1. Introduction
Vulvovaginal candidiasis (VVC) is a disease widely spread among the female population. Three-fourths of women worldwide are estimated to be affected by VVC at least once during their reproductive age. Moreover, 5-8% of them develop the recurrent form (RVVC), consisting of more than 4 episodes of VVC per year. Both VVC and RVVC have a significant impact on the life quality and mental wellbeing of the approximately 138 million affected women [
1,
2]. VVC is a symptomatic inflammation of the vagina, and it is mainly caused by
Candida albicans (
C. albicans). Infections due to other species of the genus
Candida, such as
C. parapsilosis,
C. glabrata,
C. tropicalis, and
C. krusei, are less common, but they are more frequently associated with the recurrent form. VVC symptoms, that include itching, burning, discharge, and dyspareunia, are caused by a hyperinflammatory response to
Candida rather than by the presence of the fungus, which normally dwells in the vagina as a commensal [
3]. As a colonizer of the healthy vaginal niches,
Candida dwells on the vaginal mucosa in form of yeast and its proliferation and pathogenicity are constantly inhibited by the immune system and by resident bacteria, such as
Lactobacillus spp. The pH of a normal vaginal environment typically ranges between 3.5 and 4.5, because of the lactic acid produced by lactobacilli [
4,
5]. Alterations in microbial composition and immune system dysfunctions may increase the risk of
Candida infection. Pregnancy, diabetes mellitus, hormones administered as oral contraceptives, wide-spectrum antibiotic therapy and genetic predisposition are among the main VVC predisposing factors [
6]. The VVC onset is linked to
C. albicans morphological transition from yeast to hyphal form. The subsequent epithelial invasion and cell damage trigger an intense immune and inflammatory response; the latter ultimately causes the symptoms of the disease.
VVC episodes are typically treated with oral or topical azole antifungals (mainly fluconazole). However, despite the therapy effectiveness grants relief from symptoms, in about 50% of cases reinfection occurs after 6 months. For RVVC, the elective therapy requires continuous treatment with fluconazole for 6 months. Nevertheless, more than half of the women show symptoms relapse few months after the end of fluconazole treatment. Overall, azole-based treatment has a limited long-term efficacy and consequently high costs [
7,
8]. Moreover, the occurrence of clinical isolates resistant to azoles further indicates the need to define alternative therapeutic approaches [
9].
In this regard, interest in probiotics and on their role in human health is increasing in recent years, because of their excellent performance in preventing and treating several diseases and of the increasing demand for natural medicines by consumers [
10]. Probiotics are living microorganisms that, when consumed in appropriate quantities, provide health benefits to the host, as defined by the World Health Organization Antimicrobial activity against pathogens, modulation of the immune system, and enhancement of essential nutrients bioavailability are among the major benefits granted by probiotics consumption. In addition, engineered microbes may be used as therapeutic vectors, to deliver vaccines, antibiotics, enzymes, or other drugs, representing thus a promising research field for the treatment of several diseases. Probiotics have been demonstrated to interact also with host microbiota. The latter may influence several organs, typically by immunomodulatory signals. Indeed, connections between the gut microbiota and the brain, liver, lung, skin and vagina have been already demonstrated [
11,
12]. Probiotics are being studied also in psychiatry as a novel therapeutic approach aimed to treat conditions such as mood disorders, anxiety, and depression [
13,
14].
Probiotics are typically Gram-positive bacteria, mainly belonging to the genera
Lactobacillus and
Bifidobacterium. Recently, other genera have been demonstrated to show probiotic activity [
15,
16]. Spore-producing bacteria, such as several species belonging to the genus
Bacillus, have been frequently employed in commercial formulations due to their properties. Indeed, they can survive various industrial processes, and they remain stable at room temperature over non-sporulating probiotic species [
17]. Most probiotic formulations are taken orally. In spore-based formulation probiotics, spore germination and bacterial proliferation occur on the gut mucosa surface.
Evidence on the benefits of probiotics in VVC treatment or prevention is yet to be fully demonstrated, but preclinical studies and clinical trials suggest a beneficial role of several probiotics against
Candida vaginal infections [
18,
19]. Although there is no sufficient evidence to support the replacement of antifungals with probiotics to date, the latter represent an interesting approach at least to prevent the recurrent forms and an aid to restore the vaginal eubiosis [
20,
21].
