1. Introduction
Innate immunity is the first line of defense against pathogens and enables the control of lentivirus infections in animal species, such as those in primates, cats, and small ruminants [
1]. Equine infectious anemia virus (EIAV), a member of the genus Lentivirus, family Retroviridae, induces an acute, chronic or inapparent clinical disease in horses, while the latter clinical form is predominant in donkeys and mules [
2]. The modest blood viral titers observed in animals in which infection takes an asymptomatic course (non-progressors), rule out any correlation between high antibody titers induced by the high viral loads, that is associated with acute phases, and subsequent infection control [
3]. As reported in literature, following recognition of the pathogen, the transduction signals of immune cells that characterize innate immunity (macrophages and/or dendritic cells) induce a series of events that can counteract infection, inhibiting in some cases the viral replication cycle [
1]. However, these mechanisms of viral control in EIAV infected non-progressors are not yet widely described.
The purpose of this study was to investigate the macrophage polarization and to assess the expression of genes related to the horse innate immune system following stimulation with cytokines and in vitro infection with EIAV.
2. Materials and Methods
Monocyte/macrophage isolation was from peripheral blood, collected at the slaughterhouse from one 7-year-old pony male horse and two Arabian female horses, one 9-year old and one 10-year old. All the animals were resulted seronegative for EIAV by ELISA [
4], AGID (210421_EIA_AGID_SOP_JCv2) [
5] and Immunoblotting [
6] serological techniques in use at the National Reference Center for Equine Infectious Anemia and WOAH Laboratory Reference, IZSLT, Rome, Italy.
2.1. PBMC Separation from Equine Whole Blood
The protocol developed required the collection of at least 12 ml of EDTA blood. After a clarification at 115g for four minutes, leucocytes were collected along with plasma and transferred in a new tube and centrifuged at 1500g for 15 minutes.
Plasma was discarded and the pellet was resuspended in four ml of PBS-0,004mM EDTA. This suspension was carefully layered onto four ml of Histopaque® (Sigma Aldrich, Germany). After a centrifugation at 400g for 30 minutes, the supernatant was discarded and peripheral blood monocyte cells (PBMC) were collected and resuspended in 10 ml of PBS-EDTA. The suspension then underwent two cycles of centrifugation at 800g for 10 minutes and after the second cycle the pellet was resuspended in 10 ml of cell culture medium (T-I: RPMI medium added with 10 mM Sodium Pyruvate, 1X antibiotic/antimycotic solution, 0.1X gentamicin, 1% glutamine, 50 µM 2-mercaptoethanol).
2.2. Equine Monocyte Derived Macrophages (eMDMs) Isolation and Stimulation
A protocol for isolation and stimulation of eMDMd with equine cytokines or with EIAV was adapted from Raabe et al, 1998 [
7] and Ma et al, 2014 [
8].
At each of the three different blood collections, about 12 ml of EDTA-blood samples were obtained. After separation, PBMCs were counted using a hematological analyzer to adjust their concentration to obtain 7.5 x 105 cells/ml and were thereafter dispensed at a volume of 9,6 ml of a 12 well cell culture microplate and left to adhere on the well surface. Following 18-20 h of incubation at 37 °C and at 5% CO2, non-adherent and loosely adherent cells were removed by washing quickly with cold PBS for three times and fresh complete medium T-II was added (T-I supplemented with 10% heat-inactivated horse serum). On the 3rd day, most of the adherent cells differentiated into macrophages and were either stimulated with equine recombinant cytokines IFNγ (Abcam) or IL4 (Abcam) at 50 ng/ml in T-II medium, or infected [Menarim, 2020] with Miami (IBVR VIR RE RSCIC 57) or Wyoming (IBVR VIR RE RSCIC 110) reference strains of EIAV at a multiplicity of infection (MOI) = 0,1. The cytokine treatment lasted for seven days, whereupon stimulated eMDMs were inoculated with the two EIAV reference strains on day 10th. The supernatant of the different treated eMDM cultures was collected weekly for one month.
