Submitted:
01 August 2024
Posted:
01 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Design
2.3. Preparation of Ultra-Fine Bubble Water
2.4. In Vivo WBI with X-Rays
2.5. In Vitro Irradiation with X-Rays and Electron Spin Resonance (ESR) Spectroscopy
2.6. Serum Collection
2.7. Quantitative Analysis of 8-Hydroxydeoxyguanosine (8-OHdG)
2.8. Liquid chromatography-Tandem Mass Spectrometry (LC-MS/MS)
2.9. Identification of Differentially Expressed Proteins and Enrichment Analysis
2.10. Statistical Analyses
3. Results
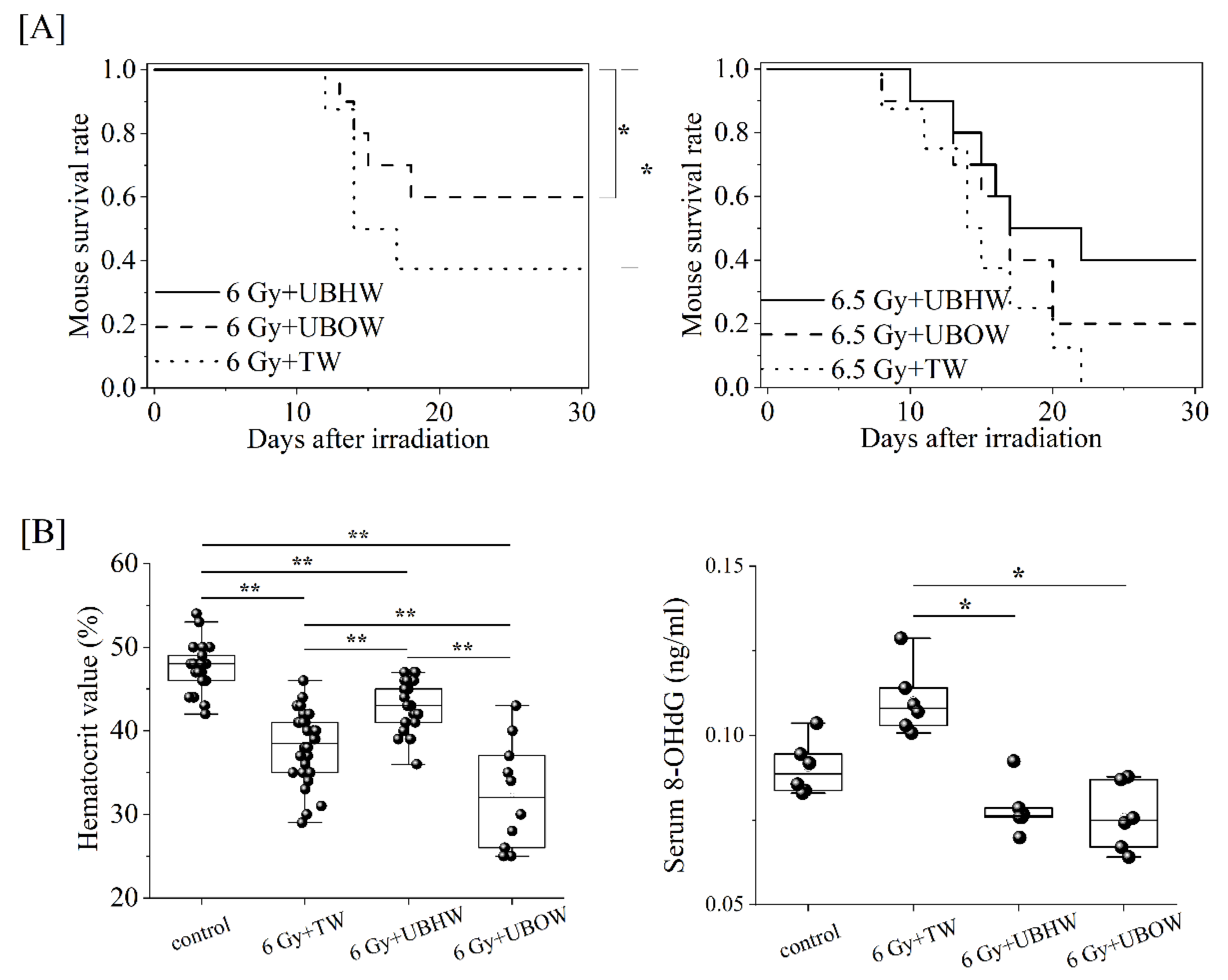
3.1. Survival of Mice Treated with Ultra-Fine Bubble Water Up To Day 30
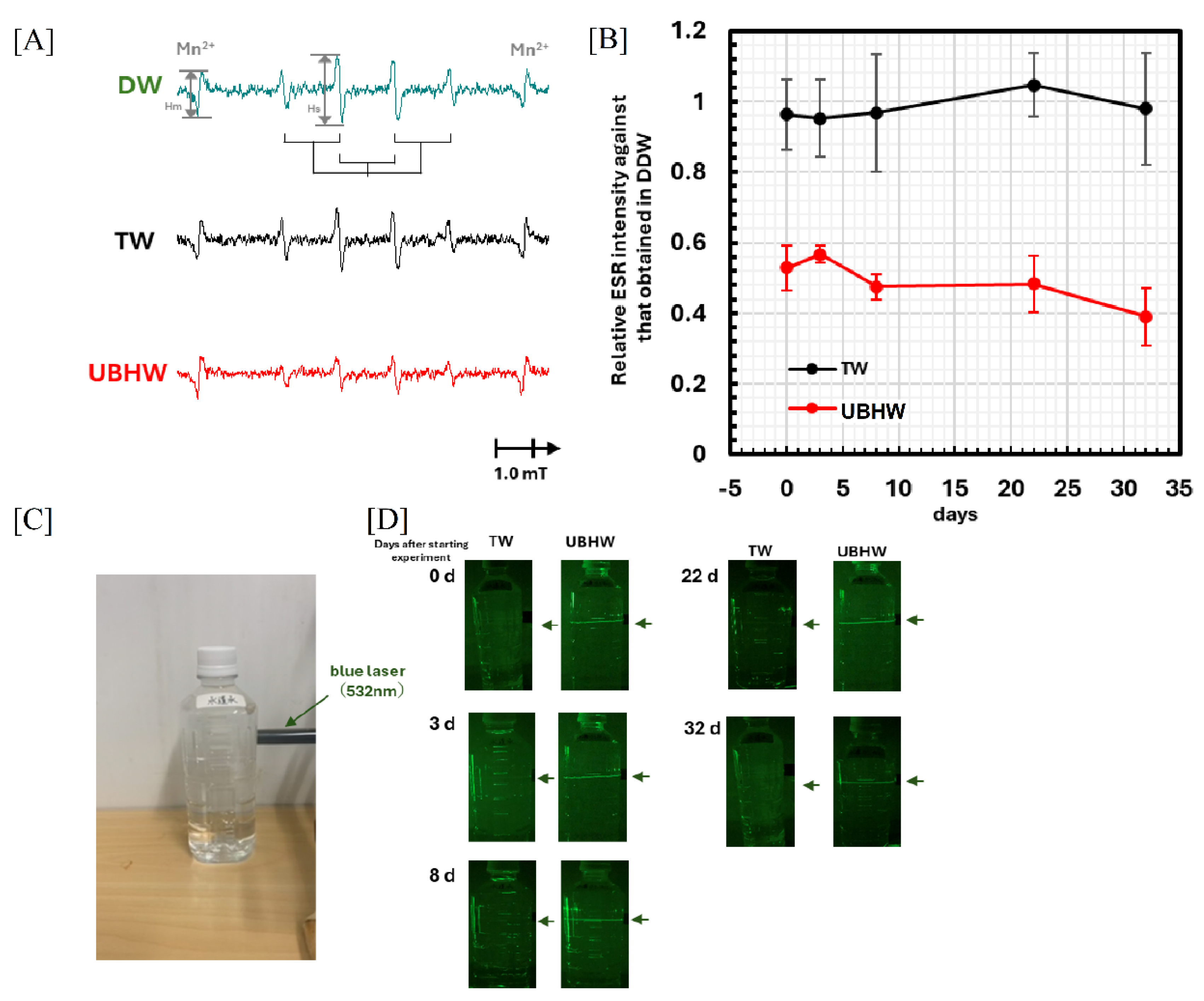
3.2. Measurement of scavenging ability of UBHW against hydroxy radicals by ESR and spin-trapping technique
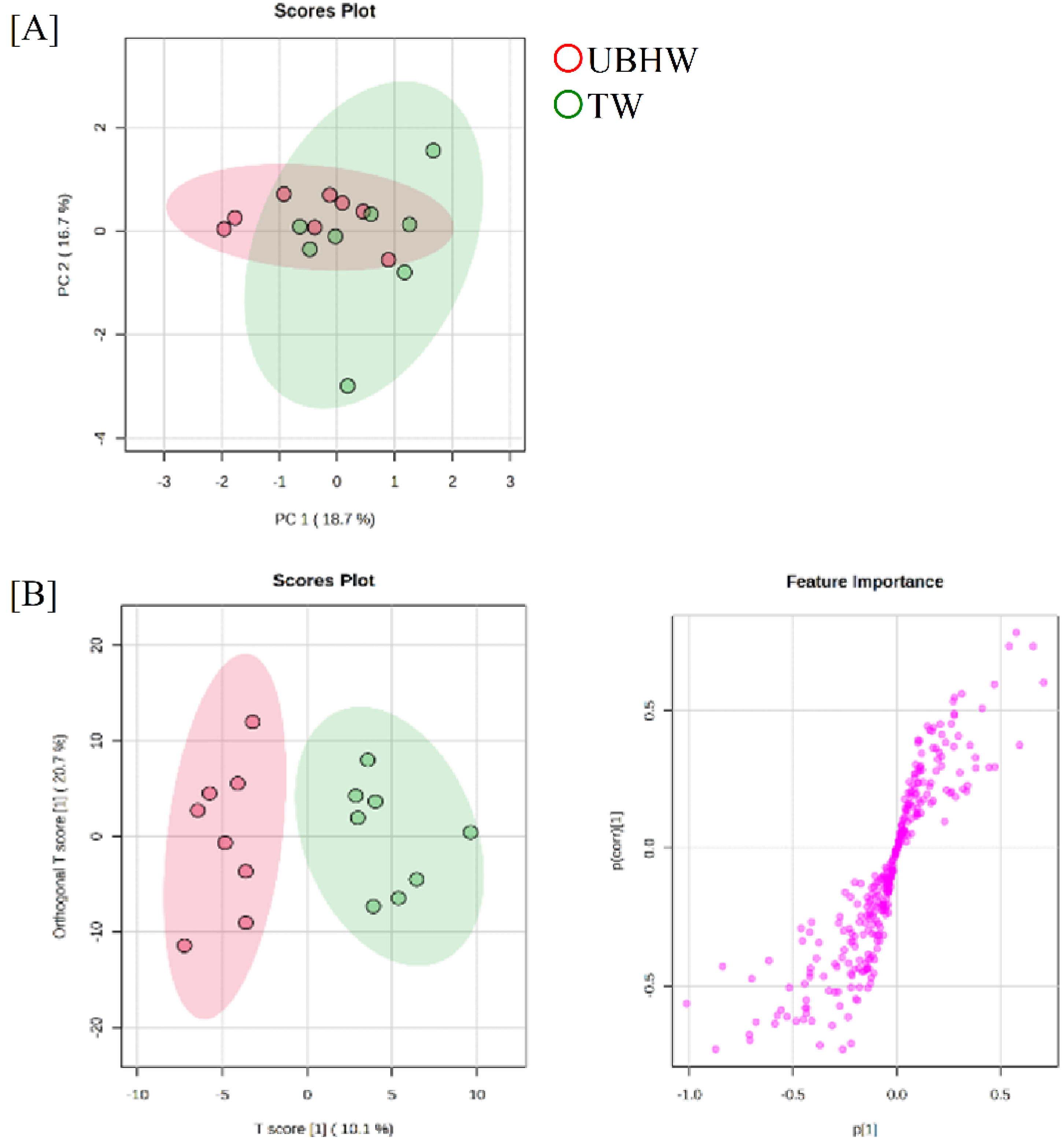
3.3. Proteome analysis of mice treated with UBHW
4. Discussion
| Mus musculus (References) | Significant proteins | Expected | Fold enrichment |
P value | |
|---|---|---|---|---|---|
| GO biological process complete | |||||
