Submitted:
01 August 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
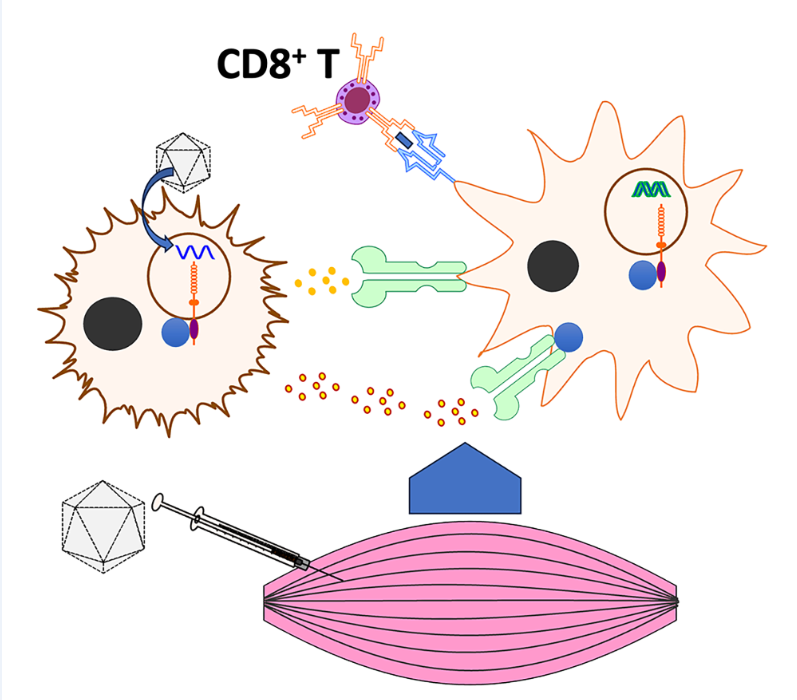
Keywords:
1. Introduction
2. Materials and Methods:
2.1. AAV Vector Production
2.2. Mouse Strains and Experiments
2.3. Flow Cytometry
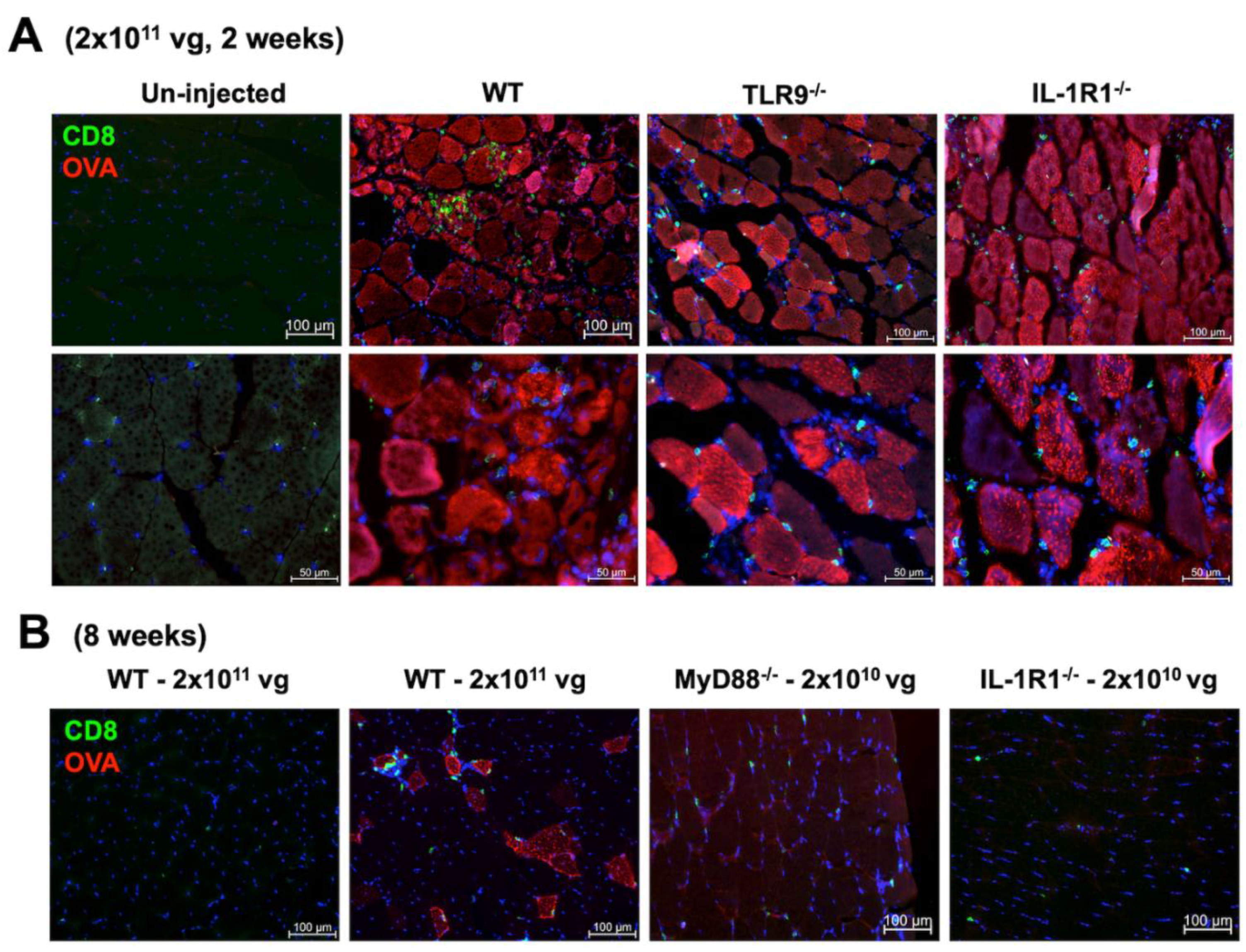
2.4. Immunohistochemistry
2.5. Antibody Assays
2.6. Statistical Analysis
3. Results
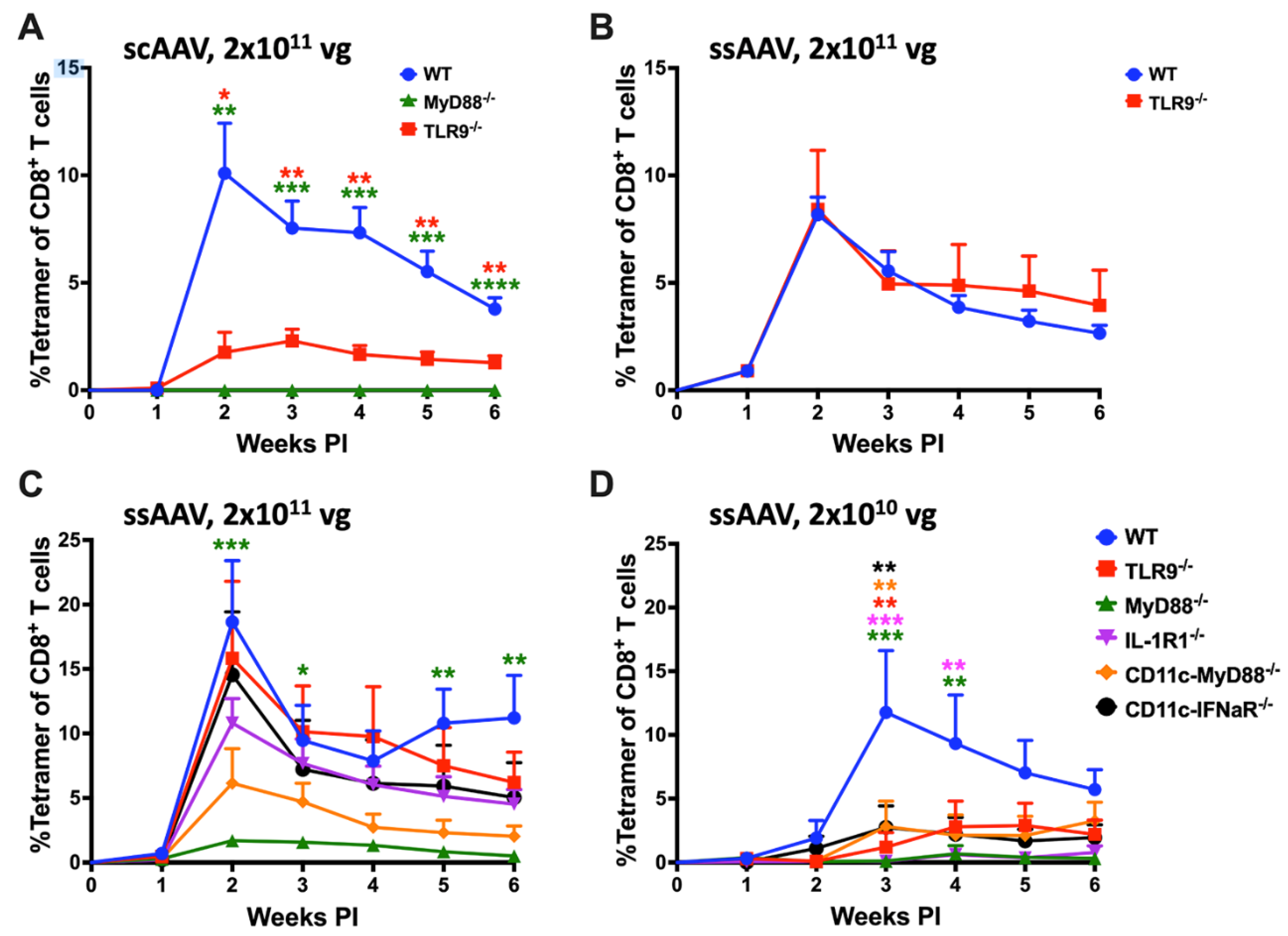
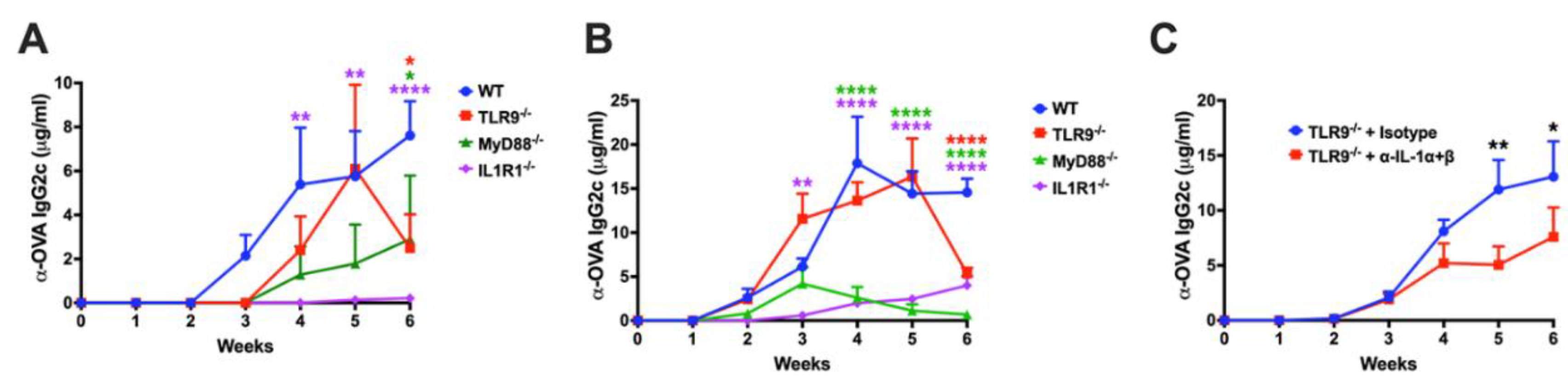
3.1. Single-Stranded AAV Vector Induces TLR9-Independent CD8+ T Cell Response Following High Dose Intramuscular Injection
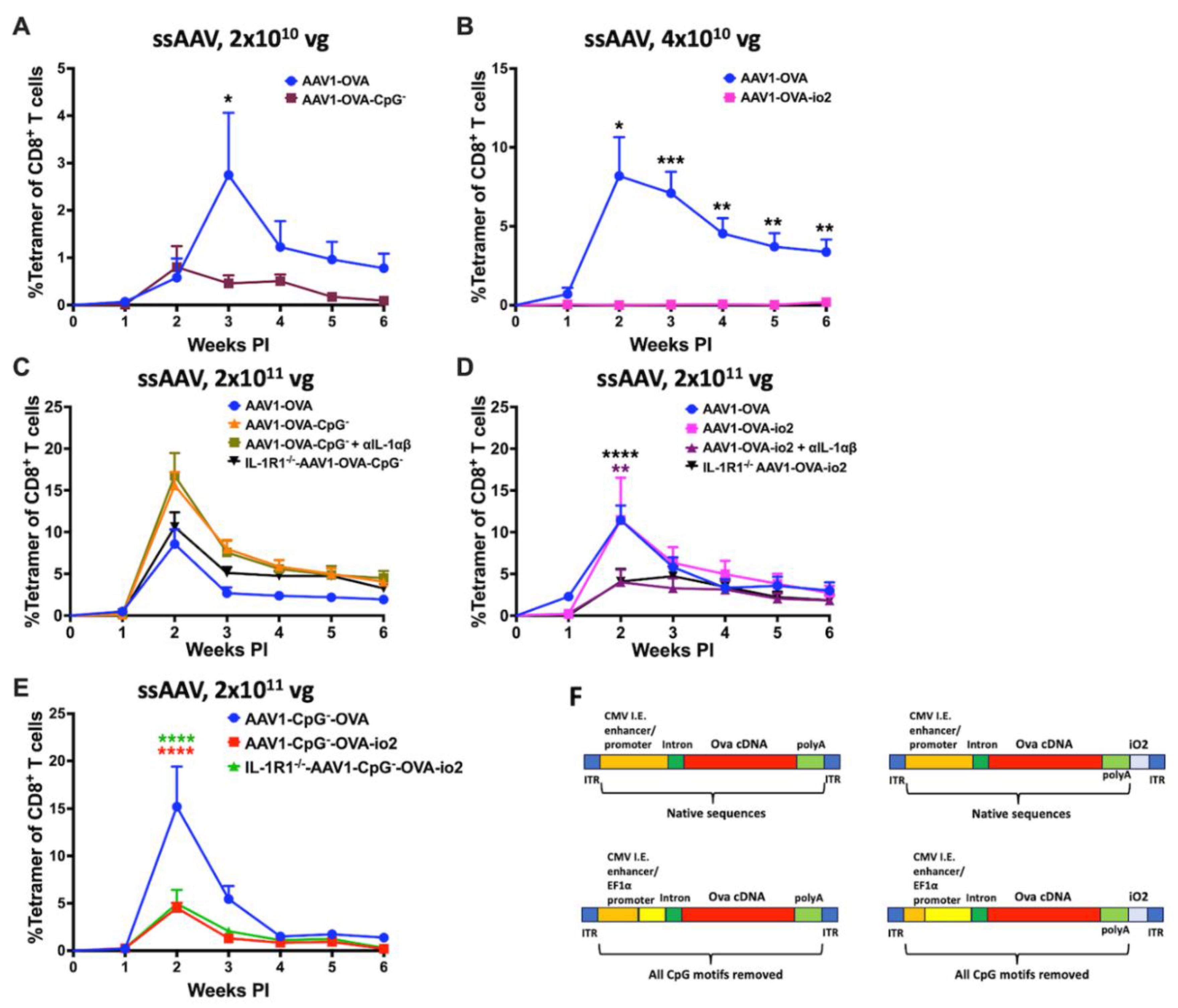
3.2. TLR9, IFN I, and IL-1R1 Are Crucial for Maximum CD8+ T Cell Response to the Transgene Product at Lower but Not at Higher Vector Doses
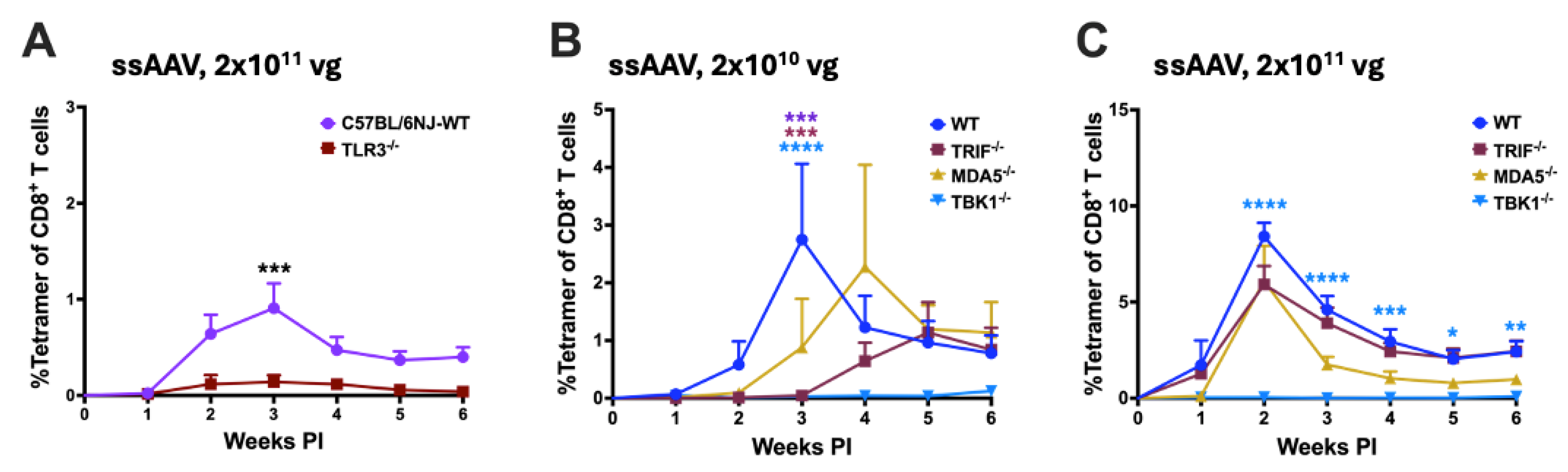
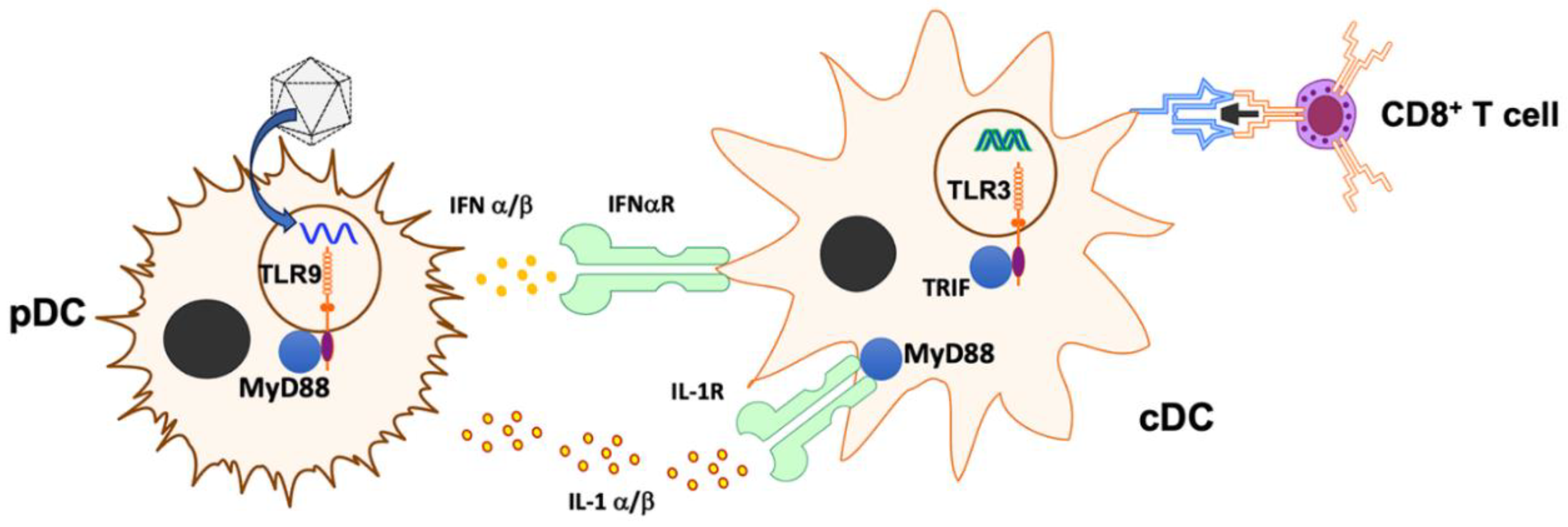
3.3. Potential Role for dsRNA Sensing
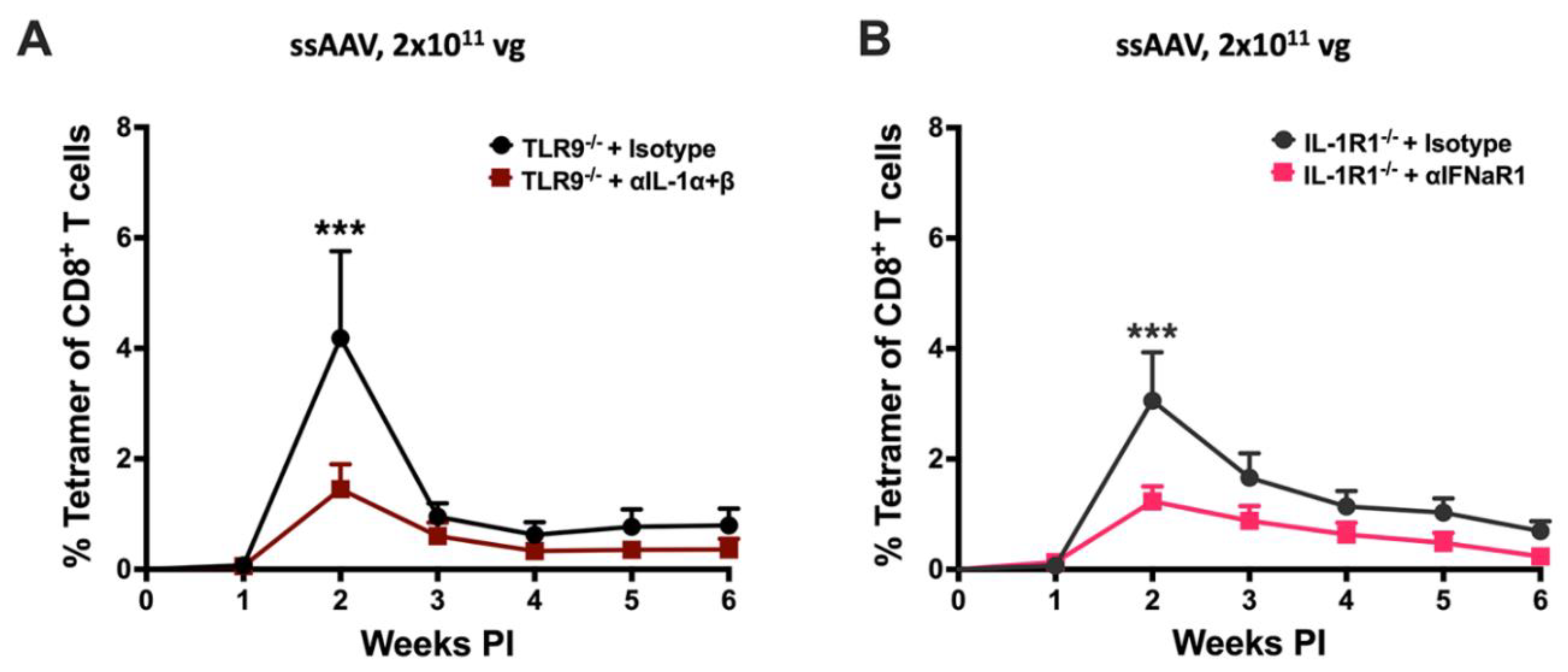
