Submitted:
01 August 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
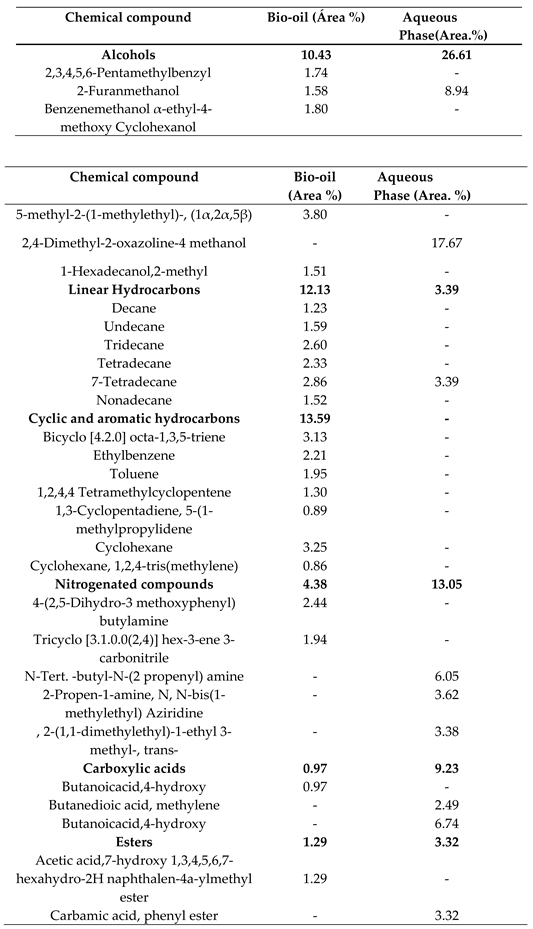
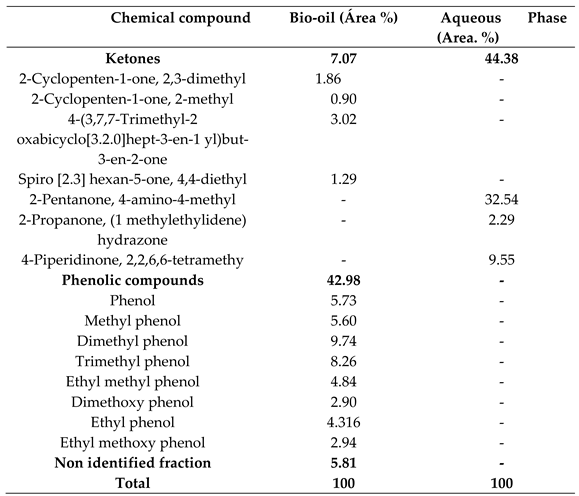
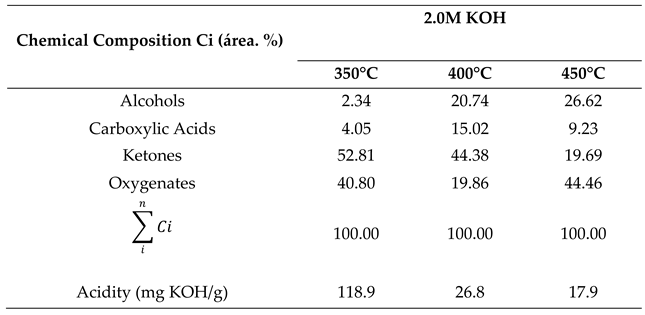
2.1. Chemical Composition of Bio-Oil by Gas Chromatography-Mass Spectrometry (GC-MS)
2.1.1. Effect of Temperature on the Chemical Composition of the Products
2.2. Evaluation of the Antioxidant Potential of the Bio-Oil
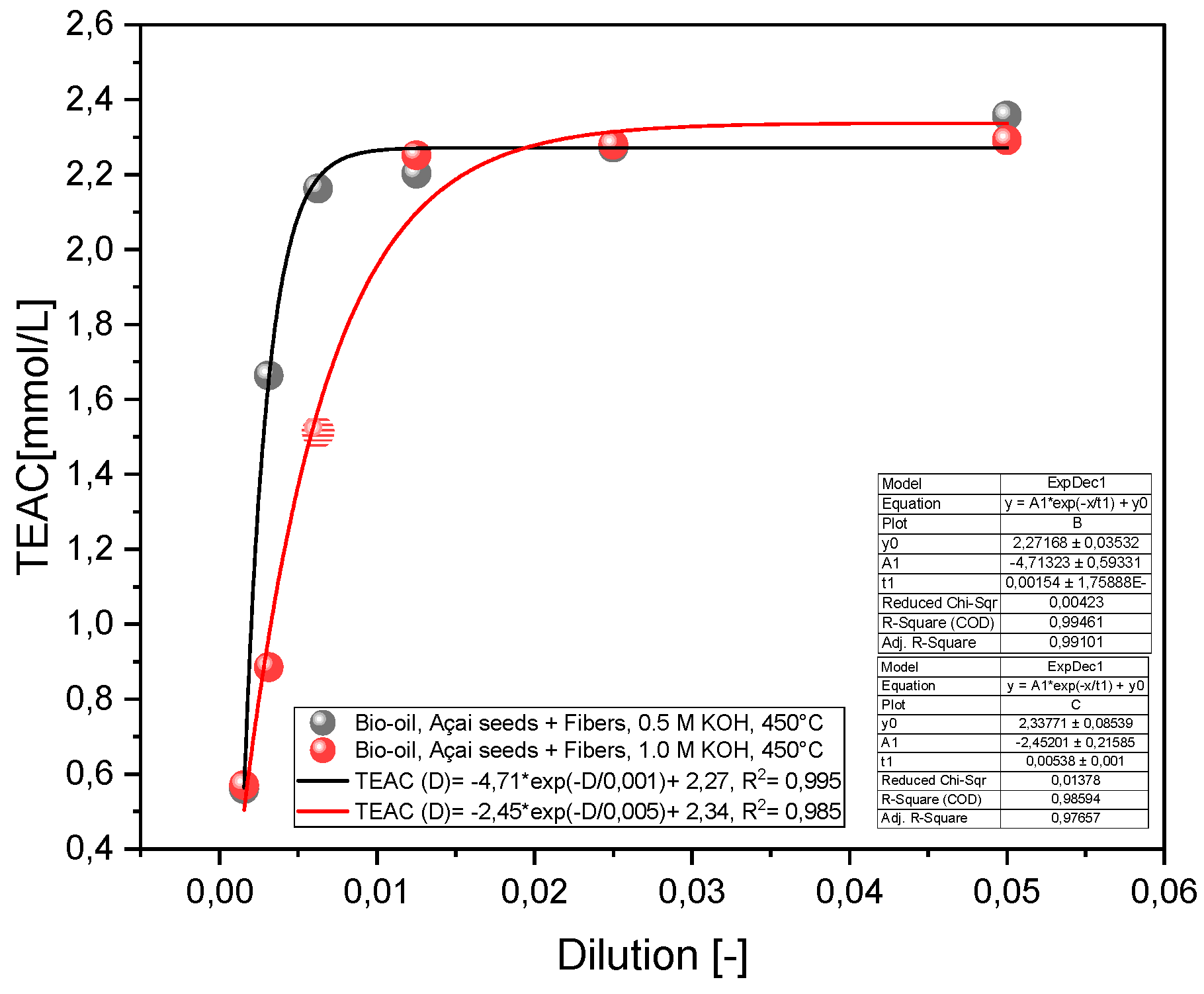
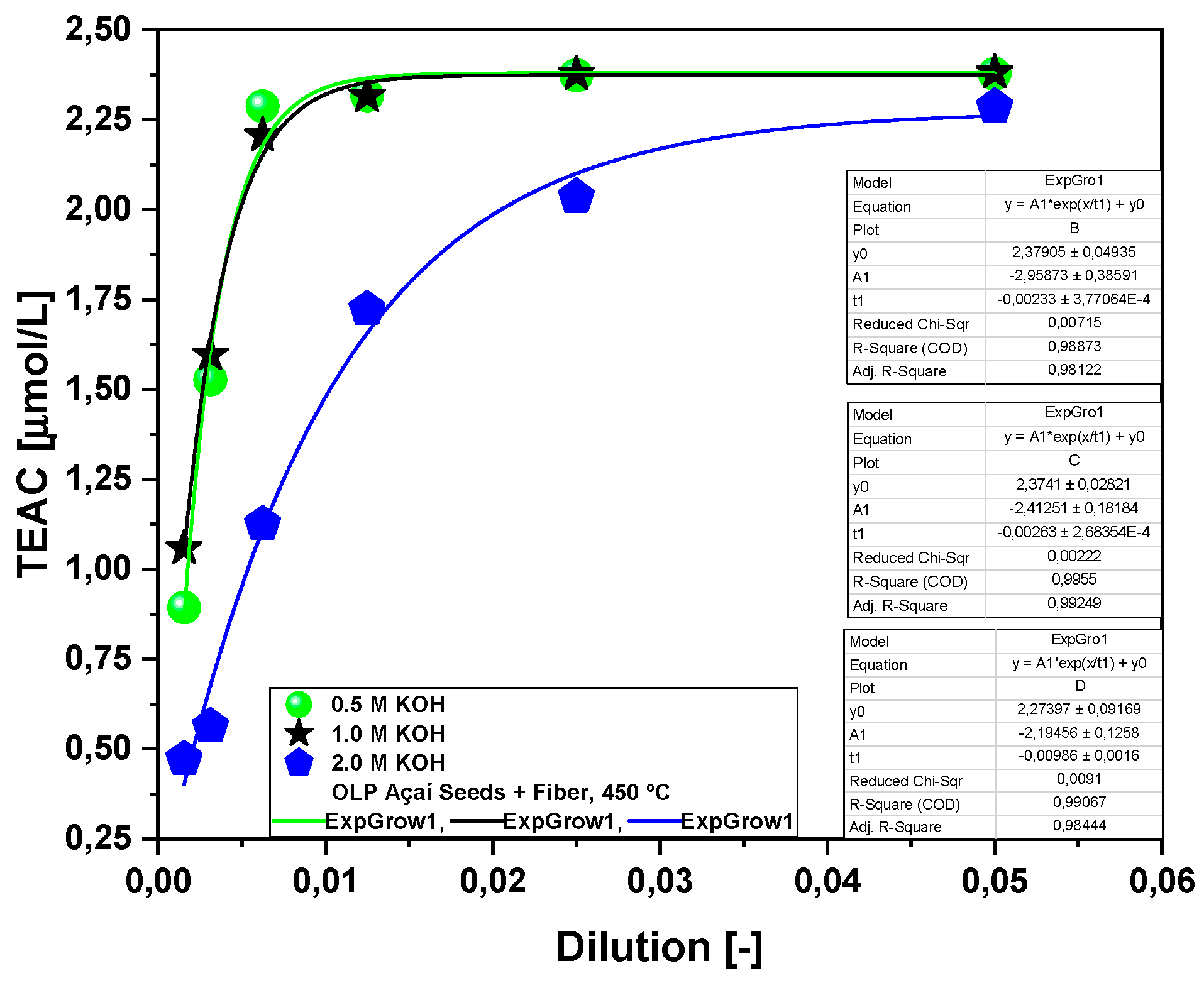
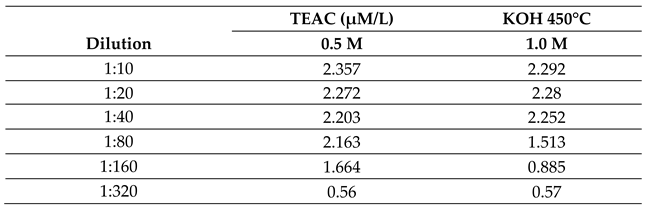
