1. Introduction
Predation is a key factor in regulating the population structure of invertebrates in aquatic ecosystems. In particular, high predation rates on early juvenile stages of bivalve mollusks and echinoderms significantly impact the survival rates of their populations [
1,
2]. The sea cucumber
Apostichopus japonicus, as a species of aquatic animal, faces a variety of predatory threats from various animals, including marine mammals, seabirds, fish, starfish (especially
Asterina pectinifera), crustaceans, and gastropods [
3]. Although starfish are primary predator of the sea cucumber due to the high overlap in their habitats, there is still a lack of in-depth studies on the impact of predators on sea cucumber [
4].
Predatory pressure can be divided into direct types (direct challenges to homeostasis, such as injury or predator attacks) and indirect types (perceived threats that require cognitive assessment, such as cues of predation risk) [
5]. Under the indirect predatory pressure, preys can response after recognizing risk signals of predation. For example, the preys must identify and assess the predator’s odor and then respond behaviorally and physiologically. Another example is the rapid behavioral and physiological response to the alarm cues of injured conspecifics or heterospecifics. Sea urchins can respond to alarm cues from injured or killed conspecifics [
6,
7,
8,
9]. However, it remains unclear whether sea cucumbers respond to signals emitted by injured conspecifics. Hamel et al. [
10] found that
Cucumaria frondosa can not only recognize threat signals (such as the odor of predators or alarm signals from injured prey) but also induce coelomocytes immune function. Despite this, there are no reported studies on the effects of predatory pressure in
A. japonicus.
Aquatic organisms with limited mobility and without distinct sensory organs, such as limbs or eyes, depend on behavioral, physiological, and immunological adaptations to counter environmental stress. Burnovicz et al. [
11] established a link between escape behavior and cardiac reactivity in crabs, highlighting the activation of defensive mechanisms in response to threats. In organisms lacking measurable physiological responses like cardiac function, immunological changes offer a viable assessment approach. Sea cucumbers
(A. japonicus) predominantly exhibit non-specific immunity, primarily through humoral defense mechanisms [
12]. Their coelomic fluid, rich in immune cells and factors, constitutes a robust immune defense that neutralizes invading substances once through the initial body wall barrier. Monitoring immune enzyme activity in sea cucumbers is essential for elucidating how they physiologically respond to stressors [
13]. The production of reactive oxygen species (ROS) escalates under environmental stress, potentially causing cellular damage. Sea cucumbers employ an antioxidant system to mitigate this oxidative stress, with key enzymatic antioxidants such as superoxide dismutase (SOD) and catalase playing critical roles [
14]. Examining the activity of these antioxidant enzymes under predatory stress provides insights into the sea cucumber’s regulatory strategies against environmental challenges.
This research employed A. japonicus to explore the effects of direct and indirect predation stress, along with alarm cues from injured conspecifics, on the enzymatic activities related to immune response and oxidative stress in sea cucumbers. Through the assessment of physiological reactions across a spectrum of predatory contexts, the study sought to reveal novel perspectives on the adaptive mechanisms sea cucumbers employ to counteract predatory challenges.
2. Materials and Methods
2.1. Animals and Materials
Sea cucumbers A. japonicus used in the study were purchased from a farm in Wafangdian city and quickly transported to the mariculture laboratory of Liaoning Ocean and Fisheries Science Research Institute for temporary cultivation. A total of 360 healthy sea cucumbers (wet weight of 0.42 ± 0.15 g, mean ± standard deviation) were randomly selected for the experiment. The sea cucumbers were placed in triangular tanks for temporary cultivation and fed with commercial sea mud powder mixed with commercial seaweed powder in the ratio of 1:1. The photoperiod for cultivation was 12 hours light: 12 hours dark (12L:12D), and the residual feed and feces at the bottom of the pool were cleaned daily, with one-third of the seawater replaced. To standardize the experiment, the sea cucumbers were fasted for three days prior to the experiment and throughout its duration.
The starfish Asterina pectinifera were collected from the offshore region in Dalian city and promptly transferred to the mariculture facility at the Liaoning Ocean and Fisheries Science Research Institute. With an average wet mass of 60.83 ± 8.57 g and a radius measuring 56.57 ± 2.73 mm, these specimens were housed in triangular tanks under fasting conditions to standardize their physiological state. A two-week pre-cultivation period was implemented to ensure a consistent level of hunger among the individuals.
2.2. Experimental Design
Following the pre-cultivation phase, healthy sea cucumbers (A. japonicus) were randomly assigned to one of four experimental groups to evaluate the effects of different predatory pressures on their immune and antioxidant enzyme activities: (1) Group Ⅰ: direct predation pressure, one hungry starfish coexisted with sea cucumbers in the same tank; (2) Group Ⅱ: indirect predation pressure, one hungry starfish sieged in a basket with small holes on all sides, co-cultivated in the tank with the sea cucumbers; (3) Group Ⅲ: injured conspecific information, a wound of about 1 cm was made on the back of a healthy sea cucumber with a dissecting knife as the source of the injured conspecific individual, and ten wounded sea cucumbers were processed and placed in a basket with holes on all sides, which was then co-cultivated with 30 healthy sea cucumbers; (4) Group 0: healthy control, only sea cucumbers were in the tank without any other biological stimulation conditions. Each experimental group was replicated three times, with each replicate containing 30 healthy sea cucumbers. The trials were carried out in triangular tanks. The environmental conditions were tightly controlled with a photoperiod of 12 hours light to 12 hours darkness (12D:12L). The experimental temperature was maintained at 22.09 ± 0.68 °C, salinity at 31.59 ± 0.18, and dissolved oxygen levels at 6.47 ± 0.13 mg/L. To maintain consistent predatory pressure, water was not exchanged, and no additional feed was provided throughout the duration of the experiment.
2.3. Sample Collection
At the onset of the application of different predation pressures for each experimental group, six healthy sea cucumbers were rapidly collected from each group at 3, 12, 72, and 96 hours after exposure to various pressures. The samples were immediately frozen in liquid nitrogen and then transferred to a -80 °C freezer for storage, to be used for subsequent enzyme activity assays.
2.4. Enzyme Activity Assay
For the enzyme activity assay, the sea cucumber juveniles preserved at -80 °C were taken out and slowly thawed in an ice bath. After completely thawing, the sea cucumbers were weighed, and a homogenate was prepared by adding homogenizing buffer at a 9:1 ratio to the weight. The tissue homogenate was centrifuged at 4 °C at 3500 ×g for 10 minutes, and the supernatant was collected for enzyme activity assays using kits from Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Specific enzyme indicators and testing methods are shown in
Table 1.
