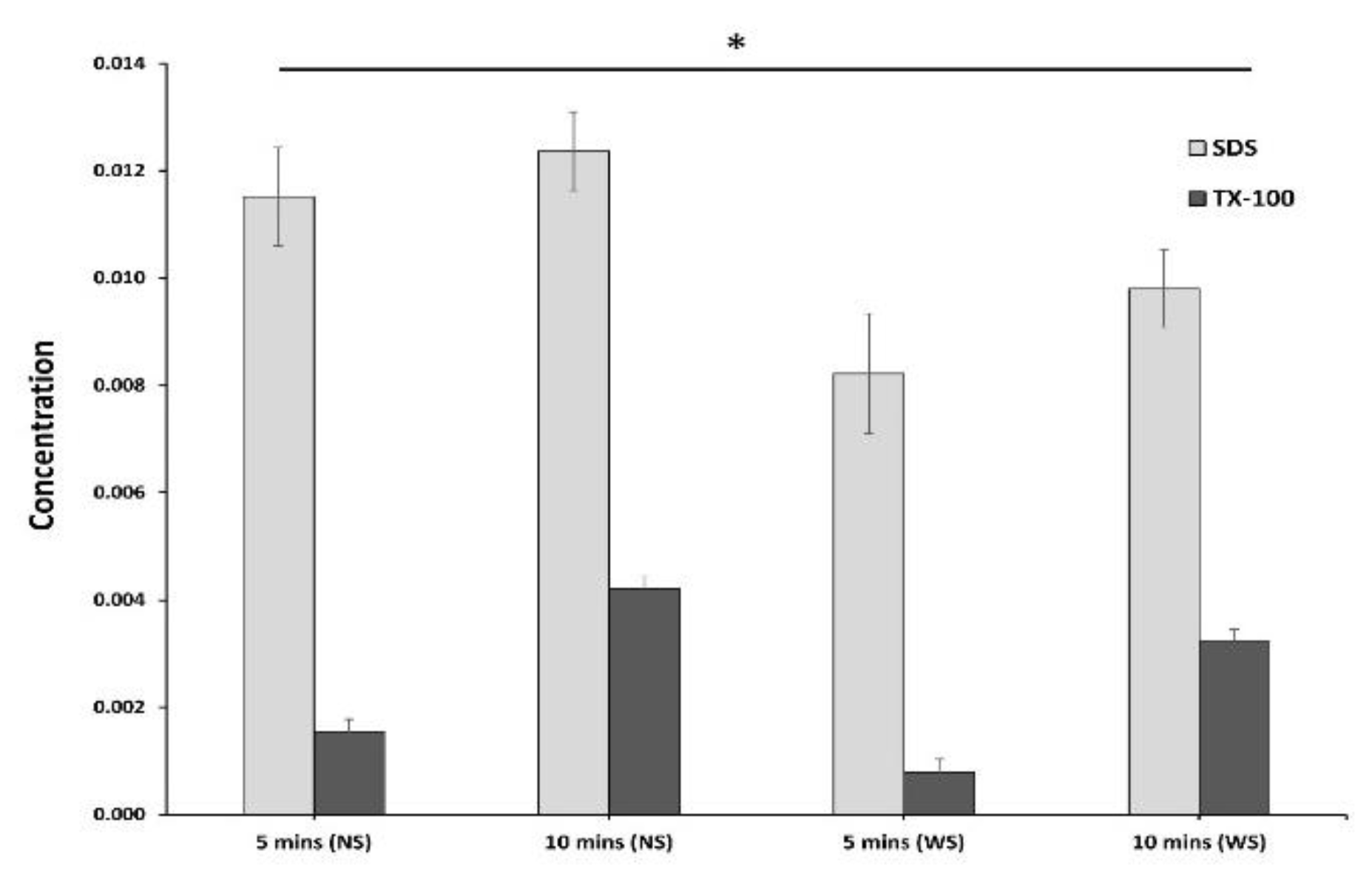
3.7. Residual Detergent Determination
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- P. Gao, L. Li, W. Xia, Y. Xu, and S. Liu, “LWT - Food Science and Technology Valorization of Nile tilapia ( Oreochromis niloticus ) fish head for a novel fish sauce by fermentation with selected lactic acid bacteria,” LWT - Food Sci. Technol., vol. 129, no. April, p. 109539, 2020. [CrossRef]
- D. Coppola, C. Lauritano, F. P. Esposito, G. Riccio, C. Rizzo, and D. de Pascale, “Fish Waste: From Problem to Valuable Resource,” Marine Drugs, vol. 19, no. 2. MDPI, Feb. 01, 2021. [CrossRef]
- Y. Sun, L. Yan, S. Chen, and M. Pei, “Functionality of decellularized matrix in cartilage regeneration: A comparison of tissue versus cell sources,” Acta Biomaterialia, vol. 74. Acta Materialia Inc, pp. 56–73, Jul. 01, 2018. [CrossRef]
- C. Frantz, K. M. Stewart, V. M. Weaver, C. Frantz, K. M. Stewart, and V. M. Weaver, “The extracellular matrix at a glance The Extracellular Matrix at a Glance,” vol. 2010, pp. 4195–4200, 2010. [CrossRef]
- G. Agmon and K. L. Christman, “Controlling stem cell behavior with decellularized extracellular matrix scaffolds,” Curr. Opin. Solid State Mater. Sci., vol. 20, no. 4, pp. 193–201, 2016. [CrossRef]
- E. V. Isaeva, E. E. Beketov, N. V. Arguchinskaya, S. A. Ivanov, P. V. Shegay, and A. D. Kaprin, “Decellularized Extracellular Matrix for Tissue Engineering (Review),” Sovrem. Tehnol. v Med., vol. 14, no. 3, pp. 57–69, 2022. [CrossRef]
- S. F. Badylak, D. O. Freytes, and T. W. Gilbert, “Extracellular matrix as a biological scaffold material: Structure and function,” Acta Biomater., vol. 5, no. 1, pp. 1–13, 2009. [CrossRef]
- M. J. Nisperos et al., “Time-Dependent Demineralization of Tilapia ( Oreochromis niloticus ) Bones Using Hydrochloric Acid for Extracellular Matrix Extraction,” 2023.
- S. Pang, F. Y. Su, A. Green, J. Salim, J. McKittrick, and I. Jasiuk, “Comparison of different protocols for demineralization of cortical bone,” Sci. Rep., vol. 11, no. 1, pp. 1–10, 2021. [CrossRef]
- S. K. Gupta and N. C. Mishra, “Decellularization Methods for Scaffold Fabrication,” 2017. [CrossRef]