2.2.1. Effect of Temperature on the Antioxidant Activity of the Bio-Oil Using the TEAC Method
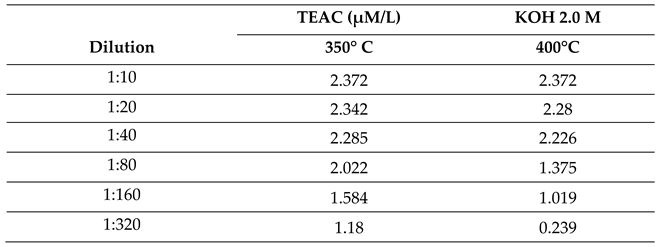
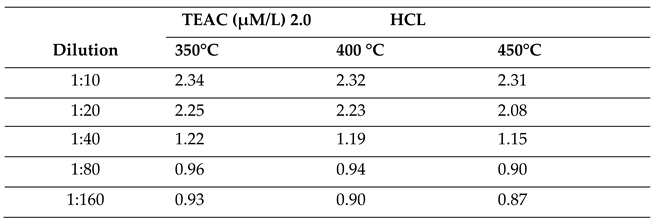
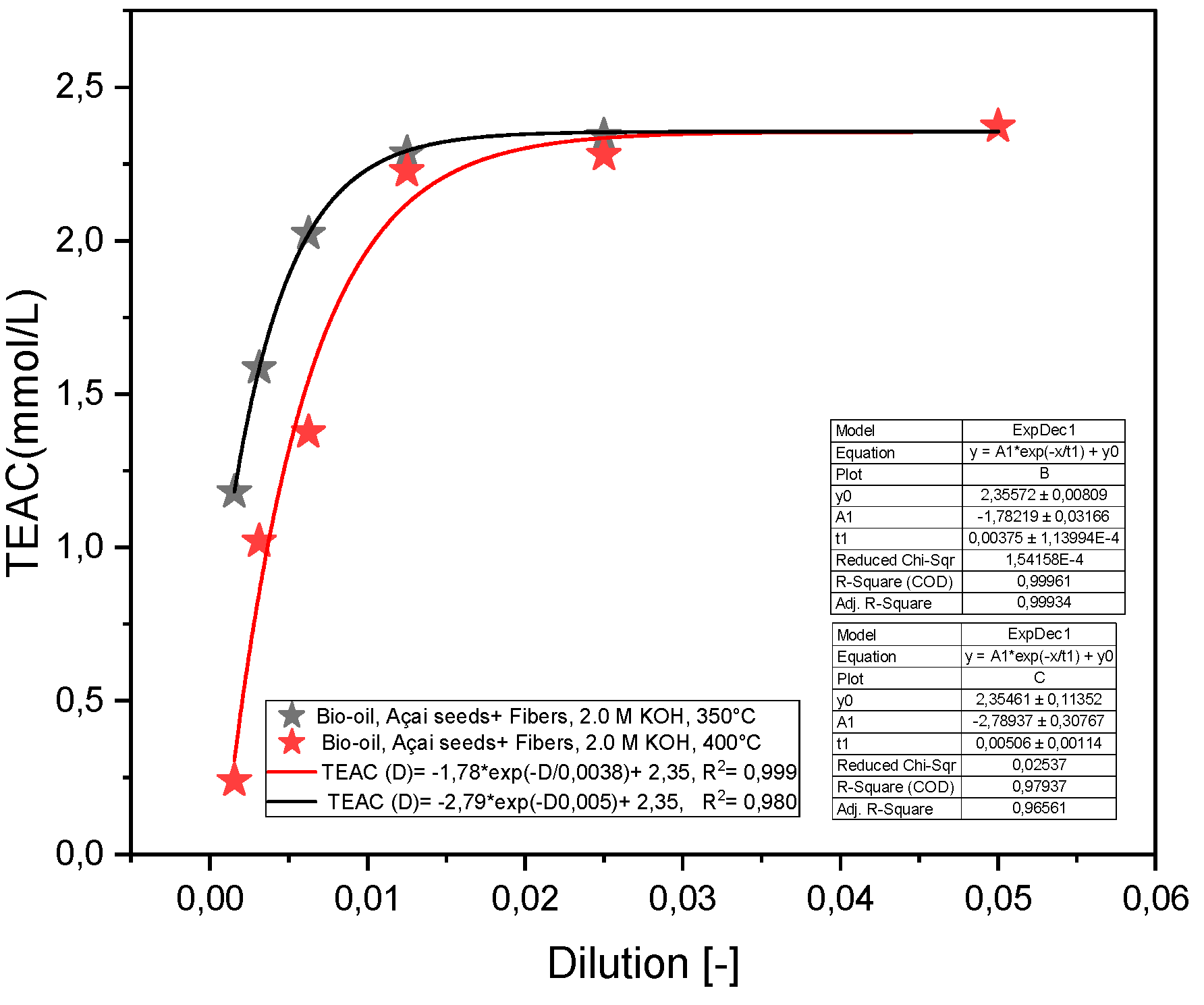
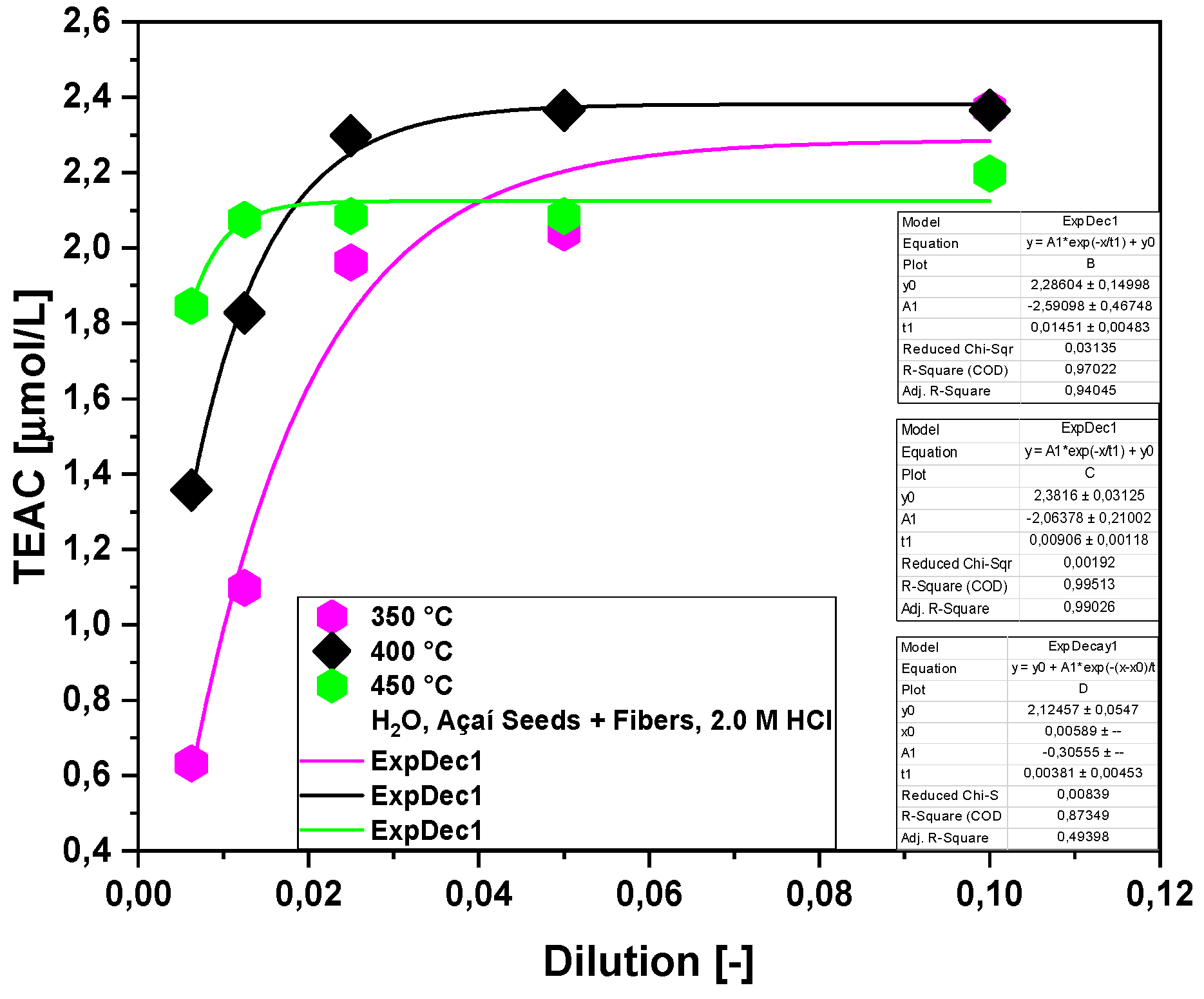
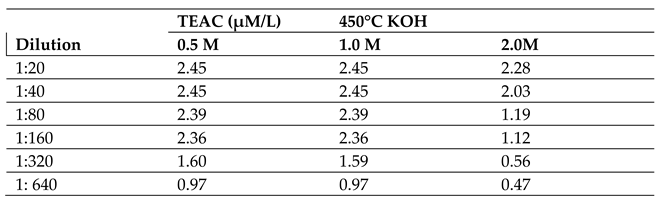
2.2.2. Effect of Molar Concentration on the Antioxidant Activity of Bio-Oil Using the TEAC Method
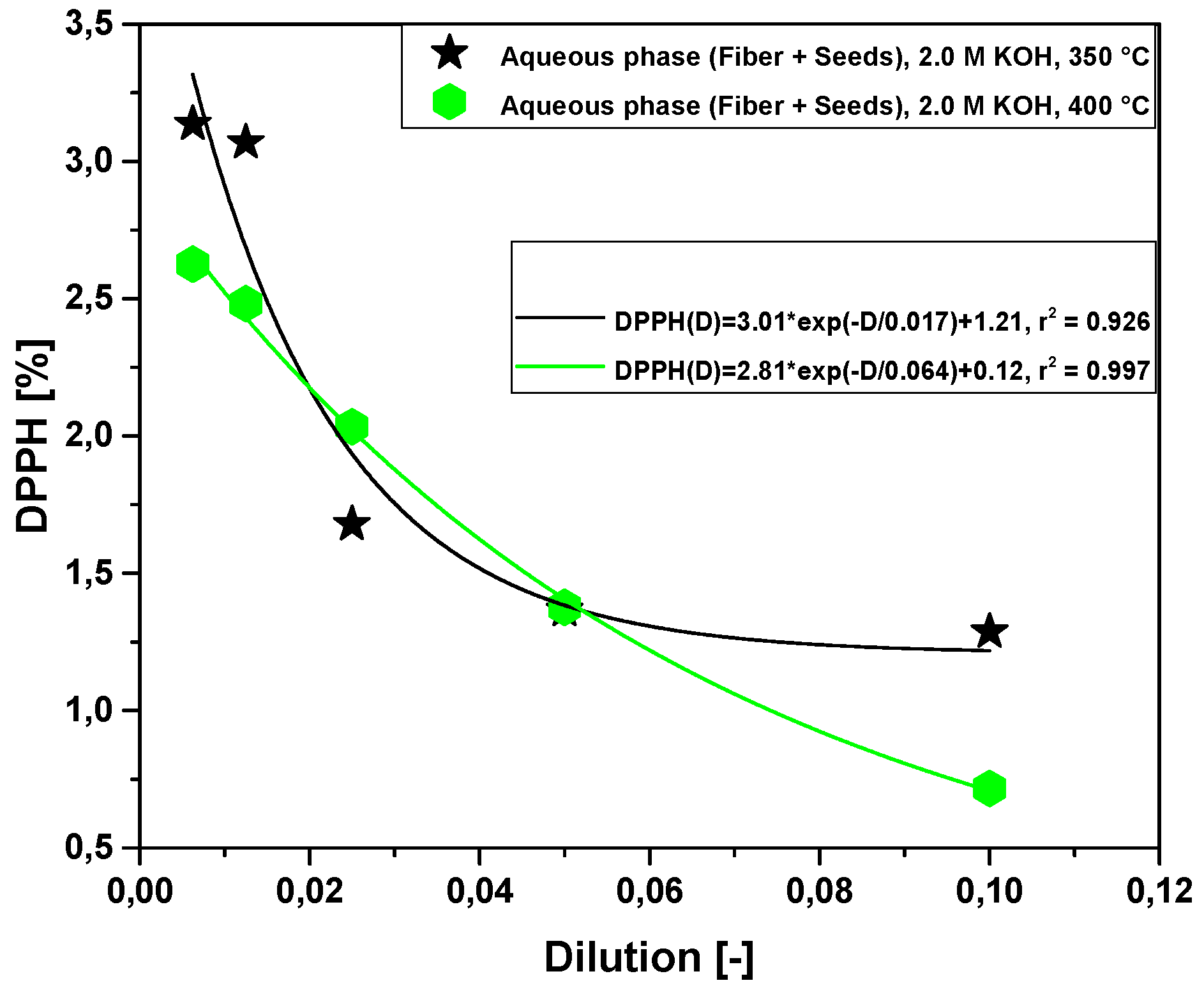
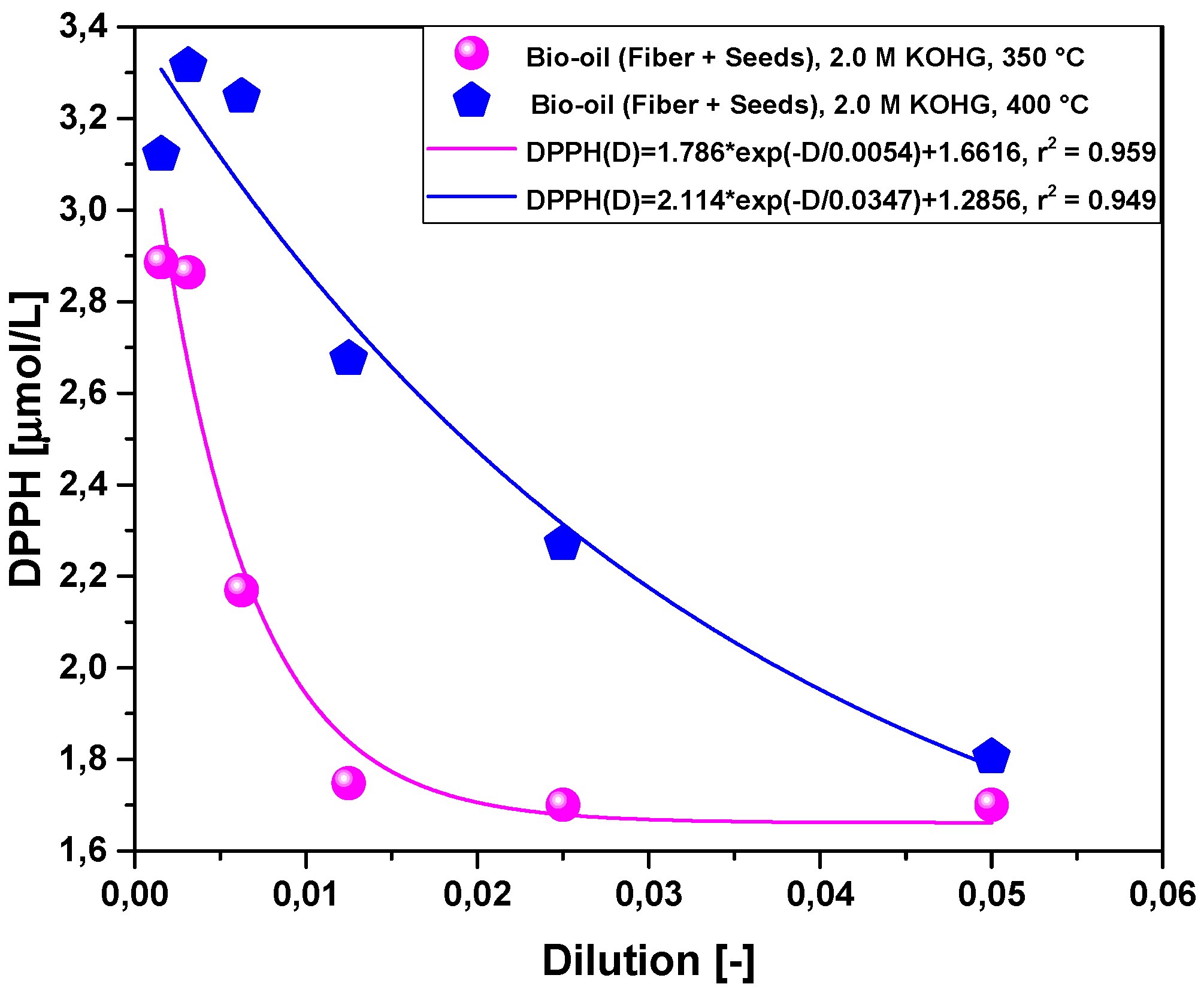
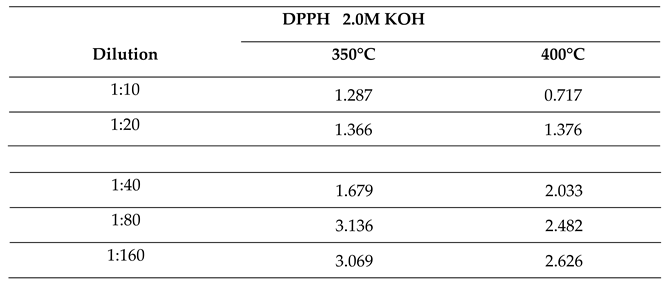
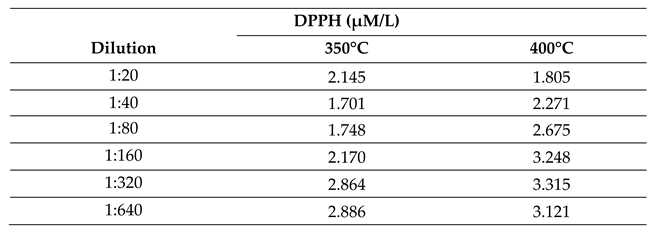
