1. Introduction
An increase in plant-sourced (PS) and decrease in animal-sourced (AS) nutrient intake is promoted in support of a sustainable diet [
1] and to maintain human and planetary health [
2]. However, PS dietary patterns as practised by vegetarians and vegans are associated with lower bone mineral density (BMD) and increased fracture risk [
3]. Therefore, a greater understanding of the functional properties of PS nutrient supplements is required to inform and support the transition to higher intake of PS nutrient intake for bone health.
Protein and calcium are major components of bone tissue. Protein provides the structural matrix of bone, occupying ~50% of bone volume and one-third of bone mass, and calcium is the dominant mineral within that matrix. Collagen and non-collagenous proteins form the organic matrix of bone, so an adequate dietary protein and calcium intake
is essential for optimal acquisition and maintenance of adult bone mass [
4]. However, at present, animal-sourced (AS) protein and dairy-based calcium predominate in the Western diet [
1,
5,
6].
Bone remodeling describes the normally balanced rate of bone resorption and bone formation that ensures 5-10% of the skeleton per annum is remodelled for essential maintenance and repair. Fracture risk is increased by high remodeling rates, or an imbalance (uncoupling) between rates of formation and resorption [
7]. Importantly, in the context of PS nutrient intake, sufficient intake of minerals and protein is required in support of bone remodeling in the adult skeleton [
8]. In this respect, legumes are an example of low-cost PS food of high nutritional value and an excellent source of good quality protein (20–45% protein), providing high amounts of the essential amino acids such as lysine and leucine [
9]. Natural plant sources of calcium are rare, but the multi-mineral extract from the cytoskeleton of the red seaweed
Lithothamnion sp (containing 32.6% calcium) is considered an effective PS of mineral for bone health [
10].
In addition to the supply of mineral and protein in support of bone remodeling, the ingestion of protein and calcium exert an active, temporal change in bone metabolism that favors a post-prandial reduction in the rate of bone resorption and potential for longer-term change in the rate of bone formation [
4]. These nutrient bioactivities are related to calcium bioavailablity, and subsequent increase in serum ionized calcium [
11] and increase in enteroendocrine peptides known to modulate osteoclast function [
12] via the entero-osseous axis [
13]. Of interest to the present study is the augmentation of post-prandial secretion of enteroendocrine peptides by co-ingestion of calcium [
14] and the development of a PS calcium+protein supplement of benefit to bone health.
The principal nutrient modulators of post-prandial bone metabolism under consideration in this report are a plant-sourced marine multi-mineral derived from the cytoskeleton of the red seaweed Lithothamnion sp containing 32.6% calcium (Aquamin F, Marigot Ltd. Cork. Ireland) co-ingested with a plant-sourced legume protein isolate (Vicia faba L., Marigot Ltd. Cork, Ireland). The study sought to determine the acute (0-4h), temporal change in ionised calcium (iCa), parathyroid hormone (PTH) and the International Osteoporosis Foundation’s (IOF) recommended biomarkers of bone resorption, C-terminal crosslinked telopeptide of type I collagen (CTX), and formation, procollagen type 1 amino-terminal propeptide (P1NP), following co-ingestion of a PS marine multi-mineral + legume protein isolate in healthy young adult men and women.
2. Materials and Methods
2.1. Ethical Approval and Participant Recruitment
The study was granted ethical approval by the University of Limerick Education and Health Sciences Research Ethics Committee (2022_03_06_EHS), conducted in accordance with the ethical standards outlined in the most recent version of the Declaration of Helsinki and registered with clinicaltrials.gov identifier NCT5533502. Potential participants were informed of the risks and benefits before providing written informed consent. Eligibility criteria: (i) aged 18 to 35 y; (ii) healthy (
i.e., no current injury, illness, medication, history of chronic disease, known allergies and intolerances, normotensive, non-obese, with normal blood chemistry). All participant volunteers were assessed for eligibility and screened using a medical questionnaire, physical examination, dietary intake record, anthropometry, and body composition analysis (Tanita MC, 180-MA, Tanita United Kingdom Ltd).
Supplementary Figure S1 provides a CONSORT flow diagram of participant enrolment, allocation, follow-up and analysis. Participant’s characteristics are presented in
Table 1.
2.2. Study Design
The study was a single-blind, block randomised, repeated measures, cross-over design comprising two study arms control (CON) and supplement (SUPP). Participant volunteers were randomised at entry to the study. Participants maintained their habitual diet and refrained from purposeful exercise and alcohol consumption for the previous 24h on two separate occasions at least three days apart. On trial days, participants arrived at the laboratory @ 8:00am following an overnight fast and remained seated throughout the trial. A cannula was inserted into a superficial vein on the dorsal surface of the hand and the hand placed in a heated hand box (air temperature 55°C) for 15 min to arterialise venous drainage. Following baseline blood draw, participants ingested, in randomised order, either CON or SUPP within 5 minutes. Serial samples of arterialised venous blood were then drawn 15, 30 ,45 ,60 ,90 ,120 ,150, 180- and 240-min post-ingestion.
