Submitted:
01 August 2024
Posted:
03 August 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Structure of the Cardiac Muscle Cell and Role of Mitochondria
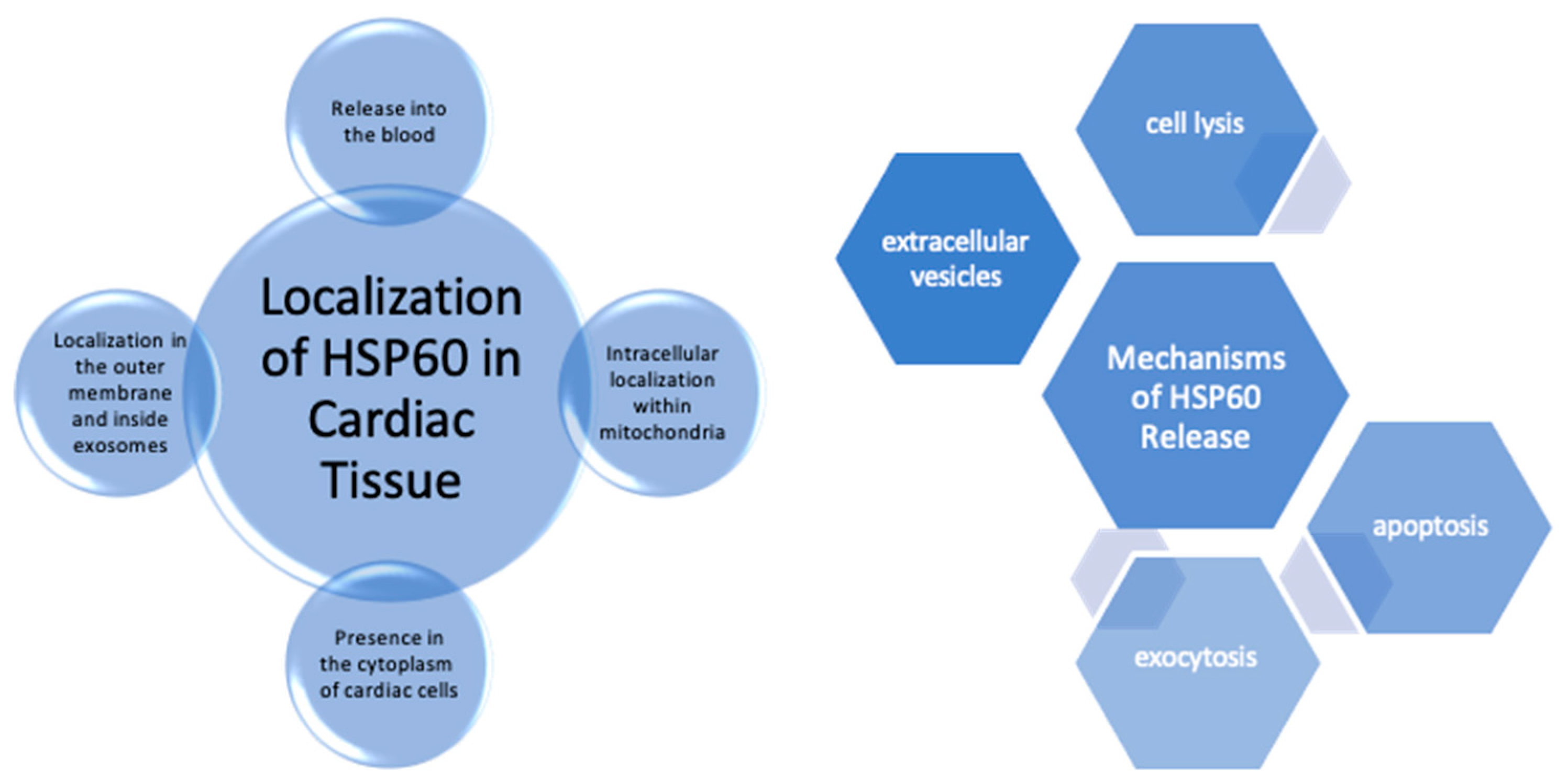
3. Hsp60 Expression and Localization in the Healthy Heart Tissue
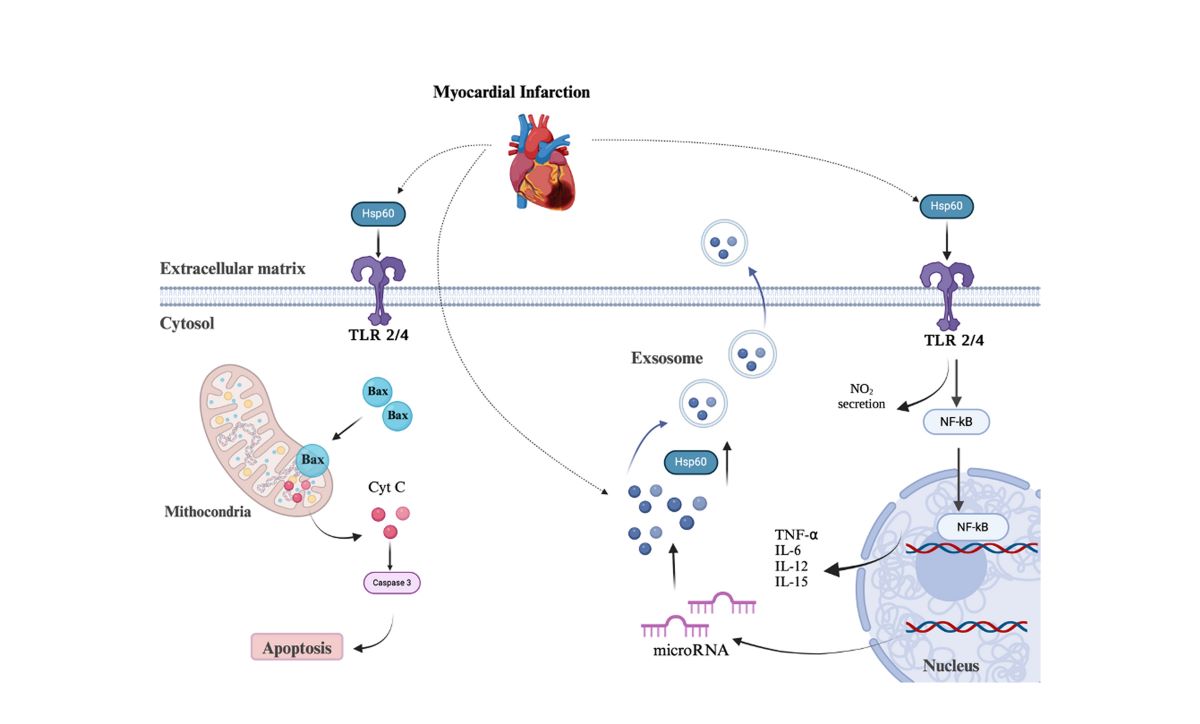
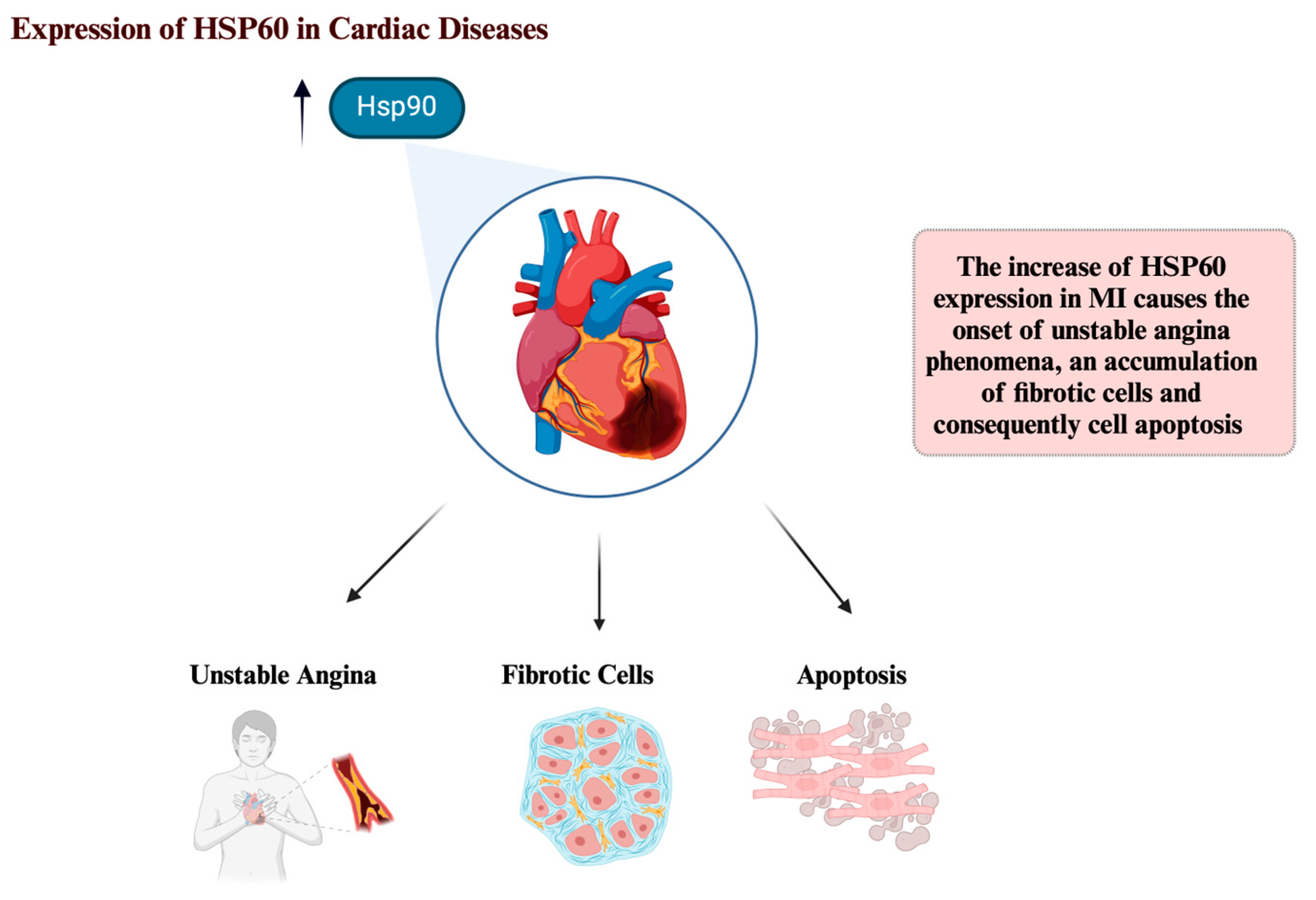
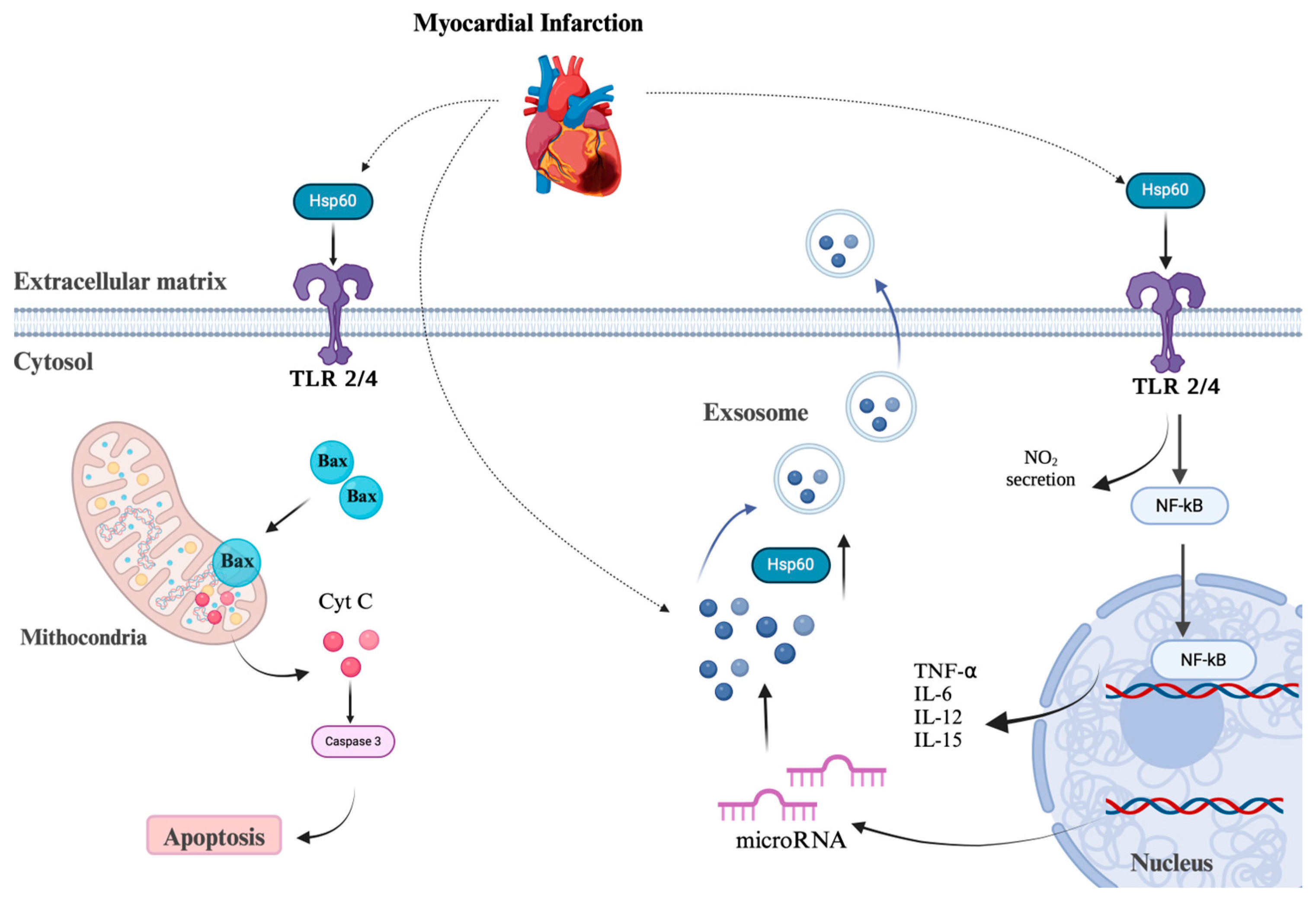
4. HSP60 and Cardiac Diseases
5. Conclusions
Conflict of Interests
Author Contributions
Funding
Abbreviations
| Oxidative phosphorylation | OXPHOS |
| Electron transport chain | ETC |
| Mitochondrial antioxidant manganese superoxide dismutase | MnSOD, SOD2 |
| Endoplasmic reticulum | ER |
| Inner mitochondrial membrane | IMM |
| Toll-like receptors | TLRs |
| Damage Associated Molecular Pattern | DAMP |
| Nitric oxide | NO |
| Interleukin | IL |
| Major histocompatibility complex | MHC |
| Antigen-presenting cells | APCs |
| Myocardial infarction | MI |
| Atrial fibrillation | AF |
| Left ventricle | LV |
| Acute myocardial infarction | AMI |
| Cardiovascular diseases | CVDs |
| Dilated cardiomyopathy | DCM |
| Chronic Heart Failure | CHF |
| Embryonic days | ED |
References
- Tzameli, I. The evolving role of mitochondria in metabolism. Trends Endocrinol. Metab. 2012, 23, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2019, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- de Souza Breda, C.N.; Davanzo, G.G.; Basso, P.J.; Câmara, N.O.S.; Moraes-Vieira, P.M.M. Mitochondria as central hub of the immune system. Redox Biol. 2019, 26, 101255. [Google Scholar]
