Submitted:
01 August 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Results
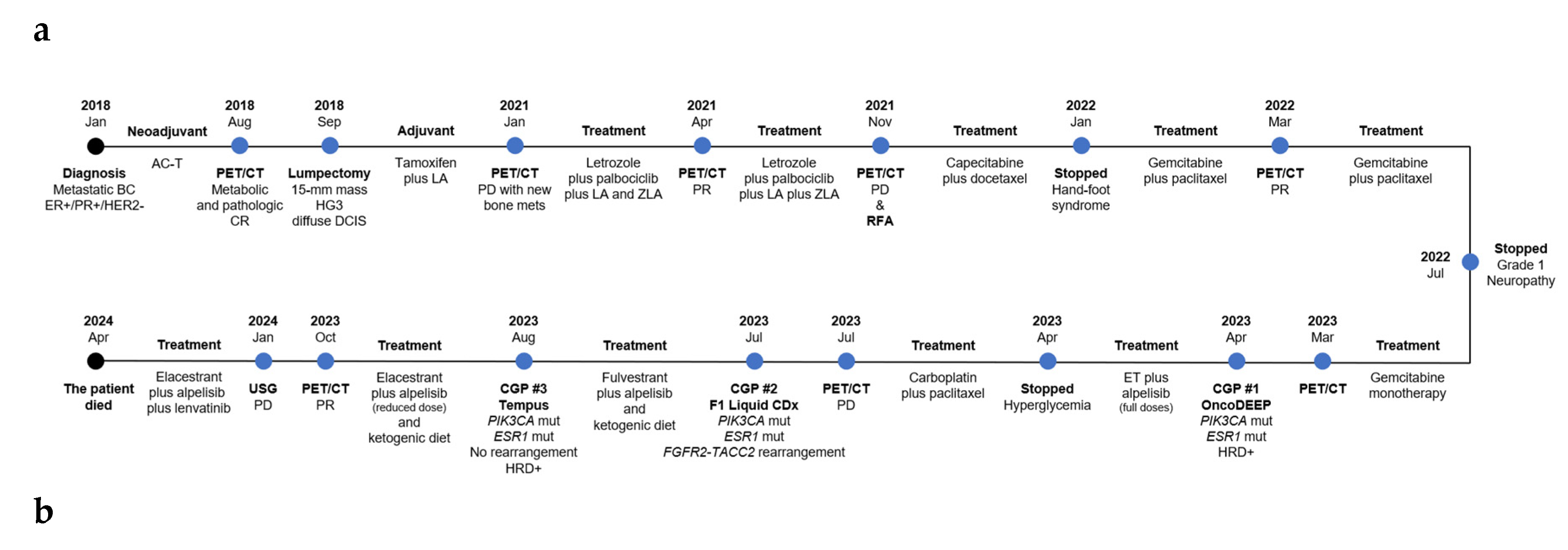
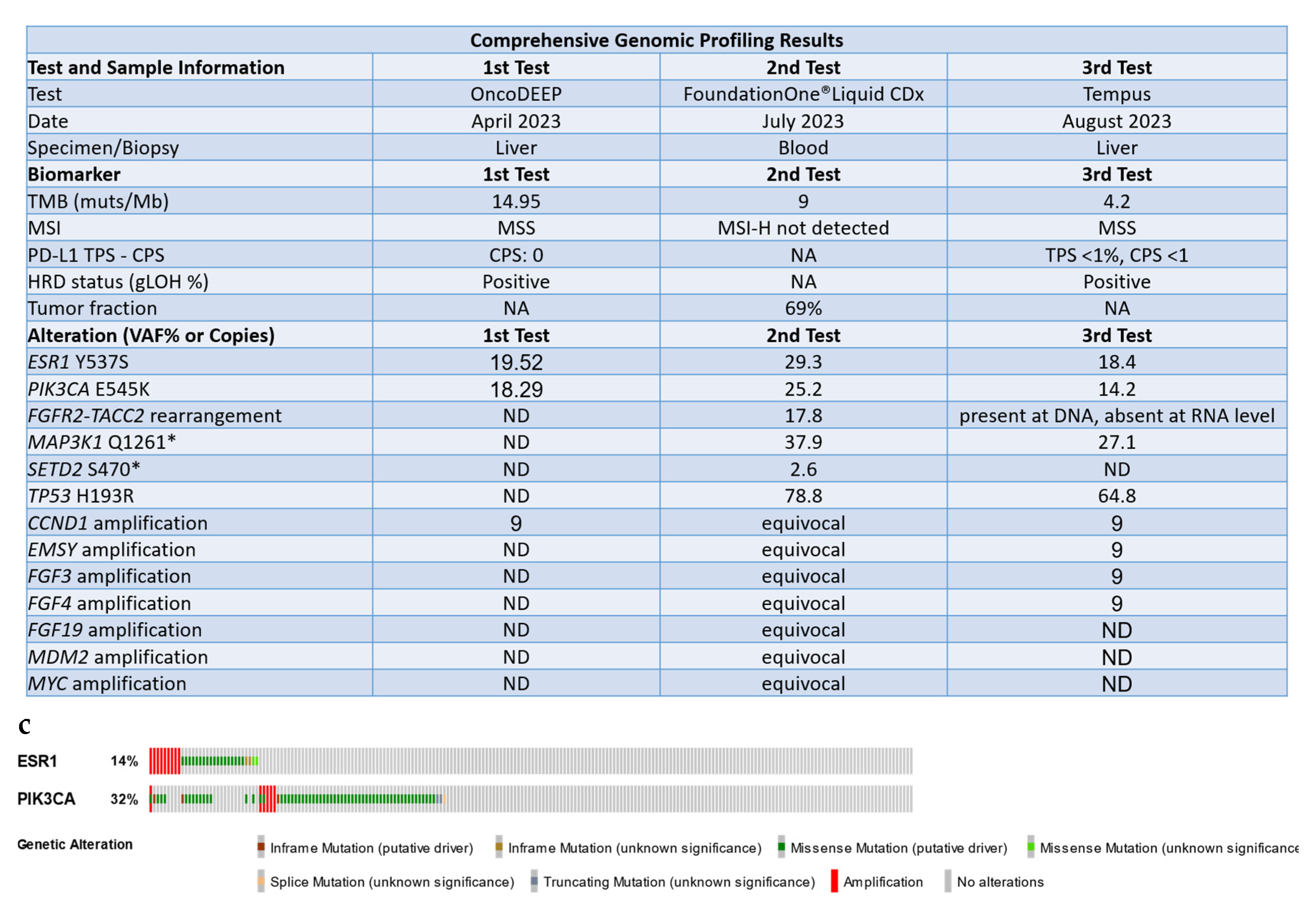
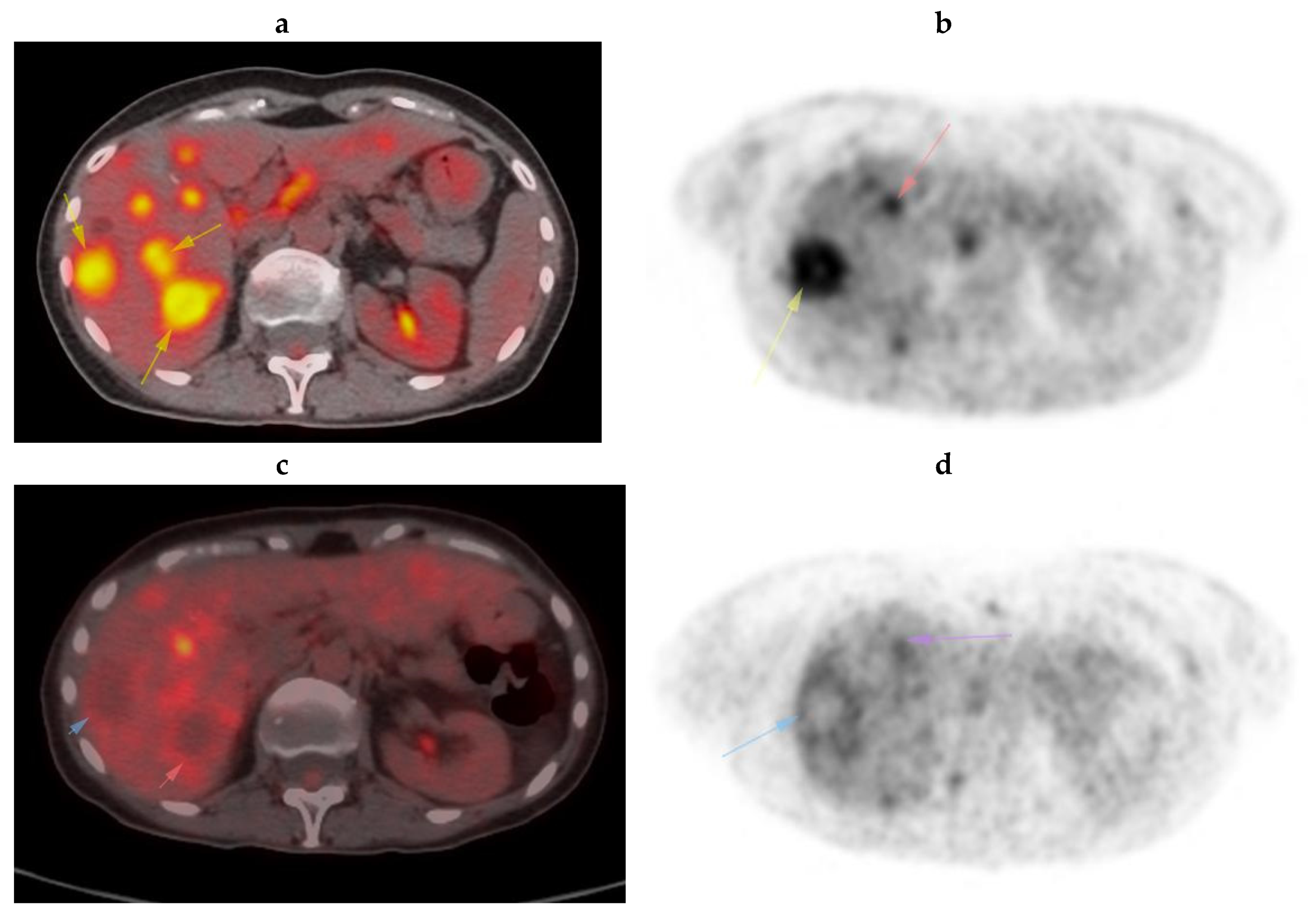
Case Presentation
Discussion
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Siegel RL, Miller KD, Wagle NS, et al.: Cancer statistics, 2023. CA Cancer J Clin 73:17-48, 2023.
- Sung H, Ferlay J, Siegel RL, et al.: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71:209-249, 2021. [CrossRef]
- Al Sukhun S, Temin S, Barrios CH, et al.: Systemic Treatment of Patients With Metastatic Breast Cancer: ASCO Resource-Stratified Guideline. JCO Glob Oncol 10:e2300285, 2024.
