1. Introduction
Next generation sequencing technologies with higher sensitivity combined with appropriate bioinformatics analysis and artificial intelligence algorithms have significantly improved our understanding of the mutational landscape of human cancers. The precise application of targeted therapies has become an increasing possibility over the last two decades as comprehensive genomic profiling (CGP) assays unravel an increasing number of mutations in targetable pathways. This ensures the important role of tumor genetic testing for the selection of effective therapeutics (Waarts et al. 2022).
Assays on next-generation sequencing (NGS) technology when developed with scientific precision can highlight intricate genetic portraits of the patient’s tumor. The comprehensive genetic profile facilitates the discernment of clinically significant genetic anomalies that may be harnessed as potential therapeutic targets. CGP assays can be applied to both tissue and liquid biopsy samples. A CGP thus enables parallel detection of a wide array of genetic alterations, encompassing insertions, deletions, fusions, amplifications, rearrangements, and gene mutations.
CGP is an emerging need in the current cancer genomics landscape (Hiemenz et al. 2022). Unlike tumor-specific targeted gene panels, CGP covers key genes in totality i.e., full exons and certain crucial intronic regions that become essential to assist in disease treatment, management, and monitoring beyond the established and emerging guidelines (Shafi et al. 2023). For advanced and complex tumors, CGP-based tests can render multiple actionable mutations for targeted therapeutics (Mathew et al. 2022). In addition, the cell free or circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and single cell genomic analysis are increasing making precision oncology the most promising tool for personalized medicine (Bharde et al. 2023). Several such actionable assays are approved for clinical practice with multiple gene panels suggesting CGP (Karol et al. 2021; Takeda et al. 2021).
The ever-evolving landscape of tumor biology, drug development, and immunotherapy is certainly driving towards swift embrace of precision oncology (Bharde et al. 2024; Krzyszczyk et al. 2018).
NGS test validation is crucial and complex, involving multiple stages such as preclinical, clinical trials, and regulatory approvals. Adequate sample selection is vital for robust validation, as it directly impacts clinical performance and the test’s purpose. Regulatory approval is essential to ensure reliability and compliance. Meeting predefined performance standards is key. Comprehensive validation, from sample preparation to reporting, ensures high-quality outcomes (Fabrizio et al. 2021). NGS test validation also needs assessment at technical and computational aspects as the amount of data output is huge. MSI as a biomarker includes over hundreds and thousands of data points, the scoring of which requires the NGS tool to study the allelic makeup at all the considered MSI sites later averaged as a single score. A threshold is based on validation samples including patients are then used to stratify into responders and non-responders (Jennings et al. 2017). In addition to predicting immunotherapy response, high-frequency MSI (MSI-H) is also recognized as a potential marker for identifying and categorizing germline mutations in certain DNA mismatch repair (MMR) genes associated with Lynch syndrome. Samples used for validation play a crucial role in establishing the detection sensitivity of the algorithm, consecutively upending necessary standards for reporting on clinical backdrop. Thus, the objective of this study is to validate the NGS-based OncoIndx® test with NGS standard references, clinical samples, and U.S. Food and Drug Administration (FDA) approved cross-laboratory samples to determine its analytical performance and precision.
We show the prediction of optimal treatment strategies (precision therapies and immunotherapies), based on the identification of biomarkers in more than 4000 clinical samples (Cohort-based publication underway) with proven and published links to approved therapies and precision medicine clinical trials (CT) within a sizable population of advanced cancer patients. The assay 339 intronic, 15719 exonic regions, 50 translocations (fusions), homologous recombination deficiency (HRD) based on 47 homologous recombination repair (HRR) markers, genome-wide loss of heterozygosity (LOH), large-scale transitions (LST) and telomeric allelic imbalance (TAI) of the 1080 genes covered in the panel. In addition, the assay covers 36 pharmacogenomic markers compatible with both liquid and tissue biopsies for a range of solid tumors. It has the capability to detect single nucleotide variants (SNVs), CNAs, structural variants, microsatellite instability (MSI), and tumor mutational burden (TMB). With a maximum coverage depth of 1000x for tissue biopsy and 5000x for liquid biopsy, it offers a comprehensive view of the cancer status, more precise than the whole genome or whole exome sequencing strategies that may yield more variants of unknown clinical significance (VUS), thus balancing comprehension and efficacy.
2. Materials and Methods
2.1. Sample Collection and Targeted Exon Sequencing
A total of 63 patient samples including industry reference standards (cfDNA and gDNA), clinical samples (blood and formalin-fixed paraffin embedded tissue (FFPE) samples), and cross-laboratory samples were utilized in the study. The ctDNA or FFPE DNA were extracted using commercially available DNA extraction kits (QIAamp minELute ccfDNA DNA kit, Qiagen, Germany). The extracted DNA was subjected to quality check (≥80% ccfDNA content) on TapeStation instrument (Agilent, USA) and the concentration was determined on Qubit 4.0 fluorometer (ThermoFisher Scientific, USA). Quality assessment was followed by preparation of Illumina-compatible libraries using target hybridization method. DNA libraries were then sequenced on the NextSeq 2000 platform in a paired-end fashion. Variant calling, and annotation was then performed using indigenously developed iCare platform. An illustration of the NGS sequencing workflow is illustrated in Figure 1.
Figure 1.
Illustration of OncoIndx® workflow. Figure presents the detailed workflow of OncoIndx NGS assay from sample collection, DNA extraction, DNA sequencing, variant calling, variant annotation, and data analysis.
Figure 1.
Illustration of OncoIndx® workflow. Figure presents the detailed workflow of OncoIndx NGS assay from sample collection, DNA extraction, DNA sequencing, variant calling, variant annotation, and data analysis.
2.2. Bioinformatic Data Processing
Post-NGS analysis involves trimming of the adapter and barcode sequences from the raw FastQ files upon thorough quality assessment (QA tests). Trimmed sequences were then aligned with the Genome Reference Consortium Human Build 38 (GRCh38) reference genome. Further, variant calling involved using the proprietary iCare software platform.
