Submitted:
31 July 2024
Posted:
05 August 2024
Read the latest preprint version here
Abstract
Keywords:
Introduction
Types of Knee Joint Injuries
Anterior Cruciate Ligament (ACL) Injuries
Mechanisms of Injury
Cellular and Molecular Responses to ACL Injury
Inflammatory Response
- Vascular Response: The immediate consequence of ACL rupture is blood vessel disruption, leading to hemorrhage and hematoma formation within the ligament. This vascular injury results in hypoxia, exacerbating cell death and tissue damage. Hypoxia-inducible factors (HIFs) are activated under low oxygen conditions, leading to the expression of genes that facilitate survival under hypoxic conditions and promote angiogenesis.
- Cellular Infiltration: The damage-associated molecular patterns (DAMPs) released from necrotic cells and exposed ECM components activate the innate immune system. Neutrophils are the first responders, arriving within hours. They release reactive oxygen species (ROS) and proteolytic enzymes, which aid in clearing damaged tissue and recruiting more immune cells. Neutrophil extracellular traps (NETs) can also be formed, capturing and neutralizing pathogens but potentially contributing to further tissue damage.
- Macrophage Activation: Following neutrophil infiltration, macrophages migrate to the injury site. These cells exhibit plasticity, initially adopting a pro-inflammatory (M1) phenotype, releasing cytokines like interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). These cytokines further enhance the inflammatory response and upregulate matrix metalloproteinases (MMPs) activity, which degrades the ECM. As inflammation progresses, macrophages shift to an anti-inflammatory (M2) phenotype, secreting growth factors and cytokines that promote tissue repair and remodeling. This phenotypic switch is crucial for resolving inflammation and initiating tissue regeneration.
Extracellular Matrix (ECM) Degradation and Remodeling
- Matrix Metalloproteinases (MMPs): MMPs are zinc-dependent proteolytic enzymes crucial for ECM remodeling. They degrade various ECM components, with MMP-1 (collagenase-1) and MMP-13 (collagenase-3) targeting type I collagen, the primary collagen type in ligaments. MMP-3 (stromelysin-1) degrades other ECM components and activates other MMPs. The expression of MMPs is upregulated by pro-inflammatory cytokines, initiating ECM breakdown.
- Tissue Inhibitors of Metalloproteinases (TIMPs): TIMPs are natural inhibitors of MMPs, playing a crucial role in regulating ECM turnover. The equilibrium between MMPs and TIMPs dictates the extent of ECM degradation and subsequent tissue remodeling. Following an ACL injury, the expression of TIMPs is often insufficient to counterbalance the heightened MMP activity, leading to excessive ECM degradation. The balance between MMPs and TIMPs is tightly regulated at the transcriptional level and influenced by various signaling pathways, including the MAPK and NF-κB pathways.
- Growth Factors: Various growth factors released in response to ACL injury are integral to ECM synthesis and remodeling. Transforming growth factor-beta (TGF-β) is a key regulator, promoting collagen synthesis and ECM formation. TGF-β signals through the Smad pathway, leading to the transcription of ECM-related genes. Fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) also play significant roles in fibroblast proliferation and ECM production, primarily through the activation of the MAPK and PI3K-Akt signaling pathways.
Fibroblast Activation and Proliferation
- Fibroblast Proliferation: The inflammatory milieu and released growth factors stimulate fibroblast proliferation. These cells migrate to the injury site, synthesizing new ECM components, predominantly collagen and proteoglycans. Fibroblast activation is mediated by growth factors like TGF-β and PDGF, which activate downstream signaling pathways such as MAPK and PI3K-Akt, leading to cell proliferation and migration.
- Collagen Synthesis: Fibroblasts produce type I collagen, vital for restoring the ligament’s structural integrity. The proper alignment and organization of collagen fibers are crucial for the mechanical properties of the healed ligament. Mechanical loading during rehabilitation influences collagen fiber alignment, promoting the formation of more organized and functional tissue. The synthesis of collagen involves the transcriptional activation of collagen genes, followed by the post-translational modification and assembly of collagen fibers, processes regulated by TGF-β and other growth factors.
- ECM Production: Beyond collagen, fibroblasts synthesize other ECM components, including elastin and proteoglycans, which contribute to the biomechanical properties of the ligament. Proteoglycans, like decorin and biglycan, bind to collagen fibers and help retain water within the tissue, maintaining its viscoelastic properties. The production of ECM components is regulated by a complex network of signaling pathways, including TGF-β/Smad, MAPK, and PI3K-Akt.
Mesenchymal Stem Cell (MSC) Recruitment and Differentiation
- MSC Recruitment: MSCs can be mobilized from the bone marrow and other sources in response to injury. Chemotactic signals released from the injury site, such as stromal cell-derived factor-1 (SDF-1) and vascular endothelial growth factor (VEGF), attract MSCs to the damaged tissue. The migration of MSCs is regulated by signaling pathways, including the SDF-1/CXCR4 axis and the VEGF/VEGFR pathway.
- Differentiation: Upon arrival at the injury site, MSCs differentiate into fibroblasts and other cell types involved in tissue repair. The local microenvironment, including mechanical cues and biochemical signals, influences MSC differentiation. TGF-β, FGF, and PDGF are among the growth factors promoting MSC differentiation into fibroblasts, chondrocytes, and other repair cells. These growth factors activate signaling pathways such as TGF-β/Smad, MAPK, and PI3K-Akt, driving MSC differentiation.
- Paracrine Effects: In addition to differentiating into repair cells, MSCs secrete various cytokines and growth factors that modulate the inflammatory response and promote tissue repair. These paracrine effects include the regulation of immune cell activity, promotion of angiogenesis, and stimulation of fibroblast activity. MSC-derived exosomes and microvesicles also play a role in intercellular communication, delivering bioactive molecules that influence the repair process.
Angiogenesis
- VEGF: Vascular endothelial growth factor (VEGF) is a key regulator of angiogenesis. VEGF is upregulated in response to hypoxia and other signals from the injury site. It promotes the proliferation and migration of endothelial cells, leading to the formation of new blood vessels. VEGF signaling through the VEGF receptor (VEGFR) activates pathways like MAPK and PI3K-Akt, driving endothelial cell proliferation and migration.
- Angiogenic Factors: Other factors involved in angiogenesis include fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF). These factors work in concert with VEGF to stimulate endothelial cell activity and vessel formation. The FGF/FGFR and PDGF/PDGFR signaling pathways activate downstream effectors, including MAPK and PI3K-Akt, promoting angiogenesis.
- Role in Repair: Newly formed blood vessels enhance oxygen and nutrient delivery to the injury site, supporting the metabolic demands of proliferating fibroblasts and other reparative cells. Angiogenesis also facilitates the removal of waste products and debris from the injury site. The interplay between angiogenic and inflammatory signals ensures a coordinated repair process, where the formation of new blood vessels is tightly regulated to meet the tissue’s needs.
Mechanotransduction and Mechanical Loading
- Integrin Signaling: Integrins are transmembrane receptors that mediate cell-ECM interactions and transmit mechanical signals to the cell interior. Mechanical loading activates integrins, leading to focal adhesion kinase (FAK) and other signaling molecules’ activation. This activation promotes cytoskeletal reorganization, cell proliferation, and ECM synthesis. Integrin signaling interacts with various pathways, including MAPK and PI3K-Akt, to regulate cellular responses to mechanical stress.
- Ion Channels: Stretch-activated ion channels, including calcium channels, respond to mechanical loading by allowing ions to influx into the cell. Increased intracellular calcium levels activate various signaling pathways, such as the calcineurin/NFAT pathway and the calmodulin-dependent kinase (CaMK) pathway. These pathways regulate gene expression and cellular responses to mechanical loading. The activation of calcium-dependent pathways plays a critical role in the adaptation of cells to mechanical stress.
- MAPK Pathway: The mitogen-activated protein kinase (MAPK) pathway is another key mechanotransduction pathway activated by mechanical loading. Activation of MAPKs, such as ERK1/2 and p38, regulates cell proliferation, differentiation, and ECM production. The MAPK pathway integrates signals from mechanical stress and growth factors, coordinating cellular responses essential for tissue repair and remodeling.
Understanding Biological and Physiological Processes
Immediate Vasoconstriction of the Blood Flow at the Molecular Level
-
Initiation by Endothelial Cells
- Role of Endothelial Cells: Endothelial cells, which line the inner walls of blood vessels, act as sentinels, detecting and responding to vascular injury. When an injury occurs, these cells are activated and release several signaling molecules, with endothelin-1 (ET-1) being one of the most significant vasoconstrictors. This response is part of the body’s immediate attempt to reduce blood loss and start the repair process.
- Endothelin-1 (ET-1) Release: ET-1 is synthesized as a precursor molecule, preproendothelin, which is subsequently processed into big endothelin by endothelin-converting enzyme (ECE). Finally, big endothelin is cleaved to form the active ET-1. The synthesis and release of ET-1 are upregulated by stimuli such as shear stress, hypoxia, and pro-inflammatory cytokines, which often accompany vascular injury.
- Endothelin Receptors: ET-1 exerts its vasoconstrictive effects by binding to specific endothelin receptors on vascular smooth muscle cells. These receptors, ETA and ETB, are G-protein coupled receptors (GPCRs). Binding of ET-1 to ETA receptors primarily results in vasoconstriction, while ETB receptors, depending on their location, can mediate both vasoconstriction (on smooth muscle cells) and vasodilation (on endothelial cells).
- 2.
-
Sympathetic Nervous System Activation
- Neurotransmitter Release: The sympathetic nervous system is immediately activated in response to injury, leading to the release of neurotransmitters such as norepinephrine (noradrenaline) from postganglionic sympathetic nerve endings. Norepinephrine is stored in synaptic vesicles within nerve terminals and is released into the synaptic cleft in response to nerve impulses.
- Adrenergic Receptors: Norepinephrine binds to alpha-1 adrenergic receptors on vascular smooth muscle cells. These receptors are part of the GPCR family and, upon activation, initiate a cascade of intracellular events that lead to muscle contraction and vasoconstriction.
- 3.
-
Intracellular Signaling Pathways
- Phospholipase C (PLC) Activation: The binding of norepinephrine to alpha-1 adrenergic receptors activates the Gq protein, which subsequently activates PLC. PLC catalyzes the hydrolysis of the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2), producing two second messengers: inositol trisphosphate (IP3) and diacylglycerol (DAG).
- Calcium Release: IP3 binds to IP3 receptors located on the membrane of the sarcoplasmic reticulum (SR), a specialized endoplasmic reticulum in muscle cells. This binding induces the release of Ca2+ ions from the SR into the cytoplasm of smooth muscle cells, leading to an increase in intracellular calcium levels, which is crucial for muscle contraction.
- Calcium-Calmodulin Complex: The elevated Ca2+ ions in the cytoplasm bind to calmodulin, a calcium-binding messenger protein. The calcium-calmodulin complex then activates myosin light-chain kinase (MLCK), an enzyme essential for initiating muscle contraction.
- 4.
-
Smooth Muscle Contraction
- Myosin Light-Chain Phosphorylation: MLCK phosphorylates the myosin light chains in smooth muscle cells, a modification necessary for myosin to interact with actin filaments. This interaction facilitates the sliding of actin and myosin filaments past each other, resulting in muscle contraction.
- Vasoconstriction: The contraction of smooth muscle cells around the blood vessels reduces the diameter (lumen) of the vessel, effectively decreasing blood flow to the injured area. This vasoconstriction is a critical immediate response to prevent excessive blood loss and maintain hemostasis.
- 5.
-
Nitric Oxide (NO) Modulation
- NO Inhibition: Under normal conditions, endothelial cells produce NO via the enzyme endothelial nitric oxide synthase (eNOS). NO diffuses into adjacent smooth muscle cells, where it activates soluble guanylate cyclase (sGC), increasing cyclic GMP (cGMP) levels and promoting muscle relaxation.
- Favoring Vasoconstriction: Following injury, the production of NO is reduced due to endothelial damage and the dominance of vasoconstrictive signaling. This reduction in NO levels shifts the balance towards vasoconstriction, further aiding the immediate narrowing of blood vessels.
Immobilization to Reduce Pain and Swelling at the Molecular Level
-
Inflammatory Response Modulation
- Cytokine Production: Upon injury, immune cells such as macrophages and mast cells release pro-inflammatory cytokines including tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6). These cytokines are pivotal in mediating the inflammatory response, promoting the recruitment of additional immune cells to the injury site and amplifying the inflammatory cascade.
- Reduced Cytokine Spread: Immobilization limits mechanical movements, thereby reducing the physical disruption that can exacerbate cytokine production and spread. By stabilizing the injured area, immobilization helps contain the inflammatory response to a localized region, preventing the spread of inflammation and reducing overall tissue damage and swelling.
- 2.
-
Pain Signaling Pathway
- Nociceptor Activation: Injuries activate nociceptors, which are sensory neurons that detect pain. These neurons release neuropeptides such as substance P and calcitonin gene-related peptide (CGRP), which further propagate pain signals and promote vasodilation and plasma extravasation, contributing to the sensation of pain.
- Prostaglandin Synthesis: Inflammation induced by injury leads to the activation of the enzyme cyclooxygenase (COX), which converts arachidonic acid into prostaglandins. Prostaglandins are lipid compounds that sensitize nociceptors, amplifying pain signals transmitted to the central nervous system.
- Immobilization Effect: By stabilizing the injured area, immobilization reduces mechanical stress and micro-movements that can activate nociceptors and enhance pain perception. This stabilization mitigates the intensity of pain signals and helps manage pain more effectively.
- 3.
-
Swelling and Fluid Dynamics
- Vascular Permeability: Pro-inflammatory mediators such as histamine and bradykinin increase vascular permeability, allowing fluids and plasma proteins to leak from blood vessels into surrounding tissues, causing swelling (edema). This leakage contributes to the inflammatory response by delivering immune cells and proteins to the site of injury.
- Immobilization and Edema: Immobilization helps reduce vascular permeability by minimizing mechanical stress on blood vessels. By limiting movement, immobilization reduces the mechanical forces that can exacerbate the leakage of fluids and proteins, thus helping to control and reduce swelling.
- 4.
-
Matrix Metalloproteinases (MMPs)
- Tissue Remodeling: MMPs are a group of enzymes that degrade various components of the extracellular matrix (ECM), playing a critical role in tissue remodeling during inflammation and repair. However, excessive MMP activity can lead to further tissue damage and delayed healing.
- Controlled MMP Activity: Immobilization helps regulate MMP activity by reducing the inflammatory response and providing a stable environment for tissue repair. By stabilizing the injury site, immobilization limits the physical stress and movement that can trigger excessive MMP activation, promoting more controlled and effective tissue remodeling.
Detailed Molecular Insights
- Endothelial Cell Activation: Endothelial cells detect mechanical and chemical signals resulting from injury and respond by upregulating genes involved in the production of vasoconstrictive peptides like ET-1. Transcription factors such as hypoxia-inducible factor (HIF) and nuclear factor-kappa B (NF-κB) play a pivotal role in this gene regulation.
- Sympathetic Nervous System: The activation of the sympathetic nervous system involves the release of catecholamines like norepinephrine, which bind to adrenergic receptors on target cells. These receptors, coupled to G-proteins, initiate a series of intracellular signaling pathways leading to vasoconstriction.
- Intracellular Calcium Dynamics: The release of calcium ions from the sarcoplasmic reticulum is tightly regulated by IP3 receptors and ryanodine receptors (RyRs). The subsequent binding of calcium to calmodulin and the activation of MLCK are critical steps in smooth muscle contraction.
- Cytokine Signaling: Pro-inflammatory cytokines like TNF-α, IL-1, and IL-6 are produced in response to injury and play central roles in mediating the inflammatory response. These cytokines activate transcription factors such as NF-κB and activator protein-1 (AP-1), which regulate the expression of genes involved in inflammation.
- Prostaglandin Pathway: The synthesis of prostaglandins is initiated by COX enzymes, with COX-1 being constitutively expressed and COX-2 being inducible and upregulated during inflammation. Prostaglandins act on specific GPCRs to enhance nociceptor sensitivity, amplifying pain perception.
- MMP Regulation: The activity of MMPs is controlled by their natural inhibitors, tissue inhibitors of metalloproteinases (TIMPs). The balance between MMPs and TIMPs is crucial for effective ECM remodeling and is influenced by cytokines and growth factors.
Vasodilatation
Endothelial Cell Activation
- Role of Endothelial Cells: Endothelial cells are integral to the regulation of vascular tone and blood flow. These cells respond to mechanical stimuli, such as shear stress from increased blood flow, as well as chemical signals including cytokines, growth factors, and inflammatory mediators. Upon activation by these stimuli, endothelial cells produce and release various vasoactive substances to induce vasodilatation, thereby increasing blood supply to the affected area and facilitating healing.
- Production of Nitric Oxide (NO): One of the primary substances produced by activated endothelial cells is nitric oxide (NO). NO is synthesized from the amino acid L-arginine by the enzyme endothelial nitric oxide synthase (eNOS). The activity of eNOS is regulated by several factors, including intracellular calcium levels, phosphorylation by kinases such as Akt, and interactions with cofactors like tetrahydrobiopterin (BH4). The production of NO is a rapid response mechanism that allows for quick adjustments in vascular tone.
Nitric Oxide (NO)
- Synthesis and Diffusion: NO is a small, highly diffusible gas that quickly moves from endothelial cells into the adjacent smooth muscle cells of the blood vessel wall. Its rapid diffusion is facilitated by its lipophilic nature, allowing it to cross cell membranes easily. Once synthesized, NO interacts with various intracellular targets in smooth muscle cells to induce relaxation and vasodilatation.
- Mechanism of Action: In smooth muscle cells, NO binds to and activates soluble guanylate cyclase (sGC), an enzyme that catalyzes the conversion of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP). This increase in cGMP levels is a key step in the vasodilation process.
Guanylate Cyclase Activation
- sGC Activation: The binding of NO to sGC causes a conformational change that significantly increases the enzyme’s activity. This activation leads to a rise in intracellular cGMP levels, which serves as a second messenger in the signaling pathway that results in smooth muscle relaxation.
- Role of cGMP: cGMP activates protein kinase G (PKG), which then phosphorylates various target proteins. These phosphorylation events lead to the opening of potassium channels and the inhibition of calcium entry into the smooth muscle cells. PKG also promotes the reuptake of calcium into the sarcoplasmic reticulum and its extrusion from the cell, further reducing intracellular calcium levels.
cGMP Pathway
- Signal Cascade: The cGMP pathway involves a series of molecular interactions that ultimately lead to the relaxation of smooth muscle cells. By lowering intracellular calcium concentrations, PKG decreases the activity of myosin light-chain kinase (MLCK), an enzyme necessary for muscle contraction. This reduction in MLCK activity results in the dephosphorylation of myosin light chains, preventing their interaction with actin and leading to muscle relaxation.
- Smooth Muscle Relaxation: The relaxation of smooth muscle cells in the vessel wall results in vasodilatation, increasing the diameter of the blood vessel and enhancing blood flow to the affected area. This process is crucial for providing the necessary oxygen and nutrients to tissues during injury and inflammation.
Prostaglandins
- Role in Vasodilatation: Prostaglandins are another group of lipid compounds that contribute significantly to vasodilatation. These molecules are produced via the cyclooxygenase (COX) pathway, which converts arachidonic acid into various prostaglandins, including PGE2.
- Mechanism of Action: Prostaglandins like PGE2 bind to specific G-protein coupled receptors (GPCRs) on smooth muscle cells. This binding activates adenylate cyclase, increasing cyclic adenosine monophosphate (cAMP) levels within the cell. Similar to cGMP, cAMP reduces intracellular calcium levels, leading to smooth muscle relaxation and vasodilatation.
Proliferation of Tissue
Growth Factors
- Key Growth Factors: Several growth factors are essential for tissue proliferation, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF). These growth factors are released by various cells, such as platelets, macrophages, and fibroblasts, in response to injury.
- Function of Growth Factors: VEGF promotes angiogenesis, the formation of new blood vessels, ensuring an adequate supply of oxygen and nutrients to proliferating tissues. FGF stimulates the proliferation and differentiation of fibroblasts and other mesenchymal cells. PDGF recruits and activates various cell types involved in the repair process, enhancing cell migration and proliferation.
Receptor Activation
- Binding and Signaling: Growth factors exert their effects by binding to specific receptors on the surface of target cells. These receptors are often receptor tyrosine kinases (RTKs) that, upon activation, initiate intracellular signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway and the phosphoinositide 3-kinase (PI3K)/Akt pathway.
- Intracellular Pathways: The MAPK pathway involves a cascade of phosphorylation events that activate transcription factors, leading to the expression of genes involved in cell proliferation and differentiation. The PI3K/Akt pathway promotes cell survival and growth by activating downstream targets involved in protein synthesis and cell cycle progression.
Cell Cycle Progression
- Regulation of Cell Division: The activation of these signaling pathways promotes cell cycle progression by upregulating genes essential for DNA synthesis and cell division. This regulation ensures that cells can proliferate efficiently, contributing to tissue repair and regeneration.
- Role of Cyclins and CDKs: Cyclins and cyclin-dependent kinases (CDKs) are key regulators of the cell cycle. Their expression and activity are tightly controlled by growth factor signaling, ensuring orderly progression through the different phases of the cell cycle.
Extracellular Matrix (ECM) Remodeling
- ECM Degradation and Synthesis: Tissue proliferation is also supported by ECM remodeling. Matrix metalloproteinases (MMPs) are enzymes that degrade ECM components, facilitating new tissue formation. Conversely, tissue inhibitors of metalloproteinases (TIMPs) regulate MMP activity to ensure balanced remodeling.
- MMP and TIMP Balance: The balance between MMPs and TIMPs is critical for proper ECM remodeling. Excessive MMP activity can lead to excessive ECM degradation and impaired tissue repair, while insufficient MMP activity can result in excessive ECM accumulation and fibrosis.
Inflammation
Initiation and Mediators
- Inflammatory Response: The body’s immediate response to tissue injury involves the activation of the innate immune system, resulting in an inflammatory response. This response is essential for containing the injury, preventing infection, and setting the stage for tissue repair.
- Cytokines and Chemokines: Pro-inflammatory cytokines such as TNF-α, IL-1, and IL-6 are released by immune cells like neutrophils, macrophages, and mast cells. These cytokines play a crucial role in amplifying the inflammatory response by recruiting additional immune cells to the site of injury.
- Histamine and Bradykinin: These mediators increase vascular permeability, allowing immune cells and plasma proteins to exit the bloodstream and enter the injured tissue. This increase in permeability contributes to the classic signs of inflammation: redness, heat, swelling, and pain.
Resolution and Healing
- Anti-Inflammatory Cytokines: As the healing process progresses, the body produces anti-inflammatory cytokines such as IL-10 and TGF-β. These cytokines help resolve inflammation by inhibiting pro-inflammatory signaling pathways and promoting tissue repair.
- Macrophage Polarization: Macrophages undergo a phenotypic switch from a pro-inflammatory (M1) phenotype to an anti-inflammatory and tissue-repair (M2) phenotype. M2 macrophages play a crucial role in cleaning up debris and promoting tissue regeneration.
Icing and Anti-Inflammatory Drugs
Impact on Healing
- Decreased Lymphatic Flow: Icing constricts blood vessels, reducing blood flow and lymphatic drainage. This can limit the removal of waste products and inflammatory mediators from the injury site, potentially slowing the healing process.
- Reduced Cell Proliferation: Cooling an injured area can decrease the activity of enzymes and cells involved in tissue repair and regeneration, slowing down processes such as cell proliferation and ECM remodeling.
- Inhibited Cell-Cell Interactions: Cold temperatures can impair cell signaling and interactions necessary for coordinated healing, such as the communication between immune cells and fibroblasts.
Anti-Inflammatory Drugs
- COX Inhibition: Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit cyclooxygenase (COX) enzymes, reducing the production of prostaglandins. While this alleviates pain and inflammation, it can also impair processes that are essential for healing, such as vasodilatation and cell proliferation.
- Delayed Healing: Chronic use of NSAIDs can interfere with the normal inflammatory response required for efficient tissue repair, leading to delayed healing.
Numbing Effects of Ice
- Pain Relief: The primary benefit of ice application is its numbing effect, which provides temporary pain relief by decreasing the nerve conduction velocity of pain signals.
- Short Duration: To avoid the negative impacts on healing, ice should be applied for only a few minutes at a time, just enough to achieve pain relief without significantly disrupting the physiological processes of inflammation and tissue repair.
- 5 days.
Type III Collagen Production
Initial Response
- Extracellular Matrix (ECM) Production: Immediately following an injury, the body initiates a complex repair process involving the production of extracellular matrix (ECM) components. This initial response is crucial for stabilizing the injured area and providing a scaffold for new tissue growth. Type III collagen is the primary ECM component produced during the early stages of wound healing, forming a soft, temporary matrix that supports cellular migration and proliferation.
- Role of Growth Factors: Key growth factors such as transforming growth factor-beta (TGF-β) and platelet-derived growth factor (PDGF) play vital roles in this process. TGF-β is particularly important for its ability to stimulate fibroblast proliferation and ECM production. It binds to TGF-β receptors on the surface of fibroblasts, initiating a signaling cascade that activates SMAD proteins, which then translocate to the nucleus to regulate gene expression. PDGF, on the other hand, promotes the recruitment and activation of fibroblasts and other reparative cells to the injury site.
- Fibroblast Activation: Once activated by these growth factors, fibroblasts become the primary producers of type III collagen. The binding of TGF-β and PDGF to their respective receptors triggers intracellular signaling pathways, notably the mitogen-activated protein kinase (MAPK) pathway and the phosphoinositide 3-kinase (PI3K)/Akt pathway. These pathways play crucial roles in cellular proliferation, survival, and the upregulation of genes involved in collagen synthesis.
- Collagen Gene Transcription: The activation of MAPK and PI3K/Akt pathways leads to the upregulation of specific genes, including COL3A1, which encodes type III collagen. Transcription factors activated by these pathways, such as AP-1, bind to the promoter regions of collagen genes, increasing their transcription and ensuring an abundant supply of type III collagen to form the initial ECM scaffold.
Transition to Type I Collagen
Remodeling Phase
- Provisional Matrix Formation: During the proliferative phase of wound healing, type III collagen forms a temporary scaffold that supports cell proliferation and tissue formation. This scaffold is essential for early wound stability and facilitates the migration and organization of cells within the healing tissue.
- Matrix Metalloproteinases (MMPs): The transition from type III to type I collagen is facilitated by matrix metalloproteinases (MMPs), a group of enzymes that degrade ECM components. MMP-1 (collagenase-1) specifically targets type III collagen, breaking it down to allow for the deposition of type I collagen. MMP-2 (gelatinase A) further degrades denatured collagen and gelatin, aiding in the remodeling process. The activity of MMPs is tightly regulated by tissue inhibitors of metalloproteinases (TIMPs) to ensure balanced ECM turnover.
- Synthesis of Type I Collagen: Concurrent with the degradation of type III collagen, fibroblasts begin synthesizing type I collagen, the most abundant collagen in the human body. Type I collagen fibers are thicker and have greater tensile strength compared to type III collagen, making them suitable for forming the final, durable ECM in healed tissues.
Collagen Cross-Linking
- Lysyl Oxidase Activity: The enzyme lysyl oxidase is crucial for the maturation and stabilization of collagen fibers. It catalyzes the oxidative deamination of lysine residues in collagen, facilitating the formation of covalent cross-links between collagen molecules. These cross-links enhance the tensile strength and stability of collagen fibers, contributing to the mechanical properties of the matured scar tissue.
Reconstruction and Orientation of Collagen Fibers
Stress and Mechanical Loading
- Mechanosensitive Cells: Fibroblasts and other cells within the healing tissue are mechanosensitive, meaning they can detect and respond to mechanical forces. These cells use mechanoreceptors, such as integrins, to sense changes in the mechanical environment and adjust their behavior accordingly.
Mechanotransduction
- Integrin Signaling: Integrins, which are transmembrane receptors, play a key role in converting mechanical signals into biochemical signals. They connect the ECM to the cytoskeleton of fibroblasts, enabling cells to sense and respond to mechanical cues. Integrin clustering at focal adhesions activates intracellular signaling pathways such as focal adhesion kinase (FAK) and the RhoA/ROCK pathway, which regulate cytoskeletal dynamics and cell contractility. FAK activation leads to the phosphorylation of various substrates that propagate signals to the nucleus, influencing gene expression and cellular behavior.
- Ion Channels: Mechanically activated ion channels, such as stretch-activated calcium channels, allow the influx of calcium ions (Ca2+). These ions act as secondary messengers in various signaling pathways. Increased intracellular Ca2+ levels can activate calmodulin, a calcium-binding protein, and downstream effectors like MLCK, promoting cytoskeletal rearrangement and enhancing the mechanical strength of the tissue.
- MAPK Pathway: Mechanical stress can activate the MAPK pathway, leading to the phosphorylation and activation of transcription factors like AP-1. These transcription factors upregulate genes involved in collagen synthesis and matrix remodeling, ensuring that the ECM is properly structured and functional. The MAPK pathway plays a crucial role in coordinating the cellular responses to mechanical stress, promoting tissue repair and regeneration.
Cellular Response
- Collagen Fiber Realignment: In response to mechanical stress, fibroblasts realign the collagen fibers along the lines of tension. This realignment involves the reorganization of the cytoskeleton and the secretion of new collagen fibers in a specific orientation, resulting in a more organized and functional tissue structure.
Importance of Full Range of Motion (ROM) and Weight Bearing Exercises
Stimulating Collagen Organization
- Mechanical Stimuli: Full ROM exercises and weight-bearing activities provide the necessary mechanical stimuli for the proper orientation and maturation of collagen fibers. These activities encourage the alignment of fibers in the direction of functional stress, enhancing the strength and elasticity of the healing tissue.
Preventing Adhesions
- Regular Movement: Regular movement helps prevent the formation of adhesions and scar tissue that can restrict mobility. By maintaining tissue pliability and flexibility, exercises ensure that the healing tissue remains functional and capable of normal movement.
Optimizing Tissue Quality
- Mechanical Loading: Mechanical loading during rehabilitation not only influences collagen orientation but also improves the overall quality of the repaired tissue. It promotes the production of type I collagen and facilitates the transition from a provisional matrix to a more durable and resilient ECM. This process is essential for restoring the full functional capacity of the tissue.
Molecular Pathways Involved in Mechanotransduction
Integrin Signaling
- Focal Adhesions: Integrins cluster at focal adhesions, connecting the ECM to the actin cytoskeleton. This clustering activates intracellular signaling pathways such as FAK and RhoA/ROCK, which regulate cytoskeletal dynamics and cell contractility. FAK activation leads to the phosphorylation of various substrates that propagate signals to the nucleus, influencing gene expression and cellular behavior.
Ion Channels
- Calcium Influx: Mechanically activated ion channels, such as stretch-activated calcium channels, allow the influx of calcium ions (Ca2+). These ions act as secondary messengers in various signaling pathways. Increased intracellular Ca2+ levels can activate calmodulin, a calcium-binding protein, and downstream effectors like MLCK, promoting cytoskeletal rearrangement and enhancing the mechanical strength of the tissue.
MAPK Pathway
- Gene Expression: Mechanical stress can activate the MAPK pathway, leading to the phosphorylation and activation of transcription factors like AP-1. These transcription factors upregulate genes involved in collagen synthesis and matrix remodeling, ensuring that the ECM is properly structured and functional. The MAPK pathway plays a crucial role in coordinating the cellular responses to mechanical stress, promoting tissue repair and regeneration.
Transformation Process
Initial Collagen Production
- Provisional ECM Formation: Immediately following tissue injury, the body responds by producing type III collagen rapidly. This collagen forms a provisional extracellular matrix (ECM), a temporary scaffold essential for stabilizing the wound and facilitating the initial stages of tissue repair. Type III collagen fibers are thinner, more loosely organized, and less tensile than type I collagen, making them suitable for forming a flexible and adaptive framework. This provisional matrix supports the migration of immune cells to the injury site, providing structural support for the proliferative activities of fibroblasts and other reparative cells, and laying the groundwork for subsequent tissue reconstruction.
Matrix Metalloproteinases (MMPs)
- Collagen Degradation: The transition from type III to type I collagen necessitates the degradation of the initial provisional matrix. Matrix metalloproteinases (MMPs) play a crucial role in this process. Specifically, MMP-1 (collagenase-1) and MMP-2 (gelatinase A) are enzymes that break down type III collagen, facilitating its removal from the ECM. This degradation process is tightly regulated by cytokines and growth factors to ensure that the breakdown of type III collagen is balanced with the synthesis of type I collagen, thereby avoiding excessive ECM degradation. Tissue inhibitors of metalloproteinases (TIMPs) also play a vital role in regulating MMP activity to maintain ECM integrity.
Synthesis of Type I Collagen
- Fibroblast Activation: Fibroblasts are the primary cells involved in producing type I collagen. They are activated by various growth factors, such as transforming growth factor-beta (TGF-β) and platelet-derived growth factor (PDGF), as well as mechanical stimuli from the healing environment. These growth factors bind to their respective receptors on fibroblasts, triggering intracellular signaling pathways such as the mitogen-activated protein kinase (MAPK) pathway and the phosphoinositide 3-kinase (PI3K)/Akt pathway. These pathways lead to the upregulation of collagen genes, particularly COL1A1 and COL1A2, which encode the α1 and α2 chains of type I collagen, respectively.
- Type I Collagen Characteristics: Type I collagen fibers are thicker, stronger, and more organized than type III collagen. They provide the tensile strength and structural integrity necessary for the long-term functionality of the repaired tissue. The transition to type I collagen ensures that the newly formed tissue can withstand mechanical stresses and maintain its structural integrity over time.
Regaining Range of Motion (ROM), Proprioception, and Contractile Function
Mechanical Stimuli and Mechanotransduction
- Role of Mechanical Load: Regaining ROM and applying mechanical load during rehabilitation are essential for stimulating fibroblasts through mechanotransduction pathways. Integrins, which are transmembrane receptors that link the ECM to the cytoskeleton, play a pivotal role in sensing mechanical changes and transmitting these signals into the cell. When mechanical stress is applied, integrins cluster at focal adhesions, activating signaling pathways such as focal adhesion kinase (FAK) and MAPK. These pathways regulate cellular functions such as adhesion, migration, proliferation, and survival, which are critical for tissue repair and remodeling.
Proprioception
- Sensory Feedback: Proprioception involves the sensory neurons and mechanoreceptors in the tissue that provide feedback on the position and movement of the body. This sensory input is essential for coordinating movement and ensuring proper tissue function. Rehabilitation exercises enhance proprioceptive input, which helps in the realignment and strengthening of collagen fibers by guiding the movement patterns that stress the tissue in beneficial ways. Enhanced proprioception ensures that movements are precise and controlled, reducing the risk of re-injury.
Contractile Information
- Muscle Contractions: During rehabilitation exercises, muscle contractions produce mechanical forces that influence collagen fiber orientation and ECM remodeling. These forces stimulate mechanosensitive pathways within fibroblasts, leading to the reorganization and alignment of collagen fibers along the lines of tension. This process is crucial for restoring the contractile properties of the tissue, ensuring that it can withstand mechanical loads and function effectively in daily activities.
