Submitted:
01 August 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Chicken Breast Preparation and Characterization
2.3. Inoculum Preparation and Samples Inoculation
2.4. UV-C Radiation of Chicken Breast Samples
2.5. Microbial Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of the Chicken Breast
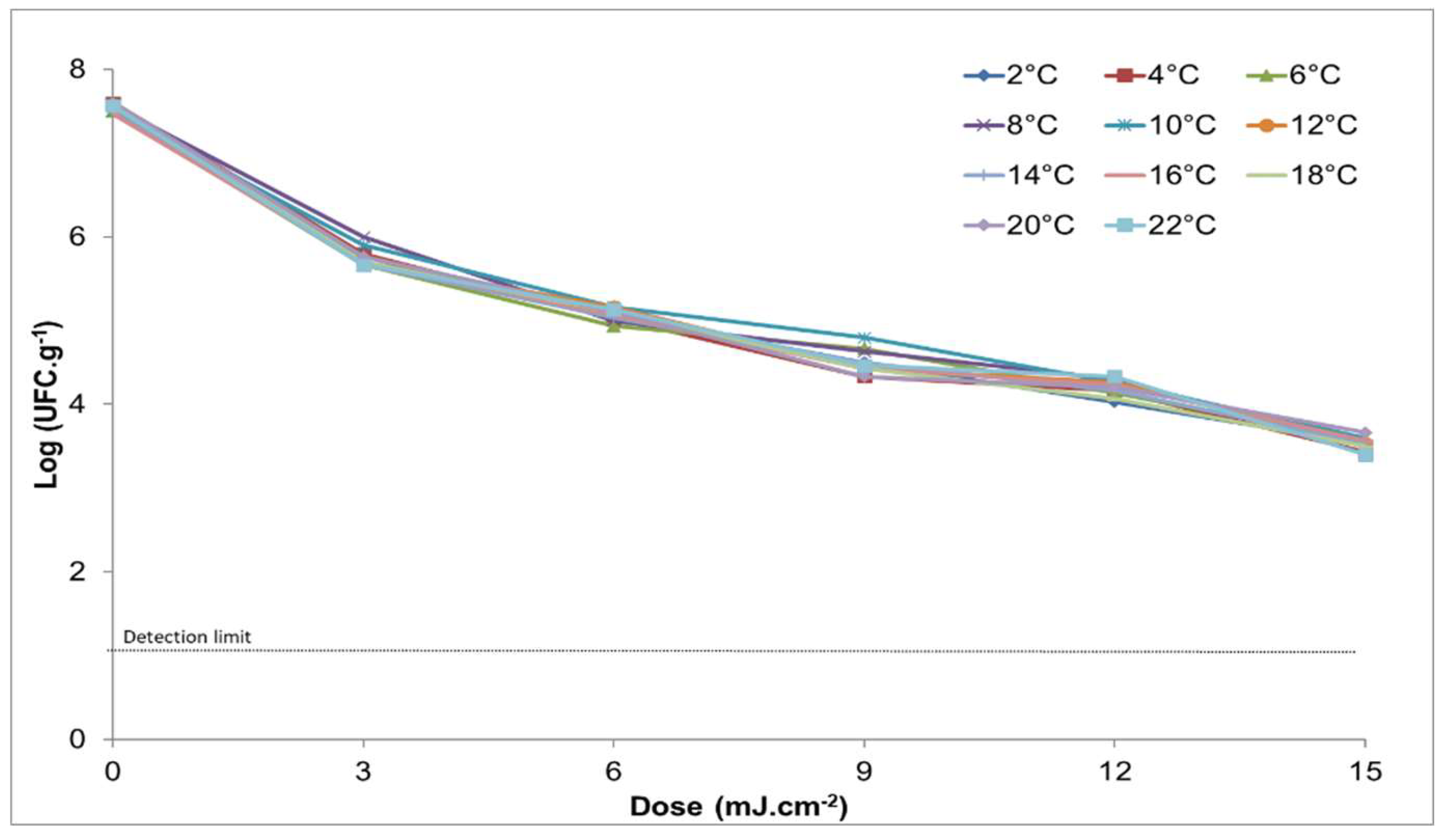
3.2. Behavior of Salmonella enteritidis at Different Doses of UV Light in Chicken Breast at Storage Temperatures from 2 to 22 ° C
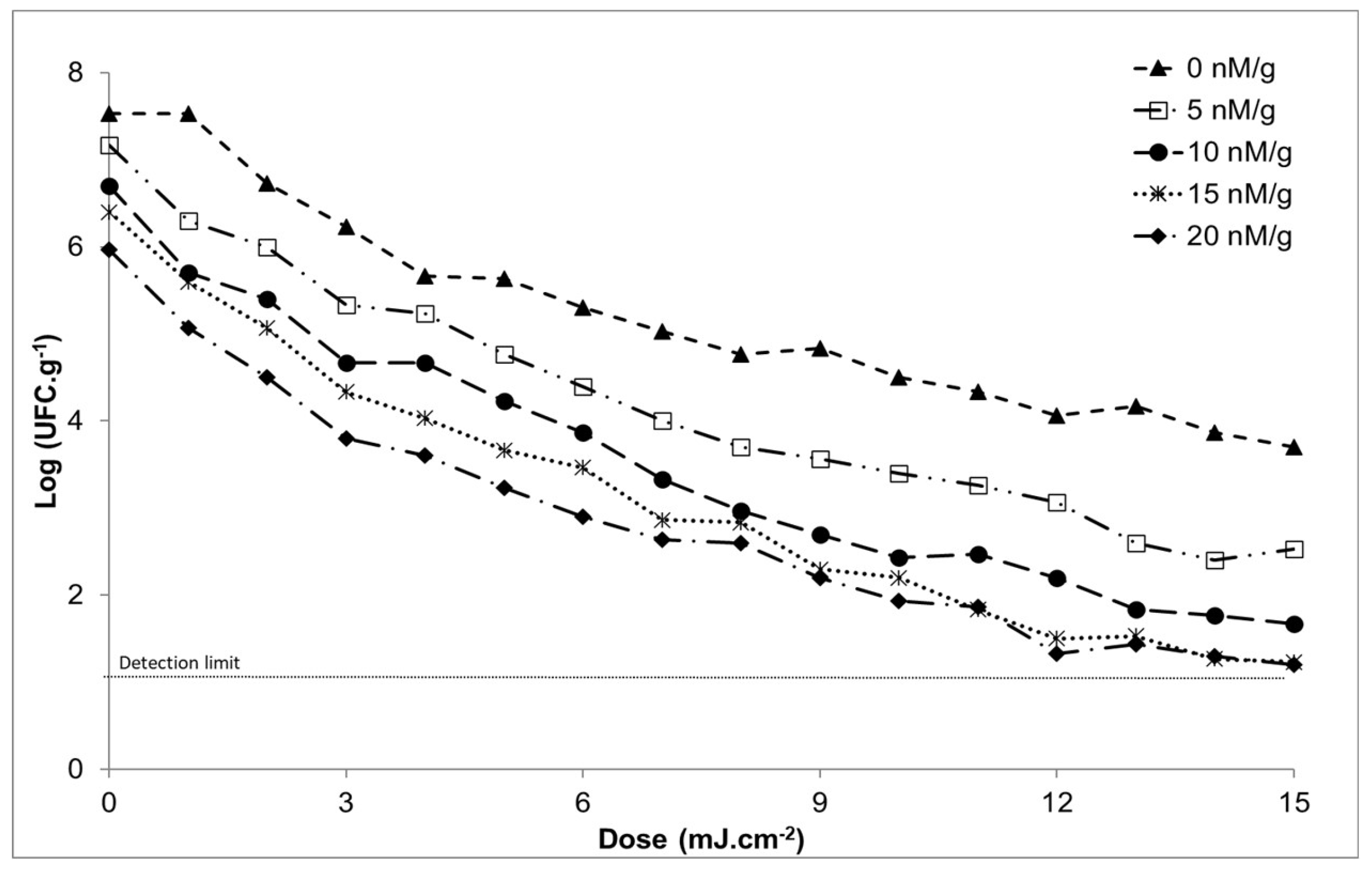
3.3. Primary Model of the Inactivation of Salmonella enteritidis at Different Doses of Caffeine in Chicken Breast at a Constant Temperature of 14ºC
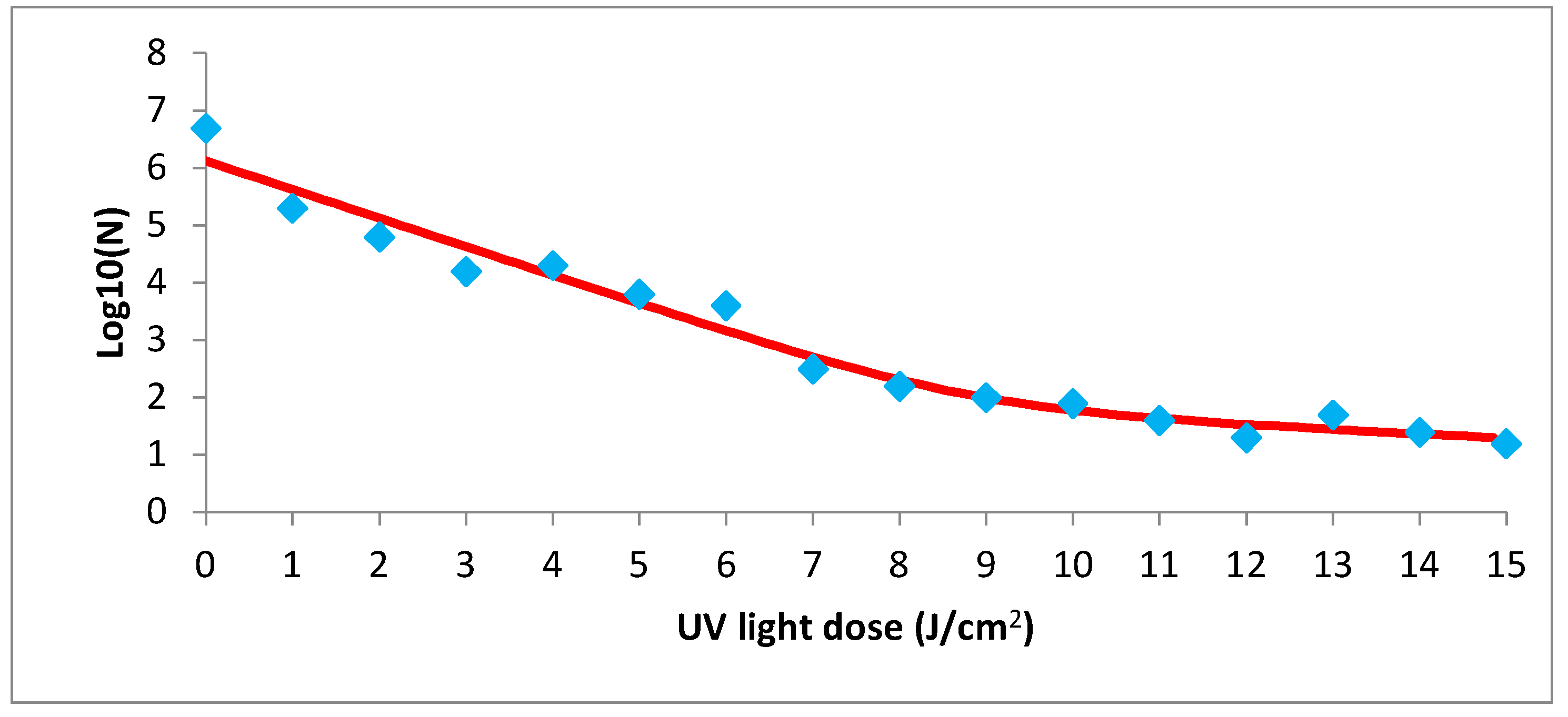
3.5. Inactivation Effect of UV and Caffein on Salmonella enteritidis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Temperature (°C) | Model | Log-linear regression | Log-linear + shoulder | Log-linear + tail | Log-linear + shoulder + tail* | Weibull | Weibull Fixed p-parameter |
Weibull + tail* | Double Weibull* | Biphasic model* | Biphasic + shoulder* |
| 2 | RMSE | 0.5378 | 0.3117 | 0.2488 | 0.2270 | 0.2314 | 0.6375 | 0.1992 | 0.3061 | 0.1115 | 0.1577 |
| R2 adj | 0.8895 | 0.9629 | 0.9764 | 0.9803 | 0.9796 | 0.8448 | 0.9848 | 0.9642 | 0.9952 | 0.9905 | |
| RMSE | 0.4814 | 0.2045 | 0.5039 | 0.2504 | 0.2490 | 0.5656 | 0.2990 | 0.2990 | 0.1897 | 0.2683 | |
| R2 adj | 0.8356 | 0.9703 | 0.8198 | 0.9555 | 0.9560 | 0.7731 | 0.9366 | 0.9366 | 0.9745 | 0.9489 | |
| RMSE | 0.4436 | 0.0544 | 0.4123 | 0.0666 | 0.0816 | 0.5277 | 0.1000 | 0.1000 | 0.0409 | 0.0548 | |
| R2 adj | 0.9163 | 0.9987 | 0.9277 | 0.9981 | 0.9972 | 0.8815 | 0.9957 | 0.9957 | 0.9993 | 0.9987 | |
| 4 | RMSE | 0.5337 | 0.4395 | 0.2810 | 0.3433 | 0.3971 | 0.6283 | 0.3441 | 0.3398 | 0.3403 | 0.4816 |
| R2 adj | 0.8555 | 0.9020 | 0.9599 | 0.9402 | 0.9200 | 0.7997 | 0.9399 | 0.9414 | 0.9412 | 0.8823 | |
| RMSE | 0.7604 | 0.2047 | 0.6000 | 0.2508 | 0.6890 | 0.8932 | 0.2752 | 0.2757 | 0.2530 | 0.3577 | |
| R2 adj | 0.7886 | 0.9847 | 0.8684 | 0.9770 | 0.8264 | 0.7083 | 0.9723 | 0.9723 | 0.9766 | 0.9532 | |
| RMSE | 0.4052 | 0.2816 | 0.4647 | 0.3449 | 0.3130 | 0.4787 | 0.3834 | 0.3834 | 0.3388 | 0.4764 | |
| R2 adj | 0.9226 | 0.9626 | 0.8983 | 0.9440 | 0.9538 | 0.8921 | 0.9307 | 0.9307 | 0.9459 | 0.8931 | |
| 6 | RMSE | 0.4762 | 0.3261 | 0.3978 | 0.3994 | 0.3049 | 0.5619 | 0.3734 | 0.3714 | 0.2976 | 0.3834 |
| R2 adj | 0.8777 | 0.9427 | 0.9147 | 0.9140 | 0.9499 | 0.8298 | 0.9248 | 0.9256 | 0.9523 | 0.9207 | |
| RMSE | 0.4121 | 0.2776 | 0.4638 | 0.3401 | 0.3362 | 0.4859 | 0.4117 | 0.4117 | 0.3033 | 0.4290 | |
| R2 adj | 0.9224 | 0.9648 | 0.9017 | 0.9472 | 0.9484 | 0.8921 | 0.9225 | 0.9225 | 0.9580 | 0.9159 | |
| RMSE | 0.7805 | 0.2751 | 0.3999 | 0.3369 | 0.5415 | 0.9133 | 0.3376 | 0.3376 | 0.2973 | 0.4183 | |
