Submitted:
01 August 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction

2. Material and Methods
2.1. Collection of Samples
2.1.1. Location no. 1: Municipal Bees (Košice - altitude 206 masl, Košice- Barca - altitude 210 masl, Michalovce - Altitude 113 masl):
2.1.2. Location no. 2: Inner Village (Lipníky - Altitude 280 masl)
2.1.3. Location no. 3: Extravillain of the Village (Okrúhle- Altitude 280 masl)
2.1.4. Location no. 4: Forest Bees (Komárnik- Altitude 420 masl)


| Habitat according to the location of bee colonies | Numbers of bee colonies |
|---|---|
| A) City bees | 22 |
| B) Rural bees in the inner city | 27 |
| C) Rural bees in extravilla | 22 |
| D) Forest habitat | 11 |
2.2. Sample Processing and Detection of Vairimorpha spp.
2.2.1. Microscopic Diagnostics
| Strength of infection | Number of spores |
|---|---|
| Negative sample | 0 in the field of view |
| Weak nozem infection + | 1-19 spores per field of view |
| Moderately strong nozem infection ++ | 20-100 spores per field of view |
| Strong nozem infection +++ | Over 100 spores per field of view |
2.2.2. Molecular Diagnostics
| Components of the PCR mix | Quantity |
|---|---|
| PCR water | 11,5 µl |
| Firepol Master Mix (Solis Biodine) | 4 µl |
| APIS FOR(5′-GGGGCCATGTGTTTGACGTACTATGTA-3′) | 0,5 µl |
| APIS REV (5′-GGGGGGCGTTTAAAAATGTGAAACAACTATG-3′) | 0,5 µl |
| MITOC FOR (5′CGGCGACGATGATGATGATGAAAATATTAA-3′) | 0,5 µl |
| MITOC REV (5′-CCCGGTCATTCTCAAAAAAAACCG-3´) | 0,5 µl |
| Template | 2,5 µl |
3. Results
3.1. Microscopic Diagnostics
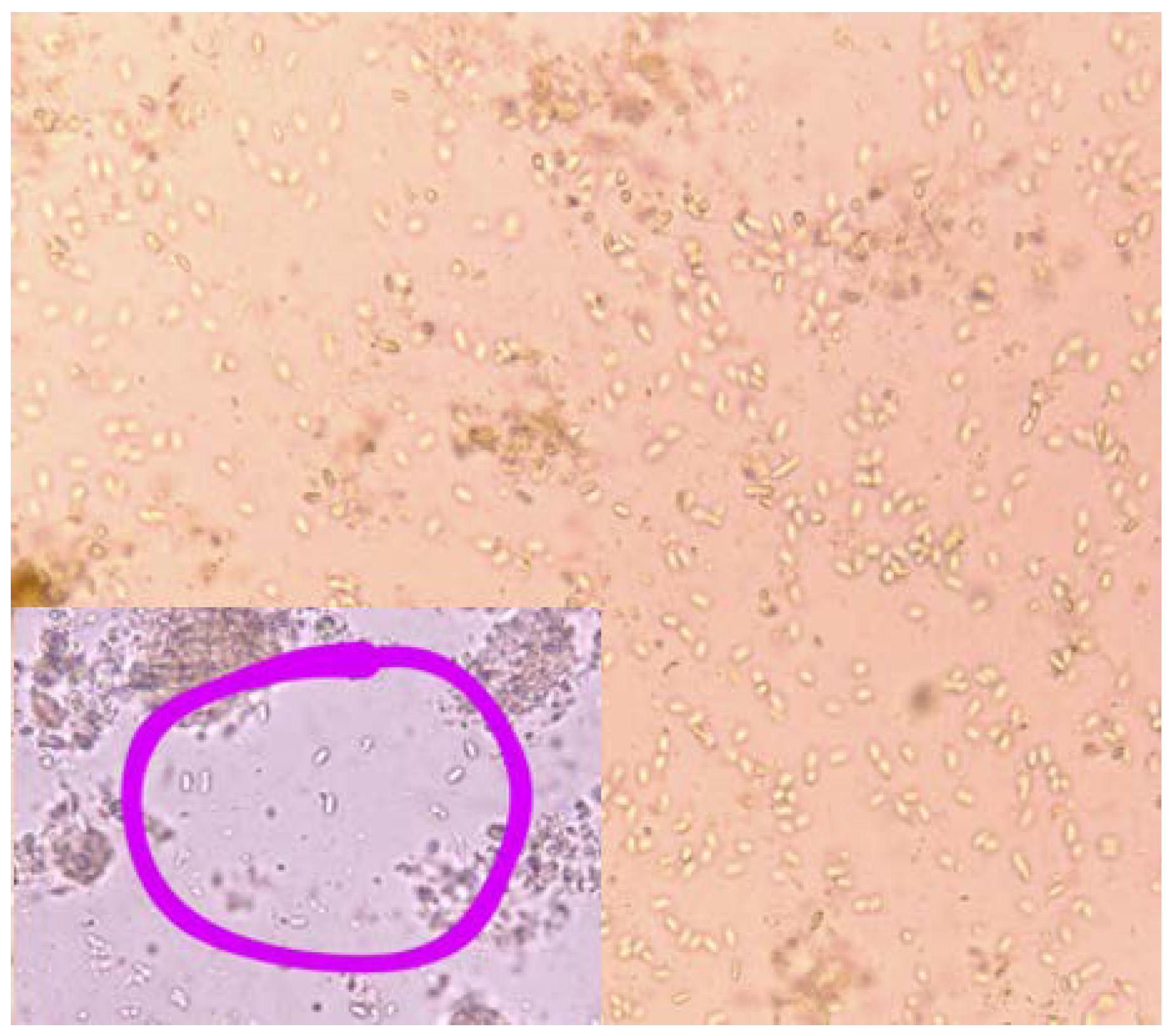
| negative | + | ++ | +++ | Positive | |
|---|---|---|---|---|---|
| City | 5 | 5 | 3 | 9 | 17/22 |
| Inner city | 7 | 6 | 6 | 8 | 20/27 |
| Extravilla | 18 | 0 | 0 | 4 | 4/22 |
| Forest | 11 | 0 | 0 | 0 | 0/11 |
| Total count | 41 | 11 | 9 | 21 | 41/82 |
3.2. Molecular Diagnostics
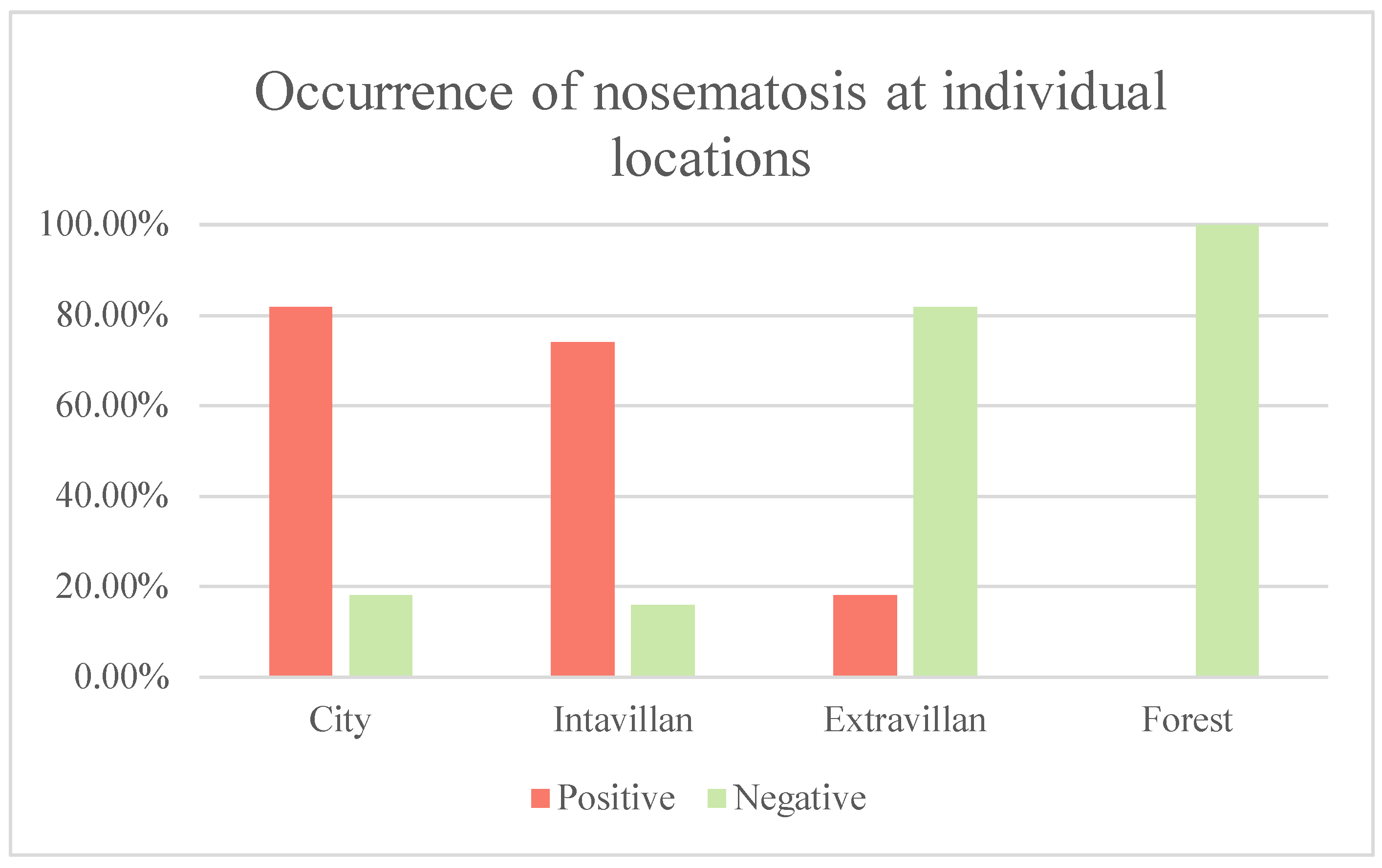
3.3. Proportion of Positive Cases with Respect to Habitat
| Location | Number of positive colonies | Number of negative colonies |
|---|---|---|
| City bees | 18 | 4 |
| Rural bees - intravillan | 20 | 7 |
| Rural bees - extravillan | 4 | 18 |