3.4. Simultaneous Block of Two Innate Pathways Indicates Redundancy at High Vector Doses and Provides Path for Targeted Therapeutic Intervention
3.5. Effect of Innate Immune Pathways on Antibody Formation against Transgene Product
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mendell, J.R.; Rodino-Klapac, L.R.; Rosales-Quintero, X.; Kota, J.; Coley, B.D.; Galloway, G.; Craenen, J.M.; Lewis, S.; Malik, V.; Shilling, C.; Byrne, B.J.; Conlon, T.; Campbell, K.J.; Bremer, W.G.; Viollet, L.; Walker, C.M.; Sahenk, Z.; Clark, K.R. , Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol 2009, 66, 290–7. [Google Scholar] [CrossRef]
- Mendell, J.R.; Rodino-Klapac, L.R.; Rosales, X.Q.; Coley, B.D.; Galloway, G.; Lewis, S.; Malik, V.; Shilling, C.; Byrne, B.J.; Conlon, T.; Campbell, K.J.; Bremer, W.G.; Taylor, L.E.; Flanigan, K.M.; Gastier-Foster, J.M.; Astbury, C.; Kota, J.; Sahenk, Z.; Walker, C.M.; Clark, K.R. , Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol 2010, 68, 629–38. [Google Scholar] [CrossRef]
