Submitted:
01 August 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anaesthetic Protocol
2.3. Anaesthesia Monitoring and Data Collection
2.4. Statistical Analysis
3. Results
| T0 min |
T1 min |
T2 min |
T3 min |
T4 min |
|
|---|---|---|---|---|---|
| Control | 1 (0) | 3 (0) | 3 (1) | 9 (1) | 9 (1) |
| Tramadol | 1 (0) | 7 (0) | 8 (1) | 16 (6) | 17 (6) |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grond, S.; Sablotzki, A. Clinical pharmacology of tramadol. Clin Pharmacokinet 2004, 43, 879–923. [Google Scholar] [CrossRef] [PubMed]
- Simon, B. T.; Steagall, P. V. The present and future of opioid analgesics in small animal practice. Journal of Veterinary Pharmacology and Therapeutics 2017, 40, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, M. Tramdol Vs Tapentadol: A new Horizon in Pain Treatment? American Journal of Animal and Veterinary Sciences 2012, 7, 7–11. [Google Scholar] [CrossRef]
- Kindu, T.; et al. Preemptive analgesic effects of tramadol for ovariohysterectomy in bitches. Journal of Veterinary Science & Technology 2018, 9. [Google Scholar] [CrossRef]
- Barbosa, J.; et al. Comparative metabolism of tramadol and tapentadol: a toxicological perspective. Drug Metabolism Reviews 2016, 48, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, M.; et al. Pharmacokinetics of tramadol and metabolites after injective administration in dogs. Polish Journal of Veterinary Sciences 2010, 13, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Giudice, E.; et al. Clinical findings in degenerative lumbosacral stenosis in ten dogs. Journal of Veterinary Behavior 2019, 33, 7–15. [Google Scholar] [CrossRef]
- Perez, T.; et al. Tramadol metabolism to o-desmethyl tramadol (M1) and N-desmethyltramadol (M2) by dog liver microsomes: species comparison and identification of responsible canine cytochrome P450s. Drug Metabolism and Disposition 2016, 44, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Oliva, A.; et al. Clinical pharmacology of tramadol and tapentadol, and their therapeutic efficacy in different models of acute and chronic pain in dogs and cats. Journal of Advanced Veterinary and Animal Research 2021, 8, 404–422. [Google Scholar] [CrossRef] [PubMed]
- Kukanich, B.; Papich, M. G. Pharmacokinetics of tramadol and the metabolite O-desmethyltramadol in dogs. Journal of Veterinary Pharmacology and Therapeutics 2004, 27, 239–246. [Google Scholar] [CrossRef]
- McMillan, C.; et al. Pharmacokinetics of intravenous tramadol in dogs. The Canadian Journal of Veterinary Research 2008, 72, 325–331. [Google Scholar]
- Kukanich, B.; Kukanich, K.; Rodriguez, J. The effects of concurrent administration of cytochrome P-450 inhibitors on the pharmacokinetics of oral methadone in healthy dogs. Veterinary Anaesthesia and Analgesia 2011, 38, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Saccò, M.; et al. The relationship between blood pressure and pain. Journal of Clinical Hypertension 2013, 15, 600–605. [Google Scholar] [CrossRef]
- Van Hagen, M.; et al. Analysis of polymorphisms of canine cytochrome P450-CYP2D15. Journal of Veterinary Pharmacology and Therapeutics 2020, 43, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Stamer, U. M.; et al. Impact of CYP2D6 genotype on postoperative tramadol analgesia. PAIN 2003, 105, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S. E.; et al. Absolute quantitation of drug-metabolizing cytochrome p450 enzymes and accessory proteins in dog liver microsomes using label-free standard-free analysis reveals interbreed variability. Drug Metabolism and Disposition 2019, 47, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Schütter, A. F.; Tünsmeyer, J; Kästner, S. B. R. Influence of tramadol on acute thermal and mechanical cutaneous nociception in dogs. Veterinary Anaesthesia and Analgesia 2017, 44, 309–316. [Google Scholar] [CrossRef] [PubMed]
- WSAVA. Guidelines for recognition, assessment and treatment of pain. Journal of Small Animal Practice 2014, 1–59.