2.3. Variant Prioritization and Interpretation
Filtered genomic variants from the variant calling pipeline were prioritized using cancer databases like ClinVar - NCBI (
https://www.ncbi.nlm.nih.gov/clinvar/), as well as our proprietary curated database on iCare software platform. Additionally, in silico prediction tools including SIFT, POLYPHEN, etc. were added for prioritizing variants. Variants were then interpreted based on the Tier level categorization from tier I, tier II, and tier III levels (as per Association for Molecular Pathology, AMP) which indicates the variants with strong clinical significance (level A and B evidence), variants with potential clinical significance (level C or D evidence), and variants with unknown clinical significance respectively (Li et al. 2017). Finally, molecular therapies were recommended along with published, recruiting and potential clinical trials.
2.4. Validating Test Outcomes
2.4.1. Level 1: Reference Standards
First level of extensive validation of the OncoIndx® comprehensive panel was performed using 43 NGS standard reference materials from Seraseq™ (USA) with known true mutations including SNV, small InDels, CNA, and translocations (fusions). Reference standard samples at tumor fractions 5%, 4%, 3%, 2%, 1%, and 0.1% were assessed to determine the detection efficiency and limit of detection (LOD). Reproducibility of variant detection across different batches of samples was also estimated for OncoIndx®. Sample analyses were repeated for 5 sequencing batches whose outcomes were then investigated.
2.4.2. Level 2: Clinical Samples
As level 2 performance validation, 14 clinical samples sequenced by OncoIndx® panel were analyzed. The concordance between the OncoIndx® test findings and clinically established hotspot findings were established for each clinical sample.
2.4.3. Level 3: Orthogonal Validation
Finally, the OncoIndx® test detection efficiency of immune-oncology biomarkers, the tumor mutation burden (TMB) and microsatellite instability (MSI) were validated against 6 cross-laboratory samples whose results were produced by accredited reference laboratory tests.
3. Results
3.1. OncoIndx® Detected Genomic Alterations from Ngs Standard Reference Samples with High Concordance and Analytical Precision
OncoIndx® was tested to detect SNVs, INDELs, CNAs, and translocations (fusion) from industry NGS reference samples which were pre-synthesized with variants. The outcomes are discussed in detail in the following sections.
3.1.1. Single Nucleotide Variants, and INDELs
Clinical implementation of genomic tests requires high analytical precision. Several parameters including accuracy, sensitivity and specificity stand vital for such investigations. Thus, OncoIndx® variant calling pipeline (iCare’s VCP)© was validated using 43 NGS reference samples with variant distributions at 5 %, 1% and 0.1% frequency. From the test outcomes, variant detection was most sensitive and accurate at 5% variant allele frequency (VAF) was reported in Table 1. CNAs were detected with 100% accuracy, sensitivity, and specificity, with no false positive and/or false negative occurrences. From a total of 264 SNVs detected by OncoIndx®, 156 were true positives and 108 rendered true negative outcomes with excellent concordance against reference standards. Highest positive and negative predictive values (NPV and PPV) obtained from OncoIndx® assay were 100% for SNVs. Maximum accuracy of 97.40% was obtained for small INDELs with 100% specificity and 95.60% sensitivity. In addition, translocations (fusions) were detected with a high accuracy of 98.48%. It also yielded 100% specificity and a sensitivity of 97.44%. OncoIndx® assay pipeline yielded no false positive hits even at 1% VAF. This highlights the reliability and sensitivity of the assay to exhibit acceptable performance for variant calling even at 1% VAF.