| Forest bees | 0 | 11 |
4. Discussion
5. Conclusions
Funding
References
- Abou-Shaara, H.F., Darwish, A.A.E. (2021). Expected prevalence of the facultative parasitoid Megaselia scalaris of honey bees in Africa and the Mediterranean region under climate change conditions. Int J Trop Insect Sci, 41, 3137–3145. [CrossRef]
- Abou-Shaara, H.F., Owayss, A.A., Ibrahim, Y.Y., et al. (2017). A review of impacts of temperature and relative humidity on various activities of honey bees. Insect. Soc., 64, 455–463. [CrossRef]
- Alberoni, D., Di Gioia, D., Baffoni, L. (2023). Alterations in the Microbiota of Caged Honeybees in the Presence of Nosema ceranae Infection and Related Changes in Functionality. Microb Ecol, 86(1), 601-616. [CrossRef]
- Al Naggar Y, Dabour K, Masry S, Sadek A, Naiem E, Giesy JP. Sublethal effects of chronic exposure to CdO or PbO nanoparticles or their binary mixture on the honey bee (Apis millefera L.). Environ Sci Pollut Res Int. 2020 Jun;27(16):19004-19015. Epub 2018 Oct 3. [CrossRef] [PubMed]
- Amado De Santis, A.A., Chacoff, N.P. (2020). Urbanization Affects Composition but Not Richness of Flower Visitors in the Yungas of Argentina. Neotrop Entomol, 49, 568–577. [CrossRef]
- Balzani P, Galeotti G, Scheggi S, Masoni A, Santini G, Baracchi D. Acute and chronic ingestion of polyethylene (PE) microplastics has mild effects on honey bee health and cognition. Environ Pollut. 2022 Jul 15;305:119318. Epub 2022 Apr 18. [CrossRef] [PubMed]
- Bass, C., & Field, L. M. (2018). Neonicotinoids. Current biology, 28(14), R772-R773. [CrossRef]
- Bass, Chris, Hayward, Angela, Troczka, Bartlomiej J., Haas, Julian, Nauen, Ralf. (2024). The molecular determinants of pesticide sensitivity in bee pollinators, Science of The Total Environment, Volume 915, 170174, ISSN 0048-9697. [CrossRef]
- Beadle, K., Singh, K. S., Troczka, B. J., Randall, E., Zaworra, M., Zimmer, C. T.,... & Bass, Blot, N., Clémencet, J., Jourda, C., Lefeuvre, P., Warrit, N., Esnault, O., Delatte, H. (2023). Geographic population structure of the honeybee microsporidian parasite Vairimorpha (Nosema) ceranae in the South West Indian Ocean. Sci Rep, 13(1), 12122. [CrossRef]