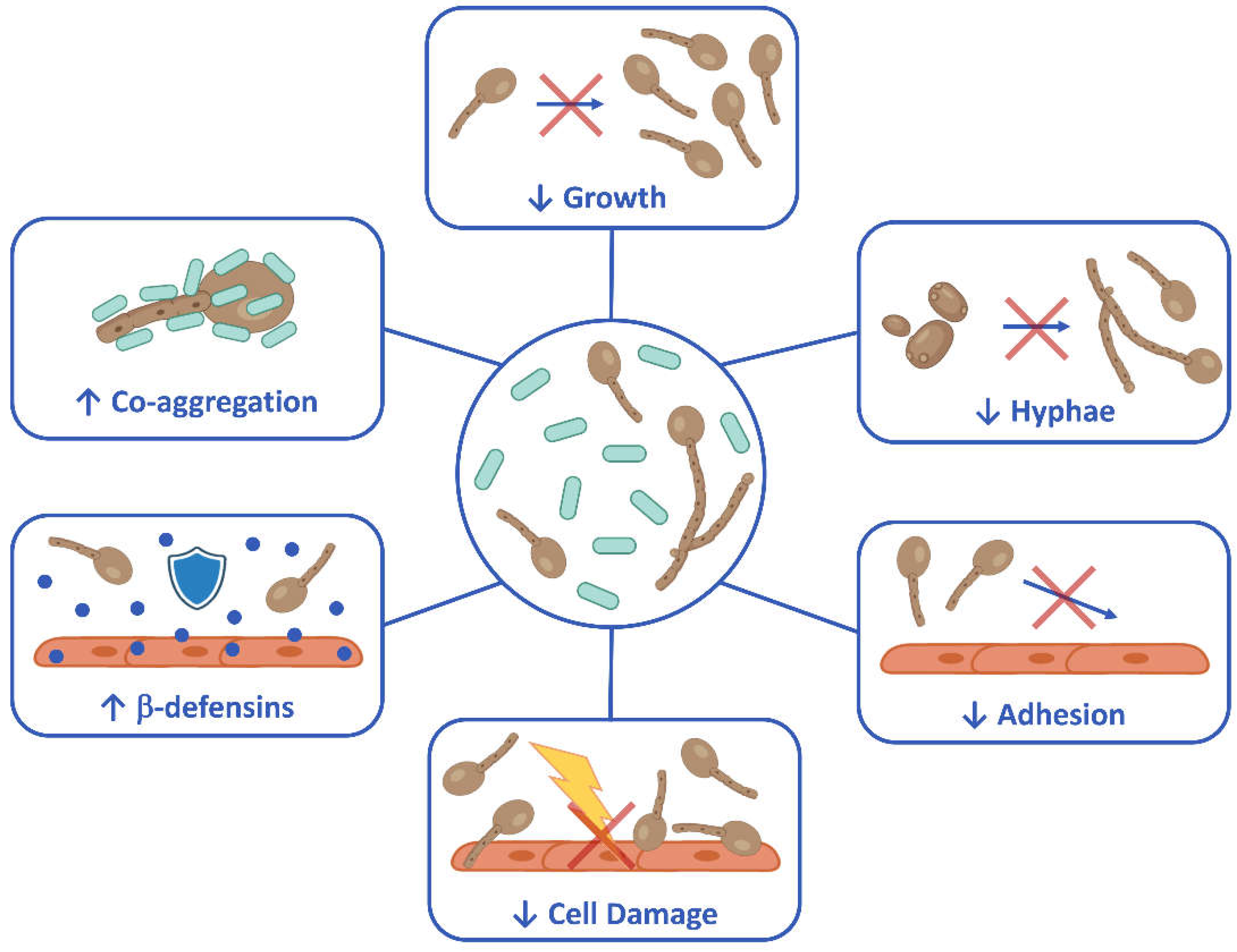
Here, we showed in vitro that Bacillus coagulans LMG S-24828 (recently reclassified as Heyndrickxia coagulans and marketed as Weizy® by Giellepi S.p.A.), counteracts Candida virulence and exerts a beneficial effect against vaginal epithelial cells infection by C. albicans. These promising preliminary data suggest that this bacterial strain has all the potentialities to be employed as a spore-forming probiotic for counteracting Candida spp. vaginal infections.
2. Materials and Methods
2.1. Microbial Strains and Growth Conditions
The strain of Bacillus coagulans LMG S-24828 (Weizy®) was provided by Giellepi SpA (Milan, Italy) in the form of lyophilized spores. Spores were stored at 4 °C and germination was obtained by incubation in Tryptic Soy Broth (TSB) (Condalab, Spain) for 24 hours at 37 °C, under agitation. Bacteria, obtained from spores’ germination, were used in their exponential growth phase for each experiment. The reference strains Candida albicans SC5314 (ATCC MYA-2876) and Candida parapsilosis CLIB214 (ATCC 22019) were employed. Both strains had been stored in frozen stocks at -80 °C in Sabouraud Dextrose Broth (SDB) (Condalab, Spain) supplemented with 15% glycerol. After thawing, the fungi were grown in Yeast Extract-Peptone-Dextrose broth (YPD) (Scharlab S.L., Spain) and incubated at 37 °C under aerobic conditions for 24 hours. Fungi in the exponential growth phase were used in the experiments.
2.2. pH Measurement of Microbial Culture
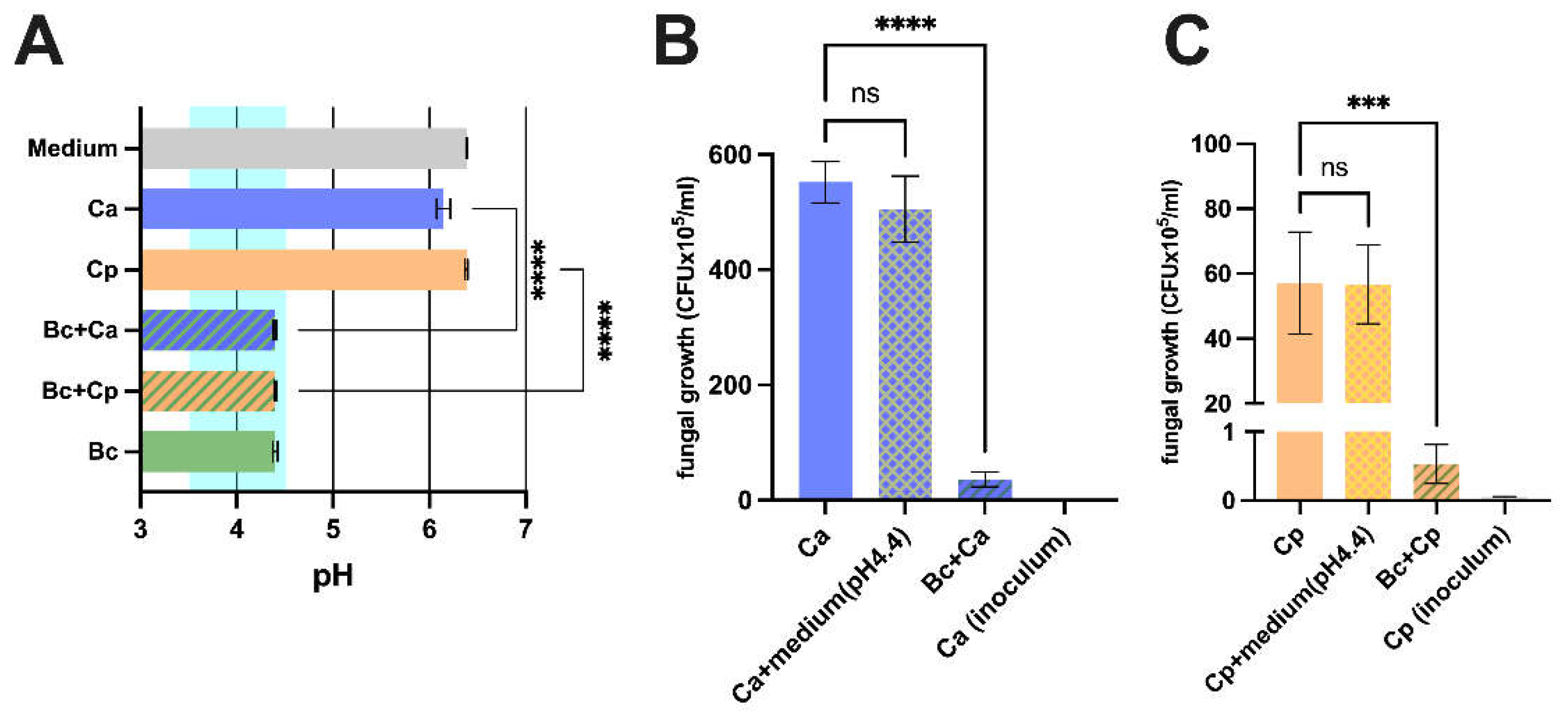
Bacillus coagulans (5x106 CFU/mL) and C. albicans or C. parapsilosis (both at 5x103 CFU/mL) were added to a medium consisting of 5 ml of YPD broth and 5 ml of TSB. The co-culture was incubated at 37 °C for 24 hours, under agitation. Samples containing only the bacterium, only the fungus, or sterile media were also included as controls. After incubation, samples were centrifuged at 4,000 rpm for 15 minutes and sterilized by filtration; then, pH was measured using a pHmeter (Hanna Instruments, Italy).
