1. Introduction
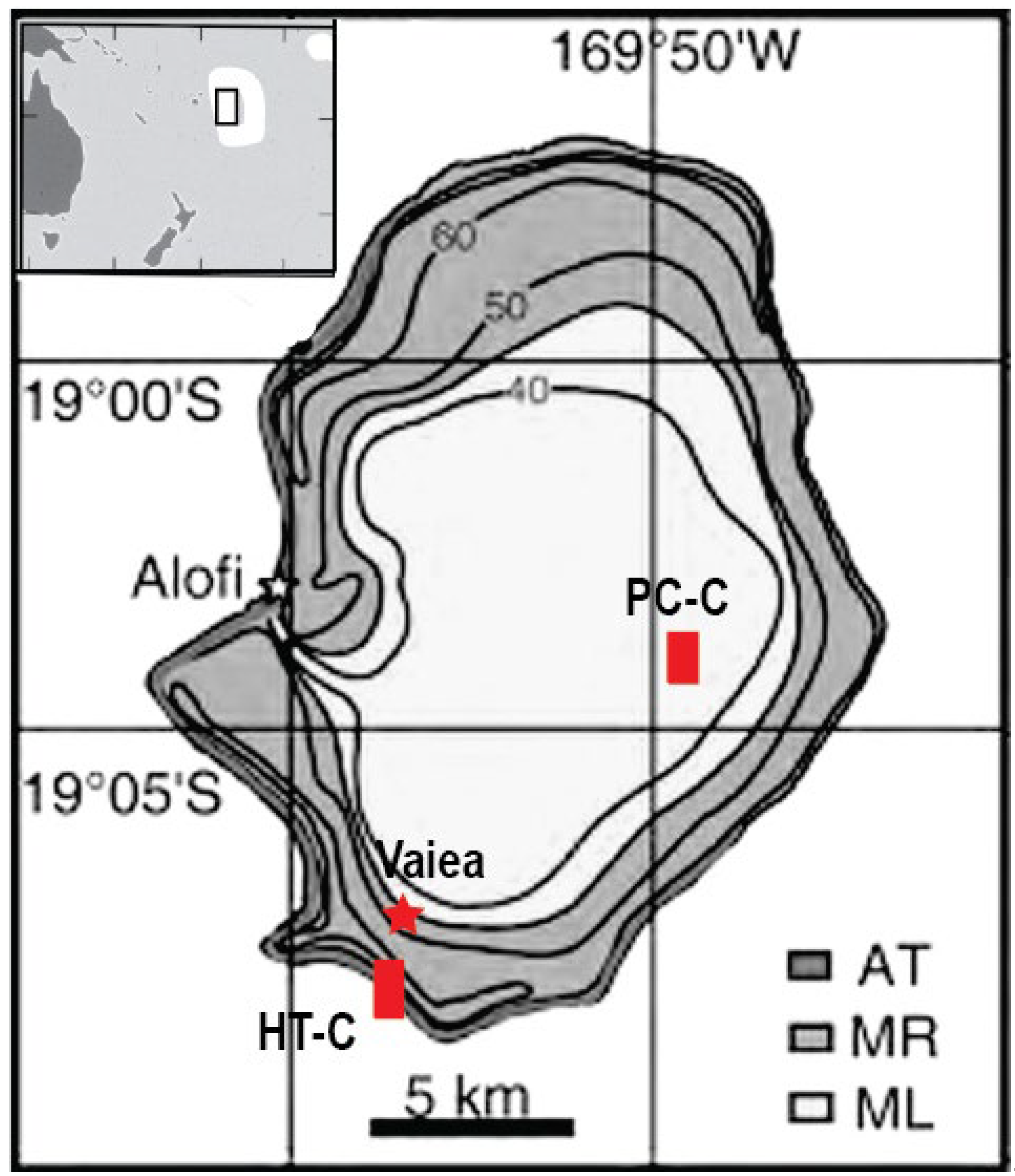
Two types of carbonate-rich calcretes have been documented from Niue Island in the Southwest Pacific (
Figure 1). One is commonly associated with the island interior soils (
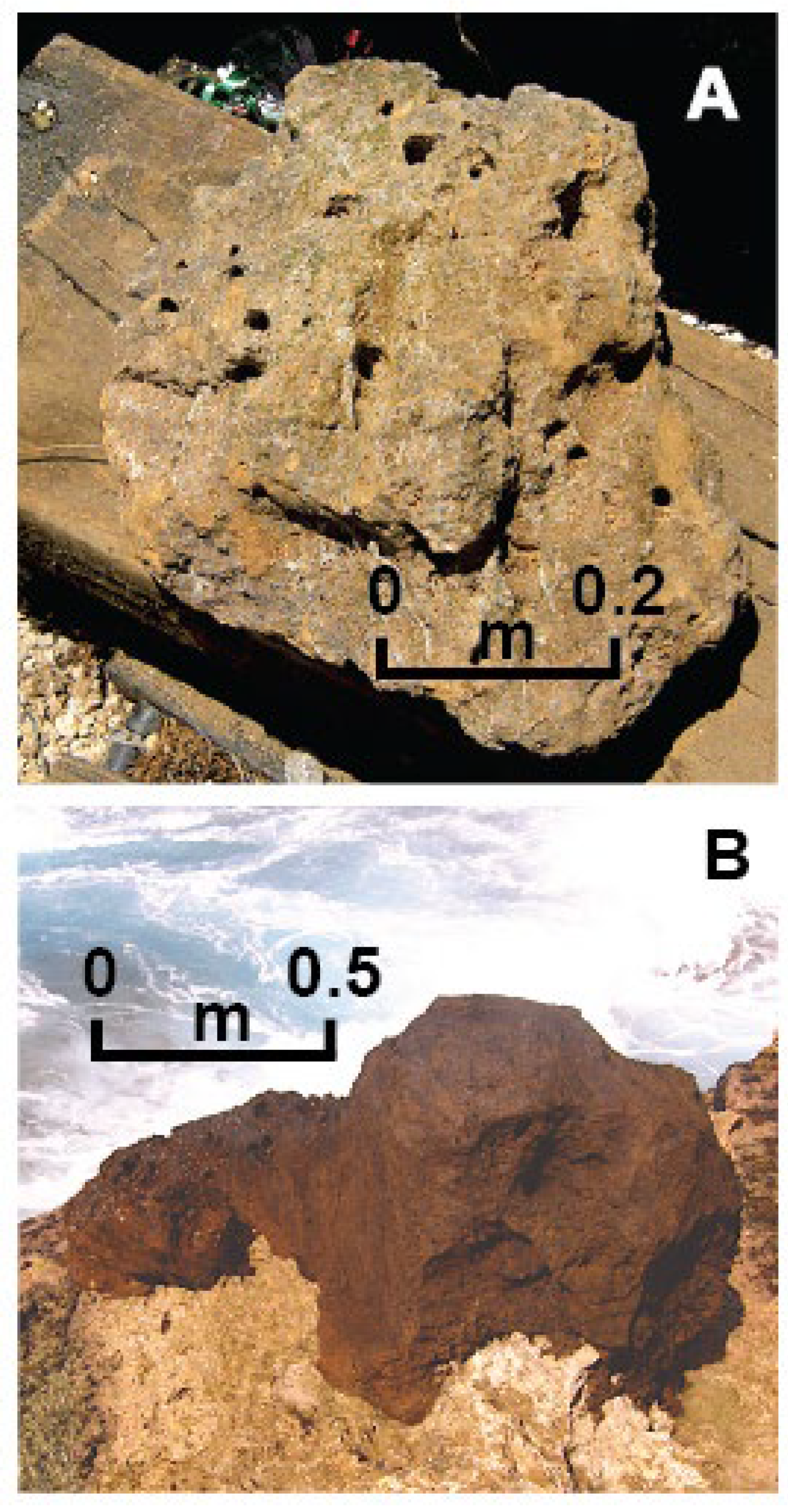
Figure 2 A) (Whitehead and Aharon, 2023). The other, confined to the coastal terrace, is uncommon and has been described from field observations as a “Palagonite tuff” by Marsden (1964) and Schofield (1967), and an “Iron-rich deposit” by Nunn and Britton (2004) (
Figure 2 B). Neither one of those studies substantiated the field observations with laboratory investigations.