- Mendell, J.R.; Campbell, K.; Rodino-Klapac, L.; Sahenk, Z.; Shilling, C.; Lewis, S.; Bowles, D.; Gray, S.; Li, C.; Galloway, G.; Malik, V.; Coley, B.; Clark, K.R.; Li, J.; Xiao, X.; Samulski, J.; McPhee, S.W.; Samulski, R.J.; Walker, C.M. , Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med 2010, 363, 1429–37. [Google Scholar] [CrossRef]
- Manno, C.S.; Chew, A.J.; Hutchison, S.; Larson, P.J.; Herzog, R.W.; Arruda, V.R.; Tai, S.J.; Ragni, M.V.; Thompson, A.; Ozelo, M.; Couto, L.B.; Leonard, D.G.; Johnson, F.A.; McClelland, A.; Scallan, C.; Skarsgard, E.; Flake, A.W.; Kay, M.A.; High, K.A.; Glader, B. , AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 2003, 101, 2963–72. [Google Scholar] [CrossRef]
- Brantly, M.L.; Spencer, L.T.; Humphries, M.; Conlon, T.J.; Spencer, C.T.; Poirier, A.; Garlington, W.; Baker, D.; Song, S.; Berns, K.I.; Muzyczka, N.; Snyder, R.O.; Byrne, B.J.; Flotte, T.R. , Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum Gene Ther 2006, 17, 1177–86. [Google Scholar] [CrossRef]
- Yla-Herttuala, S. , Endgame: glybera finally recommended for approval as the first gene therapy drug in the European union. Mol Ther 2012, 20, 1831–2. [Google Scholar] [CrossRef]
- Fuchs, S.P.; Desrosiers, R.C. , Promise and problems associated with the use of recombinant AAV for the delivery of anti-HIV antibodies. Mol Ther Methods Clin Dev 2016, 3, 16068. [Google Scholar] [CrossRef]
- Gardner, M.R. , Promise and Progress of an HIV-1 Cure by Adeno-Associated Virus Vector Delivery of Anti-HIV-1 Biologics. Front Cell Infect Microbiol 2020, 10, 176. [Google Scholar] [CrossRef]