3. Results and Analysis
3.1. Direct Predation of Sea Cucumbers by A. pectinifera
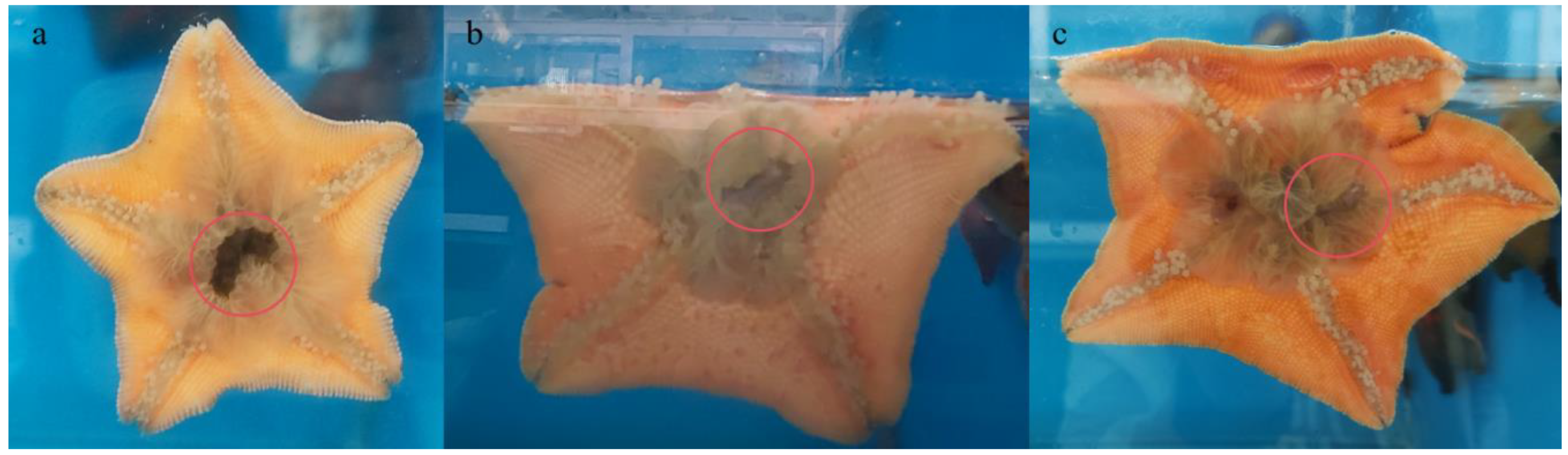
Figure 1 depicts a starfish feeding on sea cucumbers during the experimental phase, with a red circle highlighting the sea cucumber currently being predated. Throughout the predation event, the sea cucumber’s sluggish mobility allows the starfish to approach incrementally, using its tube feet to ensnare the sea cucumber adjacent to its beak. The starfish then contours its body, creating a secure enclosure against the pool’s boundary, enveloping the sea cucumber tightly before extruding its stomach to commence the digestion process. This continues until the sea cucumber’s hue progressively lightens, culminating in its complete assimilation by
A. pectinifera.
3.2. Changes in the Activity of Immune Defense Enzymes in Sea Cucumbers Under Different Predation Pressures
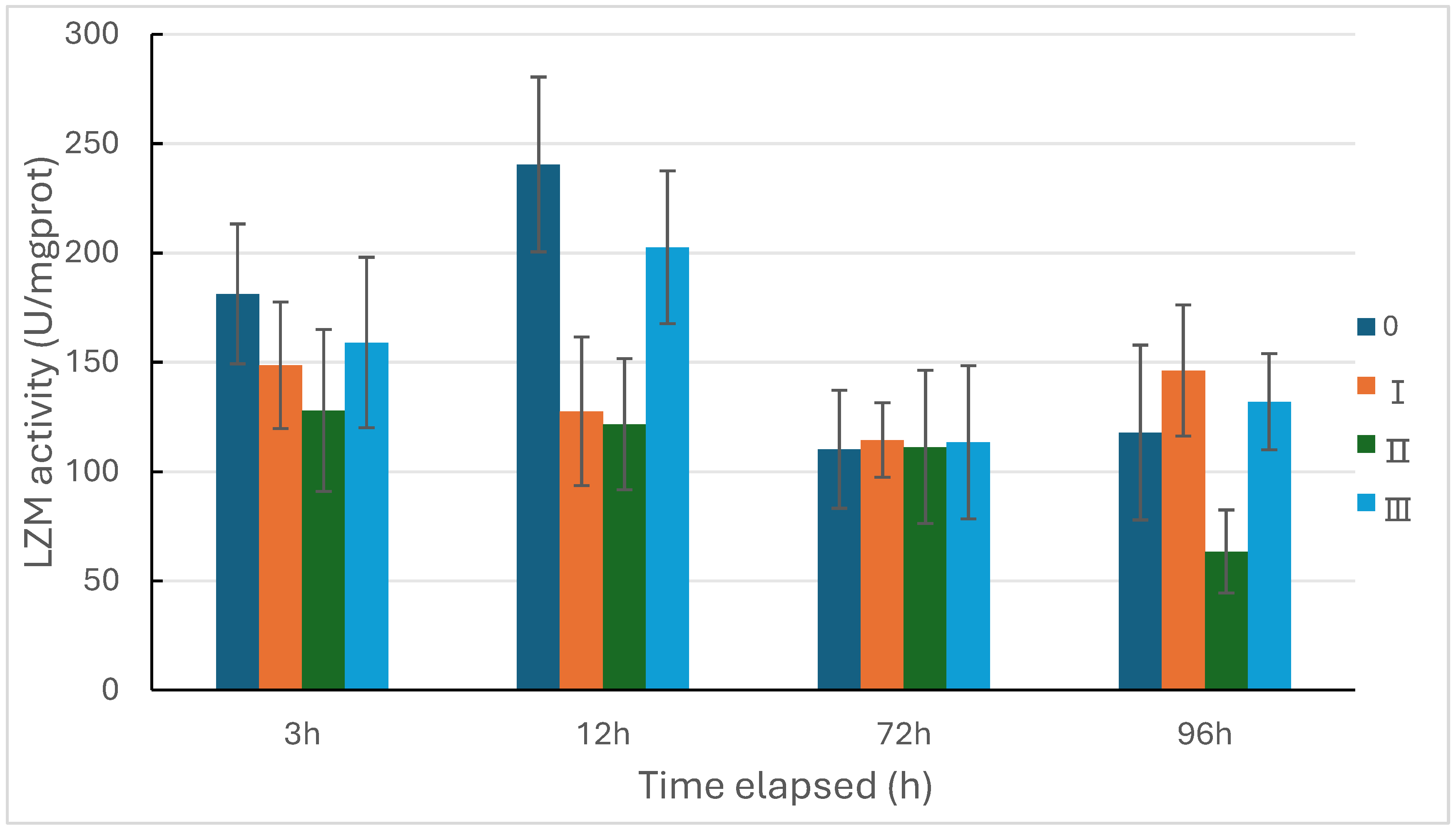
Figure 2,
Figure 3 and
Figure 4 illustrate the temporal variations in the activities of lysozyme, acid phosphatase, and alkaline phosphatase in sea cucumbers under three types of predation pressures. The trend of lysozyme activity reveals that, compared with the control group, in both the direct predation pressure group and the injury information group exhibited a decrease followed by an increase in lysozyme activity. At 3 and 12 hours, the activity in both groups was lower than the control, slightly higher at 72 hours, and showed an increasing trend by 96 hours (
Figure 2).