Here we report the results of a laboratory investigation of the two contrasting calcrete types occurring in Niue Island. The calcrete block exteriors conceal their content (
Figure 2A,B) and therefore examination in the field offers no clues as to their origin and provenance. In this study we employ X-Ray Diffraction (XRD) to identify the calcrete mineral constituents, and petrographic microscopy of thin sections to unravel the microfabrics of the minerals and organic residues. Additionally, we acquired elemental chemistry and stable carbon and oxygen isotopes from hand-size subsamples to discern origin and provenance. In conjunction, the results afforded deduction of the factors controlling deposition in a sedimentary setting atypical of calcrete formation (Milnes, 1992; Alonso-Zarza and Wright, 2010).
2. Niue Island Setting
Niue Island is an uplifted former atoll in the tropical Southwest Pacific and, at 259 km
2, one of the largest carbonate islands in the region (
Figure 1). The geology and landscape of the island have been shaped primarily by (i) early subsidence of the underlying extinct volcano during Early-Mid Miocene to Pliocene (Schofield, 1959; Hill, 1983; Lu et al., 1996); (ii) uplift since early Pleistocene resulting from upwarping of the lithospheric plate as it travels toward the Tonga Trench (Dubois et al., 1975); (iii) sea-level fluctuations since Late-Miocene time (Aharon et al., 1993; Wheeler and Aharon, 1997), and (iv) enhanced sediment transport and erosion associated with the sea-level cycles and frequent cyclones (Kennedy et al., 2012; Marsters and Kennedy, 2014). The depositional history of the carbonate sediments in Niue, comprising limestone, dolomite and remnant coral aragonite, has been investigated in outcrops, quarries (Schofield, 1959; Paulay and Spencer, 1992; Nunn and Britton, 2004; Kennedy et al., 2012; Marsters and Kennedy, 2014) and cores up to 320 m long (Aharon et al., 1993; Lu et al., 1996; Wheeler et al., 1999).
The island geology is differentiated into a modern to mid-Holocene age shore platform at sea-level (Kennedy et al., 2012), a prominent Alofi Terrace of Late Pleistocene-age rising to an elevation of about 20-23 m asl, and a Late Miocene to Pliocene-age Mutalau Atoll, consisting of a lagoon enclosed by a ridge that preserve the former coral atoll topography (Schofield, 1959).
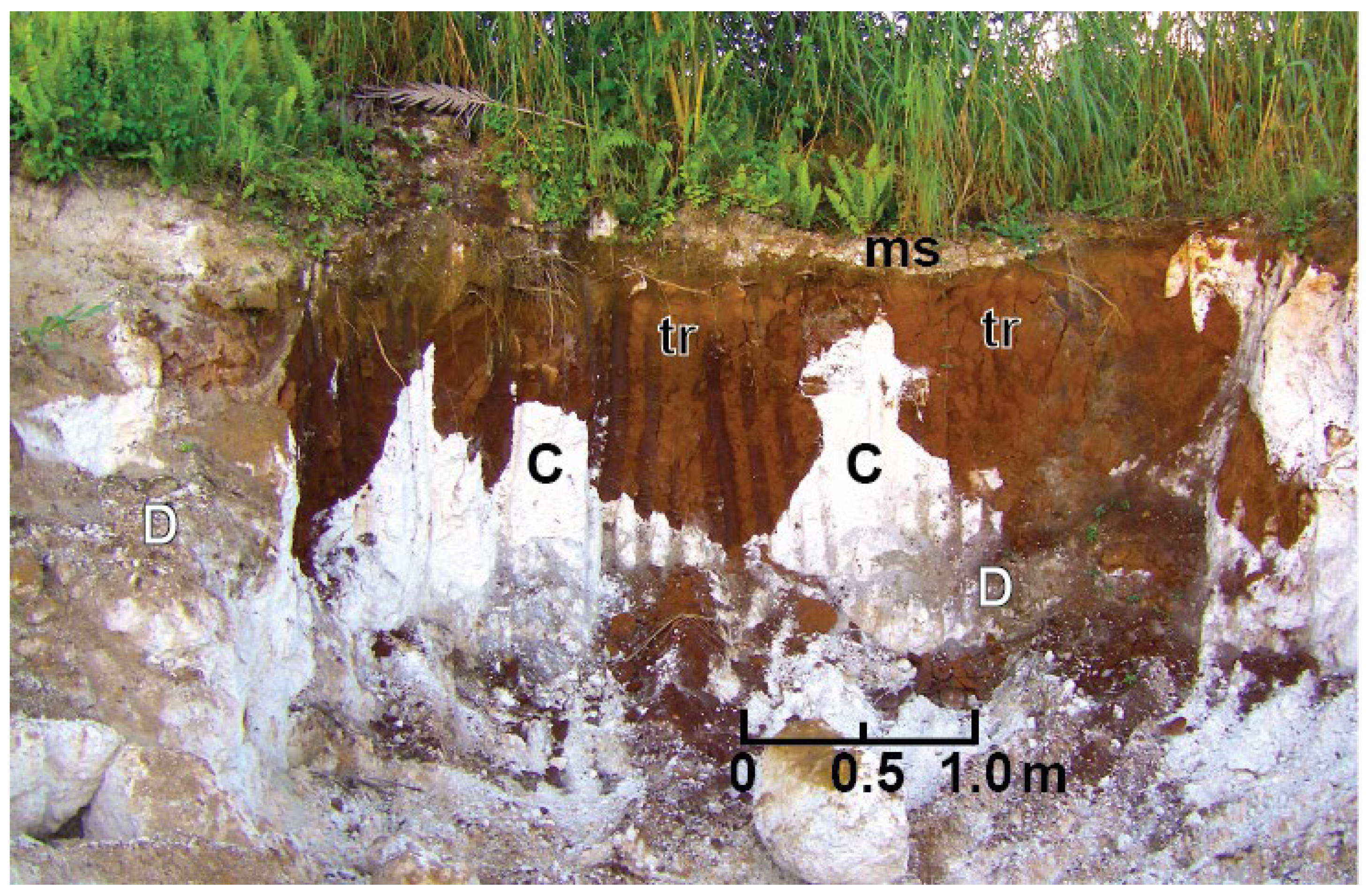
Niue is blanketed by a thin veneer of ~35 cm thick, highly weathered, soils dominated by Fe and Al minerals consisting of goethite, gibbsite and boehmite (Whitehead and Aharon, 2023). The soils have been systematically sampled and investigated in order to explain the origin of their unusually high radioactivity (Fieldes et al., 1960; Schofield, 1967; Whitehead et al., 1993, among others). The Alofi Terrace and the seaward slopes are mantled by tropical black earths (Wright and Van-Westerndorp, 1965). The soils on the remainder of the island are tropical terra rossa that are low in silica and high in iron oxide and alumina. Lithified red to light-brown colored terra rossa soil filling sinkholes in the carbonate sands and overlying massive dolomite is commonly observed in the former lagoon (
Figure 3). The provenance of the highly weathered Niuean soils has long been a subject of debate, bearing on three explicit sources: (i) volcanic ash and meteoric dust (Fields et al., 1960); limestone from the carbonate platform (Whitehead et al., 1993), and (iii) pumice rafts (Whitehead and Aharon, 2023).