| R2 adj | 0.6871 | 0.9611 | 0.9178 | 0.9417 | 0.8494 | 0.5715 | 0.9414 | 0.9414 | 0.9546 | 0.9101 | |
| 8 | RMSE | 0.3096 | 0.1820 | 0.3039 | 0.1649 | 0.1374 | 0.3692 | 0.1683 | 0.1683 | 0.1407 | 0.1871 |
| R2 adj | 0.9338 | 0.9771 | 0.9362 | 0.9812 | 0.9870 | 0.9058 | 0.9804 | 0.9804 | 0.9863 | 0.9758 | |
| RMSE | 0.5666 | 0.2787 | 0.3524 | 0.2952 | 0.2145 | 0.6709 | 0.3991 | 0.1879 | 0.1934 | 0.2735 | |
| R2 adj | 0.8815 | 0.9713 | 0.9542 | 0.9678 | 0.9830 | 0.8339 | 0.9412 | 0.9870 | 0.9862 | 0.9724 | |
| RMSE | 0.5213 | 0.3358 | 0.5927 | 0.4103 | 0.5159 | 0.6134 | 0.4429 | 0.4429 | 0.3960 | 0.5541 | |
| R2 adj | 0.8753 | 0.9482 | 0.8388 | 0.9227 | 0.8778 | 0.8273 | 0.9100 | 0.9100 | 0.9280 | 0.8591 | |
| 10 | RMSE | 0.6261 | 0.2772 | 0.6084 | 0.3395 | 0.3128 | 0.7352 | 0.3831 | 0.3831 | 0.3282 | 0.4637 |
| R2 adj | 0.8158 | 0.9639 | 0.8260 | 0.9459 | 0.9540 | 0.7460 | 0.9310 | 0.9310 | 0.9494 | 0.8990 | |
| RMSE | 0.3984 | 0.0447 | 0.3334 | 0.0498 | 0.0534 | 0.4736 | 0.0654 | 0.0654 | 0.0576 | 0.0815 | |
| R2 adj | 0.9098 | 0.9989 | 0.9368 | 0.9986 | 0.9984 | 0.8725 | 0.9976 | 0.9976 | 0.9981 | 0.9962 | |
| RMSE | 0.2008 | 0.1225 | 0.1989 | 0.1496 | 0.1312 | 0.2440 | 0.1607 | 0.1607 | 0.1485 | 0.2074 | |
| R2 adj | 0.9782 | 0.9919 | 0.9786 | 0.9879 | 0.9907 | 0.9678 | 0.9860 | 0.9860 | 0.9881 | 0.9767 | |
| 12 | RMSE | 0.4075 | 0.1954 | 0.4325 | 0.2393 | 0.2121 | 0.4851 | 0.2598 | 0.2598 | 0.2396 | 0.3388 |
| R2 adj | 0.9337 | 0.9847 | 0.9253 | 0.9771 | 0.9820 | 0.9060 | 0.9730 | 0.9730 | 0.9771 | 0.9541 | |
| RMSE | 0.5596 | 0.4233 | 0.4924 | 0.5145 | 0.4208 | 0.6560 | 0.5153 | 0.5153 | 0.5215 | 0.7376 | |
| R2 adj | 0.8112 | 0.8920 | 0.8538 | 0.8404 | 0.8933 | 0.7406 | 0.8399 | 0.8399 | 0.8360 | 0.6720 | |
| RMSE | 0.6649 | 0.2282 | 0.5477 | 0.2795 | 0.2549 | 0.7804 | 0.3122 | 0.3439 | 0.2651 | 0.3742 | |
| R2 adj | 0.7877 | 0.9750 | 0.8560 | 0.9625 | 0.9688 | 0.7076 | 0.9532 | 0.9432 | 0.9663 | 0.9328 | |
| 14 | RMSE | 0.4405 | 0.1211 | 0.4406 | 0.1483 | 0.1574 | 0.5228 | 0.1927 | 0.1927 | 0.1466 | 0.2073 |
| R2 adj | 0.9102 | 0.9932 | 0.9102 | 0.9898 | 0.9885 | 0.8736 | 0.9828 | 0.9828 | 0.9901 | 0.9801 | |
| RMSE | 0.6994 | 0.5778 | 0.8634 | 0.7076 | 0.6137 | 0.8168 | 0.7842 | 0.7516 | 0.7011 | 0.9915 | |
| R2 adj | 0.8035 | 0.8659 | 0.7006 | 0.7989 | 0.8487 | 0.7321 | 0.7530 | 0.7731 | 0.8026 | 0.6052 | |
| RMSE | 0.6937 | 0.4784 | 0.4931 | 0.4847 | 0.4561 | 0.8119 | 0.5016 | 0.5111 | 0.5832 | 0.8248 | |
| R2 adj | 0.7179 | 0.8658 | 0.8575 | 0.8623 | 0.8780 | 0.6136 | 0.8525 | 0.8469 | 0.8006 | 0.6011 | |
| 16 | RMSE | 0.6937 | 0.4784 | 0.4931 | 0.4847 | 0.4561 | 0.8090 | 0.5016 | 0.2572 | 0.5832 | 0.8248 |
| R2 adj | 0.7179 | 0.8658 | 0.8575 | 0.8623 | 0.8780 | 0.6154 | 0.8525 | 0.9611 | 0.8006 | 0.6011 | |
| RMSE | 0.5794 | 0.3891 | 0.5219 | 0.4765 | 0.3857 | 0.6813 | 0.4724 | 0.4724 | 0.4730 | 0.6690 | |