After cytokine treatment, eMDM were collected to evaluate the mRNA expression level of the pro-inflammatory genes matrix metalloproteinase 13 (MMP13) and IL6. The same collection and study analysis were performed on untreated eMDMs in two wells each infected with one of two reference viral strains at 7 and 10 days post-infection (PID).
2.3. Molecular Analyses
Molecular analyses were conducted on three blood and 29 supernatants samples, collected weekly from the three eMDM cultures stimulated with cytokines and infected with EIAV. The three blood samples were examined for the EIAV by Real Time tat PCR [
9] and Nested LTR-tat PCR [
10], both from DNA and RNA templates.
Varicellovirus equidalpha 1 and 4 (EqHV1/4) [
11],
Percavirus equidgamma 2 and 5 (EqHV-2 and EqHV-5) [
12] were also targeted. In addition, the supernatants of eMDM cultures were examined for the presence of EHV-2 and other equine
Herpesvirus which initially integrated into the cellular genome, can begin to replicate while the macrophages are in culture. In the latter case, a further Panherpesvirus PCR [
13] was used to verify the presence of an infection due to other Herpesviruses.
The DNA and RNA nucleic acid extractions were both carried out using the QIAsymphony (Qiagen) with the QIAamp® Viral RNA Mini kit (Qiagen). To improve the sensitivity for the detection of the EIAV from an RNA template, cDNA synthesis was carried out using 20 µl of RNA with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems,Thermo Fisher Scientific), following the manu-facturer’s instructions, using the Gene AmpR PCR System 9700 (Applied Biosystems, Thermo Fisher Scientific,Waltham) with a thermal cycling profile of 10 min at 25 °C and 45 min at 37 °C.
A SYBR® Green real-time PCR was used to evaluate the mRNA expression level of the pro-inflammatory genes MMP13 and IL6 in eMDMs treated with IFNγ, IL4 or infected with EIAV reference strains [
14].
The nucleic acid extractions (both DNA and RNA) were carried out using the QIAsymphony (Qiagen) with the QIAamp® Viral RNA Mini kit (Qiagen).
Three separate in-house SybrGreen PCRs were performed using three pairs of primers eMMP13, eIL6, e18S, the Hot Fire Pol Multiplex qPCR Mix (ROX) (Solis Biodyne) and the fluorescent dye Eva Green (Biotium). The amplification thermal profile used was: 95 °C for 10 minutes, 40 cycles at 95 °C for 15 seconds, 60 °C for 60 seconds. For the HRM analysis, the thermal profile used was the following: 95 °C for 15 seconds and continuous heating from 60 °C to 95 °C with a ramp of 0.05 °C/s. At the end of the PCR runs, the amplicons and melting curves obtained for each of the markers were compared to those produced by the samples.
Four replicates of the following six eMDM samples were analyzed: stimulated for seven days with IFNγ or IL4, seven and 10 days after infection with the EIAV Wyoming strain seven and 10 days after infection with the EIAV Miami strain [
14].