- Pieper, K. Perioperative schmerztherapie bei hund und katzeeine Übersicht. Tierarztliche Praxis Ausgabe K: Kleintiere – Heimtiere 2016, 44, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Grubb, T.; et al. 2020 AAHA Anesthesia and Monitoring Guidelines for Dogs and Cats. Journal of the American Animal Hospital Association 2020, 56, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Desmeules, J. A.; et al. Contribution of monoaminergic modulation to the analgesic effect of tramadol. British Journal of Clinical Pharmacology 1996, 41, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Perez Jimenez, t. E.; et al. Identification of canine cytochrome P-450s (CYPs) metabolizing the tramadol (+)-M1 and (+)-M2 metabolites to the tramadol (+)-M5 metabolite in dog liver microsomes. Journal of Veterinary Pharmacology and Therapeutics 2018, 41, 815–824. [Google Scholar] [CrossRef]
- Giorgi, M.; et al. Pharmacokinetics of tramadol and its major metabolites following rectal and intravenous administration in dogs. New Zealand Veterinary Journal 2009, 57, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, H.; et al. Distribution, pharmacokinetics and primary metabolism model of tramadol in zebrafish. Molecular Medicine Reports 2016, 14, 5644–5652. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, A.; et al. Pharmacokinetics and analgesic efficacy of intranasal administration of tramadol in dogs after ovariohysterectomy. Veterinary Anaesthesia and Analgesia 2020, 47, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Holford, S.; et al. Parent-metabolite pharmacokinetic models for tramadol – tests of assumptions and predictions. Journal of Pharmacology and Clinical Toxicology 2014, 2. [Google Scholar] [CrossRef]