- Bruckner, S., Straub, L., Neumann, P., Williams, G.R. (2023). Negative but antagonistic effects of neonicotinoid insecticides and ectoparasitic mites Varroa destructor on Apis mellifera honey bee food glands. Chemosphere, 313, 137535. [CrossRef]
- Buteler M, Alma AM, Stadler T, Gingold AC, Manattini MC, Lozada M. Acute toxicity of microplastic fibers to honeybees and effects on foraging behavior. Sci Total Environ. 2022 May 20;822:153320. Epub 2022 Jan 22. [CrossRef] [PubMed]
- Butolo, N.P., Azevedo, P., Alencar, L.D., Malaspina, O., Nocelli, R.C.F. (2021). Impact of low temperatures on the immune system of honeybees. Journal of Thermal Biology, 101, 103082. [CrossRef]
- Castelli, L., Branchiccela, B., Romero, H., et al. (2022). Seasonal Dynamics of the Honey Bee Gut Microbiota in Colonies Under Subtropical Climate. Microb Ecol, 83, 492–500. [CrossRef]
- Castaños, C.E., Boyce, M.C., Bates, T., Millar, A.H., Flematti, G., Lawler, N.G., et al. (2023). Lipidomic features of honey bee and colony health during limited supplementary feeding. Insect Molecular Biology, 32(6), 658–675. [CrossRef]
- Cho Y, Jeong S, Lee D, Kim SW, Park RJ, Gibson L, Zheng C, Park CR. Foraging trip duration of honeybee increases during a poor air quality episode and the increase persists thereafter. Ecol Evol. 2021 Jan 23;11(4):1492-1500. [CrossRef] [PubMed] [PubMed Central]
- Chrousos, G.P. (2009). Stress and disorders of the stress system. Nat Rev Endocrinol, 5, 374–381. [CrossRef]
- Corby-Harris, Vanessa, Snyder, Lucy, Meador, Charlotte, Watkins-DeJong, Emily, Obernesser, Bethany T., Brown, Nicholas, Carroll, Mark J. (2022). Diet and pheromones interact to shape honey bee (Apis mellifera) worker physiology, Journal of Insect Physiology, Volume 143, 104442, ISSN 0022-1910. [CrossRef]
- Cui, P., Kong, K., Yao, Y., Huang, Z., Shi, S., Liu, P., Huang, Y., Abbas, N., Yu, L., Zhang, Y. (2022). Community composition, bacterial symbionts, antibacterial and antioxidant activities of honeybee-associated fungi. BMC Microbiol, 22(1), 168. [CrossRef]