Figure 3 depicts the activity changes of acid phosphatase under the three types of pressures. Group Ⅰ displayed a distinctive pattern of increase, decrease, and subsequent increase, with a significant drop in activity at 72 hours compared to the control (
P<0.05), followed by a sharp rise at 96 hours that exceeded the control levels. Group Ⅱ and Ⅲ showed a slight increase in acid phosphatase activity at 3 and 12 hours, which was slightly lower than the control at 72 and 96 hours.
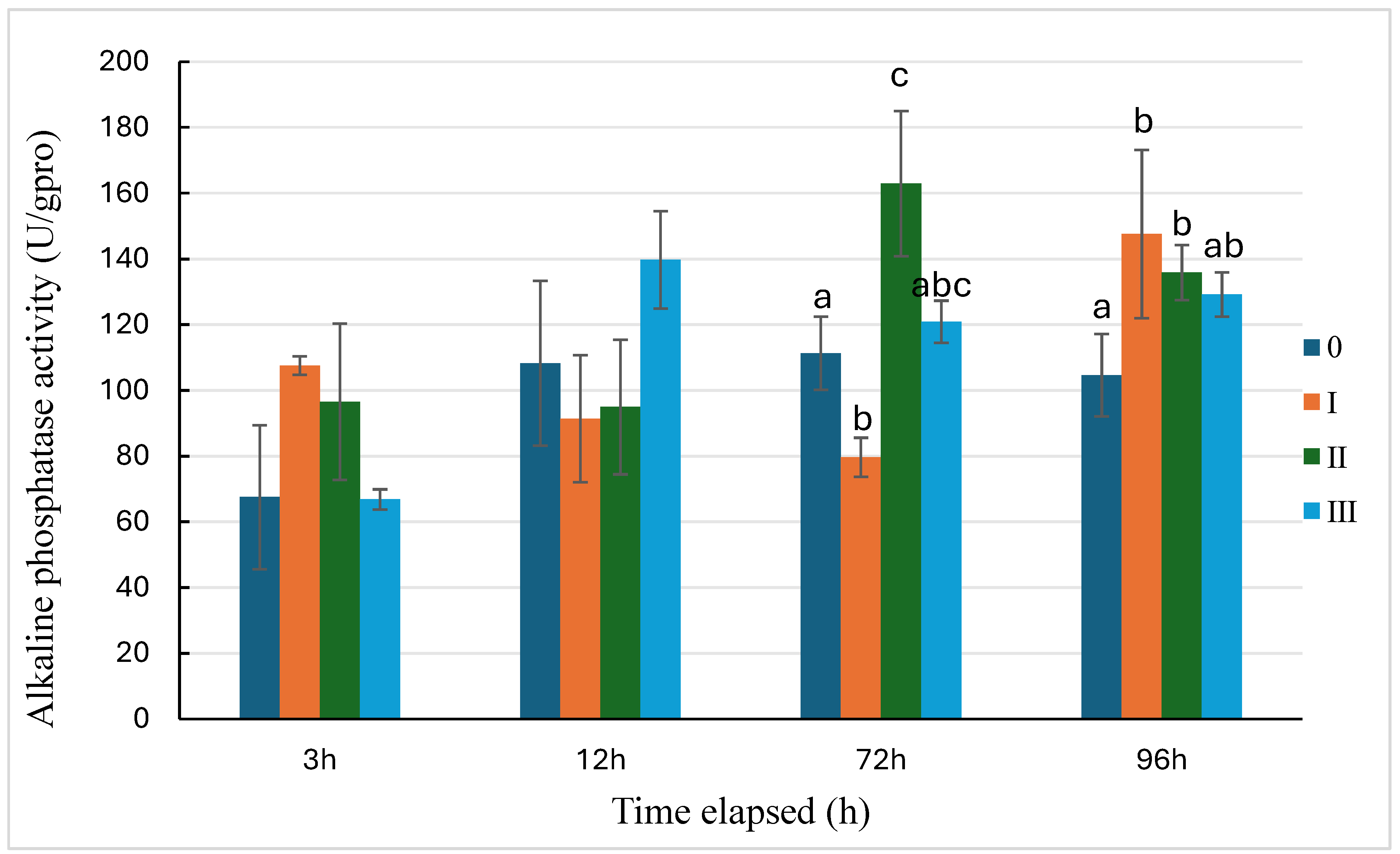
The activity trends of alkaline phosphatase under the three pressures are presented in
Figure 4. Alkaline phosphatase activity in Group Ⅰ was slightly higher at 3 hours, began to decline at 12 hours, and was significantly lower than the control at 72 hours (
P<0.05), before rapidly increasing and becoming significantly higher than the control at 96 hours (
P<0.05). Group Ⅱ showed no significant difference from the control at 3 and 12 hours, but exhibited a significant increase at 72 and 96 hours (
P<0.05). In contrast, Group Ⅲ, apart from being essentially equivalent to the control at 3 hours, showed a slight but non-distinct increase at other time points compared to the control.