- Vettorato, E.; et al. Pharmacokinetics and efficacy of intravenous and extradural tramadol in dogs. Veterinary Journal 2010, 183, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Kukanich, B. Pain Management in Veterinary Species. In Pharmacotherapeutics for Veterinary Dispensing, 1st ed.; Mealey, K. L., Ed.; John Wiley & Sons, Inc.: New Jersey, USA, 2019; pp. 173–188. [Google Scholar]
- Ronagh, A.; et al. Comparison of sedative and some cardiopulmonary effects of intramuscular medetomidine or medetomidine–tramadol in dogs. Veterinary Anaesthesia and Analgesia 2020, 47, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Itami, T.; et al. Cardiovascular effects of tramadol in dogs anesthetized with sevoflurane. Journal of Veterinary Medical Science 2011, 73, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; et al. Alpha-2 Agonists. Anesthesiology Clinics 2017, 35, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Watts, S. W.; et al. Serotonin and blood pressure regulation. Pharmacological Reviews 2012, 64, 359–388. [Google Scholar] [CrossRef] [PubMed]
- Kemme, M. J. B.; et al. No evidence for functional involvement of 5-HT2B receptors in serotonin-induced vasodilatation in the human forearm. Journal of Cardiovascular Pharmacology 2000, 36, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Bruning, T. A.; et al. No functional involvement of 5-HT receptors in nitric oxide-dependent dilation caused by serotonin in the human forearm vascular bed. Journal of Cardiovascular Pharmacology 1994, 24, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Bruning, T. A.; et al. Serotonin-induced vasodilation in the human forearm is mediated by the nitric oxide pathway. Journal of Cardiovascular Pharmacology 