Table 1.
Outcomes of statistical analysis obtained OncoIndx® comprehensive genomic panel at 5% variant allele frequency.
Table 1.
Outcomes of statistical analysis obtained OncoIndx® comprehensive genomic panel at 5% variant allele frequency.
| |
|
|
|
|
|
|
|
|
|
|
| Alteration type |
Total number of alterations |
True positives |
False positives |
True negatives |
False negatives |
*PPV |
*NPV |
Accuracy |
Specificity |
Sensitivity |
| SNVs |
264 |
156 |
0 |
108 |
0 |
100 |
100 |
100 |
100 |
100 |
| Small INDELs |
154 |
87 |
0 |
63 |
4 |
100 |
94.03 |
97.40 |
100 |
95.60 |
| CNA |
66 |
39 |
0 |
27 |
0 |
100 |
100 |
100 |
100 |
100 |
| Translocations (Fusions) |
66 |
38 |
0 |
27 |
1 |
100 |
96.43 |
98.48 |
100 |
97.44 |
3.1.2. Copy Number Alterations and Translocations (Fusions)
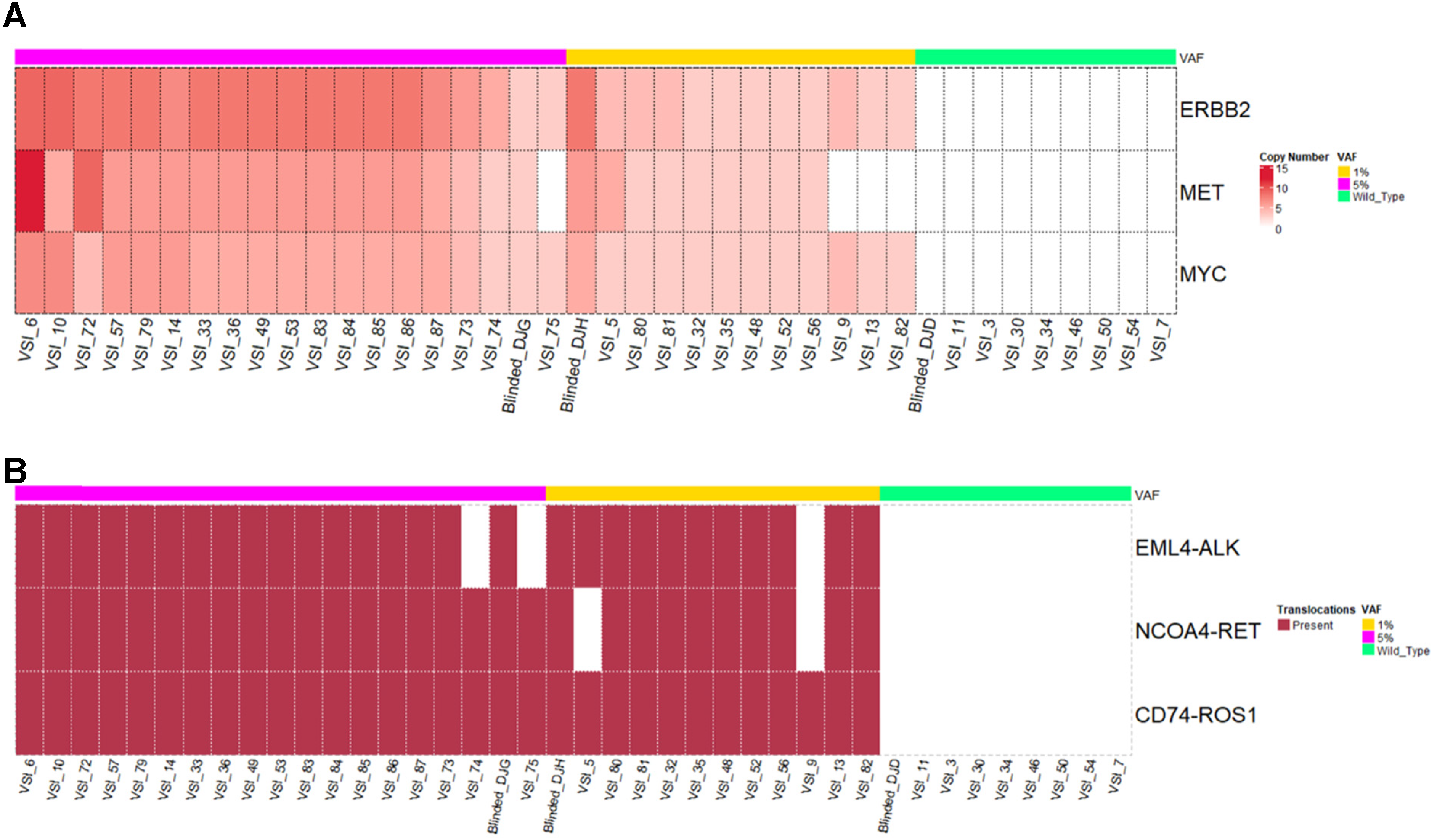
Detection of CNAs and fusions can be challenging due to inconsistencies of rearranged chromosomal regions, breakpoints, read lengths, etc. SV such as translocations, especially fusions may be important in solid tumors as it is in hematological cancers (Taniue and Akimitsu 2021). In OncoIndx®, the detection of CNAs and fusion variants were validated using customized reference materials at VAF from 5%, 1% to 0.1%. The validation set was mainly focused on 3 genes for CNAs namely, ERBB2, MET, MYC, and 3 translocations namely, EML4-ALK, NCOA4-RET, and CD74-ROS1. CNAs were detected with highest PPV, NPV, specificity and accuracy of 100%. In addition, no false positives and false negatives were detected from OncoIndx® assay for CNAs at 5% VAF, whose distributions are shown in Figure 2A. 100% of patients with ERBB2, and MYC amplification were detected at a VAF as low as 0.1% (Figure 2A). Next, for fusions, a detection accuracy of 98.48%, PPV and specificity of 100% were obtained with no false positive detection. At all three dilutions of VAF (5%, 1%, and 0.1%), fusions were detected at a specificity of 100%. NCOA4-RET and CD74-ROS1 fusions were predominantly detected at 0.1%VAF indicating the sensitivity of OncoIndx® assay at a low VAF of 0.1% (Figure 2B).
Figure 2.
Distribution of copy number alterations and translocations (fusions) detected by OncoIndx. (A) From the total number of samples in the cohort of 5% VAF, figure indicates 39 true positive copy number alterations with 0 false positives detected across the reference sample validation cohort. (B) Figure indicates the detection of fusions with 38 true positives and 1 false positive. In both A and B, no CNA/Fusions were detected in Wild-type controls.
Figure 2.
Distribution of copy number alterations and translocations (fusions) detected by OncoIndx. (A) From the total number of samples in the cohort of 5% VAF, figure indicates 39 true positive copy number alterations with 0 false positives detected across the reference sample validation cohort. (B) Figure indicates the detection of fusions with 38 true positives and 1 false positive. In both A and B, no CNA/Fusions were detected in Wild-type controls.