Biomechanical Qualities and Cross-Link Formation
Collagen Cross-Linking
- Lysyl Oxidase Activity: The enzyme lysyl oxidase is critical for the maturation and stabilization of collagen fibers. It catalyzes the oxidative deamination of lysine residues in collagen, facilitating the formation of covalent cross-links between collagen molecules. These cross-links enhance the tensile strength and stiffness of collagen fibers, contributing to the biomechanical integrity of the tissue. Lysyl oxidase activity is regulated by factors such as TGF-β, which stimulates its expression and activity, ensuring the proper formation of cross-links.
Tissue Stiffness and Strength
- Mechanical Stability: Cross-linking increases the mechanical stability of the collagen network, allowing the tissue to bear greater loads and resist deformation. This process is essential for the functional recovery of the injured tissue, as it ensures that the repaired area can handle the stresses of daily activities and prevent re-injury. The enhanced tensile strength and stiffness provided by collagen cross-linking are crucial for restoring the tissue’s structural integrity and functionality.
Support by Load and Mobilization
Load Application
- Gradual Loading: Gradual and controlled loading during rehabilitation exercises promotes the alignment and maturation of collagen fibers. Weight-bearing activities and resistance exercises stimulate fibroblasts to produce and organize type I collagen in the direction of applied stress. This process ensures that collagen fibers are properly oriented to handle mechanical loads, enhancing the strength and functionality of the tissue.
Mobilization into End of ROM
- Stretching and Mobilization: Stretching and mobilizing the tissue to the end of its ROM encourages the realignment of collagen fibers and prevents the formation of adhesions. Regular movement and stretching ensure that the tissue remains flexible and functional, reducing the risk of stiffness and restricted mobility. Mobilization exercises help maintain tissue pliability and ensure that the repaired tissue can move freely without being restricted by scar tissue.
Timeframe for Tissue Recovery
Extended Healing Period
- Long-Term Remodeling: The complete transformation and maturation of collagen fibers can take 300-500 days, reflecting the complexity of tissue remodeling and the gradual process of regaining full function. During this time, continuous and appropriate rehabilitation is crucial to ensure optimal healing. The extended healing period allows for the progressive strengthening and stabilization of the tissue, ensuring that it can handle mechanical stresses and function effectively in daily activities.
Phases of Healing
- Proliferative Phase: During the initial weeks to months, type III collagen is laid down and gradually replaced by type I collagen. Controlled loading and ROM exercises are crucial during this phase to support the transition and strengthen the new tissue. This phase is characterized by active cell proliferation and ECM synthesis, providing a scaffold for further tissue remodeling.
- Remodeling Phase: Over several months to a year or more, collagen fibers undergo continuous remodeling, cross-linking, and realignment in response to mechanical stimuli. Ongoing rehabilitation is necessary to optimize tissue strength and function, ensuring that collagen fibers are properly aligned and cross-linked to withstand mechanical stresses. This phase involves the gradual maturation and strengthening of the tissue, leading to the restoration of its full functional capacity.
Molecular Pathways Involved
Transforming Growth Factor-Beta (TGF-β)
- Collagen Synthesis: TGF-β is a key regulator of collagen synthesis and ECM remodeling. It stimulates fibroblasts to produce type I collagen by activating the SMAD signaling pathway, which upregulates collagen gene expression. TGF-β also enhances the activity of lysyl oxidase, promoting the cross-link formation necessary for strengthening collagen fibers. The regulation of collagen synthesis and ECM remodeling by TGF-β ensures that the tissue can withstand mechanical stresses and maintain its structural integrity.
Focal Adhesion Kinase (FAK) Pathway
- Mechanotransduction: FAK is activated by integrins in response to mechanical stress. This pathway regulates cell adhesion, migration, and survival, contributing to the organization and strength of the ECM. FAK activation leads to the phosphorylation of various proteins involved in cytoskeletal dynamics and gene expression, coordinating the cellular response to mechanical stimuli. The FAK pathway plays a crucial role in ensuring that cells can sense and respond to mechanical changes in the environment, promoting tissue repair and remodeling.
Mitogen-Activated Protein Kinase (MAPK) Pathway
- Cell Proliferation and Collagen Synthesis: The MAPK pathway is activated by mechanical and chemical signals, promoting cell proliferation and collagen synthesis. This pathway involves a cascade of phosphorylation events that activate transcription factors such as AP-1, which upregulate genes involved in ECM remodeling and collagen production. The MAPK pathway plays a critical role in the remodeling and maturation of the ECM, ensuring the proper structure and function of the repaired tissue. The activation of this pathway ensures that cells can proliferate and synthesize the necessary components for tissue repair and regeneration.
Meniscal Injuries
Mechanisms of Injury
Cellular Responses to Meniscal Injury
Initial Inflammatory Response
- Vascular Changes: Meniscal injuries, particularly in the peripheral (vascular) zone, result in the disruption of blood vessels, leading to hematoma formation and increased vascular permeability. This vascular disruption allows immune cells and signaling molecules to infiltrate the injury site, setting the stage for the repair process. The increased permeability facilitates the movement of plasma proteins and cells from the bloodstream into the tissue, contributing to swelling and the formation of a supportive matrix for cell migration.
- Immune Cell Recruitment: Neutrophils are among the first immune cells to arrive at the injury site. They release reactive oxygen species (ROS) and proteolytic enzymes that help clear damaged tissue and debris. This initial cleanup is followed by the recruitment of macrophages, which play a crucial role in orchestrating subsequent repair processes by releasing growth factors and cytokines. Macrophages transition from an inflammatory (M1) phenotype to a reparative (M2) phenotype, aiding in debris clearance and promoting tissue repair.
- Cytokine Release: Injured meniscal tissue releases pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). These cytokines further recruit and activate additional immune cells, amplifying the inflammatory response and initiating the healing process. These cytokines also stimulate the production of matrix metalloproteinases (MMPs), which degrade ECM components, facilitating tissue remodeling and repair. Additionally, cytokines can induce pain and contribute to the overall inflammatory milieu that characterizes the acute phase of meniscal injury.
Extracellular Matrix Degradation and Remodeling
- Matrix Metalloproteinases (MMPs): MMPs such as MMP-1, MMP-3, and MMP-13 are upregulated in response to injury and cytokine signaling. These enzymes degrade various ECM components, including collagen and aggrecan. While this degradation is necessary to remove damaged tissue and allow for new matrix synthesis, excessive MMP activity can lead to further tissue breakdown and compromised meniscal function. The regulation of MMP activity is critical for maintaining a balance between ECM degradation and synthesis, ensuring effective tissue remodeling without excessive breakdown.
- Tissue Inhibitors of Metalloproteinases (TIMPs): TIMPs regulate MMP activity by inhibiting their enzymatic function. The balance between MMPs and TIMPs is crucial for controlled ECM remodeling. In the context of meniscal injury, an imbalance favoring MMP activity can lead to excessive ECM degradation and impaired healing. TIMPs are essential for preventing uncontrolled matrix degradation and ensuring that the ECM is rebuilt in a manner that supports tissue function and integrity.
- Growth Factors: Growth factors such as TGF-β, fibroblast growth factor (FGF), and insulin-like growth factor 1 (IGF-1) are involved in promoting ECM synthesis and remodeling. These factors stimulate the production of collagen and other ECM components by meniscal cells, contributing to tissue repair and regeneration. They also help regulate the activities of MMPs and TIMPs, ensuring a balanced remodeling process. Growth factors play a pivotal role in orchestrating the repair process by modulating cellular activities and promoting the synthesis of new matrix components.
Chondrocyte and Fibrochondrocyte Activation
- Chondrocyte Activation: Chondrocytes in the meniscus, particularly in the inner avascular zone, become activated in response to injury and inflammatory signals. These cells increase the production of catabolic enzymes and pro-inflammatory cytokines, contributing to ECM degradation. This activity is necessary for clearing damaged matrix components but can be detrimental if not properly regulated, leading to further tissue breakdown. Activated chondrocytes also produce anabolic factors to balance matrix degradation with repair.
- Fibrochondrocyte Activation: Fibrochondrocytes, which are more prevalent in the vascularized outer zone of the meniscus, also become activated following injury. These cells play a crucial role in synthesizing new ECM components, including type I and type II collagen, which are essential for meniscal repair. Fibrochondrocytes respond to growth factors and mechanical stimuli by increasing their production of ECM proteins, supporting tissue regeneration. They are also involved in the repair of vascularized regions, contributing to the overall healing process.
- Cellular Hypertrophy: Both chondrocytes and fibrochondrocytes can undergo hypertrophy, characterized by an increase in cell size and metabolic activity. This hypertrophic response is associated with increased ECM production and repair but can also lead to altered tissue mechanics if not properly regulated. Cellular hypertrophy can result in stiffening of the tissue, affecting its functional properties. The balance between hypertrophy and normal cellular activity is essential for maintaining tissue flexibility and function.
Mesenchymal Stem Cell (MSC) Recruitment and Differentiation
- MSC Recruitment: Chemotactic signals such as stromal cell-derived factor 1 (SDF-1) and vascular endothelial growth factor (VEGF) attract MSCs from surrounding tissues and the bone marrow to the injury site. These MSCs migrate through the ECM and localize to areas of damage, where they contribute to tissue repair. The recruitment of MSCs is a critical step in enhancing the regenerative capacity of the meniscus, particularly in regions with limited vascular supply.
- Differentiation: Once at the injury site, MSCs can differentiate into chondrocytes and fibrochondrocytes in response to local cues, including growth factors and mechanical signals. TGF-β, IGF-1, and bone morphogenetic proteins (BMPs) are among the key factors that promote MSC differentiation into meniscal cells. These differentiated cells then produce ECM components necessary for tissue regeneration. The ability of MSCs to differentiate into multiple cell types makes them valuable for tissue engineering and regenerative medicine applications.
- Paracrine Effects: In addition to differentiating into repair cells, MSCs exert paracrine effects by secreting cytokines and growth factors that modulate the inflammatory response, promote angiogenesis, and enhance the activity of resident meniscal cells. These paracrine effects help create a supportive environment for tissue repair and regeneration. MSCs’ ability to influence the local microenvironment through paracrine signaling is crucial for coordinating the repair process and improving healing outcomes.
Angiogenesis
- Vascular Endothelial Growth Factor (VEGF): VEGF is a major regulator of angiogenesis. Its expression is upregulated in response to hypoxia and inflammatory signals at the injury site. VEGF stimulates the proliferation and migration of endothelial cells, leading to the formation of new capillaries, which enhance the blood supply to the injured tissue. VEGF plays a vital role in ensuring that the regenerating tissue receives adequate oxygen and nutrients to support cell survival and function.
- Role of Angiogenesis in Healing: Enhanced vascularization improves the delivery of reparative cells, growth factors, and nutrients to the injury site. This supports the metabolic demands of proliferating and differentiating cells and facilitates the removal of metabolic waste products, promoting a conducive environment for tissue repair. Angiogenesis also helps integrate the newly formed tissue with the existing vascular network, improving overall tissue function and viability.
- Zone-Specific Angiogenesis: The meniscus is divided into three zones based on vascularity: the red-red zone (outer third), red-white zone (middle third), and white-white zone (inner third). Angiogenesis is most prominent in the red-red zone due to its existing blood supply, while the white-white zone remains avascular and relies on diffusion for nutrient supply, limiting its healing capacity. This differential vascularity significantly influences the healing potential and repair strategies for different regions of the meniscus. Understanding the vascular distribution within the meniscus is crucial for designing targeted therapies and interventions to enhance healing.
Mechanotransduction and Mechanical Loading
- Integrin Signaling: Integrins are transmembrane receptors that facilitate cell-ECM interactions and transmit mechanical signals to the cell interior. Mechanical loading activates integrins, leading to the activation of focal adhesion kinase (FAK) and subsequent signaling pathways. These pathways regulate cytoskeletal dynamics, cell proliferation, and ECM production, promoting tissue repair and adaptation to mechanical stress. Integrin signaling is critical for maintaining the structural integrity of the meniscus and ensuring that it can withstand mechanical forces.
- Ion Channels: Stretch-activated ion channels, particularly calcium channels, respond to mechanical loading by allowing the influx of ions into the cell. Elevated intracellular calcium levels activate signaling pathways, including the calcineurin/NFAT pathway and the calmodulin-dependent kinase (CaMK) pathway. These pathways regulate gene expression and cellular responses to mechanical loading, influencing processes such as cell migration, proliferation, and ECM synthesis. Ion channel activation is essential for translating mechanical signals into biochemical responses that drive tissue repair and adaptation.
- MAPK Pathway: The mitogen-activated protein kinase (MAPK) pathway is another key mechanotransduction pathway activated by mechanical loading. Activation of MAPKs, such as ERK1/2 and p38, regulates cell proliferation, differentiation, and ECM production. This pathway plays a crucial role in coordinating the cellular response to mechanical stimuli, ensuring that the meniscus adapts to mechanical loads and maintains its functional properties. The MAPK pathway integrates mechanical and biochemical signals to regulate gene expression and cellular activities essential for tissue repair and regeneration.
Articular Cartilage and Its Functionality with Molecular Biology Insights
Cartilage Injuries: Causes and Types
Cellular Responses to Cartilage Injury
- Chondrocyte Death: Chondrocyte death can occur through two main mechanisms: necrosis and apoptosis. Necrosis is typically associated with acute trauma and results from direct mechanical damage to the cells, leading to their abrupt and uncontrolled death. This process often triggers an inflammatory response. Apoptosis, or programmed cell death, is a more regulated process that can be induced by various factors, including inflammatory cytokines, oxidative stress, and ECM degradation. Apoptosis involves a series of signaling cascades that lead to cellular shrinkage, DNA fragmentation, and ultimately cell death. The loss of chondrocytes impairs the maintenance of the ECM, exacerbating tissue damage.
- Extracellular Matrix Damage: The ECM is critical for the structural integrity and function of cartilage. It is composed of a dense network of collagen fibers and proteoglycans that provide tensile strength and compressive resistance. Injury to the cartilage disrupts the ECM, impairing its biomechanical properties. The breakdown of collagen and proteoglycans releases matrix fragments into the joint space, which can further stimulate inflammatory responses and perpetuate the cycle of degeneration.
Inflammatory Response to Cartilage Injury
- Cytokine Release: Following injury, pro-inflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) are released by chondrocytes and synovial cells. These cytokines amplify the inflammatory response and stimulate the production of catabolic enzymes that degrade ECM components. IL-1 and TNF-α play central roles in the inflammatory cascade, leading to the activation of signaling pathways that promote the expression of additional inflammatory mediators and matrix-degrading enzymes.
- Matrix Metalloproteinases (MMPs): MMPs are a family of enzymes that degrade various components of the ECM, including collagen and proteoglycans. In response to inflammatory cytokines, MMPs such as MMP-1 (collagenase), MMP-3 (stromelysin), and MMP-13 (collagenase-3) are upregulated. These enzymes break down the structural proteins of the ECM, exacerbating tissue damage and impairing the repair process.
- Aggrecanases: Aggrecanases, particularly ADAMTS-4 and ADAMTS-5 (a disintegrin and metalloproteinase with thrombospondin motifs), are enzymes that specifically degrade aggrecan, a major proteoglycan in cartilage. The activity of these enzymes increases following injury, further compromising the ECM and contributing to the loss of cartilage integrity.
Extracellular Matrix Remodeling and Repair
- Degradation: The initial phase of ECM remodeling involves the breakdown of damaged matrix components by MMPs and aggrecanases. This degradation process is necessary to clear debris and prepare the tissue for repair. However, excessive or uncontrolled degradation can lead to further tissue breakdown and hinder the repair process.
- Synthesis: In response to injury, chondrocytes increase the synthesis of ECM components, including type II collagen and aggrecan. Growth factors such as transforming growth factor-beta (TGF-β), insulin-like growth factor-1 (IGF-1), and bone morphogenetic proteins (BMPs) play key roles in promoting ECM synthesis and chondrocyte proliferation. These growth factors stimulate signaling pathways that enhance the production of matrix proteins and support tissue repair.
Chondrocyte Responses to Injury
- Proliferation and Clustering: Following injury, surviving chondrocytes can proliferate and form clusters, known as chondrocyte clones. This response aims to increase the number of cells available for matrix repair. However, these clusters can alter the biomechanical properties of the cartilage and contribute to matrix degradation if not properly regulated. The formation of chondrocyte clusters can lead to the production of fibrocartilage, which is mechanically inferior to the original hyaline cartilage.
- Phenotypic Modulation: Chondrocytes can undergo phenotypic modulation in response to injury, shifting from a quiescent, matrix-producing phenotype to a more fibroblastic phenotype characterized by increased production of type I collagen. This phenotypic shift is driven by inflammatory cytokines and mechanical stress. While it represents an attempt to repair the tissue, the resulting fibrocartilage is less durable and resilient than hyaline cartilage, compromising the long-term functionality of the joint.
- Autophagy: Autophagy is a cellular process that involves the degradation and recycling of damaged or dysfunctional cellular components. It can be activated in chondrocytes in response to injury and stress, helping to maintain cellular homeostasis and protect chondrocytes from apoptosis. Autophagy supports tissue survival and repair by promoting the clearance of damaged organelles and proteins, thereby preserving cellular function.
Mesenchymal Stem Cell (MSC) Recruitment and Differentiation
- MSC Recruitment: Chemotactic signals, such as stromal cell-derived factor-1 (SDF-1) and platelet-derived growth factor (PDGF), attract MSCs to the injured cartilage. These cells can originate from the synovium, subchondral bone, or other surrounding tissues. The recruitment of MSCs is a critical step in the repair process, as these cells have the potential to replenish the chondrocyte population and enhance ECM synthesis.
- Differentiation: Once at the injury site, MSCs can differentiate into chondrocytes in response to local cues, including growth factors like TGF-β and BMPs. This differentiation process is crucial for replenishing the chondrocyte population and restoring ECM synthesis. MSCs undergo a series of differentiation stages, ultimately adopting the phenotype and functional characteristics of mature chondrocytes.
- Paracrine Effects: MSCs also exert paracrine effects by secreting cytokines and growth factors that modulate inflammation, promote chondrocyte survival, and enhance matrix synthesis. These paracrine signals help create a favorable environment for tissue repair by reducing inflammation, inhibiting apoptosis, and stimulating the production of ECM components.
Angiogenesis and Subchondral Bone Response
- Angiogenesis: Angiogenic factors such as vascular endothelial growth factor (VEGF) stimulate the formation of new blood vessels in the subchondral bone and synovium. While increased vascularization can enhance nutrient supply and support tissue repair, excessive angiogenesis can lead to subchondral bone changes that negatively impact cartilage health. For instance, increased blood vessel formation can result in the infiltration of inflammatory cells and the release of catabolic factors that contribute to cartilage degradation.
- Subchondral Bone Remodeling: The subchondral bone responds to cartilage injury through remodeling processes that influence the overlying cartilage. Changes in subchondral bone structure, such as increased bone density or the formation of osteophytes (bone spurs), can alter the mechanical environment of the cartilage and contribute to further degeneration. Subchondral bone remodeling can result in altered load distribution across the joint, exacerbating cartilage damage and impairing the repair process.
Mechanotransduction and Mechanical Loading
- Integrin Signaling: Integrins are transmembrane receptors that mediate cell-ECM interactions and transmit mechanical signals to the cell interior. Mechanical loading activates integrins, leading to the activation of focal adhesion kinase (FAK) and downstream signaling pathways that regulate cell behavior and ECM synthesis. Integrin signaling plays a crucial role in maintaining cartilage integrity and promoting tissue repair.
- Ion Channels: Stretch-activated ion channels, including calcium channels, respond to mechanical loading by allowing the influx of ions into the cell. Elevated intracellular calcium levels activate signaling pathways, including the calcineurin/NFAT pathway and the calmodulin-dependent kinase (CaMK) pathway, which regulate gene expression and cellular responses to mechanical loading. These pathways influence chondrocyte proliferation, differentiation, and ECM production.
- MAPK Pathway: The mitogen-activated protein kinase (MAPK) pathway is another key mechanotransduction pathway activated by mechanical loading. Activation of MAPKs, such as ERK1/2 and p38, regulates cell proliferation, differentiation, and ECM production. The MAPK pathway plays a critical role in the cellular response to mechanical stress, promoting cartilage repair and maintaining tissue homeostasis.
Cellular Responses to Knee Joint Injuries
Inflammation and Immune Response
- Vascular Response: Injury to knee joint structures disrupts blood vessels, leading to hemorrhage and the formation of a hematoma. This increases vascular permeability, allowing immune cells and signaling molecules to infiltrate the injury site. Endothelial cells lining the blood vessels produce adhesion molecules like E-selectin and P-selectin, which facilitate the attachment and migration of leukocytes to the injury site. This initial response helps to contain the injury and prepare the site for subsequent repair processes. The production of vascular endothelial growth factor (VEGF) is also upregulated, promoting angiogenesis and the restoration of blood flow to the injured area.
- Neutrophil Infiltration: Neutrophils are the first immune cells to arrive at the injury site, typically within hours. They release reactive oxygen species (ROS) and proteolytic enzymes such as neutrophil elastase and matrix metalloproteinases (MMPs) that help to clear debris and damaged tissue. Neutrophils also release chemokines like IL-8, which attract other immune cells to the injury site, amplifying the inflammatory response. These cells play a key role in the initial stages of inflammation, but their activity must be tightly regulated to prevent excessive tissue damage and the development of chronic inflammation.
- Macrophage Activation: Following neutrophils, macrophages infiltrate the injury site. These cells play a dual role in inflammation and repair. Pro-inflammatory (M1) macrophages release cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), which amplify the inflammatory response and recruit additional immune cells. Later, anti-inflammatory (M2) macrophages secrete growth factors like transforming growth factor-beta (TGF-β) and vascular endothelial growth factor (VEGF), promoting tissue repair and remodeling. The transition from M1 to M2 macrophages is a critical step in resolving inflammation and initiating healing. Macrophages also engage in phagocytosis, clearing apoptotic cells and matrix debris, which is essential for tissue regeneration.
- Lymphocyte Recruitment: Lymphocytes, particularly T cells, are also involved in the inflammatory response. They release cytokines that modulate the activity of other immune cells and resident cells, influencing the overall inflammatory environment. Regulatory T cells (Tregs) help to modulate the immune response and prevent excessive inflammation, which can lead to chronic damage. These interactions between different immune cells and cytokines create a complex regulatory network that ensures a balanced and effective response to injury. The adaptive immune response, mediated by lymphocytes, also contributes to the specificity and regulation of the immune response to injury.
Extracellular Matrix (ECM) Remodeling
- Matrix Metalloproteinases (MMPs): MMPs are proteolytic enzymes that degrade various ECM components, including collagen and proteoglycans. MMP-1 (collagenase-1) and MMP-13 (collagenase-3) degrade collagen, while MMP-3 (stromelysin-1) degrades other ECM proteins and activates other MMPs. MMP activity is upregulated in response to pro-inflammatory cytokines and mechanical stress. The regulation of MMP expression and activity is complex and involves multiple signaling pathways, including the NF-κB and MAPK pathways. Excessive MMP activity can lead to the breakdown of the ECM, compromising the structural integrity of the tissue and hindering the repair process. Additionally, the overactivity of MMPs can release ECM fragments that further stimulate inflammation, creating a feedback loop that exacerbates tissue damage.
- Tissue Inhibitors of Metalloproteinases (TIMPs): TIMPs regulate MMP activity by inhibiting their enzymatic function. The balance between MMPs and TIMPs is critical for controlled ECM remodeling. An imbalance favoring MMP activity can lead to excessive ECM degradation and impaired healing. TIMPs are regulated by growth factors and cytokines, ensuring that ECM remodeling occurs in a controlled manner. Maintaining the appropriate balance between MMPs and TIMPs is crucial for effective tissue repair and regeneration. The interaction between TIMPs and MMPs is a key regulatory mechanism that ensures that ECM degradation does not exceed the synthesis of new matrix components.
- Growth Factors: Various growth factors, including TGF-β, fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF), are involved in promoting ECM synthesis and remodeling. These factors stimulate the production of collagen and other ECM components by fibroblasts and chondrocytes, contributing to tissue repair and regeneration. The signaling pathways activated by these growth factors include the SMAD pathway (for TGF-β) and the PI3K/AKT pathway (for FGF and PDGF). Growth factors play a pivotal role in orchestrating the repair process by regulating cell proliferation, differentiation, and matrix production. For example, TGF-β can induce the expression of type II collagen and aggrecan, which are critical for cartilage repair.
Chondrocyte Responses
- Chondrocyte Apoptosis and Necrosis: Injury can lead to chondrocyte death through necrosis or apoptosis. Necrosis results from direct mechanical damage and leads to the release of cellular contents that can further stimulate inflammation. Apoptosis, or programmed cell death, can be triggered by inflammatory cytokines, oxidative stress, and matrix degradation. The mitochondrial pathway and the death receptor pathway are two main mechanisms of apoptosis in chondrocytes. The loss of chondrocytes compromises the maintenance of the ECM, exacerbating tissue damage and impairing the repair process. The balance between apoptosis and survival signals, such as those mediated by the PI3K/AKT pathway, is critical for maintaining chondrocyte viability.
- Chondrocyte Proliferation and Clustering: Surviving chondrocytes can proliferate and form clusters, known as chondrocyte clones. This response aims to increase the number of cells available for matrix repair but can alter the biomechanical properties of the cartilage if not properly regulated. The proliferation of chondrocytes is regulated by growth factors such as IGF-1 and FGF. However, unregulated chondrocyte clustering can lead to the formation of fibrocartilage, which is mechanically inferior to the original hyaline cartilage. The process of chondrocyte proliferation is also influenced by mechanical loading, which can enhance the production of matrix components.
- Phenotypic Modulation: Chondrocytes can undergo phenotypic modulation in response to injury, shifting from a quiescent, matrix-producing phenotype to a more fibroblastic phenotype characterized by increased production of type I collagen. This shift can compromise the quality of the repaired matrix, leading to the formation of fibrocartilage rather than hyaline cartilage. Inflammatory cytokines like IL-1 and TNF-α play a role in this phenotypic modulation. Phenotypic modulation is a double-edged sword; while it aims to repair tissue, it often results in mechanically inferior fibrocartilage. The regulation of chondrocyte phenotype involves signaling pathways such as Wnt/β-catenin and Hedgehog, which control the expression of cartilage-specific genes.
- Autophagy: Autophagy is a cellular process that involves the degradation and recycling of cellular components. It can be activated in chondrocytes in response to injury and stress. Autophagy helps to maintain cellular homeostasis and protect chondrocytes from apoptosis, supporting tissue survival and repair. The mTOR pathway is a key regulator of autophagy in chondrocytes. By promoting the clearance of damaged organelles and proteins, autophagy helps preserve chondrocyte function and viability under stress conditions. Autophagy can also influence the secretion of extracellular vesicles, which carry signaling molecules that modulate the repair process.
Synoviocyte Activation
- Type A Synoviocytes: These macrophage-like cells are involved in phagocytosis and the clearance of debris from the joint space. Following injury, type A synoviocytes increase their activity to remove necrotic cells and ECM fragments. They also produce pro-inflammatory cytokines that amplify the immune response. The activation of type A synoviocytes is crucial for clearing the joint of debris and initiating the repair process. These cells can also present antigens to T cells, linking innate and adaptive immune responses.
- Type B Synoviocytes: These fibroblast-like cells are responsible for the production of synovial fluid and ECM components of the synovial membrane. Injury and inflammation stimulate type B synoviocytes to produce cytokines, chemokines, and matrix-degrading enzymes, contributing to the overall inflammatory environment and ECM remodeling. Type B synoviocytes are regulated by growth factors like TGF-β and FGF. Their activity is essential for maintaining the synovial fluid’s lubricating properties and supporting the ECM’s structural integrity. The production of hyaluronan by type B synoviocytes is particularly important for the viscoelastic properties of synovial fluid.
- Cytokine Production: Activated synoviocytes produce pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α, which further amplify the inflammatory response and promote the recruitment of additional immune cells. These cytokines activate signaling pathways in resident cells, leading to increased production of MMPs and other matrix-degrading enzymes. The balance of cytokine production by synoviocytes is critical for modulating the inflammatory response and facilitating tissue repair. The regulation of cytokine production involves feedback mechanisms, such as the anti-inflammatory effects of IL-10 and TGF-β.
Fibroblast Activation and Proliferation
- Fibroblast Proliferation: The local environment created by the inflammatory response and the release of growth factors stimulates the proliferation of resident fibroblasts. These cells migrate to the site of injury and begin synthesizing new ECM components, primarily collagen and proteoglycans. Growth factors such as PDGF and FGF are critical for fibroblast proliferation. The migration and proliferation of fibroblasts are essential for forming a robust repair matrix. Fibroblasts respond to mechanical signals through integrins, which activate intracellular signaling pathways like FAK and ERK, promoting their proliferation and migration.
- Collagen Synthesis: Fibroblasts produce type I collagen, which is essential for restoring the structural integrity of ligaments and tendons. The alignment and organization of collagen fibers are critical for the mechanical properties of the repaired tissue. Mechanical loading during rehabilitation can influence collagen fiber alignment, promoting the formation of more organized and functional tissue. The synthesis of collagen is regulated by the TGF-β/SMAD pathway. Proper collagen synthesis and alignment are crucial for restoring the mechanical strength and function of injured tissues. The cross-linking of collagen fibers by enzymes like lysyl oxidase also contributes to the tensile strength of the repaired matrix.
- ECM Production: In addition to collagen, fibroblasts synthesize other ECM components, including elastin and various proteoglycans, which contribute to the biomechanical properties of the tissue. Proteoglycans help retain water within the tissue, maintaining its viscoelastic properties. The synthesis of these ECM components is regulated by growth factors and mechanical signals. The production of a well-balanced ECM is essential for the functional recovery of injured ligaments and tendons. Fibroblasts also produce decorin and biglycan, which regulate collagen fibrillogenesis and matrix assembly.
Mesenchymal Stem Cell (MSC) Recruitment and Differentiation
- MSC Recruitment: Chemotactic signals, such as stromal cell-derived factor-1 (SDF-1) and VEGF, attract MSCs from surrounding tissues and the bone marrow to the injury site. These MSCs migrate through the extracellular matrix and localize to areas of damage. The recruitment of MSCs is regulated by signaling pathways involving chemokine receptors like CXCR4. Effective recruitment of MSCs is crucial for replenishing the cell population at the injury site and supporting the repair process. The homing of MSCs to the injury site involves interactions with endothelial cells and the ECM.
- Differentiation: Once at the injury site, MSCs can differentiate into chondrocytes, fibroblasts, and other cell types in response to local cues, including growth factors and mechanical signals. TGF-β, IGF-1, and BMPs are among the key factors that promote MSC differentiation. The differentiation process involves signaling pathways like SMAD (for TGF-β) and Wnt/β-catenin (for BMPs). Proper differentiation of MSCs is essential for regenerating the appropriate cell types needed for effective tissue repair. MSC differentiation is also influenced by the mechanical environment, with compressive and tensile forces guiding their fate.
- Paracrine Effects: In addition to differentiating into repair cells, MSCs exert paracrine effects by secreting cytokines and growth factors that modulate the inflammatory response, promote angiogenesis, and enhance the activity of resident cells. These paracrine effects are mediated by factors such as IL-10 and TGF-β. MSCs’ paracrine activity helps create a supportive microenvironment for tissue repair by reducing inflammation and promoting cellular proliferation and differentiation. The secretion of extracellular vesicles by MSCs also plays a role in intercellular communication and the modulation of the repair process.
Angiogenesis
- VEGF: VEGF is a major regulator of angiogenesis. Its expression is upregulated in response to hypoxia and other signals from the injury site. VEGF promotes the proliferation and migration of endothelial cells, leading to the formation of new blood vessels. The VEGF signaling pathway involves the activation of receptors like VEGFR-2 and downstream signaling molecules such as PI3K/AKT and ERK. VEGF is crucial for initiating and sustaining angiogenesis during tissue repair. The regulation of VEGF expression involves hypoxia-inducible factors (HIFs), which respond to low oxygen levels in the injured tissue.
- Angiogenic Factors: Other factors involved in angiogenesis include FGF and PDGF. These factors work in concert with VEGF to stimulate endothelial cell activity and vessel formation. The signaling pathways activated by these factors include the MAPK pathway and the PI3K/AKT pathway. The coordinated activity of multiple angiogenic factors ensures effective vascularization of the repair tissue. The balance between pro-angiogenic and anti-angiogenic signals, such as those from thrombospondin-1, is critical for proper vessel formation and function.