3. Results
3.1.1. Morphology of eMDM Following Isolation and Stimulation
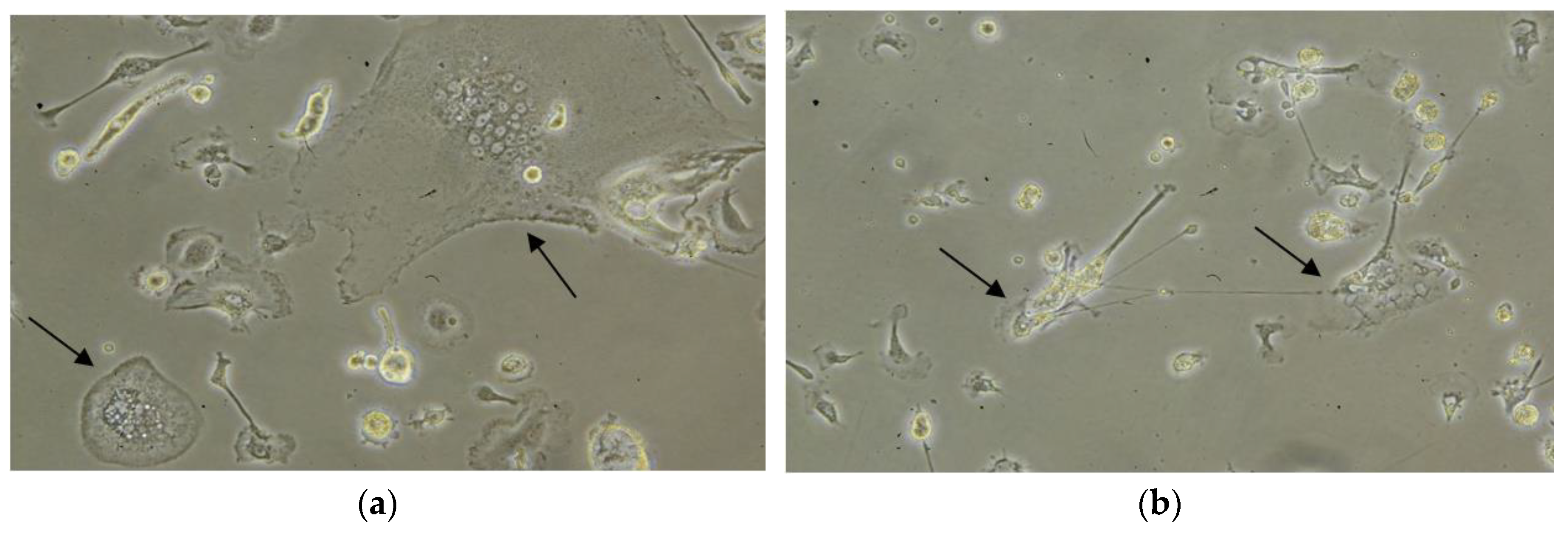
eMDMs were isolated from blood collected from three serologically EIAV negative horses. After four days from the treatment/infection, the shape and size of the macrophages started to change. Based on the morphology, stimulation with IFNγ polarized the macrophages towards an M1 (macrophage with inflammatory profile) morphology of differentiation similar to a more or less irregularly shaped fried egg, in contrast to stimulation with IL4 that induced M2 (macrophage with homeostatic functions) type shaped cells characterized by the emission of long pseudopodia, similar to fibroblastic cells (
Figure 1).
The majority of the macrophages infected with both EIAV strains showed an M1-like morphology. Interestingly, M1 eMDMs, infected with the EIAV, died before M2 eMDMs.
3.1.2. Molecular Analyses
EIAV genome was not detected in the three equine whole blood samples by Real Time tat PCR and by Nested LTR-tat PCR when using both DNA and RNA templates. Furthermore, they tested negative by EHV-1, EHV-2, EHV-4 and EHV-5 in the panherpesvirus PCR as also the 29 supernatant samples, weekly collected after stimulation with cytokines, which tested negative for EHV-2 and in the panherpesvirus PCRs.