3.3. Changes in Antioxidant Stress Enzyme Activity in Sea Cucumbers Under Different Predation Pressures
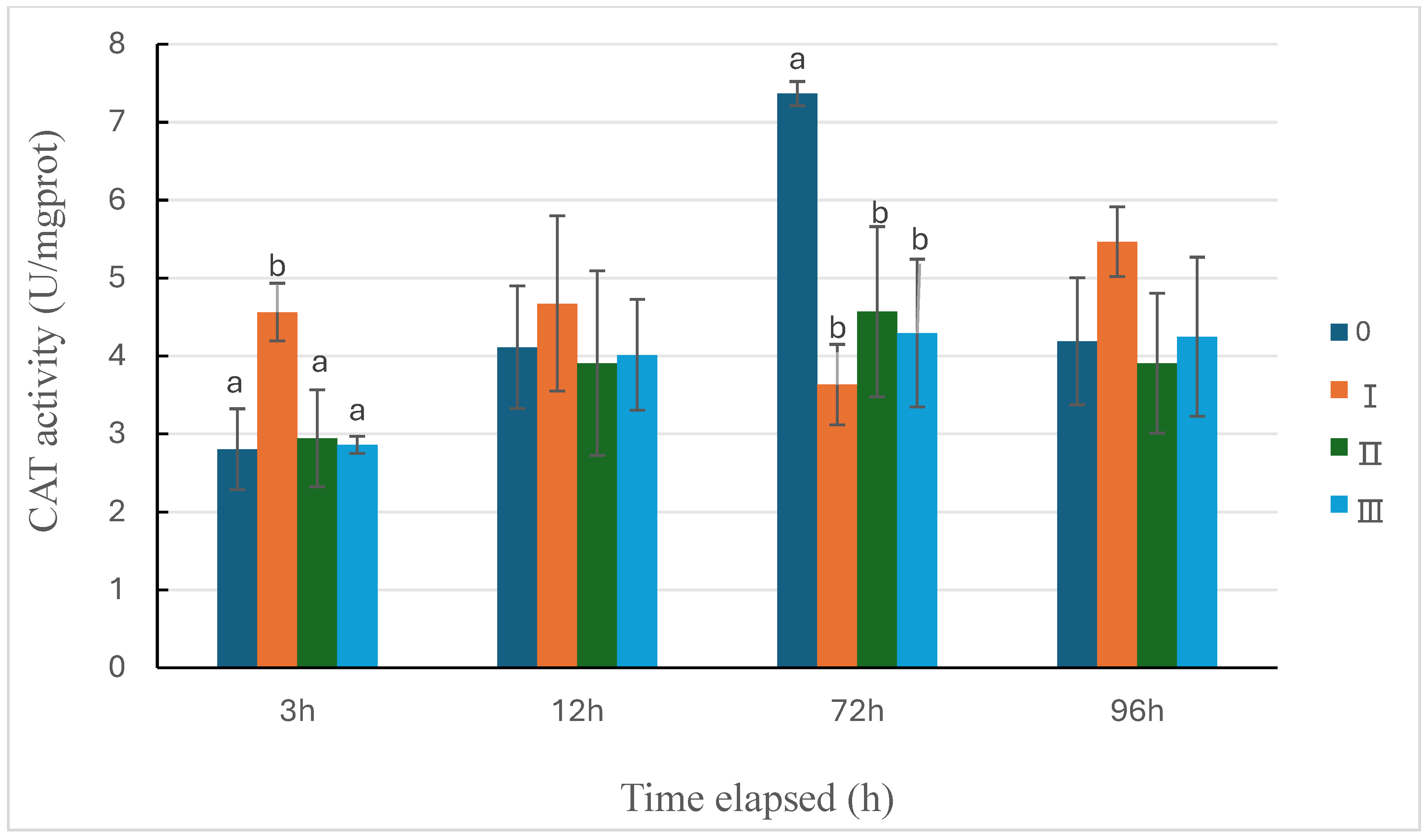
The change in catalase (CAT) activity in sea cucumbers under different predation pressures is shown in
Figure 5. Compared with the control group, the activity in Group Ⅰ significantly increased at 3 hours (
P<0.05), then began to decrease, and at 72 hours its activity was significantly lower than that of the control group (
P<0.05), but started to rise at 96 hours, slightly higher than the control group. Compared with the control group, the indirect predation group and the injured information group showed significantly reduced enzyme activity at 72 hours (
P<0.05), while it did show significant differences at other time points.
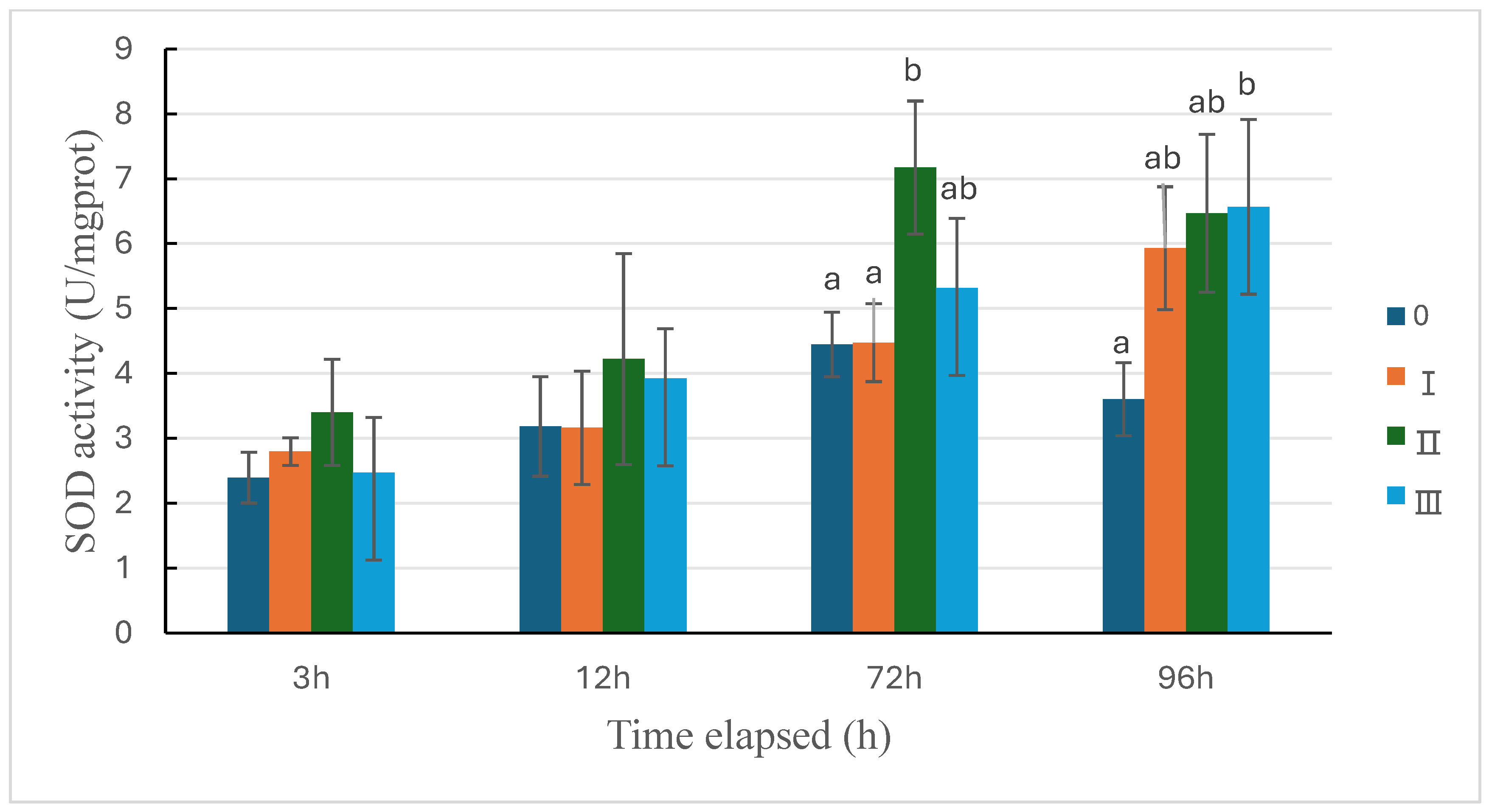
An overall upward trend in the activity of superoxide dismutase (SOD) in each group was revealed in
Figure 6. The enzyme activity in the three predation pressure groups at 3 hours and 12 hours was not significantly different from the control group, but at 72 hours and 96 hours, the enzyme activity in all three predation pressure groups was higher than that of the control group, with Group Ⅱ showing a significant increase in enzyme activity at 72 hours (
P<0.05), and Group Ⅲ showing a significant increase in enzyme activity at 96 hours (
P<0.05) (
Figure 6).