- D’Amico P, Cristofanilli M: Standard of Care in Hormone Receptor-Positive Metastatic Breast Cancer: Can We Improve the Current Regimens or Develop Better Selection Tools? JCO Oncol Pract 18:331-334, 2022.
- Ma J, Chan JJ, Toh CH, et al.: Emerging systemic therapy options beyond CDK4/6 inhibitors for hormone receptor-positive HER2-negative advanced breast cancer. NPJ Breast Cancer 9:74, 2023.
- Mittal A, Molto Valiente C, Tamimi F, et al.: Filling the Gap after CDK4/6 Inhibitors: Novel Endocrine and Biologic Treatment Options for Metastatic Hormone Receptor Positive Breast Cancer. Cancers (Basel) 15, 2023.
- Turner NC, Swift C, Kilburn L, et al.: ESR1 Mutations and Overall Survival on Fulvestrant versus Exemestane in Advanced Hormone Receptor-Positive Breast Cancer: A Combined Analysis of the Phase III SoFEA and EFECT Trials. Clin Cancer Res 26:5172-5177, 2020.
- Brett JO, Spring LM, Bardia A, et al.: ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res 23:85, 2021.
- Chaudhary N, Chibly AM, Collier A, et al.: CDK4/6i-treated HR+/HER2- breast cancer tumors show higher ESR1 mutation prevalence and more altered genomic landscape. NPJ Breast Cancer 10:15, 2024.
- Zundelevich A, Dadiani M, Kahana-Edwin S, et al.: ESR1 mutations are frequent in newly diagnosed metastatic and loco-regional recurrence of endocrine-treated breast cancer and carry worse prognosis. Breast Cancer Res 22:16, 2020.