3.1.3. Limit of Detection of OncoIndx®
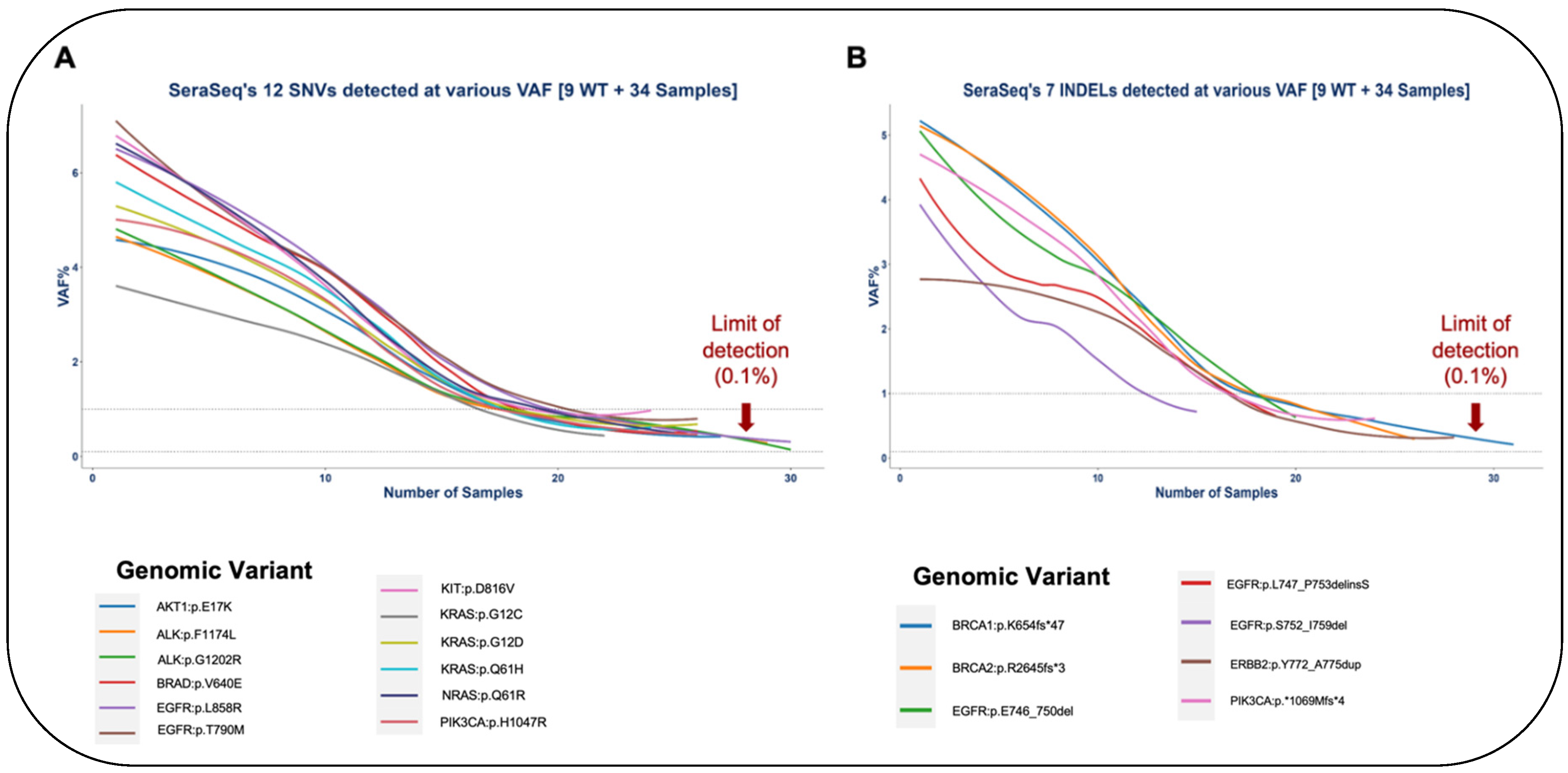
For identifying the limit of detection (LOD) of OncoIndx® assays, serial dilutions of standard reference Seraseq™ samples were performed from 0.2% to 1% VAF. Gene variants including AKT1:p.E17K, EGFR:p.L858R, EGFR:p.E746_A750del and ERBB2:p.Y772_A775dup were detected in each of the diluted samples. Expected vs. observed VAF% were visualized for all variants to further determine LOD. A total of 83.33% (n=5) of SNVs could be detected at VAF less than 1% namely, EGFR (p.T790M), NRAS (p.Q61R), PIK3CA (p.H1047R), KRAS (p.G12D), ALK (p.G1202R) respectively (Figure 3A). Similarly for small InDels, 60% (n=3) of the variants could be detected at VAF less than 1% namely, BRCA1 (p.K654fs*47), BRCA2 (p.R2645fs*3), and PIK3CA (p.*1069Mfs*4) (Figure 3B). The observed % VAF thus denotes the LOD of OncoIndx® assays which were determined for both SNVs and INDELs. Thus, the overall sensitivity of OncoIndx® appears at 0.1% from LOD values as indicated by the arrows in Figure 3A,B. List of SNVs and INDELs validated from the industrial samples are presented in Table 2.
Figure 3.
Limit of detection of OncoIndx® test. (A) and (B) Detection limit observed at 0.1% VAF for majority of SNVs and INDELs pre-established by standard reference samples tested by OncoIndx® assay (shown by arrows).
Figure 3.
Limit of detection of OncoIndx® test. (A) and (B) Detection limit observed at 0.1% VAF for majority of SNVs and INDELs pre-established by standard reference samples tested by OncoIndx® assay (shown by arrows).
Table 2.
Table presents the list of SNVs and INDELs detected and validated from the industrial samples.
Table 2.
Table presents the list of SNVs and INDELs detected and validated from the industrial samples.
| |
|
| List of SNVs and INDELs validated from the industrial samples |
| AKT1:p.E17K |
EGFR:p.T790M |
| ALK:p.F1174L |
ERBB2:p.Y772_A775dup |
| ALK:p.G1202R |
KIT:p.D816V |
| BRAF:p.V640E |
KRAS:p.G12C |
| BRCA1:p.K654fs*47 |
KRAS:p.G12D |
| BRCA2:p.R2645fs*3 |
KRAS:p.Q61H |
| EGFR:p.E746_A750del |
KRAS:p.Q61R |
| EGFR:p.L747_P753delinsS |
NRAS:p.Q61R |
| EGFR:p.L858R |
PIK3CA:p.*1069Mfs*4 |
| EGFR:p.S752_I759del |
PIK3CA:p.H1047R |
3.2. High Concordance of Genomic Alterations Obtained from Clinical Samples
Clinical sample cohort with 14 samples were tested across genomic alterations for 5 hotspot genes such as EGFR, ALK, KRAS, PIK3CA, and BRCA2. OncoIndx® assay showed 100% concordance for alterations detected in EGFR, ALK, KRAS, and BRCA2 genes, and 50% concordance for PIK3CA alterations (Table 3). In ALK positive samples, the OncoIndx® assay efficiently determined ALK fusion partners beyond EML4 including NPM1. In addition, we predict that the 50% concordance obtained for PIK3CA alteration in one clinical sample may be most likely due to tumor heterogeneity presented by testing different sample types, i.e., former clinical finding being in FFPE sample and OncoIndx® assay in ctDNA from blood sample.