2.2.3. Effect of Temperature on Antioxidant Activity of Bio-Oil Using the DPPH• Method
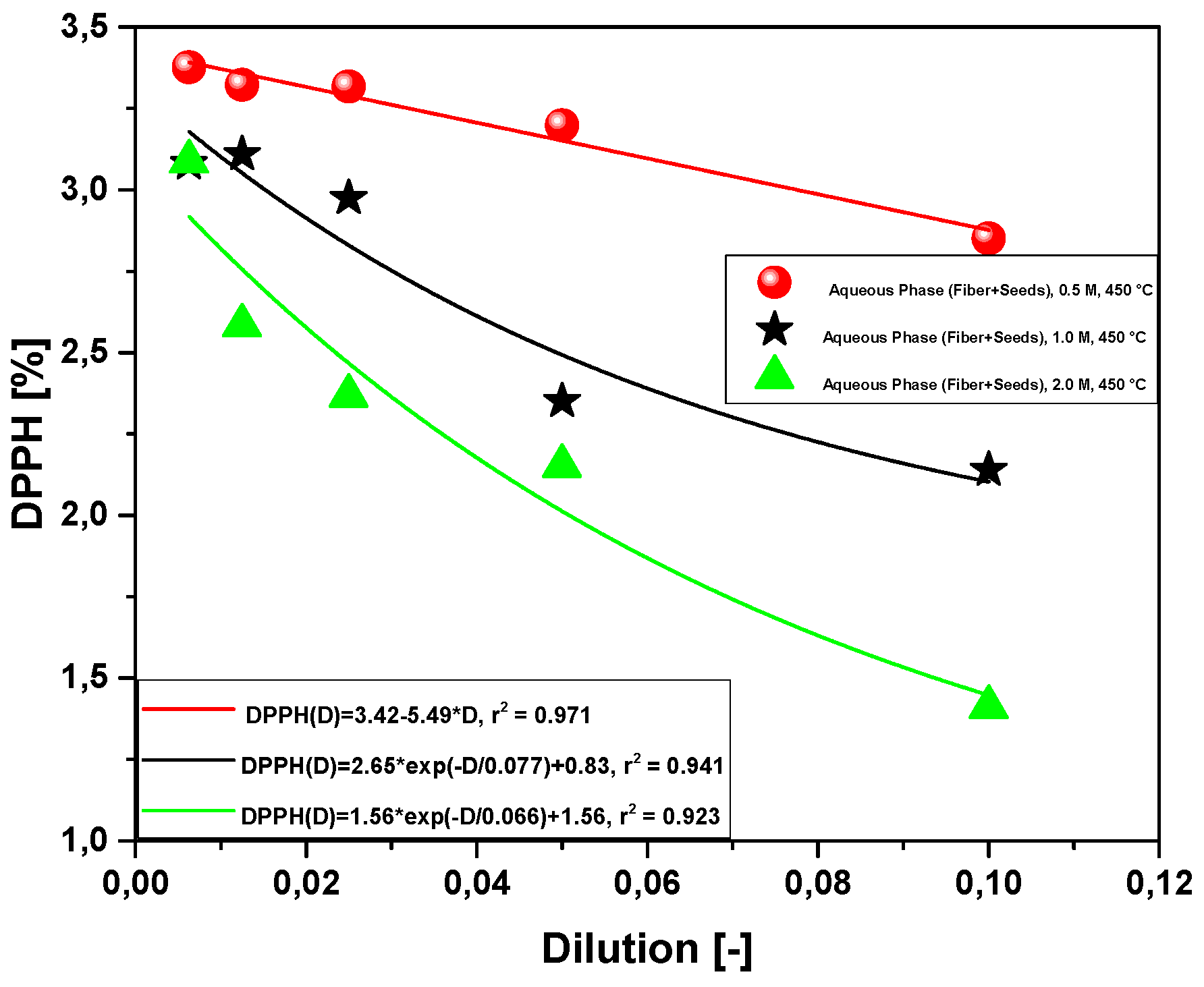
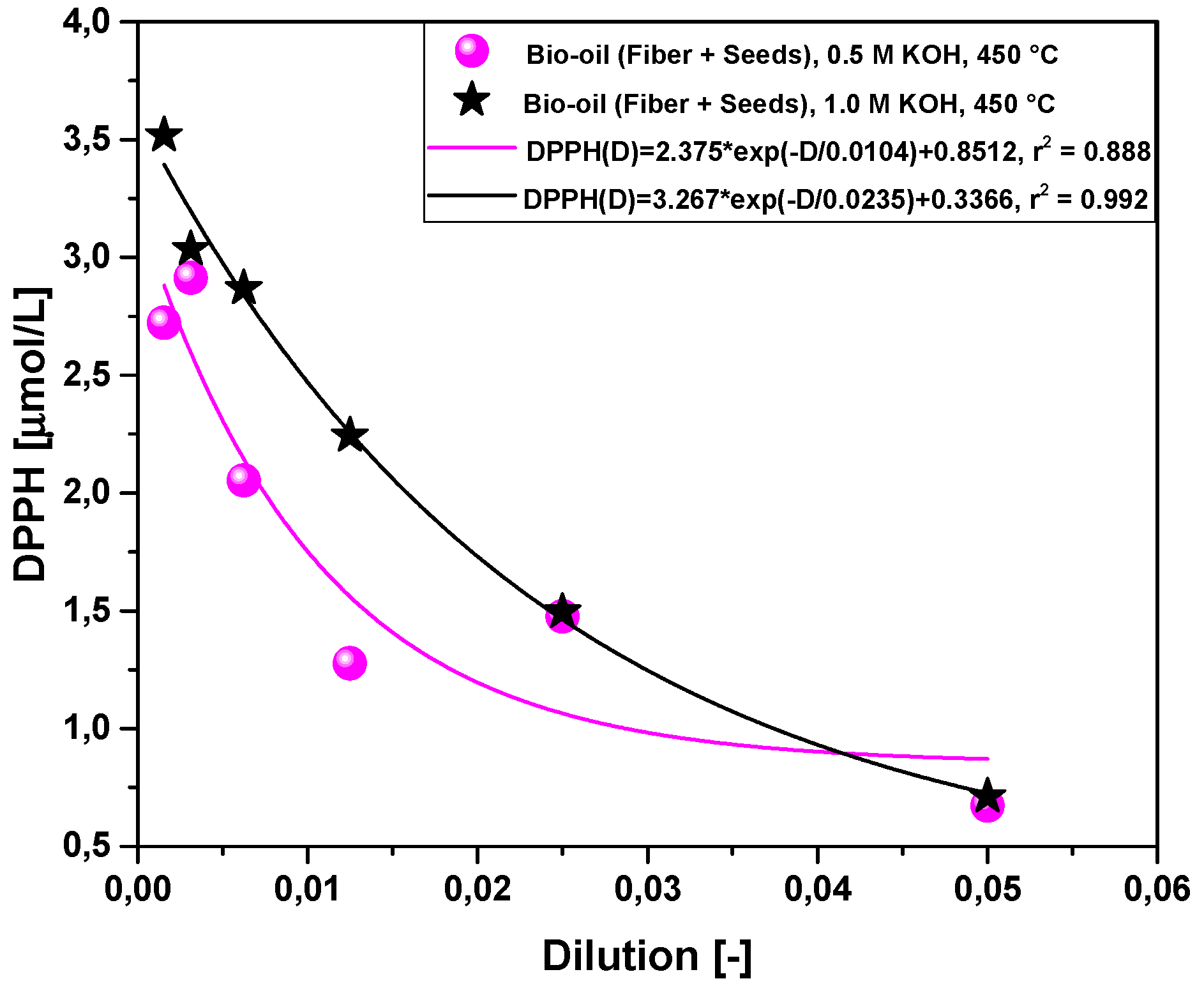
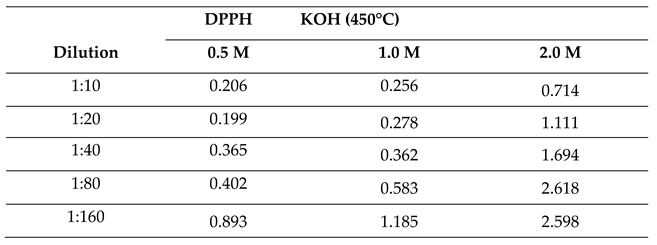
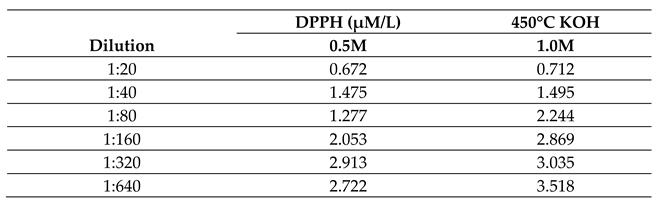
2.2.4. Effect of Molar Concentration on the Antioxidant Activity of Bio-Oil Using the DPPH Method
2.3. Evaluation of the Antimicrobial Activity of Bio-Oil
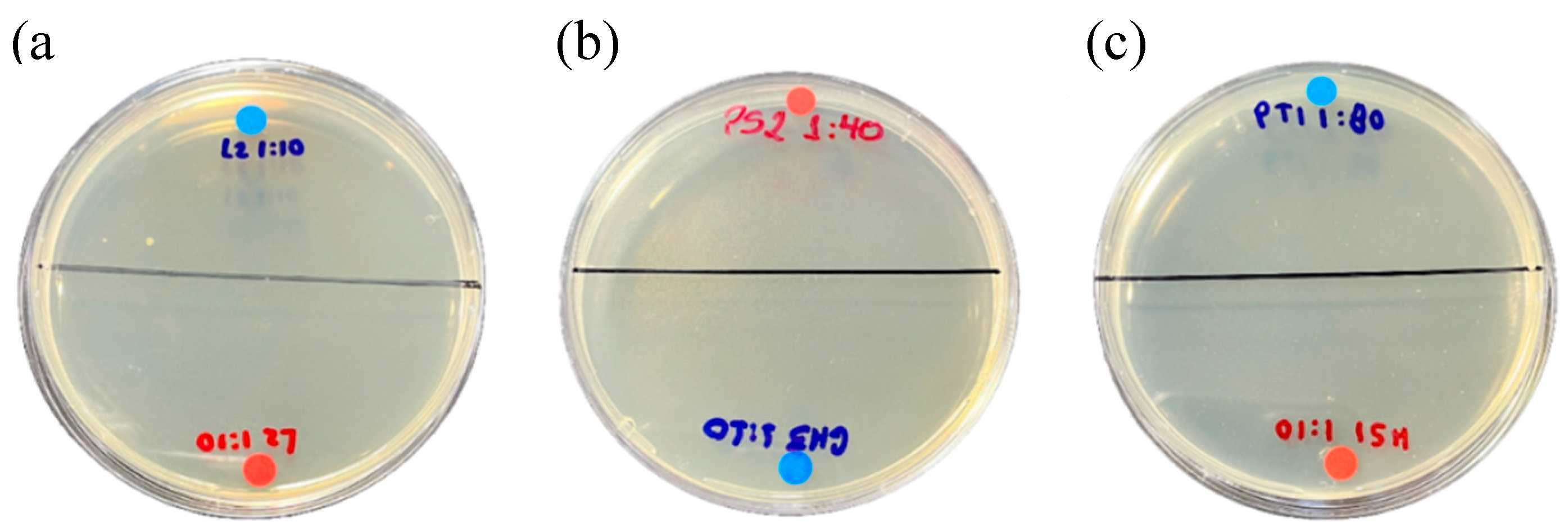
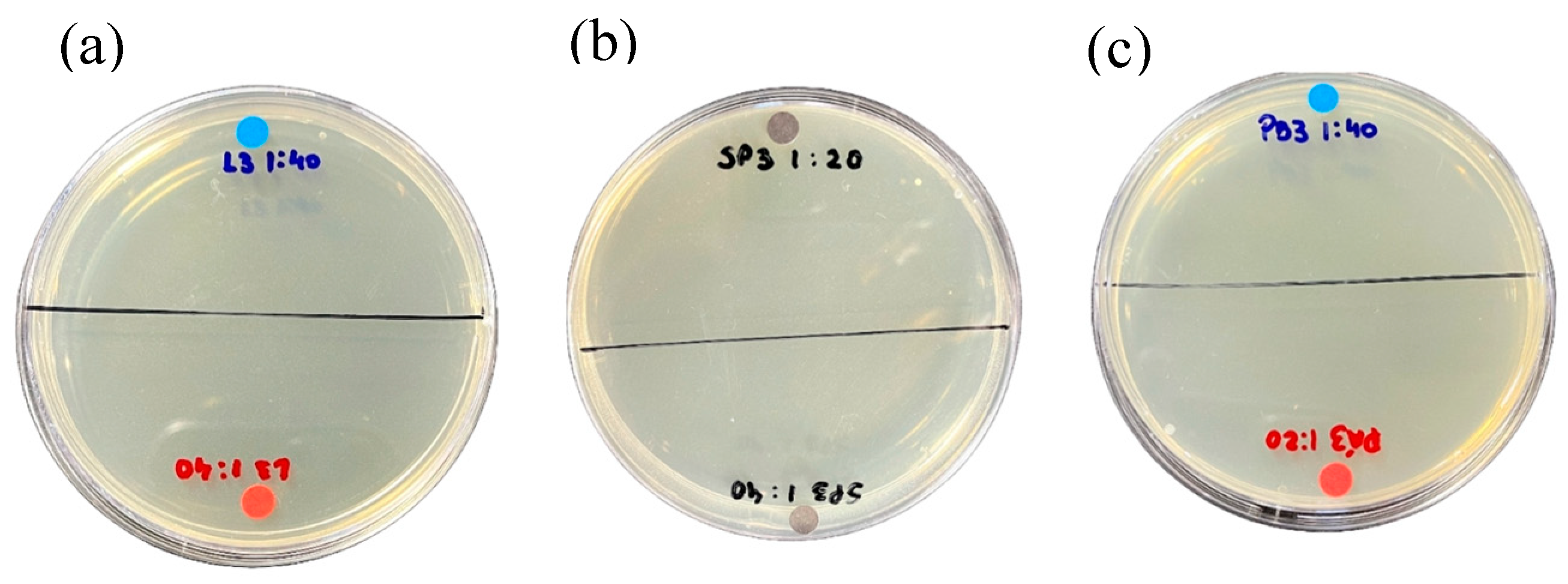
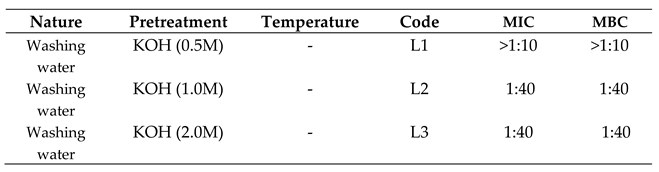
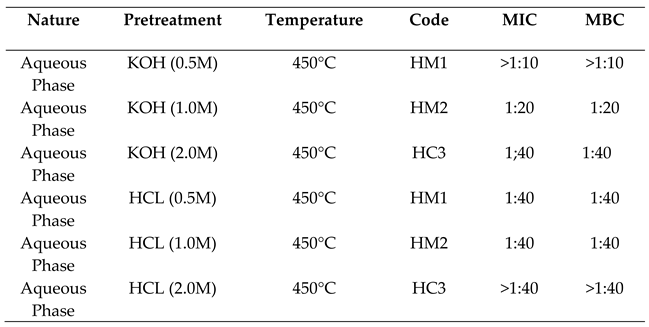
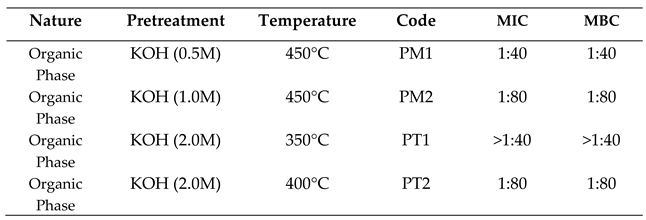