2.3. Analysis of Bacillus Coagulans Effect on Candida Growth
The impact of B. coagulans on C. albicans and C. parapsilosis growth was evaluated by incubating the fungi with or without the bacterium. Five hundred microliters of B. coagulans (5x106 CFU/mL) in TSB were seeded in a 24-well plate with 500 µL of C. albicans or C. parapsilosis (both at 5x103 CFU/mL) in YPD broth. As a control sample, both Candida species were incubated with sterile TSB without B. coagulans. To assess acidification contribution, 500 µL of both fungi were seeded with 500 µL of TSB that had been acidified with chloridric acid (Mallinckrodt Baker, USA) to pH 4.4. The plate was incubated at 37 °C for 24 hours. Then, culture supernatants were collected together with adherent fungi, that were detached by washing the wells with Soybean-Casein-Digest-Lecithin-Polysorbate-80 broth (SCDLP80, Biotec, Italy). The Candida burden was quantified by serial diluting the samples and seeding them on Sabouraud Dextrose Agar (SDA) (Condalab, Spain) supplemented with chloramphenicol (50 mg/l) (Biolife Italiana Srl, Italy). Colony Forming Units (CFU) were counted after 24-48 hours of incubation at 37 °C.
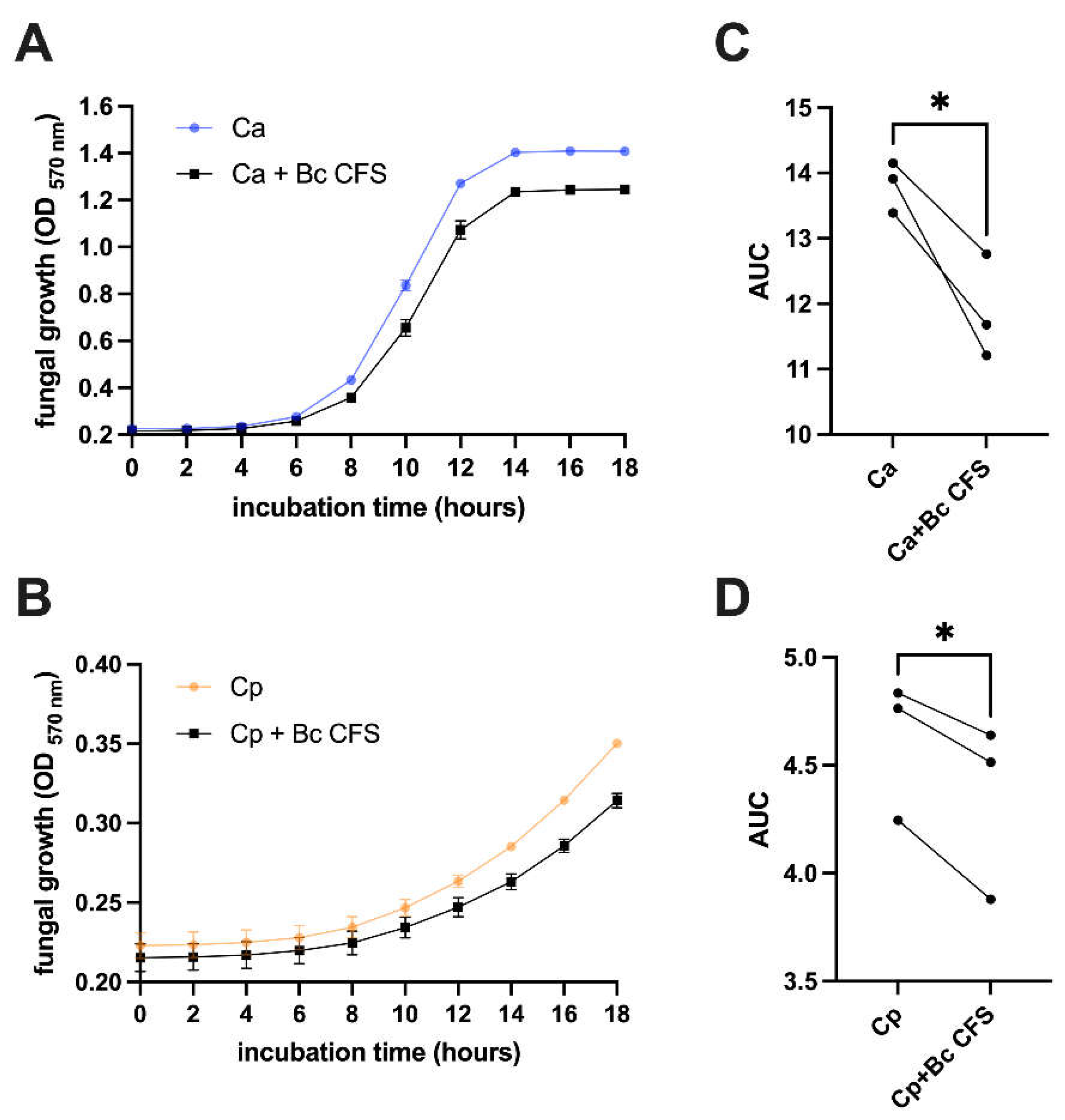
2.4. Preparation of Cell-Free-Supernatant (CFS) from Bacillus Coagulans and Its Effect on Candida Growth
The
B. coagulans broth culture obtained from spore germination, as detailed above, was centrifuged at 4,000 rpm at 4 °C for 15 minutes. Then, the supernatant was discarded, and the pellet was resuspended in 1 ml of TSB. Optical density (OD) at the wavelength of 595 nm was measured using a spectrophotometer (Sunrise, Tecan, Switzerland), and bacterial concentration was calculated from the OD values by means of a stored standard curve. Then, 5 ml of TSB containing
B. coagulans (1x10
8 CFU/mL) were incubated at 37 °C for 24 hours, under agitation. At the end of the incubation, CFS was obtained by centrifugation of the bacterial culture at 4,000 rpm at 4 °C for 15 minutes, as previously described [