- Dargas JH, Chaves SR, Fischer E. Pollination of lark daisy on roadsides declines as traffic speed increases along an Amazonian highway. Plant Biol (Stuttg). 2016 May;18(3):542-4. Epub 2016 Feb 21. [CrossRef] [PubMed]
- De Miranda, J.R., Bailey, L., Ball, B.V., Blanchard, P., Budge, G.E., Chejanovsky, N., Chen, Y., Gauthier, L., Genersch, E., de Graaf, D.C., Ribière, M., Ryabov, E., De Smet, L., van der Steen, J.J.M. (2013). Standard methods for virus research in Apis mellifera. J Apic Res, 52, 1–56. [CrossRef]
- Dequenne, I., Philippart de Foy, J. M., & Cani, P. D. (2022). Developing Strategies to Help Bee Colony Resilience in Changing Environments. Animals (Basel), 12(23), 3396. [CrossRef]
- Dhruba Naug, Nutritional stress due to habitat loss may explain recent honeybee colony collapses, Biological Conservation, Volume 142, Issue 10, 2009, Pages 2369-2372, ISSN 0006-3207. [CrossRef]
- Engel, P., Kwong, W.K., McFrederick, Q., Anderson, K.E., Barribeau, S.M., Chandler, J.A., Cornman, R.S., Dainat, J., de Miranda, J.R., Doublet, V., Emery, O., Evans, J.D., Farinelli, L., Flenniken, M.L., Granberg, F., Grasis, J.A., Gauthier, L., Hayer, J., Koch, H., Kocher, S., Martinson, V.G., Moran, N., Munoz-Torres, M., Newton, I., Paxton, R.J., Powell, E., Sadd, B.M., Schmid-Hempel, P., Schmid-Hempel, R., Song, S.J., Schwarz, R.S., vanEngelsdorp, D., Dainat, B. (2016). The Bee Microbiome: Impact on Bee Health and Model for Evolution and Ecology of Host-Microbe Interactions. mBio, 7(2), e02164-15. [CrossRef]
- Engel P, Kwong WK, McFrederick Q, Anderson KE, Barribeau SM, Chandler JA, Cornman RS, Dainat J, de Miranda JR, Doublet V, Emery O, Evans JD, Farinelli L, Flenniken ML, Granberg F, Grasis JA, Gauthier L, Hayer J, Koch H, Kocher S, Martinson VG, Moran N, Munoz-Torres M, Newton I, Paxton RJ, Powell E, Sadd BM, Schmid-Hempel P, Schmid-Hempel R, Song SJ, Schwarz RS, vanEngelsdorp D, Dainat B. The Bee Microbiome: Impact on Bee Health and Model for Evolution and Ecology of Host-Microbe Interactions. mBio. 2016 Apr 26;7(2):e02164-15. [CrossRef] [PubMed] [PubMed Central]
- Even, N., Devaud, J.M., Barron, A.B. (2012). General Stress Responses in the Honey Bee. Insects, 3(4), 1271-98. [CrossRef]