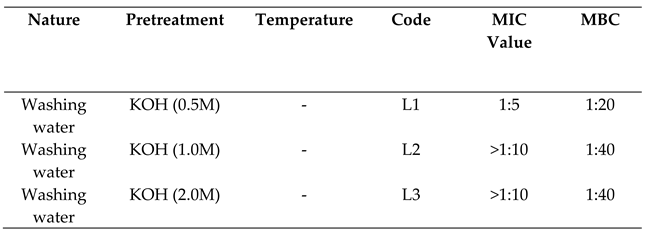
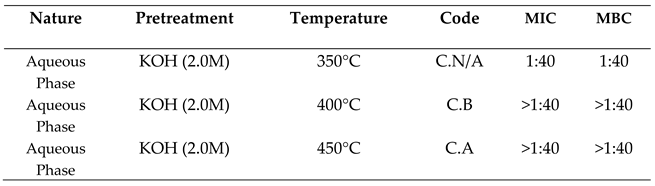
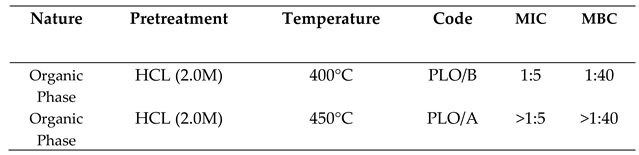
2.3.1. Activity against Escherichia Coli
2.3.2. Activity Against Staphylococcus Aureus
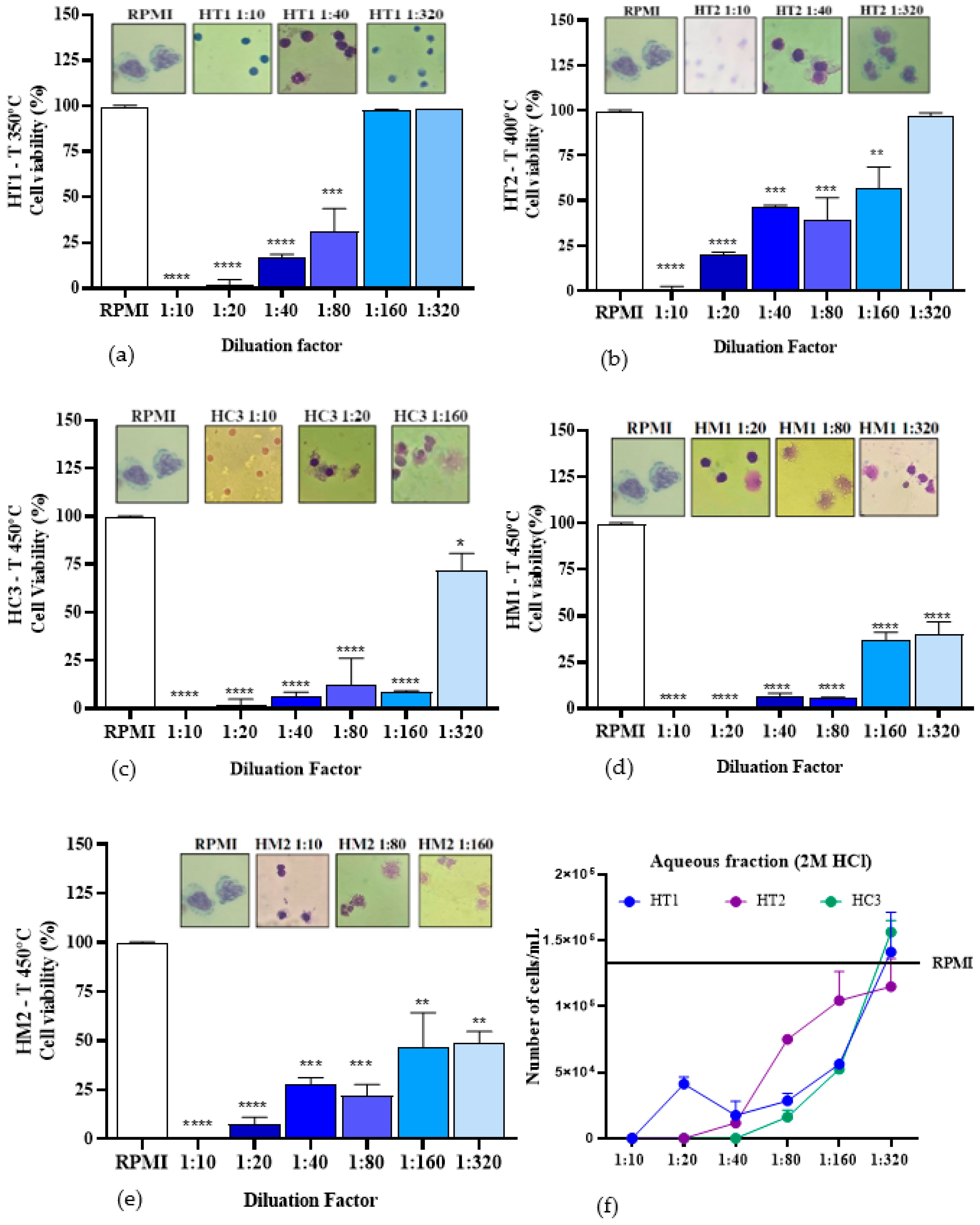
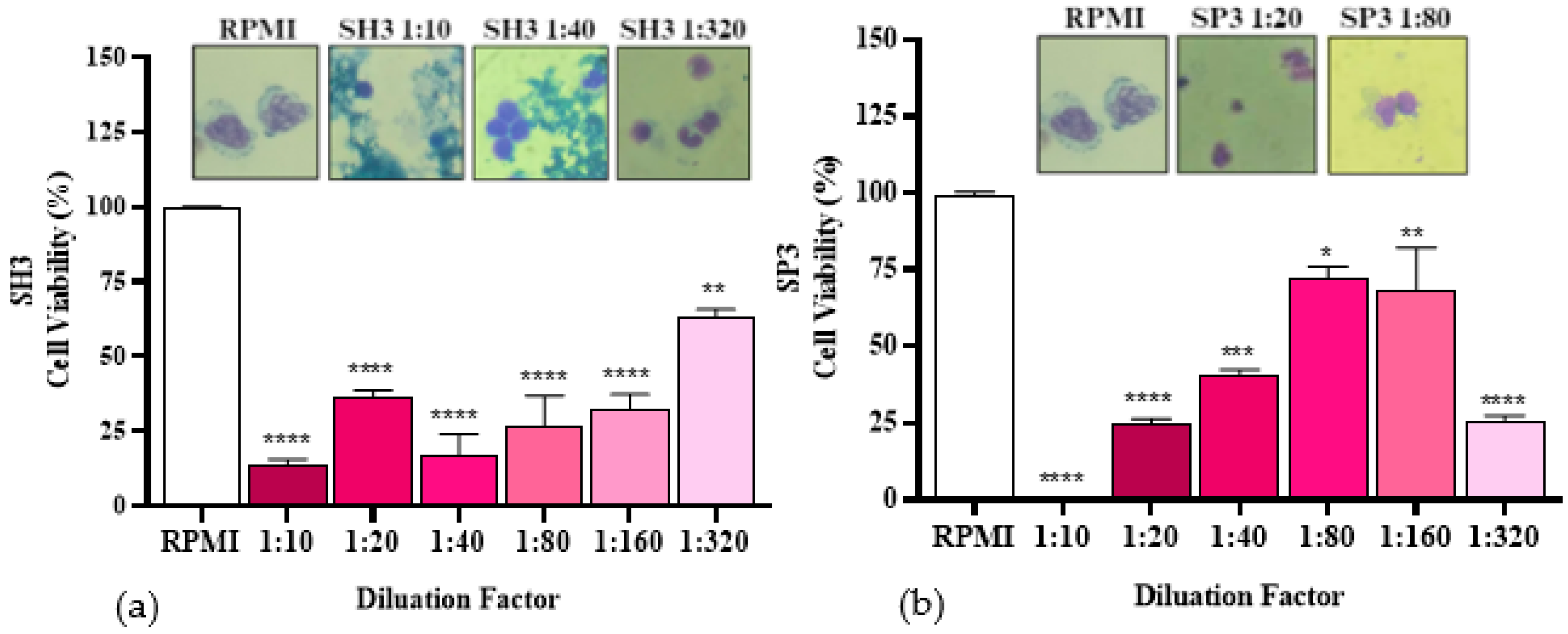
2.4. Cell Viability Assay
3. Discussion
4. Materials and Methods
4.1. Bio-Oil Production
4.2. Sample Preparation
4.2.1. Wash Water
4.2.2. Aqueous Fraction
4.2.3. Organic Fraction (PLO)
4.3. Identification of Components by Gas Chromatography (GC-MS)