Occurrence of odd, solitary rocks underlying soils and overlying sediments on Niue have long been known to the locals who nicknamed the more common yellowish-gray as “Brown Rock” (
Figure 2 A) and the scanty reddish-brown as “Black Rock” (
Figure 2 B). Marsden (1964) described a solitary “Black Rock” found on a shelf ~ 3m asl on the northwest side of Niue as an “oxidized, yellow palagonite-cemented tuff with a hard, thick dark-brown crust containing much CaCO
3 and appearing slightly permeable” (Marsden, 1964, p. 818-819). Neither the collected sample, or the field location have been ever found (Schofield, 1967). More recent, Nunn and Britton (2004) reported of another “Black Rock” type the authors examined on the southern coast near Vaiea (
Figure 1). This “Black Rock”, about 1.1 m in length, projects from the edge of the terrace at ~3.2 m asl and is frequently engulfed by the waves (Fig 2 B). These authors (op.cit.) identified the rock as an “iron-rich deposit” formed by groundwater in cracks within the limestone platform. Survival of the rock in a high-energy environment was explained by its higher resistance to erosion than the surrounding limestone (Nunn and Britton, 2004). Unlike the scarcity of the “Black Rock”, the distribution of the rectangular-flat “Brown Rock” is geographically wider and commonly occurs underlying the soil blanketing the ancient Mutalau barrier reef (i.e., “makatea”) and lagoon (Whitehead and Aharon, 2023) (
Figure 2 A).
A “Brown Rock” from the top of the lagoon interior and a “Black Rock” from the coast near Vaiea were investigated in the field and representative subsamples were taken for further studies. Field observations identified the rocks as “calcretes” (Whitehead and Aharon, 2023). In the following we report on the laboratory methods we employed and the analytical results rendering data on the mineral constituents, textural relation between components, elemental chemistry and carbon and oxygen isotopes that have a bearing on the origin and provenance of the calcretes.
3. Methods and Results
3.1. Mineral Compositions
Several grams of representative rock samples were powdered in an agate mortar, thoroughly mixed and bottled in sealed vials. The mineralogy of the randomly oriented powders was determined by X-Ray Diffraction (XRD) using a benchtop Bruker X-Ray Difractometer. XRD data was collected with Cu Kα radiation by step-scans at 0.04o/2θ increments with fixed counting time of 1.0 sec. Automated quantitative calibration of mineral weight percentages was based on integrated peak intensity procedures.
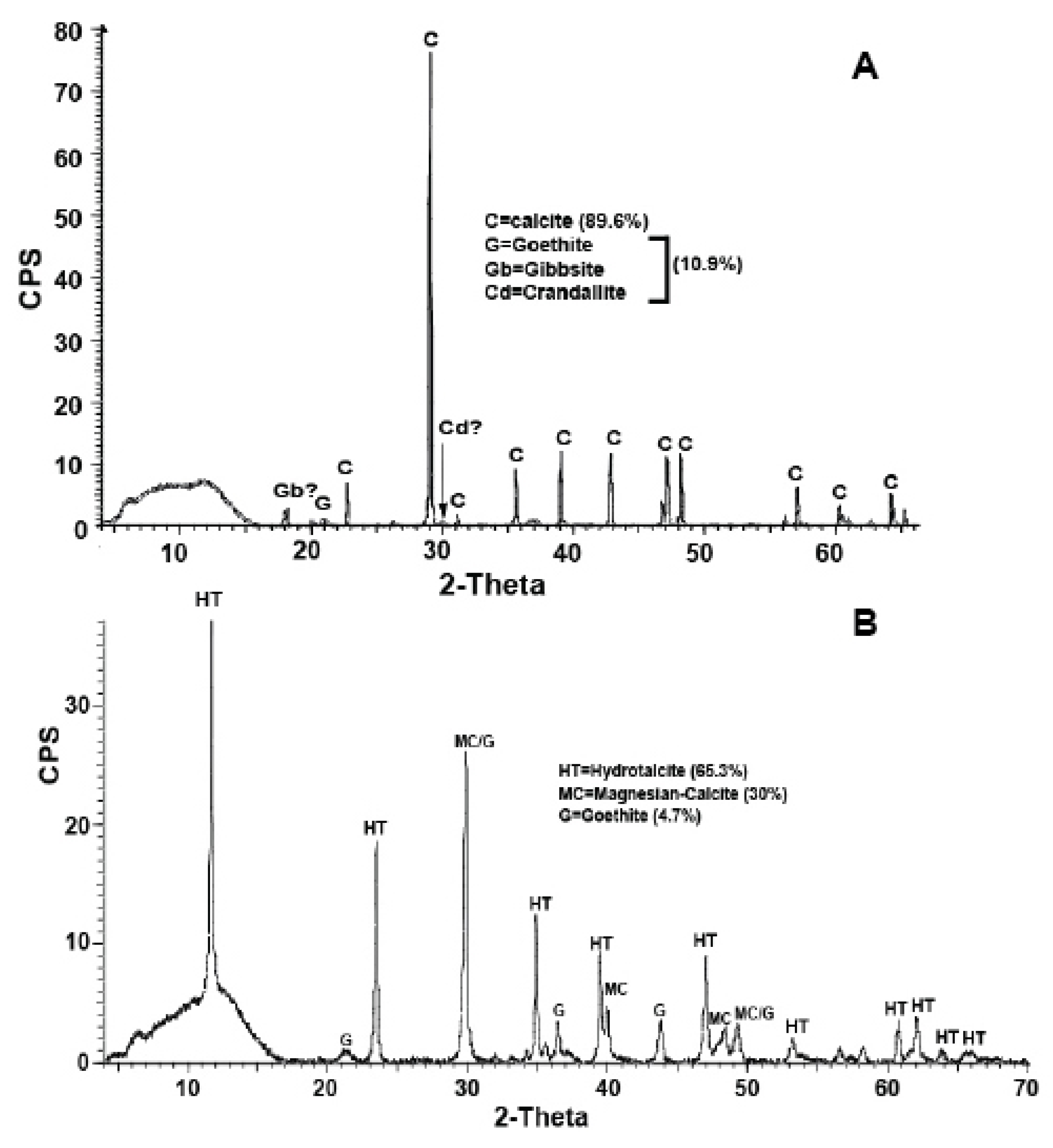
The XRD scans, shown in
Figure 4 A,B, exhibit major dissimilarities between the calcretes. The mineralogy of the “brown rock” (henceforth, PC-C) presents no surprises. It is dominated by low- magnesium calcite at ~90% abundance, followed by a small but significant assemblage of Mg-Al silicates (goethite and gibbsite) and a hydrated Ca-Al phosphate [CaAl
3(PO
4)(PO
3OH)(OH)
6] amounting to ~10% abundance. The peaks of gibbsite and crandallite-related mineral carry a significant uncertainty arising from the overshadowing of the dominant calcite peaks. In contrast, the dominant carbonate minerals in the “Black Rock” (henceforth, HT-C) are hydrotalcite and magnesian-calcite at 65% and 30% abundance, respectively. Traces of goethite are present in the XRD scan (
Figure 4 B). The carbonate minerals of powder aliquots from the two calcretes were preferentially dissolved with dilute HCl and filtered in order to examine the insoluble residue (IR). Concentration of the IRs by a magnet showed the presence of abundant magnetite clusters in the PC-C but total absence in the HT-C.