| inflammatory response | 544 | O09049, P09581, Q9Z121, P35230, Q61508 | 0.20 | 25.68 | 0.0044 |
| regulation of cell population proliferation | 1786 | O09049, P09581, Q62351, P35230, Q00724, Q61508, Q06318 | 0.65 | 10.77 | 0.0016 |
| regulation of immune system process | 1661 | O09049, P09581, Q62351, Q00724, Q61508, Q06318 | 0.60 | 9.93 | 0.0421 |
| response to organic substance | 2499 | O09049, P09581, Q9Z121, Q62351, P35230, Q00724, Q06318 | 0.91 | 7.70 | 0.0163 |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Shim, G.; de Toledo, SM.; Azzam, E.I. The translationally controlled tumor protein and the cellular response to ionizing radiation-induced DNA damage. Results Probl Cell Differ 2017, 64, 227–253. [Google Scholar] [CrossRef] [PubMed]
- Helm, J.S.; Rudel, R.A. Adverse outcome pathways for ionizing radiation and breast cancer involve direct and indirect DNA damage, oxidative stress, inflammation, genomic instability, and interaction with hormonal regulation of the breast. Arch Toxicol 2020, 94, 1511–1549. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 2017, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Dohi, K.; Satoh, K.; Miyamoto, K.; Momma, S.; Fukuda, K.; Higuchi, R.; Ohtaki, H.; Banks, WA. Molecular hydrogen in the treatment of acute and chronic neurological conditions: mechanisms of protection and routes of administration. J Clin Biochem Nutr 2017, 61, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, S.; Xu, J.; Wang, T. Hydrogen therapy in cardiovascular and metabolic diseases: from bench to bedside. Cell Physiol Biochem 2018, 47, 1–10. [Google Scholar] [CrossRef]
- Matei, N.; Camara, R.; Zhang, J.H. Emerging mechanisms and novel applications of hydrogen gas therapy. Med Gas Res 2018, 8, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, Y.; Yang, P.; Liu, J. Hydrogen as a complementary therapy against ischemic stroke: A review of the evidence. J Neurol Sci 2019, 396, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Tamura, T. Hydrogen Gas Therapy: From Preclinical Studies to Clinical Trials. Curr Pharm Des 2021, 27, 650–658. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, Y.; Wu, S.; Wu, W.; Deng, Y.; Shao, A. Molecular hydrogen: A potential radioprotective agent. Biomed Pharmacother 2020, 130, 110589. [Google Scholar] [CrossRef]
- Hirano, S.I.; Ichikawa, Y.; Sato, B.; Yamamoto, H.; Takefuji, Y.; Satoh, F. Molecular hydrogen as a potential clinically applicable radioprotective agent. Int J Mol Sci 2021, 22, 4566. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Thuyet, D.Q.; Saito, M.; Yoshimoto, T. Antioxidant activity of hydrogen nanobubbles in water with different reactive oxygen species both in vivo and in vitro. Langmuir 2018, 34, 11878–11885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, W.; Li, X.; Wang, W.X.; Liu, S. Enhanced removal of free radicals by aqueous hydrogen nanobubbles and their role in oxidative stress. Environ Sci Technol 2022, 56, 15096–15107. [Google Scholar] [CrossRef] [PubMed]
- Unites States Patent. Available online: https://image-ppubs.uspto.gov/dirsearch-public/print/downloadPdf/10500553 (accessed on 12 May 2024).