4.4. Evaluation of the Antioxidant Potential of the Bio-Oil
4.4.1. Evaluation of Antioxidant Activity Using the ABTS•+ Radical Scavenging Method (TEAC)
4.4.2. Evaluation of Antioxidant Activity by the DPPH● Radical Scavenging Method
4.5. Evaluation of the Antimicrobial Activity of Bio-Oil
4.6. Cell Viability Assay
4.6.1. Ethics Committee
4.6.2. Obtaining Peripheral Blood Mononuclear Cells (PBMCs)
4.6.3. Trypan Blue Exclusion Method and Cellular Morphology Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cedrim, Paula Cavalcante Amélio Silva; BARROS, Elenita Marinho Albuquerque; Nascimento, Ticiano Gomes do. Propriedades antioxidantes do açaí (Euterpe oleracea) na síndrome metabólica. Brazilian Journal of Food Technology, v. 21, p. e2017092, 2018.
- De Castro, D.A.R. Processo de Produção de Bio-Óleo e Bio-Adsorventes via Pirólise das Sementes do Açaí (Euterpe oleraceae, Mart). Ph.D. Thesis, PRODERNA, UFPa, Belém, Brazil, 2019.
- Valois, Flávio Pinheiro et al. Improving the Antioxidant Activity, Yield and Hydrocarbon Content of Bio-Oil from Açaí Seeds Pyrolysis by Chemical Activa-Tion: Effect of Temperature and Molarity. 2023.