22]. Briefly, the supernatants were collected, filter-sterilized with 0.22 µm syringe filters (Corning Incorporated, Germany) and stored at -80 °C until their use. The sterility of CFS was confirmed by incubating an aliquot of each CFS at 37 °C for 72 hours and then seeding it on Tryptic Soy Agar (TSA). The effect of CFS on
C. albicans and
C. parapsilosis growth was evaluated by seeding 100 µl of YPD containing
C. albicans or
C. parapsilosis (1x10
4 CFU/mL) in a 96-well plate, together with 100 µL of CFS (or the same volume of TSB in the control samples). The plate was incubated at 37°C and the cultures OD were kinetically measured (reading cycle: 2 hours) for 18 hours at the wavelength of 570 nm by a spectrophotometer (Sunrise, Tecan, Switzerland).
2.5. Bacillus Coagulans Impact on C. albicans Hyphae Formation
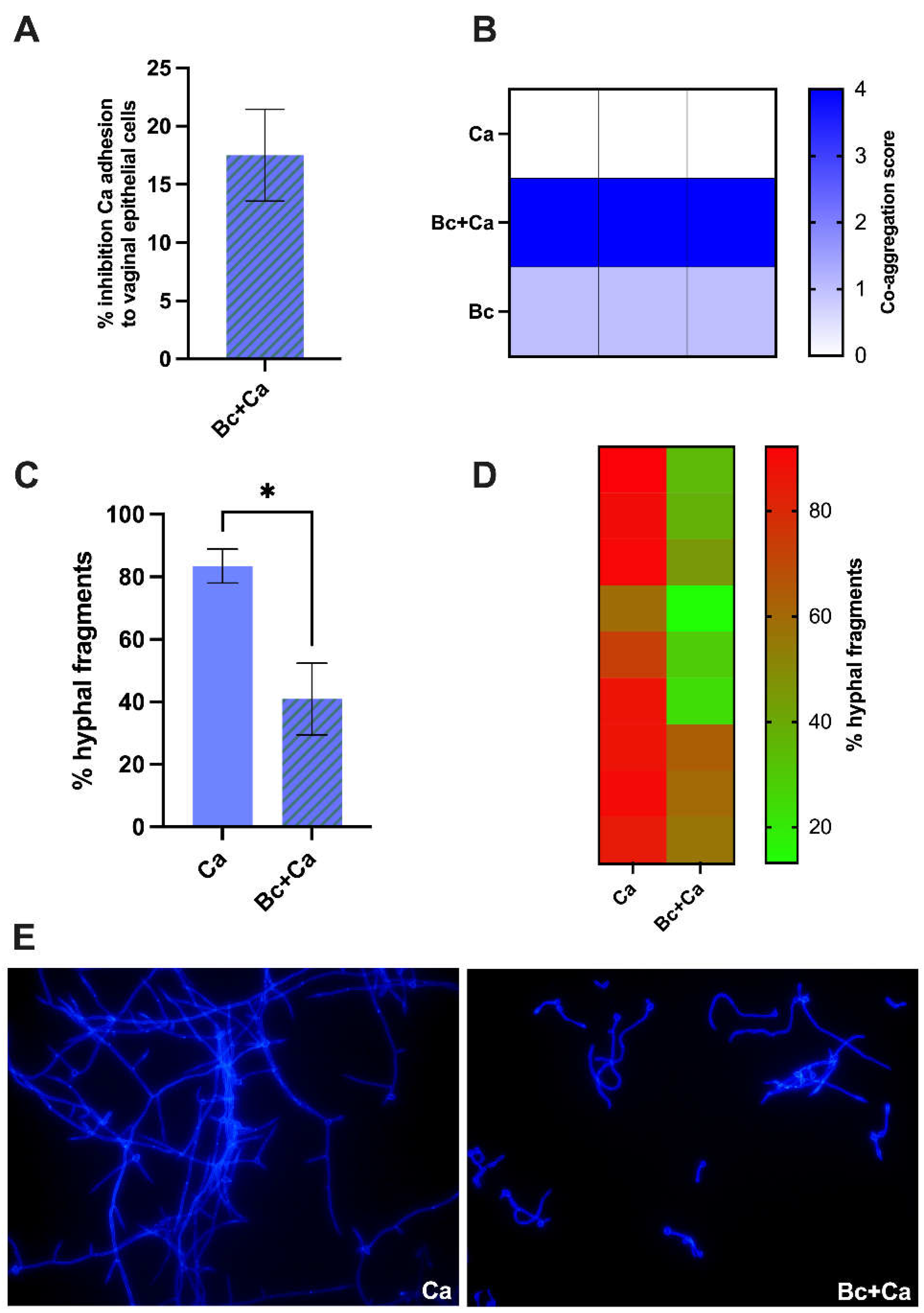
Filamentation assay was performed on chamber slides (Nunc Lab-Tek II, Thermo Fisher Scientific, USA). One hundred µL of C. albicans in YPD broth supplemented with 10% of fetal bovine serum (FBS) (SIAL Srl, Italy) were seeded in the well of the chamber slide along with 100 µl of B. coagulans in TSB supplemented with 10% FBS. As a control sample, the fungus was incubated with sterile TSB supplemented with 10% FBS. The chamber slide was placed at 37 °C with 5% CO2 for 4 hours. Fifteen minutes before the end of the incubation, 40 µL of 1% Uvitex 2B fluorescent dye (Polysciences, Inc, PA, USA) were added to each well. After the end of incubation, wells were gently washed twice with 200 µL of phosphate buffered saline (PBS) that had been warmed at 37 °C. Then, samples were fixed with 1% paraformaldehyde (200 µl/well, 30 minutes of incubation at 4 °C). Finally, wells were washed twice with 200 µL of cold PBS (4 °C) and they were allowed to dry. The chamber slide was then disassembled by removing the plastic frame that kept the wells isolated, and the slide surface was treated with ProLong Gold Antifade Reagent (Molecular Probes, Invitrogen, St. Louis, Mo, USA). The fluorescence of fungi was visualized by epifluorescence microscope Nikon Eclypse 90i (Nikon Instruments, Tokyo, Japan). Yeast cells and hyphal fragments were counted (at least three fields for each experimental condition) and the percentage of hyphal fragments in each sample was calculated.