- Forsgren, E. (2010). European foulbrood in honey bees. J Invertebr Pathol, 103(Suppl 1), S5–S9. [CrossRef]
- Galajda, R., Valenčáková, A., Sučik, M., Kandráčová, P. (2021). Nosema Disease of European Honey Bees. J Fungi (Basel), 7(9), 714. [CrossRef]
- Genersch, E. (2010). American foulbrood in honeybees and its causative agent, Paenibacillus larvae. J Invertebr Pathol, 103(Suppl 1), S10–S19. [CrossRef]
- Geslin B, Gauzens B, Thébault E, Dajoz I (2013) Plant Pollinator Networks along a Gradient of Urbanisation. PLOS ONE 8(5): e63421. [CrossRef]
- Glass, J.R., Harrison, J.F. (2024). A thermal performance curve perspective explains decades of disagreements over how air temperature affects the flight metabolism of honey bees. J Exp Biol, 227(7), jeb246926. [CrossRef]
- Goulson D, Nicholls E, Botías C, Rotheray EL. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015 Mar 27;347(6229):1255957. [CrossRef] [PubMed]
- Goulson D, Nicholls E. Anthropogenic influences on bee foraging. Science. 2022 Mar 4;375(6584):970-972. Epub 2022 Mar 3. [CrossRef] [PubMed]
- Hassler, Edgar E., Joseph A. Cazier, Brandon Hopkins, James T. Wilkes, Kiefer Smith, and Max Rünzel. (2021). "A century of discovery: Mining 100 years of honey bee research." Journal of Apicultural Research 60, no. 1: 3-12. [CrossRef]
- Hester, K.P., Stoner, K.A., Eitzer, B.D., Koethe, R.W., Lehmann, D.M. (2023). Pesticide residues in honey bee (Apis mellifera) pollen collected in two ornamental plant nurseries in Connecticut: Implications for bee health and risk assessment, Environmental Pollution, Volume 333, 2023, 122037, ISSN 0269-7491. [CrossRef]
- Higes, M., Martín, R., Meana, A. (2006). Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J Invertebr Pathol, 92(2), 93–95. [CrossRef]
- Iwasa, T., Motoyama, N., Ambrose, J. T., & Roe, R. M. (2004). Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop protection, 23(5), 371-378. [CrossRef]
- Jaworski, C. C., Geslin, B., Zakardjian, M., Lecareux, C., Boulogne, I., Raynaud, X.,... & Tison, L. (2023). In-hive disturbances linked to neonicotinoids exposure in honey bees. Scientific reports, 13(1), 3747.