- Role in Repair: The newly formed blood vessels enhance the delivery of oxygen and nutrients to the injury site, supporting the metabolic demands of proliferating fibroblasts and other reparative cells. Angiogenesis also facilitates the removal of waste products and debris from the injury site. The balance between pro-angiogenic and anti-angiogenic factors is critical for proper vascularization and tissue repair. Adequate vascularization is essential for sustaining the repair process and supporting long-term tissue regeneration. The interaction between endothelial cells and pericytes stabilizes the newly formed vessels, ensuring their functionality.
| Category | Aspect | Description |
| Inflammation and Immune Response |
Vascular Response | Injury disrupts blood vessels, leading to hemorrhage and hematoma formation, increasing vascular permeability. Endothelial cells produce adhesion molecules (E-selectin, P-selectin) and VEGF to facilitate leukocyte migration and promote angiogenesis. |
| Neutrophil Infiltration | Neutrophils arrive within hours, releasing ROS, proteolytic enzymes (neutrophil elastase, MMPs), and chemokines (IL-8) to clear debris and attract other immune cells, amplifying inflammation. | |
| Macrophage Activation | M1 macrophages release cytokines (IL-1, IL-6, TNF-α) to amplify inflammation, while M2 macrophages secrete growth factors (TGF-β, VEGF) to promote tissue repair and remodeling. Macrophages also phagocytose apoptotic cells and debris. | |
| Lymphocyte Recruitment | T cells release cytokines to modulate immune and resident cell activity. Regulatory T cells (Tregs) help prevent excessive inflammation, linking innate and adaptive immune responses. | |
| Extracellular Matrix (ECM) Remodeling |
MMPs | MMPs degrade ECM components (collagen, proteoglycans). MMP-1 and MMP-13 target collagen, while MMP-3 degrades other ECM proteins and activates additional MMPs. MMP activity is upregulated by pro-inflammatory cytokines and mechanical stress, regulated by NF-κB and MAPK pathways. |
| TIMPs | TIMPs inhibit MMP activity, balancing ECM degradation and synthesis. An imbalance favoring MMP activity leads to excessive ECM degradation. TIMPs are regulated by growth factors and cytokines. | |
| Growth Factors | Growth factors (TGF-β, FGF, PDGF) promote ECM synthesis and remodeling by stimulating fibroblasts and chondrocytes to produce collagen and other ECM components. Key pathways include SMAD (TGF-β) and PI3K/AKT (FGF, PDGF). | |
| Chondrocyte Responses |
Apoptosis and Necrosis | Chondrocytes die via necrosis or apoptosis due to mechanical damage, cytokines, oxidative stress, and matrix degradation. Apoptosis involves mitochondrial and death receptor pathways. The PI3K/AKT pathway balances apoptosis and survival. |
| Proliferation and Clustering | Surviving chondrocytes proliferate and form clusters (chondrocyte clones) to repair the matrix. Regulated by IGF-1 and FGF, but unregulated clustering can lead to fibrocartilage formation, mechanically inferior to hyaline cartilage. | |
| Phenotypic Modulation | Chondrocytes shift from a matrix-producing phenotype to a fibroblastic phenotype, producing type I collagen in response to injury. This shift, driven by IL-1 and TNF-α, compromises matrix quality, leading to fibrocartilage formation. | |
| Autophagy | Autophagy degrades and recycles cellular components, maintaining homeostasis and protecting chondrocytes from apoptosis. Regulated by the mTOR pathway, autophagy promotes the clearance of damaged organelles and proteins, preserving chondrocyte function. | |
| Synoviocyte Activation |
Type A Synoviocytes | Macrophage-like cells involved in phagocytosis and debris clearance. Post-injury, they increase activity, removing necrotic cells and ECM fragments, and producing pro-inflammatory cytokines. |
| Type B Synoviocytes | Fibroblast-like cells producing synovial fluid and ECM components. Injury stimulates them to produce cytokines, chemokines, and matrix-degrading enzymes, contributing to inflammation and ECM remodeling. | |
| Cytokine Production | Activated synoviocytes produce pro-inflammatory cytokines (IL-1, IL-6, TNF-α), amplifying inflammation and recruiting additional immune cells. Cytokines activate resident cell signaling pathways, increasing MMP production. | |
| Fibroblast Activation and Proliferation |
Proliferation | Inflammatory response and growth factors stimulate fibroblast proliferation. Fibroblasts migrate to the injury site, synthesizing ECM components (collagen, proteoglycans). Regulated by PDGF and FGF, fibroblasts respond to mechanical signals through integrins. |
| Collagen Synthesis | Fibroblasts produce type I collagen, essential for restoring ligament and tendon integrity. Mechanical loading during rehabilitation promotes collagen fiber alignment. Regulated by TGF-β/SMAD pathway, collagen cross-linking by lysyl oxidase enhances tensile strength. | |
| ECM Production | Fibroblasts synthesize ECM components (elastin, proteoglycans) that contribute to tissue biomechanics. Proteoglycans retain water, maintaining viscoelastic properties. Fibroblasts produce decorin and biglycan, regulating collagen fibrillogenesis and matrix assembly. | |
| Mesenchymal Stem Cell (MSC) Recruitment and Differentiation |
Recruitment | Chemotactic signals (SDF-1, VEGF) attract MSCs to the injury site. MSC migration involves chemokine receptors (CXCR4) and interactions with endothelial cells and ECM. Effective recruitment replenishes cells at the injury site. |
| Differentiation | MSCs differentiate into chondrocytes, fibroblasts, and other cells in response to growth factors (TGF-β, IGF-1, BMPs) and mechanical signals. Signaling pathways include SMAD (TGF-β) and Wnt/β-catenin (BMPs). Mechanical environment influences differentiation. | |
| Paracrine Effects | MSCs secrete cytokines and growth factors (IL-10, TGF-β) that modulate inflammation, promote angiogenesis, and enhance resident cell activity. MSC paracrine effects create a supportive microenvironment for tissue repair, involving extracellular vesicle secretion. | |
| Angiogenesis |
VEGF | VEGF, upregulated by hypoxia, promotes endothelial cell proliferation and migration, leading to new blood vessel formation. VEGF signaling involves receptors (VEGFR-2) and downstream molecules (PI3K/AKT, ERK), regulated by hypoxia-inducible factors (HIFs). |
| Angiogenic Factors | Factors like FGF and PDGF work with VEGF to stimulate endothelial cell activity and vessel formation. Activated pathways include MAPK and PI3K/AKT. The balance between pro-angiogenic and anti-angiogenic signals (thrombospondin-1) is critical. | |
| Role in Repair | New blood vessels enhance oxygen and nutrient delivery, supporting fibroblast and reparative cell metabolism. Angiogenesis facilitates waste removal from the injury site. Interactions between endothelial cells and pericytes stabilize vessels, ensuring functionality. |
Mechanical Loading and Cellular Mechanisms
Mechanotransduction Pathways
Integrin Signaling
- Integrin Activation: Mechanical loading induces the clustering of integrins at focal adhesion sites, which are specialized structures that link the ECM to the actin cytoskeleton within the cell. This clustering enhances the strength of the integrin-ECM bond and initiates intracellular signaling cascades. The process begins with integrins binding to ECM proteins such as fibronectin and collagen, which triggers conformational changes in integrin molecules, promoting their activation and subsequent signaling. This clustering is crucial for amplifying mechanical signals and ensuring a robust cellular response.
- Focal Adhesion Kinase (FAK): One of the first responses to integrin clustering is the activation of FAK. Activated FAK undergoes autophosphorylation at specific tyrosine residues, creating binding sites for various signaling proteins, including Src family kinases. This leads to the formation of a multi-protein signaling complex at focal adhesions. FAK plays a central role in transmitting mechanical signals by recruiting and activating other signaling molecules, thereby amplifying the mechanical signal and directing cellular responses. The activation of FAK is essential for the initiation of several downstream signaling pathways that regulate various cellular functions.
- Downstream Signaling Pathways: Activated FAK triggers several downstream signaling pathways, including the MAPK pathway, PI3K/Akt pathway, and Rho family GTPases. These pathways regulate various cellular processes, such as cell proliferation, survival, migration, and differentiation. Each of these pathways contributes to the overall cellular response to mechanical stress, ensuring that cells can adapt and maintain homeostasis under varying mechanical conditions. For example, the MAPK pathway regulates gene expression and cell differentiation, while the PI3K/Akt pathway promotes cell survival and proliferation.
- Cytoskeletal Remodeling: Integrin signaling also influences the organization of the actin cytoskeleton, which is crucial for maintaining cell shape and enabling cell movement. Mechanical loading promotes the formation of stress fibers and focal adhesions, enhancing the cell’s mechanical stability and ability to withstand further mechanical stress. The cytoskeleton’s dynamic nature allows cells to rapidly reorganize in response to mechanical signals, supporting processes such as migration, division, and structural integrity. This remodeling is essential for cells to adapt to changes in their mechanical environment and maintain tissue function.
Ion Channels and Calcium Signaling
- Stretch-Activated Ion Channels: Mechanical loading causes the deformation of the cell membrane, leading to the opening of stretch-activated ion channels. These channels are permeable to various ions, including calcium (Ca²⁺), sodium (Na⁺), and potassium (K⁺). The opening of these channels allows ions to flow into the cell, initiating a cascade of intracellular events that translate mechanical signals into biochemical responses. These channels play a key role in sensing mechanical forces and converting them into cellular responses.
- Calcium Influx: The entry of Ca²⁺ into the cell is a key event in mechanotransduction. Elevated intracellular calcium levels act as a secondary messenger, activating various signaling pathways that influence cellular functions. Calcium ions bind to and activate numerous calcium-binding proteins, triggering a range of cellular responses from muscle contraction to gene expression. This influx is tightly regulated to ensure precise cellular responses to mechanical stimuli.
- Calcineurin/NFAT Pathway: Increased Ca²⁺ levels activate calcineurin, a calcium/calmodulin-dependent phosphatase. Calcineurin dephosphorylates nuclear factor of activated T-cells (NFAT), allowing it to translocate to the nucleus and regulate gene expression. NFAT controls the expression of genes involved in cell proliferation, differentiation, and survival, making it a critical mediator of cellular responses to mechanical stress. This pathway is essential for coordinating cellular activities in response to changes in mechanical loading.
- Calmodulin-Dependent Kinase (CaMK) Pathway: Ca²⁺ binds to calmodulin, forming a complex that activates CaMK. Activated CaMK phosphorylates various target proteins, influencing gene expression and cellular responses to mechanical loading. This pathway helps regulate numerous cellular activities, including metabolism, muscle contraction, and synaptic plasticity. The CaMK pathway ensures that cells can appropriately respond to changes in calcium levels induced by mechanical stress.
MAPK Pathway
- Activation by Mechanical Loading: Mechanical stress activates MAPKs through integrin signaling and other mechanotransduction mechanisms. Key MAPKs involved in mechanotransduction include extracellular signal-regulated kinases (ERK1/2), c-Jun N-terminal kinases (JNK), and p38 MAPK. These kinases are activated by various upstream signaling molecules, which respond to mechanical cues by initiating phosphorylation cascades. The activation of these MAPKs is crucial for transmitting mechanical signals to the nucleus.
- ERK1/2 Pathway: ERK1/2 is activated by the sequential phosphorylation of upstream kinases, including Raf and MEK. Once activated, ERK1/2 translocates to the nucleus, where it phosphorylates various transcription factors, such as Elk-1 and c-Fos, leading to changes in gene expression. The ERK1/2 pathway is particularly important for regulating cell growth and differentiation in response to mechanical stress. This pathway ensures that cells can proliferate and differentiate appropriately in response to mechanical cues.
- JNK Pathway: The JNK pathway is activated in response to stress and inflammatory signals. Activated JNK translocates to the nucleus and phosphorylates transcription factors, such as c-Jun, which regulates genes involved in cell proliferation, apoptosis, and differentiation. This pathway plays a critical role in managing cellular responses to mechanical and oxidative stress. The JNK pathway ensures that cells can respond to harmful stimuli and maintain cellular integrity.
- p38 MAPK Pathway: p38 MAPK is activated by a variety of stress signals, including mechanical stress. Activated p38 MAPK phosphorylates transcription factors and other target proteins, influencing gene expression and cellular responses to mechanical loading. The p38 pathway is essential for coordinating cellular responses to inflammation and stress, promoting survival and repair mechanisms. This pathway ensures that cells can adapt to adverse conditions and maintain tissue homeostasis.
Cellular Responses to Mechanical Loading
ECM Synthesis and Remodeling
- Collagen Production: Mechanical loading stimulates the production of type I and type III collagen by fibroblasts and other cells. Collagen fibers provide structural support and tensile strength to the ECM. This enhanced collagen production helps reinforce the tissue, making it more resilient to mechanical stresses. Collagen is a major component of the ECM, and its synthesis is essential for maintaining the structural integrity of tissues.
- Proteoglycan Synthesis: Mechanical loading also enhances the synthesis of proteoglycans, such as aggrecan and decorin. Proteoglycans contribute to the compressive strength and hydration of the ECM, maintaining its viscoelastic properties. These molecules help the ECM absorb and dissipate mechanical forces, protecting cells and tissues from damage. Proteoglycans play a crucial role in maintaining the biomechanical properties of tissues.
- Matrix Degradation: Balanced matrix remodeling involves both the synthesis of new ECM components and the degradation of damaged or excess matrix. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) regulate this balance, ensuring proper ECM turnover and tissue adaptation. This dynamic process allows tissues to remodel and adapt to changing mechanical environments, maintaining optimal function. Proper regulation of ECM remodeling is essential for maintaining tissue homeostasis and preventing pathological conditions.
Cell Proliferation and Differentiation
- Fibroblast Proliferation: Mechanical loading promotes the proliferation of fibroblasts, increasing the pool of cells available for ECM synthesis and tissue repair. Growth factors such as transforming growth factor-beta (TGF-β) and platelet-derived growth factor (PDGF) play key roles in this process. Increased fibroblast proliferation helps accelerate tissue repair, replacing damaged cells and restoring structural integrity. Fibroblasts are critical for synthesizing ECM components and facilitating tissue repair.
- Mesenchymal Stem Cell (MSC) Differentiation: Mechanical loading influences the differentiation of MSCs into specific cell types, such as chondrocytes, osteoblasts, and fibroblasts. The local mechanical environment, along with biochemical signals, determines the differentiation pathway of MSCs. This process is crucial for regenerating various tissue types, including bone, cartilage, and connective tissues. MSC differentiation is essential for repairing and regenerating damaged tissues.
- Chondrocyte Activity: In cartilage, mechanical loading stimulates chondrocyte activity, promoting the synthesis of cartilage-specific ECM components, such as type II collagen and aggrecan. This enhances cartilage repair and maintenance, ensuring that the tissue remains resilient and functional under mechanical stress. Chondrocytes are essential for maintaining the structural integrity and function of cartilage.
Inflammation and Immune Response
- Cytokine Regulation: Mechanical loading affects the production of pro-inflammatory and anti-inflammatory cytokines by various cells. This modulation helps to balance the inflammatory response, reducing excessive inflammation and promoting tissue repair. Proper regulation of cytokine levels ensures that inflammation supports healing rather than causing additional tissue damage. Cytokines are critical for coordinating the immune response and facilitating tissue repair.
- Immune Cell Infiltration: Controlled mechanical loading can influence the infiltration and activity of immune cells, such as macrophages and neutrophils, at the injury site. This regulation ensures a balanced immune response that supports tissue healing. By attracting and activating the appropriate immune cells, mechanical loading helps clear damaged tissue and promote regeneration. Immune cells play a crucial role in clearing debris and facilitating tissue repair.
Angiogenesis
- Vascular Endothelial Growth Factor (VEGF) Production: Mechanical loading stimulates the production of VEGF and other angiogenic factors by fibroblasts, chondrocytes, and MSCs. VEGF promotes the proliferation and migration of endothelial cells, leading to the formation of new blood vessels. Enhanced angiogenesis ensures that regenerating tissues receive adequate blood supply, supporting their metabolic needs. VEGF is a key regulator of angiogenesis and is essential for promoting new blood vessel formation.
- Vascularization: Enhanced vascularization improves the delivery of oxygen, nutrients, and reparative cells to the injury site, supporting tissue repair and regeneration. Increased blood vessel formation facilitates efficient waste removal and provides essential support for tissue growth and repair processes. Adequate vascularization is critical for ensuring that tissues receive the necessary resources for healing and regeneration.
| Category | Subcategory | Details |
| Mechanical Loading and Cellular Mechanisms | Role |
Mechanical loading is essential for maintaining the health, function, and repair of musculoskeletal tissues, including the knee joint. It ensures the proper functioning of these tissues by continuously subjecting them to various forces during daily activities. |
| Daily Activities | Activities such as walking, running, and jumping apply mechanical forces to musculoskeletal tissues, stimulating cellular responses that contribute to tissue health and repair. | |
| Mechanotransduction | The process of converting mechanical signals into biochemical signals involves complex cellular mechanisms. These mechanotransduction processes are vital for adapting to mechanical stresses, maintaining tissue integrity, and ensuring proper cellular function. | |
| Mechanotransduction Pathways | General Process |
Mechanotransduction pathways allow cells to sense mechanical stimuli and transmit these signals from the cell surface to the nucleus. This results in changes in gene expression, protein synthesis, and cellular behavior, enabling cells to adapt to their physical environment, maintain structural integrity, and respond to mechanical stress. |
| Physiological Importance | Mechanotransduction is critical for tissue development, maintenance, and repair. It also plays a significant role in pathological conditions where mechanical forces are altered, such as in osteoarthritis or tendon injuries. The ability of cells to convert mechanical signals into biochemical responses allows them to interact dynamically with their environment and maintain homeostasis. | |
| Integrin Signaling | Integrin Structure |
Integrins are transmembrane receptors composed of alpha and beta subunits that form heterodimers, recognizing and binding specific ECM proteins. They mediate cell attachment to the ECM and other cells, playing a pivotal role in transmitting mechanical signals into the cell interior. |
| Integrin Activation | Mechanical loading induces the clustering of integrins at focal adhesion sites, specialized structures that link the ECM to the actin cytoskeleton within the cell. This clustering strengthens the integrin-ECM bond and initiates intracellular signaling cascades. Integrins bind to ECM proteins like fibronectin and collagen, triggering conformational changes that promote their activation and signaling. | |
| Focal Adhesion Kinase (FAK) | FAK is one of the first molecules activated in response to integrin clustering. It undergoes autophosphorylation at specific tyrosine residues, creating binding sites for various signaling proteins, including Src family kinases. This forms a multi-protein signaling complex at focal adhesions, amplifying mechanical signals and directing cellular responses. FAK activation is crucial for initiating downstream signaling pathways that regulate cellular functions. | |
| Downstream Pathways | Activated FAK triggers several downstream signaling pathways, including the MAPK pathway, PI3K/Akt pathway, and Rho family GTPases. These pathways regulate diverse cellular processes, such as cell proliferation, survival, migration, and differentiation. Each pathway contributes to the overall cellular response to mechanical stress, ensuring that cells can adapt and maintain homeostasis under varying mechanical conditions. | |
| Cytoskeletal Remodeling | Integrin signaling influences the organization of the actin cytoskeleton, which is essential for maintaining cell shape and enabling cell movement. Mechanical loading promotes the formation of stress fibers and focal adhesions, enhancing the cell’s mechanical stability and ability to withstand further mechanical stress. The dynamic nature of the cytoskeleton allows cells to rapidly reorganize in response to mechanical signals, supporting processes such as migration, division, and structural integrity. This remodeling is crucial for cells to adapt to changes in their mechanical environment and maintain tissue function. | |
| Ion Channels and Calcium Signaling | Stretch-Activated Channels | Mechanical loading deforms the cell membrane, leading to the opening of stretch-activated ion channels. These channels are permeable to various ions, including calcium (Ca²⁺), sodium (Na⁺), and potassium (K⁺). The opening of these channels allows ions to flow into the cell, initiating a cascade of intracellular events that translate mechanical signals into biochemical responses. These channels are key in sensing mechanical forces and converting them into cellular responses. |
| Calcium Influx | The entry of Ca²⁺ into the cell is a pivotal event in mechanotransduction. Elevated intracellular calcium levels act as a secondary messenger, activating various signaling pathways that influence cellular functions. Calcium ions bind to and activate numerous calcium-binding proteins, triggering a range of cellular responses from muscle contraction to gene expression. The influx of calcium is tightly regulated to ensure precise cellular responses to mechanical stimuli. | |
| Calcineurin/NFAT Pathway | Increased Ca²⁺ levels activate calcineurin, a calcium/calmodulin-dependent phosphatase. Calcineurin dephosphorylates nuclear factor of activated T-cells (NFAT), allowing it to translocate to the nucleus and regulate gene expression. NFAT controls the expression of genes involved in cell proliferation, differentiation, and survival, making it a critical mediator of cellular responses to mechanical stress. This pathway coordinates cellular activities in response to mechanical loading. | |
| CaMK Pathway | Ca²⁺ binds to calmodulin, forming a complex that activates calmodulin-dependent kinase (CaMK). Activated CaMK phosphorylates various target proteins, influencing gene expression and cellular responses to mechanical loading. This pathway helps regulate numerous cellular activities, including metabolism, muscle contraction, and synaptic plasticity. The CaMK pathway ensures that cells can appropriately respond to changes in calcium levels induced by mechanical stress. | |
| MAPK Pathway | Activation by Loading |
Mechanical stress activates MAPKs through integrin signaling and other mechanotransduction mechanisms. Key MAPKs involved in mechanotransduction include extracellular signal-regulated kinases (ERK1/2), c-Jun N-terminal kinases (JNK), and p38 MAPK. These kinases are activated by various upstream signaling molecules that respond to mechanical cues by initiating phosphorylation cascades, transmitting mechanical signals to the nucleus. |
| ERK1/2 Pathway | The ERK1/2 pathway is activated by the sequential phosphorylation of upstream kinases, including Raf and MEK. Once activated, ERK1/2 translocates to the nucleus, where it phosphorylates various transcription factors, such as Elk-1 and c-Fos, leading to changes in gene expression. This pathway is particularly important for regulating cell growth and differentiation in response to mechanical stress, ensuring that cells can proliferate and differentiate appropriately. | |
| JNK Pathway | The JNK pathway is activated in response to stress and inflammatory signals. Activated JNK translocates to the nucleus and phosphorylates transcription factors such as c-Jun, regulating genes involved in cell proliferation, apoptosis, and differentiation. This pathway plays a critical role in managing cellular responses to mechanical and oxidative stress, ensuring that cells can respond to harmful stimuli and maintain cellular integrity. | |
| p38 MAPK Pathway | The p38 MAPK pathway is activated by a variety of stress signals, including mechanical stress. Activated p38 MAPK phosphorylates transcription factors and other target proteins, influencing gene expression and cellular responses to mechanical loading. This pathway is essential for coordinating cellular responses to inflammation and stress, promoting survival and repair mechanisms, ensuring cells can adapt to adverse conditions and maintain tissue homeostasis. | |
| Cellular Responses to Mechanical Loading | ECM Synthesis and Remodeling |
Mechanical loading influences the synthesis and remodeling of the ECM, which is crucial for maintaining tissue integrity and function. The ECM provides structural support to tissues and regulates various cellular activities, including proliferation, differentiation, and migration. |
| Collagen Production | Mechanical loading stimulates the production of type I and type III collagen by fibroblasts and other cells. Collagen fibers provide structural support and tensile strength to the ECM. Enhanced collagen production reinforces the tissue, making it more resilient to mechanical stresses. Collagen is a major component of the ECM, and its synthesis is essential for maintaining the structural integrity of tissues. | |
| Proteoglycan Synthesis | Mechanical loading enhances the synthesis of proteoglycans such as aggrecan and decorin. Proteoglycans contribute to the compressive strength and hydration of the ECM, maintaining its viscoelastic properties. These molecules help the ECM absorb and dissipate mechanical forces, protecting cells and tissues from damage. Proteoglycans are crucial for maintaining the biomechanical properties of tissues. | |
| Matrix Degradation | Balanced matrix remodeling involves both the synthesis of new ECM components and the degradation of damaged or excess matrix. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) regulate this balance, ensuring proper ECM turnover and tissue adaptation. This dynamic process allows tissues to remodel and adapt to changing mechanical environments, maintaining optimal function and preventing pathological conditions. | |
| Cell Proliferation and Differentiation | Fibroblast Proliferation |
Mechanical loading promotes the proliferation of fibroblasts, increasing the pool of cells available for ECM synthesis and tissue repair. Growth factors such as transforming growth factor-beta (TGF-β) and platelet-derived growth factor (PDGF) play key roles in this process. Increased fibroblast proliferation accelerates tissue repair, replacing damaged cells and restoring structural integrity. Fibroblasts are critical for synthesizing ECM components and facilitating tissue repair. |
| MSC Differentiation | Mechanical loading influences the differentiation of mesenchymal stem cells (MSCs) into specific cell types such as chondrocytes, osteoblasts, and fibroblasts. The local mechanical environment, along with biochemical signals, determines the differentiation pathway of MSCs. This process is crucial for regenerating various tissue types, including bone, cartilage, and connective tissues. MSC differentiation is essential for repairing and regenerating damaged tissues. | |
| Chondrocyte Activity | In cartilage, mechanical loading stimulates chondrocyte activity, promoting the synthesis of cartilage-specific ECM components such as type II collagen and aggrecan. This enhances cartilage repair and maintenance, ensuring that the tissue remains resilient and functional under mechanical stress. Chondrocytes are essential for maintaining the structural integrity and function of cartilage. | |
| Inflammation and Immune Response | Cytokine Regulation |
Mechanical loading affects the production of pro-inflammatory and anti-inflammatory cytokines by various cells. This modulation helps balance the inflammatory response, reducing excessive inflammation and promoting tissue repair. Proper regulation of cytokine levels ensures that inflammation supports healing rather than causing additional tissue damage. Cytokines are critical for coordinating the immune response and facilitating tissue repair. |
| Immune Cell Infiltration | Controlled mechanical loading influences the infiltration and activity of immune cells such as macrophages and neutrophils at the injury site. This regulation ensures a balanced immune response that supports tissue healing. By attracting and activating the appropriate immune cells, mechanical loading helps clear damaged tissue and promote regeneration. Immune cells play a crucial role in clearing debris and facilitating tissue repair. | |
| Angiogenesis | VEGF Production |
Mechanical loading stimulates the production of vascular endothelial growth factor (VEGF) and other angiogenic factors by fibroblasts, chondrocytes, and MSCs. VEGF promotes the proliferation and migration of endothelial cells, leading to the formation of new blood vessels. Enhanced angiogenesis ensures that regenerating tissues receive adequate blood supply, supporting their metabolic needs. VEGF is a key regulator of angiogenesis and is essential for promoting new blood vessel formation. |
| Vascularization | Enhanced vascularization improves the delivery of oxygen, nutrients, and reparative cells to the injury site, supporting tissue repair and regeneration. Increased blood vessel formation facilitates efficient waste removal and provides essential support for tissue growth and repair processes. Adequate vascularization is critical for ensuring that tissues receive the necessary resources for healing and regeneration. |
Rehabilitation Strategies Based on Musculoskeletal Healing Stages: Early Mechanical Loading
Inflammation Stage
Vasodilation
-
Histamine:
- Source: Histamine is released from mast cells, basophils, and platelets upon injury.
- Receptors: It binds to H1 receptors on endothelial cells, leading to their contraction and increasing vascular permeability. This increased permeability allows immune cells and proteins to exit the bloodstream and enter the site of injury. Histamine also stimulates endothelial nitric oxide synthase (eNOS) to produce nitric oxide (NO), which diffuses into adjacent smooth muscle cells, causing them to relax and leading to vasodilation. This process helps deliver immune cells and nutrients to the site of injury and clears cellular debris.
- Role in Healing: Histamine plays a crucial role in the early stages of healing by facilitating the delivery of essential immune cells and nutrients to the injury site. This helps initiate the healing process by clearing debris and setting the stage for tissue repair.
-
Bradykinin:
- Source: Bradykinin is formed from kininogen through the action of the enzyme kallikrein, which is activated during tissue injury.
- Receptors: Bradykinin binds to B2 receptors on endothelial cells, promoting the release of NO and prostacyclin (PGI2), both potent vasodilators. Additionally, bradykinin increases the permeability of the vascular endothelium, allowing plasma proteins and immune cells to enter the tissue, further supporting the inflammatory response and aiding in tissue repair.
- Role in Pain Sensation: Bradykinin sensitizes nociceptors, contributing to pain signaling. This pain serves as a protective mechanism, encouraging the individual to limit movement and prevent further injury.
-
Prostaglandin E2 (PGE2):
- Source: PGE2 is synthesized from arachidonic acid via the cyclooxygenase (COX) pathway, with COX-2 being particularly active in response to inflammatory signals.
- Receptors: PGE2 acts on EP2 and EP4 receptors on smooth muscle cells, increasing intracellular cyclic AMP (cAMP) levels, which leads to muscle relaxation and vasodilation. PGE2 also sensitizes sensory nerves, contributing to the sensation of pain and amplifying the inflammatory response.
- Role in Inflammation: PGE2 enhances the inflammatory response by increasing vascular permeability and attracting immune cells to the site of injury. This helps clear debris and pathogens, facilitating the healing process.
Invasion of Platelets
-
Activation and Adhesion:
- Triggers: Platelet activation is triggered by the exposure of subendothelial collagen and von Willebrand factor (vWF) in the damaged endothelium. These molecules bind to glycoprotein receptors (GPVI and GPIb) on platelets, causing them to adhere to the site of injury.
- Aggregation: Once activated, platelets release adenosine diphosphate (ADP) and thromboxane A2 (TXA2), which further amplify platelet activation and aggregation, forming a platelet plug that prevents further blood loss and stabilizes the initial injury site.
- Role in Hemostasis: The formation of the platelet plug is crucial for preventing excessive blood loss and providing a temporary scaffold for subsequent tissue repair.
-
Release of Mediators:
- ADP and TXA2: These mediators promote additional platelet activation and aggregation, strengthening the initial platelet plug.
-
Growth Factors: Activated platelets release a variety of growth factors, including platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-β), which recruit and activate fibroblasts and smooth muscle cells. These growth factors are crucial for initiating the subsequent stages of tissue repair and regeneration by promoting cell proliferation and matrix synthesis.
- Role in Tissue Repair: The growth factors released by platelets play a key role in attracting and activating cells necessary for tissue repair. This sets the stage for the fibroblastic stage, where these cells will proliferate and synthesize the extracellular matrix.
Invasion of Inflammatory Cells
-
Neutrophils:
- Recruitment: Neutrophils are attracted to the injury site by chemotactic agents such as interleukin-8 (IL-8), complement component C5a, and leukotriene B4 (LTB4).
- Actions: Neutrophils perform phagocytosis to engulf and destroy pathogens, release proteolytic enzymes like elastase and collagenase to degrade damaged tissue, and generate reactive oxygen species (ROS) to kill microbes. These actions are critical for clearing cellular debris and preventing infection.
- Role in Debris Clearance: By clearing debris and pathogens, neutrophils help create a clean environment conducive to tissue repair. Their activity is essential for the initial cleanup phase of the healing process.
-
Monocytes and Macrophages:
- Recruitment: Monocytes are attracted to the injury site by chemokines like monocyte chemoattractant protein-1 (MCP-1 or CCL2) and differentiate into macrophages upon entering the tissue.
-
Macrophage Polarization:
- M1 Macrophages: These macrophages produce pro-inflammatory cytokines (IL-1, IL-6, TNF-α) and ROS, enhancing pathogen clearance and sustaining the inflammatory response.
- M2 Macrophages: These macrophages secrete anti-inflammatory cytokines (IL-10, TGF-β) and growth factors, resolving inflammation and promoting tissue repair and remodeling. The balance between M1 and M2 macrophages is crucial for the progression from inflammation to healing.
- Role in Transition to Healing: The shift from M1 to M2 macrophages is critical for transitioning from the inflammatory phase to the healing phase. M2 macrophages play a key role in tissue repair and remodeling.
Chemical Mediators
-
Histamine:
- Role: Induces vasodilation and increases vascular permeability, facilitating the influx of immune cells and nutrients to the injury site. This helps to clear debris and supports the initial stages of healing.
-
Bradykinin:
- Role: Promotes vasodilation, increases vascular permeability, and sensitizes nociceptors, contributing to pain signaling and enhancing the inflammatory response.
-
Prostaglandin E2 (PGE2):
- Role: Induces vasodilation, enhances vascular permeability, and sensitizes sensory nerves to pain. PGE2 also modulates inflammatory responses by influencing the behavior of immune cells, supporting the transition from acute inflammation to tissue repair.
-
Additional Mediators:
- Leukotrienes (e.g., LTB4): Produced from arachidonic acid by the lipoxygenase pathway, act as potent chemotactic agents for neutrophils and other leukocytes, supporting their recruitment to the injury site.
- Nitric Oxide (NO): Produced by endothelial cells (via eNOS) and macrophages (via iNOS), NO induces smooth muscle relaxation, leading to vasodilation. It also possesses antimicrobial properties, aiding in the defense against infection at the injury site.
- Role in Healing: These additional mediators support various aspects of the inflammatory response, including the recruitment of immune cells, vasodilation, and pathogen clearance. Their coordinated action is essential for effective tissue repair.
Fibroblastic Stage
TGF-β1
-
Receptors and Signaling Pathways: TGF-β1 binds to TGF-β receptors (TGF-βRI and TGF-βRII) on fibroblasts, initiating the phosphorylation of Smad2/3 proteins. These phosphorylated Smads form complexes with Smad4, which then translocate to the nucleus to regulate the transcription of ECM genes, promoting the production of ECM components.
- Intracellular Signaling: This pathway is crucial for activating fibroblasts and promoting their proliferation and ECM production. The Smad complexes act as transcription factors, driving the expression of genes necessary for ECM synthesis and organization.
- Role in Tissue Repair: By promoting the production of ECM components, TGF-β1 supports the formation of a stable matrix for tissue repair. This matrix provides structural support and facilitates the healing process.
-
Effects on ECM Production: TGF-β1 enhances the production of type I and type III collagen, fibronectin, and integrins, contributing to the assembly and stability of the ECM. It also inhibits the expression of matrix metalloproteinases (MMPs), reducing ECM degradation and supporting the formation of a stable matrix for tissue repair.
- Collagen Production: The increased production of collagen provides the necessary scaffold for tissue repair, ensuring mechanical strength and stability.
- ECM Stability: By inhibiting MMPs, TGF-β1 helps maintain the integrity of the newly formed ECM, preventing its premature degradation and ensuring effective tissue repair.
BMP
-
Receptors and Signaling Pathways: BMPs bind to BMP receptors (BMPR-I and BMPR-II), leading to the activation of Smad1/5/8 proteins. These proteins form complexes with Smad4 and translocate to the nucleus to influence gene expression, promoting the synthesis of ECM components and differentiation of fibroblasts.
- Intracellular Signaling: The activation of Smad1/5/8 proteins by BMPs drives the expression of genes involved in ECM production and fibroblast differentiation. This signaling pathway is essential for orchestrating the activities of fibroblasts during tissue repair.
- Role in Fibroblast Differentiation: BMPs promote the differentiation of fibroblasts into myofibroblasts, which are specialized for ECM production and wound contraction. This differentiation is crucial for effective tissue repair and remodeling.
-
Effects on ECM Production: BMPs promote the differentiation of fibroblasts into myofibroblasts, which are specialized for ECM production and wound contraction. They also stimulate the synthesis of collagen and other ECM proteins, contributing to the structural integrity of the healing tissue.
- Collagen Synthesis: BMPs enhance collagen production, ensuring the formation of a strong and stable ECM.
- Wound Contraction: The differentiation of fibroblasts into myofibroblasts facilitates wound contraction, reducing the size of the wound and promoting more efficient healing.
CTGF
-
Receptors and Signaling Pathways: CTGF interacts with cell surface receptors such as integrins and heparan sulfate proteoglycans, activating downstream signaling pathways like the MAPK/ERK pathway. These pathways promote cell proliferation, migration, and ECM synthesis.
- Intracellular Signaling: The activation of the MAPK/ERK pathway by CTGF drives the expression of genes involved in cell proliferation, migration, and ECM production. This signaling pathway is essential for coordinating the activities of fibroblasts during tissue repair.
- Role in Cell Migration: CTGF promotes fibroblast migration to the injury site, ensuring that sufficient cells are present to produce the ECM necessary for tissue repair.
-
Effects on ECM Production: CTGF enhances the expression of collagen, fibronectin, and proteoglycans, and improves fibroblast adhesion and migration. This facilitates ECM deposition and remodeling, contributing to the structural and functional recovery of the injured tissue.
- ECM Deposition: The increased production of ECM components by CTGF ensures the formation of a robust and stable matrix for tissue repair.
- Tissue Remodeling: By promoting fibroblast adhesion and migration, CTGF supports the dynamic remodeling of the ECM, ensuring effective tissue repair and functional recovery.
ECM Production and Organization
-
Proliferation: Activated fibroblasts proliferate extensively in response to these growth factors, increasing the number of cells capable of synthesizing ECM components. This cellular proliferation is essential for producing the ECM necessary for tissue repair.
- Cellular Expansion: The proliferation of fibroblasts ensures that a sufficient number of cells are available to produce the ECM, supporting effective tissue repair.
-
Collagen Synthesis: The fibroblasts produce large quantities of type I and type III collagen, which are critical for structural support in the newly formed tissue. This collagen forms a scaffold that provides mechanical strength and supports further cellular activities.
- Structural Support: The production of collagen provides the necessary scaffold for tissue repair, ensuring mechanical strength and stability.
-
Initial Organization: Initially, the collagen fibers are laid down in a random, haphazard manner, forming a provisional matrix that fills the wound space and provides temporary mechanical strength. This matrix serves as a foundation for subsequent tissue remodeling.
- Temporary Matrix: The initial disorganized collagen network provides immediate mechanical support, allowing for the continuation of the healing process.
-
Scar Tissue Formation: Over time, this disorganized collagen network becomes the foundation of scar tissue. The random orientation of collagen fibers leads to the characteristic stiffness and reduced functionality of scar tissue compared to normal tissue. The development of scar tissue is a critical aspect of wound healing, providing structural support but often resulting in impaired tissue function.
- Long-term Implications: The formation of scar tissue, while providing necessary structural support, can result in reduced tissue functionality and flexibility. This underscores the importance of effective rehabilitation strategies to minimize scar tissue formation and promote optimal healing.