2.6. Evaluation of Bacillus Coagulans Ability to Co-Aggregate with C. albicans
To establish if
B. coagulans was able to co-aggregate with
C. albicans, 50 µL of yeasts resuspended in PBS (2x10
7 CFU/mL) and 50 µL of
B. coagulans suspension in PBS (2x10
9 CFU/mL) were seeded in a U-bottom 96-well plate (Corning Incorporated, USA) and incubated in gentle agitation at 37 °C for 1 hour. Samples containing the bacteria, or the fungus alone were also included as controls. Co-aggregation rate was visually evaluated and scores from 0 to 4 were assigned, according to previous report [
19]. Briefly, score 0 means no aggregation; score 1 is characterized by aggregates with small clusters; score 2 is characterized by aggregates with larger numbers of yeasts; score 3 includes clumps visible with the naked eye, containing large numbers of yeast cells; score 4, the maximum, is assigned when large clumps become visible with the naked eye at the center of the well.
2.7. Long-Term Effect of Bacillus Coagulans on Candida Metabolism
To evaluate whether the B. coagulans - Candida co-incubation could determine a long-term effect on fungal metabolic activity, 500 µL of the C. albicans or C. parapsilosis (both 1x105 CFU/mL) in YPD broth were seeded in a 24-well plate (Sarstedt, Germany), 500 µL of B. coagulans (1x108 CFU/mL) in TSB were added to each well and incubation was carried out at 37 °C for 24 hours. In control samples, sterile TSB was added. At the end of incubation time, an aliquot of each sample was collected and seeded on SDA supplemented with chloramphenicol to exclude B. coagulans growth. Plates were incubated at 37°C for 24 hours. Plates were then washed to collect the grown fungi and 100 μl of both C. albicans and C. parapsilosis suspensions (1x106 CFU/mL) were seeded in DMEM supplemented with 5% FBS in a 96-well plate and incubated at 37 °C for 24 hours. Then, the medium was removed from each well, and 100 µL of 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT, Sigma Aldrich, USA) solution, supplemented with menadione (1 μM, Sigma-Aldrich, USA), were added. The plate was covered with tinfoil and incubated at 37 °C for 3 hours. Finally, 80 µL were collected from each well, transferred to another 96-well plate and the color intensity was quantified by measuring the OD at 492 nm wavelength.
2.8. Establishment of the Monolayer of Vaginal and Intestinal Epithelial Cells
Two different human epithelial cell lines were employed: A-431 cell line from vaginal epithelial squamous cell carcinoma (ATCC CLR-1555) and the CaCo-2 cell line from colon-rectal adenocarcinoma (ATCC HTB-37). Both cell lines were cultured in DMEM (SIAL Srl, Italy) supplemented with L-glutamine (2 nM) (Euroclone SpA, Italy), penicillin (100 U/ml) (Euroclone SpA, Italy), streptomycin (100 μL/ml) (Euroclone SpA, Italy), ciprofloxacin (20 mg/ml) (Euroclone SpA, Italy) and FBS (Fetal Bovine Serum, SIAL Srl, Italy). The cell lines were kept viable by sub-culturing twice a week and keeping them at 37 °C with 5% CO2. Epithelial cell monolayers were obtained by seeding the cells in a 24-well plate (Sarstedt, Germany) (5x105/mL, 1 mL/well) and by incubating the plate for 1 day (A-431) and 2 days (CaCo-2) at 37 °C with 5% CO2. Before being infected, the epithelial cell monolayers were washed with warmed PBS. DMEM supplemented with 10% FBS was employed for monolayer establishment, whereas DMEM supplemented with 5% FBS was used for the experiments. In the experiments including B. coagulans, antibiotics were not added to the medium.