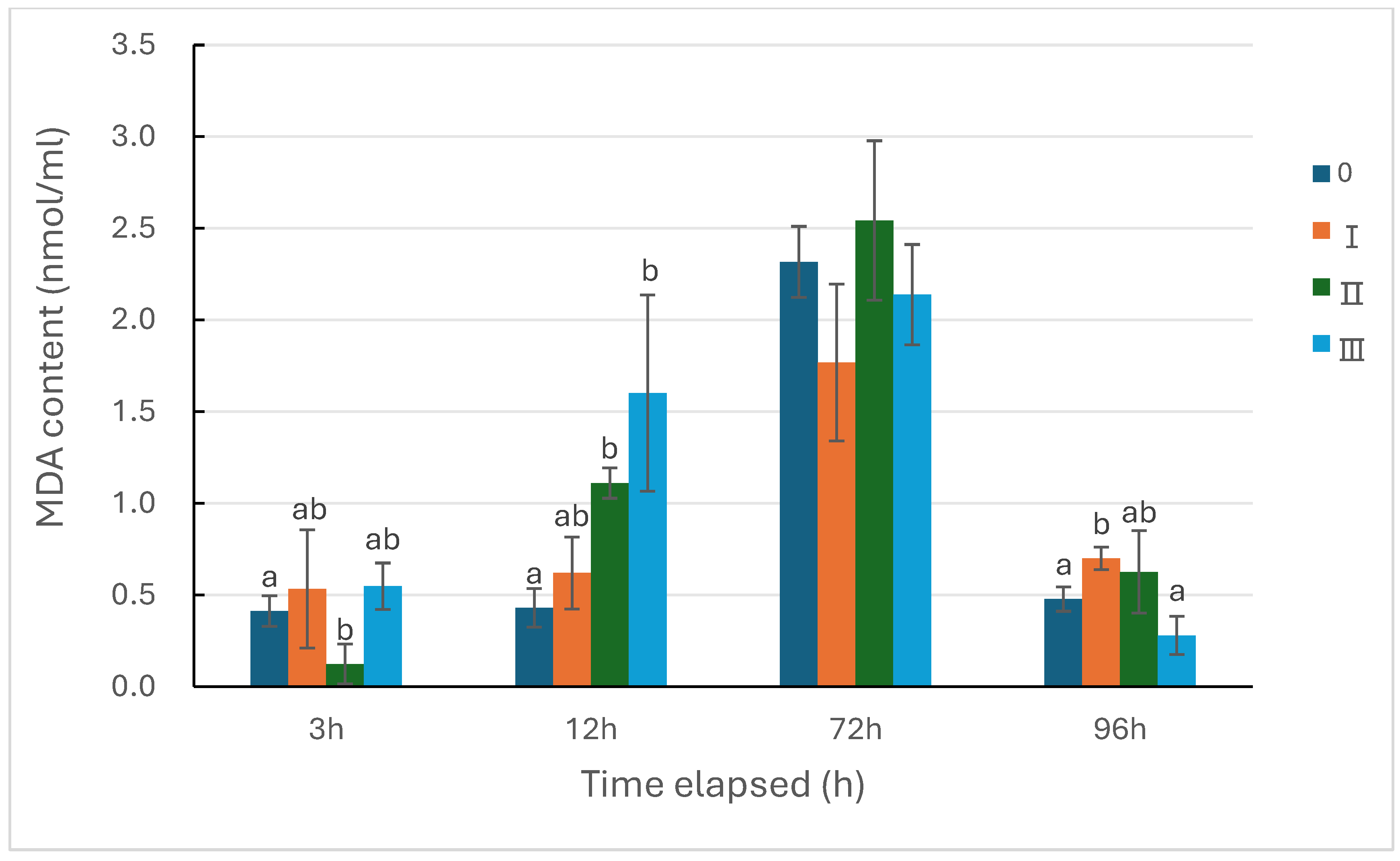
Figure 7 shows the change in malondialdehyde (MDA) activity in sea cucumbers under the three predation pressures. The overall trend of MDA activity in all groups was an initial increase followed by a decrease, but compared with the control group, the levels of the treatment groups varied. Among them, the MDA activity in Group Ⅰ at 96 hours was significantly higher than that of the control group (
P<0.05); the MDA activity in the indirect pressure group was significantly lower than that of the control group at 3 hours (
P<0.05), rapidly increased at 12 hours, and was significantly higher than the control group (
P<0.05), and as time extended, the activity remained higher than that of the control group, but the difference was not significant. Group Ⅱ, however, was significantly higher than the control group at 12 hours (
P<0.05), with no significant differences at other time points.