Table 3.
Table shows the concordance levels of genomic alterations in clinical samples as detected by OncoIndx® assay.
Table 3.
Table shows the concordance levels of genomic alterations in clinical samples as detected by OncoIndx® assay.
| |
|
|
|
| S.No. |
Genes |
Concordant Genomic findings from OncoIndx® assay |
Concordance levels obtained from OncoIndx® assay |
| 1 |
EGFR |
L858R
E746_A750del
L747_S752del |
100% |
| 2 |
ALK |
NPM1-ALK
ALK-EML4 Fusion
G1202R
G1269A |
100% |
| 3 |
KRAS |
A146T |
100% |
| 4 |
PIK3CA |
H1047R |
50% |
| 5 |
BRCA2 |
S636* |
100% |
Tumor heterogeneity is a major roadblock in effective treatment decision making which is also one of the main challenges of FFPE DNA testing. Thus, a ctDNA based genomic detection may offer benefits in these cases by analyzing the totality of tumor rather than individual sections like the former testing method. Thus, OncoIndx® may be a beneficial complementary tool in such scenarios. In addition to the concordance among genomic alterations, the OncoIndx® assay was also validated against important biomarkers including MSI, TMB, LOH, LST, and TAI whose outcomes are detailed in the next section.
3.3. Validation of Biomarker Signatures against Reference Laboratories: Microsatellite Instability and Tumor Mutation Burden
In addition to detecting genomic variants, the OncoIndx® test identifies the status of biomarkers to predict immunotherapy response in patients. From the blood samples collected, MSI, and TMB were detected. MSI in tumors has been studied to promote “immune-hot” conditions (Wang et al. 2023; Bai et al. 2021). Thus, presence of high MSI favors tumor immune responses. To validate the reproducibility of MSI detected from OncoIndx®, 2 MSI detection tools were utilized against the outcomes of clinically stratified reference laboratory samples. From our pipeline, MSI were detected with an accuracy of 95%, sensitivity of 90%, and a specificity of 100%. Overall, PPV was identified to be 100% and NPV was 90.91% (Table 4).
Table 4.
Table presents the analytical parameters of MSI detection by OncoIndx® test.
Table 4.
Table presents the analytical parameters of MSI detection by OncoIndx® test.
| |
|
| Statistics of MSI detection in OncoIndx® (Percentage %) |
| Positive Predictive Value (PPV) |
100 |
| Negative Predictive Value (PPV) |
90.91 |
| Sensitivity |
90 |
| Specificity |
100 |
| Accuracy |
95 |
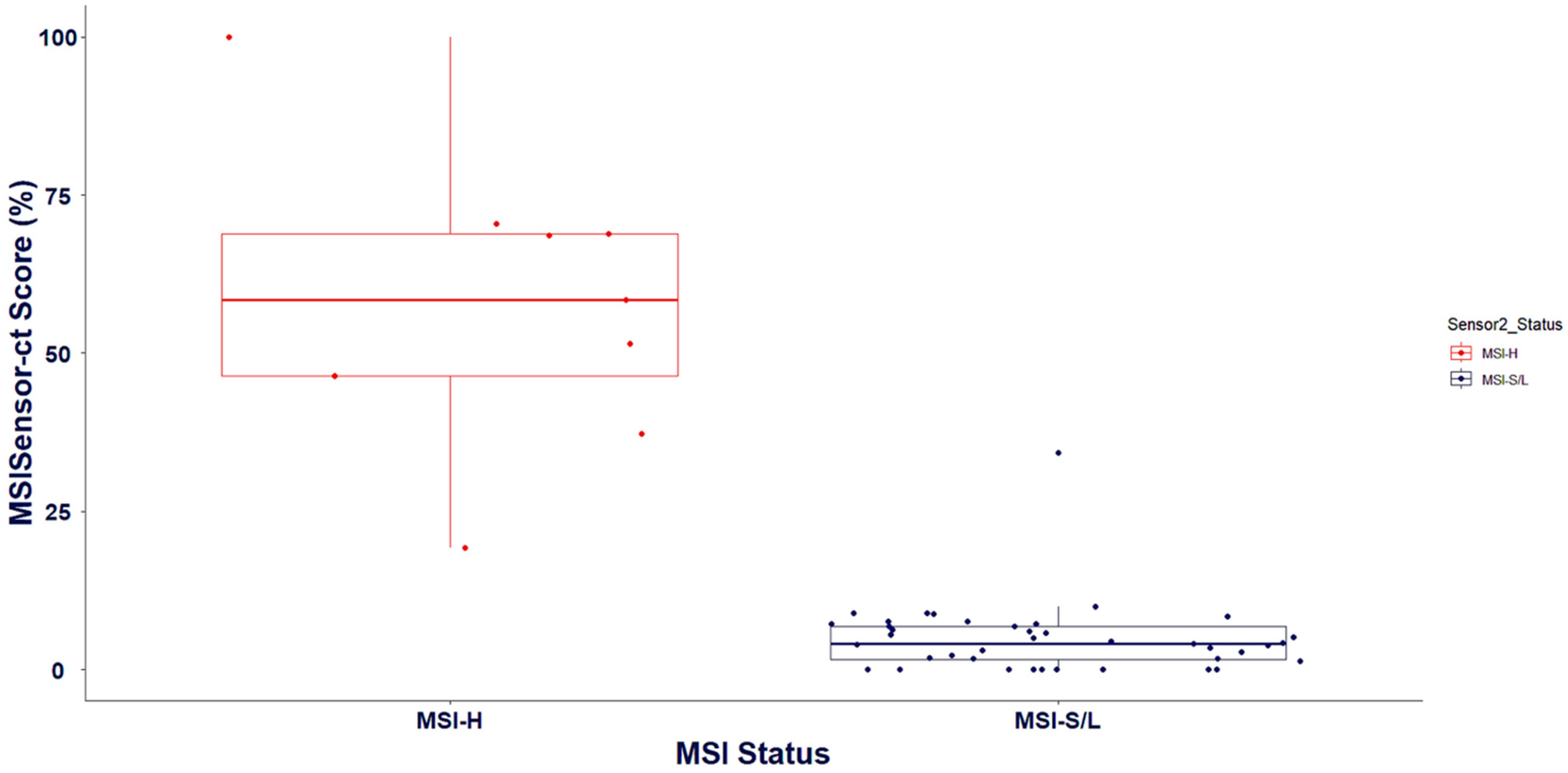
All MSI high and MSI low samples were appropriately predicted with 1 misclassified sample whose value was in the intermediate range (Figure 4). Intermediate MSI values are challenging as they are neither high nor low and are missed in many classifier tools. Thus, more optimizations are necessary and are constantly underway in OncoIndx® for better accuracy.