- Hahn, P.A.; Martins, M.A. , Adeno-associated virus-vectored delivery of HIV biologics: the promise of a "single-shot" functional cure for HIV infection. J Virus Erad 2023, 9, 100316. [Google Scholar] [CrossRef]
- Bengtsson, N.E.; Hall, J.K.; Odom, G.L.; Phelps, M.P.; Andrus, C.R.; Hawkins, R.D.; Hauschka, S.D.; Chamberlain, J.R.; Chamberlain, J.S. , Muscle-specific CRISPR/Cas9 dystrophin gene editing ameliorates pathophysiology in a mouse model for Duchenne muscular dystrophy. Nat Commun 2017, 8, 14454. [Google Scholar] [CrossRef]
- Nelson, C.E.; Hakim, C.H.; Ousterout, D.G.; Thakore, P.I.; Moreb, E.A.; Castellanos Rivera, R.M.; Madhavan, S.; Pan, X.; Ran, F.A.; Yan, W.X.; Asokan, A.; Zhang, F.; Duan, D.; Gersbach, C.A. , In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 2016, 351, 403–7. [Google Scholar] [CrossRef]
- Administration, U.F. a. D. , FDA Approves First Gene Therapy for Treatment of Certain Patients with Duchenne Muscular Dystrophy. In 06/22/2023.