2.3. Supplement Composition
The supplement (SUPP) was composed of a plant-sourced marine multi-mineral (Aquamin F, Marigot Ltd. Cork. Ireland ) + plant-sourced protein isolate (
Vicia faba L
., Marigot Ltd. Cork, Ireland). The final composition of the mineral + protein ingested by participants contained 800mg of calcium (equivalent to 2.45g of Aquamin F) and 0.33g of
Vicia faba L protein per kilogram of body mass (
Table 2) dissolved in 500ml of water. CON provided an equal volume of mineral water (<15mg/dL Ca). Detailed analysis of the supplement constituents is provided in Supplemental
Table S1.
2.4. Blood Sample Collection and Analysis
A portion of the whole-blood sample was analysed immediately after collection for iCa and K concentration by ion-selective potentiometry (I-STAT®; Abbott Laboratories). Intra-assay CV using control solutions was <1% for both iCa and K. Based on baseline samples the inter-assay CV for was <1·5% for iCa and <11% for K. The remaining blood was separated by centrifugation at 10,000 x g at 4°C for 5 min and frozen at -80°C until analysis. Twelve of the 180 blood samples were haemolysed and not analysed.
PTH, CTX and P1NP was analysed by 2-site immunometric assay using electrochemiluminescent detection (Roche Cobas e411, Roche Diagnostics, UK). The inter-assay CV was <10% for PTH, 5.3% for CTX and 4.5% for P1NP. Active plasma GLP-1(7-36) and total GIP (1-42) was measured using the MSD® metabolic assay (Meso Scale Discovery, Rockville, Maryland, USA) based on sandwich ELISA and according to the manufacturer’s instructions. The inter-assay CV was 7.3 and 16.1% for active GLP-1 and total GIP, respectively.
2.5. Treatment of Data and Statistical Analyses
Prior to statistical analysis, potential outliers were assessed by boxplot, and data were assessed for normal distribution by Shapiro-Wilk test (P > 0.05), homogeneity of variance by Levene’s test (P > 0.05) and Mauchly’s test of sphericity (P > 0.05). Data are reported as the mean (SD) unless stated otherwise. An a priori hypothesis for statistical analysis (H0) assumed the mean response for SUPP was equal to CON (i.e., µSUPP = µCON). Temporal change in serially sampled data was analysed by repeated measures ANOVA, the level of significance for post-hoc tests subject to Bonferroni correction. The overall change in analyte with respect to baseline over the 4h period post-ingestion was computed by trapezoidal integration and presented as the area under the curve (AUC0-240). Difference between treatment AUC0-240 was analysed by paired t-test. Cohen’s effect size was calculated using the standardised formula (t-score/√n). Statistical significance was set at P ≤ 0.05. SPSS Version 28 (IBM Corporation, Armonk, NY) was employed for all statistical analyses.
3. Results
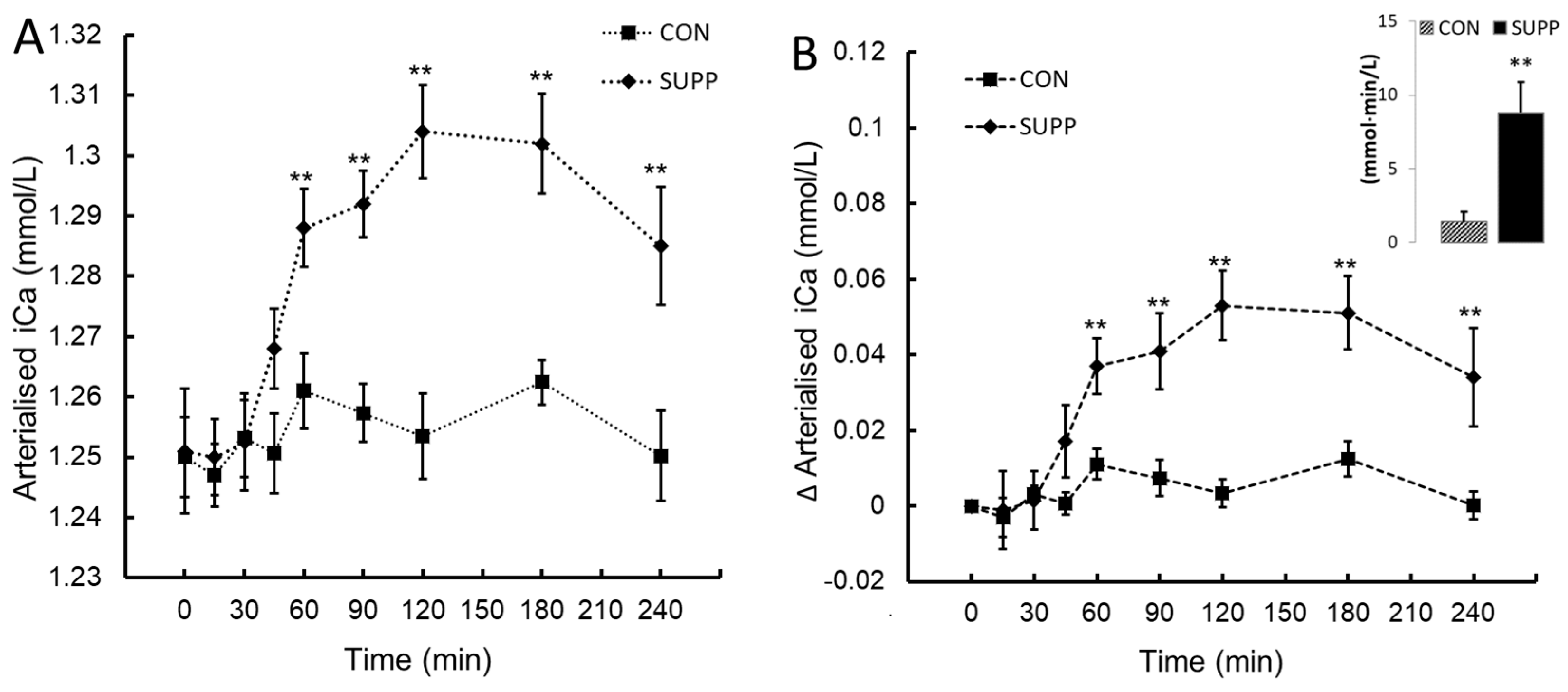
3.1. Temporal Pattern of Change in Ionised Calcium (iCa) Following Ingestion
No change in iCa was observed post-ingestion in CON. iCa increased after 60 min in SUPP with peak values attained at 120 min post-ingestion. These data shown a statistically significant two-way interaction between treatment and time,
F(8, 72) = 12.35,
P < .001, η
p2 = 0.58. The mean increase in iCa following ingestion was greater in SUPP than CON at time 60, 90, 120, 180 and 240 minutes (
Figure 1A and 1B). Assessed by the integrated AUC
0-240 SUPP increased the overall ∆iCa response > 5-fold compared to CON (mean difference 7.4 (CI
95%, 3.16 to 11.7) mmol∙min/L,
t(9) = 3.94,
P = 0.002.
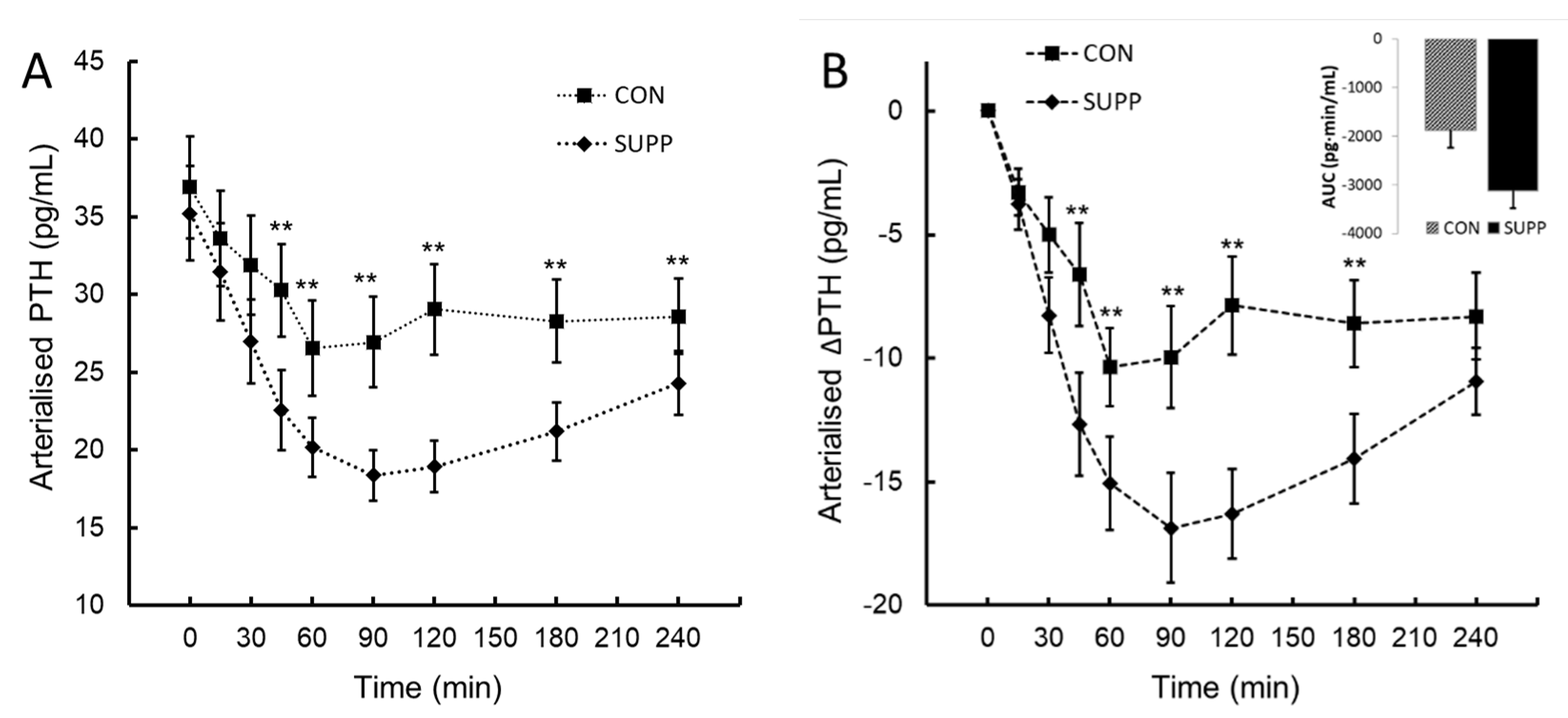
3.2. Temporal Pattern of Change in Parathyroid Hormone (PTH) Following Ingestion
An inverse PTH response to change in iCa was observed. These data shown a statistically significant two-way interaction between treatment and time,
F(8, 72) = 5.47,
P < .006, η
p2 = 0.38. The mean decrease in PTH following ingestion was greater in SUPP than CON at time 30, 45, 60, 90, 120, 180 and 240 minutes (
Figure 2A and 2B). Assessed by the integrated AUC
0-240 ∆PTH, the overall ∆PTH response to SUPP was 66% lower than CON (mean difference -1246 (95%CI
, -2365 to -126) pg∙min/mL,
t(9) = -2.517,
P = 0.016.