Hydrotalcite is a layered double Mg and Al hydroxy-carbonate hydrate (LDH) represented by the general formula [Mg6Al2(CO3)(OH)16(H2O)4] (Forano et al., 2013). It is a rare natural mineral and is commonly found in association with weathered ultramafic and serpentinite rocks (O’Neil and Barnes, 1971; Stanimirova, 2001; Oskierski et al., 2013; Turvey et al., 2018). With the two exceptions below, hydrotalcite occurrences are exceptionally rare in sedimentary rocks. The exceptions are the occurrences of HT in high-Mg mudstones from the Kozani Basin in Greece (Hall and Stamatakis, 2000) and a sparse distribution in lithified terra rossa soils from Cayman Islands (Jones, 2021).
3.2. Microfabrics
Polished thin sections were prepared from the PC-C and HT-C and examined under a Nikon SMZ800 petrographic microscope using reflected light under a range of magnifications.
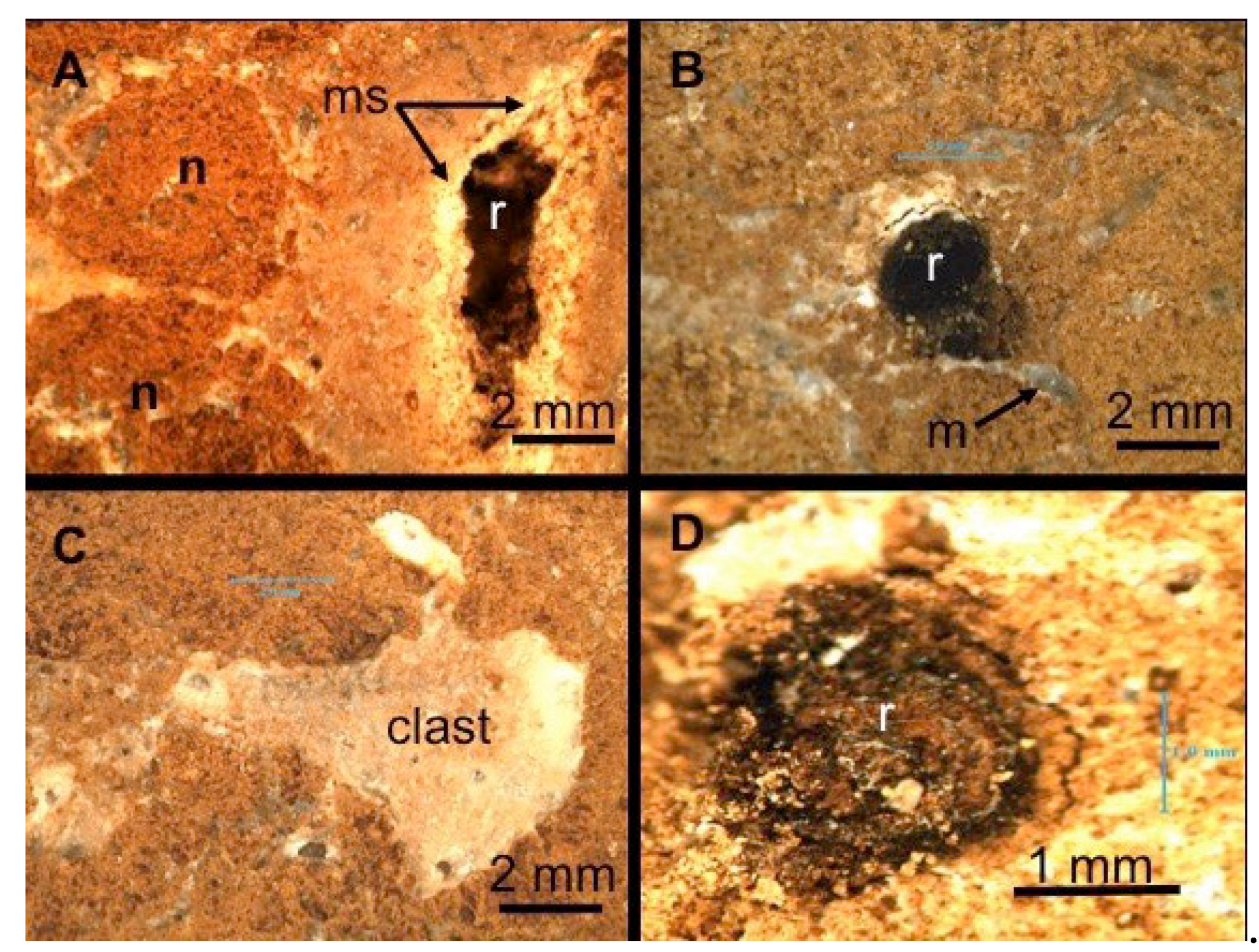
The most outstanding feature that clues in on the pedogenic origin of PC-C is the presence of organic matter-infilling voids and representing decayed root mats (
Figure 5, A and B). The root voids have locally evolved into rhizoliths (calcified roots) as displayed in
Figure 5 A, B and D. The presence of brown-micritic nodules, micrite screens around the root voids, mottled-gray calcites and calcite clasts (
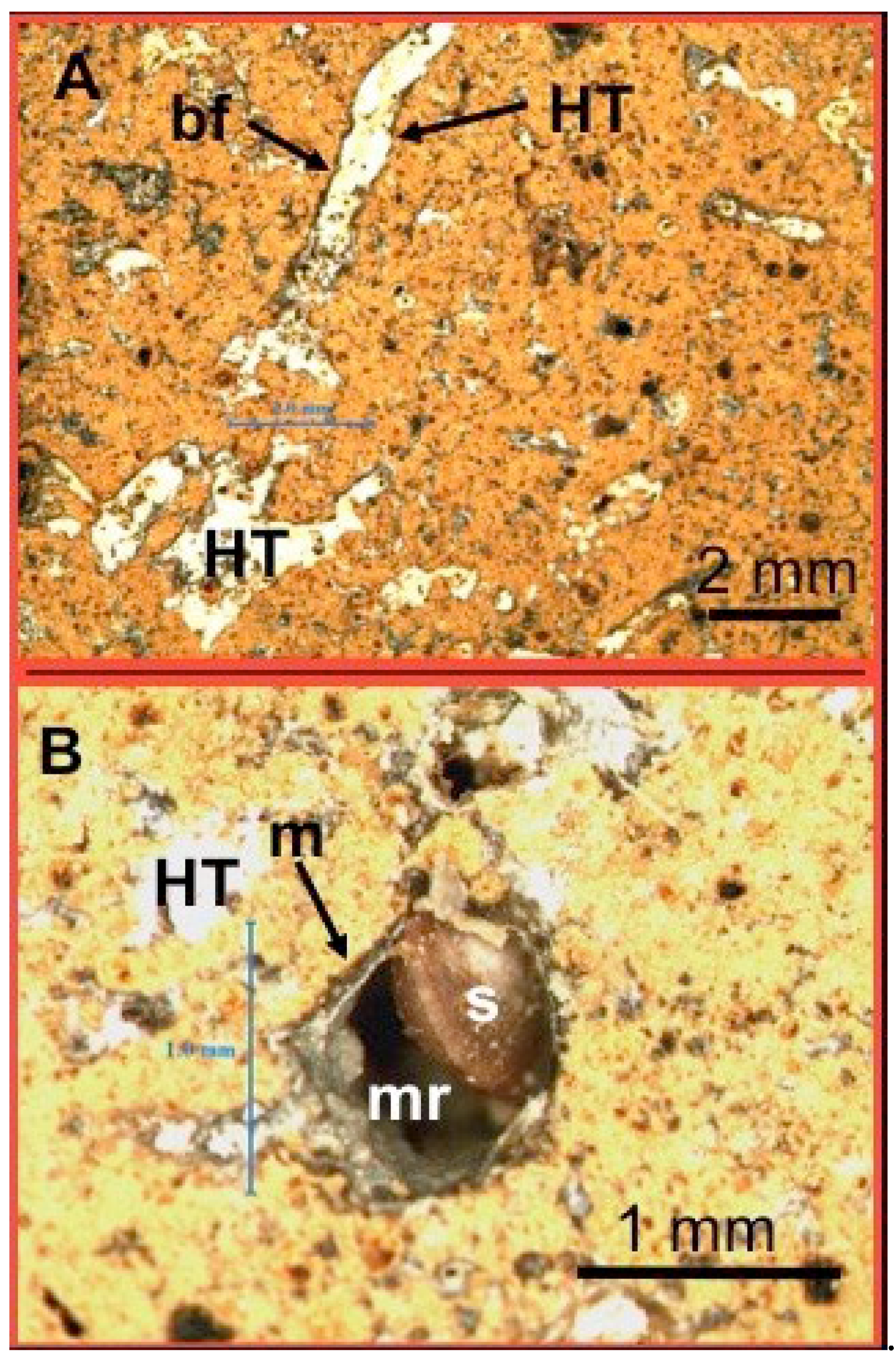
Figure 5 C) point to a pedogenic calcrete fabrics (Wright et al., 1988; Rasbury et al., 2000). Not unlike the PC-C, the HT-C also displays microfabrics typical of calcretes (
Figure 5, A and B). In contrast to PC-C that exhibits high-porosity and immature rhizoliths, the HT-C is more compacted and shows lower porosity attributed to infilling of the rhizoliths with hydrotalcite coated by microbial films (
Figure 5 A). Occasional dissolution voids are observed in
Figure 5 B. The small snail shell that found shelter in the void is likely modern. The PC-C exhibits “immature” fabrics by comparison with the HT-C’s “mature” fabrics. On this basis we surmise that the former maybe of a younger age than the latter.