- Bonomo, L. F.; Silva, D. N.; Boasquivis, P. F.; Paiva, F. A.; Guerra, J. F.; Martins, T. A.; Torres, Á. G. J.; Paula, I. T.; Caneschi, W. L.; Jacolot, P.; Grossin, N.; Tessier, F. J.; Boulanger, E.; Silva, M. E.; Pedrosa, M. L.; Oliveira, R. P. Açaí (Euterpe oleracea Mart.) modulates oxidative stress resistance in Caenorhabditis elegans by direct and indirect mechanisms. PLoS One, v. 9, n. 3, p. e89933, 2014, PMid:24594796. [CrossRef]
- Menezes, E. M. S.; Torres, A. T.; Srur, A. U. S. Valor nutricional da polpa de açaí (Euterpe oleracea Mart) liofilizada. Acta Amazonica, v. 38, n. 2, p. 311-316, 2008. [CrossRef]
- Bernaud, R. F. S.; Funchal, C. D. S. Atividade antioxidante do açaí. Nutrição Brasil, v. 10, n. 5, p. 310-316, 2011.
- Yamaguchi, K. K. L.; Pereira, L. F.; Lamarão, C. V.; Lima, E. S.; Veiga Junior, V. F. Amazon acai: chemistry and biological activities: a review. Food Chemistry, v. 179, p. 137-151, 2015, PMid:25722148. [CrossRef]
- Felipe Fernando da Costa Tavares, Marcos Danilo Costa de Almeida, João Antonio Pessoa da Silva, Ludmila Leite Araújo, Nilo 710 Sérgio Medeiros Cardozo and Ruth Marlene Campomanes Santana. Thermal treatment of açaí (Euterpe oleracea) fiber for com- 711 posite reinforcement. Polímeros, 30(1), e2020003, 2020, . [CrossRef]
- Bufalino, L.; Guimaraes, A.A.; de Silva, B.M.; de Souza, R.L.F.; de Melo, I.C.N.A.; de Oliveira, D.N.P.S.; Trugilho, P.F. Local variability of yield and physical properties of açaí waste and improvement of its energetic attributes by separation of lignocellulosic fibers and seeds. J. Renew. Sustain. Energy 2018, 10, 053102.
- Guerreiro, L.H.H.; Baia, A.C.F.; Assunção, F.P.D.C.; Rodrigues, G.D.O.; e Oliveira, R.L.; Junior, S.D.; Pereira, A.M.; de Sousa, E.M.P.; Machado, N.T.; de Castro, D.A.R.; et al. Investigation of the Adsorption Process of Biochar Açaí (Euterpea olerácea Mart.) Seeds Produced by Pyrolysis. Energies 2022, 15, 6234.
- Komaiko, J. S., & Mcclements, D. J. (2016). Formação de nanoemulsõesde grau alimentício usando métodos de baixa energia: uma revisão dos métodos disponíveis.Comprehensive Reviews in Food Science and Food Safety,15(2), 331–352.
- Dias Filho, Dário Gomes et al. Revisão de literatura sobre a atividade antioxidante do açaí. Revista Contemporânea, v. 3, n. 1, p. 240-248, 2023.
- Del Pozo-Insfran, D., Brenes, C.H., Talcottt, S.T., 2004. Phytochemical composition and pigment stability of acai (Euterpe oleracea Mart). J. Agr. Food Chem. 52, 1539 1545.
- Rocha, S. M. B. M.; Oliveira, A. G.; Costa, M. C. D. Benefícios funcionais do açaí na prevenção de doenças cardiovasculares. Journal of Amazon Health Science, v. 1, n. 1, p. 1-10, 2015.
- Zhang Qi; Chang Jie; Wang Tiejun; Xu Ying. Review of biomass pyrolysis oil properties and upgrading research. Energy Conversion and Management 48 (2007) 87-92.
- Gaurav Kumar; Achyut K. Panda; R. K. Singh. Optimization of process for the production of bio-oil from eucalyptus wood. J Fuel Chem Technol, 2010, 38(2), 162 – 167. [CrossRef]
- Hyeon Su Heo; Hyun Ju Park; Jong-In Dong; Sung Hoon Park; Seungdo Kim; Dong Jin Suh; Young-Woong Suh; Seung-Soo Kim; Young-Kwon Park. Fast pyrolysis of rice husk under different reaction conditions. Journal of industrial and Engineering Chemistry, Volume 16, Issue 1, 25 January 2010, 27-31.
- Kang, Jie et al. Anti-oxidant capacities of flavonoid compounds isolated from acai pulp (Euterpe oleracea Mart.). Food Chemistry, v. 122, n. 3, p. 610-617, 2010. [CrossRef]
- Serrão, A. C. M. et al. Process analysis of pyrolysis of Açaí (Euterpe Oleracea, Mart) seeds: Influence of temperature on the yield of reaction products and physico-chemical properties of Bio-Oil. Braz. J. Dev, v. 7, p. 18200-18220, 2021. [CrossRef]
- Sato, M.K.; de Lima, H.V.; Costa, A.N.; Rodrigues, S.; Pedroso, A.J.S.; de Freitas Maia, C.M.B. Biochar from Acai agroindustry waste: Study of pyrolysis conditions. Waste Manag. 2019, 96, 158–167. [CrossRef]
- Nascimento, B.F.D.; de Araujo, C.M.B.; Nascimento, A.C.D.; da Silva, F.L.H.; de Melo, D.J.N.; Jaguaribe, E.F.; Cavalcanti, J.V.F.L.; Sobrinho, M.A.D.M. Detoxification of sisal bagasse hydrolysate using activated carbon produced from the gasification of açaí waste. J. Hazard. Mater. 2020, 409, 124494. [CrossRef]
- Valdez, G. D.; Valois, F. P.; Bremer, S. J.; Bezerra, K. C. A.; Guerreiro, L. H.; Santos, M. C.; Machado, N. T. Improving the Bio-Oil Quality of Residual Biomass Pyrolysis by Chemical Activation: Effect of Alkalis and Acid Pre-Treatment. Energies, v. 16, n. 7, p. 3162, 2023. [CrossRef]
- Silva, Iago Castro da et al. Evaluation of the Antimicrobial Capacity of Bacteria Isolated from Stingless Bee (Scaptotrigona aff. postica) Honey Cultivated in Açai (Euterpe oleracea) Monoculture. Antibiotics, v. 12, n. 2, p. 223, 2023.
- Kuskoski, E. M.; Fett, P.; Asuero, A. G. Antocianos: un grupo de pigmentos naturales. Aislamiento, identificación y propriedades. Alimentaria, v. 2, n. 61, p. 61-74, 2002.
- Alasalvar, C.; Al-Farsi, M.; Quantick, P. C.; Shahidi, F.; Wiktorowicz, R. Effect of chill storage and modified atmosphere packaging (MAP) on antioxidant activity, anthocyanins, carotenoids, phenolics and sensory quality of ready-to-eat shredded orange and purple carrots. Food Chemistry, v. 89, p. 69–76, 2005. [CrossRef]
- Filho, André Luiz M.; Pereira, Maria Renata Rocha. Atividade antimicrobiana de óleos extraídos de açaí e de pupunha sobre o desenvolvimento de Pseudomonas aeruginosa e Staphylococcus aureus. Biosci. j.(Online), p. 598-603, 2012.
- Rogez, Hervé et al. Kinetic modeling of anthocyanin degradation and microorganism growth during postharvest storage of açai fruits (Euterpe oleracea). Journal of food science, v. 77, n. 12, p. C1300-C1306, 2012. [CrossRef]
- Abe Sato, Suenne Taynah et al. Isolation and genetic identification of endophytic lactic acid bacteria from the Amazonian açai fruits: Probiotics features of selected strains and their potential to inhibit pathogens. Frontiers in microbiology, v. 11, p. 610524, 2021.
- Barbosa, Andrezza de Melo et al. Caracterização de partículas de açaí visando seu potencial uso na construção civil. Matéria (Rio de Janeiro), v. 24, p. e12435, 2019.
- Gois, Antonia Regina dos Santos. Desenvolvimento de metodologia para quantificação de fenóis presentes em fração aquosa de pirólise por GC/MS e UHPSFC. 2021.
- Souza, rafael da silva. Avaliação da composição de bio-óleos produzidos empregando resíduos de acerola. (Trabalho de conclusão de curso). UEMGS, 2015.
- Mahadevan, R.; Adhikari, S.; Shakya, R.; Wang, K.; Dayton, D.; Lehrich, M.; Taylor, S.E. Effect of Alkali and Alkaline Earth Metals on in-Situ Catalytic Fast Pyrolysis of Lignocellulosic Biomass: A Microreactor Study. Energy Fuels 2016, 30, 3045–3056. [CrossRef]
- Yang, C.-Y.; Lu, X.-S.; Lin, W.-G.; Yang, X.-M.; Yao, J.-Z. TG-FTIR Study on Corn Straw Pyrolysis-influence of Minerals. Chem. Res. Chin. Univ. 2006, 22, 524–532. [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Influence of inorganic salts on the primary pyrolysis products of cellulose. Bioresour. Technol. 2010, 101, 4646–4655. [CrossRef]
- Chen, W.; Li, K.; Chen, Z.; Xia, M.; Chen, Y.; Yang, H.; Chen, X.; Chen, H. A new insight into chemical reactions between biomass and alkaline additives during pyrolysis process. Proc. Combust. Inst. 2021, 38, 3881–3890. [CrossRef]
- Simakova, I.L.; Murzin, D.Y. Transformation of bio-derived acids into fuel-like alkanes via ketonic decarboxylation and hy drodeoxygenation: Design of multifunctional catalyst, kinetic and mechanistic aspects. J. Energy Chem. 2016, 25, 208–224.