4. Discussion
It is currently known that the primary predators of juvenile sea cucumbers in the wild include carnivorous fish, starfish, sea urchins, crabs, and gastropods [
15,
16,
17,
18,
19,
20,
21,
22,
23]. Among these diverse predators, starfish
A. pectinifera have emerged as the main predators of juvenile sea cucumbers, which can consume sea cucumbers smaller than 5 cm, and their high numbers often lead to significant mortality in both wild and enclosed sea cucumber populations [
16,
23,
24]. Hatanaka et al. [
16] demonstrated that
A. pectinifera can prey on smaller-sized sea cucumbers, with
A. pectinifera averaging an arm length of 43.3 mm consuming 1.8, 0.5, and 0.1 sea cucumbers daily with average body lengths of 15.9, 30.1, and 40.0 mm, respectively. In this study, photographs of starfish preying on sea cucumbers were also captured, showing the sea cucumbers wrapped in the everted stomach of the starfish, with their skin already digested, further confirming the predatory behavior of starfish towards juvenile sea cucumbers. Therefore, the starfish
A. pectinifera were selected as the agents to exert predation pressure on sea cucumbers in this study.
This study measured and compared the activities of enzymes related to the immune and antioxidant defense of sea cucumbers, examining the effects of direct predation pressure, indirect predation pressure, and injury in conspecifics. The study reveals that sea cucumbers exhibit altered immune and antioxidant responses not only to direct predation stress but also to chemical signals emitted by nearby predators or injured members of their own species. Stress can be defined in various ways but can be considered the organism’s response to challenges that may pose real and potential threats to its integrity [
25]. Predation pressure can be categorized into direct stress, which involves predation by predators (including physical impacts such as contact, light and shadow, and water currents), and indirect stress, which encompasses chemical cues from nearby predators (perceived without physical contact) and chemical cues released by injured conspecifics. For vertebrates, responses to these pressures might involve fear or anxiety in the face of imminent or potential danger [
26]. The stress responses to these two scenarios likely involve a broad range of mechanisms, including changes in genetics, metabolism, energy, immunity, endocrinology, neurology, and behavior, aimed at overcoming and compensating for the imbalances caused by stressors. With these responses, animals attempt to avoid dangerous situations and any threats to their survival or integrity, ultimately striving to reintegrate into a balanced state [
25].
4.1. Changes in Immune Defense-Related Functions of Sea Cucumbers Under Different Predation Pressures
As an invertebrate, the sea cucumber lacks adaptive immunity, relying instead on innate immunity. The coelomocytes and immune markers can directly reflect the animal’s response to environmental stress [
27]. Lysozyme (LZM) is an important immune factor widely present in animal cells and coelomic fluid, playing a role in the non-specific immune system by lysing bacteria and aiding the body in resisting bacterial infections [
28]. Changes in environmental factors, such as salinity, can lead to an increase in the activity of lysozyme in the sea cucumber’s coelomic fluid [
29]. Under pH stress, the activity of lysozyme in sea cucumbers significantly decreases [
30]. Acid phosphatase (ACP) and alkaline phosphatase (AKP), as crucial enzymes in the non-specific immune response, participate in the elimination of pathogens, and their activities can change in response to environmental stimuli [
31,
32,
33,
34].
In this study, the activities of the three enzymes in the direct predation group generally followed a trend of initial decline followed by an increase. The activities of ACP and AKP were significantly lower than the control group at 72 hours, which may be due to the fear of the predator suppressing the sea cucumber’s consumption of ATP to adapt to the environment with the presence of a predator. The inorganic phosphate required for ATP synthesis is generated through the hydrolysis of phosphoesters by acid and alkaline phosphatases [
27]. This suggests that the sea cucumber’s immune defense mechanism was suppressed at 72 hours after facing the predator, increasing the likelihood of pathogen invasion. This is similar to the response in lepidopteran larvae, where predator-induced stress reduces their immunity to bacteria, leading to physiological consequences such as reduced body mass [
35]. In the indirect predation group, the activity of alkaline phosphatase was significantly higher than the control group at 72 and 96 hours. This indicates that indirect pressure, such as chemical signals from predators, has an impact on the sea cucumber’s immune defense system, enhancing its immune defense, which is beneficial for the growth and survival of the sea cucumber. Without loss of tissue integrity, the immune system of the sea cucumber has increased the activity of immune enzymes, preparing for potential damage. It is speculated that sea cucumbers can detect impending attacks and respond to increase their chances of survival in non-lethal predatory events [
10], but this preparation is relatively slow (significant differences were only shown at 72 hours). In the injury information group, the activities of lysozyme, acid phosphatase, and alkaline phosphatase fluctuated at various time points but did not show significant changes, indicating that the sea cucumber may have made some immune defense preparations for potential harm after recognizing the information from injured conspecifics. It can be seen that when sea cucumbers encounter direct predation pressure, their non-specific immunity may decrease, and this pressure may suppress the immune defense function of the sea cucumber. Under indirect pressure, the sea cucumber prepares for immune defense in the face of recognized potential danger [
10]. The recognition of predator information helps the sea cucumber to enhance non-specific immunity, which is beneficial for its survival and growth. Further exploration is needed to understand how predation pressure affects the expression of immune-related molecules in sea cucumbers.
4.2. Changes in Antioxidant Defense Functions of Sea Cucumbers Under Different Predation Pressures
Environmental stress factors primarily affect organisms by causing oxidative damage due to the accumulation of reactive oxygen species (ROS) resulting from abnormal aerobic metabolism [
36]. To survive, organisms employ a range of measures to reduce the levels of reactive oxygen species. The defense against oxidative damage in sea cucumbers mainly relies on enzymatic and non-enzymatic antioxidants, protecting them from the harmful effects of a variety of reactive oxygen species [
37]. Antioxidant enzymes are a crucial part of the sea cucumber’s resistance to ROS, including catalase (CAT) and superoxide dismutase (SOD). The activity of these antioxidant enzymes may change in response to environmental changes and stress.
SOD can reduce oxidative free radicals within the body, mitigating oxidative damage. Catalase catalyzes the decomposition of excess hydrogen peroxide in cells [
38]. Malondialdehyde (MDA) is a biomarker of oxidative damage in organisms, directly reflecting the stress caused by oxidative free radicals [
39], and its accumulation is positively correlated with the degree of oxidative stress experienced by the organism [
40].