- Y. Li, Q. Wu, L. Li, F. Chen, J. Bao, and W. Li, “Decellularization of porcine whole lung to obtain a clinical-scale bioengineered scaffold,” J. Biomed. Mater. Res. - Part A, vol. 109, no. 9, pp. 1623–1632, 2021. [CrossRef]
- J. L. Balestrini et al., “Production of decellularized porcine lung scaffolds for use in tissue engineering,” Integr. Biol. (United Kingdom), vol. 7, no. 12, pp. 1598–1610, 2015. [CrossRef]
- S. Alaee, R. Asadollahpour, A. Hosseinzadeh Colagar, and T. Talaei-Khozani, “The decellularized ovary as a potential scaffold for maturation of preantral ovarian follicles of prepubertal mice,” Syst. Biol. Reprod. Med., vol. 67, no. 6, pp. 413–427, 2021. [CrossRef]
- T. Prebeg, D. Omerčić, V. Erceg, and G. Matijašić, “Comparison of Sodium Lauryl Sulfate and Sodium Lauryl Ether Sulfate Detergents for Decellularization of Porcine Liver for Tissue Engineering Applications,” Chem. Eng. Trans., vol. 100, no. April, pp. 745–750, 2023. [CrossRef]
- C. A. M. Bondi, J. L. Marks, L. B. Wroblewski, H. S. Raatikainen, S. R. Lenox, and K. E. Gebhardt, “Human and Environmental Toxicity of Sodium Lauryl Sulfate ( SLS ): Evidence for Safe Use in Household Cleaning Products,” pp. 27–32, 2015. [CrossRef]
- T. Wang et al., “This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please c,” Laryngoscope, vol. 44, no. 0, pp. 2–31, 2016.
- T. W. Gilbert, T. L. Sellaro, and S. F. Badylak, “Decellularization of tissues and organs,” vol. 27, pp. 3675–3683, 2006. [CrossRef]
- M. A. Keshvari et al., “Decellularization of kidney tissue: comparison of sodium lauryl ether sulfate and sodium dodecyl sulfate for allotransplantation in rat,” Cell Tissue Res., vol. 386, no. 2, pp. 365–378, 2021. [CrossRef]
- Yaghoubi, N. Azarpira, S. Karbalay-Doust, S. Daneshi, Z. Vojdani, and T. Talaei-Khozani, “Prednisolone and mesenchymal stem cell preloading protect liver cell migration and mitigate extracellular matrix modification in transplanted decellularized rat liver,” Stem Cell Res. Ther., vol. 13, no. 1, pp. 1–19, 2022. [CrossRef]
- Hassanpour, T. Talaei-khozani, E. Kargar-abarghouei, and V. Razban, “Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate ( SLES ) -treated protocol , as a natural three- dimensional scaffold for construction of bioengineered ovaries,” pp. 1–13, 2018.