- Oliveira, Sara Dayan da Silva. Atividade antioxidante dos óleos essenciais de genótipos de Croton grewioides Baill, Croton tetradenius Baill e seus compostos majoritários. 2018.
- Fair, R.J., Tor, Y. (2014). Antibiotics and Bacterial Resistance in the 21st Century. Perspectives in Medicinal Chemistry, volume 6. pp. 25–64. [CrossRef]
- Monteiro, Ana Rita Pinto. Atividade antimicrobiana de óleos essenciais. 2015. Tese de Doutorado. Universidade Fernando Pessoa (Portugal).
- Daniel Valdez, G.; Valois, F.P.; Bremer, S.J.; Bezerra, K.C.A.; Hamoy Guerreiro, L.H.; Santos, M.C.; Bernar, L.P.; Feio, W.P.; Moreira, L.G.S.; Mendonça, N.M.; et al. Improving the Bio-Oil Quality of Residual Biomass Pyrolysis by Chemical Activation: Effect of Alkalis and Acid Pre-Treatment. Energies 2023, 16, 3162. [CrossRef]
- Silva, Jefferson David Oliveira da. Pirólise da biomassa residual da acerola (Malpighia emarginata) e avaliação de seus produtos. Dissertação de Mestrado. UFS ,2019.
- Costa, Diego Magalhães. Estudo de bio-óleos de sorgo biomassa obtidos via pirólise catalítica rápida. 2016.
- Espíndola, K.M.M; Ferreira, R.G; Narvaez, L.E.M; Silva Rosario, A.C.R; Da Silva, A.H.M; Silva, A.G.B; Vieira, A.P.O; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front Oncol. 21; 9:541, 2019. [CrossRef]
- Albuquerque BR, Heleno SA, Oliveira MBPP, Barros L, Ferreira ICFR. Phenolic compounds: current industrial applications, 924 limitations and future challenges. Food Funct. 2021 Jan 7;12(1):14-29. [CrossRef]
- Del Pozo C, Bartrolí J, Alier S, Puy N, Fàbregas E. Production of antioxidants and other value-added compounds from coffee 813 silver skin via pyrolysis under a biorefinery approach. Waste Manag. 2020 May 15; 109:19-27.
- Patra, Jayanta Kumar et al. Volatile compounds and antioxidant capacity of the bio-oil obtained by pyrolysis of Japanese red pine (pinus densiflora siebold and zucc.). Molecules, v. 20, n. 3, p. 3986-4006, 2015. [CrossRef]
- De Freitas, Carlos Eduardo Pereira et al. Estudo in vitro da Atividade Antioxidante por captura do radical livre DPPH e análise da atividade fotoprotetora do óleo de castanha-do-Pará extraído com solvente alternativo. Brazilian Journal of Development, v. 7, n. 5, p. 52411-52423, 2021.
- Caleja, C. et al. Phenolic compounds as nutraceuticals or functional food ingredients. Current pharmaceutical design. v. 23, n. 19, p. 2787-2806, 2017. [CrossRef]
- Beker, Sabrina Anderson. Avaliação do potencial antimicrobiano de TBHQ (terc butil-hidroquinona) e de bio-óleo para uso em biodiesel de soja (B100) e óleo diesel B (B10). 2014.
- Mourant, D.; Yang, X. D.-Q.; et al. Anti-fungal properties of the pyroligneous liquor from the pyrolysis of softwood bark. Wood Fiber Sci., v. 73, p. 542–548, 2005.
- Mazela, B. Fungicidal value of wood tar from pyrolysis of treated wood. Waste Management, v. 27, p. 461–465, 2006. [CrossRef]
- Kim, K. H.; Jeong, H. S.; Kim, J. Y.; Han, G. S.; Choi, I. G.; Choi, J. W. Evaluation of the antifungal effects of bio-oil prepared with lignocellulosic biomass using fast pyrolysis technology. Chemosphere, v. 89, p. 688–693, 2012. [CrossRef]
- Lai, P. K.; Roy, J. Antimicrobial and chemo-preventive properties of herbs and spices. Curr. Med. Chem, v. 11, p. 1451–1460, 2004.
- Xue, J.; Davidson, P. M.; Zhong, Q. Thymol nanoemulsified with whey protein isolate-maltodextrin conjugate: the enhanced emulsifying capacity and anti-listerial properties in milk by propylene glycol. Journal of Agricultural and Food Chemistry. v. 61, n. 51, p. 12720–12726, 2013.
- Farag, R. S., Daw, Z. Y., Hewedi, F. M., & El-Baroty, G. S. A. Antimicrobial activity of some Egyptian spice essential oils. Journal of Food Protection, v.52, p. 665 667, 1989. [CrossRef]
- Chapman, J. S. Biocide resistence mechanisms. International Biodeterioration & Biodegradation, v. 51, n. 2, p. 133-138, 2003.
- Puvača, N., Milenković, J., Coghill, T. G., Bursić, V., Petrović, A., Tanasković, N., Pelić, M., Pelić, D. L., & Miljković, T. (2021). Antimicrobial Activity ofSelected Essential Oils against Selected Pathogenic Bacteria: In Vitro Study, Antibiotics, 10(5), 1-14. [CrossRef]
- Trajano, Vinicius Nogueira et al. Propriedade antibacteriana de óleos essenciais de especiarias sobre bactérias contaminantes de alimentos. Food Science and Technology, v. 29, p. 542-545, 2009. [CrossRef]
- Mímica-Dukić, Neda et al. Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta médica, v. 69, n. 05, p. 413-419, 2003. [CrossRef]
- Sagdic, O. et al. Note: effect of some spice extracts on bacterial inhibition. Food science and technology international, v. 9, n. 5, p. 353-358, 2003. [CrossRef]
- Cataluña, R; Kuamoto, P; Petzhold, C.; Caramao, E.; Machado, M. E.; Silva, R. Using Bio-oil Produced by Biomass Pyrolysis as Diesel Fuel. Energy & Fuels, v. 27, p. 6831−6838, 2013. [CrossRef]
- RochadeCastro, D.A.; da Silva Ribeiro, H.J.; Hamoy Guerreiro, L.H.; Pinto Bernar, L.; Jonatan Bremer, S.; Costa Santo, M.; da Silva Almeida, H.; Duvoisin, S., Jr.; Pizarro Borges, L.E.; Teixeira Machado, N. Production of Fuel-Like Fractions by Fractional Distillation of Bio-Oil from Açaí (Euterpe oleracea Mart.). Seeds Pyrolysis. Energ. 2021, 14, 3713.
- De Sousa, J.L.; Guerreiro, L.H.H.; Bernar, L.P.; Ribeiro, H.J.D.S.; e Oliveira, R.L.; Santos, M.C.; Almeida, H.D.S.; Junior, S.D.; Borges, L.E.P.; Castro, D.A.R.; et al. Chemical Analysis of Bio-Oil Produced by Pyrolysis of Açaí (Euterpe oleracea, Mart) Seeds. Braz. J. Dev. 2021, 7, 15549–15565.
- Ferenz, Mariane et al. Ação Antimicrobiana de Óleos Essenciais Contra Staphylococcus Aureus e Pseudomonas Aeruginosa. Blucher Food Science Proceedings, v. 1, n. 1, p. 35-36, 2014.
- Mashiba, Leandro Nozomi; MAGALHÃES, Washington Luiz Esteves; MATOS, M. de. Determinação da atividade antioxidante e antimicrobiana das frações da lignina pirolítica. 2019.
- Pereira, R. R.; Gomes, A. T.; Testi, M.; Bianchera, A.; Ribeiro-Costa, R. M.; Padula, C.; Sonvico, F. Ucuùba Fat Characterization and Use to Obtain Lipid Nanoparticles by High-Pressure Homogenization with Full Factorial Design. Chemical Engineering & Technology, v. 44, n. 6, p. 1009-1016, 2021. [CrossRef]
- Costa, M.E.G.; da Costa Assunção, F.P.; Teribele, T.; Pereira, L.M.; de Castro, D.A.R.; Santo, M.C.; da Costa, C.E.F.; Shultze, M.; Hofmann, T.; Machado, N.T. Characterization of Bio-Adsorbents Produced by Hydrothermal Carbonization of Corn Stover: Application on the Adsorption of Acetic Acid from Aqueous Solutions.Enegies 2021, 14, 8154 . [CrossRef]
- Santos, Vanuza Oliveira et al. Pirólise da biomassa Amazônica: parâmetros cinéticos e termodinâmicos usando análise termogravimétrica, 2022.
- Silva, Jonas Joaquim Mangabeira da; ROGEZ, Hervé. Avaliação da estabilidade oxidativa do óleo bruto de açaí (Euterpe oleracea) na presença de compostos fenólicos puros ou de extratos vegetais amazônicos. Química Nova, v. 36, p. 400-406, 2013. [CrossRef]
- Re, R.; Pelegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Riceevans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical biology & medicine, New York, v.26, n.9-10, p.1231-1237,1999. [CrossRef]
- Brand-Williams, Wendy; Cuvelier, Marie-Elisabeth; Berset, C. L. W. T. Use of a free radical method to evaluate antioxidant activity. LWT-Food science and Technology, v. 28, n. 1, p. 25-30, 1995. [CrossRef]
- CLSI, Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved standar: M27, Four Edition, November, 2017.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