| R2 adj | 0.8474 | 0.9312 | 0.8762 | 0.8968 | 0.9324 | 0.7890 | 0.8985 | 0.8985 | 0.8983 | 0.7966 | |
| RMSE | 0.3476 | 0.2496 | 0.4014 | 0.3057 | 0.2895 | 0.4112 | 0.3546 | 0.3546 | 0.2924 | 0.4135 | |
| R2 adj | 0.9408 | 0.9695 | 0.9211 | 0.9543 | 0.9590 | 0.9172 | 0.9385 | 0.9385 | 0.9581 | 0.9163 | |
| 18 | RMSE | 0.5472 | 0.1900 | 0.5384 | 0.2327 | 0.2417 | 0.6454 | 0.2960 | 0.2960 | 0.2037 | 0.2881 |
| R2 adj | 0.8702 | 0.9843 | 0.8743 | 0.9765 | 0.9747 | 0.8194 | 0.9620 | 0.9620 | 0.9820 | 0.9640 | |
| RMSE | 0.5348 | 0.1097 | 0.4345 | 0.0836 | 0.1070 | 0.6341 | 0.1311 | 0.1311 | 0.1411 | 0.1996 | |
| R2 adj | 0.8933 | 0.9955 | 0.9296 | 0.9974 | 0.9957 | 0.8501 | 0.9936 | 0.9936 | 0.9926 | 0.9851 | |
| RMSE | 0.4688 | 0.2374 | 0.3425 | 0.2908 | 0.2221 | 0.5531 | 0.2721 | 0.1114 | 0.2048 | 0.2683 | |
| R2 adj | 0.8590 | 0.9638 | 0.9247 | 0.9457 | 0.9683 | 0.8037 | 0.9525 | 0.9920 | 0.9731 | 0.9538 | |
| 20 | RMSE | 0.4526 | 0.4866 | 0.3186 | 0.3840 | 0.4636 | 0.5310 | 0.3634 | 0.3634 | 0.3901 | 0.5431 |
| R2 adj | 0.9136 | 0.9002 | 0.9572 | 0.9378 | 0.9094 | 0.8811 | 0.9443 | 0.9443 | 0.9358 | 0.8757 | |
| RMSE | 0.8864 | 0.7444 | 0.9001 | 0.9117 | 0.7578 | 1.0330 | 1.0600 | 0.9281 | 0.9086 | 1.2849 | |
| R2 adj | 0.6931 | 0.7836 | 0.6836 | 0.6753 | 0.7757 | 0.5831 | 0.5611 | 0.6636 | 0.6775 | 0.3551 | |
| RMSE | 0.8106 | 0.4411 | 0.2494 | 0.3054 | 0.3199 | 0.9479 | 0.3062 | 0.1801 | 0.2368 | 0.3318 | |
| R2 adj | 0.6465 | 0.8953 | 0.9665 | 0.9498 | 0.9450 | 0.5165 | 0.9496 | 0.9826 | 0.9698 | 0.9408 | |
| 22 | RMSE | 0.7309 | 0.4878 | 0.8440 | 0.5974 | 0.6194 | 0.8531 | 0.6927 | 0.6750 | 0.5527 | 0.7817 |
| R2 adj | 0.7647 | 0.8952 | 0.6863 | 0.8428 | 0.8311 | 0.6795 | 0.7887 | 0.7994 | 0.8655 | 0.7309 | |
| RMSE | 0.4208 | 0.2650 | 0.4531 | 0.3246 | 0.4211 | 0.4996 | 0.3983 | 0.3605 | 0.2931 | 0.4099 | |
| R2 adj | 0.9262 | 0.9707 | 0.9144 | 0.9561 | 0.9261 | 0.8959 | 0.9338 | 0.9458 | 0.9642 | 0.9299 | |
| RMSE | 0.4889 | 0.1759 | 0.2487 | 0.0997 | 0.1263 | 0.5780 | 0.0948 | 0.1169 | 0.1552 | 0.2194 | |
| R2 adj | 0.8596 | 0.9818 | 0.9637 | 0.9942 | 0.9906 | 0.8038 | 0.9947 | 0.9920 | 0.9859 | 0.9717 |
| UVC (J / cm2) |
Model | Log-linear regression | Log-linear ± shoulder |
log-linear ± tail | Log-linear ± shoulder ± tail* |
Weibull* | Weibull Fixed p-parameter* |
Weibull ± tail* | Biphasic model* |
| o | RMSE | 0.0399 | 0.0485 | 0.0488 | 0.0686 | 0.0486 | 0.0502 | 0.0688 | 0.0684 |
| R2 adj | 0.9958 | 0.9938 | 0.9937 | 0.9877 | 0.9938 | 0.9934 | 0.9876 | 0.9877 | |
| 1 | RMSE | 0.1292 | 0.1458 | 0.1543 | 0.2051 | 0.1461 | 0.1615 | 0.2066 | 0.2034 |
| R2 adj | 0.9602 | 0.9493 | 0.9432 | 0.8997 | 0.9491 | 0.9378 | 0.8982 | 0.9013 | |
| 2 | RMSE | 0.1026 | 0.0942 | 0.1256 | 0.1333 | 0.0938 | 0.1199 | 0.1325 | 0.1776 |
| R2 adj | 0.9786 | 0.9819 | 0.9679 | 0.9639 | 0.9821 | 0.9708 | 0.9643 | 0.9358 | |