In this study, facing direct predation pressure, the activity of CAT in sea cucumbers significantly increased at 3 hours, catalyzing the decomposition of excess H
2O
2 produced by the direct predation pressure [
38]. We speculate that CAT may serve as an indicator of the sea cucumber’s response to predation pressure. At 96 hours, although the activity of MDA in the direct predation group decreased, it remained significantly higher than the control group, indicating that sea cucumbers may still be affected by the oxidative stress brought about by direct predation pressure. When facing indirect predation pressure, the activities of SOD and CAT in sea cucumbers both showed a trend of increasing and then decreasing with time, similar to the trend observed under pH stress [
30]. Significant differences were shown in both enzymes compared with the control group at 72 hours. The increase in SOD activity is similar to the results of studies where pollutants stimulate sea cucumbers [
41], suggesting that the increase in SOD activity may be due to oxidative stress in sea cucumbers after recognizing the information from predators, and this increase may lead to an increase in H
2O
2 levels. However, in this experiment, the activity of CAT did not compensate for the increase in SOD activity. Sun et al. [
42] also found in a study on Yesso scallops that the changes in SOD and CAT in the serum of Yesso scallops after feeding with
Antheraea pernyi cecropin were not consistent, indicating that the mechanisms of these two antioxidant enzymes are not the same. From the trend of MDA activity changes, at 12 hours, the MDA activity in all pressure groups increased rapidly, indicating that the body was stimulated by oxidative stress. When the time progressed to 72 hours, with the increase in SOD activity in all pressure groups, the levels of MDA in each group returned to levels similar to the control group. Further research found that the change in CAT activity in sea cucumbers exposed to injured conspecific information was similar to that in the indirect pressure group, but the change in SOD activity was similar to that in the direct pressure group. This may be due to the slow recognition of injured conspecific information by sea cucumbers, leading to a significant increase in SOD activity only at 96 hours, similar to the results of immune enzymes. Moreover, the MDA value in the injured information group at 96 hours had decreased to the lowest among all groups, corresponding to the highest SOD enzyme activity at this time point. It is speculated that the sea cucumber’s response to the information from injured conspecifics is slow and weak, leading to a delayed but more pronounced induction of SOD enzyme synthesis, which clears the oxidative stress damage caused by the accumulation of MDA.
The changes in enzyme activity indicate that the immune and antioxidant systems of sea cucumbers produce different responses under different predation pressures. A series of comprehensive enzyme adjustments can help sea cucumbers regulate their physiology under predation pressure, and then improve their adaptability to different predation pressure environments by adjusting their own state.
5. Conclusion
This study reveals the complexity of the impact of predator pressure on the immune and antioxidant functions of sea cucumbers. Facing different types of predation pressures, sea cucumbers can respond to direct and indirect predation pressures by regulating their immune and antioxidant enzyme activities at 72 hours. The physiological response of sea cucumbers to predation pressure shows their survival strategy in the natural environment, which is of great significance for the aquaculture of sea cucumbers. By monitoring the immune and antioxidant enzyme activities of sea cucumbers, their health status can be assessed, providing a scientific basis for disease prevention and aquaculture management, thereby improving breeding efficiency and survival rate.
Author Contributions
Methodology, Wenbin Kang and Chong Zhao; Investigation, Liang Qu; Resources, Meng Wang and Jian Song; Writing – original draft, Chong Wang; Writing – review & editing, Yongxin Sun and Shuo Wang; Funding acquisition, Qingzhi Wang.
Funding
This work was supported by Technology Foundation of Dalian Jinshiwan Laboratory (Dljswsf202402), China Scholarship Council, Science and Technology Foundation of Dalian (2021YF16SN015, 2021JB11SN035), National Key Research and Development Program of China (2022YFD2400305) and Foundation of Liaoning Academy of Agricultural Sciences (2024XKJS5249).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data, models and code generated or used during the study appear in the submitted article.
Acknowledgments
We would like to thank Dr. Xi Xie for his valuable suggestions on this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bell, J.D.; Munro, J.L.; Nash, W.J.; Rothlisberg, P.C.; Loneragan, N.R.; Ward, R.D.; Andrew, N.L. Restocking and stock enhancement of marine invertebrate fisheries. Adv Mar Biol. 2005, 49, xi–374. [Google Scholar] [PubMed]
- Gosselin, L.A.; Qian, P.Y. Juvenile mortality in benthic marine invertebrates. Mar Ecol Prog Ser. 1997, 146, 265–282. [Google Scholar] [CrossRef]
- Francour, P. Predation on holothurians: A literature review. Invertebr Biol. 1997, 116, 52–60. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, H.; Hamel, J.F. Larval, juvenile, and adult predators. In The Sea Cucumber Apostichopus japonicus: History, Biology and Aquaculture; Academic Press: Amsterdam, 2015; pp. 243–256. [Google Scholar]
- Boonstra, R. Reality as the leading cause of stress: Rethinking the impact of chronic stress in nature. Funct Ecol. 2013, 27, 11–23. [Google Scholar] [CrossRef]
- Campbell, A.C.; Coppard, S.; Tudor-Tomas, C.D. Escape and aggregation responses of three echinoderms to conspecific stimuli. Biol Bull-us. 2001, 201, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Hu, F.; Qin, C.; Huang, X.; Zhao, C. Conspecific alarm cues are a potential effective barrier to regulate foraging behavior of the sea urchin Mesocentrotus nudus. Mar Environ Res. 2021, 171, 105476. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yang, M.; Hu, F.; Huang, X.; Zhao, C. Foraging behavior of the sea urchin Mesocentrotus nudus exposed to conspecific alarm cues in various conditions. Sci Rep-uk 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhadan, P.; Vaschenk, M. Long-term study of behaviors of two cohabiting sea urchin species, Mesocentrotus nudus and Strongylocentrotus intermedius, under conditions of high food quantity and predation risk in situ. PeerJ, 2019, 7, e8087. [Google Scholar] [CrossRef] [PubMed]
- Hamel, J.F.; Jobson, S.; Caulier, G.; Mercier, A. Evidence of anticipatory immune and hormonal responses to predation risk in an echinoderm. Sci Rep-uk 2020. [Google Scholar] [CrossRef]
- Burnovicz, A.; Oliva, D.; Hermitte, G. The cardiac response of the crab Chasmagnathus granulatus as an index of sensory perception. J Exp Biol. 2009, 212, 313–324. [Google Scholar] [CrossRef]
- Zhang, F. Progresses in research on defence mechanism of echinodems (in Chinese). Journal of DaLian Fisheries University. 2005, 340–344. [Google Scholar]
- Ye, H.B.; Fan, Y.; Li, T.B.; Li, L.; Wang, X.L. Study progress on immunity defense mechanism of Apostichopus japonicus (in Chinese). Journal of Anhui Agricultural Sciences. 2018, 46, 27–29. [Google Scholar]
- Li, G.; Ren, L.; Sun, G.; Yang, J.; Wei, X.; Liu, Z.; Li, S.; Jiang, H. Effects of hypoxic stress on oxidative stress Indices in Apostichopus japonicus (in Chinese). Progress in Fishery Sciences. 2016, 37, 133–139. [Google Scholar]
- Wright, A.; Hill, L. Nearshore marine resources of the south pacific: information for fisheries development, management. Institute of Pacific Studies Forum Fisheries Agency International Centre for Ocean Development. 1993, 371–401. [Google Scholar]
- Hatanaka, H.; Uwaoku, H.; Yasuda, T. Experimental studies on the predation of juvenile sea cucumber, Apostichopus japonicus by sea star, Asterina pectinifera. Suisanzoshoku. 1994, 42, 563–566. [Google Scholar]
- Sui, X. The status and prospects for artificial breeding and enhancement of sea cucumber, Apostichopus japonicus (Selenka) (in Chinese). Modern Fisheries Information. 1996, 1–4. [Google Scholar]
- Liao, Y. Zoology of China: Echinodermata: Sea cucumbers. Science press: China, 1997.