- Khan KA, Al-Ghamdi AA, Ghramh HA, Ansari MJ, Ali H, Alamri SA, Al-Kahtani SN, Adgaba N, Qasim M, Hafeez M. (2020). Structural diversity and functional variability of gut microbial communities associated with honey bees. Microb Pathog. Jan;138:103793. Epub 2019 Oct 15. [CrossRef] [PubMed]
- Kit S. Prendergast, Sean Tomlinson, Kingsley W. Dixon, Philip W. Bateman, Myles H.M. (2022). Menz, Urban native vegetation remnants support more diverse native bee communities than residential gardens in Australia's southwest biodiversity hotspot, Biological Conservation, Volume 265, 109408, ISSN 0006-3207. [CrossRef]
- Klocek D, Grybchuk D, Macedo DH, Galan A, Votýpka J, Schmid-Hempel R, Schmid-Hempel P, Yurchenko V, Kostygov AY. (2023). RNA viruses of Crithidia bombi, a parasite of bumblebees. J Invertebr Pathol. Nov;201:107991. Epub 2023 Sep 13. [CrossRef] [PubMed]
- Langowska, A., Zawilak, M., Sparks, T.H. et al. (2017). Long-term effect of temperature on honey yield and honeybee phenology. Int J Biometeorol 61, 1125–1132. [CrossRef]
- Laurino, D., Manino, A., Patetta, A., Porporato, M. (2011). Toxicity of neonicotinoid insecticides to honey bees: laboratory tests. Bulletin of insectology, 64(1), 107-113.
- Levin, M.D., Bohart, G.E. (1955). Effect of Cold Storage on the Activity and Life Span of Two Osmia Lignaria Subspecies (Hymenoptera: Megachilidae). Journal of Economic Entomology, 48(3), 326–329. [CrossRef]
- Li YH, Chen YH, Chang FM, Wu MC, Nai YS. (2024). Monitoring the Season-Prevalence Relationship of Vairimorpha ceranae in Honey Bees (Apis mellifera) over One Year and the Primary Assessment of Probiotic Treatment in Taichung, Taiwan. Insects. Mar 19;15(3):204. [CrossRef] [PubMed] [PubMed Central]
- Lin Z, Shen S, Wang K, Ji T (2024). Biotic and abiotic stresses on honeybee health. Integrative Zoology 19, 442–457. [CrossRef]
- Mahé, C., Jumarie, C., Boily, M. (2021). The countryside or the city: Which environment is better for the honeybee?, Environmental Research, Volume 195, 110784, ISSN 0013-9351. [CrossRef]
- Manjon, C., Troczka, B.J., Zaworra, M., Beadle, K., Randall, E., Hertlein, G.,... & Bass, C. (2018). Unravelling the molecular determinants of bee sensitivity to neonicotinoid insecticides. Current Biology, 28(7), 1137-1143.
- Manlik, O., Mundra, S., Schmid-Hempel, R., & Schmid-Hempel, P. (2023). Impact of climate change on parasite infection of an important pollinator depends on host genotypes. Global Change Biology, 29, 69–80. [CrossRef]
- Mayack, Christopher, Naug, Dhruba (2009). Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection, Journal of Invertebrate Pathology, Volume 100, Issue 3, Pages 185-188, ISSN 0022-2011. [CrossRef]
- McDermott A. (2021). News Feature: To understand the plight of insects, entomologists look to the past. Proc Natl Acad Sci U S A. Jan 12;118(2):e2018499117. Epub 2020 Dec 16. [CrossRef] [PubMed] [PubMed Central]
- McMenamin AJ, Genersch E. (2015). Honey bee colony losses and associated viruses. Curr Opin Insect Sci 8:121–129. [CrossRef]
- Mutinelli, F. (2011). The spread of pathogens through trade in honey bees and their products (including queen bees and semen): overview and recent developments. Rev Sci Tech, 30(1), 257-271. [CrossRef]
- Murilhas, A. (2005). Aethina tumida arrives in Portugal. Will it be eradicated? Eur Bee Newsl, 7, 7–9.