| 3 | RMSE | 0.0869 | 0.1032 | 0.1065 | 0.1428 | 0.1036 | 0.1047 | 0.1434 | 0.1506 |
| R2 adj | 0.9866 | 0.9812 | 0.9800 | 0.9639 | 0.9810 | 0.9806 | 0.9637 | 0.9599 | |
| 4 | RMSE | 0.0710 | 0.0719 | 0.0868 | 0.0194 | 0.0763 | 0.0824 | 0.0121 | 0.1229 |
| R2 adj | 0.9928 | 0.9926 | 0.9892 | 0.9995 | 0.9917 | 0.9903 | 0.9998 | 0.9783 | |
| 5 | RMSE | 0.0380 | 0.0417 | 0.0235 | 0.0219 | 0.0399 | 0.0526 | 0.0210 | 0.0332 |
| R2 adj | 0.9979 | 0.9975 | 0.9992 | 0.9993 | 0.9977 | 0.9960 | 0.9994 | 0.9984 | |
| 6 | RM5E | 0.0678 | 0.0509 | 0.0785 | 0.0720 | 0.0546 | 0.0907 | 0.0772 | 0.0686 |
| R2 adj | 0.9932 | 0.9962 | 0.9909 | 0.9924 | 0.9956 | 0.9879 | 0.9912 | 0.9931 | |
| 7 | RMSE | 0.1970 | 0.1234 | 0.0225 | 0.0121 | 0.1100 | 0.2511 | 0.0100 | 0.0060 |
| R2 adj | 0.9488 | 0.9799 | 0.9993 | 0.9998 | 0.9841 | 0.9168 | 0.9999 | 1.0000 | |
| 8 | RMSE | 0.3864 | 0.1616 | 0.1104 | 0.1343 | 0.2113 | 0.4834 | 0.1289 | 0.0767 |
| R2 adj | 0.8186 | 0.9683 | 0.9852 | 0.9781 | 0.9457 | 0.7161 | 0.9798 | 0.9929 | |
| 9 | RMSE | 0.3342 | 0.2330 | 0.0178 | 0.0184 | 0.2127 | 0.4198 | 0.0197 | 0.0250 |
| R2 adj | 0.8817 | 0.9425 | 0.9997 | 0.9996 | 0.9521 | 0.8134 | 0.9996 | 0.9993 | |
| 10 | RMSE | 0.3360 | 0.2380 | 0.1128 | 0.1567 | 0.2191 | 0.4220 | 0.1587 | 0.1324 |
| R2 adj J | 0.8854 | 0.9425 | 0.9871 | 0.9751 | 0.9513 | 0.8191 | 0.9744 | 0.9822 | |
| 11 | RMSE | 0.3088 | 0.2764 | 0.0988 | 0.1138 | 0.2582 | 0.3873 | 0.1055 | 0.1397 |
| R2 adj J | 0.8966 | 0.9172 | 0.9894 | 0.9860 | 0.9277 | 0.8373 | 0.9879 | 0.9788 | |
| 12 | RM5E | 0.3199 | 0.2281 | 0.0528 | 0.0444 | 0.1942 | 0.4047 | 0.0507 | 0.0742 |
| R2 adj | 0.9261 | 0.9624 | 0.9980 | 0.9986 | 0.9728 | 0.8817 | 0.9981 | 0.9960 | |
| 13 | RMSE | 0.4358 | 0.1895 | 0.0823 | 0.0271 | 0.2460 | 0.5451 | 0.0204 | 0.0401 |
| R2 adj | 0.8143 | 0.9649 | 0.9934 | 0.9993 | 0.9408 | 0.7094 | 0.9996 | 0.9984 | |
| 14 | RMSE | 0.4375 | 0.2009 | 0.1448 | 0.1113 | 0.1834 | 0.5471 | 0.1206 | 0.1861 |
| R2 adj | 0.8130 | 0.9606 | 0.9795 | 0.9879 | 0.9671 | 0.7076 | 0.9858 | 0.9662 | |
| 15 | RM5E | 0.3691 | 0.2540 | 0.0187 | 0.0255 | 0.2321 | 0.4628 | 0.0249 | 0.1502 |
| R2 adj | 0.8627 | 0.9350 | 0.9996 | 0.9993 | 0.9457 | 0.7841 | 0.9994 | 0.9773 |
| Caffeine (nM/g) |
Model | Log-linear regression | Log-linear + shoulder | Log-linear + tail | Log-linear + shoulder + tail | Weibull | Weibull Fixed p-parameter |
Weibull + tail | Double Weibull | Biphasic model | Biphasic + shoulder |
| 0 | RMSE | 0.3197 | 0.1481 | 0.2739 | 0.1518 | 0.1273 | 0.3398 | 0.1304 | 0.1127 | 0.1133 | 0.1159 |
| R2 adj | 0.9224 | 0.9833 | 0.9430 | 0.9825 | 0.9877 | 0.9123 | 0.9871 | 0.9903 | 0.9902 | 0.9898 | |
| RMSE | 0.4962 | 0.4084 | 0.5130 | 0.4251 | 0.4063 | 0.5204 | 0.4531 | 0.4229 | 0.4126 | 0.4310 | |
| R2 adj | 0.8446 | 0.8947 | 0.8339 | 0.8859 | 0.8958 | 0.8290 | 0.8704 | 0.8871 | 0.8925 | 0.8827 | |