- Papa, L.; Djedaini, M.; Hoffman, R. Mitochondrial Role in Stemness and Differentiation of Hematopoietic Stem Cells. Stem Cells Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Ardehali, H.; Balaban, R.S.; DiLisa, F.; Dorn, G.W.; Kitsis, R.N. , et al. Mitochondrial function, biology, and role in disease: a scientific statement from the American Heart Association. Circ. Res. 2016, 118, 1960–1991. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Wu, D.; Huang, Z.; Hou, T.; Jian, C. , et al. Mitochondrial flashes regulate ATP homeostasis in the heart. Elife 2017, 6. [Google Scholar]
- Yan, J.; Bao, E.; Yu, J. Heat shock protein 60 expression in heart, liver and kidney of broilers exposed to high temperature. Res. Veter- Sci. 2008, 86, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Snoeckx, L.H.E.H.; Cornelussen, R.N.; Van Nieuwenhoven, F.A.; Reneman, R.S.; Van der Vusse, G.J. Heat Shock Proteins and Cardiovascular Pathophysiology. Physiol. Rev. 2001, 81, 1461–1497. [Google Scholar] [CrossRef]
- Njemini, R.; Bautmans, I.; Lambert, M.; Demanet, C.; Mets, T. Heat shock proteins and chemokine/cytokine secretion profile in ageing and inflammation. Mech. Ageing Dev. 2007, 128, 450–454. [Google Scholar] [CrossRef]