Molecular Interactions and Remodeling
-
Cross-linking: Enzymes such as lysyl oxidase (LOX) mediate the cross-linking of collagen fibers, increasing the tensile strength of the ECM. This cross-linking process is essential for stabilizing the newly formed tissue and ensuring its mechanical integrity.
- Tensile Strength: The cross-linking of collagen fibers enhances the tensile strength of the ECM, providing stability and durability to the repaired tissue.
-
ECM Remodeling: Despite the initial random organization, subsequent remodeling processes involve the reorganization of collagen fibers. Fibroblasts and myofibroblasts exert mechanical forces that attempt to align collagen fibers along the lines of tension, although this reorganization is often incomplete in scar tissue. This remodeling improves the mechanical properties of the tissue but may not fully restore its original functionality.
- Mechanical Realignment: The reorganization of collagen fibers along lines of tension improves the mechanical properties of the tissue, enhancing its functionality and resilience.
-
Regulation by Other Cytokines and Growth Factors: Other molecules, such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), also contribute to the wound healing process by promoting angiogenesis and further fibroblast recruitment and activation. These factors support the formation of new blood vessels, ensuring an adequate supply of oxygen and nutrients to the healing tissue.
- Angiogenesis: The formation of new blood vessels ensures an adequate supply of oxygen and nutrients to the healing tissue, supporting effective tissue repair and regeneration.
Remodeling Stage
Molecular Details of Scar Remodeling
-
Collagen Remodeling:
- Collagen Cross-Linking: Enzymes such as lysyl oxidase (LOX) catalyze the formation of covalent cross-links between collagen molecules, enhancing the tensile strength and stability of the ECM. This process is crucial for ensuring the durability and mechanical integrity of the remodeled tissue.
- Matrix Metalloproteinases (MMPs): MMPs, particularly MMP-1 (collagenase) and MMP-9 (gelatinase), are involved in the degradation of disorganized collagen fibers. Their activity is regulated by tissue inhibitors of metalloproteinases (TIMPs), maintaining a balance between collagen synthesis and degradation. This balance is essential for effective tissue remodeling and functional recovery.
- Collagen Reorganization: Myofibroblasts exert contractile forces on the ECM, aligning collagen fibers along the lines of mechanical stress. This reorganization improves the structural integrity and functional properties of the tissue, making it more resilient and capable of withstanding mechanical loads.
- Tissue Strengthening: The reorganization and cross-linking of collagen fibers enhance the tensile strength and mechanical properties of the tissue, supporting its long-term functionality.
-
Cellular Mechanisms:
- Fibroblast and Myofibroblast Activity: These cells continue to proliferate and produce ECM components. Myofibroblasts, characterized by the expression of alpha-smooth muscle actin (α-SMA), generate contractile forces that facilitate tissue contraction and collagen fiber alignment. This cellular activity is crucial for the reorganization and strengthening of the remodeled tissue.
- Integrin Signaling: Cell-ECM interactions mediated by integrins trigger intracellular signaling pathways, such as the focal adhesion kinase (FAK) and the MAPK/ERK pathways. These pathways promote cell migration, survival, and ECM production, supporting the dynamic remodeling of the tissue.
- Cell-ECM Interaction: The interaction between cells and the ECM is essential for coordinating cellular activities during tissue repair, ensuring effective remodeling and functional recovery.
-
Growth Factors and Cytokines:
- Transforming Growth Factor-beta (TGF-β): TGF-β is a pivotal regulator of ECM remodeling. It stimulates fibroblast proliferation, myofibroblast differentiation, and the synthesis of collagen and other ECM proteins. TGF-β also modulates the expression of MMPs and TIMPs, influencing ECM turnover and ensuring a balanced remodeling process.
- Connective Tissue Growth Factor (CTGF): CTGF enhances the fibrotic response by promoting collagen synthesis and fibroblast adhesion to the ECM. This growth factor plays a significant role in the regulation of tissue remodeling and the maintenance of ECM integrity.
- Platelet-Derived Growth Factor (PDGF): PDGF attracts fibroblasts to the wound site and stimulates their proliferation and ECM production. PDGF is essential for sustaining the remodeling process and ensuring adequate cellular activity.
- Role in Remodeling: These growth factors and cytokines are crucial for orchestrating the activities of fibroblasts and myofibroblasts, supporting the dynamic remodeling of the tissue and ensuring its long-term functionality.
-
Extracellular Matrix Components:
- Fibronectin and Elastin: These ECM proteins contribute to the structural integrity and elasticity of the tissue. Fibronectin facilitates cell adhesion and migration, while elastin provides resilience to the ECM, allowing it to withstand mechanical stress and maintain its functional properties.
- Proteoglycans and Glycosaminoglycans (GAGs): These molecules retain water within the ECM, maintaining its viscoelastic properties and providing a hydrated environment conducive to cellular activities. The presence of these components ensures the flexibility and functionality of the remodeled tissue.
- ECM Integrity: The coordinated production of these ECM components ensures the structural integrity and functional resilience of the remodeled tissue, supporting its long-term performance.
Consequences of Continuous Collagen Synthesis
-
Scar Formation:
- Fibrosis: Persistent activation of fibroblasts and continuous collagen synthesis result in excessive ECM deposition, leading to fibrosis. The resulting scar tissue is often stiffer and less elastic than the original tissue due to the dense collagen network. This fibrosis can impair the functional recovery of the tissue and lead to long-term complications.
- Mechanical Properties: Although the scar tissue provides immediate tensile strength, it lacks the intricate organization and biomechanical properties of the native tissue. This can impair function, leading to reduced flexibility, strength, and overall tissue performance.
- Impact on Functionality: The formation of scar tissue, while necessary for immediate structural support, often results in impaired tissue functionality and flexibility. This highlights the importance of effective rehabilitation strategies to minimize scar tissue formation and promote optimal healing.
-
Tendon Adhesions:
- Adhesion Formation: Continuous collagen deposition around tendons can lead to adhesions, where the tendons adhere to surrounding tissues. This restricts tendon gliding and impairs joint mobility, leading to functional limitations and discomfort.
- Functional Impairment: Tendon adhesions can result in reduced range of motion, stiffness, and pain, significantly affecting the functional recovery of the affected limb. This can hinder the rehabilitation process and lead to long-term functional deficits.
- Clinical Implications: Tendon adhesions pose significant challenges for rehabilitation, often requiring interventions to restore mobility and function. Addressing these adhesions is crucial for achieving successful long-term recovery.
-
Molecular Pathways in Adhesion Formation:
- Inflammatory Mediators: Persistent inflammation, characterized by elevated levels of pro-inflammatory cytokines (e.g., IL-1, TNF-α), promotes fibrosis and adhesion formation. The chronic inflammatory state sustains the activation of fibroblasts and myofibroblasts, leading to excessive ECM deposition and adhesion development.
- Fibroblast-to-Myofibroblast Transition: TGF-β and other growth factors induce the differentiation of fibroblasts into myofibroblasts, which produce large amounts of collagen and contract the ECM, contributing to adhesion development. This transition is a key factor in the formation of restrictive adhesions and the subsequent impairment of tissue function.
- Role in Adhesion Development: Understanding the molecular pathways involved in adhesion formation is crucial for developing strategies to prevent and treat these adhesions, ensuring effective rehabilitation and functional recovery.
| Healing stage | Cellular phase | Biophysical characteristics | Therapeutic intervention |
| Inflammation Stage | Vasodilation, invasion of platelets, and inflammatory cells (neutrophils, monocytes, and macrophages) are crucial processes in the body’s response to injury. These events are orchestrated by a complex interplay of chemical mediators, including histamine, bradykinin, and PGE2, each playing specific roles at the molecular level. to injury, facilitating effective tissue repair and restoration of function. |
Swelling, erythema, warmth, pain | Cryotherapy, preferably with compression NSAIDs (unless contraindicated) Manual therapy |
| The strength of the scar depends on the temporary clot and stitches | Methods: electrical stimulation, laser therapy, ultrasound, PEMF, ESWT, isometric and BFR training. | ||
| Fibroblastic stage. |
Growth factors such as Transforming Growth Factor-beta 1 (TGF-β1), Bone Morphogenetic Proteins (BMP), and Connective Tissue Growth Factor (CTGF) play critical roles in wound healing by activating fibroblastic cells. Upon activation, these fibroblastic cells undergo proliferation and upregulate the synthesis of extracellular matrix (ECM) components including collagen, fibronectin, and proteoglycans. |
Expression of inflammatory markers | Manual therapy: passive range of motion, soft tissue mobilization, joint mobilization |
| The scar begins to gain tensile strength | Methods: electrical stimulation, laser therapy, ultrasound, PEMF, ESWT Therapeutic exercises: prescribed to achieve the goal of full weight bearing on the surgical limb while protecting the tissues (slow eccentric tempo) |
||
| Remodelling stage. |
The remodeling of the scar improves the organization and mechanical properties of the extracellular matrix (ECM) through a dynamic process involving the coordinated activity of various cells, enzymes, and signaling pathways. Fibroblasts and myofibroblasts play key roles in this process by synthesizing and remodeling collagen and other ECM components. |
The inflammation should subside; pain, if present, may be due to osteoarthritis, DOMS, re-damage to healing tissue | Manual therapy depending on needs, based on the patient’s assessment of the operated limb and the rest of the body; passive and active range of motion, soft tissue mobilization, including scar mobilization, joint mobilization |
| Methods: Typically discontinued at this stage unless patient assessment indicates special requirements for the surgical limb or rest of the body Therapeutic exercises: prescribed to increase active ROM and flexibility, build muscle strength and endurance, improve proprioception, motor control, and improve cardiovascular fitness | |||
| Abbreviations: BMP, bone morphogenetic protein; CTGF, connective tissue growth factor; DOMS, delayed onset muscle soreness; ECM, extracellular matrix; ESWT, extracorporeal shock wave therapy; NSAIDs, non-steroidal anti-inflammatory drugs; PEMF, pulsed electromagnetic field therapy; BFR, blood flow restriciton; PGE2, prostaglandin E2; ROM, range of motion; TGF-β1, transforming growth factor-β1. | |||
Early Mechanical Loading: Benefits and Risks
Benefits of Early Mechanical Loading
Risks and Considerations
Clinical Guidelines and Recommendations
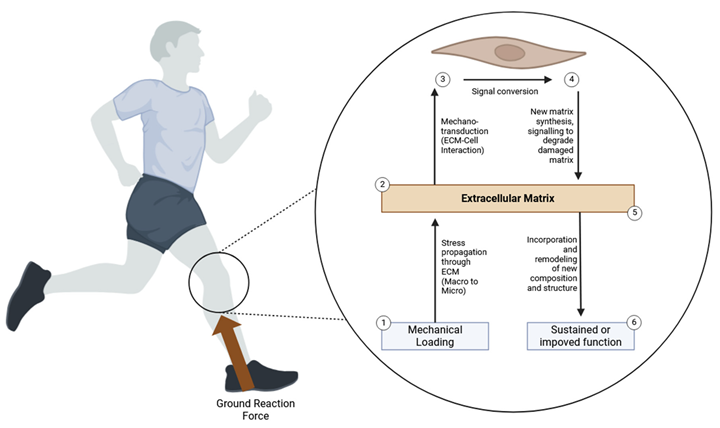
Signaling Pathways Involved in Mechanical Loading
Integrin Signaling Pathway
- Integrin Activation: Mechanical loading causes integrins to cluster at focal adhesion sites. These sites are specialized structures that connect the ECM to the actin cytoskeleton within the cell. The clustering enhances the integrin-ECM bond, initiating intracellular signaling cascades.
- 2.
- Focal Adhesion Kinase (FAK): One of the first responses to integrin clustering is the activation of FAK. Activated FAK undergoes autophosphorylation at specific tyrosine residues, creating binding sites for various signaling proteins, including Src family kinases. This leads to the formation of a multi-protein signaling complex at focal adhesions.
- 3.
- Downstream Signaling: Activated FAK triggers several downstream signaling pathways, including the MAPK pathway, PI3K/Akt pathway, and Rho family GTPases. These pathways regulate cellular processes such as proliferation, survival, migration, and differentiation.
- 4.
- Cytoskeletal Remodeling: Integrin signaling influences the organization of the actin cytoskeleton, which is crucial for maintaining cell shape and enabling cell movement. Mechanical loading promotes the formation of stress fibers and focal adhesions, enhancing the cell’s mechanical stability and ability to withstand further mechanical stress.
Ion Channels and Calcium Signaling
- Stretch-Activated Ion Channels: Mechanical loading deforms the cell membrane, leading to the opening of stretch-activated ion channels. These channels are permeable to various ions, including calcium (Ca²⁺), sodium (Na⁺), and potassium (K⁺).
- 2.
- Calcium Influx: The entry of Ca²⁺ into the cell is a pivotal event in mechanotransduction. Elevated intracellular calcium levels act as a secondary messenger, activating various signaling pathways that influence cellular functions.
- 3.
- Calcineurin/NFAT Pathway: Increased Ca2+ levels activate calcineurin, a calcium/calmodulin-dependent phosphatase. Calcineurin dephosphorylates nuclear factor of activated T-cells (NFAT), allowing it to translocate to the nucleus and regulate gene expression. NFAT controls the expression of genes involved in cell proliferation, differentiation, and survival.
- 4.
- Calmodulin-Dependent Kinase (CaMK) Pathway: Ca²⁺ binds to calmodulin, forming a complex that activates CaMK. Activated CaMK phosphorylates various target proteins, influencing gene expression and cellular responses to mechanical loading.
Mitogen-Activated Protein Kinase (MAPK) Pathway
- Activation by Mechanical Loading: Mechanical stress activates MAPKs through integrin signaling and other mechanotransduction mechanisms. Key MAPKs involved in mechanotransduction include extracellular signal-regulated kinases (ERK1/2), c-Jun N-terminal kinases (JNK), and p38 MAPK.
- 2.
- ERK1/2 Pathway: ERK1/2 is activated by the sequential phosphorylation of upstream kinases, including Raf and MEK. Once activated, ERK1/2 translocates to the nucleus, where it phosphorylates various transcription factors, such as Elk-1 and c-Fos, leading to changes in gene expression.
- 3.
- JNK Pathway: The JNK pathway is activated in response to stress and inflammatory signals. Activated JNK translocates to the nucleus and phosphorylates transcription factors, such as c-Jun, which regulates genes involved in cell proliferation, apoptosis, and differentiation.
- 4.
- p38 MAPK Pathway: p38 MAPK is activated by a variety of stress signals, including mechanical stress. Activated p38 MAPK phosphorylates transcription factors and other target proteins, influencing gene expression and cellular responses to mechanical loading.
Wnt/β-Catenin Signaling Pathway
- Wnt Ligands: Mechanical loading can enhance the expression of Wnt ligands, which bind to Frizzled receptors and co-receptors on the cell surface.
- 2.
- β-Catenin Stabilization: Binding of Wnt ligands inhibits the degradation of β-catenin, leading to its accumulation in the cytoplasm and subsequent translocation to the nucleus.
- 3.
- Gene Expression: In the nucleus, β-catenin interacts with transcription factors to regulate the expression of target genes involved in cell proliferation, differentiation, and ECM synthesis.
YAP/TAZ Signaling Pathway
- Hippo Pathway Inhibition: Mechanical loading inhibits the Hippo pathway, which normally suppresses YAP/TAZ activity. Inhibition of the Hippo pathway leads to the activation and nuclear translocation of YAP/TAZ.
- 2.
- Nuclear Translocation: Activated YAP/TAZ translocate to the nucleus, where they interact with transcription factors to regulate gene expression.
- 3.
- Regulation of Cell Behavior: YAP/TAZ regulate the expression of genes involved in cell proliferation, survival, and differentiation, contributing to tissue repair and regeneration.
Interactions Among Signaling Pathways
- Integrin and MAPK Pathways: Integrin signaling through FAK can activate the MAPK pathway, leading to changes in gene expression and cellular behavior. The crosstalk between these pathways enhances the ability of cells to respond to mechanical stimuli.
- 2.
- Calcium Signaling and MAPK Pathway: Calcium influx can influence the MAPK pathway by activating calcium-sensitive enzymes and kinases. This integration helps to fine-tune cellular responses to mechanical loading.
- 3.
- Wnt and YAP/TAZ Signaling: Both Wnt/β-catenin and YAP/TAZ signaling pathways can be activated by mechanical loading, and they may work together to regulate gene expression and cellular responses. The interaction between these pathways can enhance tissue repair and regeneration.
Molecular and Cellular Biology Aspects of Differences between Meniscus, Cartilage, Ligament, and Subchondral Bone in Rehabilitation and Injury
Meniscus
-
Cell Types:
- Fibrochondrocytes: These specialized cells produce both collagen and proteoglycans, which are crucial for the meniscus’s structural integrity and function. Fibrochondrocytes exhibit characteristics of both fibroblasts and chondrocytes, allowing them to adapt to the mixed fibrocartilaginous nature of the tissue. They are responsible for maintaining the extracellular matrix (ECM) and responding to mechanical stimuli by adjusting the production of matrix components. The dual characteristics of fibrochondrocytes make them versatile in synthesizing both fibrous and cartilaginous matrix, ensuring the meniscus can resist a combination of compressive and tensile forces. This adaptability is vital for the meniscus to function effectively in the highly dynamic environment of the knee joint, where it must cushion impacts, distribute loads, and stabilize the joint during movement. Fibrochondrocytes also play a role in maintaining the mechanical properties of the meniscus, which are essential for the overall stability and function of the knee.
- Extracellular Matrix (ECM): The ECM of the meniscus contains a high concentration of type I collagen in the outer regions and type II collagen in the inner regions. Proteoglycans like aggrecan are also present, providing compressive strength and contributing to the tissue’s ability to absorb shock and distribute loads across the knee joint. The varying ECM composition is essential for the different mechanical roles of the meniscus, with type I collagen providing durability and tensile strength, while type II collagen and proteoglycans ensure resilience against compression and enhance shock absorption. This complex ECM organization allows the meniscus to maintain its structural integrity and functional performance under various mechanical stresses. The presence of glycosaminoglycans (GAGs) within the ECM helps retain water, which is crucial for maintaining the viscoelastic properties of the meniscus.
-
Injury Response:
- Inflammatory Mediators: Injury to the meniscus triggers the release of inflammatory cytokines such as IL-1β, TNF-α, and PGE2. These cytokines lead to ECM degradation through increased metalloproteinase (MMP) activity, particularly MMP-13, which breaks down collagen. This inflammatory response can compromise the structural integrity of the meniscus and hinder its function. The catabolic environment induced by these mediators accelerates the breakdown of critical ECM components, weakening the tissue and impairing its load-bearing capacity. This degradation process not only compromises the mechanical properties of the meniscus but also creates a hostile environment for healing cells, further limiting repair potential. The inflammatory response is characterized by an increase in catabolic enzymes and a decrease in anabolic activities, leading to a net loss of ECM components.
- Healing Capacity: The meniscus has a unique vascularity pattern that significantly affects its healing capacity. The outer third of the meniscus, known as the red-red zone, is well vascularized, allowing for better healing through cellular infiltration and ECM production. This zone can heal relatively well due to the presence of blood vessels that supply essential nutrients and cells for tissue repair. In contrast, the inner two-thirds, known as the red-white and white-white zones, are avascular, severely limiting the healing response. These regions rely on the limited diffusion of nutrients from the synovial fluid, which is often insufficient for significant repair. As a result, injuries in these avascular zones frequently require surgical intervention to promote healing and restore function. The limited blood supply in these areas hinders the delivery of reparative cells and growth factors, making natural healing processes less effective.
-
Rehabilitation Considerations:
- Biomechanical Loading: Controlled, progressive loading is essential to stimulate fibrochondrocyte activity and ECM synthesis. Techniques such as partial weight-bearing exercises and proprioceptive training are beneficial in promoting meniscal healing and restoring knee function. Progressive loading helps adapt the meniscus to increasing mechanical demands without causing further damage. These exercises ensure that the meniscus is conditioned to handle everyday activities and sports-related stresses, reducing the likelihood of re-injury. Proprioceptive training, which enhances the body’s ability to sense joint position and movement, is particularly important for preventing future injuries and maintaining joint stability. Controlled loading regimes are designed to gradually increase the mechanical stress on the meniscus, encouraging adaptation and strengthening without causing damage.
- Growth Factors: Administration of growth factors like TGF-β and PDGF can enhance fibrochondrocyte proliferation and matrix production. These biologic therapies support the healing process by promoting cell growth and ECM synthesis, improving the structural integrity of the meniscus. Growth factors also modulate the inflammatory response, reducing ECM degradation and promoting tissue repair. This approach can accelerate recovery and enhance the biomechanical properties of the meniscus, making it more resilient to future injuries. Growth factors can be administered through various methods, including direct injection into the joint or incorporation into scaffolds used in meniscal repair surgeries. The application of growth factors aims to create a more favorable environment for tissue repair and regeneration, potentially improving the outcomes of meniscal injuries.
Cartilage
-
Cell Types:
- Chondrocytes: The only cell type found in cartilage, chondrocytes are responsible for maintaining the ECM. They are embedded within lacunae and produce type II collagen and aggrecan, which are crucial for the cartilage’s structure and function. Chondrocytes have limited capacity for proliferation and migration, contributing to the poor healing potential of cartilage. These cells are essential for the synthesis and turnover of the ECM, ensuring the cartilage maintains its load-bearing properties and smooth surface for joint articulation. Chondrocytes are highly specialized for a low-oxygen environment and are adapted to produce the components necessary for a resilient and durable cartilage matrix. They regulate the balance between anabolic and catabolic activities within the cartilage, maintaining tissue homeostasis.
- Extracellular Matrix (ECM): The ECM of cartilage is composed primarily of type II collagen fibers and proteoglycans such as aggrecan. Aggrecan attracts water, providing resistance to compressive forces and contributing to the cartilage’s ability to absorb impacts and facilitate smooth joint movement. The highly organized structure of the ECM provides both strength and flexibility, crucial for the cartilage’s load-bearing and frictionless properties. The presence of water-binding proteoglycans ensures that the cartilage remains resilient under compressive loads, maintaining joint function. This unique composition allows cartilage to function effectively as a shock absorber and load distributor in joints. The ECM also includes other molecules like hyaluronan and link proteins that help stabilize the matrix and retain its functional properties.
-
Injury Response:
- Inflammatory Mediators: Injury to cartilage leads to the release of catabolic cytokines (e.g., IL-1, TNF-α) and aggrecanases (e.g., ADAMTS-4, ADAMTS-5) that degrade the ECM. This inflammatory response further impairs the already limited healing capacity of cartilage. The breakdown of aggrecan and collagen by these enzymes leads to a loss of cartilage integrity and function, making the tissue more susceptible to further damage and degeneration. The inflammatory milieu not only degrades the ECM but also inhibits the anabolic activities of chondrocytes, exacerbating tissue breakdown. This degradation process compromises the structural integrity and biomechanical properties of the cartilage, leading to progressive joint dysfunction and pain. The chronic inflammatory environment can perpetuate a cycle of degradation and insufficient repair, exacerbating cartilage damage.
- Limited Repair Capacity: The lack of blood vessels, nerves, and lymphatics in cartilage impedes its intrinsic healing capacity. Chondrocytes exhibit limited migratory and proliferative abilities, which severely restricts the tissue’s ability to repair itself after injury. This avascular nature means that damaged cartilage relies on the slow and often insufficient diffusion of nutrients and reparative cells from the surrounding synovial fluid. The poor regenerative capacity necessitates interventions that can introduce new cells or stimulate the existing chondrocytes to enhance repair. The inability to effectively repair and regenerate can lead to chronic joint issues, including osteoarthritis, where the progressive loss of cartilage results in pain and functional impairment. The avascular and aneural nature of cartilage makes it less responsive to traditional healing mechanisms, necessitating innovative therapeutic approaches.
-
Rehabilitation Considerations:
- Mechanical Stimulation: Controlled mechanical loading can enhance chondrocyte metabolism and ECM synthesis. Methods such as hydrotherapy and continuous passive motion (CPM) machines can provide the necessary mechanical stimuli to support cartilage maintenance and repair. These interventions can help maintain cartilage health and function by promoting the production of ECM components and improving the mechanical environment of the joint. Mechanical loading encourages chondrocytes to produce more ECM, thereby maintaining the tissue’s functional integrity and delaying degeneration. Hydrotherapy, for example, allows for gentle joint movement and loading in a buoyant environment, reducing stress on the cartilage while promoting healing. Continuous passive motion can help maintain joint flexibility and reduce stiffness, enhancing the overall rehabilitation process.
- Biologic Therapies: Cell-based therapies like autologous chondrocyte implantation (ACI) or mesenchymal stem cell (MSC) therapy can support cartilage repair by introducing new cells capable of producing ECM components, thereby enhancing the tissue’s regenerative capacity. These therapies aim to overcome the limited intrinsic healing potential of cartilage by providing a source of cells that can restore the damaged ECM and improve joint function. ACI involves harvesting and expanding chondrocytes from the patient, which are then implanted into the defect, while MSC therapy utilizes stem cells that can differentiate into chondrocytes, providing a versatile approach to cartilage repair. These advanced therapies are often combined with scaffolds or matrices that support cell growth and integration into the existing cartilage. Biologic therapies offer the potential to regenerate damaged cartilage and restore joint function, addressing the limitations of traditional treatments.
Ligament
-
Cell Types:
- Fibroblasts: The primary cell type in ligaments, responsible for the production and maintenance of collagen fibers and other ECM components. Fibroblasts play a crucial role in the ligament’s response to mechanical stress and injury. These cells are highly active in synthesizing and organizing collagen fibers, which provide the tensile strength necessary for ligament function. Fibroblasts are responsive to mechanical signals, adjusting their activity to reinforce the ECM and ensure the ligament can withstand varying levels of stress. They are pivotal in the ligament’s ability to adapt and repair following injury, producing the necessary components for new collagen fiber formation. Fibroblasts also secrete growth factors and cytokines that modulate the healing process and influence the behavior of other cells involved in repair.
- Extracellular Matrix (ECM): The ECM in ligaments is dominated by type I collagen, providing high tensile strength essential for resisting stretching forces. Other ECM components include elastin and proteoglycans, which contribute to the ligament’s viscoelastic properties and ability to withstand mechanical loads. The hierarchical structure of collagen fibers, from fibrils to bundles, ensures that ligaments can resist stretching and provide stability to joints. The presence of elastin allows ligaments to return to their original shape after stretching, while proteoglycans help maintain the tissue’s hydration and resilience. This intricate ECM organization is crucial for the ligament’s role in stabilizing joints and guiding movement. The organized arrangement of collagen fibers ensures that ligaments can handle the mechanical demands placed upon them, providing structural support and flexibility.
-
Injury Response:
- Inflammatory Phase: Following injury, fibroblasts and immune cells release cytokines such as IL-6, IL-1β, and TNF-α, initiating the inflammatory response. This phase is characterized by increased vascular permeability, leukocyte infiltration, and the release of inflammatory mediators that prepare the tissue for repair. The inflammatory response is crucial for clearing debris and initiating the healing process but can also lead to pain and swelling. This phase sets the stage for subsequent healing by attracting cells and molecules necessary for tissue repair. The inflammatory response also involves the removal of damaged ECM components and the initiation of new tissue formation. The initial inflammatory phase creates an environment conducive to repair by mobilizing reparative cells and signaling molecules to the injury site.
- Healing Phases: The repair process in ligaments involves three overlapping phases: inflammation, proliferation (characterized by fibroblast proliferation and ECM synthesis), and remodeling (involving collagen fiber realignment and maturation). Each phase is critical for restoring the ligament’s structural integrity and function. During the proliferative phase, fibroblasts produce new collagen fibers, which gradually replace the damaged tissue. The remodeling phase involves the reorganization and strengthening of these fibers to match the functional demands of the ligament. This phased approach ensures that the ligament gradually regains its mechanical properties, allowing for safe and effective recovery. The remodeling phase can continue for months to years, as collagen fibers realign and mature to restore the ligament’s full strength and function. The extended remodeling phase is essential for achieving optimal mechanical properties and functionality in the healed ligament.
-
Rehabilitation Considerations:
- Early Mobilization: Gradual, controlled loading promotes fibroblast activity and collagen synthesis without overstressing the tissue. Examples include isometric exercises and gradual progression to isotonic and plyometric exercises, which help strengthen the ligament and improve its functional properties. Early mobilization can enhance the quality of the newly formed collagen fibers and reduce the risk of adhesion formation and joint stiffness. Controlled exercises stimulate fibroblasts to produce robust and well-aligned collagen fibers, improving the ligament’s strength and elasticity. Early movement also helps to maintain joint range of motion and function, preventing long-term stiffness and functional deficits. Gradual loading ensures that the healing ligament is exposed to increasing levels of mechanical stress, promoting adaptation and strengthening.
- Proprioceptive Training: Enhancing neuromuscular control through balance and coordination exercises helps prevent re-injury and improve joint stability. Proprioceptive training is essential for restoring joint function and preventing future injuries by improving the body’s ability to sense and respond to joint position and movement. This type of training can enhance the functional stability of the joint, reducing the likelihood of ligament re-injury. Proprioceptive exercises improve the interaction between the nervous system and the musculoskeletal system, ensuring that movements are precise and coordinated. These exercises are critical for athletes and individuals engaging in high-demand activities, as they help to restore confidence and functional performance. Proprioceptive training involves activities such as balance exercises, agility drills, and sport-specific movements to enhance joint stability and neuromuscular control.
Subchondral Bone
-
Cell Types:
- Osteoblasts: Cells responsible for bone formation. They produce the bone matrix and are involved in the mineralization process, which is essential for maintaining bone strength and integrity. Osteoblasts secrete collagen and other matrix proteins that form the scaffold for bone mineralization. These cells play a vital role in synthesizing new bone tissue, ensuring the subchondral bone remains robust and supportive. Osteoblasts are highly responsive to mechanical stimuli, which can enhance their activity and promote bone formation. Osteoblasts also produce growth factors and cytokines that regulate bone metabolism and coordinate the activities of other bone cells.
- Osteoclasts: Cells involved in bone resorption. They break down bone tissue, allowing for the removal of old or damaged bone and facilitating bone remodeling. Osteoclasts play a key role in maintaining the balance between bone formation and resorption, ensuring bone homeostasis. By resorbing old or damaged bone, osteoclasts make way for new bone formation, maintaining the structural integrity of the subchondral bone. This resorption process is tightly regulated to prevent excessive bone loss and maintain skeletal health. Osteoclasts are regulated by various signaling molecules, including RANKL and osteoprotegerin, which control their activity and lifespan.
- Osteocytes: Mature bone cells embedded within the bone matrix, involved in mechanotransduction and regulation of bone remodeling. Osteocytes sense mechanical stress and coordinate the activity of osteoblasts and osteoclasts to maintain bone homeostasis. They communicate through a network of canaliculi, allowing them to regulate bone remodeling in response to mechanical loads. Osteocytes are crucial for detecting changes in mechanical stress and orchestrating appropriate responses to ensure bone strength and health. These cells play a central role in adapting bone structure to mechanical demands, maintaining the functional integrity of the subchondral bone. Osteocytes produce signaling molecules such as sclerostin and RANKL that influence the activities of osteoblasts and osteoclasts, thereby regulating bone remodeling.
-
Injury Response:
- Inflammatory Mediators: Injury to the subchondral bone leads to the release of cytokines such as IL-1, IL-6, and TNF-α, which promote bone resorption and remodeling. These inflammatory mediators can disrupt the balance between bone formation and resorption, leading to conditions like subchondral sclerosis and cyst formation. The inflammatory response can also contribute to pain and swelling, complicating the healing process. The cytokines released during inflammation can activate osteoclasts, leading to increased bone resorption and potential weakening of the subchondral bone. This imbalance can result in a compromised structural foundation for the overlying cartilage, exacerbating joint degeneration. Inflammatory mediators also inhibit osteoblast activity, further disrupting bone formation and remodeling.
- Bone Remodeling: Bone remodeling involves a coordinated effort between osteoclasts and osteoblasts. Injury often triggers an imbalance in this process, leading to conditions such as subchondral sclerosis (abnormal hardening of the bone) and cyst formation (fluid-filled cavities within the bone). Effective bone remodeling is crucial for maintaining joint health and function. An imbalance can result in weakened bone structure and increased susceptibility to further injury or degenerative changes. Bone remodeling ensures that old or damaged bone is replaced with new bone, maintaining the integrity and strength of the subchondral bone. This process is essential for adapting the bone structure to mechanical demands and ensuring joint stability. Disruptions in bone remodeling can lead to suboptimal bone quality, affecting the overall health and function of the joint.
-
Rehabilitation Considerations:
- Load Management: Gradual reintroduction of weight-bearing activities stimulates bone remodeling without causing further damage. Techniques include progressive resistance training and low-impact exercises like swimming or cycling, which help maintain bone density and strength while minimizing the risk of re-injury. Proper load management is essential to avoid overstressing the bone while promoting healthy remodeling and strengthening. Weight-bearing activities stimulate osteoblasts to form new bone, enhancing the density and resilience of the subchondral bone. Gradual progression in loading allows the bone to adapt and strengthen, supporting overall joint health. Load management strategies must balance the need for mechanical stimulation with the risk of overloading the healing bone.
- Pharmacological Interventions: Bisphosphonates or other drugs can regulate bone turnover and reduce pain. These medications can help manage bone loss and improve the structural integrity of subchondral bone, supporting overall joint health. Pharmacological interventions can be used in conjunction with physical rehabilitation to optimize recovery and maintain bone health. These drugs can inhibit osteoclast activity, reducing bone resorption and helping to preserve bone density. In some cases, anabolic agents that stimulate bone formation may also be used to enhance bone repair and regeneration. Pharmacological treatments aim to restore the balance between bone formation and resorption, ensuring optimal bone health and function.