| RMSE | 0.4871 | 0.4191 | 0.4027 | 0.3896 | 0.4022 | 0.5107 | 0.3974 | 0.4271 | 0.4261 | 0.4451 | |
| R2 adj | 0.8103 | 0.8595 | 0.8703 | 0.8786 | 0.8706 | 0.7915 | 0.8737 | 0.8542 | 0.8548 | 0.8416 | |
| 5 | RMSE | 0.2630 | 0.1764 | 0.2711 | 0.1836 | 0.1915 | 0.2808 | 0.2038 | 0.1993 | 0.1842 | 0.1924 |
| R2 adj | 0.9683 | 0.9857 | 0.9663 | 0.9846 | 0.9832 | 0.9639 | 0.9810 | 0.9818 | 0.9845 | 0.9830 | |
| RMSE | 0.4241 | 0.3509 | 0.3412 | 0.3107 | 0.3107 | 0.4489 | 0.4035 | 0.3060 | 0.3273 | 0.3418 | |
| R2 adj | 0.9239 | 0.9479 | 0.9507 | 0.9592 | 0.9591 | 0.9147 | 0.9311 | 0.9604 | 0.9547 | 0.9505 | |
| RMSE | 0.4881 | 0.4057 | 0.2958 | 0.2934 | 0.3486 | 0.5149 | 0.2935 | 0.2909 | 0.2943 | 0.3073 | |
| R2 adj | 0.8743 | 0.9131 | 0.9538 | 0.9546 | 0.9359 | 0.8601 | 0.9545 | 0.9553 | 0.9543 | 0.9501 | |
| 10 | RMSE | 0.3585 | 0.2517 | 0.2320 | 0.1685 | 0.2055 | 0.3821 | 0.3124 | 0.1814 | 0.2332 | 0.2435 |
| R2 adj | 0.9425 | 0.9717 | 0.9758 | 0.9873 | 0.9811 | 0.9347 | 0.9564 | 0.9853 | 0.9757 | 0.9735 | |
| RMSE | 0.3575 | 0.2671 | 0.2505 | 0.2042 | 0.2267 | 0.3810 | 0.2119 | 0.2120 | 0.2442 | 0.2551 | |
| R2 adj | 0.9479 | 0.9709 | 0.9744 | 0.9830 | 0.9791 | 0.9408 | 0.9817 | 0.9817 | 0.9757 | 0.9735 | |
| RMSE | 0.5428 | 0.4842 | 0.4793 | 0.4753 | 0.4482 | 0.5713 | 0.4721 | 0.4831 | 0.4536 | 0.4738 | |
| R2 adj | 0.8914 | 0.9136 | 0.9153 | 0.9167 | 0.9259 | 0.8797 | 0.9178 | 0.9140 | 0.9242 | 0.9173 | |
| 15 | RMSE | 0.3702 | 0.3166 | 0.3647 | 0.3244 | 0.3147 | 0.3917 | 0.3276 | 0.3276 | 0.3029 | 0.3072 |
| R2 adj | 0.9524 | 0.9652 | 0.9538 | 0.9635 | 0.9656 | 0.9467 | 0.9627 | 0.9627 | 0.9681 | 0.9672 | |
| RMSE | 0.4405 | 0.3679 | 0.2684 | 0.3225 | 0.2853 | 0.4675 | 0.3674 | 0.2129 | 0.3125 | 0.2181 | |
| R2 adj | 0.9169 | 0.9420 | 0.9691 | 0.9554 | 0.9651 | 0.9064 | 0.9422 | 0.9806 | 0.9649 | 0.9796 | |
| RMSE | 0.5291 | 0.3820 | 0.3091 | 0.2341 | 0.3112 | 0.5600 | 0.5696 | 0.2630 | 0.3125 | 0.3264 | |
| R2 adj | 0.8995 | 0.9476 | 0.9657 | 0.9803 | 0.9652 | 0.8874 | 0.8835 | 0.9752 | 0.9649 | 0.9617 | |
| 20 | RMSE | 0.4977 | 0.3750 | 0.3231 | 0.3128 | 0.3017 | 0.5258 | 0.2914 | 0.2395 | 0.2415 | 0.2523 |
| R2 adj | 0.8747 | 0.9288 | 0.9472 | 0.9505 | 0.9539 | 0.8601 | 0.9570 | 0.9710 | 0.9705 | 0.9678 | |
| RMSE | 0.5530 | 0.3819 | 0.4105 | 0.3561 | 0.3043 | 0.5837 | 0.2890 | 0.2587 | 0.2598 | 0.2713 | |
| R2 adj | 0.8589 | 0.9327 | 0.9222 | 0.9415 | 0.9573 | 0.8427 | 0.9614 | 0.9691 | 0.9688 | 0.9660 | |
| RMSE | 0.3976 | 0.3359 | 0.3571 | 0.3244 | 0.3519 | 0.4188 | 0.3524 | 0.3533 | 0.3717 | 0.4890 | |
| R2 adj | 0.9296 | 0.9498 | 0.9432 | 0.9531 | 0.9449 | 0.9219 | 0.9447 | 0.9444 | 0.9385 | 0.8935 |
References
- Townshend, A. Food and Nutritional Analysis - Meat and Meat Products. In Encyclopedia of Analytical Science; Elsevier Applied Science, 2019; pp 436–450.