- Kumar, S.R.P.; Duan, D.; Herzog, R.W. , Immune Responses to Muscle-Directed Adeno-Associated Viral Gene Transfer in Clinical Studies. Hum Gene Ther 2023, 34, 365–371. [Google Scholar] [CrossRef]
- Duan, D. , Lethal immunotoxicity in high-dose systemic AAV therapy. Mol Ther 2023, 31, 3123–3126. [Google Scholar] [CrossRef]
- Lek, A.; Keeler, A.; Flotte, T.R. , Death after High-Dose rAAV9 Gene Therapy in a Patient with Duchenne's Muscular Dystrophy. Reply. N Engl J Med 2023, 389, 2211. [Google Scholar] [CrossRef]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. , Immune Responses to Viral Gene Therapy Vectors. Mol Ther 2020, 28, 709–722. [Google Scholar] [CrossRef]
- Verdera, H.C.; Kuranda, K.; Mingozzi, F. , AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Mol Ther 2020, 28, 723–746. [Google Scholar] [CrossRef]
- Salabarria, S.M.; Corti, M.; Coleman, K.E.; Wichman, M.B.; Berthy, J.A.; D'Souza, P.; Tifft, C.J.; Herzog, R.W.; Elder, M.E.; Shoemaker, L.R.; Leon-Astudillo, C.; Tavakkoli, F.; Kirn, D.H.; Schwartz, J.D.; Byrne, B.J. , Thrombotic microangiopathy following systemic AAV administration is dependent on anti-capsid antibodies. J Clin Invest 2024, 134. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, X.; Yang, Y. , The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest 2009, 119, 2388–98. [Google Scholar] [CrossRef]
- Rogers, G.L.; Shirley, J.L.; Zolotukhin, I.; Kumar, S.R.P.; Sherman, A.; Perrin, G.Q.; Hoffman, B.E.; Srivastava, A.; Basner-Tschakarjan, E.; Wallet, M.A.; Terhorst, C.; Biswas, M.; Herzog, R.W. , Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8(+) T cells. Blood 2017, 129, 3184–3195. [Google Scholar] [CrossRef]
- Shirley, J.L.; Keeler, G.D.; Sherman, A.; Zolotukhin, I.; Markusic, D.M.; Hoffman, B.E.; Morel, L.M.; Wallet, M.A.; Terhorst, C.; Herzog, R.W. , Type I IFN Sensing by cDCs and CD4(+) T Cell Help Are Both Requisite for Cross-Priming of AAV Capsid-Specific CD8(+) T Cells. Mol Ther 2020, 28, 758–770. [Google Scholar] [CrossRef]
- Bertolini, T.B.; Shirley, J.L.; Zolotukhin, I.; Li, X.; Kaisho, T.; Xiao, W.; Kumar, S.R.P.; Herzog, R.W. , Effect of CpG Depletion of Vector Genome on CD8(+) T Cell Responses in AAV Gene Therapy. Front Immunol 2021, 12, 672449. [Google Scholar] [CrossRef]
- Eickhoff, S.; Brewitz, A.; Gerner, M.Y.; Klauschen, F.; Komander, K.; Hemmi, H.; Garbi, N.; Kaisho, T.; Germain, R.N.; Kastenmuller, W. , Robust Anti-viral Immunity Requires Multiple Distinct T Cell-Dendritic Cell Interactions. Cell 2015, 162, 1322–37. [Google Scholar] [CrossRef]
- Mays, L.E.; Vandenberghe, L.H.; Xiao, R.; Bell, P.; Nam, H.J.; Agbandje-McKenna, M.; Wilson, J.M. , Adeno-associated virus capsid structure drives CD4-dependent CD8+ T cell response to vector encoded proteins. J Immunol 2009, 182, 6051–60. [Google Scholar] [CrossRef]
- Kumar, S.R.P.; Biswas, M.; Cao, D.; Arisa, S.; Munoz-Melero, M.; Lam, A.K.; Pineros, A.R.; Kapur, R.; Kaisho, T.; Kaufman, R.J.; Xiao, W.; Shayakhmetov, D.M.; Terhorst, C.; de Jong, Y.P.; Herzog, R.W. , TLR9-independent CD8(+) T cell responses in hepatic AAV gene transfer through IL-1R1-MyD88 signaling. Mol Ther 2024, 32, 325–339. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Quinn, T.J.; Thorne, P.S.; Sayeed, S.; Yi, A.K.; Krieg, A.M. , CpG motifs in bacterial DNA cause inflammation in the lower respiratory tract. J Clin Invest 1997, 100, 68–73. [Google Scholar] [CrossRef]
- Krieg, A.M. , CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 2002, 20, 709–60. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. , Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–50. [Google Scholar] [CrossRef]
- Faust, S.M.; Bell, P.; Cutler, B.J.; Ashley, S.N.; Zhu, Y.; Rabinowitz, J.E.; Wilson, J.M. , CpG-depleted adeno-associated virus vectors evade immune detection. J Clin Invest 2013, 123, 2994–3001. [Google Scholar] [CrossRef]
- Wright, J.F. , Codon Modification and PAMPs in Clinical AAV Vectors: The Tortoise or the Hare? Mol Ther 2020, 28, 701–703. [Google Scholar] [CrossRef]
- Konkle, B.A.; Walsh, C.E.; Escobar, M.A.; Josephson, N.C.; Young, G.; von Drygalski, A.; McPhee, S.W.J.; Samulski, R.J.; Bilic, I.; de la Rosa, M.; Reipert, B.M.; Rottensteiner, H.; Scheiflinger, F.; Chapin, J.C.; Ewenstein, B.; Monahan, P.E. , BAX 335 hemophilia B gene therapy clinical trial results: potential impact of CpG sequences on gene expression. Blood 2021, 137, 763–774. [Google Scholar] [CrossRef]
- Wright, J.F. , Quantification of CpG Motifs in rAAV Genomes: Avoiding the Toll. Mol Ther 2020, 28, 1756–1758. [Google Scholar] [CrossRef]