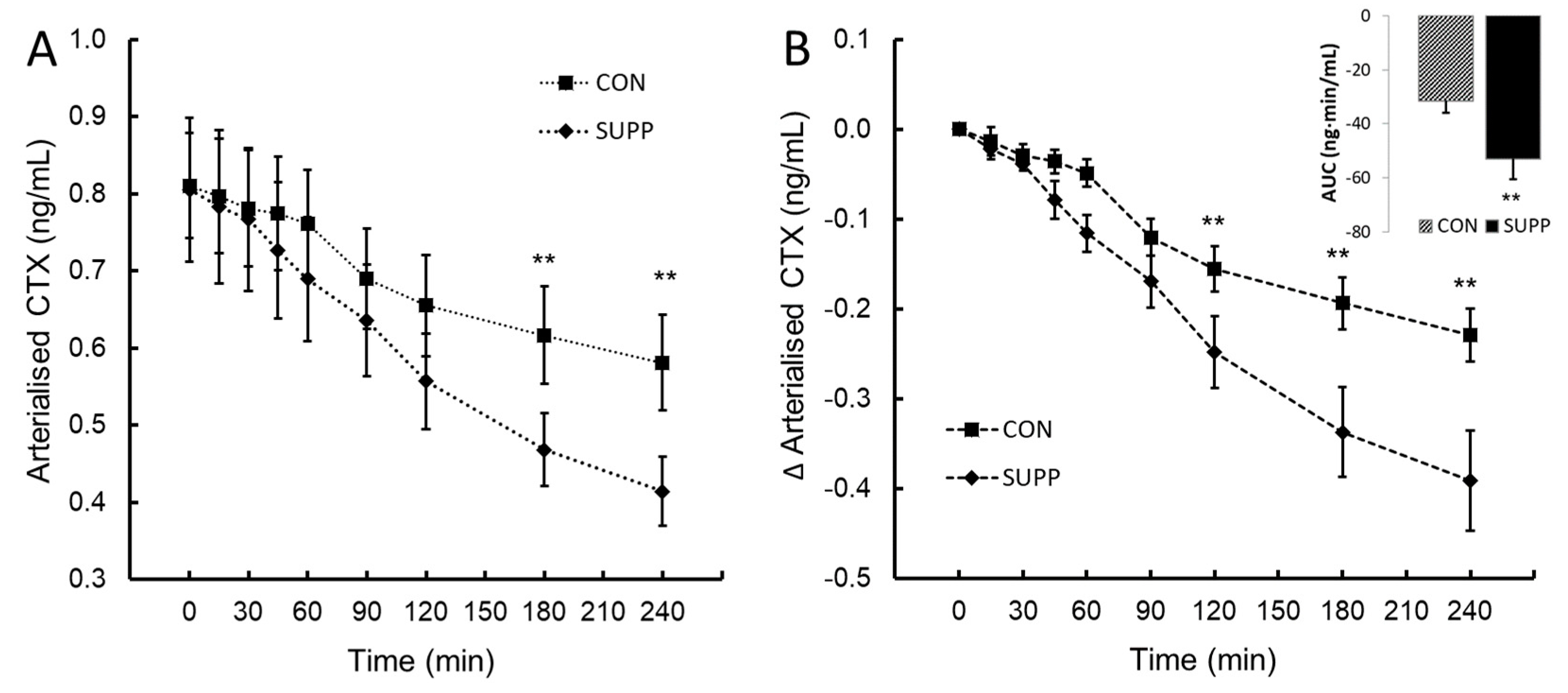
3.3. Temporal Change in C-Terminal Peptide of Type I Collagen (CTX) and Procollagen Type 1 Amino-Terminal Propeptide (P1NP) Following Ingestion
CTX followed the normal, mid-morning diurnal decrease in CON. A statistically significant two-way interaction between treatment and time,
F(8, 72) = 6.19,
P < .013, η
p2 = 0.41 was observed post-ingestion. The mean decrease in CTX following ingestion was greater in SUPP than CON at time 120, 180 and 240 minutes (
Figure 3A and 3B). Assessed by the integrated AUC
0-240 ∆CTX, SUPP decreased the overall ∆CTX response by 70% compared to CON (mean difference -22 (95%CI
, -39.7 to -3.30) ng∙min/mL,
t(9) = -2.673,
P = 0.013.