3.3. Elemental Chemistry
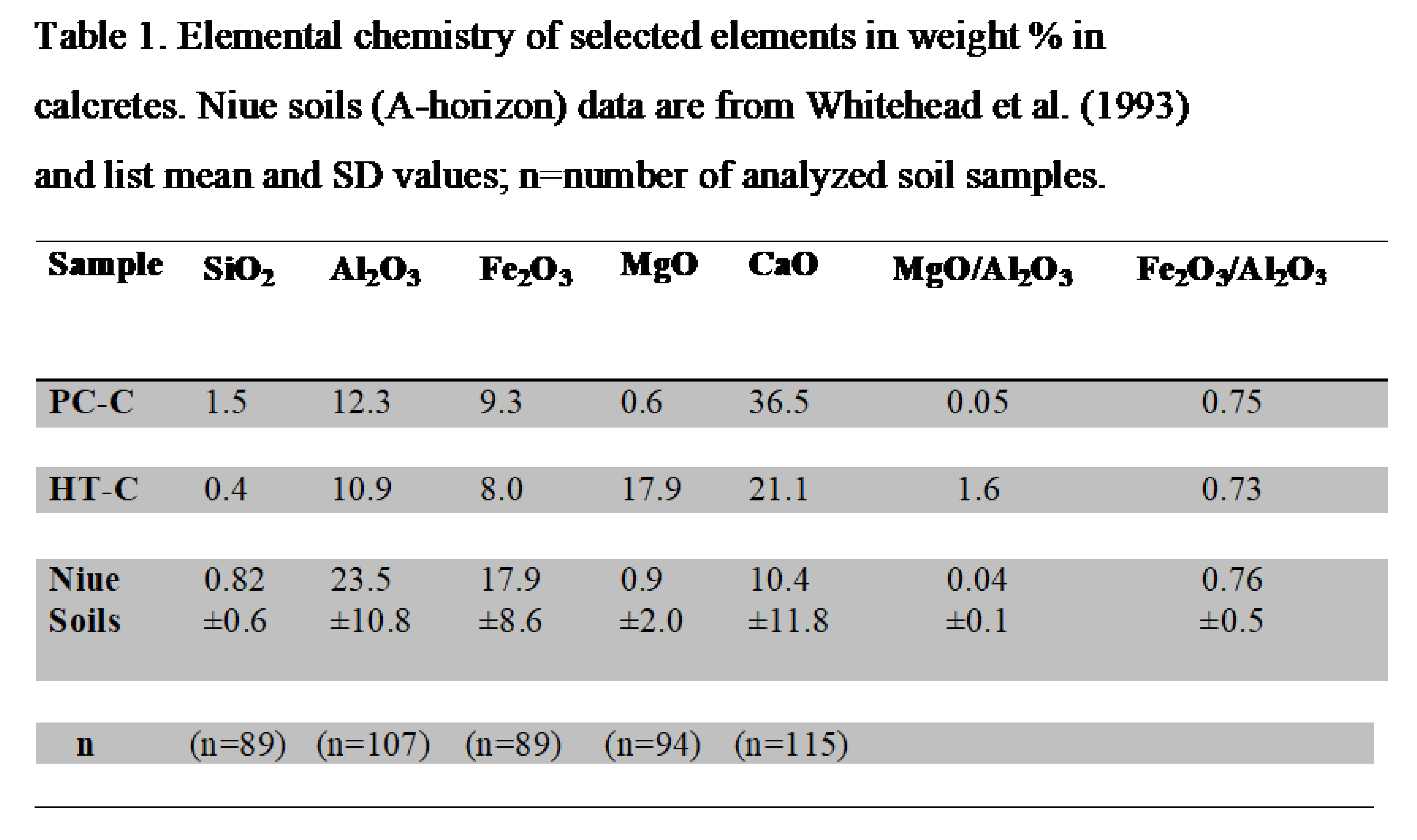
Major oxides composition of PC-C and HT-C were determined by XRF on powdered samples prepared as fused disks, and the results are listed in Table 1. Table 1 also lists
Silica is ~3.7 times higher in PC-C relative to HT-C whereas the latter has ~30 times more Mg than the former (Table 1). The discrepancies are attributed to higher abundance of detrital silica-rich minerals inherited from the soils in PC-C, and the Mg enrichment in HT-C is explained by the dominant presence of Mg-rich hydrotalcite and magnesian calcite.
A Weathering Index (WI) developed by Whitehead and Aharon (2023) serves as a useful indicator of the preferential silica removal during weathering relative to the sum of iron and aluminum concentration: (SiO2/(Al2O3+Fe2O3)). Both calcrete types exhibit exceptionally low WIs values of 0.069 and 0.021 suggesting a high degree of weathering that compares well with the mean WI of 0.02 of the highly weathered Niue soils (Table 1).
3.4. Carbon and Oxygen Isotopes
Well mixed powdered aliquots of ~1.0 mg by weight of PC-C and HT-C were reacted with 100% orthophosphoric acid at 50
oC. The carbon and oxygen isotope values of the evolved and purified CO
2 were determined in a continuous flow mode (CF) using a Gasbench coupled to a modified Delta-Plus Isotope Ratio Mass Spectrometer (CF-IRMS). The
13C/
12C and
18O/
16O ratios are reported in the conventional delta (δ) notation in per mil (‰) relative to the Vienna-Pee Dee Belemnite (V-PDB). Analytical precision for both oxygen and carbon based on standard and sample repeats is ±0.1‰ (1σ). Water δ
18O values listed below are reported on the conventional V-SMOW scale. Conversion from the V-PDB to V-SMOW scale is given by the equation (Kim et al., 2015):
Because the NBS-19 standard is a calcite, no kinetic fractionation factor correction associated with the acid reaction to liberate CO2 was necessary for δ 18O of calcite-dominated PC-C samples. In contrast, the kinetic fractionation factor correction for hydrocalcite-dominated HT-C samples is unknown and hence no correction to the δ 18O values was performed. The total range of the correction factor for a wide range of carbonates is only 1‰ and therefore ignoring it should introduce only a small uncertainty in δ 18O values of hydrocalcite, not exceeding several tenths of a permil (O’Neil and Barnes, 1971).