- Eisenhauer EA, Therasse P, Bogaerts J, et al.: New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer 45:228-247, 2009. [CrossRef]
- Yang SX, Hewitt SM, Yu J: Locoregional tumor burden and risk of mortality in metastatic breast cancer. NPJ Precis Oncol 6:22, 2022.
- McAndrew NP, Finn RS: Clinical Review on the Management of Hormone Receptor-Positive Metastatic Breast Cancer. JCO Oncol Pract 18:319-327, 2022. [CrossRef]
- Belachew EB, Sewasew DT: Molecular Mechanisms of Endocrine Resistance in Estrogen-Positive Breast Cancer. Front Endocrinol (Lausanne) 12:599586, 2021.
- O’Leary B, Cutts RJ, Huang X, et al.: Circulating Tumor DNA Markers for Early Progression on Fulvestrant With or Without Palbociclib in ER+ Advanced Breast Cancer. J Natl Cancer Inst 113:309-317, 2021.
- Turner NC, Liu Y, Zhu Z, et al.: Cyclin E1 Expression and Palbociclib Efficacy in Previously Treated Hormone Receptor-Positive Metastatic Breast Cancer. J Clin Oncol 37:1169-1178, 2019.
- Herzog SK, Fuqua SAW: ESR1 mutations and therapeutic resistance in metastatic breast cancer: progress and remaining challenges. Br J Cancer 126:174-186, 2022.
- Toy W, Weir H, Razavi P, et al.: Activating ESR1 Mutations Differentially Affect the Efficacy of ER Antagonists. Cancer Discov 7:277-287, 2017.
- Chandarlapaty S, Chen D, He W, et al.: Prevalence of ESR1 Mutations in Cell-Free DNA and Outcomes in Metastatic Breast Cancer: A Secondary Analysis of the BOLERO-2 Clinical Trial. JAMA Oncol 2:1310-1315, 2016.
- O’Leary B, Cutts RJ, Liu Y, et al.: The Genetic Landscape and Clonal Evolution of Breast Cancer Resistance to Palbociclib plus Fulvestrant in the PALOMA-3 Trial. Cancer Discov 8:1390-1403, 2018.
- Kingston B, Pearson A, Herrera-Abreu MT, et al.: ESR1 F404 mutations and acquired resistance to fulvestrant in the plasmaMATCH study. Journal of Clinical Oncology 40, 2022.
- Ma W, Hermida-Prado F, Guarducci C, et al.: Combination of fulvestrant and chemotherapy in ESR1 Y537S mutant breast cancer cells and potential synergy mechanism related to p53 wildtype. Journal of Clinical Oncology 38, 2020.
- Dong M, Shan B, Han X, et al.: Baseline Mutations and Up-Regulation of PI3K-AKT Pathway Serve as Potential Indicators of Lack of Response to Neoadjuvant Chemotherapy in Stage II/III Breast Cancer. Front Oncol 11:784985, 2021.
- Pergialiotis V, Nikolaou C, Haidopoulos D, et al.: PIK3CA Mutations and Their Impact on Survival Outcomes of Patients with Cervical Cancer: A Systematic Review. Acta Cytol 64:547-555, 2020.
- Wang Q, Shi YL, Zhou K, et al.: PIK3CA mutations confer resistance to first-line chemotherapy in colorectal cancer. Cell Death Dis 9:739, 2018.
- Bidard FC, Kaklamani VG, Neven P, et al.: Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J Clin Oncol 40:3246-3256, 2022.
- Bardia A, O’Shaughnessy J, Bidard FC, et al.: Abstract PS17-02: Elacestrant vs standard-of-care in ER+/HER2- advanced or metastatic breast cancer (mBC) with ESR1 mutation: key biomarkers and clinical subgroup analyses from the phase 3 EMERALD trial. Cancer Res 84, 2024.
- Damodaran S, O’Sullivan CC, Elkhanany A, et al.: Open-label, phase II, multicenter study of lasofoxifene plus abemaciclib for treating women with metastatic ER+/HER2- breast cancer and an ESR1 mutation after disease progression on prior therapies: ELAINE 2. Ann Oncol 34:1131-1140, 2023.