1993, 22, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Blauw, G.; et al. Effects of 5-HT on capillary and arteriovenous anastomotic blood flow in the human hand and forearm and in the pig hind leg. Journal of Cardiovascular Pharmacology 1991, 17, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Blauw, G. J.; et al. Regional Vascular Effects of Serotonin and Ketanserin in Young, Healthy Subjects. Hypertension 1988, 11, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Calama, E.; et al. Effect of 5-hydroxytrytamine on neurogenic vasoconstriction in the isolated, autoperfused hindquarters of the rat. Clinical and Experimental Pharmacology and Physiology 2005, 32, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Calama, E.; et al. 5-Hydroxytryptamine-induced vasodilator responses in the hindquarters of the anaesthetized rat, involve β2-adrenoceptors. Journal of Pharmacy and Pharmacology 2010, 55, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C. A.; et al. Mechanisms involved in the vasodilator action of 5-hydroxytryptamine in the dog femoral arterial circulation in vivo. European Journal of Pharmacology 1985, 113, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Grimm, K. A.; et al. Lumb and Jone’s Veterinary Anesthesia and Analgesia, 5th ed.; Wiley Blackwell: Iowa, USA, 2015; p. 111. [Google Scholar]
- Giovannitti, J. A.; Thoms, S. M.; Crawford, J. J. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesthesia Progress 2015, 62, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M. D. A review of the physiological effects of alpha2-agonists related to the clinical use of medetomidine in small animal practice. Canine Veterinary Journal 2003, 44, 885–897. [Google Scholar]