The δ 13C and δ 18O composition of the paired calcrete subsamples are listed in Table 2. Significant isotope differences observed between the PC-C and HT-C can be summarized as follows. PC-C exhibits substantially greater 13C and 18O depletions (-10.5 to -10.7‰ V-PDB and -5.2 to -5.4‰ V-PDB, respectively) relative to the HT-C (0.8 to 1.7‰ and -1.0 to -1.6‰ V-PDB, respectively).
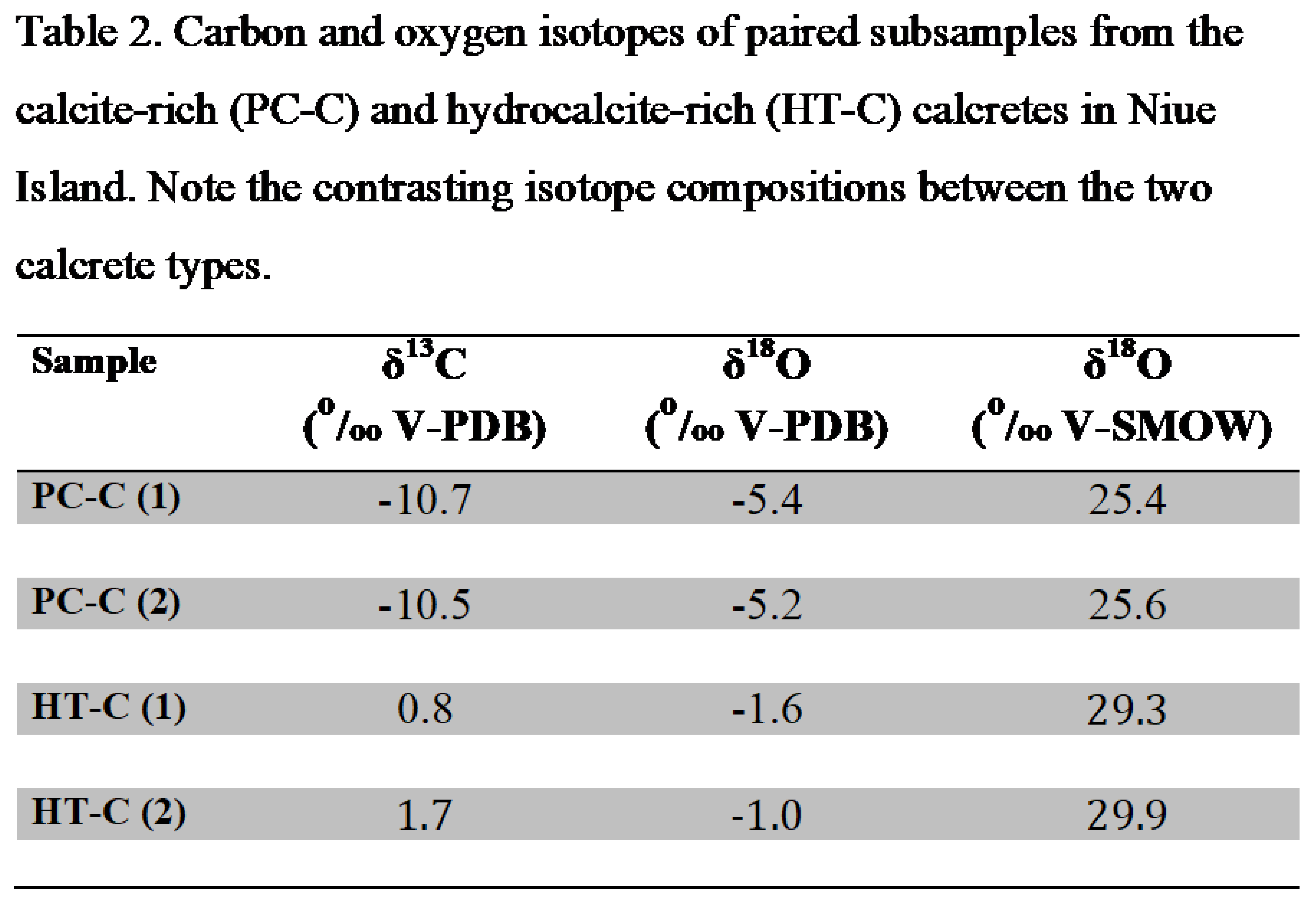
The ambient conditions conducive to the deposition of PC-C can be established on the basis of the δ 13C and δ 18O values because equilibrium isotope fractionations between calcite and Dissolved Inorganic Carbon (DIC) and calcite-H2O are well known (Salomons and Mook, 1986; Kim and O’Neil, 1997). We contend that the PC-C calcrete was formed in isotope equilibrium with the soil fluids on the basis of the following considerations. Measured δ 13C of DIC from Niue are -11.7 ±2.3‰ V-PDB (n=6) in drips from caves representing water in the vadose zone, and -9.1 ±2.7‰V-PDB (n=18) in groundwater (Aharon et al., 2006). Assuming an ambient soil temperature of 25oC which is the annual average air temperature at Niue, the predicted δ 13C composition of a calcite in isotope equilibrium with the DIC would be -8.2 to -10.8‰V-PDB that are, given the uncertainties, in good agreement with the measured δ 13C values in PC-C (Table 2). On the basis of a radiocarbon material mass balance, Aharon et al. (2006) estimated that up to 91% of the carbon in the vadose zone is derived from soil CO2 microbial respiration (δ 13C=-29.4‰V-PDB) and the remainder of 9% from dissolution of the underlying limestone/dolomite (δ 13C=-0.4 ±0.9‰V-PDB, n=149).
Drips from tips of stalactites in coastal caves and groundwater wells on Niue yield δ 18O compositions of -4.5 ±0.1‰ V-SMOW (n=5) and -4.5 ±0.14‰ V-SMOW (n=18), respectively, that represent the mean values of annual rainfall (Aharon et al., 2006). Using the temperature-depended CaCO3-H2O experimental equation of Kim and O’Neil (1997), a predicted calcite deposited in isotope equilibrium with the Niuean waters at the ambient annual temperature of 25oC would yield a δ 18O of -7.1‰V-PDB. The predicted value is roughly close to the measured δ 18O values of the paired PC-C samples (Table 2) considering the likely effect of slight evaporation of the soil fluids that will cause 18O-enrichment in the water, and the uncertainty of soil temperature.
Reconstruction of the ambient conditions conducive to hydrotalcite deposition from carbon and oxygen isotopes is complicated. That is because, with the singular exception below, no studies have reported stable C and O isotope data for pure hydrotalcite, or samples containing hydrotalcite in sedimentary rocks. Exceptional are the δ
13C and δ
18O reports of one hydrotalcite associated with serpentinite (O’Neil and Barnes, 1971) and hydrotalcite mineralization of ultramafic mine tailings (Oskierski et al., 2013; Turvey et al., 2018). Importantly, the isotope fractionations, either equilibrium or kinetic, during hydrotalcite uptake of labile carbonate ions, structural OH groups and interlayer H
2O molecules are unknown. However, given the exposure of the HT-C to atmosphere and seawater (
Figure 2 B) and the association with the Niuean dolomite as a principal source of Mg, we conjecture that the
13C and
18O enrichments in the hydrotalcite (Table 2) were controlled by mixing of three-sources (i) atmospheric CO
2 (δ
13C=-8.5 ±1‰, Mavromatis et al., 2015); (ii) HCO
3 source in dolomite dissolution
(δ 13C and δ 18O of 1.2 to 2.8‰ and 2.8 to 4.2‰, respectively) (Wheeler et al., 1999), and (iii) structural OH and interlayer H2O of seawater provenance (seawater δ 18O=-0.15‰ V-SMOW, Aharon et al., 2006).