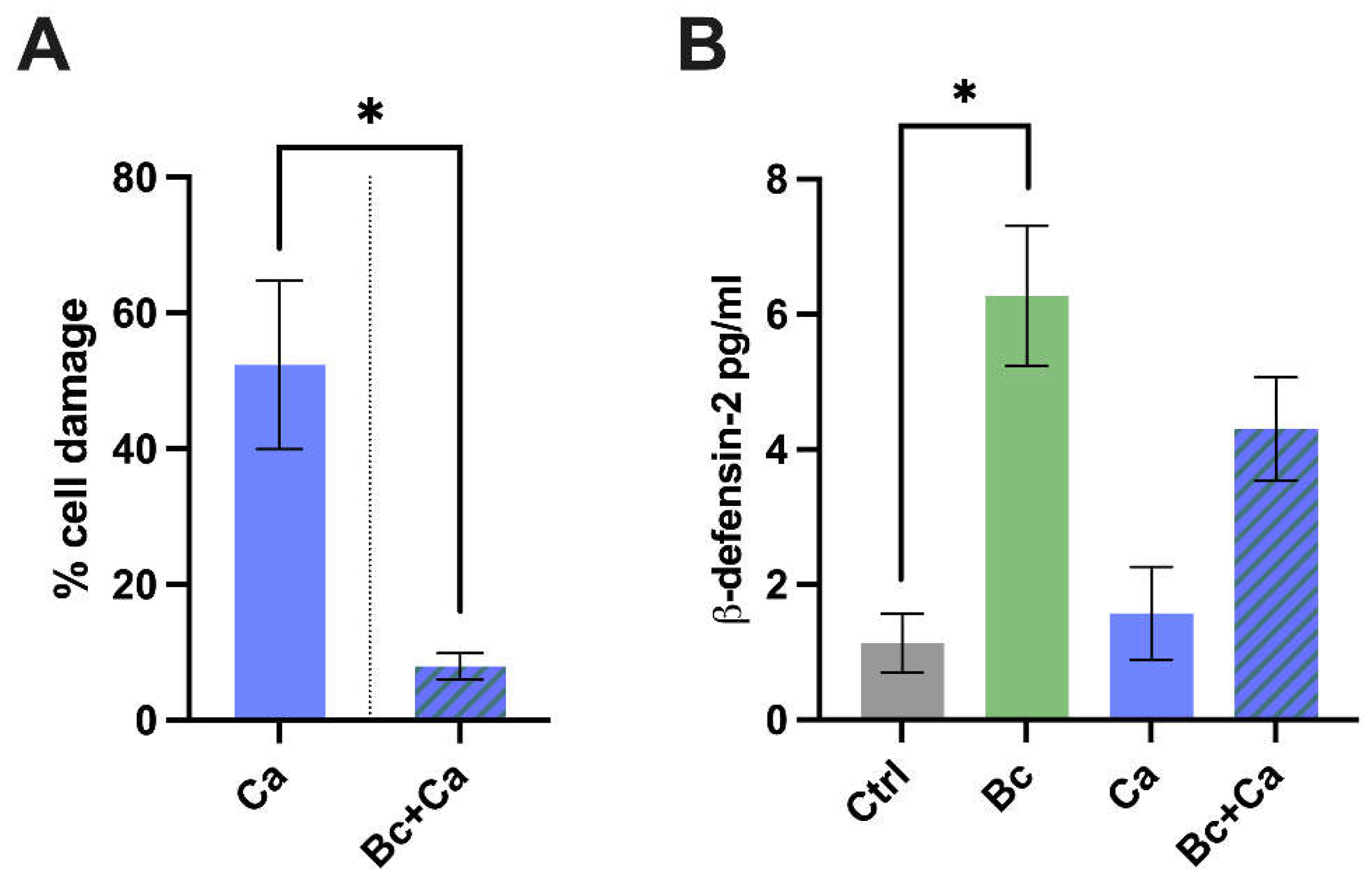
2.9. Effect of Bacillus Coagulans on C. albicans-Induced Vaginal Epithelial Cell Damage
Five hundred microliters of
B. coagulans were seeded on a vaginal epithelial cell monolayer (bacteria/vaginal cells, Multiplicity-Of-Infection (MOI 100/1) and incubated at 37 °C with 5% CO
2 for 6 hours. Five hundred microliters of
C. albicans were then added to each well (
C. albicans/vaginal cells, MOI 1/1), then the plate was incubated at 37 °C with 5% CO
2 for further 18 hours. After the incubation, lactate dehydrogenase (LDH) release in the surrounding medium was measured by a commercial kit (Hoffmann-La Roche, Switzerland). The percentage of damage was calculated following the manufacturer's instructions. Due to LDH activity impairment caused by acidification, a bacteria-acid-producer-specific protocol was employed for samples containing
B. coagulans, as previously described [
23]. Briefly, the culture medium of each well was removed, and wells were washed with warmed HBSS (Gibco, USA). Vaginal epithelial cells were then lysed with 0.1% Triton X-100 and the LDH assay was performed. Cell damage was calculated as the complementary percentage of the cell viability obtained by considering OD values from uninfected cells as 100%.
2.10. Impact of Bacillus Coagulans on C. albicans Adhesion to Vaginal Epithelial Cells
Five hundred microliters of B. coagulans (bacteria/vaginal cells, MOI 100/1) and 500 µL of C. albicans (C. albicans/vaginal cells, MOI 1/1) were simultaneously added to a vaginal epithelial cells monolayer and incubated at 37 °C with 5% CO2 for 2 hours. Cells devoid of bacteria and infected with the fungus were used as control. After the incubation, the culture medium was removed, and the wells were gently washed with warm PBS to remove non-adherent fungi. Vaginal epithelial cells were then lysed by adding 1 mL of 0.2% Triton X-100. Then, serial dilutions were performed and plated on SDA supplemented with chloramphenicol. CFU were counted after 24-48 hours of incubation at 37 °C. The percentage of fungal adhesion in samples containing C. albicans and B. coagulans was calculated by considering the mean CFU values of C. albicans control samples as 100%. Adhesion inhibition value was calculated by subtracting the percentage of adhesion from 100.
2.11. β-Defensin-2 Production after C. albicans Infection in the Presence of Bacillus Coagulans
The release of β-defensin-2 by vaginal epithelial cells, that had been pre-colonized with B. coagulans for 6 hours (bacteria/vaginal cells, MOI 100/1) and then infected with C. albicans (C. albicans/vaginal cells, MOI 1/1) for further 18 hours, were quantified by using a commercial ELISA kit (MyBioSource, USA) according to Manufacturers’ instructions.