Conclusion
| Tissue Type | Molecular and Cellular Characteristics | Injury Response | Rehabilitation Considerations |
| Meniscus | - Cell Types: 1. Fibrochondrocytes: Produce collagen and proteoglycans, maintaining ECM and responding to mechanical stimuli. 2. Extracellular Matrix (ECM): Contains type I collagen in outer regions, type II in inner regions, and proteoglycans like aggrecan |
- Inflammatory Mediators: Release of IL-1β, TNF-α, and PGE2, leading to ECM degradation. - Healing Capacity: Outer third (red-red zone) is vascularized and heals better; inner two-thirds (red-white and white-white zones) are avascular and have limited healing. |
- Biomechanical Loading: Controlled, progressive loading stimulates fibrochondrocyte activity and ECM synthesis. - Growth Factors: TGF-β and PDGF enhance fibrochondrocyte proliferation and matrix production. |
| Cartilage | - Cell Types: 1. Chondrocytes: Maintain ECM, producing type II collagen and aggrecan. 2. Extracellular Matrix (ECM): Composed of type II collagen fibers and proteoglycans like aggrecan. |
- Inflammatory Mediators: Release of catabolic cytokines (e.g., IL-1, TNF-α) and aggrecanases (e.g., ADAMTS-4, ADAMTS-5). - Limited Repair Capacity: Avascular nature impedes intrinsic healing. |
- Mechanical Stimulation: Controlled loading enhances chondrocyte metabolism and ECM synthesis. - Biologic Therapies: ACI and MSC therapy support cartilage repair. |
| Ligament | - Cell Types: 1. Fibroblasts: Produce and maintain collagen fibers and other ECM components. 2. Extracellular Matrix (ECM): Dominated by type I collagen, with elastin and proteoglycans. |
- Inflammatory Phase: Release of IL-6, IL-1β, and TNF-α, initiating inflammation. - Healing Phases: Involves inflammation, proliferation (fibroblast proliferation and ECM synthesis), and remodeling (collagen fiber realignment and maturation). |
- Early Mobilization: Gradual, controlled loading promotes fibroblast activity and collagen synthesis. - Proprioceptive Training: Enhances neuromuscular control and joint stability. |
| Subchondral Bone | - Cell Types: 1. Osteoblasts: Responsible for bone formation. 2. Osteoclasts: Involved in bone resorption. 3. Osteocytes: Regulate bone remodeling and mechanotransduction. |
- Inflammatory Mediators: Release of cytokines like IL-1, IL-6, and TNF-α. - Bone Remodeling: Involves coordinated activity between osteoclasts and osteoblasts. |
- Load Management: Gradual reintroduction of weight-bearing activities stimulates bone remodeling. - Pharmacological Interventions: Bisphosphonates and other drugs regulate bone turnover and reduce pain. |
Implications for Treatment and Rehabilitation
Rehabilitation Protocols
- Progressive Loading: Rehabilitation should start with low-intensity exercises and gradually increase in intensity and duration. This progressive loading allows tissues to adapt and strengthen over time. Initial activities might include gentle range-of-motion exercises to maintain joint flexibility and prevent stiffness. As the patient progresses, isometric strengthening exercises, which do not involve joint movement, can be introduced to build muscle strength without placing undue stress on the injured area. Gradually, dynamic exercises that involve movement and load-bearing activities are incorporated, tailored to the patient’s tolerance and healing progress. This staged approach ensures that the tissues are gradually conditioned to handle increasing loads, reducing the risk of re-injury. Additionally, this progression helps to build the patient’s confidence and reduce fear-avoidance behaviors, which can be barriers to effective rehabilitation.
- Functional Exercises: Incorporating functional exercises that mimic daily activities and sport-specific movements can help restore joint mobility, strength, and coordination. These exercises should be varied and progressively challenging to improve the functional stability and performance of the knee joint. Functional exercises might include activities such as squatting, lunging, and step-ups, which mimic common movements required in daily life and sports. These exercises help to rebuild the neuromuscular pathways necessary for coordinated movement and can be progressively loaded to increase their intensity and challenge. Over time, incorporating sport-specific drills and dynamic movements such as cutting, jumping, and pivoting can help athletes regain the specific skills and confidence needed for their sport.
- Joint-Specific Loading: Tailoring the loading regimen to the specific joint and injury type is crucial. For example, weight-bearing exercises are beneficial for cartilage repair, as they stimulate the production of cartilage matrix components and promote joint lubrication. In contrast, proprioceptive exercises, which involve balance and coordination training, are important for ligament healing as they help restore the sensory and motor pathways that control joint stability. Specific activities such as balance training on unstable surfaces, plyometrics, and agility drills can also be included based on the injury and rehabilitation stage. These exercises help to restore the dynamic stability of the joint and prepare the patient for the functional demands of their daily activities or sports. Additionally, using tools such as balance boards, foam pads, and resistance bands can provide varied stimuli that enhance proprioceptive training and neuromuscular control.
- Biomechanical Assessments: Assessments such as gait analysis, joint kinematics, and muscle strength testing can provide valuable information for tailoring rehabilitation programs. These assessments help identify compensatory movement patterns and muscle imbalances that need to be addressed. Gait analysis can reveal abnormalities in walking patterns that may result from the injury or develop as compensatory mechanisms. Joint kinematics can provide insights into the range of motion and movement dynamics of the knee, helping to identify specific deficits that need to be addressed. Muscle strength testing can highlight weaknesses in specific muscle groups that may need targeted strengthening exercises. By addressing these biomechanical issues, rehabilitation can be more effective in restoring normal movement patterns and preventing re-injury.
- Patient-Specific Goals: Rehabilitation should be aligned with the patient’s specific goals, whether returning to high-level athletic performance or regaining basic functional mobility. Goal-setting is a collaborative process that involves the patient, physiotherapist, and possibly other healthcare providers. For athletes, this might include sport-specific drills and conditioning to prepare for return to play. For non-athletes, goals might focus on restoring the ability to perform daily activities such as walking, climbing stairs, or participating in recreational activities. Setting realistic and attainable goals helps to motivate the patient and provides clear milestones to gauge progress. Additionally, involving the patient in the goal-setting process can increase their engagement and adherence to the rehabilitation program.
- Adjustable Protocols: Rehabilitation protocols should be flexible and adjustable based on the patient’s progress and response to treatment. Regular monitoring and reassessment are essential to ensure optimal outcomes. Adjustments may include modifying exercise intensity, duration, or type based on the patient’s feedback and clinical findings. For example, if a patient experiences increased pain or swelling, the intensity of exercises may be reduced, or alternative exercises that place less stress on the injured area may be introduced. Conversely, if the patient demonstrates good progress, the rehabilitation program can be intensified to continue challenging the tissues and promoting further healing and strengthening. This adaptability ensures that the rehabilitation program remains effective and responsive to the patient’s needs, maximizing the chances of a successful recovery.
- Biomarker Monitoring: Measuring biomarkers associated with inflammation, tissue repair, and mechanotransduction can provide insights into the biological response to rehabilitation. Biomarkers such as cytokines, growth factors, and ECM components can be assessed through blood or synovial fluid analysis to gauge the healing process. For example, elevated levels of inflammatory cytokines may indicate ongoing inflammation that needs to be managed, while increased levels of growth factors may signal active tissue repair. Regular monitoring of these biomarkers can help guide the timing and intensity of rehabilitation interventions. By understanding the biological processes occurring during rehabilitation, clinicians can make more informed decisions about the best course of treatment.
- Functional Assessments: Regular functional assessments, including range of motion, strength, and proprioception tests, can help evaluate the effectiveness of the rehabilitation protocol and guide adjustments. These assessments can be performed at regular intervals to track progress and inform necessary changes to the rehabilitation plan. Range of motion tests can identify improvements in joint flexibility, while strength tests can measure gains in muscle power. Proprioception tests, which assess the body’s ability to sense joint position and movement, can help determine the restoration of neuromuscular control and joint stability. Incorporating advanced assessment tools such as motion capture systems, force plates, and wearable sensors can provide detailed data on movement patterns and loading, further enhancing the ability to tailor rehabilitation programs.
Pharmacological Interventions
- Non-Steroidal Anti-Inflammatory Drugs (NSAIDs): NSAIDs can reduce pain and inflammation, facilitating early mobilization and mechanical loading. However, their use should be carefully managed to avoid potential side effects such as gastrointestinal issues or delayed tissue healing. NSAIDs work by inhibiting the enzymes that produce prostaglandins, which are mediators of inflammation and pain. By reducing the production of these mediators, NSAIDs help to alleviate pain and swelling, making it easier for patients to engage in early mobilization and rehabilitation activities. It is important to balance the benefits of NSAIDs with their potential risks, and to use the lowest effective dose for the shortest duration necessary to achieve pain relief and reduce inflammation.
- Cytokine Inhibitors: Targeting specific pro-inflammatory cytokines such as IL-1 and TNF-α with inhibitors can help reduce excessive inflammation and promote a more favorable environment for tissue repair. These inhibitors can be administered systemically or locally, depending on the severity and location of the inflammation. By blocking the activity of these cytokines, these inhibitors can help to reduce the inflammatory response and prevent the progression of tissue damage, thereby promoting more effective healing. Localized administration of cytokine inhibitors can help to target the site of inflammation directly, minimizing systemic side effects and enhancing their therapeutic efficacy.
- Selective MMP Inhibitors: Using selective inhibitors that target specific MMPs involved in pathological ECM degradation can help maintain tissue integrity while allowing for necessary remodeling. These inhibitors can be particularly useful in managing chronic conditions where excessive ECM breakdown is a concern. MMP inhibitors work by blocking the enzymatic activity of MMPs, preventing them from degrading the ECM components. This helps to preserve the structural integrity of the tissue and supports the natural repair processes. By selectively targeting specific MMPs, it is possible to reduce unwanted ECM degradation while allowing for normal tissue remodeling and repair.
- Localized Delivery: Localized delivery of growth factors directly to the injury site can enhance their effectiveness and reduce systemic side effects. Techniques such as direct injection or incorporation into biomaterial scaffolds can be used to achieve localized delivery. Localized delivery ensures that the growth factors reach the target tissue in sufficient concentrations to exert their effects, while minimizing the risk of side effects that can occur with systemic administration. This approach can be particularly beneficial in promoting targeted tissue repair and regeneration in the knee joint.
- Controlled Release Systems: Developing controlled release systems, such as hydrogels or scaffolds, can provide sustained delivery of growth factors, improving their therapeutic efficacy. These systems can be engineered to release growth factors in a controlled manner, matching the natural healing process. Controlled release systems can be designed to release growth factors at specific rates and durations, providing a steady supply of bioactive molecules to support tissue repair over an extended period. This sustained delivery can help to maintain optimal levels of growth factors at the injury site, enhancing their therapeutic effects and promoting more effective tissue regeneration.
- Integrin Modulators: Modulating integrin signaling can enhance cell-ECM interactions and promote mechanotransduction. This can be achieved through small molecule inhibitors or activators that target specific integrins, thereby enhancing the cellular response to mechanical stimuli. Integrins are transmembrane receptors that mediate the attachment of cells to the ECM and play a critical role in transmitting mechanical signals from the ECM to the cell interior. Modulating integrin activity can influence various cellular processes, including migration, proliferation, and differentiation, which are essential for tissue repair. By enhancing integrin signaling, it is possible to improve the cellular responses to mechanical loading and support more effective tissue regeneration.
- FAK Inhibitors: Inhibiting FAK activity can help regulate downstream signaling pathways involved in cell proliferation and ECM synthesis, providing a more controlled repair process. FAK inhibitors can be used to fine-tune the cellular responses to mechanical loading. FAK is a key mediator of integrin signaling and plays a central role in mechanotransduction. By inhibiting FAK activity, it is possible to modulate the cellular responses to mechanical cues, promoting more efficient and controlled tissue repair. FAK inhibitors can be used in combination with other therapies to enhance their effectiveness and support more targeted tissue regeneration.
- Calcium Signaling Modulators: Modulating calcium signaling can influence various mechanotransduction pathways, enhancing cellular responses to mechanical loading. Calcium signaling plays a crucial role in cellular activities such as contraction, secretion, and gene expression, making it a key target for therapeutic modulation. Calcium ions act as second messengers in various signaling pathways and can influence processes such as cell proliferation, differentiation, and apoptosis. Modulating calcium signaling can help to optimize the cellular responses to mechanical loading and promote tissue repair. This can be achieved through the use of calcium channel blockers or other agents that influence calcium signaling pathways, enhancing the effectiveness of rehabilitation and other therapeutic interventions.
- Biomaterial Scaffolds: Scaffolds made from biocompatible materials such as collagen, hyaluronic acid, or synthetic polymers can support tissue regeneration. These scaffolds can be designed to mimic the mechanical properties of native tissues, providing appropriate mechanical cues to promote cell proliferation and differentiation. Additionally, these scaffolds can be functionalized with bioactive molecules to enhance their regenerative potential. The choice of biomaterial and scaffold design is crucial for creating an optimal environment for tissue regeneration, as the mechanical properties and bioactivity of the scaffold can influence cellular behavior and tissue formation. Scaffold design can include features such as porosity, surface texture, and mechanical strength to provide the necessary support and guidance for tissue growth.
- 3D Bioprinting: Advanced 3D bioprinting techniques can create complex tissue constructs that closely resemble native joint structures. These constructs can incorporate multiple cell types and ECM components, enhancing their regenerative potential. Bioprinting allows for precise control over scaffold architecture, enabling the creation of customized constructs that match the patient’s anatomy. This technology can be used to fabricate scaffolds with complex geometries and gradients of mechanical properties, providing a more physiologically relevant environment for tissue regeneration. By integrating different cell types and bioactive molecules, 3D bioprinting can produce tissue constructs that mimic the native tissue’s structure and function, enhancing the potential for successful tissue repair and regeneration.
- Autologous MSCs: Using MSCs derived from the patient’s own tissues can reduce the risk of immune rejection and enhance the effectiveness of the therapy. These cells can be harvested from bone marrow, adipose tissue, or synovium and injected directly into the injury site. This approach leverages the patient’s own regenerative potential, minimizing the risk of adverse reactions. Autologous MSC therapy involves isolating MSCs from the patient’s own tissues, expanding them in culture, and then reintroducing them into the injured area to promote tissue repair. This personalized approach ensures compatibility and reduces the risk of immune response, enhancing the potential for successful outcomes.
- Allogeneic MSCs: Allogeneic MSCs from donor sources can provide an off-the-shelf solution for stem cell therapy. These cells can be expanded and cryopreserved for future use, providing a readily available source of reparative cells. This approach offers the advantage of immediate availability and standardized quality. Allogeneic MSCs can be sourced from healthy donors, expanded in culture, and stored for future use. These cells can be used in various applications, including treating acute injuries or chronic conditions. The availability of allogeneic MSCs allows for rapid intervention and the potential to treat a larger number of patients with standardized cell products.
- Paracrine Effects: In addition to differentiating into repair cells, MSCs exert paracrine effects by secreting cytokines and growth factors that modulate the inflammatory response, promote angiogenesis, and enhance the activity of resident cells. These paracrine effects can be harnessed to create a favorable environment for tissue repair. MSCs secrete a wide range of bioactive molecules that influence various cellular processes, including inflammation, tissue remodeling, and vascularization. By modulating the local microenvironment, MSCs can enhance the body’s natural healing processes and promote more effective tissue repair. Harnessing these paracrine effects can complement the differentiation potential of MSCs and enhance their therapeutic efficacy.
- Gene Overexpression: Overexpressing genes involved in mechanotransduction, ECM synthesis, or anti-inflammatory responses can enhance the cellular responses to mechanical loading and promote more effective tissue repair. Gene overexpression can be achieved using viral vectors or CRISPR/Cas9 technology. This approach involves introducing genetic material that encodes for specific proteins or signaling molecules that promote tissue repair. By increasing the expression of these genes, it is possible to enhance the cellular responses to mechanical loading and support more effective tissue regeneration. This technique can be used to boost the production of beneficial proteins and signaling molecules that are crucial for tissue repair and regeneration.
- Gene Knockdown: Silencing genes that negatively regulate tissue repair, such as those involved in excessive inflammation or ECM degradation, can create a more favorable environment for healing. Techniques such as RNA interference (RNAi) or CRISPR/Cas9 can be used to achieve gene knockdown. Gene knockdown involves reducing the expression of specific genes that inhibit tissue repair. By silencing these genes, it is possible to create a more supportive environment for tissue regeneration and enhance the effectiveness of other therapeutic interventions. This approach can help to mitigate the negative effects of certain genes that may impede the healing process.
- CRISPR/Cas9 Technology: Advanced gene-editing techniques like CRISPR/Cas9 offer precise control over gene expression, allowing for targeted modifications that enhance tissue repair and regeneration. This technology can be used to introduce specific genetic changes that promote healing while minimizing off-target effects. CRISPR/Cas9 technology involves using a guide RNA to target specific genetic sequences and the Cas9 enzyme to create precise cuts in the DNA. This allows for the introduction or deletion of specific genetic material, enabling precise control over gene expression and enhancing the potential for tissue repair. The versatility and precision of CRISPR/Cas9 make it a powerful tool for developing targeted gene therapies that can enhance tissue regeneration and repair.
Combination Therapies
- Synergistic Effects: Combining pharmacological agents with mechanical loading can enhance tissue repair and reduce inflammation, improving overall outcomes. For example, using anti-inflammatory drugs in conjunction with controlled mechanical loading can reduce pain and swelling, allowing for more effective rehabilitation. This approach leverages the strengths of both modalities to achieve better results. The combination of pharmacological agents and mechanical loading can help to modulate the inflammatory response, enhance tissue repair, and improve functional outcomes. By reducing inflammation and pain, pharmacological agents can facilitate early mobilization and mechanical loading, enhancing the overall rehabilitation process.
- Timing and Dosage: The timing and dosage of pharmacological agents should be optimized to achieve synergistic effects with mechanical loading. Careful coordination of drug administration and rehabilitation exercises is essential for maximizing benefits. This involves designing treatment schedules that align with the body’s natural healing processes. For example, anti-inflammatory drugs may be administered during the early phases of rehabilitation to reduce pain and swelling, while growth factor therapy may be introduced during later stages to enhance tissue repair and regeneration. Optimizing the timing and dosage of pharmacological agents ensures that they complement the mechanical loading and support the overall rehabilitation strategy.
- Biologics and Mechanical Loading
- Stem Cell and Growth Factor Therapy: Combining stem cell therapy or growth factor administration with mechanical loading can enhance the regenerative potential of these therapies. Mechanical loading provides the necessary mechanical cues to promote cell differentiation and ECM synthesis, while biologics provide the biochemical signals that support tissue repair. This combination can be tailored to address specific injury types and stages of healing. For example, stem cells may be injected into the injured area, followed by controlled mechanical loading exercises that stimulate the cells to differentiate and produce ECM components. Growth factors may be delivered through scaffolds or direct injection to support the healing process and enhance tissue regeneration. The synergy between mechanical cues and biochemical signals can create an optimal environment for tissue repair and regeneration.
- Scaffold-Based Therapies: Using scaffolds that release growth factors or support stem cell attachment can be combined with mechanical loading to enhance tissue regeneration. These scaffolds can be designed to mimic the mechanical properties of native tissues, providing both structural support and biochemical signals to promote repair. The integration of mechanical and biochemical cues within the scaffold can create an optimal environment for tissue regeneration. Scaffold-based therapies involve using biomaterials to create a supportive structure for tissue repair. These scaffolds can be functionalized with growth factors or other bioactive molecules to enhance their regenerative potential. By combining scaffolds with mechanical loading, it is possible to create a synergistic effect that promotes more effective tissue repair and regeneration. This approach leverages the structural and biochemical support provided by the scaffold, combined with the mechanical stimuli from loading, to optimize the healing process.
Advanced Research and Future Directions
- Rodent Models: Rodent models, such as mice and rats, are commonly used due to their cost-effectiveness, ease of handling, and well-characterized genetics. These models are particularly valuable for genetic manipulation, enabling the study of specific genes’ roles in injury and repair processes. For example, the use of transgenic mice with gene knockouts helps to elucidate the function of particular proteins in the healing process. Additionally, rodent models can be used to study the impact of different types of mechanical loading on joint tissues, providing insights into optimal rehabilitation strategies.
- Large Animal Models: Larger animals, such as sheep, pigs, and dogs, offer a closer approximation to human knee joint anatomy and biomechanics. These models are crucial for evaluating the translational potential of therapeutic interventions developed in rodents. For instance, sheep models have been used extensively to study the healing of ligament and meniscal injuries, while porcine models are valuable for assessing cartilage repair techniques. Large animal models can also help to assess the efficacy and safety of new surgical techniques and rehabilitation protocols, providing data that is more relevant to human clinical conditions.
- Human Trials: Clinical studies involving human subjects are necessary to validate the efficacy and safety of early mechanical loading protocols and other therapeutic interventions. RCTs are considered the gold standard for evaluating clinical interventions due to their ability to minimize bias and provide robust evidence. For example, RCTs can compare the outcomes of different rehabilitation protocols, helping to determine the most effective strategies for promoting healing and reducing the risk of re-injury. In addition to RCTs, pilot studies and feasibility trials can help to refine intervention protocols and identify potential challenges before larger trials are conducted.
- Longitudinal Studies: Long-term follow-up studies are needed to assess the durability and effectiveness of mechanical loading protocols and other therapeutic interventions over extended periods. These studies can help identify factors that influence long-term outcomes, such as patient adherence, comorbidities, and the nature of the injury. Longitudinal studies can also provide insights into the natural history of knee joint injuries and the long-term impact of different treatment strategies on joint health and function. This information is crucial for developing strategies to prevent recurrence and promote sustained recovery.
Biomarker Discovery
- Cytokines and Chemokines: Pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) are key mediators of the inflammatory response following injury. Elevated levels of these cytokines can indicate active inflammation and ongoing tissue damage. Anti-inflammatory cytokines like interleukin-10 (IL-10) help to modulate the inflammatory response and promote healing. Chemokines, such as CCL2 (MCP-1), play a role in recruiting immune cells to the injury site, facilitating the removal of debris and the initiation of repair processes.
- Acute Phase Proteins: Proteins such as C-reactive protein (CRP) and serum amyloid A (SAA) are produced by the liver in response to inflammation and can serve as systemic markers of inflammatory activity. Elevated levels of these proteins can indicate a heightened inflammatory state, which may necessitate modifications to the treatment protocol to address excessive inflammation and promote healing.
- Matrix Metalloproteinases (MMPs): MMPs such as MMP-1, MMP-3, and MMP-13 are enzymes that degrade the extracellular matrix (ECM), facilitating tissue remodeling and repair. Their activity is tightly regulated by tissue inhibitors of metalloproteinases (TIMPs), and the balance between MMPs and TIMPs is critical for successful tissue repair. Elevated levels of MMPs can indicate active tissue remodeling, while imbalances between MMPs and TIMPs can suggest dysregulated repair processes.
- Growth Factors: Growth factors such as transforming growth factor-beta (TGF-β), insulin-like growth factor-1 (IGF-1), and bone morphogenetic proteins (BMPs) promote cell proliferation, differentiation, and ECM synthesis, making them important markers of tissue repair. Monitoring the levels of these growth factors can provide insights into the progression of the healing process and the effectiveness of therapeutic interventions designed to enhance tissue repair.
- Collagen Fragments: Degradation products of collagen, such as C-terminal telopeptide of type I collagen (CTX-I) and type II collagen (CTX-II), can indicate ECM turnover and the extent of tissue remodeling. Elevated levels of these fragments can suggest active degradation of the ECM, which may be a sign of ongoing tissue damage or a normal part of the remodeling process.
- Integrins and Focal Adhesion Proteins: Integrins are transmembrane receptors that facilitate cell-ECM interactions and play a critical role in mechanotransduction. Focal adhesion kinase (FAK) is a key signaling protein involved in the formation of focal adhesions, which are complexes that mediate the attachment of cells to the ECM. Monitoring the levels of integrins and FAK can provide insights into the cellular responses to mechanical loading and the effectiveness of rehabilitation protocols.
- Calcium Signaling Molecules: Calcium-binding proteins like calmodulin and calcium/calmodulin-dependent protein kinases (CaMKs) are involved in calcium signaling pathways activated by mechanical stress. Changes in the levels of these proteins can indicate alterations in cellular signaling pathways that are critical for mechanotransduction and tissue repair.
- MAPK Pathway Components: The mitogen-activated protein kinase (MAPK) pathway is a key signaling pathway involved in cellular responses to mechanical loading. Components of the MAPK pathway, including extracellular signal-regulated kinases (ERK1/2), c-Jun N-terminal kinases (JNK), and p38 MAPK, can indicate the activation of mechanotransduction pathways in response to mechanical loading. Monitoring these components can provide insights into the cellular responses to mechanical stimuli and the effectiveness of rehabilitation protocols.
Biomarker Discovery
- Genomics: High-throughput sequencing technologies, such as next-generation sequencing (NGS), can identify genetic variations and expression profiles associated with injury and repair processes. Genomic studies can uncover genes that are differentially expressed in response to mechanical loading and injury, providing insights into the genetic factors that influence healing and the potential for personalized treatment strategies.
- Proteomics: Mass spectrometry-based proteomics allows for the comprehensive analysis of protein expression, modification, and interaction. Proteomic studies can identify proteins and peptides that serve as potential biomarkers for inflammation, tissue repair, and mechanotransduction. This information can be used to develop targeted therapies and monitor the effectiveness of treatment strategies.
- Metabolomics: Metabolomics involves the study of small molecules (metabolites) in biological samples. Metabolomic profiling can provide insights into the metabolic changes associated with injury and the healing process, identifying potential biomarkers that reflect cellular metabolism. This information can be used to develop interventions that support optimal metabolic conditions for healing.
- Data Mining: Bioinformatics tools can analyze large datasets generated by omics technologies to identify patterns and correlations that may indicate potential biomarkers. Machine learning algorithms can help in the identification and validation of biomarkers by analyzing complex data and uncovering hidden relationships that traditional statistical methods might miss.
- Pathway Analysis: Integrating data from genomics, proteomics, and metabolomics with known biological pathways can help identify key molecules involved in the response to injury and mechanical loading. Pathway analysis can highlight potential biomarkers and their roles in cellular processes, providing a more comprehensive understanding of the mechanisms underlying tissue repair and the effects of therapeutic interventions.
- In Vitro Studies: Cell culture models can be used to validate the function and relevance of potential biomarkers identified through omics studies. Manipulating the expression of candidate biomarkers in vitro can help determine their roles in cellular responses to mechanical loading and injury. This information can be used to develop targeted therapies and optimize rehabilitation protocols.
- In Vivo Models: Animal models can be used to validate the relevance of biomarkers in a more complex and clinically relevant context. These models can help assess the temporal dynamics of biomarker expression and their correlation with tissue repair and functional outcomes. This information can be used to develop more effective treatment strategies and monitor their effectiveness in clinical settings.
Clinical Application
- Early Detection: Biomarkers can be used for the early detection of knee joint injuries, allowing for timely intervention and treatment. For example, elevated levels of inflammatory cytokines or MMPs in synovial fluid can indicate ongoing tissue damage, prompting early intervention to prevent further injury and promote healing.
- Severity Assessment: Biomarker levels can help assess the severity of an injury and predict the likely course of recovery. For instance, high levels of collagen degradation products may indicate extensive ECM damage and a longer recovery time. This information can be used to develop personalized treatment plans that are tailored to the severity of the injury and the patient’s individual needs.
- Real-Time Monitoring: Point-of-care testing devices can provide real-time measurements of biomarker levels, allowing clinicians to monitor the patient’s response to treatment and adjust rehabilitation protocols accordingly. This approach can help to optimize treatment strategies and ensure that interventions are effective and appropriate for the patient’s condition.
- Treatment Efficacy: Biomarkers can be used to evaluate the efficacy of different treatment modalities, such as pharmacological interventions or mechanical loading protocols. Changes in biomarker levels can indicate whether a treatment is effectively promoting tissue repair or reducing inflammation. This information can be used to refine treatment strategies and develop more effective therapies.
- Tailored Protocols: Biomarker profiles can inform the design of personalized rehabilitation protocols that are optimized for the individual patient’s biological response. For example, patients with high levels of pro-inflammatory cytokines may benefit from early anti-inflammatory interventions and gradual mechanical loading. This approach can help to ensure that rehabilitation protocols are tailored to the patient’s specific needs and promote optimal healing.
- Adaptive Management: Regular monitoring of biomarkers can guide adaptive management of rehabilitation protocols. If biomarkers indicate excessive inflammation or inadequate tissue repair, the protocol can be adjusted to better meet the patient’s needs. This approach can help to ensure that rehabilitation protocols are dynamic and responsive to the patient’s changing condition, promoting optimal outcomes.
Technologies for Biomarker Measurement
- Principle: ELISA is a widely used technique for quantifying proteins and other molecules in biological samples. It uses specific antibodies to detect and quantify the target biomarker.
- Applications: ELISA is commonly used to measure cytokines, growth factors, and other protein biomarkers in blood, synovial fluid, and tissue samples. This technique is valuable for monitoring inflammatory responses, tissue repair processes, and the effectiveness of therapeutic interventions.
- Principle: Mass spectrometry provides high-resolution analysis of the molecular composition of biological samples. It can identify and quantify proteins, peptides, and metabolites with high sensitivity.
- Applications: Mass spectrometry is used in proteomics and metabolomics to discover and validate biomarkers. It can also be used to measure post-translational modifications and protein-protein interactions. This technique is valuable for identifying potential biomarkers and understanding the molecular mechanisms underlying tissue repair and the effects of therapeutic interventions.
- Principle: NGS allows for the high-throughput sequencing of DNA and RNA, providing comprehensive information on genetic variations and gene expression profiles.
- Applications: NGS is used in genomics to identify genetic biomarkers and study the gene expression changes associated with injury and repair processes. This technique is valuable for understanding the genetic factors that influence healing and developing personalized treatment strategies based on an individual’s genetic profile.
- Principle: Multiplex assays allow for the simultaneous measurement of multiple biomarkers in a single sample. These assays use different detection methods, such as bead-based or array-based technologies.
- Applications: Multiplex assays are useful for studying complex biological processes and identifying biomarker panels that reflect different aspects of tissue repair and inflammation. This approach is valuable for developing comprehensive biomarker profiles that can inform personalized treatment strategies and monitor the effectiveness of therapeutic interventions.
Future Directions in Biomarker Research
- Holistic View: Integrating data from genomics, proteomics, and metabolomics can provide a more comprehensive understanding of the biological processes underlying knee joint injuries and their repair. Multi-omics approaches can identify biomarker networks and their interactions, leading to more robust and predictive biomarker panels.
- Systems Biology: Applying systems biology approaches to integrate multi-omics data can help identify key regulatory nodes and pathways involved in mechanotransduction and tissue repair. This holistic view can inform the development of targeted therapies and personalized rehabilitation protocols.
- Machine Learning and Artificial Intelligence: Advanced analytical techniques, such as machine learning and artificial intelligence, can analyze large and complex datasets to identify novel biomarkers and predict treatment outcomes. These techniques can uncover hidden patterns and correlations that traditional statistical methods might miss, providing new insights into the mechanisms underlying tissue repair and the effects of therapeutic interventions.
- Single-Cell Analysis: Single-cell sequencing and proteomics can provide detailed insights into the cellular heterogeneity and dynamic changes within the injured tissue. This level of resolution can identify cell-specific biomarkers and their roles in tissue repair, providing a deeper understanding of the cellular mechanisms underlying healing and informing the development of targeted therapies.
- Clinical Trials: Translating biomarker discoveries into clinical practice requires rigorous validation through clinical trials. These trials can establish the clinical utility of biomarkers for diagnostics, monitoring, and treatment optimization. Rigorous clinical trials are essential for demonstrating the effectiveness and safety of biomarker-based interventions and ensuring their adoption in clinical practice.
- Regulatory Approval: Developing standardized protocols and obtaining regulatory approval for biomarker assays are essential for their widespread adoption in clinical settings. Collaboration with regulatory agencies can facilitate the translation of biomarker research into approved diagnostic and therapeutic tools, ensuring that new discoveries are accessible to patients and clinicians.
- Portable Devices: Developing portable and user-friendly devices for point-of-care testing can facilitate the rapid and accurate measurement of biomarkers in clinical and field settings. These devices can enable real-time monitoring and personalized management of rehabilitation protocols, providing immediate feedback to clinicians and patients and supporting optimal treatment strategies.
- Wearable Sensors: Wearable sensors that continuously monitor biomarkers in bodily fluids, such as sweat or interstitial fluid, can provide continuous feedback on the patient’s physiological state. These sensors can enhance the precision of rehabilitation programs and improve patient adherence by providing real-time data on the patient’s condition and allowing for dynamic adjustments to treatment protocols.
Novel Therapeutic Targets
Integrin Signaling Modulators
- Purpose: Activating integrins can enhance cell adhesion and survival, promoting tissue repair and regeneration. This is crucial for improving the structural integrity of the tissue and supporting the healing process.
- Mechanisms: Integrin activators can increase the affinity of integrins for their ECM ligands, strengthen focal adhesions, and activate downstream signaling pathways such as FAK and PI3K/Akt. These signaling pathways play pivotal roles in regulating cell survival, proliferation, and differentiation.
- Potential Applications: Integrin activators can be used in combination with mechanical loading to enhance the cellular responses required for effective tissue repair. They can also be used to promote the integration of engineered tissues and scaffolds in regenerative medicine. For instance, in surgical procedures involving tissue grafts, integrin activators could improve the integration and functionality of the grafts, leading to better clinical outcomes.
- Purpose: Inhibiting integrins can be beneficial in conditions where excessive cell adhesion and migration contribute to pathology, such as fibrosis or chronic inflammation. By reducing integrin activity, it is possible to mitigate these pathological processes and promote a healthier tissue environment.
- Mechanisms: Integrin inhibitors can block integrin-ECM interactions, reducing cell adhesion, migration, and downstream signaling. They can be designed to specifically target integrins involved in pathological processes, ensuring that the therapeutic effect is focused and effective.
- Potential Applications: Integrin inhibitors can be used to prevent fibrosis in injured tissues or to reduce inflammation in chronic joint diseases. They can also be combined with anti-inflammatory therapies to enhance their efficacy. For example, in chronic conditions like rheumatoid arthritis, integrin inhibitors could help manage inflammation and tissue damage, improving the quality of life for patients.
Focal Adhesion Kinase (FAK) Inhibitors
- Purpose: Enhancing FAK activity can promote cell proliferation, survival, and ECM synthesis, supporting tissue repair. By stimulating FAK, cells are better able to respond to the mechanical cues that promote healing and regeneration.
- Mechanisms: FAK activators can increase the autophosphorylation of FAK and the activation of downstream signaling pathways such as MAPK and PI3K/Akt. These pathways are essential for cellular processes that underpin tissue repair and regeneration.
- Potential Applications: FAK activators can be used to enhance the regenerative potential of stem cell therapies or to improve the integration and function of tissue-engineered constructs. For example, in treatments involving stem cells for cartilage repair, FAK activators could enhance the cells’ ability to proliferate and integrate with existing tissue.
- Purpose: Inhibiting FAK can reduce excessive cell proliferation and migration, which can be beneficial in conditions such as cancer or fibrosis. By blocking FAK activity, it is possible to slow down or halt the progression of these pathological processes.
- Mechanisms: FAK inhibitors can block FAK autophosphorylation and downstream signaling, reducing cell proliferation, survival, and migration. This can help to contain or reduce pathological tissue growth.
- Potential Applications: FAK inhibitors can be used to prevent fibrosis in injured tissues or to reduce the progression of cancer. They can also be combined with other therapies to enhance their efficacy in targeting pathological cell behaviors. For instance, in the treatment of fibrotic diseases, FAK inhibitors could be used alongside antifibrotic drugs to improve overall treatment outcomes.
Modulators of Mechanotransduction Pathways
- Integrin Activators and Inhibitors: As discussed, modulating integrin signaling can influence cell-ECM interactions and downstream signaling pathways. These modulators can be fine-tuned to either promote or inhibit specific cellular responses based on the therapeutic goals.
- FAK Modulators: Activating or inhibiting FAK can influence various cellular responses to mechanical loading. By targeting FAK, it is possible to either enhance tissue repair processes or inhibit pathological processes such as fibrosis.
- Calcium Channel Modulators: Modulating the activity of stretch-activated ion channels can influence intracellular calcium levels and downstream signaling pathways. This can enhance cellular responses to mechanical loading, promoting tissue repair and regeneration.
- Calcium-Binding Proteins: Modulating the activity of calcium-binding proteins such as calmodulin and CaMK can influence calcium signaling and cellular responses to mechanical loading. This can be particularly useful in enhancing the cells’ ability to respond to mechanical stimuli during rehabilitation.
- Potential Applications: Calcium signaling modulators can be used to enhance the cellular responses to mechanical loading, promoting tissue repair and regeneration. For example, in physical therapy for knee injuries, these modulators could enhance the effectiveness of exercise regimens by improving cellular responsiveness to mechanical stress.