- Kamimura, C.; Ohba, R.; Yamaguchi, M.; Hosoda, M.; Kashiwakura, I. Functional characteristics of antioxidant long-life ultra-fine bubble hydrogen water. Inorganics 2024, 12, 141. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Tatara, Y.; Nugraha, E.D.; Ramadhani, D.; Tamakuma, Y.; Sato, Y.; Miura, T.; Hosoda, M.; Yoshinaga, S.; Syaifudin, M.; Kashiwakura, I.; Tokonami, S. Detection of biological responses to low-dose radiation in humans. Free Radic Biol Med 2022, 184, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R. Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med 1987, 3, 259–303. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.; Rosen, G.M.; Rauckman, E.J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys 1980, 200, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Farhataziz, Ross, A. B. Selected specific rates of reactions of transients from water in aqueous solution. III. Hydroxyl radical and perhydroxyl radical and their radical ions. NSRDS-NBS 1977, 59, 126. [Google Scholar]
- Nirmalkar, N.; Pacek, A.W.; Barigou, M. On the Existence and Stability of Bulk Nanobubbles. Langmuir 2018, 34, 10964–10973. [Google Scholar] [CrossRef]
- Qian, L.; Shen, J.; Chuai, Y.; Cai, J. Hydrogen as a new class of radioprotective agent. Int J Biol Sci 2013, 9, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhao, D.; Lei, X.; Zhao, H.; Yang, Y.; Zhang, P.; Liu, P.; Xu, Y.; Zhu, M.; Liu, H.; Chen, Y.; Chuai, Y.; Li, B.; Gao, F.; Cai, J. Protective Effects of Hydrogen against Low-Dose Long-Term Radiation-Induced Damage to the Behavioral Performances, Hematopoietic System, Genital System, and Splenic Lymphocytes in Mice. Oxid Med Cell Longev 2016, 2016, 1947819. [Google Scholar] [CrossRef]
- Qiu, X.; Dong, K.; Guan, J.; He, J.M. Hydrogen attenuates radiation-induced intestinal damage by reducing oxidative stress and inflammatory response. Int Immunopharmacol 2020, 84, 106517. [Google Scholar] [CrossRef]
- Guan, B.; Li, D.; Meng, A. Development of radiation countermeasure agents for acute radiation syndromes. Animal Model Exp Med 2023, 6, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Cui, P.; Zhou, S.; Qiu, L.; Huang, H.; Wang, C.; Wang, J. Advances of Amifostine in Radiation Protection: Administration and Delivery. Mol Pharm 2023, 20, 5383–5395. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Joseph, S.; Albert, A.; Thomas, T.V.; Nittala, M.R.; Woods, W.C.; Vijayakumar, S.; Packianathan, S. Use of Amifostine for Cytoprotection during Radiation Therapy: A Review. Oncology 2020, 98, 61–80. [Google Scholar] [CrossRef]