Figure 4.
Validation of MSI status from cross-laboratory documentations across MSI prediction in OncoIndx®.
Figure 4.
Validation of MSI status from cross-laboratory documentations across MSI prediction in OncoIndx®.
The summarized outcomes from reference laboratory validation of MSI against the food and drug administration (FDA) approved companion diagnostic tests are presented in Table 5. These outcomes suggest high sensitivity and specificity of OncoIndx® assay in detecting MSI from NGS.
With rapidly emerging immune-oncological biomarkers, MSI stands important in predicting immunotherapy and a highly sensitive assay like OncoIndx® can improve responsive standards of immunotherapy in cancer. MSI threshold criteria utilized for our CGP test is presented in Table 6.
Table 5.
OncoIndx® test prediction of MSI status validated against FDA approved test outcomes.
Table 5.
OncoIndx® test prediction of MSI status validated against FDA approved test outcomes.
| |
| Sample Type |
FDA approved test prediction |
OncoIndx® test prediction |
| Blood |
MSS |
3.2 (MSI-low) |
| Blood |
MSS |
1.55 (MSI-low) |
| Blood |
MSS |
0.79 (MSI-low) |
| Blood |
MSS |
3.07 (MSI-low) |
| Blood |
MSS |
3.61 (MSI-low) |
Table 6.
MSI thresholds of high, Intermediate, Low, and stable scores utilized for various sample types in OncoIndx® test.
Table 6.
MSI thresholds of high, Intermediate, Low, and stable scores utilized for various sample types in OncoIndx® test.
| |
|
|
|
| Biomarker/s |
Outcome |
Blood/Pleural Effusion |
FFPE/RNALater |
| MSI |
MSI-H |
≥ 20 |
≥ 20 |
| MSI-I |
≥ 10 |
≥ 10 |
| MSI-L |
< 10 |
< 10 |
| MSI-S |
0 |
0 |
Likewise, TMB predictions from OncoIndx® assay were cross validated with reference standards like Seraseq™ and the College of American pathologists (CAP) accredited genomic DNA lab reference samples. Reference laboratory validations against the FDA-approved tests were also performed using OncoIndx® CGP (Table 7, Table 8).
Table 7.
TMB validation performed against NGS reference standards.
Table 7.
TMB validation performed against NGS reference standards.
| |
|
|
|
| Sample |
Sample type |
True prediction |
OncoIndx® test prediction |
| Control |
Healthy control |
Negative control |
1.5 |
| Healthy control |
Negative control |
1.5 |
| Healthy control |
Negative control |
1.5 |
| Healthy control |
Negative control |
2.5 |
| Healthy control |
Negative control |
1.67 |
| Healthy control |
Negative control |
0 |
| Healthy control |
Negative control |
0 |
| Healthy control |
Negative control |
0.5 |
| SeraSeqTM reference samples |
TMB Mix Score 7 (0%) |
5.8-9.2 |
8.67 |
| TMB Mix Score 7 (0.5%) |
10.5-15.7 (d=3.5-7.7) |
10.83 |
| TMB Mix Score 7 (2%) |
16.6-19.2 (d=3.5-7.7) |
6.67 |
| TMB Mix Score 20 (0%) |
6.1-8.9 |
6.5 |
| TMB Mix Score 20 (0.5%) |
23.7-28.3 (d=15.8-21.2) |
8.17 |
| TMB Mix Score 20 (2%) |
34.6-36.6 (d=26.4-29.8) |
5.83 |
| CAP gDNA samples |
gDNA |
9 |
11.5 |
| gDNA |
26 |
19.5 |
| gDNA |
9 |
9.83 |
| gDNA |
26 |
5.67 |
Table 8.
Cross-laboratory validation of TMB with OncoIndx® test predictions.
Table 8.
Cross-laboratory validation of TMB with OncoIndx® test predictions.
| |
|
|
| Sample Type |
FDA approved test prediction |
OncoIndx® test prediction |
| Blood |
1 |
3.2 |
| Blood |
7.26 |
1.55 |
| Blood |
6.7 |
0.79 |
| Blood |
3 |
3.07 |
| Blood |
4 |
3.61 |
| Blood |
4.77 |
3.167 |
TMB thresholds utilized in the CGP assay is presented in Table 9. The outcomes have been categorized as low and High TMB scores which are then predictive of immunotherapy benefits.
Table 9.
TMB thresholds of OncoIndx® test.
Table 9.