There was no change in values of procollagen type 1 amino-terminal propeptide (P1NP) at baseline or with respect to time post-ingestion for CON or SUPP. Baseline values averaged 67.6 (18.7) and 68.1 (2.4) pM for CON and SUPP, respectively.
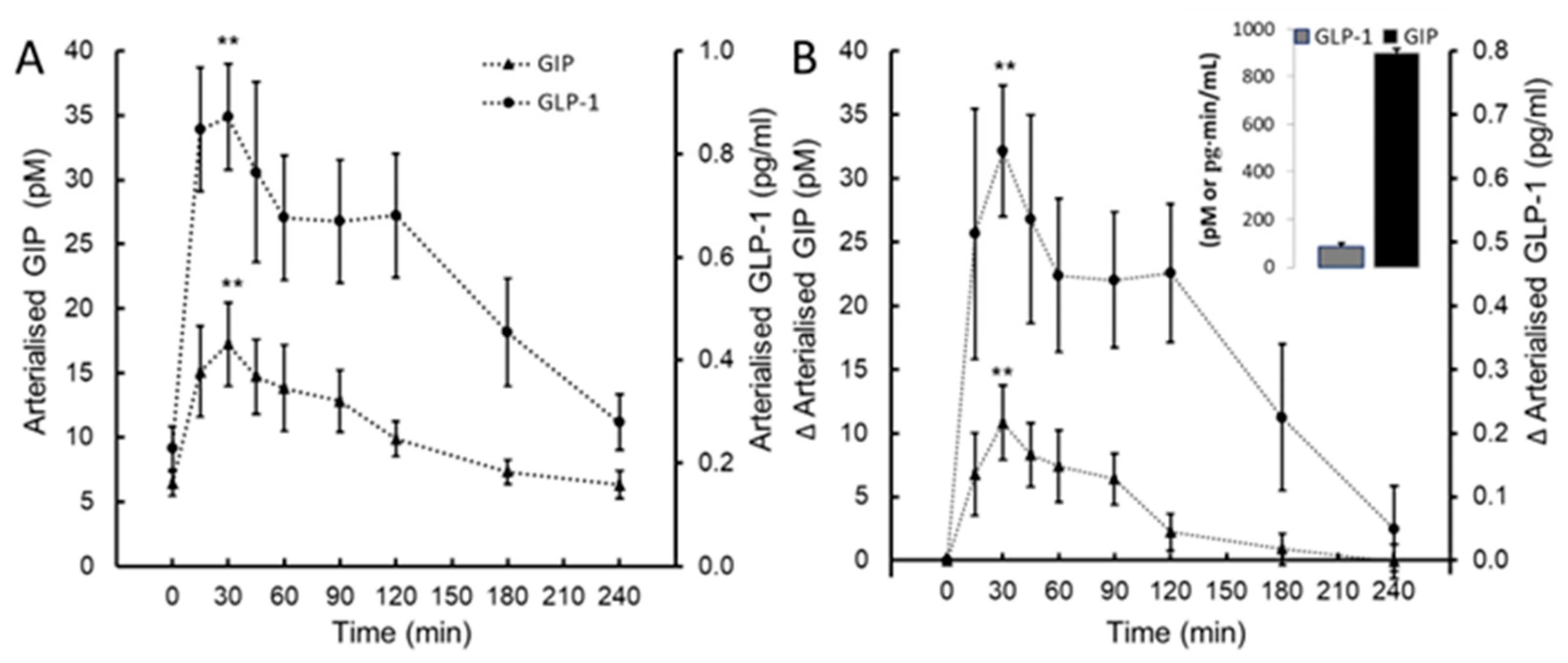
3.4. Temporal Change in Glucose-Dependent Insulinotropic Peptide (GIP) and Glucagon-like Peptide-1(GLP-1) Following Ingestion
A statistically significant effect of time was observed post-ingestion for GIP (
F(8, 72) = 4.78,
P < .001, η
p2 = 0.407) and GLP-1 (
F(8, 72) = 5.22,
P < .001, η
p2 = 0.43). Mean GIP increased 3-fold from baseline, from 6.4 (2.6) pmol/L to a peak concentration of 17.2 (9.1) pmol/L 30 min post-ingestion (
Figure 4A and 4B). Similarly, the mean GLP-1 increased 4-fold from 0.22 (0.12) pg/mL to a peak concentration of 0.87 (0.28) pg/mL 30 min post-ingestion (
Figure 4A and 4B).