- MAPK Activators: Activating MAPK pathways such as ERK1/2, JNK, and p38 can promote cell proliferation, differentiation, and ECM synthesis. This can support tissue repair and regeneration by enhancing key cellular processes.
- MAPK Inhibitors: Inhibiting MAPK pathways can reduce excessive cell proliferation and inflammation, which can be beneficial in conditions such as cancer or chronic inflammation. This can help to manage pathological processes and support a healthier tissue environment.
- Potential Applications: MAPK pathway modulators can be used to enhance the regenerative potential of stem cell therapies or to reduce inflammation in chronic joint diseases. For instance, in treatments for osteoarthritis, MAPK inhibitors could help to reduce inflammation and slow disease progression.
- Wnt Activators: Activating Wnt signaling can promote cell proliferation, differentiation, and ECM synthesis, enhancing tissue repair and regeneration. This pathway is critical for many developmental processes and can be harnessed to support tissue repair.
- Wnt Inhibitors: Inhibiting Wnt signaling can reduce excessive cell proliferation and differentiation, which can be beneficial in conditions such as cancer or fibrosis. This can help to manage pathological tissue growth and promote healthier tissue function.
- Potential Applications: Wnt signaling modulators can be used to enhance the regenerative potential of stem cell therapies or to prevent fibrosis in injured tissues. For example, in therapies aimed at cartilage repair, Wnt activators could enhance the differentiation of stem cells into cartilage-producing cells.
- YAP/TAZ Activators: Activating YAP/TAZ signaling can promote cell proliferation, survival, and differentiation, supporting tissue repair and regeneration. These pathways are involved in mechanotransduction and play a key role in cellular responses to mechanical stress.
- YAP/TAZ Inhibitors: Inhibiting YAP/TAZ signaling can reduce excessive cell proliferation and migration, which can be beneficial in conditions such as cancer or fibrosis. This can help to control pathological tissue growth and support healthier tissue function.
- Potential Applications: YAP/TAZ signaling modulators can be used to enhance the regenerative potential of stem cell therapies or to prevent fibrosis in injured tissues. For instance, in the treatment of fibrotic conditions, YAP/TAZ inhibitors could help to reduce excessive tissue growth and improve overall tissue health.
Gene Therapy Approaches
- Purpose: Overexpressing genes involved in mechanotransduction, ECM synthesis, or anti-inflammatory responses can enhance tissue repair and regeneration. By increasing the expression of beneficial genes, it is possible to promote cellular processes that support healing.
- Mechanisms: Gene overexpression can be achieved through viral or non-viral vector delivery systems. This approach can increase the expression of target genes and enhance their biological effects. For example, using adenoviral vectors, genes that promote collagen synthesis can be overexpressed to enhance tissue repair.
- Potential Applications: Gene overexpression can be used to enhance the regenerative potential of stem cell therapies or to improve the integration and function of tissue-engineered constructs. For instance, in regenerative medicine, overexpressing growth factors in stem cells could enhance their ability to repair damaged tissues.
- Purpose: Silencing genes that negatively regulate tissue repair, such as those involved in excessive inflammation or ECM degradation, can create a more favorable environment for healing. By reducing the expression of detrimental genes, it is possible to mitigate negative cellular processes and promote healing.
- Mechanisms: Gene knockdown can be achieved through RNA interference (RNAi) or CRISPR/Cas9 technology. This approach can reduce the expression of target genes and mitigate their negative effects. For example, using RNAi, genes that promote inflammatory responses can be silenced to reduce inflammation.
- Potential Applications: Gene knockdown can be used to reduce inflammation in chronic joint diseases or to prevent fibrosis in injured tissues. For instance, in conditions like rheumatoid arthritis, gene knockdown could reduce the inflammatory response and improve joint health.
- Purpose: CRISPR/Cas9 technology offers precise control over gene expression, allowing for targeted modifications that enhance tissue repair and regeneration. By editing specific genes, it is possible to enhance beneficial processes and inhibit detrimental ones.
- Mechanisms: CRISPR/Cas9 can be used to edit specific genes, either by knocking out deleterious genes or by introducing beneficial genetic modifications. This technology provides a high degree of precision, enabling targeted interventions at the genetic level.
- Potential Applications: CRISPR/Cas9 can be used to create genetically modified stem cells with enhanced regenerative potential or to correct genetic defects that impair tissue repair. For example, in therapies for genetic disorders affecting joint health, CRISPR/Cas9 could be used to correct the underlying genetic defects.
Combination Therapies
- Synergistic Effects: Combining pharmacological agents with mechanical loading can enhance tissue repair and reduce inflammation, improving overall outcomes. For example, using anti-inflammatory drugs in conjunction with controlled mechanical loading can reduce pain and swelling, allowing for more effective rehabilitation. This combination can help to ensure that inflammation is managed while promoting the mechanical stimuli needed for tissue repair.
- Timing and Dosage: The timing and dosage of pharmacological agents should be optimized to achieve synergistic effects with mechanical loading. Careful coordination of drug administration and rehabilitation exercises is essential for maximizing benefits. This ensures that the interventions complement each other and do not interfere with the healing process.
- Potential Applications: Combining pharmacological agents with mechanical loading can be used to enhance the effectiveness of rehabilitation protocols and improve patient outcomes. For instance, in the treatment of knee injuries, combining anti-inflammatory drugs with specific physical therapy exercises could lead to faster recovery and better overall joint function. This approach can be tailored to the individual needs of patients, providing a more personalized treatment plan.
- Stem Cell and Growth Factor Therapy: Combining stem cell therapy or growth factor administration with mechanical loading can enhance the regenerative potential of these therapies. Mechanical loading provides the necessary mechanical cues to promote cell differentiation and ECM synthesis, while biologics provide the biochemical signals that support tissue repair. This combination can help to optimize the environment for tissue regeneration, improving the effectiveness of the therapies.
- Scaffold-Based Therapies: Using scaffolds that release growth factors or support stem cell attachment can be combined with mechanical loading to enhance tissue regeneration. These scaffolds can be designed to mimic the mechanical properties of native tissues, providing both structural support and biochemical signals to promote repair. This approach can help to ensure that the regenerative process is supported by both mechanical and biochemical cues, improving the overall effectiveness of the treatment.
- Potential Applications: Combining biologics with mechanical loading can be used to enhance the effectiveness of regenerative medicine approaches and improve patient outcomes. For example, in treatments for cartilage damage, using a scaffold that releases growth factors in combination with physical therapy could significantly improve tissue regeneration and joint function. This combination can be tailored to the specific needs of patients, providing a more comprehensive and effective treatment plan.
Personalized Rehabilitation Strategies
Integration of Patient-Specific Data
- Gait Analysis: Gait analysis involves the assessment of walking patterns, including stride length, cadence, and joint angles. This information can help identify biomechanical abnormalities and guide the design of targeted interventions to correct these issues. For example, gait analysis can reveal deviations in walking patterns that may contribute to knee pain or instability, allowing for the development of specific exercises to address these problems. Advanced gait analysis may use high-tech equipment such as motion capture systems and force plates to provide detailed insights into the patient’s walking mechanics. The data collected can also be used to create customized orthotics or footwear that correct abnormal gait patterns. Additionally, digital gait analysis platforms can provide visual feedback to patients, helping them understand and correct their walking mechanics. Continuous gait monitoring can help track progress over time and adjust rehabilitation protocols as needed.
- Joint Kinematics: Joint kinematics refers to the study of joint movements and angles during different activities. Assessing joint kinematics can provide insights into the functional limitations and mechanical loading patterns that need to be addressed during rehabilitation. This can help to identify abnormal joint movements that may increase the risk of re-injury and guide the development of exercises to improve joint function. Techniques like three-dimensional motion analysis and wearable sensors can capture precise joint movements during various activities, offering a comprehensive view of joint mechanics. These insights can be particularly valuable for developing exercises that enhance joint stability and prevent future injuries. Real-time kinematic feedback systems can also help patients improve their movement patterns during exercises and daily activities. Regular assessments can track improvements and highlight areas needing further focus.
- Muscle Strength Testing: Measuring muscle strength, particularly in the quadriceps and hamstrings, is essential for understanding the extent of muscle weakness and imbalance. Strength testing can inform the development of exercises aimed at restoring muscle function and preventing re-injury. For instance, patients with weak quadriceps may benefit from targeted strength training exercises to improve knee stability and function. Isokinetic dynamometers and handheld dynamometers are often used to provide accurate and objective measurements of muscle strength. Regular strength assessments can help track progress and make necessary adjustments to the exercise regimen to ensure continuous improvement. These assessments can also identify muscle imbalances that may need to be addressed to optimize recovery and prevent re-injury. Personalized strength training programs can be designed to address specific weaknesses and imbalances identified during testing.
- Magnetic Resonance Imaging (MRI): MRI provides detailed images of soft tissues, including ligaments, menisci, and cartilage. MRI can be used to assess the extent of injury, monitor tissue healing, and detect complications such as fibrosis or osteoarthritis. For example, MRI can reveal subtle changes in cartilage integrity that may not be visible on other imaging modalities, helping to guide treatment decisions. Functional MRI (fMRI) can also assess changes in blood flow and muscle activation patterns, providing additional insights into the rehabilitation process. Repeated MRI scans can track the progress of healing over time, allowing for timely interventions if complications arise. MRI can also be used to evaluate the effectiveness of different therapeutic interventions, helping to optimize treatment plans. Advanced MRI techniques, such as diffusion-weighted imaging, can provide additional information about tissue health and repair.
- Ultrasound: Ultrasound imaging can be used to assess soft tissue structures in real-time, allowing for dynamic evaluation of joint function and tissue repair. It is particularly useful for guiding interventions such as injections and monitoring the progress of rehabilitation. Ultrasound can provide real-time feedback on the effectiveness of treatments, helping to make adjustments as needed. Doppler ultrasound can also assess blood flow and inflammation in the affected area, offering further diagnostic information. Portable ultrasound devices make it possible to perform these assessments conveniently in various settings, including at the patient’s home or in a clinical environment. Ultrasound can also be used for biofeedback during rehabilitation exercises, helping patients to visualize and correct their movements. Elastography, an advanced ultrasound technique, can assess tissue stiffness, providing further insights into the healing process.
- Computed Tomography (CT): CT scans provide detailed images of bone structures and can be used to assess the alignment and integrity of the knee joint. CT imaging is helpful for diagnosing fractures and other bone-related issues. For example, CT can reveal the extent of bone damage in complex fractures, informing surgical planning and post-operative rehabilitation. Dual-energy CT can also differentiate between different tissue types, such as bone and soft tissue, providing more comprehensive diagnostic information. 3D reconstructions from CT scans can help in planning surgical interventions and ensuring accurate alignment and fixation of bone fragments. CT imaging can also be used to assess the outcomes of surgical procedures and guide post-operative rehabilitation. Low-dose CT protocols can reduce radiation exposure while still providing high-quality images.
- Inflammatory Markers: Measuring levels of pro-inflammatory cytokines (e.g., IL-1, IL-6, TNF-α) and acute phase proteins (e.g., CRP, SAA) can provide insights into the inflammatory status of the patient and guide the use of anti-inflammatory therapies. For instance, high levels of these markers may indicate ongoing inflammation that requires targeted anti-inflammatory treatment. Serial measurements can track changes in inflammation over time, helping to adjust treatment protocols accordingly. By regularly monitoring these markers, clinicians can determine the effectiveness of anti-inflammatory treatments and make necessary adjustments to ensure optimal outcomes. Anti-inflammatory therapies can be personalized based on the patient’s biomarker profile, enhancing their effectiveness. Biomarker-guided treatment can help minimize side effects by avoiding unnecessary medication use.
- Markers of Tissue Repair: Biomarkers such as growth factors (e.g., TGF-β, IGF-1), collagen degradation products (e.g., CTX-II), and MMPs can indicate the extent of tissue remodeling and repair. These markers can help monitor the effectiveness of rehabilitation protocols and guide adjustments. For example, elevated levels of collagen degradation products may suggest active tissue remodeling that needs to be supported with appropriate interventions. Monitoring these biomarkers can provide early indications of successful tissue healing or potential complications. Personalized treatment plans can be developed based on the biomarker profiles, ensuring that the rehabilitation process is tailored to the patient’s specific needs. Regular biomarker monitoring can help optimize the timing and intensity of rehabilitation interventions, enhancing their effectiveness. Biomarker profiles can also help identify patients at risk of delayed healing or complications, allowing for early intervention.
- Mechanotransduction Markers: Assessing levels of integrins, FAK, and components of the MAPK pathway can provide information on the cellular responses to mechanical loading and the effectiveness of mechanotherapy. These markers can help to tailor rehabilitation exercises to enhance tissue repair. Understanding the mechanotransduction pathways can also provide insights into how different types of mechanical loading affect cellular responses, guiding the design of optimal rehabilitation protocols. This data can be used to adjust the intensity and type of mechanical loading exercises to maximize their therapeutic benefits. Personalized mechanotherapy plans can be developed based on the patient’s mechanotransduction marker profile, optimizing the effectiveness of these interventions. Mechanotransduction markers can also help identify the most effective types of mechanical stimuli for individual patients, enhancing the precision of rehabilitation protocols.
- Pain Assessment: Self-reported pain levels using scales such as the Visual Analog Scale (VAS) or the Numeric Rating Scale (NRS) can help monitor the patient’s pain experience and guide pain management strategies. Regular pain assessment can help to ensure that pain is being effectively managed and that rehabilitation exercises are not exacerbating discomfort. Tracking pain levels over time can also provide insights into the effectiveness of different interventions and guide adjustments to the treatment plan. Pain management strategies can be adapted based on the patient’s feedback, ensuring that they remain comfortable and motivated throughout the rehabilitation process. Pain diaries and mobile apps can facilitate continuous monitoring and provide detailed data on pain patterns. Integrating pain assessment with other clinical data can help provide a comprehensive view of the patient’s condition, informing more effective treatment plans.
- Functional Assessments: Patient-reported outcome measures (PROMs) such as the Knee Injury and Osteoarthritis Outcome Score (KOOS) or the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) can provide insights into the patient’s functional status and quality of life. These assessments can help to track progress over time and identify areas that need improvement. PROMs can capture aspects of the patient’s daily life and overall well-being that may not be evident through clinical assessments alone. This comprehensive view of the patient’s functional status can guide the development of more effective and personalized rehabilitation strategies. PROMs can also help identify specific activities or tasks that the patient finds challenging, allowing for targeted interventions to address these issues. Regularly updating PROMs can help track improvements and adjust rehabilitation goals as needed.
- Activity Levels: Monitoring the patient’s activity levels and adherence to rehabilitation exercises can help identify barriers to progress and inform the design of more effective interventions. For example, tracking activity levels can reveal if a patient is struggling to adhere to their exercise regimen, allowing for adjustments to be made to improve compliance. Wearable activity trackers and mobile apps can provide detailed and continuous data on the patient’s activity patterns, offering valuable insights for optimizing rehabilitation protocols. Regular feedback and encouragement can help patients stay motivated and committed to their rehabilitation program. Activity tracking data can also be shared with healthcare providers, facilitating remote monitoring and timely adjustments to the treatment plan. Detailed activity logs can help identify patterns and trends that may affect rehabilitation outcomes, allowing for more informed decision-making.
Predictive Models and Decision-Support Systems
- Predictive Analytics: Machine learning algorithms can analyze large datasets to identify patterns and correlations that can predict treatment outcomes. For example, predictive models can be developed to identify patients at risk of poor outcomes based on their baseline characteristics and early responses to treatment. This can help to personalize treatment plans and optimize outcomes. Predictive analytics can also identify factors that contribute to successful rehabilitation, guiding the development of more effective protocols. By continuously learning from new data, these models can become increasingly accurate and reliable over time. Predictive models can also help identify patients who may benefit from more intensive or specialized rehabilitation programs, ensuring that resources are allocated effectively. Integrating predictive analytics with clinical decision-making can help improve the precision and effectiveness of rehabilitation strategies.
- Classification Algorithms: Classification algorithms can be used to categorize patients into different risk groups or treatment pathways based on their individual profiles. This can help tailor rehabilitation protocols to the specific needs of each patient, ensuring that they receive the most appropriate interventions for their condition. These algorithms can consider various factors, such as demographic data, clinical history, and genetic information, to provide a comprehensive assessment of the patient’s rehabilitation needs. Classification algorithms can also help identify patients who may benefit from more intensive or specialized rehabilitation programs, ensuring that resources are allocated effectively. By segmenting patients into distinct categories, classification algorithms can help personalize treatment plans and improve outcomes.
- Clinical Decision Support: Decision-support systems can integrate patient-specific data, predictive models, and clinical guidelines to provide evidence-based recommendations for treatment and rehabilitation. These systems can assist clinicians in making informed decisions and optimizing care for each patient. For instance, a decision-support system might recommend specific exercises or interventions based on the patient’s biomechanical assessments and biomarker profiles. This can help to ensure that the treatment plan is based on the latest evidence and tailored to the patient’s unique needs. Decision-support systems can also provide alerts and reminders to clinicians, helping to ensure that all aspects of the patient’s care are addressed in a timely manner. These systems can also facilitate communication and coordination among the healthcare team, ensuring a cohesive and integrated approach to patient care. Integrating decision-support systems with electronic health records can help streamline the workflow and enhance clinical decision-making.
- Real-Time Monitoring: Decision-support systems can incorporate real-time data from wearable sensors, biomarker measurements, and patient-reported outcomes to continuously monitor the patient’s progress. This allows for timely adjustments to rehabilitation protocols based on the patient’s response to treatment. For example, if a patient is not responding well to a particular exercise regimen, the system can suggest modifications to improve effectiveness. Real-time monitoring can also detect early signs of complications, allowing for prompt intervention and potentially preventing more severe issues. Continuous data collection and analysis can provide a comprehensive view of the patient’s progress, helping to optimize rehabilitation protocols. Real-time monitoring can also enhance patient engagement by providing immediate feedback and encouragement, helping to keep patients motivated and on track with their rehabilitation program. Automated alerts and reminders can help ensure that patients adhere to their rehabilitation plans.
- Adaptive Protocols: Rehabilitation protocols should be flexible and adaptive, allowing for adjustments based on the patient’s progress and response to treatment. Regular reassessment and monitoring are essential to ensure that the rehabilitation program remains effective and responsive to the patient’s needs. For instance, if a patient shows signs of improvement, the protocol can be adjusted to include more challenging exercises. Conversely, if a patient is struggling, the protocol can be modified to reduce the intensity or frequency of exercises. This adaptive approach ensures that the rehabilitation program remains aligned with the patient’s evolving needs and goals. Adaptive protocols can also incorporate feedback from the patient, ensuring that their preferences and experiences are considered in the design and implementation of the rehabilitation plan. Regular check-ins and assessments can help track progress and identify areas needing adjustment.
- Tailored Exercises: The design of exercise programs should be tailored to address the specific deficits and goals of each patient. For example, patients with muscle weakness may benefit from resistance training, while those with joint instability may require proprioceptive and balance exercises. This individualized approach ensures that the exercises are targeting the patient’s unique needs and promoting optimal recovery. Tailored exercises can also consider the patient’s preferences and lifestyle, making it more likely that they will adhere to the rehabilitation program. By involving patients in the design of their exercise programs, clinicians can enhance their engagement and motivation. Tailored exercises can also be adjusted based on the patient’s progress and response to treatment, ensuring that the rehabilitation program remains effective and responsive to their needs. Personalized exercise plans can help optimize outcomes by addressing the patient’s specific strengths and weaknesses.
- Multidisciplinary Approach: Collaboration among healthcare professionals, including physical therapists, orthopedic surgeons, sports medicine specialists, and nutritionists, is essential for providing comprehensive and coordinated care. A multidisciplinary approach ensures that all aspects of the patient’s rehabilitation are addressed. For instance, a nutritionist might work with the patient to optimize their diet for tissue healing, while a physical therapist focuses on improving mobility and strength. Regular communication and collaboration among the healthcare team can help to ensure that the treatment plan is cohesive and effective. Multidisciplinary meetings and case conferences can facilitate the exchange of information and the development of integrated care plans. This collaborative approach can also help identify and address any potential barriers to progress, ensuring that the patient receives the most comprehensive and effective care possible. Coordinated care plans can help optimize rehabilitation outcomes by addressing the patient’s needs from multiple perspectives.
Advanced Technologies for Monitoring and Adjustment
- Activity Monitoring: Wearable sensors can track the patient’s activity levels, movement patterns, and adherence to rehabilitation exercises. This data can provide insights into the patient’s functional status and identify areas that need improvement. For example, sensors can detect if a patient is not performing exercises correctly, allowing for timely intervention. Activity monitors can also provide feedback to the patient, helping to motivate them and encourage adherence to the rehabilitation program. The data collected can be shared with healthcare providers, facilitating remote monitoring and timely adjustments to the treatment plan. Wearable sensors can also provide detailed data on the patient’s daily activity patterns, helping to identify areas where they may need additional support or guidance. Continuous activity monitoring can help track progress and ensure that patients are meeting their rehabilitation goals.
- Physiological Monitoring: Wearable sensors can also monitor physiological parameters such as heart rate, muscle activity, and joint angles. This information can help assess the patient’s response to exercise and guide the adjustment of rehabilitation protocols. For instance, if a patient’s heart rate is consistently elevated during exercises, adjustments can be made to ensure that the intensity is appropriate. Physiological monitoring can also provide early warnings of potential issues, such as overexertion or improper technique, allowing for prompt correction. By continuously tracking these parameters, clinicians can gain a deeper understanding of the patient’s physical responses to rehabilitation and make data-driven decisions. Wearable sensors can also provide real-time feedback to patients, helping them adjust their movements and exercises to optimize their effectiveness. Integrating physiological monitoring with other data sources can provide a comprehensive view of the patient’s health and progress.
- Virtual Consultations: Telemedicine platforms allow for virtual consultations with healthcare professionals, providing patients with access to care regardless of their location. Virtual consultations can be used for regular check-ins, progress assessments, and adjustments to rehabilitation protocols. This ensures that patients receive continuous support and guidance throughout their rehabilitation journey. Telemedicine can also help to reduce barriers to care, such as travel difficulties or time constraints, making it easier for patients to access the support they need. Video consultations can provide a face-to-face interaction, fostering a strong patient-provider relationship and enhancing communication. Telemedicine platforms can also facilitate the sharing of diagnostic images and other data, ensuring that healthcare providers have all the information they need to make informed decisions. Telemedicine can also enable multidisciplinary teams to collaborate more effectively, ensuring comprehensive care for the patient.
- Remote Monitoring: Remote monitoring systems can collect data from wearable sensors and other devices, allowing healthcare professionals to track the patient’s progress in real-time. This enables timely interventions and adjustments to rehabilitation protocols based on the patient’s needs. For example, remote monitoring can detect early signs of complications, allowing for prompt intervention. Remote monitoring can also provide continuous feedback to the patient, helping to ensure that they are following the rehabilitation program correctly and making progress towards their goals. These systems can include automated alerts and reminders to keep patients on track and engaged in their rehabilitation. Remote monitoring can also facilitate communication between patients and healthcare providers, ensuring that any concerns or issues are addressed promptly. Integrating remote monitoring with telemedicine can enhance the continuity and quality of care.
- Kinematic Analysis: 3D motion analysis systems can provide detailed assessments of joint kinematics and movement patterns during different activities. This information can be used to identify biomechanical abnormalities and guide the design of targeted interventions. For example, motion analysis can reveal compensatory movements that may increase the risk of re-injury, allowing for the development of corrective exercises. Kinematic analysis can also provide objective data on the effectiveness of different interventions, helping to optimize rehabilitation protocols. The use of advanced motion capture technology can provide highly accurate and detailed data, enhancing the precision of the analysis. Kinematic analysis can also help identify specific movement patterns or activities that may be contributing to pain or discomfort, allowing for targeted interventions to address these issues. Regular motion analysis can track improvements in movement patterns and help refine rehabilitation protocols over time.
- Feedback and Training: 3D motion analysis systems can also provide real-time feedback to patients during rehabilitation exercises, helping them improve their movement patterns and achieve better outcomes. This feedback can enhance the effectiveness of exercises by ensuring that they are performed correctly and safely. Real-time feedback can also help to motivate patients and improve adherence to the rehabilitation program. Interactive training sessions using motion analysis data can provide patients with visual feedback on their movements, making it easier for them to understand and correct their technique. These systems can also track the patient’s progress over time, providing valuable data on the effectiveness of the rehabilitation program and helping to guide adjustments as needed. Motion analysis can be integrated with other rehabilitation tools to create a comprehensive and dynamic treatment plan.
Future Directions in Personalized Rehabilitation
- Comprehensive Profiling: Integrating data from genomics, proteomics, metabolomics, and other omics technologies can provide a comprehensive understanding of the biological processes underlying knee joint injuries and their response to treatment. Multi-omics approaches can identify biomarker networks and their interactions, leading to more robust and predictive biomarker panels. This information can help to develop more targeted and effective rehabilitation strategies. For example, genomics can identify genetic predispositions to certain types of injuries, while proteomics can reveal protein changes associated with tissue repair. By combining these data sources, researchers can gain a holistic view of the factors influencing rehabilitation outcomes. Multi-omics data can also help identify new therapeutic targets and inform the development of personalized treatment plans. Integrating multi-omics data with clinical and patient-reported outcomes can provide a comprehensive view of the patient’s health and response to treatment.
- Systems Biology: Applying systems biology approaches to integrate multi-omics data can help identify key regulatory nodes and pathways involved in tissue repair and mechanotransduction. This holistic view can inform the development of targeted therapies and personalized rehabilitation protocols. For instance, systems biology can reveal how different biological pathways interact during the healing process, providing insights for developing more effective interventions. Understanding these interactions can help to identify new therapeutic targets and optimize treatment strategies. Systems biology can also model complex biological processes, allowing for the simulation and prediction of treatment responses. This can help to refine and optimize rehabilitation protocols, ensuring that they are tailored to the patient’s unique needs and biological profile. Systems biology can also help identify potential biomarkers for monitoring treatment progress and outcomes.
- Predictive Modeling: Advanced analytics and machine learning can develop more accurate predictive models for treatment outcomes, identifying patients at risk of poor outcomes and guiding the design of personalized rehabilitation protocols. These models can help to ensure that patients receive the most appropriate and effective treatments based on their individual profiles. Predictive modeling can also identify key factors that contribute to successful rehabilitation, guiding the development of more effective protocols. By analyzing large datasets, machine learning algorithms can uncover patterns and relationships that may not be evident through traditional statistical methods. Predictive models can also help identify patients who may benefit from more intensive or specialized rehabilitation programs, ensuring that resources are allocated effectively. Integrating predictive analytics with clinical decision-making can help improve the precision and effectiveness of rehabilitation strategies. Advanced analytics can also help optimize resource allocation and planning for rehabilitation programs.
- Dynamic Adjustment: Machine learning algorithms can continuously learn from patient data and adjust rehabilitation protocols in real-time, ensuring that the treatment remains effective and responsive to the patient’s needs. This dynamic adjustment can help to optimize rehabilitation outcomes by ensuring that protocols are tailored to the patient’s evolving condition. Real-time data analysis can also provide early warnings of potential issues, allowing for prompt intervention and adjustment of the treatment plan. By leveraging the power of machine learning, clinicians can make data-driven decisions that enhance the effectiveness of rehabilitation. Continuous learning and adaptation can help to ensure that rehabilitation protocols remain up-to-date with the latest evidence and best practices. Dynamic adjustment can also enhance patient engagement and adherence by providing personalized and responsive care.
- Education and Training: Educating patients about their condition and the importance of adherence to rehabilitation protocols can enhance engagement and compliance. Providing patients with the knowledge and tools to manage their rehabilitation can empower them to take an active role in their recovery. For example, educational resources and training sessions can help patients understand the benefits of specific exercises and motivate them to adhere to their rehabilitation plan. Ongoing education and support can also help to address any concerns or misconceptions that patients may have about their treatment. Patient education can be delivered through various formats, including online modules, printed materials, and one-on-one consultations. Educational programs can also include practical training on how to perform exercises correctly and safely. Patient education can help build confidence and reduce anxiety about the rehabilitation process.
- Gamification: Incorporating gamification elements into rehabilitation programs can increase patient motivation and adherence. Using game-based exercises and tracking progress through rewards and challenges can make rehabilitation more engaging and enjoyable. For instance, rehabilitation exercises can be designed as interactive games that reward patients for completing tasks and achieving milestones, making the process more fun and motivating. Gamification can also provide a sense of accomplishment and progress, helping to keep patients engaged and motivated throughout their rehabilitation journey. By incorporating elements of competition and social interaction, gamified programs can create a supportive and motivating environment for patients. Gamification can also include personalized goals and challenges that are tailored to the patient’s abilities and progress, ensuring that the program remains challenging and rewarding. Integrating gamification with real-time feedback and monitoring can enhance the effectiveness of rehabilitation programs.