- Nguyen, B.Y.; Ruiz-Velasco, A.; Bui, T.; Collins, L.; Wang, X.; Liu, W. Mitochondrial function in the heart: the insight into mechanisms and therapeutic potentials. Br. J. Pharmacol. 2018, 176, 4302–4318. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Singh, H. Mitochondrial ion channels in cardiac function. Am. J. Physiol. Physiol. 2021, 321, C812–C825. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells 2019, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Ardehali, H.; Balaban, R.S.; DiLisa, F.; Dorn, G.W., 2nd; Kitsis, R.N. , et al. Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement From the American Heart Association. Circ Res. 2016, 118, 1960–1991. [Google Scholar] [CrossRef] [PubMed]
- Giannessi, D.; Colotti, C.; Maltinti, M.; Del Ry, S.; Prontera, C.; Turchi, S.; L'Abbate, A.; Neglia, D. Circulating heat shock proteins and inflammatory markers in patients with idiopathic left ventricular dysfunction: their relationships with myocardial and microvascular impairment. Cell Stress Chaperon 2007, 12, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Knowlton, A.A. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am. J. Physiol. Circ. Physiol. 2007, 292, H3052–H3056. [Google Scholar] [CrossRef]
- Pockley, A.G.; Wu, R.; Lemne, C.; Kiessling, R.; de Faire, U.; Frostegård, J. Circulating Heat Shock Protein 60 Is Associated With Early Cardiovascular Disease. Hypertension 2000, 36, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, J.; Chen, H.; Adam, A.; Tang, S.; Kemper, N.; Hartung, J.; Bao, E. Expression and location of HSP60 and HSP10 in the heart tissue of heat-stressed rats. Exp. Ther. Med. 2016, 12, 2759–2765. [Google Scholar] [CrossRef]