- EU Parliament and Council. Regulation (EC) No. 853/2004 of the European Parliament and of the Council of April 29, 2004, which establishes specific hygiene rules for food of animal origin. DO L 2004, 139, 55. [Google Scholar]
- Chouliara, E.; Karatapanis, A.; Savvaidis, I. N.; Kontominas, M. G. Combined Effect of Oregano Essential Oil and Modified Atmosphere Packaging on Shelf-Life Extension of Fresh Chicken Breast Meat, Stored at 4 °C. Food Microbiol 2007, 24, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Barbut, S. Poultry Products Processing. CRC Press eBooks, 2016.
- Yang, S.; Park, S. Y.; Ha, S. Do. A Predictive Growth Model of Aeromonas hydrophila on Chicken Breasts under Various Storage Temperatures. LWT - Food Science and Technology 2016, 69, 98–103. [Google Scholar] [CrossRef]
- Boelaert, F.; Tegegne, H. A.; Stoicescu, A.; Messens, W.; Hempen, M.; Rossi, M.; Sarno, E.; Rizzi, V.; Aznar, I.; Antoniou, S. E.; Baldinelli, F.; Van der Stede, Y.; Niskanen, T.; Young, J.; Merk, H.; Quinten, C.; Barco, L.; Mancin, M.; Garbo, A.; Patuzzi, I.; Lettini, A. A.; Losasso, C.; Mantovani, C.; Morabito, S.; Scavia, G.; Knijn, A.; Tozzoli, R.; Moro, O.; Gianfranceschi, M.; Suffredini, E.; Di Bartolo, I.; Delibato, E.; Anniballi, F.; Ianiro, G.; Altieri, I.; Maugliani, A.; Morales, M. A. G.; Casulli, A.; Cacciò, S.; Albert, D. The European Union One Health 2018 Zoonoses Report. EFSA Journal 2019, 17. [Google Scholar] [CrossRef]
- Aymerich, T.; Picouet, P. A.; Monfort, J. M. Decontamination Technologies for Meat Products. Meat Sci 2008, 78, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. H.; Ren, Y.; Seow, J.; Liu, T.; Bang, W. S.; Yuk, H. G. Intervention Technologies for Ensuring Microbiological Safety of Meat: Current and Future Trends. Comprehensive Reviews in Food Science and Food Safety. March 2012, pp 119–132. [CrossRef]
- Troy, D. J.; Ojha, K. S.; Kerry, J. P.; Tiwari, B. K. Sustainable and Consumer-Friendly Emerging Technologies for Application within the Meat Industry: An Overview. Meat Sci 2016, 120, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Beltrán, J. A.; Barbosa-Cánovas, G. V. Review: Advantages and Limitations on Processing Foods by UV Light. Food Science and Technology International. June 2004, pp 137–147. [CrossRef]
- Gabriel, A. A. Inactivation of Escherichia coli O157:H7 and Spoilage Yeasts in Germicidal UV-C-Irradiated and Heat-Treated Clear Apple Juice. Food Control 2012, 25, 425–432. [Google Scholar] [CrossRef]
- Amanina, A. K. Z.; Rosnah, S.; Noranizan, M. A.; Alifdalino, S. UV-C Effect on Microbial Disinfection of Pineapple-Mango Juice Blend Using Dean-Vortex Technology. Food Res 2019, 3, 285–294. [Google Scholar] [CrossRef]
- Caminiti, I. M.; Palgan, I.; Muñoz, A.; Noci, F.; Whyte, P.; Morgan, D. J.; Cronin, D. A.; Lyng, J. G. The Effect of Ultraviolet Light on Microbial Inactivation and Quality Attributes of Apple Juice. Food Bioproc Tech 2012, 5, 680–686. [Google Scholar] [CrossRef]
- Koutchma, T.; Parisi, B. Biodosimetry of Escherichia Coli UV Inactivation in Model Juices with Regard to Dose Distribution in Annular UV Reactors. J Food Sci 2004, 69. [Google Scholar] [CrossRef]
- Chun, H. H.; Kim, J. Y.; Lee, B. D.; Yu, D. J.; Song, K. B. Effect of UV-C Irradiation on the Inactivation of Inoculated Pathogens and Quality of Chicken Breasts during Storage. Food Control 2010, 21, 276–280. [Google Scholar] [CrossRef]
- Barba, F. J.; Koubaa, M.; do Prado-Silva, L.; Orlien, V.; Sant’Ana, A. Mild Processing Applied to the Inactivation of the Main Foodborne Bacterial Pathogens: A Review. Trends in Food Science and Technology. Elsevier Ltd. August 1, 2017, pp 20–35. [CrossRef]
- Reichel, J.; Kehrenberg, C.; Krischek, C. UV-C Irradiation of Rolled Fillets of Ham Inoculated with Yersinia enterocolitica and Brochothrix thermosphacta. Foods 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Porciúncula, L. O.; Sallaberry, C.; Mioranzza, S.; Botton, P. H. S.; Rosemberg, D. B. The Janus Face of Caffeine. Neurochem Int 2013, 63, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Madusanka, N.; Eddleston, M. D.; Arhangelskis, M.; Jones, W. Polymorphs, Hydrates and Solvates of a Co-Crystal of Caffeine with Anthranilic Acid. Acta Crystallogr B Struct Sci Cryst Eng Mater 2014, 70, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S. A.; Salameh, M. M.; Phetsomphou, S.; Yang, H.; Seo, C. W. Application of Caffeine, 1,3,7-Trimethylxanthine, to Control Escherichia coli O157:H7. Food Chem 2006, 99, 645–650. [Google Scholar] [CrossRef]
- Possas, A.; Valero, A.; García-Gimeno, R. M.; Pérez-Rodríguez, F.; Mendes de Souza, P. Combining UV-C Technology and Caffeine Application to Inactivate Escherichia Coli on Chicken Breast Fillets. Food Control 2021, 129. [Google Scholar] [CrossRef]
- Abosede, O. O.; Gordon, A. T.; Dembaremba, T. O.; Lorentino, C. M. A.; Frota, H. F.; Santos, A. L. S.; Hosten, E. C.; Ogunlaja, A. S. Trimesic Acid-Theophylline and Isopthalic Acid-Caffeine Cocrystals: Synthesis, Characterization, Solubility, Molecular Docking, and Antimicrobial Activity. Cryst Growth Des 2020, 20, 3510–3522. [Google Scholar] [CrossRef]
- Maletta, A. B.; Were, L. M. Effect of Coffee Filtrate, Methylglyoxal, Glyoxal, and Caffeine on Salmonella Typhimurium and S. Enteritidis Survival in Ground Chicken Breasts. J Food Sci 2012, 77. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.; Rose, J.; Gerba, C. Quantitative Microbial Risk Assessment, 2nd Edition.; John Wiley & Sons Inc: Hoboken, New Jersey, 2014. [Google Scholar]
- Dean, K.; Wissler, A.; Hernandez-Suarez, J. S.; Nejadhashemi, A. P.; Mitchell, J. Modeling the Persistence of Viruses in Untreated Groundwater. Science of the Total Environment 2020, 717. [Google Scholar] [CrossRef] [PubMed]
- McMeekin, T.; Olley, J.; Ratkowsky, D.; Corkrey, R.; Ross, T. Predictive Microbiology Theory and Application: Is It All about Rates? Food Control 2013, 29, 290–299. [Google Scholar] [CrossRef]
- Possas, A.; Valero, A.; García-Gimeno, R. M.; Pérez-Rodríguez, F.; de Souza, P. M. Influence of Temperature on the Inactivation Kinetics of Salmonella Enteritidis by the Application of UV-C Technology in Soymilk. Food Control 2018, 94, 132–139. [Google Scholar] [CrossRef]
- Keklik, N. M.; Demirci, A.; Puri, V. M.; Heinemann, P. H. Modeling the Inactivation of Salmonella Typhimurium, Listeria monocytogenes, and Salmonella Enteritidis on Poultry Products Exposed to Pulsed UV Light. J Food Prot 2012, 75, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Hessel, C. T.; de Freitas Costa, E.; Boff, R. T.; Pessoa, J. P.; Tondo, E. C. A Systematic Review and Bayesian Meta-Analysis about Salmonella spp. Prevalence on Raw Chicken Meat. Microb Risk Anal 2022, 21, 100205. [Google Scholar] [CrossRef]
- Pagal, G. A.; Gabriel, A. A. Individual and Combined Mild Heat and UV-C Processes for Orange Juice against Escherichia coli O157:H7. LWT 2020, 126. [Google Scholar] [CrossRef]
- AOAC International; Latimer, G. W. AOAC International. In Official methods of analysis of AOAC International; 2012.