TMB thresholds of OncoIndx® test.
| |
|
|
|
| Biomarker/s |
Outcomes |
Blood/Pleural effusion |
FFPE/RNALater |
| TMB |
TMB-H |
≥ 10 |
≥ 10 |
| |
TMB-L |
< 10 |
< 10 |
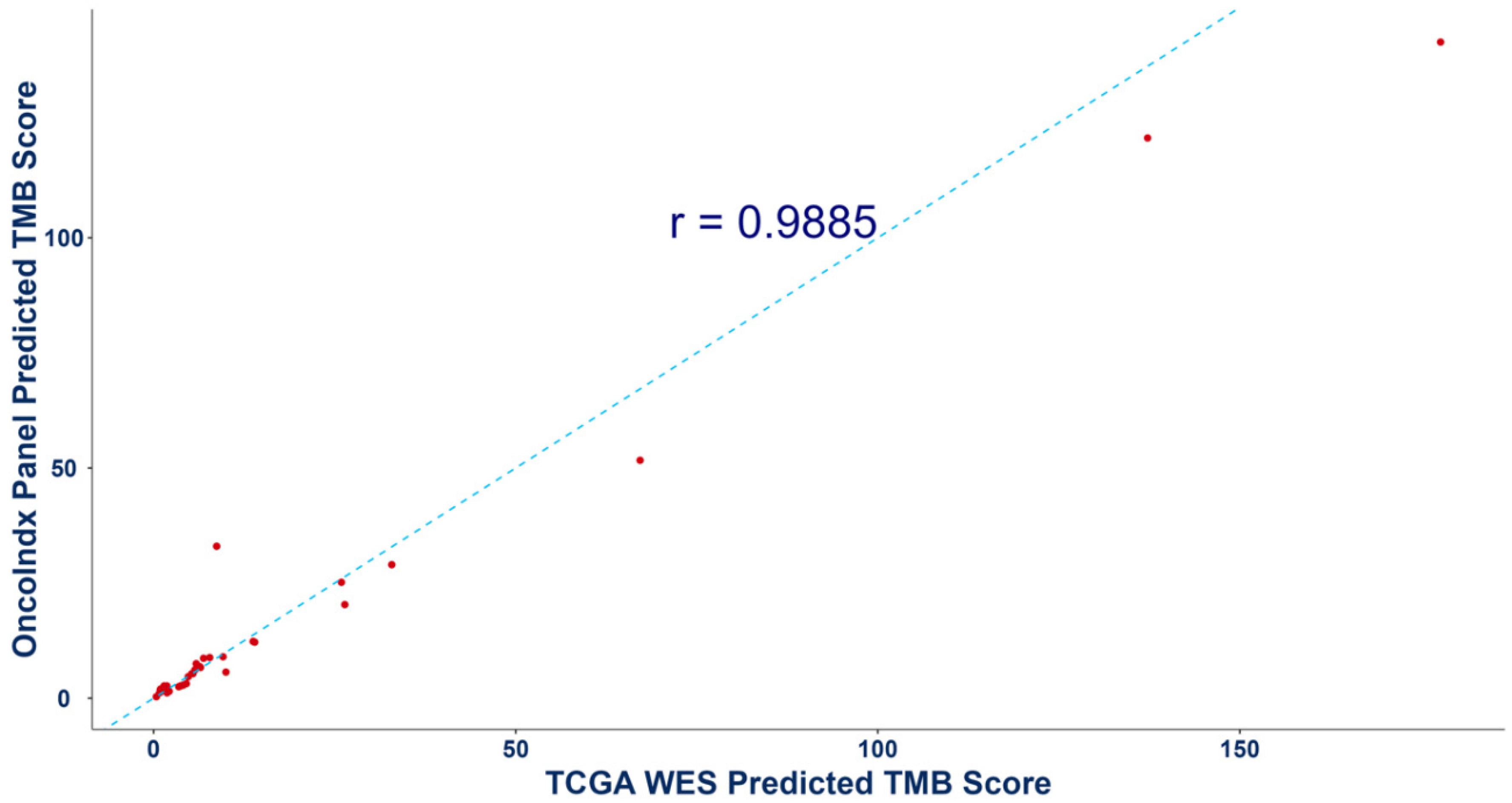
In addition, the predicted TMB values from targeted OncoIndx® panel was also validated by comparing with whole exome sequencing (WES) data from the cancer genome atlas program (TCGA) database. Pearson correlation coefficient of r=0.9885 was obtained (Figure 5). Overall, these outcomes indicate concordance between the OncoIndx® CGP against established FDA-approved companion diagnostic tests.
Figure 5.
Pearson correlation outcomes for Tumor mutation burden from Whole exome sequencing and OncoIndx gene panel. Figure shows a high correlation between the predicted TMB scores of whole exome sequencing (WES) vs. the targeted comprehensive gene panel from OncoIndx.
Figure 5.
Pearson correlation outcomes for Tumor mutation burden from Whole exome sequencing and OncoIndx gene panel. Figure shows a high correlation between the predicted TMB scores of whole exome sequencing (WES) vs. the targeted comprehensive gene panel from OncoIndx.
4. Conclusions
In conclusion, the performance of OncoIndx® assay for SNVs, small INDELs, CNAs, and translocations (fusions) were optimally validated against NGS reference standards for analytical validation, patient samples for clinical validation, and cross-laboratory testing for orthogonal validation. The outcomes prove OncoIndx® assay to be an effective tool for NGS analysis. In addition, the test performance was evaluated at multiple VAF, and the results show that OncoIndx® can detect variants and perform optimally at VAF as less as 0.1%.
Overall, OncoIndx® comprises of markers for precisely targeted therapies, immunotherapy, selected chemotherapies which are all designed to interrupt oncogenic processes and regulate molecular pathways that either drive the disease or imbibe resistance. Integrating the comprehensive capabilities of OncoIndx®] with rigorous clinical validation required for NGS tests, we ensure a deep understanding of cancer genetics as well as the highest standards of accuracy and reliability in clinical practice.
Funding Information: The study did not receive any external funding.
Author Contributions
Conceptualization, GS; JK; AV; Methodology, GS; Software, GS; Validation, GS; HK; Formal Analysis, GS; AR; Investigation, GS; MD; Resources, GS; JK; HK; AD; SP; BJ; Data Curation, KH; MB; Writing—Original Draft Preparation, AR; Writing—Review & Editing, AR; GS; SI; JK; AB; Visualization, AR; SH; Supervision, GS; Project Administration, GS; HK; MU.