- M. F. C. Miranda et al., “Effects of chemical and physical methods on decellularization of murine skeletal muscles,” An. Acad. Bras. Cienc., vol. 93, no. 2, pp. 1–11, 2021. [CrossRef]
- K. Sawada, D. Terada, T. Yamaoka, S. Kitamura, and T. Fujisato, “Cell removal with supercritical carbon dioxide for acellular artificial tissue,” J. Chem. Technol. Biotechnol., vol. 83, no. 6, pp. 943–949, Jun. 2008. [CrossRef]
- Lin et al., “Sonication-Assisted Method for Decellularization of Human Umbilical Artery for Small-Caliber Vascular Tissue Engineering,” 2021.
- Azhim, N. Syazwani, Y. Morimoto, K. S. Furukawa, and T. Ushida, “The use of sonication treatment to decellularize aortic tissues for preparation of bioscaffolds,” vol. 29, no. 1, pp. 130–141, 2015. [CrossRef]
- W. Shen, K. Berning, S. W. Tang, and Y. W. Lam, “Rapid and detergent-free decellularization of cartilage,” Tissue Eng. - Part C Methods, vol. 26, no. 4, pp. 201–206, Apr. 2020. [CrossRef]
- Azhim et al., “The use of sonication treatment to completely decellularize aorta tissue,” IFMBE Proc., vol. 39 IFMBE, pp. 1987–1990, 2013. [CrossRef]
- M. Kawecki et al., “A review of decellurization methods caused by an urgent need for quality control of cell-free extracellular matrix’ scaffolds and their role in regenerative medicine,” J. Biomed. Mater. Res. - Part B Appl. Biomater., vol. 106, no. 2, pp. 909–923, 2018. [CrossRef]
- T. W. Gilbert, T. L. Sellaro, and S. F. Badylak, “Decellularization of tissues and organs,” Biomaterials, vol. 27, no. 19. pp. 3675–3683, Jul. 2006. [CrossRef]
- C. Oliveira et al., “Evaluation of Small Intestine Grafts Decellularization Methods for Corneal Tissue Engineering,” PLoS One, vol. 8, no. 6, Jun. 2013. [CrossRef]
- H. Ijima, S. Nakamura, R. Bual, N. Shirakigawa, and S. Tanoue, “Physical Properties of the Extracellular Matrix of Decellularized Porcine Liver,” pp. 1–15, 2018. [CrossRef]
- M. S. Immunoassay et al., “Omics Technologies Applied to Agriculture and Food Species Differentiation and Quantification of Processed Animal Proteins and Blood Products in Fish Feed using Article Species Differentiation and Quantification of Processed Animal Proteins and Blood Prod,” 2018. [CrossRef]
- P. Barajan, S. Sujithra, N. Kiruthiga, M. J. Prabhu, and R. Kumeresan, “Isolation and Determination of Type I Collagen from Tilapia (Oreochromis niloticus) Waste”.
- laemmli, U. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 227, 680–685 (1970). [CrossRef]
- B. Pavlović, N. Cvijetić, L. Dragačević, B. Ivković, Z. Vujić, and V. Kuntić, “Direct UV Spectrophotometry and HPLC determination of triton X-100 in split virus influenza vaccine,” J. AOAC Int., vol. 99, no. 2, pp. 396–400, 2016. [CrossRef]
- M. Alizadeh, L. Rezakhani, M. Soleimannejad, E. Sharifi, M. Anjomshoa, and A. Alizadeh, “Evaluation of vacuum washing in the removal of SDS from decellularized bovine pericardium: method and device description,” Heliyon, vol. 5, no. 8, 2019. [CrossRef]
- R. Bual et al., “Characterization of Decellularized Extracellular Matrix from Milkfish (Chanos chanos) Skin,” Biomimetics, vol. 7, no. 4, pp. 1–13, 2022. [CrossRef]
- S. Arvanitoyannis and A. Kassaveti, “Fish industry waste: Treatments, environmental impacts, current and potential uses,” Int. J. Food Sci. Technol., vol. 43, no. 4, pp. 726–745, Apr. 2008. [CrossRef]
- Sockalingam and H. Z. Abdullah, “Extraction of high value added gelatin biopolymer from black tilapia (Oreochromis mossambicus) head bones,” in AIP Conference Proceedings, Jul. 2015, vol. 1669. [CrossRef]
- Suslick, K.S. (2000). Sonochemistry. In Kirk-Othmer Encyclopedia of Chemical Technology, (Ed.). [CrossRef]
- Gilpin and Y. Yang, “Decellularization Strategies for Regenerative Medicine : From Processing Techniques to Applications,” vol. 2017, 2017.
| Decellularizing Agent | Treatment | Contact time |
|---|---|---|
| 1% SDS, 1% TX-100, Deionized water (DEW) |
Sonication assisted (WS), Without sonication (NS) | 5 mins, 10 mins |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