- Bahat, Z.; Cobanoglu, U.; Ulku, C.; Kalyoncu, N.İ.; Caner, K.S.; Yavuz, M.N. Could Amifostine Prevent Experimental Radiotherapy-Induced Acute Pericarditis? Asian Pac J Cancer Prev 2022, 23, 3209–3213. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Maltezos, E. Amifostine administration during radiotherapy for cancer patients with genetic, autoimmune, metabolic and other diseases. Anticancer Drugs 2006, 17, 133–138. [Google Scholar] [CrossRef]
- Kouvaris, J.R.; Kouloulias, V.E.; Vlahos, L.J. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist 2007, 12, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, T.H.; Brizel, D.M. The role of amifostine as a radioprotector. Oncology (Williston Park) 2001, 15, 1349–1354. [Google Scholar] [PubMed]
- Hirano, S.I.; Ichikawa, Y.; Kurokawa, R.; Takefuji, Y.; Satoh, F.A. “philosophical molecule,” hydrogen may overcome senescence and intractable diseases. Med Gas Res 2020, 10, 47–49. [Google Scholar] [CrossRef]
- Hirano, S.; Ichikawa, Y.; Sato, B.; Satoh, F.; Takefuji, Y. Hydrogen is promising for medical applications. Clean Technol 2020, 2, 529–541. [Google Scholar] [CrossRef]
- Dobryszycka, W. Biological functions of haptoglobin--new pieces to an old puzzle. Eur J Clin Chem Clin Biochem 1997, 35, 647–654. [Google Scholar] [PubMed]
- Karlaftis, V.; Perera, S.; Monagle, P.; Ignjatovic, V. Importance of post-translational modifications on the function of key haemostatic proteins. Blood Coagul Fibrinolysis 2016, 27, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Beishuizen, A.; Thijs, L.G.; Haanen, C.; Vermes, I. Macrophage migration inhibitory factor and hypothalamo-pituitary-adrenal function during critical illness. J Clin Endocrinol Metab 2011, 86, 2811–2816. [Google Scholar] [CrossRef] [PubMed]
- Labat-Robert, J. Cell-matrix interactions in aging: role of receptors and matricryptins. Ageing Res Rev 2004, 3, 233–247. [Google Scholar] [CrossRef]
- Pintér, P.; Alpár, A. The Role of Extracellular Matrix in Human Neurodegenerative Diseases. Int J Mol Sci 2022, 23, 11085. [Google Scholar] [CrossRef] [PubMed]
- Pakianathan, D.R. Extracellular matrix proteins and leukocyte function. J Leukoc Biol 1995, 57, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Chang, T.S.; Bae, Y.S.; Lee, S.R.; Kang, S.W. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol 2003, 14, S211–S215. [Google Scholar] [CrossRef]
- Radyuk, S.N. Mechanisms Underlying the Biological Effects of Molecular Hydrogen. Curr Pharm Des 2021, 27, 626–735. [Google Scholar] [CrossRef]
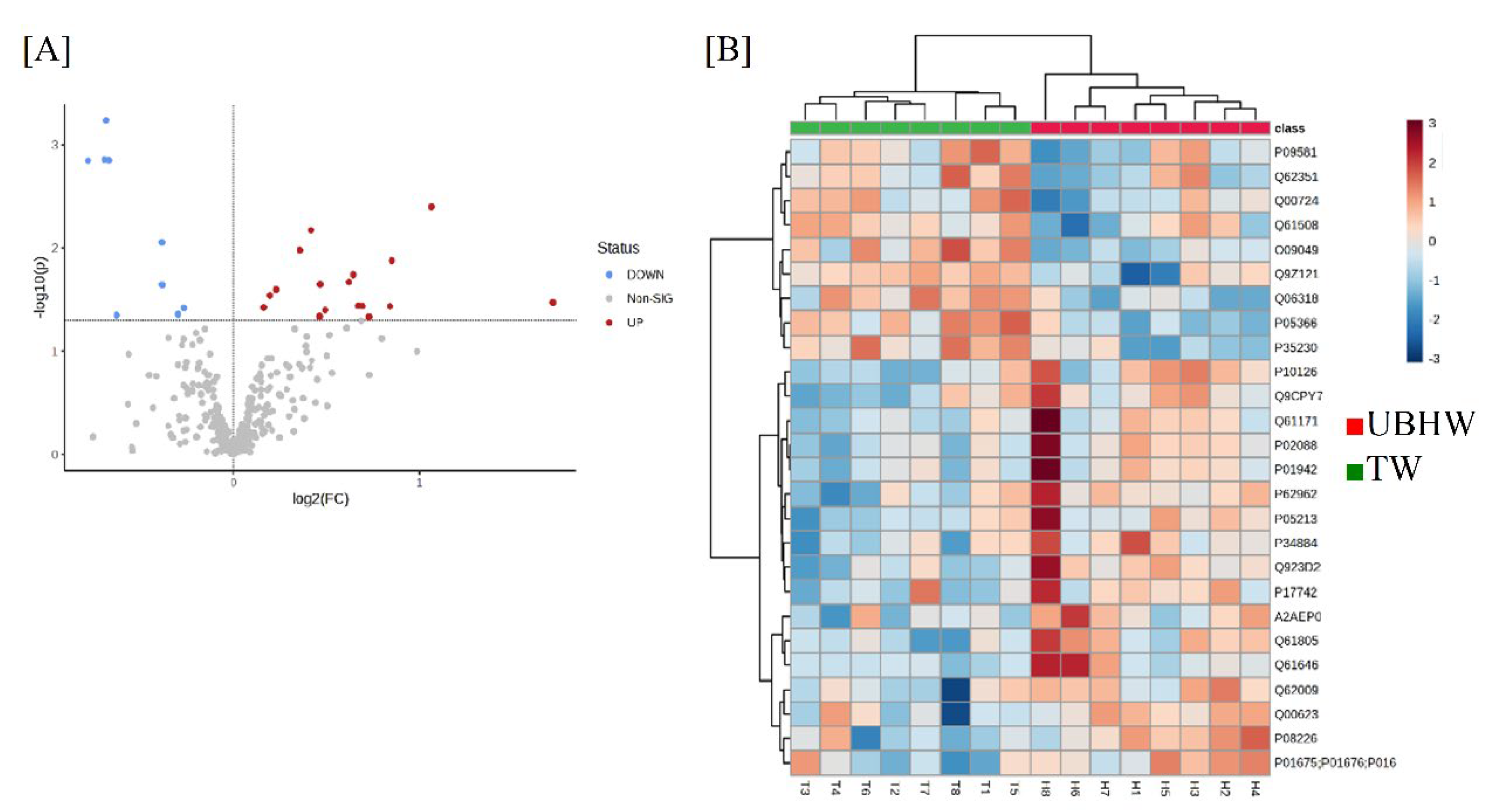
| No. | UniProt ID | Gene name | Protein name | FC | P |