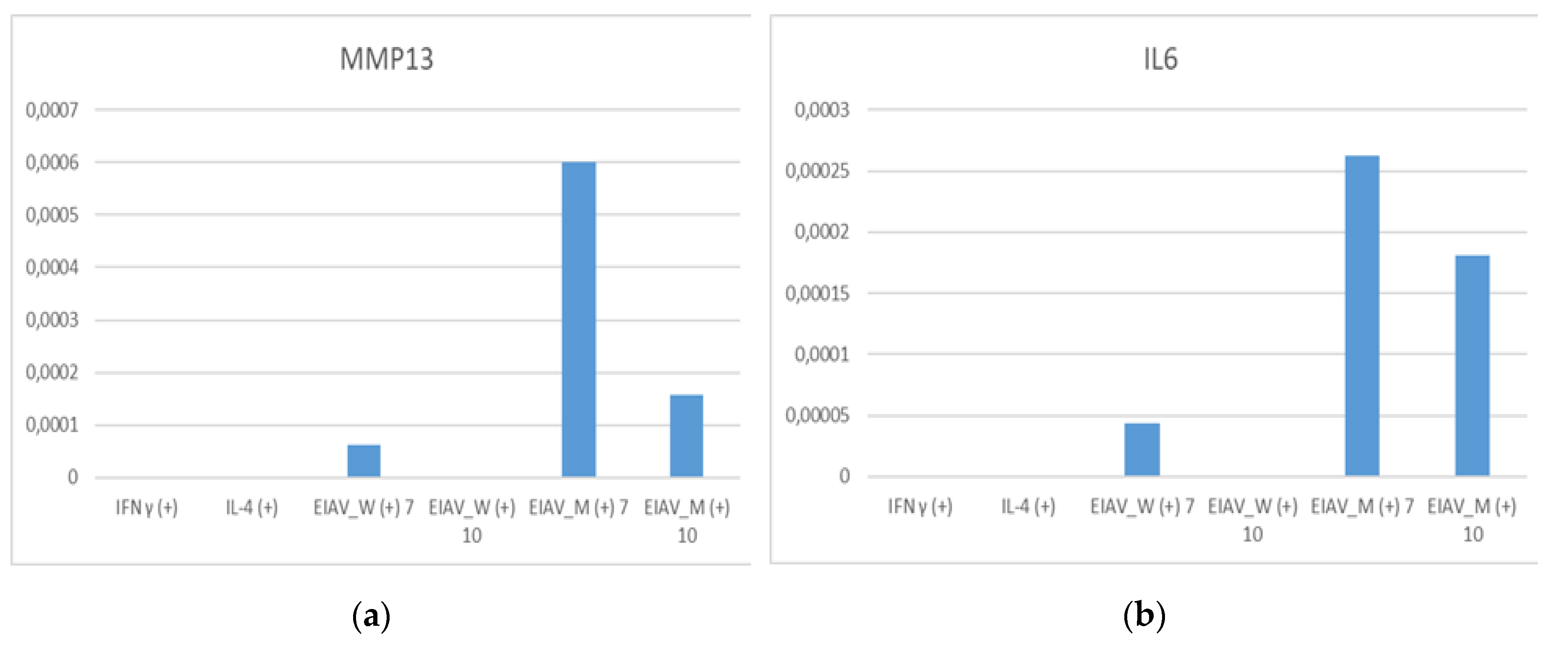
mRNA expression level of the pro-inflammatory genes MMP13 and IL6 in eMDMs treated with IFN-γ, IL4 were generally low in cytokine stimulated eMDMs while higher expression levels were observed in macrophages infected with EIAV reference strains (
Figure 2).
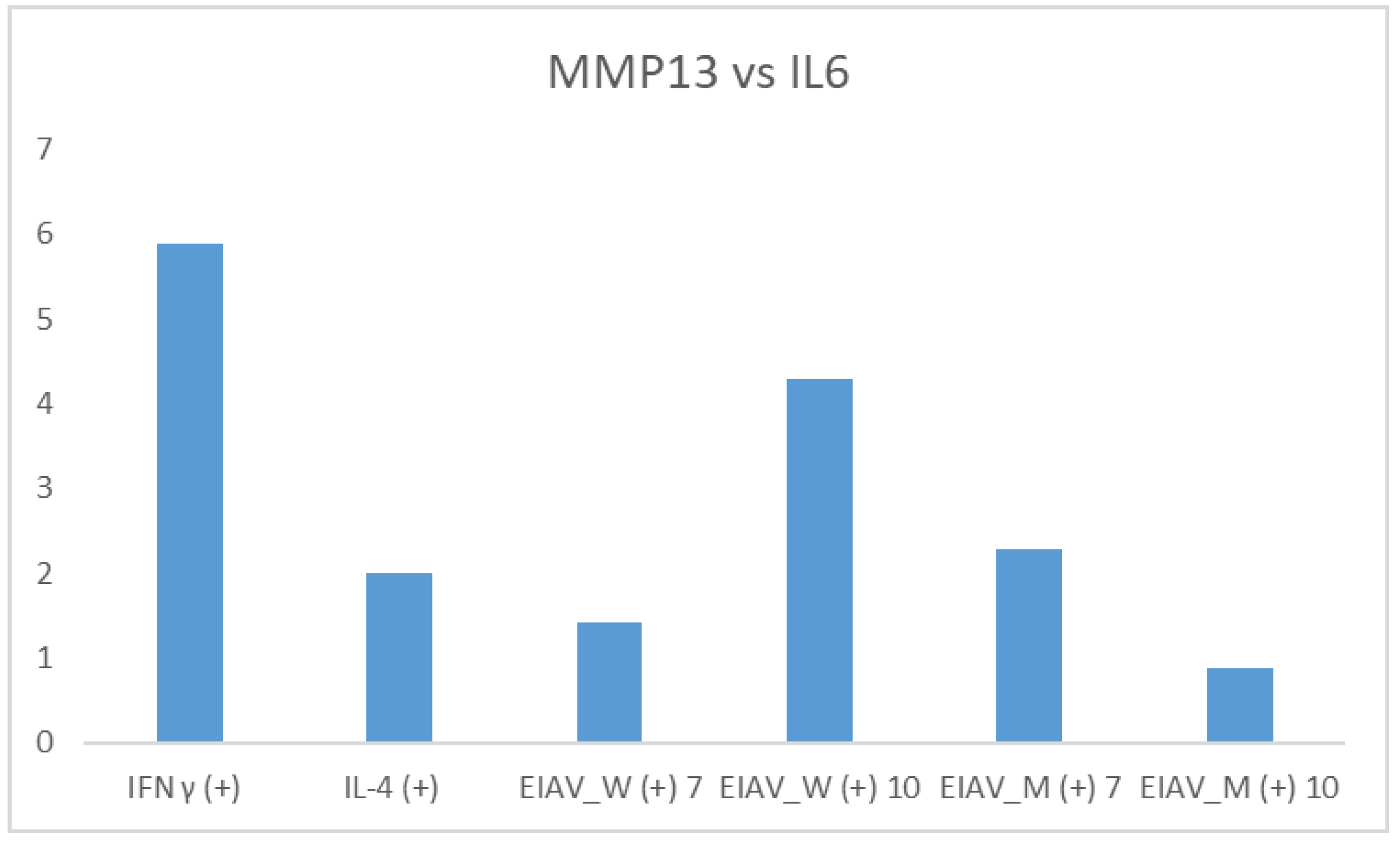
Comparing the relative expression of pro-inflammatory markers, MMP13 and IL6, the levels of the former were up to a maximum of six times higher than the levels of IL6 (
Figure 3).
In particular, MMP13 was over expressed when compared to IL6 in the following cases: stimulation with IFNγ (6 times), 10 days after infection with the EIAV Wyoming strain (four times), seven days after infection with the Miami strain two and a half times), and seven days after infection with the EIAV Wyoming (one and a half times). On the contrary, after 10 days from the infection with the Miami strain, the two markers were expressed in an equivalent manner.
4. Discussion
According to the results of this preliminary study, horse monocytes from peripheral blood can be differentiated in vitro into macrophages, that generally gives rise to mixed populations, morphologically referable to M1 and M2. The addition of specific cytokines or a viral infection results in a more specific differentiation. As stated for other species [
1], stimulation with equine recombinant cytokine IFNγ polarized horse macrophages towards the M1 phenotype while equine recombinant cytokine IL4 polarized towards the M2 phenotype. Viral infection (EIAV Wyoming strain and Miami strain) resulted in morphological transformation of macrophages compatible with the M1 pattern of differentiation.