- Nagaraja, N., Raja, D.S. (2021). Honey bee diseases and pests management strategies in the Indian subcontinent: An update. J Invertebr Pathol, 184, 107648. [CrossRef]
- Nazzi, F., Pennacchio, F. (2014). Disentangling multiple interactions in the hive ecosystem. Trends Parasitol, 30(12), 556–561. [CrossRef]
- Nguyen TT, Yoo MS, Lee HS, Truong AT, Youn SY, Lee SJ, Kim J, Cho YS. First detection and prevalence of Apis mellifera filamentous virus in Apis mellifera and Varroa destructor in the Republic of Korea. Sci Rep. 2024 Jun 19;14(1):14105. [CrossRef] [PubMed] [PubMed Central]
- Oddie MAY, Lanz S, Dahle B, Yañez O, Neumann P. (2023). Virus infections in honeybee colonies naturally surviving ectoparasitic mite vectors. PLoS One. 2023 Dec 15;18(12):e0289883. [CrossRef] [PubMed] [PubMed Central]
- Palmer-Young EC, Markowitz LM, Huang W-F, Evans JD. High temperatures augment inhibition of parasites by a honey bee gut symbiont. Appl Environ Microbiol. 2023 Oct 31;89(10):e0102323. Epub 2023 Oct 4. [CrossRef] [PubMed] [PubMed Central]
- Papa G, Capitani G, Capri E, Pellecchia M, Negri I. Vehicle-derived ultrafine particulate contaminating bees and bee products. Sci Total Environ. 2021 Jan 1;750:141700. Epub 2020 Aug 14. [CrossRef] [PubMed]
- Parrella P, Elikan AB, Kogan HV, Wague F, Marshalleck CA, Snow JW. (2024). Bleomycin reduces Vairimorpha (Nosema) ceranae infection in honey bees with some evident host toxicity. Microbiol Spectr. Feb 6;12(2):e0334923. Epub 2024 Jan 5. [CrossRef] [PubMed] [PubMed Central]
- Phillips, Benjamin B., James M. Bullock, Kevin J. Gaston, Karen A. Hudson-Edwards, Meg Bamford, Dave Cruse, Lynn V. Dicks, Carmen Falagan, Claire Wallace, and Juliet L. Osborne. "Impacts of multiple pollutants on pollinator activity in road verges." Journal of Applied Ecology 58, no. 5 (2021): 1017-1029. [CrossRef]
- Pohl, F., 2005. Bienen- krankheiten. Franckh- Kosmos Verlags- GmbH & Co KG, Stuttgart. P115-116, ISBN 978-3-440-10407-1.
- Potts, S. G., Roberts, S. P. M., Dean, R., Marris, G., Brown, M. A., Jones, R., Neumann, P., & Settele, J. (2010). Declines of managed honey bees and beekeepers in Europe. Journal of Apicultural Research, 49(1), 15-22. [CrossRef]
- Potts, S.G., Biesmeijer, J.C., Kremen, C., et al. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol, 25, 345–353. [CrossRef]
- Prendergast, K.S., Dixon, K.W. & Bateman, P.W. (2022) A global review of determinants of native bee assemblages in urbanised landscapes. Insect Conservation and Diversity, 15(4), 385–405. [CrossRef]
- Raine NE, Rundlöf M. (2023). Pesticide Exposure and Effects on Non-Apis Bees. Annu Rev Entomol. 2024 Jan 25;69:551-576. Epub 2023 Oct 12. [CrossRef] [PubMed]
- Ramsey, SD, Ochoa, R, Bauchan, G, Gulbronson, C, Mowery, JD, Cohen A. et al. (2019).