Data Availability Statement
Data are not publicly available due to privacy restrictions. The data that support the findings of this study are available from the corresponding authors, GS and JK, upon reasonable request.
Conflicts of Interest
Authors declare no conflicts of interest.
References
- Karol, D., McKinnon, M., Mukhtar, L., Awan, A., Lo, B. and Wheatley-Price, P., 2021. The impact of foundation medicine testing on cancer patients: a single academic centre experience. Frontiers in oncology, 11, p.687730.
- Bai, J., Chen, H. and Bai, X., 2021. Relationship between microsatellite status and immune microenvironment of colorectal cancer and its application to diagnosis and treatment. Journal of Clinical Laboratory Analysis, 35(6), p.e23810.
- Bharde, A., Bhonde, M., Bose, C., Raut, N.V., Kothavade, H., D’Souza, A., Kad, T., Jadhav, B., Prajapati, S., Jadhav, V.B.L. and Joshi, S.K., Hariramani, K., Uttarwar, M., Khandare, J., Shafi, G., 2023. Comprehensive ctDNA analysis to identify genome-instability and actionable mutation landscape in gynecologic cancers. Journal of Clinical Oncology, 41, p.e17503.
- Bharde, A., Noronha, V., Ratnaparkhi, M., Patil, V., Jadhav, B., Prajapati, S., Choughule, A., Chandrani, P., Haldar, S., Moubeen, F. and Menon, N., Hariramani K., Basavalingegowda, M., Ramesh, A., Khandare, J., Chaturvedi, P., Shafi, G., Prabhash, K., 2024. Comprehensive genomic profiling of ctDNA reveals distinct genomic signatures and therapeutic implications for immunotherapy response in advanced head and neck cancer. Cancer Research, 84(6_Supplement), pp.2408-2408.
- Fabrizio, D., Cristescu, R., Albacker, L., Snyder, A., Ward, A., Lunceford, J., Aurora-Garg, D., Jin, F., Hopkins, J., Rubin, E. and Hegde, P., 2021. Real-world prevalence across 159 872 patients with cancer supports the clinical utility of TMB-H to define metastatic solid tumors for treatment with pembrolizumab. Annals of Oncology, 32(9), pp.1193-1194.
- Hiemenz, M.C., Graf, R.P., Schiavone, K., Harries, L., Oxnard, G.R., Ross, J.S. and Huang, R.S., 2022. Real-world comprehensive genomic profiling success rates in tissue and liquid prostate carcinoma specimens. The Oncologist, 27(12), pp.e970-e972.
- Jennings, L.J., Arcila, M.E., Corless, C., Kamel-Reid, S., Lubin, I.M., Pfeifer, J., Temple-Smolkin, R.L., Voelkerding, K.V. and Nikiforova, M.N., 2017. Guidelines for validation of next-generation sequencing–based oncology panels: a joint consensus recommendation of the Association for Molecular Pathology and College of American Pathologists. The Journal of molecular diagnostics, 19(3), pp.341-365.
- Kadri, S., Long, B.C., Mujacic, I., Zhen, C.J., Wurst, M.N., Sharma, S., McDonald, N., Niu, N., Benhamed, S., Tuteja, J.H. and Seiwert, T.Y., 2017. Clinical validation of a next-generation sequencing genomic oncology panel via cross-platform benchmarking against established amplicon sequencing assays. The Journal of Molecular Diagnostics, 19(1), pp.43-56.
- Krzyszczyk, P., Acevedo, A., Davidoff, E.J., Timmins, L.M., Marrero-Berrios, I., Patel, M., White, C., Lowe, C., Sherba, J.J., Hartmanshenn, C. and O’Neill, K.M., 2018. The growing role of precision and personalized medicine for cancer treatment. Technology, 6(03n04), pp.79-100.
- Li, M.M., Datto, M., Duncavage, E.J., Kulkarni, S., Lindeman, N.I., Roy, S., Tsimberidou, A.M., Vnencak-Jones, C.L., Wolff, D.J., Younes, A. and Nikiforova, M.N., 2017. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. The Journal of molecular diagnostics, 19(1), pp.4-23.
- Mathew, A., Joseph, S., Boby, J., Benny, S., Veedu, J., Rajappa, S., Rohatgi, N., Sirohi, B., Jain, R., Agarwala, V. and Shukla, D.K., 2022. Clinical benefit of comprehensive genomic profiling for advanced cancers in India. JCO Global Oncology, 8, p.e2100421.
- Shafi, G., Dongare, M., Bharde, A., Fauzul, M., Hariramani, K., D’Souza, A., Jadhav, B., Kad, T., Prajapati, S., Jadhav, V. and Kumaran, M., 2023. Abstract PR007: Comprehensive ctDNA profiling reveals potential metastatic genomic signatures in treatment-naive early-stage breast cancer patients. Cancer Research, 83(2_Supplement_2), pp.PR007-PR007.
- Takeda, M., Takahama, T., Sakai, K., Shimizu, S., Watanabe, S., Kawakami, H., Tanaka, K., Sato, C., Hayashi, H., Nonagase, Y. and Yonesaka, K., 2021. Clinical application of the FoundationOne CDx assay to therapeutic decision-making for patients with advanced solid tumors. The oncologist, 26(4), pp.e588-e596.
- Taniue, K. and Akimitsu, N., 2021. Fusion genes and RNAs in cancer development. Non-coding RNA, 7(1), p.10.
- Waarts, M.R., Stonestrom, A.J., Park, Y.C. and Levine, R.L., 2022. Targeting mutations in cancer. The Journal of clinical investigation, 132(8).
- Wang, L., Geng, H., Liu, Y., Liu, L., Chen, Y., Wu, F., Liu, Z., Ling, S., Wang, Y. and Zhou, L., 2023. Hot and cold tumors: Immunological features and the therapeutic strategies. MedComm, 4(5), p.e343.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).