4. Discussion
The interaction between dietary calcium and protein intake on bone health has been reviewed extensively and report a positive association between increased dietary protein (AS or PS) intake and BMD in healthy men and women and a reduction in the rate of bone loss by supplemental calcium intake [
15,
16]. Previous research from this research group has evaluated the acute (post-prandial) [
17] and long-term (24 week) [
18] effect of animal sourced (AS) mineral and protein nutrient supplements on bone metabolism and bone health. Prompted by the global challenge to attain a sustainable diet and to inform and support an increase in plant-sourced (PS) and decrease in animal-sourced (AS) nutrient intake this study characterised the bone-related nutrient bioactivity following co-ingestion of a novel PS mineral and protein supplement.
Dietary calcium (Ca) is a key regulator of bone metabolism and bone health, with chronic Ca deficiency being a major contributor to reduced bone mass and risk of fracture [
8,
11,
19]. The absorption of dietary Ca determines the bioavailability of Ca and novel PS calcium ingredients that positively influence Ca absorption and Ca bioavailability are desirable, particularly in those who fail to achieve the dietary recommended level of Ca [
12,
13,
20]. The homeostatic control of Ca absorption, excretion, and secretion and storage in bone is governed by the requirement to maintain a plasma concentration of ionised Ca (iCa) within a range of 1·1–1·3 mmol/L. This is achieved by the interaction of the calcitropic parathyroid hormone (PTH), 1,25 dihydroxycholecalciferol (1,25 (OH)
2D
3) and calcitonin. Counter-regulatory responses, i.e., negative feedback between PTH and 1,25 (OH)2D3 when iCa decreases, and calcitonin when iCa increases, maintain a tight regulatory control of calcium in the circulation.
A pilot study comparing the pharmacokinetic and pharmacodynamic responses to the ingestion of an equivalent (720mg elemental calcium) of plant-sourced marine multi-mineral derived from the cytoskeleton of the red seaweed
Lithothamnion sp (Aquamin F) and non-PS (calcium carbonate) report no difference in ionized or total serum calcium, but a greater lowering of PTH post-ingestion and calciuric response for PS
vs. non-PS calcium in young (mean age 28.8y), health women [
10]. In the present study, the ingestion of a matrix of PS protein and calcium (SUPP) led to an early increase in iCa after 30 min, a rapid rise to a mean peak concentration of 1.30 (∆ 0.053) mmol/L after 120 min post-ingestion, and a 5.3-fold overall 4h (AUC
0-240) mean increase in circulating iCa of 8.79 mmol∙min/L compared to CON. The counterregulatory effect of an increase in iCa was a suppression of PTH (and presumably renal production of 1,25(OH)
2D
3) to reduce the stimulus for osteoclastic bone resorption. Indeed, our observations confirm a temporal pattern of decrease in PTH that mirrored inversely the increase in iCa, resulting in a mean decrease of 66% (-3120 pg∙min/mL) in the overall 4h (AUC
0-240) circulating PTH compared to CON. Though direct comparison to other sources of calcium + protein intake was not undertaken, the appearance and rise in circulating iCa for the PS mineral + protein supplement occurred earlier in comparison to that of a dairy-based protein and calcium matrix [
21]. Incorporation of PS protein isolates that positively influence PS Ca absorption may ensure that the Ca bioavailability is optimized [
11].
The acute effect of PS Ca + Protein supplementation on bone turnover was measured by the temporal change in validated biomarkers of bone resorption, C-terminal peptide of type I collagen (CTX), and formation, procollagen type 1 amino-terminal propeptide (P1NP). Defined by circadian variation in bone turnover markers, bone remodeling exhibits a unimodal diurnal rhythm with a nocturnal peak and daytime nadir [
22,
23,
24]. The diurnal amplitude of bone resorption (CTX) is greater than bone formation and considered the prominent and sensitive biomarker of an acute change in bone remodeling. CTX in the circulation is derived from osteoclastic degradation of type 1 collagen and is widely employed to assess osteoclastic bone resorption and the dynamic change in bone remodeling, e.g., sensitivity to modulation by feeding and specific nutrient intake [
25]. In this study, the diurnally matched acute effect of SUPP invoked a 68% decrease in the overall post-prandial AUC
0-240 for CTX and a statistically significant temporal reduction in CTX from 120 through to 240 min. Though not measured, the trend in the observed temporal response suggests that the reduction in bone resorption would extend beyond 240 min. Although SUPP ingestion evoked a transient inhibition of bone resorption, there was no evidence of a postprandial change in the biomarker of bone collagen formation, P1NP, suggesting a transient shift toward skeletal deposition of calcium in the postprandial period
The reduction of appearance of CTX in the circulation probably reflects the calcium-induced reduction in circulating calcitropic PTH. However, the response of bone remodeling to food ingestion
per se is also linked to the secretion of the enterogastric hormones glucose-dependent insulinotropic peptide (GIP
1–42) and glucagon-like peptide-1 (GLP-1
7–36). Following intake of a standardized mixed meal (498 kcal) or granola bar (260 kcal) levels of GIP and GLP-1 were found to be positively associated with a decrease in CTX [
26] and constitute a link between food intake and bone homeostasis acting via an entero-osseous axis to decrease osteoclast activity [
27] These actions are supported by human studies of intravenous infusion of GIP that report a ~50% reduction in CTX at 90 min [
28]. To affirm the magnitude of enteroendocrine response to co-ingestion of a PS protein and mineral supplement that provided 117 (75) kcal total energy we observed a temporal increase in GIP and GLP-1 rising ~ 3 and 4-fold, respectively from baseline to a peak at 60 min post-ingestion. In the development of a nutrient supplement for bone health, co-ingestion of nutrients that act favorably on bone metabolism via separate, but complimentary, mechanism of action is an attractive option. Though the present study design could not determine whether co-ingestion of 800mg PS calcium augmented the postprandial rise in GIP and GLP-1 evoked by ingestion 0.33g/kg body mass of PS protein, or vice versa, a previous study found that co-ingestion of calcium (~1240 mg) with a standardized mixed meal (~300 kcal) resulted in a post-prandial increase of 47% in GIP and 22% increase in GLP-1 [
14].