- Gal-Yam EN, Levanon K: Lasofoxifene Monotherapy Induces Durable Complete Remission in a Patient with Estrogen Receptor-Positive, Metastatic Breast Cancer with an ESR1 Mutation. JCO Precis Oncol 7:e2300097, 2023.
- Wong NZH, Yap DWT, Ong RJM, et al.: Efficacy of Oral SERDs in the treatment of ER+, HER2 - metastatic breast cancer, a stratified analysis of the ESR1 wild type and mutant subgroups. Ann Oncol, 2023.
- Oliveira M, Pominchuk D, Hamilton EP, et al.: Clinical activity of camizestrant, a next-generation SERD, versus fulvestrant in patients with a detectable ESR1 mutation: Exploratory analysis of the SERENA-2 phase 2 trial. Journal of Clinical Oncology 41.
- Berger AC, Korkut A, Kanchi RS, et al.: A Comprehensive Pan-Cancer Molecular Study of Gynecologic and Breast Cancers. Cancer Cell 33:690-705 e9, 2018. [CrossRef]
- Pereira B, Chin SF, Rueda OM, et al.: The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun 7:11479, 2016.
- Fillbrunn M, Signorovitch J, Andre F, et al.: PIK3CA mutation status, progression and survival in advanced HR + /HER2- breast cancer: a meta-analysis of published clinical trials. BMC Cancer 22:1002, 2022.
- Damodaran S, Cristofanilli M, Goetz M, et al.: Abstract PO2-14-09: Baseline genomic alterations and the activity of lasofoxifene (LAS) plus abemaciclib (Abema) in patients with ER+/HER2-metastatic breast cancer (mBC): the ELAINE 2 study. Cancer Research 84:PO2-14-09-PO2-14-09, 2024.
- Martinez-Saez O, Chic N, Pascual T, et al.: Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Breast Cancer Res 22:45, 2020.
- Vernieri C, Corti F, Nichetti F, et al.: Everolimus versus alpelisib in advanced hormone receptor-positive HER2-negative breast cancer: targeting different nodes of the PI3K/AKT/mTORC1 pathway with different clinical implications. Breast Cancer Res 22:33, 2020.
- Turner NC, Oliveira M, Howell SJ, et al.: Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 388:2058-2070, 2023.
- Baselga J, Campone M, Piccart M, et al.: Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520-9, 2012.
- Varella L, Cristofanilli M: Evaluating Elacestrant in the Management of ER-Positive, HER2-Negative Advanced Breast Cancer: Evidence to Date. Onco Targets Ther 16:189-196, 2023.
- Llombart-Cussac A, Pérez-Garcia JM, Borrego MR, et al.: Preventing alpelisib-related hyperglycaemia in HR+/HER2−/PIK3CA-mutated advanced breast cancer using metformin (METALLICA): a multicentre, open-label, single-arm, phase 2 trial. Eclinicalmedicine 71, 2024.
- Howarth KD, Mirza T, Cooke SL, et al.: NRG1 fusions in breast cancer. Breast Cancer Res 23:3, 2021.
- Liu J, Tokheim C, Lee JD, et al.: Genetic fusions favor tumorigenesis through degron loss in oncogenes. Nat Commun 12:6704, 2021.
- An S, Koh HH, Chang ES, et al.: Unearthing novel fusions as therapeutic targets in solid tumors using targeted RNA sequencing. Front Oncol 12:892918, 2022.
- Subbiah V, Iannotti NO, Gutierrez M, et al.: FIGHT-101, a first-in-human study of potent and selective FGFR 1-3 inhibitor pemigatinib in pan-cancer patients with FGF/FGFR alterations and advanced malignancies. Ann Oncol 33:522-533, 2022.
- Wheler JJ, Janku F, Naing A, et al.: TP53 Alterations Correlate with Response to VEGF/VEGFR Inhibitors: Implications for Targeted Therapeutics. Mol Cancer Ther 15:2475-2485, 2016.
- Li AM, Boichard A, Kurzrock R: Mutated TP53 is a marker of increased VEGF expression: analysis of 7,525 pan-cancer tissues. Cancer Biol Ther 21:95-100, 2020.
- Uehara Y, Ikeda S, Kim KH, et al.: Targeting the FGF/FGFR axis and its co-alteration allies. ESMO Open 7:100647, 2022.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