- Di Felice, V.; Barone, R.; Trovato, E.; D’amico, D.; Macaluso, F.; Campanella, C.; Gammazza, A.M.; Muccilli, V.; Cunsolo, V.; Cancemi, P.; et al. Physiactisome: A New Nanovesicle Drug Containing Heat Shock Protein 60 for Treating Muscle Wasting and Cachexia. Cells 2022, 11, 1406. [Google Scholar] [CrossRef]
- Osterloh, A.; Meier-Stiegen, F.; Veit, A.; Fleischer, B.; von Bonin, A.; Breloer, M. Lipopolysaccharide-free Heat Shock Protein 60 Activates T Cells. J. Biol. Chem. 2004, 279, 47906–47911. [Google Scholar] [CrossRef] [PubMed]
- Pockley, A.G.; Muthana, M.; Calderwood, S.K. The dual immunoregulatory roles of stress proteins. Trends Biochem. Sci. 2008, 33, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Swaroop, S.; Sengupta, N.; Suryawanshi, A.R.; Adlakha, Y.K.; Basu, A. HSP60 plays a regulatory role in IL-1β-induced microglial inflammation via TLR4-p38 MAPK axis. J. Neuroinflammation 2016, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.H.; Mathur, S.; Wang, Y.; Bateman, R.M.; Walley, K.R. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc Res. 2006, 72, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, M.; Cheng, L.; Chen, Y.; Zhou, L.; Zeng, H.; Pockley, A.G.; Hu, F.B.; Wu, T. Elevated Heat Shock Protein 60 Levels Are Associated With Higher Risk of Coronary Heart Disease in Chinese. Circulation 2008, 118, 2687–2693. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, B.; Zhou, Q.; Wang, Y.; Liu, X.; Liu, Z.; Zhan, Z. MicroRNA-21 prevents excessive inflammation and cardiac dysfunction after myocardial infarction through targeting KBTBD7. Cell Death Dis. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, X.; Li, X.; Li, Z.; Diao, H.; Liu, L.; Zhang, J.; Ju, J.; Wen, L.; Liu, X.; et al. MicroRNA-1 downregulation induced by carvedilol protects cardiomyocytes against apoptosis by targeting heat shock protein 60. Mol. Med. Rep. 2019, 19, 3527–3536. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.M.; Fynn, S.P.; Walden, A.P.; Hobbs, W.J.; Arya, S.; Garratt, C.J. Repetitive 4-week periods of atrial electrical remodeling promote stability of atrial fibrillation: time course of a second factor involved in the self-perpetuation of atrial fibrillation. Circulation 2004, 109, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Krishnan-Sivadoss, I.; Mijares-Rojas, I.A.; Villarreal-Leal, R.A.; Torre-Amione, G.; Knowlton, A.A.; Guerrero-Beltrán, C.E. Heat shock protein 60 and cardiovascular diseases: An intricate love-hate story. Med. Res. Rev. 2020, 41, 29–71. [Google Scholar] [CrossRef]
- Saha, A.; Ahmed, S. The Link Between Heat Shock Proteins, Renin-Angiotensin System, and the Coagulation Cascade in the Pathogenesis of the Coronavirus-19 Disease. Adv Exp Med Biol. 2023, 1409, 161–171. [Google Scholar]
- Kim SC, Stice JP, Chen L, Jung JS, Gupta S, Wang Y, et al. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res. 2009;105(12):1186-95.