- Varroa destructor feeds primarily on the fat tissue of the bee body and not on the hemolymph Proc Natl Acad Sci USA, 116 ( 2019 ), s. 1792 - 1801. [CrossRef]
- Reid, R. J., Troczka, B. J., Kor, L., Randall, E., Williamson, M. S., Field, L. M.,... & Davies, T. E. (2020). Assessing the acute toxicity of insecticides to the buff-tailed bumblebee (Bombus terrestris audax). Pesticide biochemistry and physiology, 166, 104562. [CrossRef]
- Ryabov EV, Nearman AJ, Nessa A, Grubbs K, Sallmann B, Fahey R, Wilson ME, Rennich KD, Steinhauer N, Fauvel AM, Chen Y, Evans JD, vanEngelsdorp D. (2023). Apis mellifera Solinvivirus-1, a Novel Honey Bee Virus That Remained Undetected for over a Decade, Is Widespread in the USA. Viruses. 2023 Jul 21;15(7):1597. [CrossRef] [PubMed] [PubMed Central]
- Streicher T, Brinker P, Tragust S, Paxton RJ. Host Barriers Limit Viral Spread in a Spillover Host: A Study of Deformed Wing Virus in the Bumblebee Bombus terrestris. Viruses. 2024 Apr 15;16(4):607. [CrossRef] [PubMed] [PubMed Central]
- Switanek, Matthew, Crailsheim, Karl, Truhetz, Heimo, Brodschneider, Robert. (2017). Modelling seasonal effects of temperature and precipitation on honey bee winter mortality in a temperate climate, Science of The Total Environment, Volume 579, Pages 1581-1587, ISSN 0048-9697. [CrossRef]
- Thimmegowda GG, Mullen S, Sottilare K, Sharma A, Mohanta R, Brockmann A, Dhandapany PS, Olsson SB. (2020). A field-based quantitative analysis of sublethal effects of air pollution on pollinators. Proc Natl Acad Sci U S A. 2020 Aug 25;117(34):20653-20661. Epub 2020 Aug 10. [CrossRef] [PubMed] [PubMed Central]
- Tong L, Nieh JC, Tosi S. Combined nutritional stress and a new systemic pesticide (flupyradifurone, Sivanto®) reduce bee survival, food consumption, flight success, and thermoregulation. Chemosphere. 2019 Dec;237:124408. Epub 2019 Jul 20. [CrossRef] [PubMed]
- USEPA, Health Canada Pest Management Regulatory Agency, and the California Department of Pesticide Regulation, 2014. Guidance for Assessing Pesticide Risks to Bees. https://www.epa.gov/sites/production/files/2014-06/documents/pollinator_risk_assessment_guidance_06_19_14.pdf 59.
- Vanderplanck, M., Gerbaux, P., Mougel, F., Logan, M., Moerman, R., Garofalo, F., Rasmont, P., De Pauw, E., Michez, D. (2019). Influence of pollen quality on male and female reproductive performance in a solitary bee. Sci Rep, 9, 11688. [CrossRef]
- Van Espen, Marie, Williams, James H., Alves, Fátima, Hung, Yung, de Graaf, Dirk C., Verbeke, Wim (2023). Beekeeping in Europe facing climate change: A mixed methods study on perceived impacts and the need to adapt according to stakeholders and beekeepers, Science of The Total Environment, Volume 888, 164255, ISSN 0048-9697.
- Wang K, Li J, Zhao L, Mu X, Wang C, Wang M, Xue X, Qi S, Wu L. (2021). Gut microbiota protects honey bees (Apis mellifera L.) against polystyrene microplastics exposure risks. J Hazard Mater. 2021 Jan 15;402:123828. Epub 2020 Sep 5. [CrossRef] [PubMed]
- Yun JH, Jung MJ, Kim PS, Bae JW. (2018). Social status shapes the bacterial and fungal gut communities of the honey bee. Sci Rep. 2018 Jan 31;8(1):2019. [CrossRef] [PubMed] [PubMed Central]
- Zapata-Hernández, G., Gajardo-Rojas, M., Calderón-Seguel, M., Muñoz, A. A., Yáñez, K. P., Requier, F., Fontúrbel, F. E., Ormeño-Arriagada, P. I., & Arrieta, H. (2024). Advances and knowledge gaps on climate change impacts on honey bees and beekeeping: A systematic review. Global Change Biology, 30, e17219. [CrossRef]
- Zbrozek M, Fearon ML, Weise C, Tibbetts EA. (2023). Honeybee visitation to shared flowers increases Vairimorpha ceranae prevalence in bumblebees. Ecol Evol. Sep 20;13(9):e10528. [CrossRef] [PubMed] [PubMed Central]
- Zhang Y, Su M, Wang L, Huang S, Su S, Huang WF. (2021). Vairimorpha (Nosema) ceranae Infection Alters Honey Bee Microbiota Composition and Sustains the Survival of Adult Honey Bees. Biology (Basel). Sep 13;10(9):905. [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