5. Conclusion
Ingestion of the PS mineral + protein supplement evoked a 70% decrease in the diurnally matched 4h post-prandial rate of bone resorption and no change in the rate of bone formation. PS nutrient intake is promoted in support of a sustainable diet and to maintain human and planetary health. Adequate dietary protein and calcium intake is essential for optimal acquisition and maintenance of adult bone mass. Incorporation of PS mineral and protein into foods as an ingredient, or as a supplement to the diet, is considered beneficial to promote adequate Ca and protein intake and encourage individuals to meet their Ca and protein requirements. The temporal pattern of the response ingestion of the PS mineral + protein supplement ingestion indicates the mineral extract from the marine red algae, Lithothamnion sp. contains a readily bioavailable source of calcium that may be enhanced by the co-ingestion of the Vicia faba L. protein isolate to ensure that the Ca bioavailability from foods can be optimized. In addition, protein-rich legumes are a good source of bioactive peptides that are yet to be fully explored.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: CONSORT flow diagram of participant enrolment, allocation, follow-up and analysis; Table S1: Compositional analysis of the PS mineral + protein matrix.
Author Contributions
Author contribution to the study was as follows. Conceptualization: P.M.J., M.K.; methodology, M.K., A.O.D., S.O.C.; validation, M.K., and P.M.J.; formal analysis, M.K., P.M.J and S.O.C.; investigation, M.K.; resources, P.M.J ; data curation, M.K., P.M.J, A.O.D., M.R.; writing—original draft preparation, P.J.K.; writing—review and editing, M.K., A.O.D., S.O.C., M.K, ; supervision, P.M.J., S.O.C.; project administration, P.M.J.; funding acquisition, P.M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Marie Curie (M-F2021-0180) and Enterprise Ireland, Innovation Partnership Grant (IP/2019/0870) with Marigot Ltd. (Cork, Ireland).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Education and Health Sciences Research Ethics Committee of the University of Limerick (2022_03_06_EHS).
Informed Consent Statement
Written, informed consent was obtained from all participants involved in the study.
Data Availability Statement
Data available upon request to corresponding author.
Acknowledgments
The authors would like to acknowledge Elaine Ahern for contributing to the data collection and thank all volunteers for participation in the study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; Declerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [CrossRef]
- Tilman, D.; Clark, M. Global diets link environmental sustainability and human health. Nature 2014, 515, 518–522. [CrossRef]
- Rizzoli, R; Chevalley, T. Bone health: biology and nutrition. Current Opinion in Clinical Nutrition and Metabolic Care 2024, 27(1), 24-30. [CrossRef]
- Dawson-Hughes B. (2003). Interaction of dietary calcium and protein in bone health in humans. J Nutr 2003,133(3), 852S–854S. [CrossRef]
- Hone, M.; Nugent, A.P.; Walton, J.; McNulty, B.A, Egan, B. Habitual protein intake, protein distribution patterns and dietary sources in Irish adults with stratification by sex and age. J Hum Nutr Diet 2020, 33(4), 465-476. [CrossRef]
- Pasiakos, S.M.; Agarwal, S.; Lieberman, H.R.; Fulgoni, V.L. Sources and Amounts of Animal, Dairy, and Plant Protein Intake of US Adults in 2007-2010. Nutrients 2015, 7(8), 7058-69. [CrossRef]
- Heaney RP. Is the paradigm shifting? Bone 2003,33(4),457-65. [CrossRef]
- Reid, D.M.; New, S.A. Nutritional influences on bone mass. Proc Nutr Soc 1997, 56(3), 977-987. [CrossRef]
-
Pulses: nutritious seeds for a sustainable future. FAO Rome. 2016; pp. 35-37. [CrossRef]
- Zenk, J. L., Frestedt, J. L., & Kuskowski, M. A. Effect of Calcium Derived from Lithothamnion sp. on Markers of Calcium Metabolism in Premenopausal Women. Journal of medicinal food 2018, 21(2), 154–158. [CrossRef]