|---|---|---|---|---|---|
| 1 | Q61646 | Hp | Haptoglobin | 3.287 | 0.034 |
| 2 | Q923D2 | Blvrb | Flavin reductase | 2.090 | 0.004 |
| 3 | P02088 | Hbb-b1 | Hemoglobin subunit beta-1 | 1.803 | 0.013 |
| 4 | P01942 | Hba | Hemoglobin subunit alpha | 1.790 | 0.037 |
| 5 | Q61171 | Prdx2 | Peroxiredoxin-2 | 1.656 | 0.046 |
| 6 | P34884 | Mif | Macrophage migration inhibitory factor | 1.616 | 0.037 |
| 7 | A2AEP0 | Obp1b | Odorant-binding protein 1b | 1.592 | 0.036 |
| 8 | P10126 | Eef1a1 | Elongation factor 1-alpha 1 | 1.562 | 0.018 |
| 9 | P62962 | Pfn1 | Profilin-1 | 1.536 | 0.021 |
| 10 | P05213 | Tuba1b | Tubulin alpha-1B chain | 1.406 | 0.040 |
| 11 | P01675 | - | Ig kappa chain V-VI region XRPC 44 | 1.380 | 0.022 |
| 12 | P17742 | Ppia | Peptidyl-prolyl cis-trans isomerase A | 1.377 | 0.046 |
| 13 | Q61805 | Lbp | Lipopolysaccharide-binding protein | 1.334 | 0.007 |
| 14 | P08226 | Apoe | Apolipoprotein E | 1.280 | 0.010 |
| 15 | Q62009 | Postn | Periostin | 1.172 | 0.025 |
| 16 | Q00623 | Apoa1 | Apolipoprotein A1 | 1.144 | 0.029 |
| 17 | Q9CPY7 | Lap3 | Cytosol aminopeptidase | 1.119 | 0.038 |
| 18 | Q61508 | Ecm1 | Extracellular matrix protein 1 | 0.831 | 0.038 |
| 19 | P09581 | Csf1r | Macrophage colony-stimulating factor 1 receptor | 0.812 | 0.044 |
| 20 | Q00724 | Rbp4 | Retinol-binding protein 4 | 0.766 | 0.023 |
| 21 | Q9Z121 | Ccl8 | C-C motif chemokine 8 | 0.765 | 0.009 |
| 22 | Q62351 | Tfrc | Transferrin receptor protein 1 | 0.646 | 0.045 |
| 23 | O09049 | Reg3g | Regenerating islet-derived protein 3-gamma | 0.628 | 0.001 |
| 24 | P05366 | Saa1 | Serum amyloid A-1 protein | 0.622 | 0.001 |
| 25 | Q06318 | Scgb1a1 | Uteroglobin | 0.618 | 0.001 |
| 26 | P35230 | Reg3b | Regenerating islet-derived protein 3-beta | 0.581 | 0.001 |
| Mus musculus (References) | Significant proteins | Expected | Fold enrichment |
P value | |
|---|---|---|---|---|---|
| GO biological process complete | |||||
| positive regulation of phospholipid efflux | 4 | Q00623, P08226 |
0.00 | > 100 | 0.0314 |
| hydrogen peroxide catabolic process | 27 | P02088, Q61171, P01942 | 0.02 | > 100 | 0.0103 |
| Reactome pathways | |||||
| scavenging by class A receptors | 8 | Q00623, P08226 | 0.01 | > 100 | 0.0265 |
| HDL remodeling | 9 | Q00623, P08226 | 0.01 | > 100 | 0.0341 |
| chylomicron assembly | 10 | Q00623, P08226 | 0.01 | > 100 | 0.0426 |
| chylomicron remodeling | 10 | Q00623, P08226 | 0.01 | > 100 | 0.0426 |
| cellular responses to stress | 451 | P10126, P05213, Q923D2, Q61171, P01942, Q00623 | 0.35 | 17.20 | 0.0012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