Furthermore, in this study MMP13 represented a reliable target gene to evaluate proinflammatory status of macrophages in horses since IFNγ and EIAV infection considerably increased its expression. In fact, MMP13 is related to aspecific inflammation [
15] but is reported also as being involved in infectious diseases such as bovine respiratory disease [
16].
A more in-depth study of the expression genes of both cytokine-induced and virus-induced markers of eMDM polarization may help us to understand whether these are the same in the horse as those found in other animal species, such as pigs, sheep, goats [
1], men [
17] with similar modes of activation of innate immunity. The identification of the markers of each population of macrophages would allow us to analyze the differentiation profiles, and to check the control of virus infectivity in both populations envisioning therapeutic strategies through the use of this information.
Author Contributions
Conceptualization, G.C. and M.T.S.; methodology, G.C., G.M., A.C., R.N., S.R. and R.R.; investigation, G.C., G.M., A.C., R.N., R.R., M.C., S.S., A.A. and G.A.M.; data curation, G.C., G.M., A.C., R.N., S.R., R.R.; writing—original draft preparation, G.C., G.M., A.C., R.N., S.R., R.R., M.C., S.S., A.A. and G.A.M.; writing—review and editing, G.M., A.C., R.N., S.R., R.R. and M.T.S.; major contribution in writing the manuscript, G.C.; supervision, M.T.S.; project administration, M.T.S.; funding acquisition, M.T.S. All authors read and approved the final manuscript.
Funding
This research was funded by Italian Ministry of Health, grant number IZSLT 09/17 RC and The APC was funded by Istituto Zooprofilattico Sperimentale del Lazio e della Toscana “M.Aleandri”.
Institutional Review Board Statement
Ethical review and approval for this study were not taken into consideration because the samples were taken from animals at the slaughterhouse, already checked for equine infectious anemia in our laboratories.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We acknowledge Olga Lai and Gabriella Loffredo (Production Hygiene and Animal Health Department, Istituto Zooprofilattico Sperimentale del Lazio e della Toscana “M.Aleandri”, Italy) for kindly contributing in blood analyses (leukocyte and monocyte counts).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Crespo, H.; Bertolotti, L.; Juganaru, M.; Glaria, I.; de Andrés, D.; Amorena, B.; Rosati, S.; Reina, R. Small Ruminant Macrophage Polarization May Play a Pivotal Role on Lentiviral Infection. Vet Res 2013, 44, 83. [Google Scholar] [CrossRef] [PubMed]
- Câmara, R.J.F.; Bueno, B.L.; Resende, C.F.; Balasuriya, U.B.R.; Sakamoto, S.M.; Reis, J.K.P.D. Viral Diseases that Affect Donkeys and Mules. Animals (Basel) 2020, 10, 2203. [Google Scholar] [CrossRef] [PubMed]
- Leroux, C.; Cadore’, J.; Montelaro, R.C. Equine Infectious Anemia Virus (EIAV): What Has HIV’s Country Cousin Got to Tell us? Vet Res 2004, 35, 485–512. [Google Scholar] [CrossRef] [PubMed]
- Nardini, N.; Autorino, G.L.; Ricci, I.; Frontoso, R.; Rosone, F.; Simula, M.; Scicluna, M.T. Validation According to OIE Criteria of a Monoclonal, Recombinant p26-based, Serologic Competitive Enzyme-linked Immunosorbent Assay as Screening Method in Surveillance Programs for the Detection of Equine Infectious Anemia Antibodies. Journal of Veterinary Diagnostic Investigation 2016, 28, 88–97. [Google Scholar] [CrossRef] [PubMed]
- EURL European Union Reference Laboratory for Equine Diseases ANSES. Available online: https://sitesv2.anses.fr/en/minisite/equine-diseases/sop (accessed on 1 May 2024).