- De Witte, J. L.; et al. The analgesic efficacy of tramadol is impaired by concurrent administration of ondansetron. Anesthesia and Analgesia 2001, 92, 1319–1321. [Google Scholar] [CrossRef]
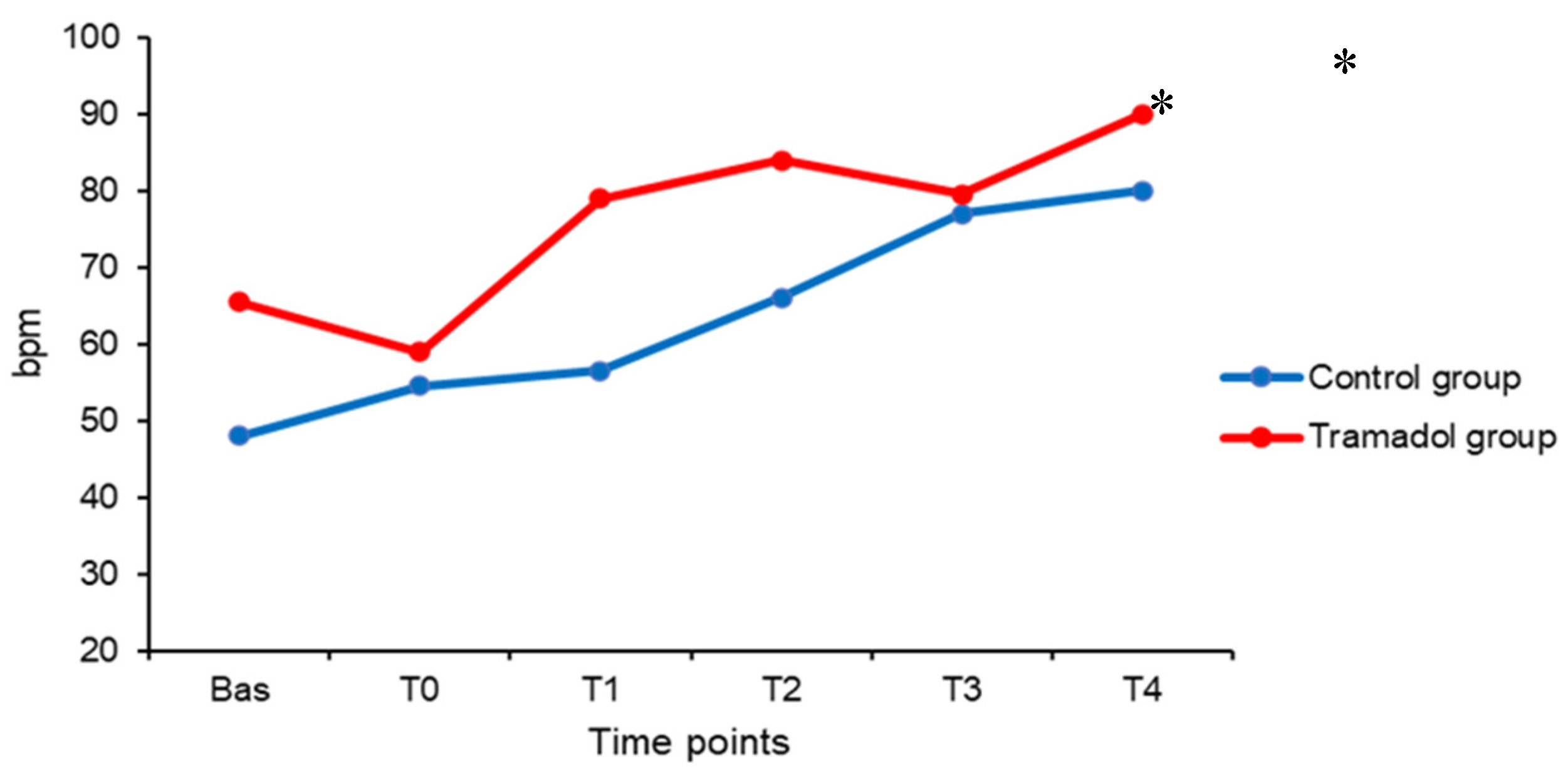
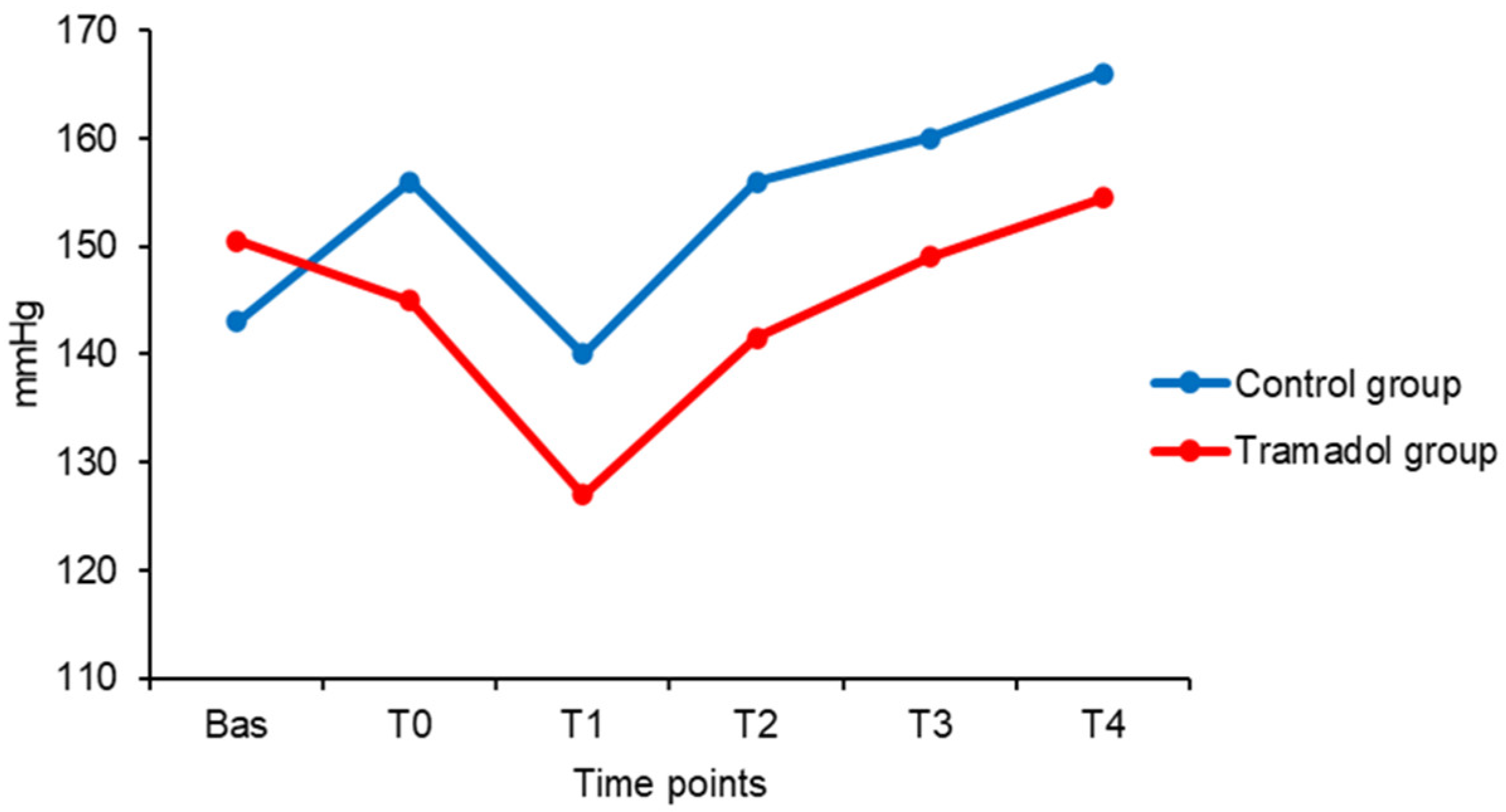
| HR bpm |
SBP mmHg |
DBP mmHg |
MBP mmHg |
RR rpm |
EtCO2 mmHg |
SpO2 % |
TºC ºC |
Iso% % |
|
|---|---|---|---|---|---|---|---|---|---|
| Bas | 48 (21) | 143 (39) | 89 (17) | 116 (24) | 12 (0) | 44 (8) | 99 (3) | 38 (1) | 1.5 (0) |
| T0 | 55 (17) | 156 (21) | 90 (24) | 115 (21) | 12 (0) | 47 (8) | 97 (5) | 38 (1) | 1.5 (0) |
| T1 | 57 (17) * | 140 (25) | 90 (32) | 107 (19) | 12 (0) | 48 (8) | 96 (5) | 38 (1) | 1.5 (0) |
| T2 | 66 (11) * | 156 (22) | 92 (18) | 117 (20) | 12 (0) | 46 (10) | 94 (6) | 38 (1) | 1.5 (0) |
| T3 | 77 (33) | 160 (8) | 108 (26) | 122 (25) | 12 (0) | 48 (1) | 98 (5) | 37 (1) | 1.5 (0) |
| T4 | 80 (27) | 166 (23) | 94 (17) | 120 (6) | 12 (0) | 47 (4) | 97 (4) | 37 (1) | 1.5 (0) |
| HR bpm |
SBP mmHg |
DBP mmHg |
MBP mmHg |
RR rpm |
EtCO2 mmHg |
SpO2 % |
TºC ºC |
Iso% % |
|
|---|---|---|---|---|---|---|---|---|---|
| Bas | 66 (22) | 151 (32) | 98 (13) | 117 (19) | 12 (0) | 44 (11) | 97 (4) | 38 (0) | 1.5 (0) |
| T0 | 59 (22) | 145 (37) | 95 (16) | 108 (31) | 12 (0) | 44 (13) | 97 (4) | 38 (0) | 1.5 (0) |
| T1 | 79 (24) | 127 (29) | 94 (24) | 103 (29) | 12 (0) | 45 (11) | 96 (4) | 37 (1) | 1.5 (0) |
| T2 | 84 (24) * | 142 (29) | 96 (17) | 110 (16) | 12 (0) | 44 (12) | 95 (6) | 37 (0) | 1.5 (0) |
| T3 | 80 (14) * | 149 (32) | 93 (20) | 108 (23) | 12 (0) | 44 (7) | 96 (2) | 37 (0) | 1.5 (1) |
| T4 | 90 (10) | 155 (36) | 92 (17) | 119 (20) | 12 (0) | 44 (7) | 96 (3) | 37 (0) | 1.5 (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