- Geeraerd, A. H.; Valdramidis, V. P.; Van Impe, J. F. GInaFiT, a Freeware Tool to Assess Non-Log-Linear Microbial Survivor Curves. Int J Food Microbiol 2005, 102, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Soro, A. B.; Harrison, S. M.; Whyte, P.; Bolton, D. J.; Tiwari, B. K. Impact of Ultraviolet Light and Cold Plasma on Fatty Acid Profile of Raw Chicken and Pork Meat. Journal of Food Composition and Analysis 2022, 114. [Google Scholar] [CrossRef]
- Kim, Y. H.; Jeong, S. G.; Back, K. H.; Park, K. H.; Chung, M. S.; Kang, D. H. Effect of Various Conditions on Inactivation of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes in Fresh-Cut Lettuce Using Ultraviolet Radiation. Int J Food Microbiol 2013, 166, 349–355. [Google Scholar] [CrossRef]
- Baysal, A. H.; Molva, C.; Unluturk, S. UV-C Light Inactivation and Modeling Kinetics of Alicyclobacillus acidoterrestris Spores in White Grape and Apple Juices. Int J Food Microbiol 2013, 166, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A. A.; Vera, D. D.; Lazo, O. M. Y.; Azarcon, V. B.; De Ocampo, C. G.; Marasigan, J. C.; Sandel, G. T. Ultraviolet-C Inactivation of Escherichia coli O157:H7, Listeria monocytogenes, Pseudomonas aeruginosa, and Salmonella enterica in Liquid Egg White. Food Control 2017, 73, 1303–1309. [Google Scholar] [CrossRef]
- Albert, I.; Mafart, P. A Modified Weibull Model for Bacterial Inactivation. In International Journal of Food Microbiology; Elsevier, 2005; Vol. 100, pp 197–211. [CrossRef]
- Mutz, Y. S.; Rosario, D. K. A.; Bernardes, P. C.; Paschoalin, V. M. F.; Conte-Junior, C. A. Modeling Salmonella Typhimurium Inactivation in Dry-Fermented Sausages: Previous Habituation in the Food Matrix Undermines UV-C Decontamination Efficacy. Front Microbiol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Siguemoto, É. S.; Gut, J. A. W.; Martinez, A.; Rodrigo, D. Inactivation Kinetics of Escherichia coli O157:H7 and Listeria monocytogenes in Apple Juice by Microwave and Conventional Thermal Processing. Innovative Food Science & Emerging Technologies 2018, 45, 84–91. [Google Scholar] [CrossRef]
- CERF, O. A Review Tailing of Survival Curves of Bacterial Spores. Journal of Applied Bacteriology 1977, 42, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gündüz, G. T.; Korkmaz, A. UV-C Treatment for the Inhibition of Molds Isolated from Dried Persimmons (Diospyros Kaki L.) and Modelling of UV-C Inactivation Kinetics. LWT 2019, 115. [Google Scholar] [CrossRef]
- Haughton, P. N.; Lyng, J. G.; Cronin, D. A.; Morgan, D. J.; Fanning, S.; Whyte, P. Efficacy of UV Light Treatment for the Microbiological Decontamination of Chicken, Associated Packaging, and Contact Surfaces. J Food Prot 2011, 74, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Adkins, A.; C. Minor, R.; Ibrahim, S. A. Behavior and Changes in Cell Morphology of Escherichia coli O157:H7 in Liquid Medium and Skim Milk in the Presence of Caffeine. CYTA - Journal of Food 2014, 12, 235–241. [CrossRef]
- Almeida, A. A. P.; Naghetini, C. C.; Santos, V. R.; Antonio, A. G.; Farah, A.; Glória, M. B. A. Influence of Natural Coffee Compounds, Coffee Extracts and Increased Levels of Caffeine on the Inhibition of Streptococcus mutans. Food Research International 2012, 49, 459–461. [Google Scholar] [CrossRef]
- Bigelow, W. D.; Esty, J. R. The Thermal Death Point in Relation to Time of Typical Thermophilic Organisms. J Infect Dis 1920, 27, 602–617. [Google Scholar] [CrossRef]
- Melnik, M.; Sprusansky, O.; Musil, P. Bio-Medical Aspects of Purine Alkaloids. Adv Biol Chem 2014, 04, 274–280. [Google Scholar] [CrossRef]