4. Discussion
Pedogenic calcretes composed of low-Mg calcite are terrestrial deposits of widespread occurrence that form in soil profiles within the vadose zone (Milnes, 1992; Alonzo-Zarza and Wright, 2010). Calcrete-bearing soils are most common in arid and semi-arid continental settings where annual rainfall amount is <760 mm and where evaporation exceeds rainfall (e.g., in Mediterranean climate) causing moist deficits (Alonzo-Zarza and Wright, 2010). Niue Island, located in the tropics, receives an annual rainfall of 2036 ±538 mm (mean and SD over a 92 yrs period, Rasbury and Aharon, 2006), and as such it seems to be an atypical setting for calcrete formation. In the following we discuss the abiotic and biotic factors involved in the formation of the two contrasting PC-C and HT-C calcretes documented in this study.
4.1. The Rainfall Factor
Rainfall on Niue Island is highly variable on inter-seasonal and inter-annual time scales (Rasbury and Aharon, 2006; Aharon et al., 2006). The inter-seasonal variability is driven by the South Pacific Convergent Zone (SPCZ) that moves seasonally over Niue as an appendix of the Intertropical Convergence Zone (ITCZ). It results in a monsoon season from December to April with a mean monthly rainfall of 307 mm and an average monthly air temperature of 26oC, and a dry season from May to November with a mean monthly rainfall of 84 mm and a mean air temperature of 24oC. The El-Niño-Southern Oscillation (ENSO) phase changes with 4.3 to 6.0 years periodicity exerts a dominant role on rainfall variability on inter-annual intervals. Severe droughts are associated with El-Niño events, respectively (Aharon et al., 2006). The regularity of rainfall switches is interrupted periodically by powerful cyclones accompanied by torrential rainfall. Twelve tropical cyclones struck Niue in the time intervals 1970-1975 and 1980-1985 (Rasbury and Aharon, 2006). Importantly, there is no surface drainage on the island and consequently rapid rainfall infiltration occurs as a result of the high porosity of the carbonate sediments. It can be concluded, therefore, that large annual rainfall values are concealing the droughts on inter-seasonal and inter-annual time scales that are conducive to calcretes formation in Niue.
4.2. Abiotic and Biotic Factors
The data presented in the preceding section suggest that both abiotic and biotic processes are controlling the deposition of the PC-C calcretes. These are (i) excess evaporation over rainfall during droughts; (ii) dissolution of the carbonates and leaching of Ca in the vadose zone (iii) invasion of atmospheric CO2; (iv) microbial and roots respiration in the soil rhizosphere controlling the pCO2; (v) CO2 degassing, and (vi) elevated Ca and HCO3 solutes from biogenic and abiotic sources give rise to supersaturation and precipitation of low-Mg calcite.
The data acquired from the HT-C contradict previous identifications as a palagonite tuff (Marsden, 1964; Schofield, 1967) or iron-rich rock ( Nunn and Britton, 2004) and establish pertinence to an exceptionally rare class of calcretes not previously acknowledged that are dominated by hydrotalcite. The hydrotalcite abundance in the HT-C calcrete rises questions on the source of Mg and the factors controlling its deposition. Association of hydrotalcite mineralization with serpentinite and ultramafic rocks and experimental studies prompted the suggestion that Mg released in the groundwater by weathering and leaching of Mg-silicates (e.g., olivine) and /or Mg-hydroxides (e.g., brucite), react with atmospheric-derived CO
2 to produce layered double hydroxides (LDH) (Oskierski et al., 2013; Turvey et al. (2018; Scott et al., 2021). Absence of ultramafic rocks in the Niue shallow subsurface makes such a Mg-source unattainable. Here we propose that the Mg source is in the abundant dolomite underlying the Niuean soils (
Figure 3).
On the basis of our observations and analytical data we propose the following model of HT-C calcrete formation: (i) erosion and transport of MgO-rich soil material from the nearest Mutalau crest to the terraced coast; (ii) dolomite dissolution and CO2 uptake from atmospheric and seawater sources, and (iii) induration of the terra rossa soil with hydrotalcite and Mg-calcite. Our proposed HT-C depositional model is supported by the following arguments:
(i) Kennedy et al. (2012) study established that Niue Island has a high potential for sediment erosion and transport. The highly dynamic environment will account for the transport of terra rossa (
Figure 3) to the coast (
Figure 2 B).
(ii) Dolomite serves as the principal source of Mg.
(iii) The 18O-enrichment exhibited by the hydrotalcite+Mg-calcite minerals is consistent with deposition from seawater whose waves regularly flood the calcrete,
(iv) The 13C-enrichment is compatible with mixed carbon sources from microbial respiration, kinetic invasion of atmospheric CO2, HCO3 from seawater and CaMg(CO3)2 dissolution. Our proposed model of hydrotalcite precipitation by dolomite-seawater interaction is supported by Kameda et al. (2000) laboratory synthesis of hydrotalcite by reaction of dolomite with seawater.
5. Summary
(i) Droughts occurring on inter-seasonal and inter-annual frequencies frame the uplifted former atoll of Niue Island in the Southwest Pacific as a suitable setting for calcretes deposition.
(ii) Two types of calcretes have been documented on the island: (i) a “common” calcite-rich calcrete associated with the soils in the paleo-lagoon, and (ii) a unique hydrotalcite-rich calcrete occurring on the coastal terrace and bathed by high waves.
(iii) Biotic and abiotic factors promoting deposition of the calcretes were identified from application of geochemical assays of XRD, optical petrography, elemental chemistry and carbon and oxygen isotopes.
(iv) Evaporation of vadose groundwater, CO2 derived from microbial and root respiration lowering the fluid pH and causing limestone dissolution, release of Ca in solution and calcite supersaturation are the primary factors controlling the pedogenic calcrete formation.
(v) Hydrotalcite minerals [Mg6Al2(CO3)(OH)16(H2O)4] are exceptionally rare in nature and therefore the occurrence of a hydrotalcite-rich calcrete is unique.
(vi) The plausible mechanism of hydrotalcite deposition is interaction between dolomite and seawater, a process analogous to the hydrotalcite synthesis in the laboratory.
(vii) Calcretes are important archives of the ambient terrestrial environments and ecosystems. A systematic sampling and laboratory studies of the calcretes in Niue Island offer valuable recorders of past rainfall variability and soil development changes in a understudied region of the Southwest Pacific impacted by droughts attributed to SPCZ and ENSO events.
6. Acknowledgements
We thank Mr. Kyle Liu, Section Managing Editor of the journal “Minerals” for the invitation to contribute a paper to the journal, James Donahoe for assistance with XRD identification of the minerals in the calcretes, and Joe Lambert for assistance with stable isotope determinations. Ms. B. K. Whitehead is thanked for assistance in the field investigation.
References
- Whitehead, N. E. and Aharon, P. (2023) New geochemical diagnostic methods point to pumice rafts as the most likely source for Niue soils. Pacific Science 77 (4), 1-13.
- Marsden, E. (1964) Radioactivity of some rocks, soils, plants, and bones. The University of Chicago Press, Chicago Ill, USA, 807-824.