- Liu, S. Handbook of aquaculture seedling breeding techniques. China agricultural press: China, 2000.
- Tanaka, M. Diminution of sea cucumber Stichopus japonicus juveniles released on artificial reefs. Bulletin of the Ishikawa Prefecture Fish Research Center. 2000, 2, 19–29. [Google Scholar]
- Kang, K. H.; Kim, J.M. The predation of trumpet shell, Charonia sp., on eight different marine invertebrate species. Aquaculture Research. 2004, 35, 1202–1206. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Z.; Wan, C.; Xin, M.; Lu, B. Factors affecting the survival rate of Apostichopus japonicus in pond culture and their solutions (in Chinese). Chinese Fisheries. 2007, 10, 30–31. [Google Scholar]
- Uekusa, R.; Yoshida, N.; Kashio, S.; Tokaji, H. Low discovery rate of sea cucumber Apostichopus japonicus juveniles after seed release in the field. Bulletin of Fisheries Sciences. 2012, 62, 43–49. [Google Scholar]
- Jiang, H.; Liu, Y.; Xin, X.; Yu, L.; Zhang, X. Starfish (Asterina pectinifera), an enemy of sea cucumbers (in Chinese). Shandong Fisheries. 2008, 25, 22–23. [Google Scholar]
- Tort, L. Stress and immune modulation in fish. Developmental & Comparative Immunology. 2011, 35, 1366–1375. [Google Scholar]
- Öhman, A. Fear and anxiety: Overlaps and dissociations. In Handbook of Emotions; The Guilford Press: New York, NY, 2008; pp. 709–728. [Google Scholar]
- Li, L.; Tian, X.; Yu, X.; Dong, S. Effects of acute and chronic heavy metal (Cu, Cd, and Zn) exposure on sea cucumbers (Apostichopus japonicus). Biomed Res Int 2016, 1–13. [Google Scholar] [CrossRef]
- Canicatti, C.R.; Parrinello, N. Studies on Holothuria polii (Echinodermata) antibacterial proteins. I. Evidence for and activity of a coelomocyte lysozyme. Cell Mol Life Sci. 1989, 45, 756–759. [Google Scholar] [CrossRef]
- Zheng, H.; Li, B.; Rong, X.; Liao, M.; Chen, G.; Zhang, Z.; Wang, L.; Wang, Y.; Zou, A. Effects of salinity and dissolved oxygen variation on the nonspecific immune response of Apostichopus japonicus (in Chinese). Progress in Fishery Sciences. 2014, 30, 118–124. [Google Scholar]
- Han, S.; Zhao, B.; Li, C.; Hu, W.; Liu, Z. Effeets of acute pH stress on immunity enzyme of sea cucumber, Apostichopus japonicus (in Chinese). Journal of Fujian Aguiculture and Forestry University (Natural Science Edition) 2020, 49, 666–670. [Google Scholar] [CrossRef]
- Xing, J.; Lin, T.; Zhan, W. Variations of enzyme activities in the haemocytes of scallop Chlamys farreri after infection with the acute virus necrobiotic virus (AVNV). Fish & Shellfish Immunology. 2008, 25, 847–852. [Google Scholar]
- Rajalakshmi, S.; Mohandas, A. Copper-induced changes in tissue enzyme activity in a freshwater mussel. Ecotoxicology & Environmental Safety. 2005, 62, 140–143. [Google Scholar]
- Song, X.; Zhang, L.; Gao, W.; Pan, B. Effect of Vibrio anguillarum on activity of phosphatase in Cyclina sinensis (in Chinese). Oceanologia Et Limnologia Sinica, 2010, 41, 254–258. [Google Scholar]
- Pipe, R.K.; Porte, C.; Livingstone, D.R. Antioxidant enzymes associated with the blood cells and hemolymph of the mussel. Mytilus edulis. Fish & Shellfish Immunology. 1993, 3, 221–233. [Google Scholar]
- Sun, J.; Bai, Y. Predator-induced stress influences fall armyworm immune response to inoculating bacteria. Invertebrate Pathology. 2020, 172, 107352. [Google Scholar] [CrossRef] [PubMed]
- Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M.K.; Ryter, S.W.; Kim, H.P. Mechanisms of cell death in oxidative stress. Antioxidants & Redox Signaling. 2007, 9, 49–89. [Google Scholar] [CrossRef]
- Elstner, E.F. Oxygen activation and oxygen toxicity. Annual Review of Plant Physiology. 1982, 33, 73–96. [Google Scholar] [CrossRef]
- Zhang, K.; Tian, H. Researh and functon of catalase in organism. Food Science and Technology 2007, 8–11. [Google Scholar]
- Del, R.D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as a toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovas. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Sui, Y.; Wang, X.; Luo, Y.; Ji, L. Hydroxyl radical production and oxidative damage induced by cadmium and naphthalene in liver of Carassius auratus. Comparative Biochemistry and Physiology C: Toxicology & Pharmacology. 2005, 140, 115–121. [Google Scholar]
- Cleoni dos, S.C.; Vanessa, A.B.; Heloísa, S.S.A.; Evaldo, L.G.E.; Marisa, N.F. Biomarker responses as indication of contaminant effects in Oreochromis niloticus. Chemosphere. 2012, 89. [Google Scholar] [CrossRef]
- Sun, Y.; Li, D.; Zhang, H.; Tian, B.; Ma, S.; Chen, X.; Wang, Q.; Du, X.; Xu, Y.; Javed, M.T. Dietary supplement of Antheraea pernyi cecropin enhances the growth rate and disease resistance of the Yesso scallop, Patinopecten yessoensis. Aquaculture Reports. 2023, 31, 101634. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).