- Lin, L.; Kim, S.C.; Wang, Y.; Gupta, S.; Davis, B.; Simon, S.I.; Torre-Amione, G.; Knowlton, A.A. HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am. J. Physiol. Circ. Physiol. 2007, 293, H2238–H2247. [Google Scholar] [CrossRef] [PubMed]
- Cappello F, Logozzi M, Campanella C, Bavisotto CC, Marcilla A, Properzi F, et al. Exosome levels in human body fluids: A tumor marker by themselves? Eur J Pharm Sci. 2017;96:93-8.
- Eckhardt, A.; Kulhava, L.; Miksik, I.; Pataridis, S.; Hlavackova, M.; Vasinova, J.; Kolar, F.; Sedmera, D.; Ostadal, B. Proteomic analysis of cardiac ventricles: baso-apical differences. Mol. Cell. Biochem. 2018, 445, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Oyama, J.-I.; Maeda, T.; Sasaki, M.; Higuchi, Y.; Node, K.; Makino, N. Repetitive hyperthermia attenuates progression of left ventricular hypertrophy and increases telomerase activity in hypertensive rats. Am. J. Physiol. Circ. Physiol. 2012, 302, H2092–H2101. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tan, H.; Cheng, L.; He, M.; Wei, Q.; Tanguay, R.M.; Wu, T. Expression of heat shock proteins in myocardium of patients with atrial fibrillation. Cell Stress Chaperon- 2007, 12, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Toga, W.; Tanonaka, K.; Takeo, S. Changes in Hsp60 Level of the Failing Heart Following Acute Myocardial Infarction and the Effect of Long-Term Treatment with Trandolapril. Biol. Pharm. Bull. 2007, 30, 105–110. [Google Scholar] [CrossRef]
- Williams, J.W.; Huang, L.-H.; Randolph, G.J. Cytokine Circuits in Cardiovascular Disease. Immunity 2019, 50, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Duan, Y.; Yang, F.; Trexler, C.; Wang, H.; Huang, L.; Li, Y.; Tang, H.; Wang, G.; Fang, X.; et al. Deletion of heat shock protein 60 in adult mouse cardiomyocytes perturbs mitochondrial protein homeostasis and causes heart failure. Cell Death Differ. 2019, 27, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, A.A.; Kapadia, S.; Torre-Amione, G.; Durand, J.-B.; Bies, R.; Young, J.; Mann, D.L. Differential Expression of Heat Shock Proteins in Normal and Failing Human Hearts. J. Mol. Cell. Cardiol. 1998, 30, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Niizeki, T.; Takeishi, Y.; Watanabe, T.; Nitobe, J.; Miyashita, T.; Miyamoto, T.; Kitahara, T.; Suzuki, S.; Sasaki, T.; Bilim, O.; et al. Relation of Serum Heat Shock Protein 60 Level to Severity and Prognosis in Chronic Heart Failure Secondary to Ischemic or Idiopathic Dilated Cardiomyopathy. Am. J. Cardiol. 2008, 102, 606–610. [Google Scholar] [CrossRef]
- Al-Zghoul, M.-B.; Ismail, Z.B.; Dalab, A.E.S.; Al-Ramadan, A.; Althnaian, T.A.; Al-Ramadan, S.Y.; Ali, A.M.; Albokhadaim, I.F.; Al Busadah, K.A.; Eljarah, A.; et al. Hsp90, Hsp60 and HSF-1 genes expression in muscle, heart and brain of thermally manipulated broiler chicken. Res. Veter- Sci. 2015, 99, 105–111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