- Cashman K. D. Calcium intake, calcium bioavailability and bone health. J Nutr 2002, 87 Suppl 2, S169–S177. [CrossRef]
- Zhong, Q.; Itokawa, T.; Sridhar, S.; Ding, K.H.; Xie, D.; Kang, B.; Bollag, W.B.; Bollag, R.J.; Hamrick, M.; Insogna, K.; Isales, C.M. Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am J Physiol Endocrinol Metab 2007, 292, E543–E548. [CrossRef]
- Stensen, S.; Gasbjerg, L. S.; Helsted, M. M.; Hartmann, B.; Christensen, M. B.; Knop, F. K. GIP and the gut-bone axis - Physiological, pathophysiological and potential therapeutic implications. Peptides 2020, 125, 170197. [CrossRef]
- Gonzalez, J. T.; Stevenson, E. J. Calcium co-ingestion augments postprandial glucose-dependent insulinotropic peptide(1-42), glucagon-like peptide-1 and insulin concentrations in humans. Eur J Nutr, 2014, 53(2), 375–385. [CrossRef]
- Shams-White, M. M.; Chung, M.; Du, M.; Fu, Z;, Insogna, K. L;, Karlsen, M. C.; LeBoff, M. S.; Shapses, S. A.; Sackey, J.; Wallace, T. C.; Weaver, C. M. Dietary protein and bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. Am J Clin Nutr, 2014,105(6), 1528–1543. [CrossRef]
- Dawson-Hughes, B; Harris, S. S. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr, 2002, 75(4), 773–779. [CrossRef]
- Hettiarachchi, M.; Cooke, R.; Norton, C.; Jakeman, P. Temporal Change in Biomarkers of Bone Turnover Following Late Evening Ingestion of a Calcium-Fortified, Milk-Based Protein Matrix in Postmenopausal Women with Osteopenia. Nutrients 2019, 11, 1413. [CrossRef]
- Norton, C.; Hettiarachchi, M.; Cooke, R.; Kozior, M.; Kontro, H.; Daniel, R.; Jakeman, P. Effect of 24-Week, Late-Evening Ingestion of a Calcium-Fortified, Milk-Based Protein Matrix on Biomarkers of Bone Metabolism and Site-Specific Bone Mineral Density in Postmenopausal Women with Osteopenia. Nutrients 2022, 14, 3486. [CrossRef]
- Weaver CM. The growing years and prevention of osteoporosis in later life. Proc Nutr Soc 2000, 59(2), 303-306. [CrossRef]
- British Nutrition Foundation (1989) Calcium. London: British Nutrition Foundation.
- Amigo-Benavent, M.; Power-Grant, O.; FitzGerald, R.J.; Jakeman, P. The insulinotropic and incretin response to feeding a milk-based protein matrix in healthy young women. Journal of Functional Foods 2020, 72, 1040-56. [CrossRef]
- Redmond, J.; Fulford, A.J.; Jarjou, L.; Zhou, B.; Prentice, A.; Schoenmakers, I. Diurnal Rhythms of Bone Turnover Markers in Three Ethnic Groups. J Clin Endocrinol Metab 2016, 101, 3222-3230. Bone 2002, 31, 57-61. [CrossRef]
- Qvist, P.; Christgau, S.; Pedersen B.J.; Schlemmer, A.; Christiansen, C. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone 2002, 31, 57-61. [CrossRef]
- Pedersen, B.J.; Schlemmer, A.; Rosenquist, C.; Hassager, C.; Christiansen, C. Circadian rhythm in type I collagen formation in postmenopausal women with and without osteopenia. Osteoporos Int 1995, 5(6), 472–477. [CrossRef]
- Heaney, R.P. The bone remodeling transient: interpreting interventions involving bone-related nutrients. Nutr Rev 2001, 59, 327-34. [CrossRef]
- Jensen, N.W.; Clemmensen, K.K.B.; Jensen, M.M.; Pedersen, H.; Færch, K.; Diaz, L.J.; Quist, J.S.; Størling, J. Associations between Postprandial Gut Hormones and Markers of Bone Remodeling. Nutrients 2021, 13, 3197. [CrossRef]
- Nissen, A.; Christensen, M.; Knop, F.K.; Vilsboll, T.; Holst, J.J.; Hartmann, B. Glucose-dependent insulinotropic polypeptide inhibits bone resorption in humans. J Clin Endocrinol Metab 2014, 99, E2325–E2329. [CrossRef]
- Christensen, M. B.; Lund, A.; Calanna, S.; Jørgensen, N. R.; Holst, J. J.; Vilsbøll, T.; Knop, F. K. Glucose-Dependent Insulinotropic Polypeptide (GIP) Inhibits Bone Resorption Independently of Insulin and Glycemia. J Clin Endocrinol Metab 2018, 103(1), 288–294. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).