Conclusion
| Aspect | Cartilage | Ligaments | Tendons | Meniscus |
| Biophysical Stimulation | - Compression - Hydrostatic pressure - Shear stress - Dynamic loading to mimic joint movements |
- Tensile loading - Cyclic stretching - Controlled dynamic loading to prevent overstretching |
- Tensile loading - Cyclic stretching - Gradual progressive loading |
- Compression - Shear stress - Tensile loading - Cyclic and static loading for comprehensive stimulation |
| Mechanotransduction | - Integrin signaling (e.g., α5β1 integrin) - Ion channels (e.g., stretch-activated Ca²⁺ channels) - MAPK pathway (ERK1/2, JNK, p38) - Wnt/β-Catenin signaling - YAP/TAZ activation |
- Integrin signaling (e.g., αvβ3 integrin) - Focal Adhesion Kinase (FAK) activation - Rho family GTPases (RhoA, Rac1) - MAPK pathway (ERK1/2, JNK, p38) |
- Integrin signaling (e.g., α5β1 integrin) - Ion channels (e.g., stretch-activated Ca²⁺ channels) - FAK activation - MAPK pathway (ERK1/2, JNK, p38) |
- Integrin signaling (e.g., α5β1 and α6β1 integrins) - Ion channels (e.g., stretch-activated Ca²⁺ channels) - MAPK pathway (ERK1/2, JNK, p38) - Wnt/β-Catenin signaling - YAP/TAZ activation |
| Stress/Strain | - Moderate compressive stress (optimal to stimulate chondrocytes) - Cyclic loading to promote ECM production - Avoid excessive stress to prevent chondrocyte apoptosis |
- Tensile stress aligned with ligament fibers - Gradual increase in load to stimulate fibroblasts - Cyclic loading to enhance collagen synthesis |
- Tensile stress aligned with tendon fibers - Gradual increase in load to stimulate tenocytes - Cyclic loading to enhance collagen synthesis |
- Combination of compressive and tensile stress - Cyclic loading to stimulate chondrocytes and fibrochondrocytes - Avoid excessive stress to prevent further tearing |
| Stress-Relaxation | - Gradual application and release of load - Allows time for ECM adaptation - Prevents cell damage and apoptosis |
- Gradual relaxation phases - Reduces risk of re-injury - Enhances ligament compliance and function |
- Gradual relaxation phases - Reduces risk of tendinopathy - Enhances tendon compliance and function |
- Gradual relaxation phases - Allows time for ECM adaptation - Prevents further damage and promotes healing |
| Hysteresis | - Minimizes energy loss during loading/unloading - Maintains cartilage resilience and function - Promotes efficient load-bearing capacity |
- Reduces energy loss during cyclic loading - Enhances ligament elasticity and function - Promotes efficient load transfer and shock absorption |
- Reduces energy loss during cyclic loading - Enhances tendon elasticity and function - Promotes efficient force transmission and load-bearing capacity |
- Minimizes energy loss during loading/unloading - Maintains meniscal resilience and function - Promotes efficient load distribution and shock absorption |
| Cell Biology of Early Mechanical Loading | - Chondrocyte proliferation and differentiation - ECM synthesis (collagen II, aggrecan) - Autophagy activation for cell survival - Modulation of inflammatory response (decreased IL-1, TNF-α) - Enhanced synthesis of proteoglycans and glycosaminoglycans |
- Fibroblast proliferation and migration - Collagen synthesis (type I and III) - ECM remodeling and organization - Modulation of inflammatory response (decreased IL-6, MMPs) - Enhanced ligament strength and flexibility |
- Tenocyte proliferation and alignment - Collagen synthesis (type I) - ECM remodeling and organization - Modulation of inflammatory response (decreased MMPs, increased TIMPs) - Enhanced tendon strength and flexibility |
- Chondrocyte and fibrochondrocyte activity - ECM synthesis (collagen I and II, proteoglycans) - MSC recruitment and differentiation - Modulation of inflammatory response (decreased pro-inflammatory cytokines) - Enhanced meniscal function and integration |
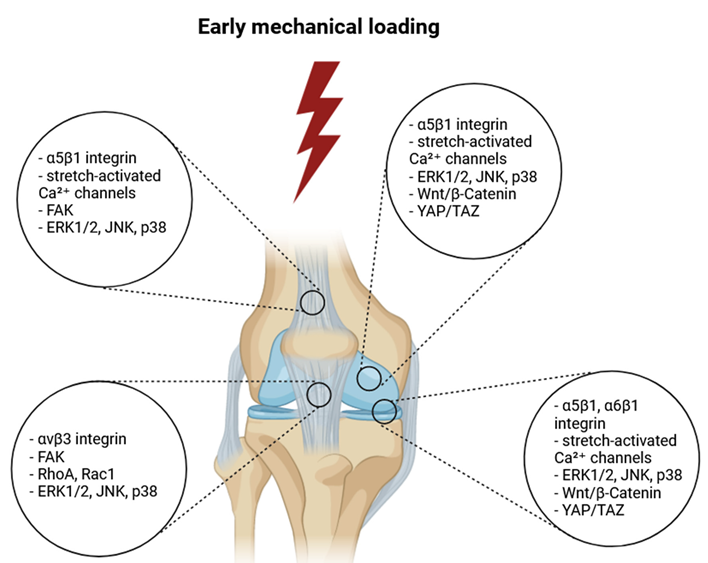
References
- Achenbach L, Bloch H, Klein C, Damm T, Obinger M, Rudert M, Krutsch W, Szymski D. Four distinct patterns of anterior cruciate ligament injury in women’s professional football (soccer): a systematic video analysis of 37 match injuries. Br J Sports Med. 2024 Jun 20;58(13):709-716. [CrossRef] [PubMed]
- Gholipour Aghdam GM, Alizadeh MH, Minoonejad H, Shirzad E, Wilke J. Knee Biomechanics During Neurocognitively Challenged Drop Landings in Male Elite Soccer Players with Anterior Cruciate Ligament Reconstruction. Sports Med Open. 2024 Feb 27;10(1):19. PMCID: PMC10899557. [CrossRef] [PubMed]
- Aizawa J, Hirohata K, Ohji S, Ohmi T, Mitomo S, Koga H, Yagishita K. Cross-sectional study on relationships between physical function and psychological readiness to return to sport after anterior cruciate ligament reconstruction. BMC Sports Sci Med Rehabil. 2022 Jun 1;14(1):97. PMCID: PMC9161472. [CrossRef] [PubMed]
- Ananías J, Vidal C, Ortiz-Muñoz L, Irarrázaval S, Besa P. Use of electromyographic biofeedback in rehabilitation following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Physiotherapy. 2024 Jun;123:19-29. Epub 2023 Dec 21. [CrossRef] [PubMed]
- Andrushko JW, Carr JC, Farthing JP, Lepley LK, DeFreitas JM, Goodall S, Hendy AM, Howatson G, Grooms DR, Zult T, Hortobagyi T, Harput G, Papandreou M, Nosaka K, Carson RG, Manca A, Deriu F, Behm DG, Kidgell DJ, Clark NC, Boyd LA. Potential role of cross-education in early-stage rehabilitation after anterior cruciate ligament reconstruction. Br J Sports Med. 2023 Dec;57(23):1474-1475. Epub 2023 Oct 11. [CrossRef] [PubMed]
- Armento A, Keeter C, Gagliardi A, Rossing H, Giachino C, VandenBerg C, Howell D, Albright J. Association of Grit With Postoperative Knee Outcomes and Physical Function After ACL Reconstruction in Adolescent Athletes. Am J Sports Med. 2023 Sep;51(11):2900-2907. Epub 2023 Jul 31. [CrossRef] [PubMed]
- Baez S, Harkey M, Birchmeier T, Triplett A, Collins K, Kuenze C. Psychological Readiness, Injury-Related Fear, and Persistent Knee Symptoms After Anterior Cruciate Ligament Reconstruction. J Athl Train. 2023 Nov 1;58(11-12):998-1003. PMCID: PMC10784889. [CrossRef] [PubMed]
- Bahr, R. Why screening tests to predict injury do not work-and probably never will…: a critical review. Br J Sports Med. 2016 Jul;50(13):776-80. Epub 2016 Apr 19. [CrossRef] [PubMed]
- Bakhsh HR, Metikala S, Billy GG, Vairo GL. Association Between Self-Reported Kinesiophobia and Single-Leg Hop for Distance in Patients With ACL Reconstruction: A Systematic Review. Sports Health. 2022 Sep-Oct;14(5):674-680. Epub 2021 Oct 15. PMCID: PMC9460087. [CrossRef] [PubMed]
- Balendra G, Jones M, Borque KA, Willinger L, Pinheiro VH, Williams A. Factors affecting return to play and graft re-rupture after primary ACL reconstruction in professional footballers. Knee Surg Sports Traumatol Arthrosc. 2022 Jul;30(7):2200-2208. Epub 2021 Oct 12. [CrossRef] [PubMed]
- Banios K, Raoulis V, Fyllos A, Chytas D, Mitrousias V, Zibis A. Anterior and Posterior Cruciate Ligaments Mechanoreceptors: A Review of Basic Science. Diagnostics (Basel). 2022 Jan 27;12(2):331. PMCID: PMC8870829. [CrossRef] [PubMed]
- Belcher S, Whatman C, Brughelli M. A systematic video analysis of 21 anterior cruciate ligament injuries in elite netball players during games. Sports Biomech. 2022 Feb 7:1-18. Epub ahead of print. [CrossRef] [PubMed]
- Bencke J, Aagaard P, Zebis MK. Muscle Activation During ACL Injury Risk Movements in Young Female Athletes: A Narrative Review. Front Physiol. 2018 May 15;9:445. PMCID: PMC5962681. [CrossRef] [PubMed]
- Benjaminse A, Otten B, Gokeler A, Diercks RL, Lemmink KAPM. Motor learning strategies in basketball players and its implications for ACL injury prevention: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017 Aug;25(8):2365-2376. Epub 2015 Aug 11. PMCID: PMC5522510. [CrossRef] [PubMed]
- Bertozzi F, Fischer PD, Hutchison KA, Zago M, Sforza C, Monfort SM. Associations Between Cognitive Function and ACL Injury-Related Biomechanics: A Systematic Review. Sports Health. 2023 Nov-Dec;15(6):855-866. Epub 2023 Jan 20. PMCID: PMC10606969. [CrossRef] [PubMed]
- Betsch M, Darwich A, Chang J, Whelan D, Ogilvie-Harris D, Chahal J, Theodoropoulos J. Wide Variability in Return-to-Sport Criteria used by Team Physicians After Anterior Cruciate Ligament Reconstruction in Elite Athletes-A Qualitative Study. Arthrosc Sports Med Rehabil. 2022 Aug 15;4(5):e1759-e1766. PMCID: PMC9596898. [CrossRef] [PubMed]
- Beynnon BD, Tourville TW, Hollenbach HC, Shultz S, Vacek P. Intrinsic Risk Factors for First-Time Noncontact ACL Injury: A Prospective Study of College and High School Athletes. Sports Health. 2023 May;15(3):433-442. Epub 2022 Sep 25. PMCID: PMC10170220. [CrossRef] [PubMed]
- Boden BP, Sheehan FT. Mechanism of non-contact ACL injury: OREF Clinical Research Award 2021. J Orthop Res. 2022 Mar;40(3):531-540. Epub 2022 Jan 6. PMCID: PMC8858885. [CrossRef] [PubMed]
- Bodkin, SG. Time to Reflect on Return to Sport Timing Following ACL Reconstruction. Sports Med. 2024 Mar 30. Epub ahead of print. [CrossRef] [PubMed]
- Bolt R, Heuvelmans P, Benjaminse A, Robinson MA, Gokeler A. An ecological dynamics approach to ACL injury risk research: a current opinion. Sports Biomech. 2021 Aug 10:1-14. Epub ahead of print. [CrossRef] [PubMed]
- Brophy RH, Wojtys EM, Mack CD, Hawaldar K, Herzog MM, Owens BD. Factors Associated With the Mechanism of ACL Tears in the National Football League: A Video-Based Analysis. Orthop J Sports Med. 2021 Nov 8;9(11):23259671211053301. PMCID: PMC8579343. [CrossRef] [PubMed]
- Buckthorpe M, Gokeler A, Herrington L, Hughes M, Grassi A, Wadey R, Patterson S, Compagnin A, La Rosa G, Della Villa F. Optimising the Early-Stage Rehabilitation Process Post-ACL Reconstruction. Sports Med. 2024 Jan;54(1):49-72. Epub 2023 Oct 3. [CrossRef] [PubMed]
- Byrne S, Lay B, Staynor J, Alderson J, Donnelly CJ. The effect of planning time on penultimate and ultimate step kinematics and subsequent knee moments during sidestepping. Scand J Med Sci Sports. 2022 Sep;32(9):1366-1376. Epub 2022 Jun 8. [CrossRef] [PubMed]
- Cacolice PA, Starkey BE, Carcia CR, Higgins PE. Research Dominance Definitions May Not Identify Higher Risk Limb for Anterior Cruciate Ligament Injury in NCAA D3 Student-Athletes. Int J Sports Phys Ther. 2022 Jun 1;17(4):622-627. PMCID: PMC9159722. [CrossRef] [PubMed]
- Carlson VR, Sheehan FT, Boden BP. Video Analysis of Anterior Cruciate Ligament (ACL) Injuries: A Systematic Review. JBJS Rev. 2016 Nov 29;4(11):e5. PMCID: PMC5865503. [CrossRef] [PubMed]
- Chaaban CR, Hearn D, Goerger B, Padua DA. Are Elite Collegiate Female Athletes PRIME for a Safe Return to Sport after ACLR? An Investigation of Physical Readiness and Integrated Movement Efficiency (PRIME). Int J Sports Phys Ther. 2022 Apr 1;17(3):445-455. PMCID: PMC8975580. [CrossRef] [PubMed]
- Chaaban CR, Turner JA, Padua DA. Think outside the box: Incorporating secondary cognitive tasks into return to sport testing after ACL reconstruction. Front Sports Act Living. 2023 Feb 15;4:1089882. PMCID: PMC9975395. [CrossRef] [PubMed]
- Chaput M, Onate JA, Simon JE, Criss CR, Jamison S, McNally M, Grooms DR. Visual cognition associated with knee proprioception, time to stability, and sensory integration neural activity after ACL reconstruction. J Orthop Res. 2022 Jan;40(1):95-104. Epub 2021 Mar 8. [CrossRef] [PubMed]
- Larson D, Vu V, Ness BM, Wellsandt E, Morrison S. A Multi-Systems Approach to Human Movement after ACL Reconstruction: The Musculoskeletal System. Int J Sports Phys Ther. 2021 Dec 1;17(1):27-46. PMCID: PMC8856762. [CrossRef] [PubMed]
- Chen Z, Li Y, Zhang Y, Zhang Z, Wang J, Deng X, Liu C, Chen N, Jiang C, Li W, Song B. Analysis of Visual Risk Factors of Anterior Cruciate Ligament Injury of Knee Joint. J Clin Med. 2022 Sep 23;11(19):5602. PMCID: PMC9573435. [CrossRef] [PubMed]
- Ciceklidag M, Kaya I, Ayanoglu T, Ayas IH, Ozer M, Ataoglu MB, Kanatli U. Proprioception After Primary Repair of the Anterior Cruciate Ligament. Am J Sports Med. 2024 Apr;52(5):1199-1208. Epub 2024 Mar 4. Erratum in: Am J Sports Med. 2024 Apr 30:3635465241253575. 10.1177/03635465241253575. [CrossRef] [PubMed]
- Colapietro M, Portnoff B, Miller SJ, Sebastianelli W, Vairo GL. Effects of Blood Flow Restriction Training on Clinical Outcomes for Patients With ACL Reconstruction: A Systematic Review. Sports Health. 2023 Mar-Apr;15(2):260-273. Epub 2022 Feb 8. [CrossRef] [PubMed]
- Collings TJ, Diamond LE, Barrett RS, Timmins RG, Hickey JT, DU Moulin WS, Williams MD, Beerworth KA, Bourne MN. Strength and Biomechanical Risk Factors for Noncontact ACL Injury in Elite Female Footballers: A Prospective Study. Med Sci Sports Exerc. 2022 Aug 1;54(8):1242-1251. Epub 2022 Mar 12. [CrossRef] [PubMed]
- Crotti M, Heering T, Lander N, Fox A, Barnett LM, Duncan MJ. Extrinsic Risk Factors for Primary Noncontact Anterior Cruciate Ligament Injury in Adolescents Aged between 14 and 18 years: A Systematic Review. Sports Med. 2024 Apr;54(4):875-894. Epub 2024 Jan 18. [CrossRef] [PubMed]
- Crotty NMN, Daniels KAJ, McFadden C, Cafferkey N, King E. Relationship Between Isokinetic Knee Strength and Single-Leg Drop Jump Performance 9 Months After ACL Reconstruction. Orthop J Sports Med. 2022 Jan 5;10(1):23259671211063800. PMCID: PMC8738888. [CrossRef] [PubMed]
- Culvenor AG, Girdwood MA, Juhl CB, Patterson BE, Haberfield MJ, Holm PM, Bricca A, Whittaker JL, Roos EM, Crossley KM. Rehabilitation after anterior cruciate ligament and meniscal injuries: a best-evidence synthesis of systematic reviews for the OPTIKNEE consensus. Br J Sports Med. 2022 Dec;56(24):1445-1453. Epub 2022 Jun 29. PMCID: PMC9726950. [CrossRef] [PubMed]
- Cunha J, Solomon DJ. ACL Prehabilitation Improves Postoperative Strength and Motion and Return to Sport in Athletes. Arthrosc Sports Med Rehabil. 2022 Jan 28;4(1):e65-e69. PMCID: PMC8811524. [CrossRef] [PubMed]
- Cuyul-Vásquez I, Álvarez E, Riquelme A, Zimmermann R, Araya-Quintanilla F. Effectiveness of Unilateral Training of the Uninjured Limb on Muscle Strength and Knee Function of Patients With Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis of Cross-Education. J Sport Rehabil. 2022 Mar 12;31(5):605-616. [CrossRef] [PubMed]
- D’Hooghe P, Grassi A, Villa FD, Alkhelaifi K, Papakostas E, Rekik R, Marin T, Tosarelli F, Zaffagnini S. The injury mechanism correlation between MRI and video-analysis in professional football players with an acute ACL knee injury reveals consistent bone bruise patterns. Knee Surg Sports Traumatol Arthrosc. 2023 Jan;31(1):121-132. Epub 2022 Jun 13. PMCID: PMC9859865. [CrossRef] [PubMed]
- Daggett MC, Witte KA, Cabarkapa D, Cabarkapa DV, Fry AC. Evidence-Based Data Models for Return-to-Play Criteria after Anterior Cruciate Ligament Reconstruction. Healthcare (Basel). 2022 May 18;10(5):929. PMCID: PMC9141289. [CrossRef] [PubMed]
- Dalvandpour N, Zareei M, Abbasi H, Abdoli B, Mohammadian MA, Rommers N, Rössler R. Focus of Attention During ACL Injury Prevention Exercises Affects Improvements in Jump-Landing Kinematics in Soccer Players: A Randomized Controlled Trial. J Strength Cond Res. 2023 Feb 1;37(2):337-342. Epub 2021 Dec 23. [CrossRef] [PubMed]
- D’Argenio EM, Eckard TG, Frank BS, Prentice WE, Padua DA. Examining the Dynamic Nature of Anterior Cruciate Ligament Injury Risk Factors in Women’s Collegiate Soccer. J Sport Rehabil. 2022 Mar 1;31(3):286-293. Epub 2021 Nov 12. [CrossRef] [PubMed]
- Das L, Johri AS, Abdusamad V, Schuh A, Goyal T. Joint awareness and return to pre-injury level of activities after ACL reconstruction in athletes vs non-athletes. Eur J Orthop Surg Traumatol. 2023 May;33(4):819-827. Epub 2022 Feb 4. [CrossRef] [PubMed]
- Dauty M, Crenn V, Louguet B, Grondin J, Menu P, Fouasson-Chailloux A. Anatomical and Neuromuscular Factors Associated to Non-Contact Anterior Cruciate Ligament Injury. J Clin Med. 2022 Mar 3;11(5):1402. PMCID: PMC8911271. [CrossRef] [PubMed]
- Davies WT, Ryu JH, Graham-Smith P, Goodwin JE, Cleather DJ. Stronger Subjects Select a Movement Pattern That May Reduce Anterior Cruciate Ligament Loading During Cutting. J Strength Cond Res. 2022 Jul 1;36(7):1853-1859. Epub 2021 Mar 8. [CrossRef] [PubMed]
- Della Villa F, Buckthorpe M, Grassi A, Nabiuzzi A, Tosarelli F, Zaffagnini S, Della Villa S. Systematic video analysis of ACL injuries in professional male football (soccer): injury mechanisms, situational patterns and biomechanics study on 134 consecutive cases. Br J Sports Med. 2020 Dec;54(23):1423-1432. Epub 2020 Jun 19. [CrossRef] [PubMed]
- Detherage JP, Divine JG, Donaworth MA, Palmer TG, Hagen JA, Hasselfeld KA, Eifert-Mangine M, Mangine RE, Clark JF, Grawe BM. Physiological Monitoring Detected Changes During Women’s Soccer Anterior Cruciate Ligament Injury. Cureus. 2021 May 4;13(5):e14838. PMCID: PMC8191855. [CrossRef] [PubMed]
- Devana SK, Solorzano CA, Vail J, Jackson N, Pham D, Jones KJ. Outcomes of Blood Flow Restriction Training After ACL Reconstruction in NCAA Division I Athletes. Orthop J Sports Med. 2024 May 13;12(5):23259671241248589. PMCID: PMC11092532. [CrossRef] [PubMed]
- Alejandra Díaz M, Smeets A, Hagen M, Sankey SP, Verschueren S, Vanrenterghem J. Postural balance strategies during landing at the moment of return-to-sports after anterior cruciate ligament reconstruction. J Biomech. 2022 Dec;145:111381. Epub 2022 Nov 8. [CrossRef] [PubMed]
- Di Paolo S, Bragonzoni L, Della Villa F, Grassi A, Zaffagnini S. Do healthy athletes exhibit at-risk biomechanics for anterior cruciate ligament injury during pivoting movements? Sports Biomech. 2022 Jun 2:1-14. Epub ahead of print. [CrossRef] [PubMed]
- Dischiavi SL, Wright AA, Heller RA, Love CE, Salzman AJ, Harris CA, Bleakley CM. Do ACL Injury Risk Reduction Exercises Reflect Common Injury Mechanisms? A Scoping Review of Injury Prevention Programs. Sports Health. 2022 Jul-Aug;14(4):592-600. Epub 2021 Aug 25. PMCID: PMC9214897. [CrossRef] [PubMed]
- Drole K, Paravlic AH. Interventions for increasing return to sport rates after an anterior cruciate ligament reconstruction surgery: A systematic review. Front Psychol. 2022 Aug 22;13:939209. PMCID: PMC9443932. [CrossRef] [PubMed]
- Dos’Santos T, McBurnie A, Donelon T, Thomas C, Comfort P, Jones PA. A qualitative screening tool to identify athletes with ‘high-risk’ movement mechanics during cutting: The cutting movement assessment score (CMAS). Phys Ther Sport. 2019 Jul;38:152-161. Epub 2019 May 23. [CrossRef] [PubMed]
- Duthon VB, Barea C, Abrassart S, Fasel JH, Fritschy D, Ménétrey J. Anatomy of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2006 Mar;14(3):204-13. Epub 2005 Oct 19. [CrossRef] [PubMed]
- Emami F, Negahban H, Sinaei E, Mostafaee N, Shahtahmassebi B, Ebrahimzadeh MH, Mehravar M. The Effects of Various Cognitive Tasks Including Working Memory, Visuospatial, and Executive Function on Postural Control in Patients With Anterior Cruciate Ligament Injury. Motor Control. 2024 Jan 22;28(2):193-209. [CrossRef] [PubMed]
- Fältström A, Hägglund M, Hedevik H, Kvist J. Poor Validity of Functional Performance Tests to Predict Knee Injury in Female Soccer Players With or Without Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2021 May;49(6):1441-1450. Epub 2021 Apr 12. [CrossRef] [PubMed]
- Fausett WA, Reid DA, Larmer PJ. Current perspectives of New Zealand physiotherapists on rehabilitation and return to sport following anterior cruciate ligament reconstruction: A survey. Phys Ther Sport. 2022 Jan;53:166-172. Epub 2021 Oct 21. [CrossRef] [PubMed]
- Figueroa D, Arce G, Espregueira-Mendes J, Maestu R, Mosquera M, Williams A, Parker D, Cohen M, Karahan M, Ochoa Perea GA, Zaffagnini S, Neyret P, Karlsson J, Musahl V, Radice F, van der Merwe WM, Landreau P, Imhoff A, Menetrey J, Ayeni OR, Arliani GG, Sherman SL, Monllau JC, D’Hooghe P, Pinczewski L, Feller J, Patnaik S. Return to sport soccer after anterior cruciate ligament reconstruction: ISAKOS consensus. J ISAKOS. 2022 Dec;7(6):150-161. Epub 2022 Aug 23. [CrossRef] [PubMed]
- Fleming, BC. Fifty Years of ACL Biomechanics: What’s Next? Am J Sports Med. 2022 Dec;50(14):3745-3748. [CrossRef] [PubMed]
- García-Rodríguez P, Pecci J, Vázquez-González S, Pareja-Galeano H. Acute and Chronic Effects of Blood Flow Restriction Training in Physically Active Patients With Anterior Cruciate Ligament Reconstruction: A Systematic Review. Sports Health. 2023 Nov 9:19417381231208636. Epub ahead of print. [CrossRef] [PubMed]
- Gholami F, Letafatkar A, Moghadas Tabrizi Y, Gokeler A, Rossettini G, Ghanati HA, Schöllhorn WI. Comparing the Effects of Differential and Visuo-Motor Training on Functional Performance, Biomechanical, and Psychological Factors in Athletes after ACL Reconstruction: A Randomized Controlled Trial. J Clin Med. 2023 Apr 13;12(8):2845. PMCID: PMC10142379. [CrossRef] [PubMed]
- Giesche F, Vieluf S, Wilke J, Engeroff T, Niederer D, Banzer W. Cortical Motor Planning and Biomechanical Stability During Unplanned Jump Landings in Men With Anterior Cruciate Ligament Reconstruction. J Athl Train. 2022 Jun 1;57(6):547-556. PMCID: PMC9387379. [CrossRef] [PubMed]
- Gokeler A, Seil R, Kerkhoffs G, Verhagen E. A novel approach to enhance ACL injury prevention programs. J Exp Orthop. 2018 Jun 18;5(1):22. PMCID: PMC6005994. [CrossRef] [PubMed]
- Gokeler A, Neuhaus D, Benjaminse A, Grooms DR, Baumeister J. Principles of Motor Learning to Support Neuroplasticity After ACL Injury: Implications for Optimizing Performance and Reducing Risk of Second ACL Injury. Sports Med. 2019 Jun;49(6):853-865. Erratum in: Sports Med. 2019 Jun;49(6):979. 10.1007/s40279-019-01078-w PMCID: PMC6548061. [CrossRef] [PubMed]
- Gokeler A, Dingenen B, Hewett TE. Rehabilitation and Return to Sport Testing After Anterior Cruciate Ligament Reconstruction: Where Are We in 2022? Arthrosc Sports Med Rehabil. 2022 Jan 28;4(1):e77-e82. PMCID: PMC8811523. [CrossRef] [PubMed]
- Gokeler A, Grassi A, Hoogeslag R, van Houten A, Lehman T, Bolling C, Buckthorpe M, Norte G, Benjaminse A, Heuvelmans P, Di Paolo S, Tak I, Villa FD. Return to sports after ACL injury 5 years from now: 10 things we must do. J Exp Orthop. 2022 Jul 30;9(1):73. Erratum in: J Exp Orthop. 2022 Nov 17;9(1):111. 10.1186/s40634-022-00548-x PMCID: PMC9339063. [CrossRef] [PubMed]
- Gokeler A, Tosarelli F, Buckthorpe M, Della Villa F. Neurocognitive Errors and Noncontact Anterior Cruciate Ligament Injuries in Professional Male Soccer Players. J Athl Train. 2024 Mar 1;59(3):262-269. PMCID: PMC10976343. [CrossRef] [PubMed]
- Golberg E, Sommerfeldt M, Pinkoski A, Dennett L, Beaupre L. Anterior Cruciate Ligament Reconstruction Return-to-Sport Decision-Making: A Scoping Review. Sports Health. 2024 Jan-Feb;16(1):115-123. Epub 2023 Jan 27. PMCID: PMC10732109. [CrossRef] [PubMed]
- Gopinatth V, Smith MV, Matava MJ, Brophy RH, Knapik DM. Most Anterior Cruciate Ligament Injuries in Professional Athletes Occur Without Contact to the Injured Knee: A Systematic Review of Video Analysis Studies. Arthroscopy. 2024 Apr 23:S0749-8063(24)00275-5. Epub ahead of print. [CrossRef] [PubMed]
- Grooms DR, Page SJ, Nichols-Larsen DS, Chaudhari AM, White SE, Onate JA. Neuroplasticity Associated With Anterior Cruciate Ligament Reconstruction. J Orthop Sports Phys Ther. 2017 Mar;47(3):180-189. Epub 2016 Nov 5. [CrossRef] [PubMed]
- Grooms D, Appelbaum G, Onate J. Neuroplasticity following anterior cruciate ligament injury: a framework for visual-motor training approaches in rehabilitation. J Orthop Sports Phys Ther. 2015 May;45(5):381-93. Epub 2015 Jan 10. [CrossRef] [PubMed]
- Grooms DR, Chaput M, Simon JE, Criss CR, Myer GD, Diekfuss JA. Combining Neurocognitive and Functional Tests to Improve Return-to-Sport Decisions Following ACL Reconstruction. J Orthop Sports Phys Ther. 2023 Aug;53(8):415–419. PMCID: PMC10847844. [CrossRef] [PubMed]
- Gureck AE, Crockett Z, Barsky BW, Samuels S, Frank JS, Storer SK, Fazekas ML. Do Differences Exist in Impact Test Domains between Youth Athletes with and without an Anterior Cruciate Ligament Injury? Healthcare (Basel). 2023 Oct 19;11(20):2764. PMCID: PMC10606848. [CrossRef] [PubMed]
- Hamdan M, Haddad B, Alshrouf MA, Azzam MI, Isleem U, Hamasha R, Albtoush OM, Alhusban MT, Mubarak N, Alryalat SA. Can MRI knee joint measurements predict the population at risk of ACL injury? BMC Sports Sci Med Rehabil. 2022 Jun 2;14(1):98. PMCID: PMC9161517. [CrossRef] [PubMed]
- Hamoongard M, Hadadnezhad M, Abbasi A. Effect of combining eight weeks of neuromuscular training with dual cognitive tasks on landing mechanics in futsal players with knee ligament dominance defect: a randomized controlled trial. BMC Sports Sci Med Rehabil. 2022 Nov 22;14(1):196. PMCID: PMC9682735. [CrossRef] [PubMed]
- Harput G, Demirci S, Soylu AR, Bayrakci Tunay V. Association between quadriceps muscle thickness and knee function in anterior cruciate ligament reconstructed athletes: a cross-sectional study. Physiother Theory Pract. 2023 Oct 3;39(10):2171-2179. Epub 2022 Apr 20. [CrossRef] [PubMed]
- Henderson FJ, Konishi Y, Shima N, Shimokochi Y. Effects of 8-Week Exhausting Deep Knee Flexion Flywheel Training on Persistent Quadriceps Weakness in Well-Trained Athletes Following Anterior Cruciate Ligament Reconstruction. Int J Environ Res Public Health. 2022 Oct 14;19(20):13209. PMCID: PMC9602677. [CrossRef] [PubMed]
- Hewett TE, Myer GD, Ford KR, Paterno MV, Quatman CE. Mechanisms, prediction, and prevention of ACL injuries: Cut risk with three sharpened and validated tools. J Orthop Res. 2016 Nov;34(11):1843-1855. Epub 2016 Sep 19. PMCID: PMC5505503. [CrossRef] [PubMed]
- Homan MD, Braaten JA, Banovetz MT, Monson JK, Kennedy NI, LaPrade RF. Principles for optimizing anterior cruciate ligament reconstruction outcomes in elite athletes: a review of current techniques. Ann Jt. 2024 Apr 7;9:19. PMCID: PMC11061659. [CrossRef] [PubMed]
- Hoogeslag RAG, Huis In ‘t Veld R, Brouwer RW, de Graaff F, Verdonschot N. Acute Anterior Cruciate Ligament Rupture: Repair or Reconstruction? Five-Year Results of a Randomized Controlled Clinical Trial. Am J Sports Med. 2022 Jun;50(7):1779-1787. Epub 2022 Apr 29. [CrossRef] [PubMed]
- Hurley ET, Mojica ES, Haskel JD, Mannino BJ, Alaia M, Strauss EJ, Jazrawi LM, Gonzlaez-Lomas G. Return to play testing following anterior cruciate reconstruction - A systematic review & meta-analysis. Knee. 2022 Jan;34:134-140. Epub 2021 Dec 9. [CrossRef] [PubMed]
- Imai S, Harato K, Morishige Y, Kobayashi S, Niki Y, Sato K, Nagura T. Effects of Dual Task Interference on Biomechanics of The Entire Lower Extremity During the Drop Vertical Jump. J Hum Kinet. 2022 Feb 10;81:5-14. PMCID: PMC8884875. [CrossRef] [PubMed]
- Jack RA 2nd, Lambert BS, Hedt CA, Delgado D, Goble H, McCulloch PC. Blood Flow Restriction Therapy Preserves Lower Extremity Bone and Muscle Mass After ACL Reconstruction. Sports Health. 2023 May;15(3):361-371. Epub 2022 Jun 27. PMCID: PMC10170230. [CrossRef] [PubMed]
- Jenkins SM, Guzman A, Gardner BB, Bryant SA, Del Sol SR, McGahan P, Chen J. Rehabilitation After Anterior Cruciate Ligament Injury: Review of Current Literature and Recommendations. Curr Rev Musculoskelet Med. 2022 Jun;15(3):170-179. Epub 2022 Apr 6. PMCID: PMC9107547. [CrossRef] [PubMed]
- Jiang L, Zhang L, Huang W, Zeng Q, Huang G. The effect of proprioception training on knee kinematics after anterior cruciate ligament reconstruction: A randomized control trial. J Back Musculoskelet Rehabil. 2022;35(5):1085-1095. [CrossRef] [PubMed]
- Johnston JT, Mandelbaum BR, Schub D, Rodeo SA, Matava MJ, Silvers-Granelli HJ, Cole BJ, ElAttrache NS, McAdams TR, Brophy RH. Video Analysis of Anterior Cruciate Ligament Tears in Professional American Football Athletes. Am J Sports Med. 2018 Mar;46(4):862-868. Epub 2018 Feb 21. [CrossRef] [PubMed]
- Jones HSR, Moore IS, King E, Stiles VH, Laudani L, McCarthy-Ryan M, McFadden C, Daniels KAJ. Movement strategy correspondence across jumping and cutting tasks after anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2022 Mar;32(3):612-621. Epub 2021 Nov 25. [CrossRef] [PubMed]
- Josse, CM. Gym-Based Training Interventions for Anterior Cruciate Ligament Injury Reduction in American Football Players. HSS J. 2023 Aug;19(3):285-291. Epub 2023 Jan 23. PMCID: PMC10331268. [CrossRef] [PubMed]
- Kadlec D, Miller-Dicks M, Nimphius S. Training for “Worst-Case” Scenarios in Sidestepping: Unifying Strength and Conditioning and Perception-Action Approaches. Sports Med Open. 2023 Apr 5;9(1):22. PMCID: PMC10076474. [CrossRef] [PubMed]
- Kakavas G, Malliaropoulos N, Pruna R, Traster D, Bikos G, Maffulli N. Neuroplasticity and Anterior Cruciate Ligament Injury. Indian J Orthop. 2020 Jan 31;54(3):275-280. PMCID: PMC7205971. [CrossRef] [PubMed]
- Kakavas G, Forelli F, Malliaropoulos N, Hewett TE, Tsaklis P. Periodization in Anterior Cruciate Ligament Rehabilitation: New Framework Versus Old Model? A Clinical Commentary. Int J Sports Phys Ther. 2023 Apr 1;18(2):541-546. PMCID: PMC10069386. [CrossRef] [PubMed]
- Kellis E, Sahinis C, Baltzopoulos V. Is hamstrings-to-quadriceps torque ratio useful for predicting anterior cruciate ligament and hamstring injuries? A systematic and critical review. J Sport Health Sci. 2023 May;12(3):343-358. Epub 2022 Jan 19. PMCID: PMC10199143. [CrossRef] [PubMed]
- King E, Richter C, Daniels KAJ, Franklyn-Miller A, Falvey E, Myer GD, Jackson M, Moran R, Strike S. Can Biomechanical Testing After Anterior Cruciate Ligament Reconstruction Identify Athletes at Risk for Subsequent ACL Injury to the Contralateral Uninjured Limb? Am J Sports Med. 2021 Mar;49(3):609-619. Epub 2021 Feb 9. PMCID: PMC9938948. [CrossRef] [PubMed]
- Koc BB, Truyens A, Heymans MJLF, Jansen EJP, Schotanus MGM. Effect of Low-Load Blood Flow Restriction Training After Anterior Cruciate Ligament Reconstruction: A Systematic Review. Int J Sports Phys Ther. 2022 Apr 1;17(3):334-346. PMCID: PMC8975583. [CrossRef] [PubMed]
- Kotsifaki R, Korakakis V, King E, Barbosa O, Maree D, Pantouveris M, Bjerregaard A, Luomajoki J, Wilhelmsen J, Whiteley R. Aspetar clinical practice guideline on rehabilitation after anterior cruciate ligament reconstruction. Br J Sports Med. 2023 May;57(9):500-514. Epub 2023 Feb 2. [CrossRef] [PubMed]
- Kiani Haft Lang M, Mofateh R, Orakifar N, Goharpey S. Differences in Neurocognitive Functions Between Healthy Controls and Anterior Cruciate Ligament-Reconstructed Male Athletes Who Passed or Failed Return to Sport Criteria: A Preliminary Study. J Sport Rehabil. 2023 Apr 25;32(6):645-654. [CrossRef] [PubMed]
- Larson D, Vu V, Ness BM, Wellsandt E, Morrison S. A Multi-Systems Approach to Human Movement after ACL Reconstruction: The Musculoskeletal System. Int J Sports Phys Ther. 2021 Dec 1;17(1):27-46. PMCID: PMC8856762. [CrossRef] [PubMed]
- Liew BXW, Feller JA, Webster KE. Understanding the psychological mechanisms of return to sports readiness after anterior cruciate ligament reconstruction. PLoS One. 2022 Mar 24;17(3):e0266029. PMCID: PMC8946672. [CrossRef] [PubMed]
- Louw Q, Gillion N, van Niekerk SM, Morris L, Baumeister J. The effect of vision on knee biomechanics during functional activities - A systematic review. J Sci Med Sport. 2015 Jul;18(4):469-74. Epub 2014 Jun 26. [CrossRef] [PubMed]
- Lucarno S, Zago M, Buckthorpe M, Grassi A, Tosarelli F, Smith R, Della Villa F. Systematic Video Analysis of Anterior Cruciate Ligament Injuries in Professional Female Soccer Players. Am J Sports Med. 2021 Jun;49(7):1794-1802. Epub 2021 May 14. [CrossRef] [PubMed]
- Maestroni L, Turner A, Papadopoulos K, Sideris V, Read P. Total Score of Athleticism: Profiling Strength and Power Characteristics in Professional Soccer Players After Anterior Cruciate Ligament Reconstruction to Assess Readiness to Return to Sport. Am J Sports Med. 2023 Oct;51(12):3121-3130. Epub 2023 Sep 8. PMCID: PMC10543956. [CrossRef] [PubMed]