- Chan, Y.K.; Wang, S.K.; Chu, C.J.; Copland, D.A.; Letizia, A.J.; Costa Verdera, H.; Chiang, J.J.; Sethi, M.; Wang, M.K.; Neidermyer, W.J., Jr.; Chan, Y.; Lim, E.T.; Graveline, A.R.; Sanchez, M.; Boyd, R.F.; Vihtelic, T.S.; Inciong, R.; Slain, J.M.; Alphonse, P.J.; Xue, Y.; Robinson-McCarthy, L.R.; Tam, J.M.; Jabbar, M.H.; Sahu, B.; Adeniran, J.F.; Muhuri, M.; Tai, P.W.L.; Xie, J.; Krause, T.B.; Vernet, A.; Pezone, M.; Xiao, R.; Liu, T.; Wang, W.; Kaplan, H.J.; Gao, G.; Dick, A.D.; Mingozzi, F.; McCall, M.A.; Cepko, C.L.; Church, G.M. , Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci Transl Med 2021, 13. [Google Scholar] [CrossRef]
- Martino, A.T.; Suzuki, M.; Markusic, D.M.; Zolotukhin, I.; Ryals, R.C.; Moghimi, B.; Ertl, H.C.; Muruve, D.A.; Lee, B.; Herzog, R.W. , The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood 2011, 117, 6459–68. [Google Scholar] [CrossRef]
- Rogers, G.L.; Suzuki, M.; Zolotukhin, I.; Markusic, D.M.; Morel, L.M.; Lee, B.; Ertl, H.C.; Herzog, R.W. , Unique Roles of TLR9- and MyD88-Dependent and -Independent Pathways in Adaptive Immune Responses to AAV-Mediated Gene Transfer. J Innate Immun 2015, 7, 302–14. [Google Scholar] [CrossRef]
- Herzog, R.W.; Cooper, M.; Perrin, G.Q.; Biswas, M.; Martino, A.T.; Morel, L.; Terhorst, C.; Hoffman, B.E. , Regulatory T cells and TLR9 activation shape antibody formation to a secreted transgene product in AAV muscle gene transfer. Cell Immunol 2019, 342, 103682. [Google Scholar] [CrossRef]
- Butterfield, J.S.S.; Biswas, M.; Shirley, J.L.; Kumar, S.R.P.; Sherman, A.; Terhorst, C.; Ling, C.; Herzog, R.W. , TLR9-Activating CpG-B ODN but Not TLR7 Agonists Triggers Antibody Formation to Factor IX in Muscle Gene Transfer. Hum Gene Ther Methods 2019, 30, 81–92. [Google Scholar] [CrossRef]
- Cao, D.; Byrne, B.J.; de Jong, Y.P.; Terhorst, C.; Duan, D.; Herzog, R.W.; Kumar, S.R.P. , Innate Immune Sensing of Adeno-Associated Virus Vectors. Hum Gene Ther 2024, 35, 451–463. [Google Scholar] [CrossRef]
- Flanigan, K.M.; Campbell, K.; Viollet, L.; Wang, W.; Gomez, A.M.; Walker, C.M.; Mendell, J.R. , Anti-dystrophin T cell responses in Duchenne muscular dystrophy: prevalence and a glucocorticoid treatment effect. Hum Gene Ther 2013, 24, 797–806. [Google Scholar] [CrossRef]
- Anthony, K.; Ala, P.; Catapano, F.; Meng, J.; Domingos, J.; Perry, M.; Ricotti, V.; Maresh, K.; Phillips, L.C.; Servais, L.; Seferian, A.M.; De Lucia, S.; de Groot, I.; Krom, Y.D.; Verschuuren, J.G.M.; Niks, E.H.; Straub, V.; Guglieri, M.; Voit, T.; Morgan, J.; Muntoni, F. , T Cell Responses to Dystrophin in a Natural History Study of Duchenne Muscular Dystrophy. Hum Gene Ther 2023, 34, 439–448. [Google Scholar] [CrossRef]
- Flotte, T.R.; Trapnell, B.C.; Humphries, M.; Carey, B.; Calcedo, R.; Rouhani, F.; Campbell-Thompson, M.; Yachnis, A.T.; Sandhaus, R.A.; McElvaney, N.G.; Mueller, C.; Messina, L.M.; Wilson, J.M.; Brantly, M.; Knop, D.R.; Ye, G.J.; Chulay, J.D. , Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: interim results. Hum Gene Ther 2011, 22, 1239–47. [Google Scholar] [CrossRef]
- Calcedo, R.; Somanathan, S.; Qin, Q.; Betts, M.R.; Rech, A.J.; Vonderheide, R.H.; Mueller, C.; Flotte, T.R.; Wilson, J.M. , Class I-restricted T-cell responses to a polymorphic peptide in a gene therapy clinical trial for alpha-1-antitrypsin deficiency. Proc Natl Acad Sci U S A 2017, 114, 1655–1659. [Google Scholar] [CrossRef]
- Bonnemann, C.G.; Belluscio, B.A.; Braun, S.; Morris, C.; Singh, T.; Muntoni, F. , Dystrophin Immunity after Gene Therapy for Duchenne's Muscular Dystrophy. N Engl J Med 2023, 388, 2294–2296. [Google Scholar] [CrossRef]
- Hakim, C.H.; Kumar, S.R.P.; Perez-Lopez, D.O.; Wasala, N.B.; Zhang, D.; Yue, Y.; Teixeira, J.; Pan, X.; Zhang, K.; Million, E.D.; Nelson, C.E.; Metzger, S.; Han, J.; Louderman, J.A.; Schmidt, F.; Feng, F.; Grimm, D.; Smith, B.F.; Yao, G.; Yang, N.N.; Gersbach, C.A.; Chen, S.J.; Herzog, R.W.; Duan, D. , Cas9-specific immune responses compromise local and systemic AAV CRISPR therapy in multiple dystrophic canine models. Nat Commun 2021, 12, 6769. [Google Scholar] [CrossRef]
- Cao, O.; Hoffman, B.E.; Moghimi, B.; Nayak, S.; Cooper, M.; Zhou, S.; Ertl, H.C.; High, K.A.; Herzog, R.W. , Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol Ther 2009, 17, 1733–42. [Google Scholar] [CrossRef]
- Kumar, S.R.P.; Hoffman, B.E.; Terhorst, C.; de Jong, Y.P.; Herzog, R.W. , The Balance between CD8(+) T Cell-Mediated Clearance of AAV-Encoded Antigen in the Liver and Tolerance Is Dependent on the Vector Dose. Mol Ther 2017, 25, 880–891. [Google Scholar] [CrossRef]
- Crosson, S.M.; Dib, P.; Smith, J.K.; Zolotukhin, S. , Helper-free Production of Laboratory Grade AAV and Purification by Iodixanol Density Gradient Centrifugation. Mol Ther Methods Clin Dev 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Copenhaver, A.M.; Casson, C.N.; Nguyen, H.T.; Duda, M.M.; Shin, S. , IL-1R signaling enables bystander cells to overcome bacterial blockade of host protein synthesis. Proc Natl Acad Sci U S A 2015, 112, 7557–62. [Google Scholar] [CrossRef]