- Scicluna, M.T.; Autorino, G.L.; Cook, S.J.; Issel, C.J.; Cook, R.F.; Nardini, R. Validation of an Immunoblot Assay Employing an Objective Reading System and Used as a Confirmatory Test in Equine Infectious Anaemia Surveillance Programs. J Virol Methods 2019, 266, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Raabe, M.R. , Issel, C.J.; Montelaro, R.C. Equine Monocyte-derived Macrophage Cultures and their Applications for Infectivity and Neutralization Studies of Equine Infectious Anemia Virus. Journal of Virological Methods 1998, 71, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, S.S.; Lin, Y.Z.; Liu, H.F.; Liu, Q.; Wei, H.M.; Wang, X.F.; Wang, Y.H.; Du, C.; Kong, X.G.; Zhou, J.H.; Wang, X. Infection of Equine Monocyte-Derived Macrophages with an Attenuated Equine Infectious Anemia Virus (EIAV) Strain Induces a Strong Resistance to the Infection by a Virulent EIAV Strain. Veterinary Research 2014, 45, 82. [Google Scholar] [CrossRef] [PubMed]
- Scicluna, M.T.; Issel, C.J.; Cook, F.R.; Manna, G.; Cersini, A.; Rosone, F.; Frontoso, R.; Caprioli, A.; Antognetti, V.; Autorino, G.L. Is a Diagnostic System Based Exclusively on Agar Gel Immunodiffusion Adequate for Controlling the Spread of Equine Infectious Anaemia? Veterinary Microbiology 2013, 165, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.B.; Zhu, W.; Cook, F.R.; Goto, Y.; Horii, Y.; Haga, T. Development of a Nested PCR Assay to Detect Equine Infectious Anemia Proviral DNA from Peripheral Blood of Naturally Infected Horses. Arch Virol 2012, 157, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Damiani, A.; Ciabatti, I.; Lorenzetti, R.; Scicluna, M.T.; Autorino, G.L. Development of Equine Herpesvirus Type 1 and Type 4. Proceedings of 45th National Congress of the Italian Society of Virology (SIV), Orvieto (TR), Italy, 19th - 21th September 2005, pp: 115 – 116.
- Hue, H.S.; Fortier, G.D.; Fortier, C.I.; Leon, A.M.; Richard, E.A.; Legrand, L.J.; Pronost, S.L. Detection and Quantitation of Equid Gammaherpesviruses (EHV-2, EHV-5) in Nasal Swab Using an Accredited Standardised Quantitative PCR Method. Journal of Virological Methods 2014, 198, 18–25. [Google Scholar] [CrossRef] [PubMed]
- VanDevander, D.R.; Warrener, P.; Bennet, L.; Schultz, E.R.; Coulter, S.; Garber, R.L.; Rose, T.M. Detection and Analysis of Diverse Herpesviral Species by Consensus Primer PCR. Journal of Clinical Microbiology 1996, 34, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Menarim, B.C.; Gillis, K.H.; Oliver, A.; Ngo, Y.; Were, S.R.; Barrett, S.H.; Rodgerson, D.H.; Dahlgren, L.A. Macrophage Activation in the Synovium of Healthy and Osteoarthritic Equine Joints. Front Vet Sci 2020, 7, 568756. [Google Scholar] [CrossRef] [PubMed]
- Lange-Consiglio, A.; Perrini, C.; Tasquier, R.; Deregibus, M.C.; Camussi, G.; Pascucci, L.; Marini, M.G.; Corradetti, B.; Bizzaro, D.; De Vita, B.; Romele, P.; Parolini, O.; Cremonesi, F. Equine Amniotic Microvesicles and their Anti-Inflammatory Potential in a Tenocyte Model in Vitro. Stem Cells Dev 2016, 25, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Lakritz, J.; Marsh, A.E.; Cockrell, M.; Smith, M.F. : Tyler, J.W. Characterization of Gelatinases in Bronchoalveolar Lavage Fluid and Gelatinases Produced by Alveolar Macrophages Isolated from Healthy Calves. Am J Vet Res 2004, 65, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional Profiling of the Human Monocyte-to-Macrophage Differentiation and Polarization: New Molecules and Patterns of Gene Expression. J Immunol 2006, 177, 7303–7311. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).