- Apshingekar, P. P.; Aher, S.; Kelly, A. L.; Brown, E. C.; Paradkar, A. Synthesis of Caffeine/Maleic Acid Co-Crystal by Ultrasound-Assisted Slurry Co-Crystallization. J Pharm Sci 2017, 106, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Daurio, D.; Medina, C.; Saw, R.; Nagapudi, K.; Alvarez-Núñez, F. Application of Twin Screw Extrusion in the Manufacture of Cocrystals, Part I: Four Case Studies. Pharmaceutics 2011, 3, 582–600. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, T.; Tumanova, N.; Robeyns, K.; Candoni, N.; Veesler, S. Solution Cocrystallization, an Effective Tool to Explore the Variety of Cocrystal Systems: Caffeine/Dicarboxylic Acid Cocrystals. CrystEngComm 2014, 16, 9603–9611. [Google Scholar] [CrossRef]
- El Hamdani, H.; El Amane, M.; Duhayon, C. Studies on the Syntheses, Structural Characterization, Antimicrobial of the CO-CRYSTAL 1,10-Phenanthrolin-1-IUM(1,10-PhenH+)-Caffeine(Caf)-Hexafluorophosphate. J Mol Struct 2018, 1155, 789–796. [Google Scholar] [CrossRef]
- Ross, T. Indices for Performance Evaluation of Predictive Models in Food Microbiology. Journal of Applied Bacteriology 1996, 81, 501–508. [Google Scholar] [CrossRef] [PubMed]
| Caffeine (nM / g) |
Protein (%) |
Grease (%) |
Humidity (%) |
Ash (%) |
Total acidity (%) |
pH | Absorption coefficient (cm-1) |
| 0 | 20.82 ± 0.18 | 2.85 ± 0.08 | 74.85 ± 2.28 | 1.69 ± 0.05 | 0.21 ± 0.05 | 5.87 ± 0.08 | 959.2 ± 46.7 |
| 5 | 20.97 ± 1.48 | 2.84 ± 0.07 | 76.56 ± 2.40 | 1.66 ± 0.10 | 0.24 ± 0.05 | 5.84 ± 0.06 | 961.5 ± 31.2 |
| 10 | 21.13 ± 2.00 | 2.86 ± 0.12 | 75.40 ± 2.18 | 1.67 ± 0.06 | 0.22 ± 0.03 | 5.82 ± 0.08 | 965.8 ± 42.9 |
| 15 | 20.77 ± 1.07 | 2.80 ± 0.09 | 76.69 ± 1.70 | 1.65 ± 0.08 | 0.23 ± 0.03 | 5.84 ± 0.05 | 961.3 ± 79.7 |
| 20 | 21.18 ± 1.70 | 2.86 ± 0.05 | 74.94 ± 2.71 | 1.67 ± 0.04 | 0.24 ± 0.02 | 5.82 ± 0.12 | 962.6 ± 49.1 |
| Dose (J / cm2) | ||||||
|---|---|---|---|---|---|---|
| T (° C) | 0.0 | 3.0 | 6.0 | 9.0 | 12.0 | 15.0 |
| 2 | 7.5 ± 0.2 | 5.8 ± 0.2 | 5.1 ± 0.2 | 4.5 ± 0.3 | 4.0 ± 0.2 | 3.5 ± 0.2 |
| 4 | 7.6 ± 0.2 | 5.8 ± 0.3 | 5.0 ± 0.2 | 4.3 ± 0.4 | 4.2 ± 0.2 | 3.4 ± 0.3 |
| 6 | 7.5 ± 0.1 | 5.7 ± 0.3 | 4.9 ± 0.4 | 4.7 ± 0.1 | 4.1 ± 0.2 | 3.6 ± 0.2 |
| 8 | 7.6 ± 0.2 | 6.0 ± 0.1 | 5.0 ± 0.2 | 4.6 ± 0.3 | 4.3 ± 0.2 | 3.5 ± 0.3 |
| 10 | 7.5 ± 0.2 | 5.9 ± 0.1 | 5.2 ± 0.2 | 4.8 ± 0.1 | 4.3 ± 0.2 | 3.6 ± 0.1 |
| 12 | 7.6 ± 0.2 | 5.7 ± 0.1 | 5.2 ± 0.2 | 4.4 ± 0.3 | 4.3 ± 0.2 | 3.5 ± 0.3 |
| 14 | 7.5 ± 0.2 | 5.7 ± 0.1 | 5.0 ± 0.0 | 4.5 ± 0.1 | 4.2 ± 0.5 | 3.5 ± 0.5 |
| 16 | 7.5 ± 0.2 | 5.7 ± 0.2 | 5.1 ± 0.2 | 4.4 ± 0.2 | 4.2 ± 0.3 | 3.6 ± 0.4 |
| 18 | 7.6 ± 0.1 | 5.7 ± 0.2 | 5.1 ± 0.2 | 4.4 ± 0.2 | 4.1 ± 0.4 | 3.5 ± 0.3 |
| 20 | 7.6 ± 0.2 | 5.8 ± 0.3 | 5.1 ± 0.5 | 4.3 ± 0.4 | 4.2 ± 0.6 | 3.7 ± 0.3 |
| 22 | 7.6 ± 0.2 | 5.7 ± 0.2 | 5.1 ± 0.2 | 4.5 ± 0.2 | 4.3 ± 0.2 | 3.4 ± 0.3 |
| Caffeine dose (nM / g) | Without caffeine* | With caffeine* | Inactivation rate |
| 0 | 7.5 ± 0.2 | 7.5 ± 0.2 | 0.23 |
| 5 | 7.6 ± 0.2 | 7.2 ± 0.0 | 0.30 |
| 10 | 7.6 ± 0.2 | 6.7 ± 0.1 | 0.32 |
| 15 | 7.6 ± 0.2 | 6.4 ± 0.2 | 0.33 |
| 20 | 7.5 ± 0.2 | 6.0 ± 0.2 | 0.29 |
| Caffeine (nM / g) | |||||
| Dose (J / cm2) | 0 | 5 | 10 | 15 | 20 |
| 0 | 7.5 ± 0.2 | 7.2 ± 0.0 | 6.7 ± 0.1 | 6.4 ± 0.2 | 6.0 ± 0.2 |
| 1 | 6.7 ± 0.2 | 6.3 ± 0.1 | 5.7 ± 0.1 | 5.6 ± 0.2 | 5.1 ± 0.2 |
| 2 | 6.2 ± 0.2 | 6.0 ± 0.1 | 5.4 ± 0.1 | 5.1 ± 0.2 | 4.5 ± 0.3 |
| 3 | 5.7 ± 0.1 | 5.3 ± 0.2 | 4.7 ± 0.2 | 4.3 ± 0.1 | 3.8 ± 0.2 |
| 4 | 5.6 ± 0.2 | 5.2 ± 0.2 | 4.7 ± 0.2 | 4.0 ± 0.2 | 3.6 ± 0.2 |
| 5 | 5.3 ± 0.1 | 4.8 ± 0.1 | 4.2 ± 0.2 | 3.7 ± 0.1 | 3.2 ± 0.2 |
| 6 | 5.0 ± 0.0 | 4.4 ± 0.1 | 3.9 ± 0.0 | 3.5 ± 0.1 | 2.9 ± 0.3 |
| 7 | 4.8 ± 0.3 | 4.0 ± 0.1 | 3.3 ± 0.2 | 2.9 ± 0.2 | 2.6 ± 0.3 |
| 8 | 4.8 ± 0.2 | 3.7 ± 0.3 | 3.0 ± 0.2 | 2.8 ± 0.6 | 2.6 ± 0.4 |
| 9 | 4.5 ± 0.1 | 3.6 ± 0.4 | 2.7 ± 0.5 | 2.3 ± 0.2 | 2.2 ± 0.1 |
| 10 | 4.3 ± 0.3 | 3.4 ± 0.2 | 2.4 ± 0.4 | 2.2 ± 0.2 | 1.9 ± 0.2 |
| 11 | 4.1 ± 0.3 | 3.3 ± 0.3 | 2.5 ± 0.5 | 1.8 ± 0.2 | 1.9 ± 0.4 |
| 12 | 4.2 ± 0.5 | 3.1 ± 0.3 | 2.2 ± 0.2 | 1.5 ± 0.1 | 1.3 ± 0.2 |
| 13 | 3.9 ± 0.4 | 2.6 ± 0.4 | 1.8 ± 0.2 | 1.5 ± 0.4 | 1.4 ± 0.3 |
| 14 | 3.7 ± 0.2 | 2.4 ± 0.2 | 1.8 ± 0.1 | 1.3 ± 0.2 | 1.3 ± 0.3 |
| 15 | 3.5 ± 0.5 | 2.5 ± 0.4 | 1.7 ± 0.3 | 1.2 ± 0.2 | 1.2 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