- Schofield, J. C. (1959) The geology and hydrology of Niue Island, South Pacific. New Zealand Geological Survey Bulletin 62, 28 p.
- Schofield, J. C. (1967) Origin of radioactivity at Niue Island. J. Geol. Geophys. 10 (6), 1362-1371.
- Nunn, P. D. and Britton, M. R. (2004) The long term evolution of Niue Island. Geographical perspectives on the rock of Polynesia. INSULA, Paris, 31-74.
- Milnes, A. R. (1992) Chapter 13-Calcrete. Developments in Earth Surface Processes 2, 309-347.
- Alonso-Zarza, A. M. and Wright, V. P. (2010) Calcretes. Developments in Sedimentology 61 (5), 225-267.
- Hill, P. J. (1983) Volcanic core of Niue Island, Southwest Pacific Ocean. Journal of Australian Geology and Geophysics 8, 323-328.
- Lu, G. , Aharon, P., Wheeler, C. W., McCabe, C. (1996) Magnetostratigraphy of the uplifted former atoll of Niue, South Pacific: Implications for accretion history and carbonate diagenesis. Sed. Geol. 105, 259-274.
- Dubois, J., Launay, J., Recy, J. (1975) Some new evidence on lithospheric bulges close to island arcs. Tectonophysics 26, 189-196.
- Aharon, P. , Goldstein, S. L., Wheeler, C. W., Jacobsen, G. (1993) Sea-level events in the South Pacific linked with the Messinian Salinity Crisis. Geology 21, 771-775.
- Wheeler, C. and Aharon, P. (1997) Geology and Hydrology of Niue. In Geology and Hydrogeology of Carbonate Islands, Developments in Sedimentology 54 (Eds. H. L. Vacher and T. Quinn), Elsevier Science, Chapter 17, 537-564.
- Kennedy, D. M. , Marsters, T. H., Woods, J. L. D., Woodroffe, C. D. (2012) Shore platform development on an uplifting limestone island over multiple sea-level cycles, Niue, South Pacific. Geomorphology 141-142, 170-182.
- Marsters, T. H. and Kennedy, D. M. (2014) Beach development on an uplifted coral atoll: Niue, south west Pacific. Geomorphology 222, 82-91.
- Pauley, G. and Speenser, T. (1992) Niue Island: geologic and faunatic history of a Pliocene atoll. Pacific Science Association Bulletin 44, 21-23.
- Wheeler, C. W. , Aharon, P., Ferrell, R. E. (1999) Successions of Late Cenozoic platform dolomites distinguished by texture, geochemistry, and crystal chemistry: Niue, South Pacific. J. Sed. Res. 69 (1), 239-255.
- Fieldes, M., Bealing, G., Claridge, G. G., Wells, N, Taylor, N. H. (1960) Mineralogy and radioactivity of Niue Island soils. N. Z. Journal of Science 3(4), 658-675.
- Whitehead, N. E., Hunt, J., Leslie, D., Rankin, P. (1993) The elemental content of Niue Island soils as an indicator of their origin. N. Z. Journal of Geology and Geophysics 36 (2), 243-254.
- Wright, A. C. S. and Van-Westerndorp, F. J. (1965) Soils and agriculture of Niue Island. N.Z. Soil Bur. Bull Dep. Sci. & Ind. Res. 17, 80 pp.
- Forano, C., Costantino, U., Prévot, V., Taviot-Gueho, C. (2013) Layered Double Hydroxides (LDH). Developments in Clay Science 5, Ch. 14.1, 745-782.
- O’Neil, J. R. and Barnes, I. (1971) 13C and 18O compositions in some fresh-water carbonates associated with ultramafic rocks and serpentinites: western United States. Geochim. Cosmochim. Acta 35, 687-697.
- Stanimirova, T. (2001) Hydrotalcite polytypes from Snarum, Norway. Annuaire de L’Universite de Sofia “St. Kliment Ohridski”, Faculte de Geologie et Geographie 1 (94), 73-80.
- Oskierski, H. C. , Dlugogorski, B. Z., Jacobsen, G. (2013) Sequestration of atmospheric CO2 in chrysotile mine tailings of the Woodreef Asbestos Mine, Australia: Quantitative mineralogy, isotopic fingerprinting and carbonation rates. Chem. Geol. 358, 156-169.
- Turvey, C. C. , Wilson, S., Hamilton, J. L., Tait, A. W., McCutcheon, J., Beinlich, A., Fallon, S. J., Dipple, G. M., Southam, G. (2018) Hydrotalcites and hydrated Mg-carbonates as carbon sinks in serpentinite mineral wastes from the Woodsreef chrysotile mine, New South Weles, Australia: Controls on carbonate mineralogy and efficiency of CO2 air capture in mine tailings. Int. J. of Greenhouse Gas Control 79, 38-60.
- Hall, A. and Stamatakis, M. G. (2000) Hydrotalcite and an amorphous clay mineral in high-magnesium mudstones from Kozani Basin, Greece. J. Sed. Res. 70 (3), 549-558.
- Jones, B. (2021) Formation, dispersion and accumulation of terra rossa on the Cayman Islands. Sedimentology 68, 1964-2008.
- Wright, V. P. , Platt, N. H., Wimbledon, W. (1988) Biogenic laminar calcretes: evidence of calcified root mat horizons in paleosols. Sedimentology 35, 603-620.
- Rasbury, E. T., Meyers, W. J., Hanson, G. N., Goldstein, R. H., Saller, A. H. (2000) Relationship of Uranium to petrography of caliche paleosols with application to precisely dating the time of sedimentation. J. Sed. Res. 70 (3), 604-618.
- Kim, S.-T. , Coplen, T. B., Horita, J. (2015) Normalization of stable isotope data for carbonate minerals: Implementation of IUPAC guidelines. Geochim. Cosmochim. Acta 158, 276-289.
- Salomons, W. and Mook, W. G. (1986) Isotope geochemistry of carbonates in the weathering zone. Handbook of environmental isotope geochemistry 2, 239-269.
- Kim, S.-T. and O’Neil, J. R. (1997) Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates. Geochim. Cosmochim. Acta 61 (16), 3461-3475.
- Aharon, P. , Rasbury, M., Murgulet, V. (2006) Caves of Niue Island, South Pacific: Speleothems and water geochemistry. In Harmon, R. S. and Wicks, C. (eds.), Perspectives on karst geomorphology, hydrology, and geochemistry. Geol. Soc. Am. Spec. Paper 404, 283-295.
- Mavromatis, V. , Bundeleva, I. A., Shirokova, L. S., Millo, C., Pokrovsky, O. S., Benezeth, P., Ader, M., Oelkers, E. H. (2015) The continuous re-equilibration of carbon isotope compositions of hydrous Mg carbonates in the presence of cyanobacteria. Chem. Geol. 404, 41-51.
- Rasbury, M. and Aharon, Paul (2006) ENSO-controlled rainfall variability records archived in tropical stalagmites from the mid-ocean island of Niue, South Pacific. Geochem. Geophys. Geosyst. 7(7). [CrossRef]
- Scott, A. , Oze, C., Shah, V., Yang, N., Shanks, B., Cheeseman, C., Marshall, A., Watson, M. (2021) Transformation of abundant magnesium silicate minerals for enhanced CO2 sequestration. Comm. Earth and Environ. doi.org/10.1038/s43247-021-00099-6.
- Kameda, T., Yoshioka, T., Uchida, M., Okuwaki, A. (2006) Synthesis of hydrocalcite using magnesium from seawater and dolomite. Molecular Crystals and Liquid Crystals Science and Technology. Section A. Molecular Crystals and Liquid Crystals 341(2), 407-412. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).