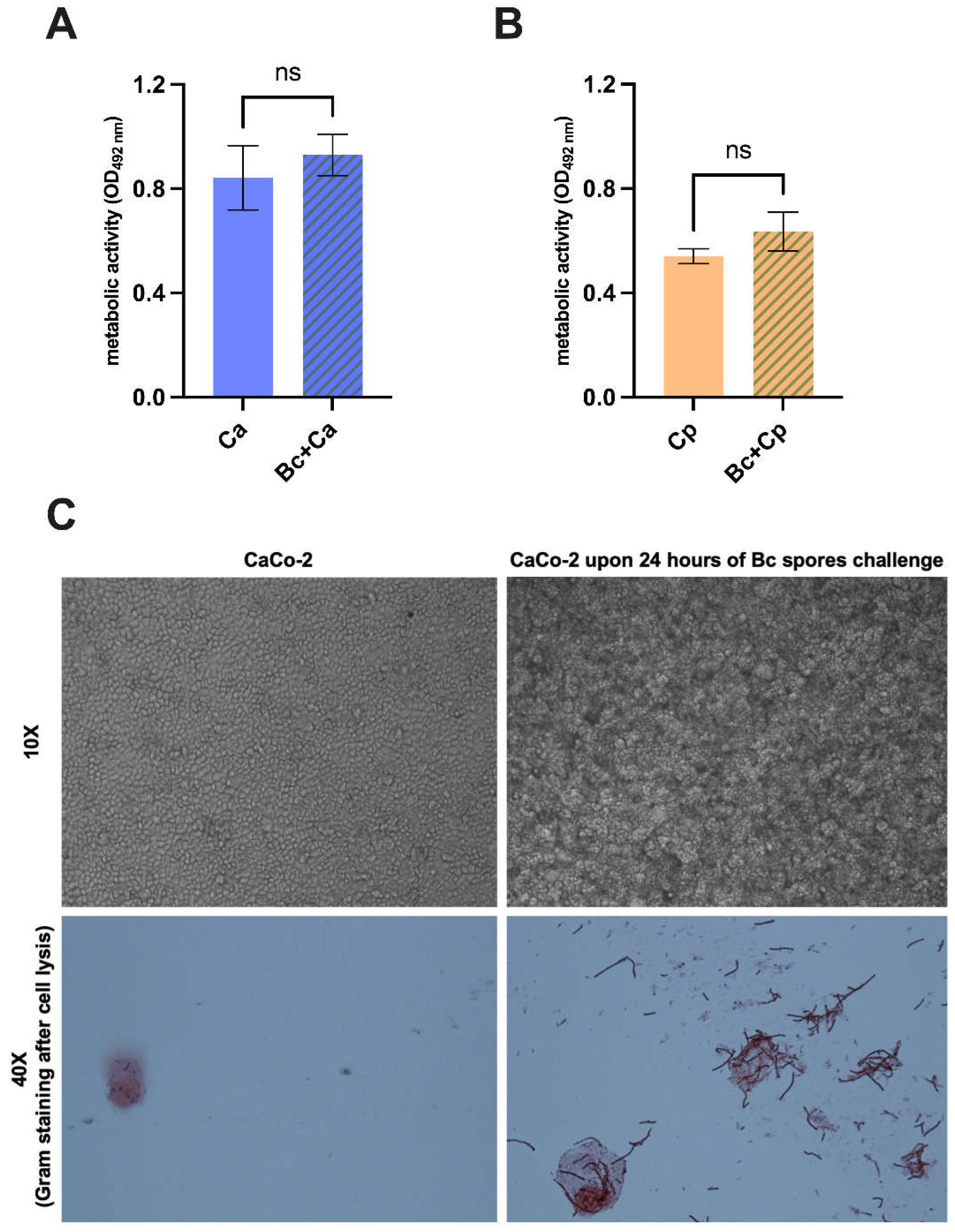
2.12. Evaluation of Bacillus Coagulans Spores’ Capacity to Germinate on the Intestinal Epithelial Cells
The CaCo-2 intestinal epithelial cell monolayer was challenged with 1 mL of DMEM-TSB (1:1) containing B. coagulans spores and incubated at 37 °C plus 5% CO2 for 24 hours. Cells incubated with sterile medium were included as control. After incubation, wells were visualized by an inverted-optical microscope (Nikon Eclipse TS100, Japan). Then, monolayers were lysed by adding 100 μl of 0.2% Triton X-100 to each well. After pipetting, 20 μl were deposited on a microscope glass slide and a Gram staining (Liofilchem, Italy) was performed. Slides were visualized using the Nikon Eclypse 90i (Nikon Instruments, Tokyo, Japan) microscope.
2.13. Statistical Analysis
All statistical analyses were carried out using GraphPad Prism 10 software. Gaussian distribution of data was confirmed by the Shapiro-Wilk test. Statistical analysis of normally distributed data was performed by using the unpaired, two-tailed, Student’s t-test or the one-way ANOVA test followed by the uncorrected Fisher’s LSD multiple comparisons tests. Data with no Gaussian distribution was analyzed by the Kruskal-Wallis test followed by the uncorrected Dunn’s multiple comparisons test. The specific statistical analysis performed in each experiment has been detailed in the figure legends. Values of *p < 0.05, ***p < 0.001, and ****p < 0.0001 were considered statistically significant.
4. Discussion
Probiotics are living microbes that confer a health benefit to the human host. The anti-pathogen activity, the immunomodulatory effect, and the tolerated proliferation within the human organism are some of the acknowledged key features of probiotics. Research on probiotics has rapidly increased over the last few decades, and probiotic administration is considered as a promising therapeutic approach to treat various diseases. Yet very little information is available to date on probiotics efficacy in treatment and prevention of VVC. In the present study, we have investigated the probiotic potentiality of
Bacillus coagulans (
B. coagulans) LMG S-24828 (Weizy®) in the contest of
Candida-associated vaginal infections. We started by evaluating the capacity of
B. coagulans to affect
Candida virulence traits related to invasiveness and pathogenicity, such as fungal growth, adhesion to epithelial cells and dimorphic transition. By employing a vaginal epithelial cell infection model, we assessed the potential beneficial and immunomodulatory properties of
B. coagulans in the context of vaginal infection by
C. albicans. A healthy vaginal environment is characterized by an acidic pH with values around 4. The acidification is granted by resident bacteria, such as lactobacilli, that produce lactic acid as the end-product of their fermentative metabolism. In addition, several other species, included in commercial probiotic formulation contribute to the acidification of the vaginal environment, thus maintaining or restoring eubiotic conditions. Recently, it has been demonstrated that
B. coagulans too is able to produce lactic acid [
24,
25]. In line with such evidence, here we show that
B. coagulans is capable to induce acidification to pH values similar to those occurring in an eubiotic vaginal milieu; in addition, such acidification capacity is not hindered by co-culturing
B. coagulans with
C. albicans or
C. parapsilosis. Such capacity to provide an acidic environment is a necessary feature (albeit not sufficient
per se) of every probiotic formulation intended for the recovery of vaginal eubiosis, which explains the importance of this result.