- Mangine R, Tersak J, Palmer T, Hill-Lindsay A, Patton B, Eifert-Mangine M, Jacobs B, Colosimo AJ. The Longitudinal Neurophysiological Adaptation of a Division I Female Lacrosse Player Following Anterior Cruciate Rupture and Repair: A Case Report. Int J Sports Phys Ther. 2023 Apr 1;18(2):467-476. PMCID: PMC10069340. [CrossRef] [PubMed]
- Maniar N, Cole MH, Bryant AL, Opar DA. Muscle Force Contributions to Anterior Cruciate Ligament Loading. Sports Med. 2022 Aug;52(8):1737-1750. Epub 2022 Apr 18. PMCID: PMC9325827. [CrossRef] [PubMed]
- Meierbachtol A, Obermeier M, Yungtum W, Bottoms J, Paur E, Nelson BJ, Tompkins M, Russell HC, Chmielewski TL. Injury-Related Fears During the Return-to-Sport Phase of ACL Reconstruction Rehabilitation. Orthop J Sports Med. 2020 Mar 26;8(3):2325967120909385. PMCID: PMC7099672. [CrossRef] [PubMed]
- Mok AC, Fancher AJ, Vopat ML, Baker J, Tarakemeh A, Mullen S, Schroeppel JP, Templeton K, Mulcahey MK, Vopat BG. Sex-Specific Outcomes After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. Orthop J Sports Med. 2022 Feb 23;10(2):23259671221076883. PMCID: PMC8873558. [CrossRef] [PubMed]
- Momaya AM, Wood AS, Benson EM, Kwapisz AL. The Influence of Psychosocial Factors on Patients Undergoing Anterior Cruciate Ligament Reconstruction. Sports Health. 2024 Mar-Apr;16(2):230-238. Epub 2024 Jan 31. PMCID: PMC10916773. [CrossRef] [PubMed]
- Mørtvedt AI, Krosshaug T, Bahr R, Petushek E. I spy with my little eye … a knee about to go ‘pop’? Can coaches and sports medicine professionals predict who is at greater risk of ACL rupture? Br J Sports Med. 2020 Feb;54(3):154-158. Epub 2019 Oct 14. PMCID: PMC7029251. [CrossRef] [PubMed]
- Nae JÄ, Cronström A. Association between sensorimotor function and visual assessment of postural orientation in patients with ACL injury. Phys Ther Sport. 2022 May;55:160-167. Epub 2022 Apr 12. [CrossRef] [PubMed]
- Nessler T, Denney L, Sampley J. ACL Injury Prevention: What Does Research Tell Us? Curr Rev Musculoskelet Med. 2017 Sep;10(3):281-288. PMCID: PMC5577417. [CrossRef] [PubMed]
- Ngatuvai MS, Yang J, Kistamgari S, Collins CL, Smith GA. Epidemiological Comparison of ACL Injuries on Different Playing Surfaces in High School Football and Soccer. Orthop J Sports Med. 2022 May 5;10(5):23259671221092321. 5 May; PMCID: PMC9083053. [CrossRef] [PubMed]
- Nilstad A, Petushek E, Mok KM, Bahr R, Krosshaug T. Kiss goodbye to the ‘kissing knees’: no association between frontal plane inward knee motion and risk of future non-contact ACL injury in elite female athletes. Sports Biomech. 2023 Jan;22(1):65-79. Epub 2021 Apr 28. [CrossRef] [PubMed]
- Oladeji L, Reynolds G, Gonzales H, DeFroda S. Anterior Cruciate Ligament Return to Play: Where Are We Now? J Knee Surg. 2024 Jul;37(8):586-592. Epub 2023 Jul 17. [CrossRef] [PubMed]
- Mohammadi Orangi B, Dehghani M, Jones PA. Manipulation of task constraints is the most effective motor learning method for reducing risk factors for ACL injury during side-step cutting in both male and female athletes. Res Sports Med. 2024 Jul-Aug;32(4):631-647. Epub 2023 May 9. [CrossRef] [PubMed]
- Parsons JL, Coen SE, Bekker S. Anterior cruciate ligament injury: towards a gendered environmental approach. Br J Sports Med. 2021 Sep;55(17):984-990. Epub 2021 Mar 10. [CrossRef] [PubMed]
- Piskin D, Benjaminse A, Dimitrakis P, Gokeler A. Neurocognitive and Neurophysiological Functions Related to ACL Injury: A Framework for Neurocognitive Approaches in Rehabilitation and Return-to-Sports Tests. Sports Health. 2022 Jul-Aug;14(4):549-555. Epub 2021 Jul 8. PMCID: PMC9214902. [CrossRef] [PubMed]
- Rambaud AJ, Neri T, Dingenen B, Parker D, Servien E, Gokeler A, Edouard P. The modifying factors that help improve anterior cruciate ligament reconstruction rehabilitation: A narrative review. Ann Phys Rehabil Med. 2022 Jun;65(4):101601. Epub 2021 Nov 20. [CrossRef] [PubMed]
- Read PJ, Pedley JS, Eirug I, Sideris V, Oliver JL. Impaired Stretch-Shortening Cycle Function Persists Despite Improvements in Reactive Strength After Anterior Cruciate Ligament Reconstruction. J Strength Cond Res. 2022 May 1;36(5):1238-1244. 1 May; Epub 2022 Jan 5. [CrossRef] [PubMed]
- Read PJ, Davies WT, Bishop C, McAuliffe S, Wilson MG, Turner AN. Residual Deficits in Reactive Strength After Anterior Cruciate Ligament Reconstruction in Soccer Players. J Athl Train. 2023 May 1;58(5):423-429. [CrossRef] [PubMed]
- Reiche E, Collins K, Genoese F, Walaszek M, Triplett A, Kuenze C, Harkey M, Baez S. Lower Extremity Reaction Time in Individuals With Contact Versus Noncontact Anterior Cruciate Ligament Injuries After Reconstruction. J Athl Train. 2024 Jan 1;59(1):66-72. PMCID: PMC10783466. [CrossRef] [PubMed]
- Reyes MA, Probasco MO, Worby TN, Loertscher DE, Soderbeck LK, Huddleston WE. Lower Kinetic Chain, Meet the Thinking Brain: A Scoping Review of Cognitive Function and Lower Extremity Injury Risk. Int J Sports Phys Ther. 2022 Aug 1;17(5):787-815 PMCID: PMC9340845. [PubMed]
- Robey NJ, Buchholz KO, Murphy SP, Smith JD, Heise GD. Do Athletes With a Reconstructed Anterior Cruciate Ligament Respond Differently Than Controls to Visual Challenges When Dynamic Postural Stability is Assessed? J Appl Biomech. 2021 Dec 1;37(6):611-618. [CrossRef] [PubMed]
- Rolley T, Gill SD, Keast M, Reade T, Page R, Bonacci J, Stella J, Johnson B, Fox A. Anticipatory effects on side-step cutting biomechanics in Women’s Australian Football League players. BMJ Open Sport Exerc Med. 2023 Jun 14;9(2):e001587. PMCID: PMC10277520. [CrossRef] [PubMed]
- Romanchuk NJ, Livock H, Lukas KJ, Del Bel MJ, Benoit DL, Carsen S. Criteria Used to Determine Unrestricted Return to Activity After ACL Reconstruction in Pediatric and Adolescent Patients: A Systematic Review. Orthop J Sports Med. 2023 Mar 7;11(3):23259671231154540. PMCID: PMC9996745. [CrossRef] [PubMed]
- Saadat S, Stephenson ML, Gillette JC. Entry angle during jump landing changes biomechanical risk factors for ACL injury. Sports Biomech. 2022 Jun 17:1-13. Epub ahead of print. [CrossRef] [PubMed]
- Saadat S, Bricarell KM, Gillette JC. Dual tasking increases kinematic and kinetic risk factors of ACL injury. Sports Biomech. 2023 Oct 26:1-14. Epub ahead of print. [CrossRef] [PubMed]
- Schilaty ND, McPherson AL, Nagai T, Bates NA. Differences in psychological readiness for return to sport after anterior cruciate ligament injury is evident in thigh musculature motor unit characteristics. BMJ Open Sport Exerc Med. 2023 Jul 5;9(3):e001609. PMCID: PMC10335479. [CrossRef] [PubMed]
- Schweizer N, Strutzenberger G, Franchi MV, Farshad M, Scherr J, Spörri J. Screening Tests for Assessing Athletes at Risk of ACL Injury or Reinjury-A Scoping Review. Int J Environ Res Public Health. 2022 Mar 1;19(5):2864. PMCID: PMC8910677. [CrossRef] [PubMed]
- Schwery NA, Kiely MT, Larson CM, Wulf CA, Heikes CS, Hess RW, Giveans MR, Solie BS, Doney CP. Quadriceps Strength following Anterior Cruciate Ligament Reconstruction: Normative Values based on Sex, Graft Type and Meniscal Status at 3, 6 & 9 Months. Int J Sports Phys Ther. 2022 Apr 1;17(3):434-444. PMCID: PMC8975560. [CrossRef] [PubMed]
- Sheean AJ, DeFoor MT, Spindler KP, Arner JW, Athiviraham A, Bedi A, DeFroda S, Ernat JJ, Frangiamore SJ, Nuelle CW, Sheean AJ, Spindler KP, Bedi A. The Psychology of ACL Injury, Treatment, and Recovery: Current Concepts and Future Directions. Sports Health. 2024 Feb 19:19417381241226896. Epub ahead of print. [CrossRef] [PubMed]
- Shekhar A, Pilar A, Ponnanna KM, Tapasvi S. ACL repair for athletes? J Orthop. 2022 Apr 7;31:61-66. PMCID: PMC9018522. [CrossRef] [PubMed]
- Sherman DA, Baumeister J, Stock MS, Murray AM, Bazett-Jones DM, Norte GE. Inhibition of Motor Planning and Response Selection after Anterior Cruciate Ligament Reconstruction. Med Sci Sports Exerc. 2023 Mar 1;55(3):440-449. Epub 2022 Oct 19. [CrossRef] [PubMed]
- Shibata S, Takemura M, Miyakawa S. Kinematics, Kinetics and Muscle Activity Analysis during Single-leg Drop-jump Landing Followed by an Unanticipated Task: Focusing on Differences in Neurocognitive Function. Int J Sports Phys Ther. 2023 Oct 1;18(5):1085-1093. PMCID: PMC10547070. [CrossRef] [PubMed]
- Sim K, Rahardja R, Zhu M, Young SW. Optimal Graft Choice in Athletic Patients with Anterior Cruciate Ligament Injuries: Review and Clinical Insights. Open Access J Sports Med. 2022 Jul 1;13:55-67. PMCID: PMC9255990. [CrossRef] [PubMed]
- Slater LV, Hart JM. Quantifying the relationship between quadriceps strength and aerobic fitness following anterior cruciate ligament reconstruction. Phys Ther Sport. 2022 May;55:106-110. Epub 2022 Mar 16. [CrossRef] [PubMed]
- Smeets A, Willems M, Gilson L, Verschueren S, Staes F, Vandenneucker H, Claes S, Vanrenterghem J. Neuromuscular and biomechanical landing alterations persist in athletes returning to sport after anterior cruciate ligament reconstruction. Knee. 2021 Dec;33:305-317. Epub 2021 Nov 4. [CrossRef] [PubMed]
- Song Y, Li L, Hughes G, Dai B. Trunk motion and anterior cruciate ligament injuries: a narrative review of injury videos and controlled jump-landing and cutting tasks. Sports Biomech. 2023 Jan;22(1):46-64. Epub 2021 Mar 4. [CrossRef] [PubMed]
- Song Y, Li L, Layer J, Fairbanks R, Jenkins M, Hughes G, Smith D, Wilson M, Zhu Q, Dai B. Indirect contact matters: Mid-flight external trunk perturbation increased unilateral anterior cruciate ligament loading variables during jump-landings. J Sport Health Sci. 2023 Jul;12(4):534-543. Epub 2022 Dec 8. PMCID: PMC10362484. [CrossRef] [PubMed]
- Stearns KM, Powers CM. Improvements in hip muscle performance result in increased use of the hip extensors and abductors during a landing task. Am J Sports Med. 2014 Mar;42(3):602-9. Epub 2014 Jan 24. [CrossRef] [PubMed]
- Stojanović E, Terrence Scanlan A, Radovanović D, Jakovljević V, Faude O. A multicomponent neuromuscular warm-up program reduces lower-extremity injuries in trained basketball players: a cluster randomized controlled trial. Phys Sportsmed. 2023 Oct;51(5):463-471. Epub 2022 Oct 12. [CrossRef] [PubMed]
- Straub RK, Powers CM. Is muscular strength a predictor for primary or secondary ACL injury? A scoping review of prospective studies. Phys Ther Sport. 2023 May;61:91-101. Epub 2023 Mar 20. [CrossRef] [PubMed]
- Sugarman BS, Sullivan ZB, Le D, Killelea C, Faherty MS, Diehl LH, Wittstein JR, Riboh JC, Toth AP, Amendola A, Taylor DC, Sell TC. Isometric Knee Strength is Greater in Individuals Who Score Higher on Psychological Readiness to Return to Sport After Primary Anterior Cruciate Ligament Reconstruction. Int J Sports Phys Ther. 2022 Dec 1;17(7):1330-1339. PMCID: PMC9718725. [CrossRef] [PubMed]
- Taberner M, van Dyk N, Allen T, Jain N, Richter C, Drust B, Betancur E, Cohen DD. Physical preparation and return to performance of an elite female football player following ACL reconstruction: a journey to the FIFA Women’s World Cup. BMJ Open Sport Exerc Med. 2020 Dec 1;6(1):e000843. PMCID: PMC8323467. [CrossRef] [PubMed]
- Tourillon, Romain & Fourchet, Francois. (2023). The Role of Foot-Ankle Complex in Rehabilitation After ACLR - Make Miracles Happen. 12. 310-316.
- Van Cant J, Pairot de Fontenay B, Douaihy C, Rambaud A. Characteristics of return to running programs following an anterior cruciate ligament reconstruction: A scoping review of 64 studies with clinical perspectives. Phys Ther Sport. 2022 Sep;57:61-70. Epub 2022 Jul 19. [CrossRef] [PubMed]
- van Melick N, Pronk Y, Nijhuis-van der Sanden M, Rutten S, van Tienen T, Hoogeboom T. Meeting movement quantity or quality return to sport criteria is associated with reduced second ACL injury rate. J Orthop Res. 2022 Jan;40(1):117-128. Epub 2021 Mar 11. [CrossRef] [PubMed]
- Vitharana TN, King E, Moran K. Sensorimotor Dysfunction Following Anterior Cruciate Ligament Reconstruction- an Afferent Perspective: A Scoping Review. Int J Sports Phys Ther. 2024 Jan 1;19(1):1410-1437. PMCID: PMC10761632. [CrossRef] [PubMed]
- Weingart A, Rynecki N, Pereira D. A Review of Neuromuscular Training and Biomechanical Risk Factor Screening for ACL Injury Prevention Among Female Soccer Players. Bull Hosp Jt Dis (2013). 2022 Sep;80(3):253-259. [PubMed]
- Weir, G. Anterior cruciate ligament injury prevention in sport: biomechanically informed approaches. Sports Biomech. 2021 Dec 29:1-21. Epub ahead of print. [CrossRef] [PubMed]
- Welling W, Frik L. On-Field Tests for Patients After Anterior Cruciate Ligament Reconstruction: A Scoping Review. Orthop J Sports Med. 2022 Jan 3;10(1):23259671211055481. PMCID: PMC8727834. [CrossRef] [PubMed]
- Welling, W. Return to sports after an ACL reconstruction in 2024 - A glass half full? A narrative review. Phys Ther Sport. 2024 May;67:141-148. Epub 2024 May 10. [CrossRef] [PubMed]
- Wilk K, Thomas ZM, Arrigo CA, Davies GJ. The Need To Change Return to Play Testing in Athletes Following ACL Injury: A Theoretical Model. Int J Sports Phys Ther. 2023 Feb 1;18(1):272-281. PMCID: PMC9897012. [CrossRef] [PubMed]
- Wohl TR, Criss CR, Grooms DR. Visual Perturbation to Enhance Return to Sport Rehabilitation after Anterior Cruciate Ligament Injury: A Clinical Commentary. Int J Sports Phys Ther. 2021 Apr 1;16(2):552-564. PMCID: PMC8016421. [CrossRef] [PubMed]
- An YW, Kim KM, DiTrani Lobacz A, Baumeister J, Higginson JS, Rosen J, Swanik CB. Cognitive Training Improves Joint Stiffness Regulation and Function in ACLR Patients Compared to Healthy Controls. Healthcare (Basel). 2023 Jun 28;11(13):1875. PMCID: PMC10340207. [CrossRef] [PubMed]
- Zago M, Esposito F, Stillavato S, Zaffagnini S, Frigo CA, Della Villa F. 3-Dimensional Biomechanics of Noncontact Anterior Cruciate Ligament Injuries in Male Professional Soccer Players. Am J Sports Med. 2024 Jun;52(7):1794-1803. Epub 2024 May 14. [CrossRef] [PubMed]
- Zebis MK, Aagaard P, Andersen LL, Hölmich P, Clausen MB, Brandt M, Husted RS, Lauridsen HB, Curtis DJ, Bencke J. First-time anterior cruciate ligament injury in adolescent female elite athletes: a prospective cohort study to identify modifiable risk factors. Knee Surg Sports Traumatol Arthrosc. 2022 Apr;30(4):1341-1351. Epub 2021 May 7. PMCID: PMC9007777. [CrossRef] [PubMed]
- Zhang L, Hacke JD, Garrett WE, Liu H, Yu B. Bone Bruises Associated with Anterior Cruciate Ligament Injury as Indicators of Injury Mechanism: A Systematic Review. Sports Med. 2019 Mar;49(3):453-462. [CrossRef] [PubMed]
- Kacprzak, Bartłomiej & Rosińska, Karolina & Siuba-Jarosz, Natalia. (2023). Hyalofast Cartilage Repair Surgery with a Full Load-Bearing Rehabilitation Program One Day after Operation Reduces the Time for Professional Athletes to Return to Play. Medicina. 59. 804. 10.3390/medicina59040804.
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Gldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum 2012, 64, 1697–1707 [Google Scholar] [CrossRef] [PubMed]. [Google Scholar] [CrossRef] [PubMed]
- Osteoarthritis Society International—OA Definition. Available online: https://oarsi.org/ (accessed on 4 February 2023).
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249 [Google Scholar] [CrossRef] [PubMed]. [Google Scholar] [CrossRef]
- Fenwick, S.A.; Gregg, P.J.; Rooney, P. Osteoarthritic cartilage loses its ability to remain avascular. Osteoarthr. Cart. 1999, 7, 441–452 [Google Scholar] [CrossRef] [PubMed]. [Google Scholar] [CrossRef] [PubMed]
- Fahy, N.; de Vries-van, M.M.I.; Lehmann, W.; Grotenhuis, N.; Farrel, E.; van der Kraan, P.M.; Murphy, J.M.; Bastiaansen-Jenniskens, Y.M.; van Osch, G.J.V.M. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarization state. Osteoarthr. Cart. 2014, 22, 1167–1175 [Google Scholar] [CrossRef]. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aristizábal, A.; Gadhi, R.; Mahomed, N.N.; Marshall, K.W.; Viswanathan, S. Synovial fluid monocyte/macrophage subsets and their correlation to patient-reported outcomes in osteoarthritic patients: A cohort study. Arthritis Res. Ther. 2019, 21, 26 [Google Scholar] [CrossRef]. [Google Scholar] [CrossRef]
- Kemble, S.; Croft, A.P. Critical Role of Synovial Tissue–Resident Macrophage and Fibroblast Subsets in the Persistence of Joint Inflammation. Front. Immunol. 2021, 12, 715894 [Google Scholar] [CrossRef]. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, M.; Kami, K.; Nishimura, Y.; Minami, K.; Senba, E.; Umemoto, Y.; Kinoshita, T.; Tajima, F. Exercise-induced increase in M2 macrophages accelerates wound healing in young mice. Physiol. Rep. 2022, 10, e15447 [Google Scholar] [CrossRef]. [Google Scholar] [CrossRef]
- Jang, J.S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef] [PubMed]
- Katsuola, G.; Kreitmaier, P.; Zeggini, E. Insights into the molecular landscape of osteoarthritis in human tissues. Curr. Opin. Rheumatol. 2022, 34, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Jeong, S.; Kim, H.; Kang, D.; Lee, J.; Kang, S.-J.; Kim, J.-H. Disease-modifying therapeutic strategies in osteoarthritis: Current status and future directions. Exp. Mol. Med. 2021, 53, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, X.; Su, S.; Du, S.S.H. Identifying the shared genes and KEGG pathways of Resolvin D1-targeted network and osteoarthritis using bioinformatics. Bioengineered 2022, 13, 9839–9854. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N.Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Rausch Osthoff, A.-K.; Niedermann, K.; Braun, J.; Adams, J.; Brodin, N.; Dagfinrud, H.; Duruoz, T.; Esbensen, B.A.; Günther, K.-P.; Hurkmans, E.; et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann. Rheum. Dis. 2018, 77, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.J.-S.; Hasni, S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Elagizi, A.; Kachur, S.; Carbone, S.; Lavie, C.J.; Blair, S.N. A Review of Obesity, Physical Activity and Cardiovascular Disease. Curr. Obes. Rep. 2020, 9, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Boutron, I.; Tubach, F.; Giradeau, B.; Ravaud, P. Blinding was judged more difficult to achieve and maintain in nonpharmacologic than pharmacologic trials. J. Clin. Epidemiol. 2004, 57, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Imamura, M.; Chien, H.F.; Lew, H.L.; Boggio, P.; Kaptchuk, T.J.; Riberto, M.; Hsing, W.T.; Battistella, L.R.; Furlan, A. Challenges and Recommendations for Placebo Controls in Randomized Trials in Physical and Rehabilitation Medicine: A Report of the International Placebo Symposium Working Group. Am. J. Phys. Med. Rehabil. 2010, 89, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Villamar, M.F.; Contreras, V.S.; Kuntz, R.E.; Fregni, F. The Reporting of Blinding in Physical Medicine and Rehabilitation Randomized Controlled Trials: A Systematic Review. J. Rehabil. Med. 2013, 45, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Yang, Y.X.; Li, J.; Fei, Y.; Guo, H.; Sun, Z.; Lu, J.; Xu, X.; Jiang, Q.; Ikegawa, S.; et al. Molecular Classification of Knee Osteoarthritis Front. Cell Dev. Biol. 2021, 9, 725568. [Google Scholar]
- Vassao, P.G.; de Souza, A.C.F.; da Silveira Campos, R.M.; Garcia, L.A.; Tucci, H.T.; Renno, A.C.M. Effects of photobiomodulation and a physical exercise program on the expression of inflammatory and cartilage degradation biomarkers and functional capacity in women with knee osteoarthritis: A randomized blinded study. Adv. Rheumatol. 2021, 61. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-R.; Yoo, J.J.; Kim, H.A. Therapeutics in Osteoarthritis Based on an Understanding of Its Molecular Pathogenesis. Int. J. Mol. Sci. 2018, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- American Kinesiotherapy Association. Available online: https://akta.org/about/history (accessed on 4 February 2023).
- .
- Alves, M.D.d.J.; Silva, D.d.S.; Pereira, E.V.M.; Pereira, D.D.; de Sousa Fernandes, M.S.; Santos, D.F.C.; Oliveira, D.P.M.; Vieira-Souza, L.M.; Aidar, F.J.; de Souza, R.F. Changes in Cytokines Concentration Following Long-Distance Running: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13, 838069. [Google Scholar] [CrossRef] [PubMed]
- Balan, E.; De Groote, E.; Bouillon, M.; Vieconte, N.; Mahieu, M.N.; Aslain, D.; Nielens, H.; Decottignies, A.; Deldicque, L. No effect of the endurance training status on senescence despite reduced inflammation in skeletal muscle of older individuals. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E447–E454. [Google Scholar] [CrossRef] [PubMed]
- Lehner, C., et al. Tenophages: a novel macrophage-like tendon cell population expressing CX3CL1 and CX3CR1. DMM Dis Model Mech. 2019, 12. [CrossRef]
- Bi, Y. , Ehirchiou, D., Kilts, T.M., Inkson, C.A., Embree, M.C., Sonoyama, W., Li, L., Leet, A.I., Seo, B.-M., Zhang, L., Shi, S., Young, M.F. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007, 13(10), 1219–1227. [CrossRef]
- Rui, Y.F. , Lui, P.P.Y., Ni, M., Chan, L.S., Lee, Y.W., Chan, K.M. Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. J Orthop Res. 2011, 29(3), 390–396. [CrossRef]
- Spiesz, E. , Thorpe, C.T., Chaudhry, S., Riley, G.P., Birch, H.L., Clegg, P.D., Screen, H.R.C. Tendon extracellular matrix damage, degradation and inflammation in response to in vitro overload exercise. J Orthop Res. 2015, 33(6), 889–897. [CrossRef]
- Cadby, J.A., Buehler, E., Godbout, C., Van Weeren, P.R., Snedeker, J.G., Lionetti, V. Differences between the cell populations from the peritenon and the tendon core with regard to their potential implication in tendon repair. PLoS One. 2014; 9, 3, e92474. [CrossRef]
- Dyment, N.A., Hagiwara, Y., Matthews, B.G., Li, Y., Kalajzic, I., Rowe, D.W. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS One. 2014, 9(4), e96113. [CrossRef]
- Yoshida, R., Alaee, F., Dyrna, F., Kronenberg, M.S., Maye, P., Kalajzic, I., Rowe, D.W., Mazzocca, A.D., Dyment, N.A. Murine supraspinatus tendon injury model to identify the cellular origins of rotator cuff healing. Connect Tissue Res. 2016, 57(6), 507–515. [CrossRef] [PubMed]
- Carroll, C.C., Dickinson, J.M., Haus, J.M., Lee, G.A., Hollon, C.J., Aagaard, P., Magnusson, S.P., Trappe, T.A. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol. 2008, 105(6), 1907–1915. [CrossRef] [PubMed]
- Light, N.D. , Bailey, A. J. Changes in crosslinking during aging in bovine tendon collagen. 1979.
- Li, Y. , Fessel, G., Georgiadis, M., Snedeker, J.G. Advanced glycation end-products diminish tendon collagen fiber sliding. Matrix Biol. 2013, 32(3-4), 169–177. [CrossRef]
- Thorpe, C.T. , Riley, G.P., Birch, H.L., Clegg, P.D., Screen, H.R.C. Fascicles from energy-storing tendons show an age-specific response to cyclic fatigue loading. J R Soc Interface. 2014, 11(92), 20131058. [CrossRef]
- Zuskov, A. , et al. Tendon biomechanics and crimp properties following fatigue loading are influenced by tendon type and age in mice. J Orthop Res. 2020, 54(1), 36–42. [Google Scholar] [CrossRef]
- Thorpe, C.T., McDermott, B.T., Goodship, A.E., Clegg, P.D., Birch, H.L. Ageing does not result in a decline in cell synthetic activity in an injury prone tendon. Scand J Med Sci Sports. 2016, 26(6), 684–693. [CrossRef]
- Connizzo, B.K. , Piet, J.M., Shefelbine, S.J., Grodzinsky, A.J. Age-associated changes in the response of tendon explants to stress deprivation is sex-dependent. Connect Tissue Res. 2020, 61(1), 48–62. [CrossRef]
- Zhou, Z., Akinbiyi, T., Xu, L., Ramcharan, M., Leong, D.J., Ros, S.J., Colvin, A.C., Schaffler, M.B., Majeska, R.J., Flatow, E.L., Sun, H.B. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell. 2010, 9(5), 911–915. [CrossRef] [PubMed]
- Veres, S.P., Harrison, J.M., Lee, J.M. Repeated subrupture overload causes progression of nanoscaled discrete plasticity damage in tendon collagen fibrils. J Orthop Res. 2013, 31(5), 731–737. [CrossRef]
- Egerbacher, M. , Arnoczky, S.P., Caballero, O., Lavagnino, M., Gardner, K.L. Loss of homeostatic tension induces apoptosis in tendon cells: an in vitro study. Clin Orthop Relat Res. 2008, 466(7), 1562–1568. [CrossRef]
- Thorpe, C.T. , et al. Tendon overload results in alterations in cell shape and increased markers of inflammation and matrix degradation. Scand J Med Sci Sports. 2015, 25(4), e381–e391. [Google Scholar] [CrossRef]
- Lavagnino, M. , Arnoczky, S.P., Gardner, K.L. In situ deflection of tendon cell-cilia in response to tensile loading: an in vitro study. J Orthop Res. 2011, 29(6), 925–930. [CrossRef]
- Donnelly, E., Ascenzi, M.-G., Farnum, C. Primary cilia are highly oriented with respect to collagen direction and long axis of extensor tendon. J Orthop Res. 2009, 28. [CrossRef]
- Gardner, K. , Arnoczky, S.P., Lavagnino, M. Effect of in vitro stress-deprivation and cyclic loading on the length of tendon cell cilia in situ. J Orthop Res. 2011, 29(4), 582–587. [CrossRef]
- Rowson, D.T. , Shelton, J.C., Screen, H.R.C., Knight, M.M. Mechanical loading induces primary cilia disassembly in tendon cells via TGFβ and HDAC6. Sci Rep. 2018, 8(1), 11107. [CrossRef]
- Rowson, D., Knight, M.M., Screen, H.R.C. Zonal variation in primary cilia elongation correlates with localized biomechanical degradation in stress deprived tendon. J Orthop Res. 2016, 34(12), 2146–2153. [CrossRef]
- Fang, F., Schwartz, A.G., Moore, E.R., Sup, M.E., Thomopoulos, S. Primary cilia as the nexus of biophysical and hedgehog signaling at the tendon enthesis. Sci Adv. 2020, 6(44), eabc1799. [CrossRef]
- Cohen, M. , Kam, Z., Addadi, L., Geiger, B. Dynamic study of the transition from hyaluronan- to integrin-mediated adhesion in chondrocytes. EMBO J. 2006, 25(2), 302–311. [CrossRef]
- Chopra, A. , Murray, M.E., Byfield, F.J., Mendez, M.G., Halleluyan, R., Restle, D.J., Raz-Ben Aroush, D., Galie, P.A., Pogoda, K., Bucki, R., Marcinkiewicz, C., Prestwich, G.D., Zarembinski, T.I., Chen, C.S., Puré, E., Kresh, J.Y., Janmey, P.A. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials. 2014, 35(1), 71–82. [CrossRef]
- Smith, S.M. , Thomas, C.E., Birk, D.E. Pericellular proteins of the developing mouse tendon: a proteomic analysis. Connect Tissue Res. 2012, 53(1), 2–13. [CrossRef]
- Docheva, D. , Popov, C. , Alberton, P., Aszodi, A. Integrin signaling in skeletal development and function. Birth Defects Res Part C Embryo Today Rev. 2014, 102, 13–36. [Google Scholar]
- Wang, D., Pun, C.C.M., Huang, S., Tang, T.C.M., Ho, K.K.W., Rothrauff, B.B., Yung, P.S.H., Blocki, A.M., Ker, E.D.F., Tuan, R.S. Tendon-derived extracellular matrix induces mesenchymal stem cell tenogenesis via an integrin/transforming growth factor-β crosstalk-mediated mechanism. FASEB J. 2020, 34(6), 8172–8186. [CrossRef] [PubMed]
- Mousavizadeh, R. , Hojabrpour, P., Eltit, F., McDonald, P.C., Dedhar, S., McCormack, R.G., Duronio, V., Jafarnejad, S.M., Scott, A. β1 integrin, ILK and mTOR regulate collagen synthesis in mechanically loaded tendon cells. Sci Rep. 2020, 10(1). [CrossRef]
- Popov, C. , Burggraf, M., Kreja, L., Ignatius, A., Schieker, M., Docheva, D. Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases. BMC Mol Biol. 2015, 16(1), 6. [CrossRef]
- Hannafin, J.A., Attia, E.A., Henshaw, R., Warren, R.F., Bhargava, M.M. Effect of cyclic strain and plating matrix on cell proliferation and integrin expression by ligament fibroblasts. J Orthop Res. 2006, 24(2), 149–158. [CrossRef]
- Kaneko, D., Sasazaki, Y., Kikuchi, T., Ono, T., Nemoto, K., Matsumoto, H., Toyama, Y. Temporal effects of cyclic stretching on distribution and gene expression of integrin and cytoskeleton by ligament fibroblasts in vitro. Connect Tissue Res. 2009, 50(4), 263–269. [CrossRef] [PubMed]
- Bayer, M.L. , Schjerling, P., Herchenhan, A., Zeltz, C., Heinemeier, K.M., Christensen, L., Krogsgaard, M., Gullberg, D., Kjaer, M. Release of tensile strain on engineered human tendon tissue disturbs cell adhesions, changes matrix architecture, and induces an inflammatory phenotype. PLoS One. 2014, 9(1), e86078. [CrossRef]
- Sun, H.B. , Andarawis-Puri, N., Li, Y., Fung, D.T., Lee, J.Y., Wang, V.M., Basta-Pljakic, J., Leong, D.J., Sereysky, J.B., Ros, S.J., Klug, R.A., Braman, J., Schaffler, M.B., Jepsen, K.J., Flatow, E.L. Cycle-dependent matrix remodeling gene expression response in fatigue-loaded rat patellar tendons. J Orthop Res. 2010, 28(10), 1380–1386. [CrossRef]
- McNeilly, C.M. , Banes, A. J., Benjamin, M., Ralphs, J.R. Tendon cells in vivo form a three-dimensional network of cell processes linked by gap junctions. J Anat. 1996, 189, 593–600. [Google Scholar] [CrossRef]
- Waggett, A.D., Benjamin, M., Ralphs, J.R. Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur J Cell Biol. 2006, 85(11), 1145–1154. [CrossRef]
- Shen, H., Schwartz, A.G., Civitelli, R., Thomopoulos, S. Connexin 43 is necessary for murine tendon enthesis formation and response to loading. J Bone Miner Res. 2020, 35(2), 4018. [CrossRef]
- Foolen, J. , Wunderli, S. L., Loerakker, S., Snedeker, J.G. Tissue alignment enhances remodeling potential of tendon-derived cells - Lessons from a novel microtissue model of tendon scarring. Matrix Biol. 2018, 65, 14–29. [Google Scholar] [CrossRef]
- Wang, N. , Tytell, J.D., Ingber, D.E. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009, 10(1), 75–82. [CrossRef]
- Lavagnino, M. , Arnoczky, S. P. In vitro alterations in cytoskeletal tensional homeostasis control gene expression in tendon cells. J Orthop Res. 2005, 23(5), 1211–1218. [Google Scholar] [CrossRef]
- Lavagnino, M. , Gardner, K.L., Arnoczky, S.P. High magnitude, in vitro, biaxial, cyclic tensile strain induces actin depolymerization in tendon cells. Muscles Ligaments Tendons J. 2015, 5(2), 124–128. [CrossRef]
- Lavagnino, M. , Brooks, A.E., Oslapas, A.N., Gardner, K.L., Arnoczky, S.P. Crimp length decreases in lax tendons due to cytoskeletal tension, but is restored with tensional homeostasis. J Orthop Res. 2017, 35(3), 573–579. [CrossRef]
- Lavagnino, M., Gardner, K., Arnoczky, S.P. Age-related changes in the cellular, mechanical, and contractile properties of rat tail tendons. Connect Tissue Res. 2013, 54(1), 70–75. [CrossRef] [PubMed]
- Lavagnino, M. , Bedi, A., Walsh, C.P., Sibilsky Enselman, E.R., Sheibani-Rad, S., Arnoczky, S.P. Tendon contraction after cyclic elongation is an age-dependent phenomenon: in vitro and in vivo comparisons. Am J Sports Med. 2014, 42(6), 1471–1477. [CrossRef]
- Arnoczky, S.P. , Lavagnino, M., Whallon, J.H., Hoonjan, A. In situ cell nucleus deformation in tendons under tensile load; a morphological analysis using confocal laser microscopy. J Orthop Res. 2002, 20(1), 29–35. [CrossRef]
- Henderson, J.T., Shannon, G., Veress, A.I., Neu, C.P. Direct measurement of intranuclear strain distributions and RNA synthesis in single cells embedded within native tissue. Biophys J. 2013, 105(10), 2252–2261. [CrossRef]
| Inflammation phase | |
| Injury/ incident | Immediate vasoconstriction of the blood flow. Immobilization to reduce pain and swelling |
| 24–48 h post |
Vasodilatation and proliferation of tissue. Inflammation. Icing is not recommended as it slows down healing by decreasing lymphatic flow, proliferation, and cell– cell-interactions. Same rules apply for anti-inflammatory drugs. Ice has numbing effects and should only be used for a few minutes for pain relief |
| Proliferation phase | |
| 5 days |
Type III collagen is produced and will be transferred to type I over time. Reconstruction and orientation of the type III fibers depend on stress of movement and weight bearing. That is why exercises in full range of motion allowed and weight bearing are so important to guarantee good healing of tissue and scars |
| Remodeling/ maturation | |
| ~3 weeks |
Type III collagen is transferred into type I. regaining range of motion, proprioceptive and contractile information to allow good healing. Regaining biomechanical qualities of the tissue. Formation of cross-links for greater stiffness. This process is supported by load and mobilization into the end of ROM in exercises. 300–500 days until tissue regains its former function |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