- Rogers, G.L.; Hoffman, B.E. , Optimal Immunofluorescent Staining for Human Factor IX and Infiltrating T Cells following Gene Therapy for Hemophilia B. J Genet Syndr Gene Ther 2012, S1. [Google Scholar] [CrossRef]
- Rogers, G.L.; Martino, A.T.; Zolotukhin, I.; Ertl, H.C.; Herzog, R.W. , Role of the vector genome and underlying factor IX mutation in immune responses to AAV gene therapy for hemophilia B. J Transl Med 2014, 12, 25. [Google Scholar] [CrossRef]
- Shao, W.; Earley, L.F.; Chai, Z.; Chen, X.; Sun, J.; He, T.; Deng, M.; Hirsch, M.L.; Ting, J.; Samulski, R.J.; Li, C. , Double-stranded RNA innate immune response activation from long-term adeno-associated virus vector transduction. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Chen, Y.G.; Hur, S. , Cellular origins of dsRNA, their recognition and consequences. Nat Rev Mol Cell Biol 2022, 23, 286–301. [Google Scholar] [CrossRef]
- Herzog, R.W.; Fields, P.A.; Arruda, V.R.; Brubaker, J.O.; Armstrong, E.; McClintock, D.; Bellinger, D.A.; Couto, L.B.; Nichols, T.C.; High, K.A. , Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther 2002, 13, 1281–91. [Google Scholar] [CrossRef]
- Wang, L.; Cao, O.; Swalm, B.; Dobrzynski, E.; Mingozzi, F.; Herzog, R.W. , Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther 2005, 12, 1453–64. [Google Scholar] [CrossRef]
- Wang, L.; Dobrzynski, E.; Schlachterman, A.; Cao, O.; Herzog, R.W. , Systemic protein delivery by muscle-gene transfer is limited by a local immune response. Blood 2005, 105, 4226–34. [Google Scholar] [CrossRef]
- Arjomandnejad, M.; Dasgupta, I.; Flotte, T.R.; Keeler, A.M. , Immunogenicity of Recombinant Adeno-Associated Virus (AAV) Vectors for Gene Transfer. BioDrugs 2023, 37, 311–329. [Google Scholar] [CrossRef]
- Bentler, M.; Hardet, R.; Ertelt, M.; Rudolf, D.; Kaniowska, D.; Schneider, A.; Vondran, F.W.R.; Schoeder, C.T.; Delphin, M.; Lucifora, J.; Ott, M.; Hacker, U.T.; Adriouch, S.; Buning, H. , Modifying immune responses to adeno-associated virus vectors by capsid engineering. Mol Ther Methods Clin Dev 2023, 30, 576–592. [Google Scholar] [CrossRef]
- Tasfaout, H.; Halbert, C.L.; McMillen, T.S.; Allen, J.M.; Reyes, T.R.; Flint, G.V.; Grimm, D.; Hauschka, S.D.; Regnier, M.; Chamberlain, J.S. , Split intein-mediated protein trans-splicing to express large dystrophins. Nature 2024. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, C.; Xiao, W.; Herzog, R.W.; Han, R. , Systemic delivery of full-length dystrophin in Duchenne muscular dystrophy mice. Nat Commun 2024, 15, 6141. [Google Scholar] [CrossRef]
- Suriano, C.M.; Kumar, N.; Verpeut, J.L.; Ma, J.; Jung, C.; Dunn, C.E.; Carvajal, B.V.; Nguyen, A.V.; Boulanger, L.M. , An innate immune response to adeno-associated virus genomes decreases cortical dendritic complexity and disrupts synaptic transmission. Mol Ther 2024, 32, 1721–1738. [Google Scholar] [CrossRef]
- Muhuri, M.; Levy, D.I.; Schulz, M.; McCarty, D.; Gao, G. , Durability of transgene expression after rAAV gene therapy. Mol Ther 2022, 30, 1364–1380. [Google Scholar] [CrossRef]
- Muhuri, M.; Zhan, W.; Maeda, Y.; Li, J.; Lotun, A.; Chen, J.; Sylvia, K.; Dasgupta, I.; Arjomandnejad, M.; Nixon, T.; Keeler, A.M.; Manokaran, S.; He, R.; Su, Q.; Tai, P.W.L.; Gao, G. , Novel Combinatorial MicroRNA-Binding Sites in AAV Vectors Synergistically Diminish Antigen Presentation and Transgene Immunity for Efficient and Stable Transduction. Front Immunol 2021, 12, 674242. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Muhuri, M.; Li, S.; Qin, W.; Xu, G.; Luo, L.; Li, J.; Letizia, A.J.; Wang, S.K.; Chan, Y.K.; Wang, C.; Fuchs, S.P.; Wang, D.; Su, Q.; Nahid, M.A.; Church, G.M.; Farzan, M.; Yang, L.; Wei, Y.; Desrosiers, R.C.; Mueller, C.; Tai, P.W.; Gao, G. , Circumventing cellular immunity by miR142-mediated regulation sufficiently supports rAAV-delivered OVA expression without activating humoral immunity. JCI Insight 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Melero, M.; Biswas, M. , Role of FoxP3(+) Regulatory T Cells in Modulating Immune Responses to Adeno-Associated Virus Gene Therapy. Hum Gene Ther 2024, 35, 439–450. [Google Scholar] [CrossRef] [PubMed]
| Vector dose | Low | High |
|---|---|---|
| IL-1 blockade | ↓ | - |
| TLR9i (io2) | ↓ | - |
| TLR9i (io2) + IL-1 blockade | nd | ↓ |
| CpG depletion | ↓ | - |
| CpG depletion + IL-1 blockade | nd | - |
| CpG depletion + TLR9i (io2) | nd | ↓ |
| CpG depletion + TLR9i (io2) + IL-1 blockade | nd | ↓ (but not lower than CpG depletion + TLR9i without IL- blockade) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