Candida can physiologically colonize a healthy vaginal mucosa without inducing symptoms, thanks to a constant inhibition of its overgrowth granted by the immune system and by the resident microbiota [
26]. Therefore, antiproliferative activity against
Candida is another pivotal attribute of probiotic formulations intended to treat or prevent vaginal infections. Our results show that
B. coagulans massively affects
Candida growth capacity, and this effect is not due only to the mere acidification of the medium, since both
C. albicans and
C. parapsilosis have been able to grow on
B. coagulans-free medium, acidified to pH of 4.4. Interestingly, the evaluation of anti-proliferative activity mediated by Cell-Free Supernatant (CFS) obtained from
B. coagulans culture, showed that even metabolites produced by this bacterial strain can reduce fungal growth. Therefore, it is possible that the acidification induced by
B. coagulans per se may affect
Candida, but that other metabolites must be involved to obtain an effective impairment of fungal growth. This would explain why
Candida growth is not inhibited in a
B. coagulans-free acidified medium. A similar effect has been described also for
C. parapsilosis [
22]. Besides supporting the probiotic features of
B. coagulans LMG S-24828, these findings also suggest possible postbiotic characteristics.
The capacity to adhere is the first step for
Candida to begin the infection process. Here, we show that
B. coagulans impairs
C. albicans adhesion and this seems to be due mostly to its capacity to co-aggregate
Candida. Indeed, co-aggregation is an important mechanism used by probiotics to clear microbial pathogens: through co-aggregation, the probiotics can create a competitive microenvironment that compromises the capacity of pathogens to adhere to epithelial cells. This is, ultimately, an effective means for preventing
Candida adhesion and infection [
19].
As a dimorphic fungus,
C. albicans can exist in two different morphologies: yeast cells or hyphal forms. When
C. albicans dwells in the vaginal niche of healthy women as a harmless colonizer, the yeast form is prevalent and, in such form,
Candida is well tolerated by the genital mucosa. Differently, the hyphal form becomes prevalent during symptomatic VVC episodes. The elongation of hyphal structures causes an invasion of epithelial cells, induces neutrophil recruitment and triggers a wide inflammatory response due to the high levels of cellular damage [
27]. Moreover, the presence of hyphae is linked to the production of candidalysin, the only toxin demonstrated to be produced by
C. albicans hyphae, that exerts cytolytic activity and induces cell damage through the induction of mtROS by vaginal epithelial cells [
28,
29]. Here, we demonstrate that the co-culture of
C. albicans with
B. coagulans dampens the hyphal elongation capacity, as shown by the observation of shorter and fewer hyphal fragments by fluorescence microscopy. In such impairment effect on
C. albicans dimorphic transition, an important role may be played by the acidification induced by
B. coagulans. Indeed, several studies report that acidic pH values inhibit
C. albicans to switch from yeast to hyphal form [
30,
31]
To contextualize the observed anti-
Candida effects of
B. coagulans in the VVC scenario, we have employed an
in vitro infection model consisting of a vaginal epithelial cell monolayer infected with
C. albicans in the presence or absence of
B. coagulans. By this model, we have demonstrated that upon fungal challenge the presence of the
Bacillus correlates with a reduction of epithelial cell damage induced by the fungus. In addition, we have assessed the production of AMPs by vaginal epithelial cells, in order to establish if
B. coagulans is capable to alert them of an impending danger and to stimulate a response. Indeed, our data show that following pre-colonization with
B. coagulans, the epithelial cells get activated and increase the release of the alarmin β-defensin-2, a molecule with a potent antimicrobial activity against Gram negative bacteria and
Candida [
32,
33].
VVC cases due to non-
albicans Candida species have always been uncommon, yet they are progressively increasing. Non-
albicans Candida species have been demonstrated to become resistant to antifungals at a higher rate than
C. albicans [
34]; hence, to prevent a
Candida infection by the use of probiotics may provide beneficial effects especially against drug-resistant species. For this reason, notwithstanding the main goal of this project is the demonstration of the beneficial effects of
B. coagulans LMG S-24828 against
C. albicans, a
C. parapsilosis reference strain was also included in some of the experiments described above. Interestingly, the effects of
B. coagulans on
C. parapsilosis are superimposable to the effects on
C. albicans. Indeed, the hindering of both
C. albicans and
C. parapsilosis growth to a similar extent, suggests that the effects of
B. coagulans LMG S-24828 are not species-specific, and they might affect a broad spectrum of
Candida species.
A complex crosstalk links the gut to the vaginal microbiota, involving a continuous bacterial species translocation through these body sites. Indeed, the origin of vagina colonizer bacteria has been traced to the rectum, which also acts as a reservoir. In addition to the migration made possible by the anatomical proximity, also bacterial hematogenous transfer from the gut to the uterus has been described [
12]. Such crosstalk is an essential feature, because most probiotic formulations are typically assumed by ingestion, and the proliferation of beneficial bacteria, as well as the germination of the spores, occur on the surface of intestinal mucosa. For spore-based probiotic formulations in particular, an efficient germination on intestinal epithelial cells is essential in targeting beneficial bacteria to the gut. Our results show that the spores of
B. coagulans LMG S-24828 can germinate on the intestinal epithelial cell line CaCo-2, which makes this strain suitable to be employed as an effective probiotic to prevent and treat vaginal epithelial infections.